Abstract
1. Starch-gel electrophoretograms of myosin and tropomyosin preparations in 8m-urea, from longissimus dorsi and psoas muscles of the pig, were characterized by laser densitometry. 2. The typical pattern for freshly prepared myosin from both muscles was similar, there being five electrophoretically distinct components. 3. The number of electrophoretically distinct components in both muscles increased after freeze-drying, but the effect of freeze-drying was more marked in longissimus dorsi. 4. Extraction with 8m-urea containing 2% β-mercaptoethanol decreased the number of major electrophoretically distinct components of the fresh myosin of both muscles to four. 5. Although there was also some simplification of the patterns after freeze-drying the greater susceptibility of the myosin from longissimus dorsi was still evident. 6. The typical pattern for freshly prepared tropomyosin in 8m-urea differed in the two muscles: in each case it was more complex than that of the corresponding myosins. 7. The pattern of tropomyosin from neither longissimus dorsi nor psoas was altered significantly after freeze-drying. 8. Electrophoretograms of pig longissimus dorsi tropomyosin in 8m-urea differed from those of longissimus dorsi tropomyosin from sheep, ox and rabbit. 9. Extraction of the tropomyosins in 8m-urea and 2% β-mercaptoethanol simplified the electrophoretic pattern to two major components with samples from pig, sheep and ox, and to one major component with samples from rabbit. 10. It was concluded that classification of skeletal muscles as `red' or `white' is insufficient to account for the degree of functional specialization which the electrophoretograms suggest.
