Abstract
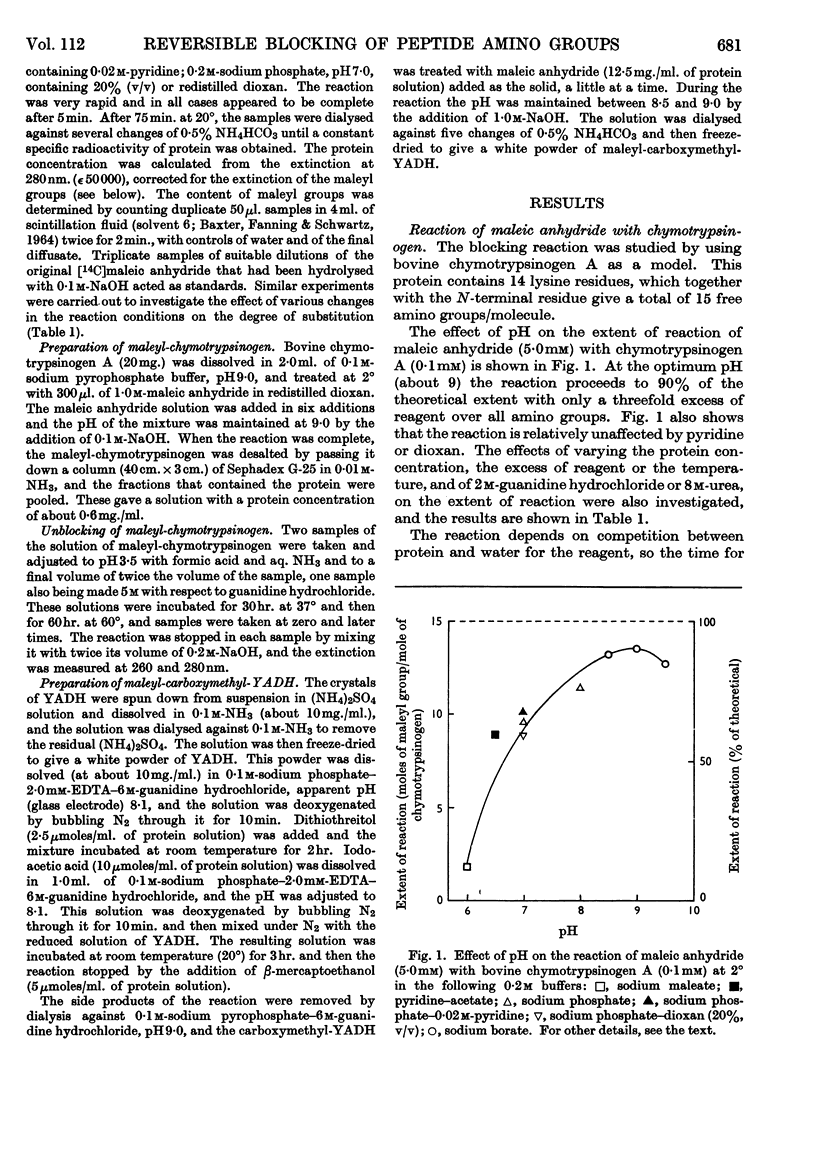
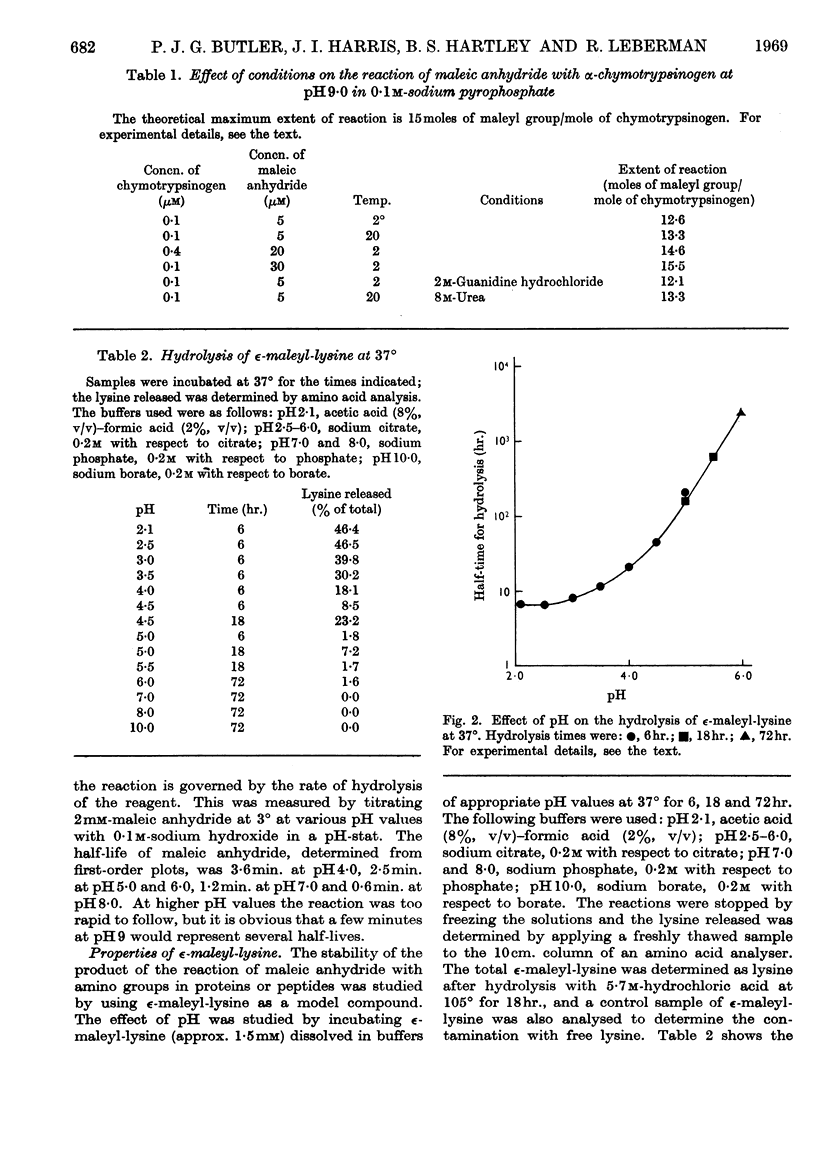