Full text
PDF

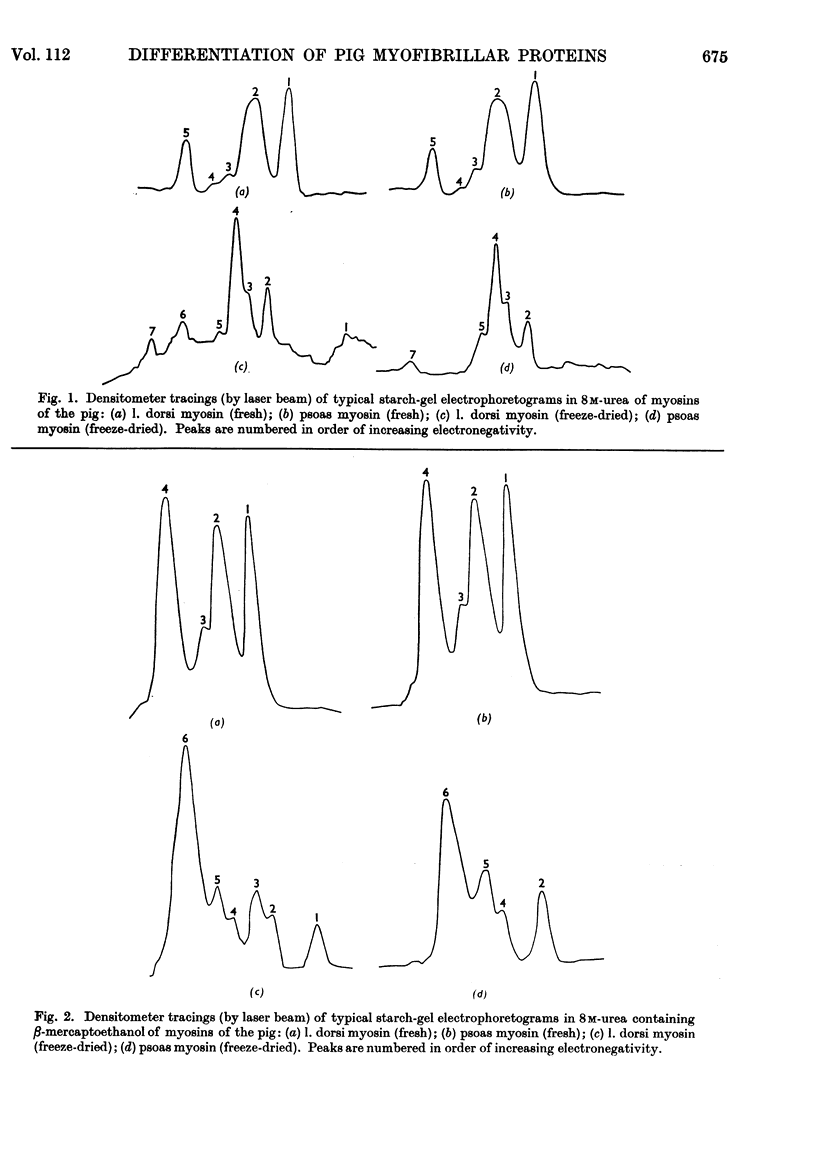

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
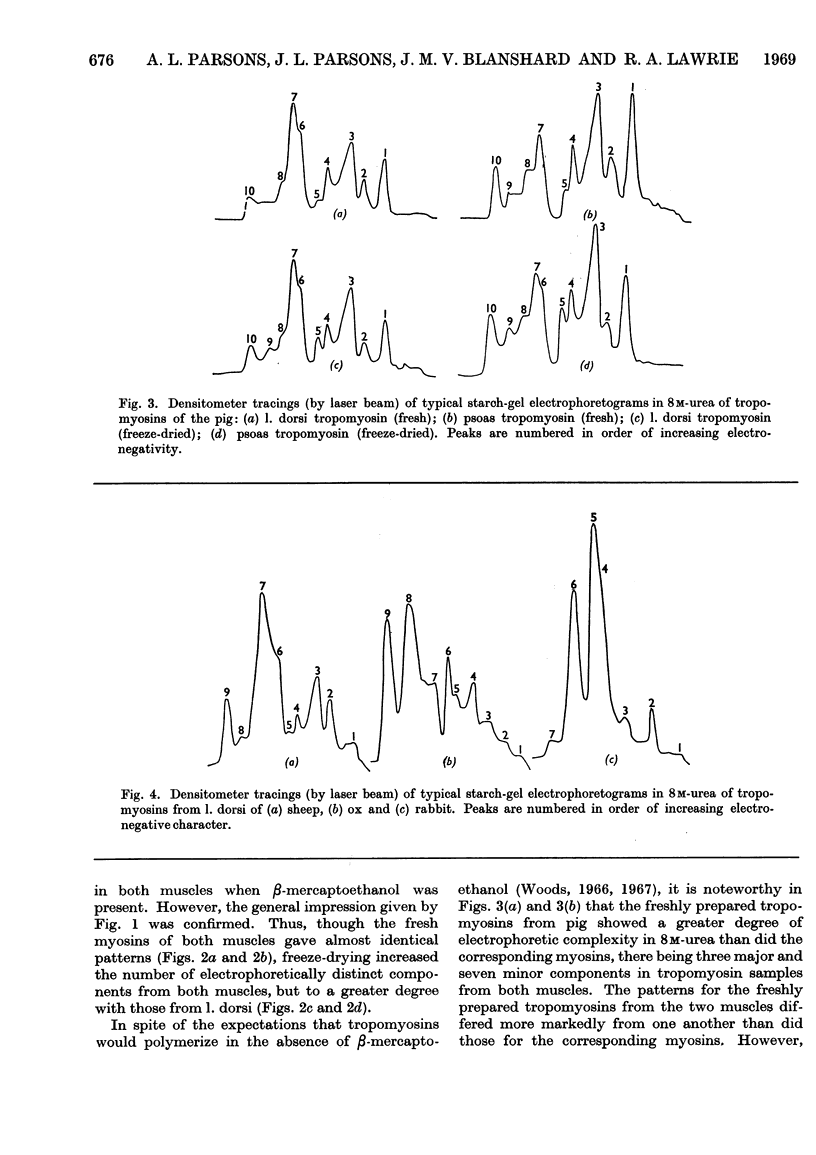
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNELL J. J. The relative stabilities of the skeletal-muscle myosins of some animals. Biochem J. 1961 Sep;80:503–509. doi: 10.1042/bj0800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMOIR G., LASZT L. [Tropomyosin B of bovine carotids]. Biochim Biophys Acta. 1962 May 21;59:365–375. doi: 10.1016/0006-3002(62)90186-5. [DOI] [PubMed] [Google Scholar]
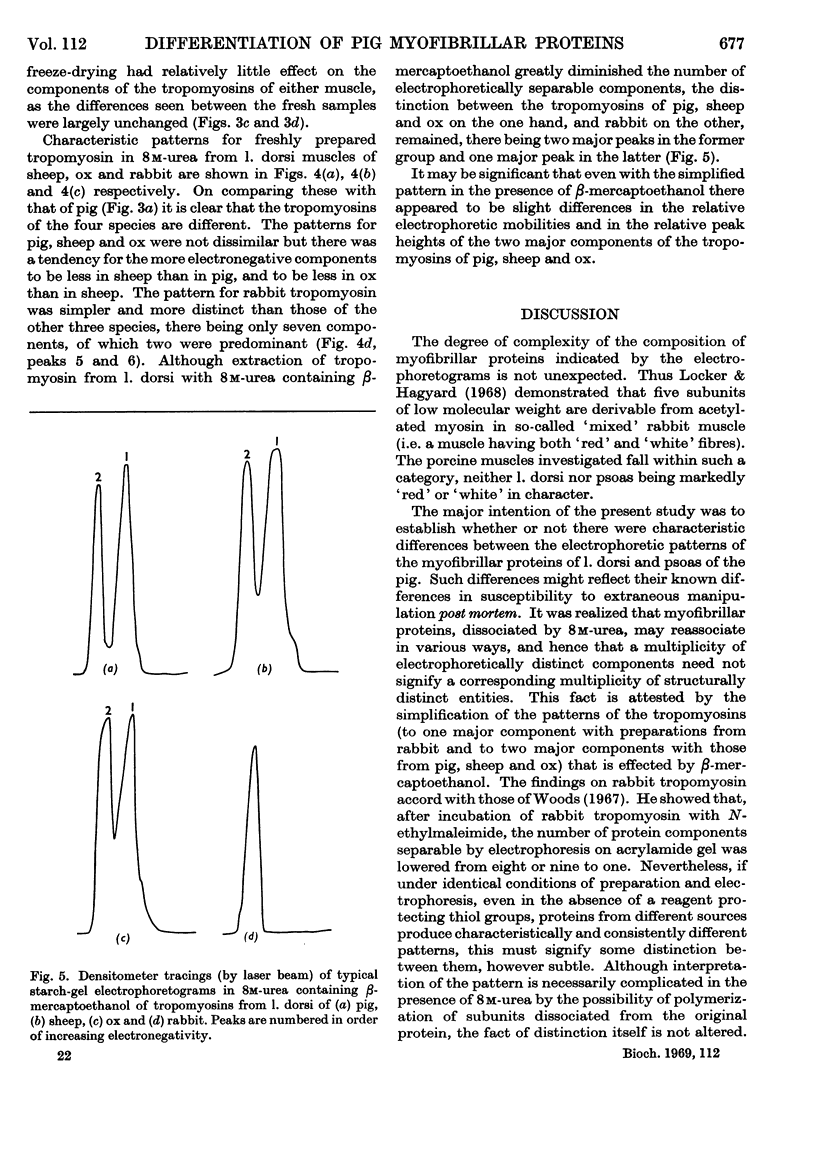
- Hartshorne D. J., Mueller H. Fractionation of troponin into two distinct proteins. Biochem Biophys Res Commun. 1968 Jun 10;31(5):647–653. doi: 10.1016/0006-291x(68)90610-4. [DOI] [PubMed] [Google Scholar]
- LAWRIE R. A. The activity of the cytochrome system in muscle and its relation to myoglobin. Biochem J. 1953 Sep;55(2):298–305. doi: 10.1042/bj0550298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWRIE R. A. The onset of rigor mortis in various muscles of the draught horse. J Physiol. 1953 Aug;121(2):275–288. doi: 10.1113/jphysiol.1953.sp004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker R. H., Hagyard C. J. The myosin of rabbit red muscles. Arch Biochem Biophys. 1968 Sep 20;127(1):370–375. doi: 10.1016/0003-9861(68)90238-5. [DOI] [PubMed] [Google Scholar]