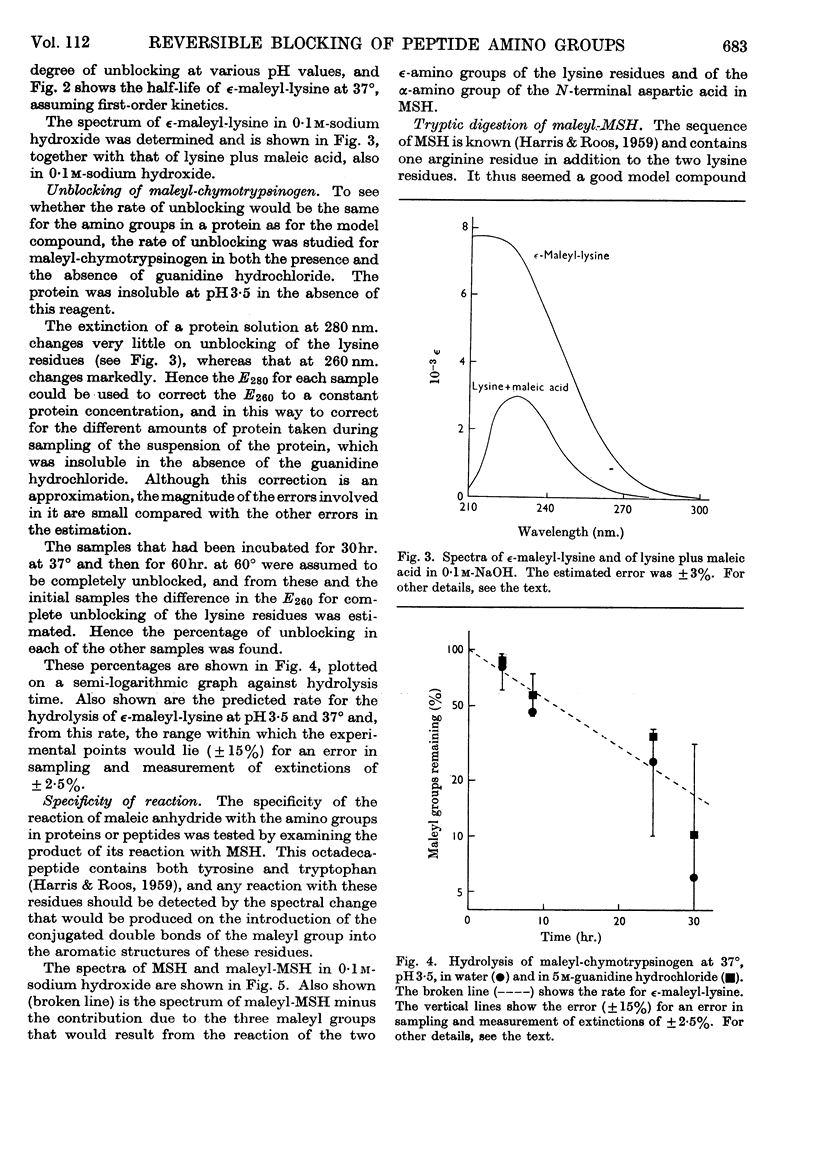
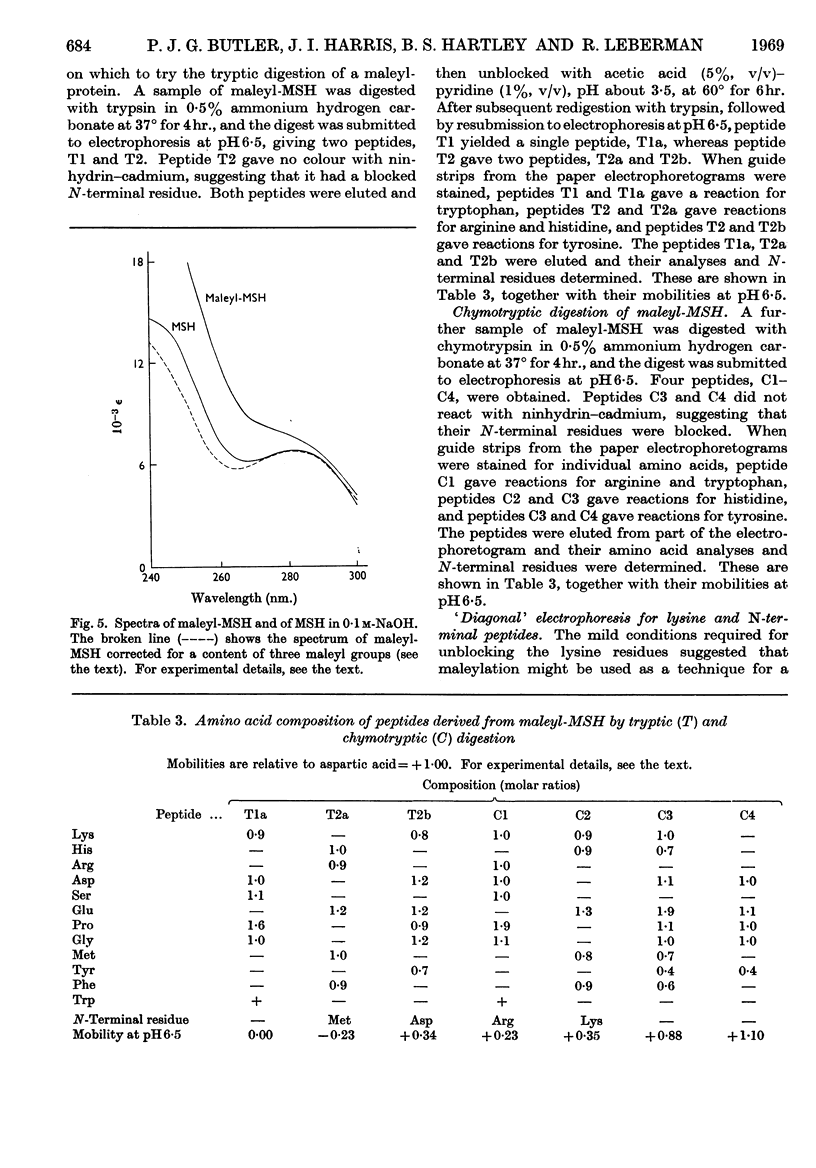