- MUELLER H., PERRY S. V. The degradation of heavy meromyosin by trypsin. Biochem J. 1962 Dec;85:431–439. doi: 10.1042/bj0850431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITHIES O. An improved procedure for starch-gel electrophoresis: further variations in the serum proteins of normal individuals. Biochem J. 1959 Mar;71(3):585–587. doi: 10.1042/bj0710585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopes R. K. Methods for starch-gel electrophoresis of sarcoplasmic proteins. An investigation of the relative mobilities of the glycolytic enzymes from the muscles of a variety of species. Biochem J. 1968 Mar;107(2):139–150. doi: 10.1042/bj1070139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopes R. K. The influence of post-mortem conditions on the solubilities of muscle proteins. Biochem J. 1964 Apr;91(1):201–207. doi: 10.1042/bj0910201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreter F. A., Seidel J. C., Gergely J. Studies on myosin from red and white skeletal muscles of the rabbit. I. Adenosine triphosphatase activity. J Biol Chem. 1966 Dec 25;241(24):5772–5776. [PubMed] [Google Scholar]
- Woods E. F. Molecular weight and subunit structure of tropomyosin B. J Biol Chem. 1967 Jun 25;242(12):2859–2871. [PubMed] [Google Scholar]
- Woods E. F. The dissociation of tropomyosin by urea. J Mol Biol. 1966 Apr;16(2):581–584. doi: 10.1016/s0022-2836(66)80199-7. [DOI] [PubMed] [Google Scholar]