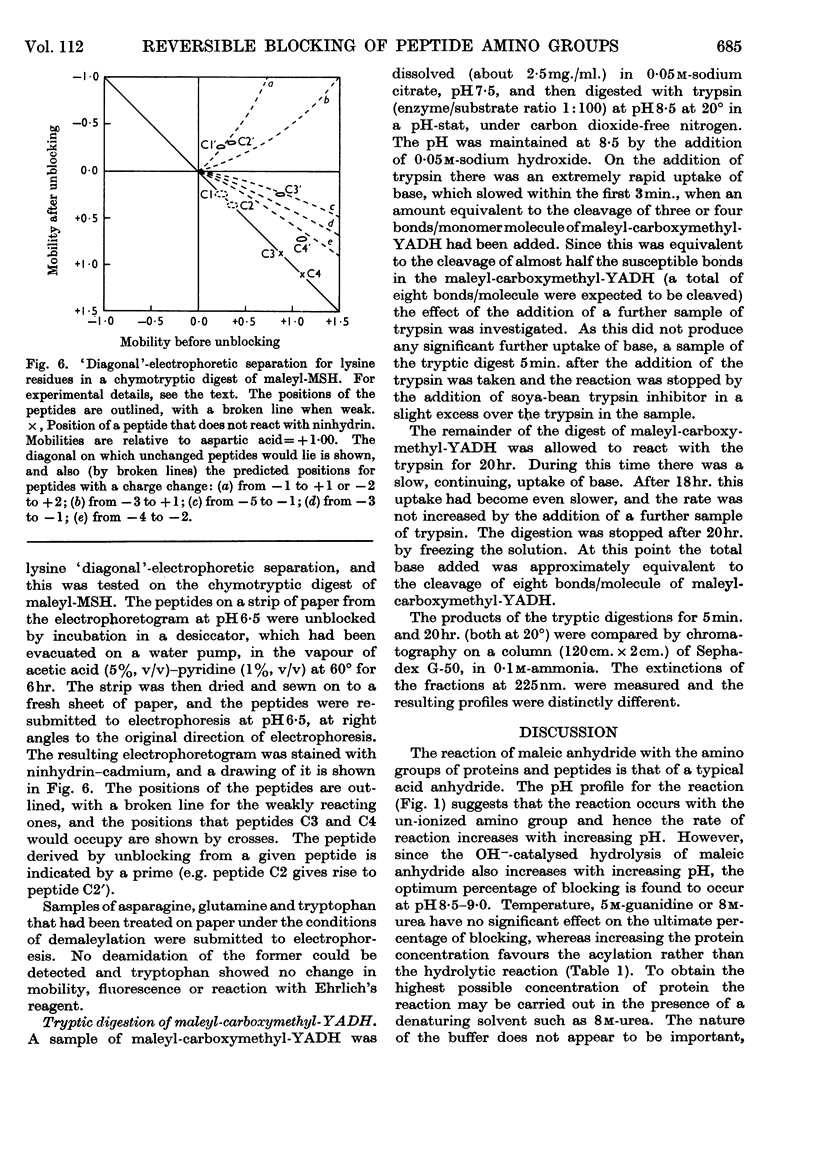
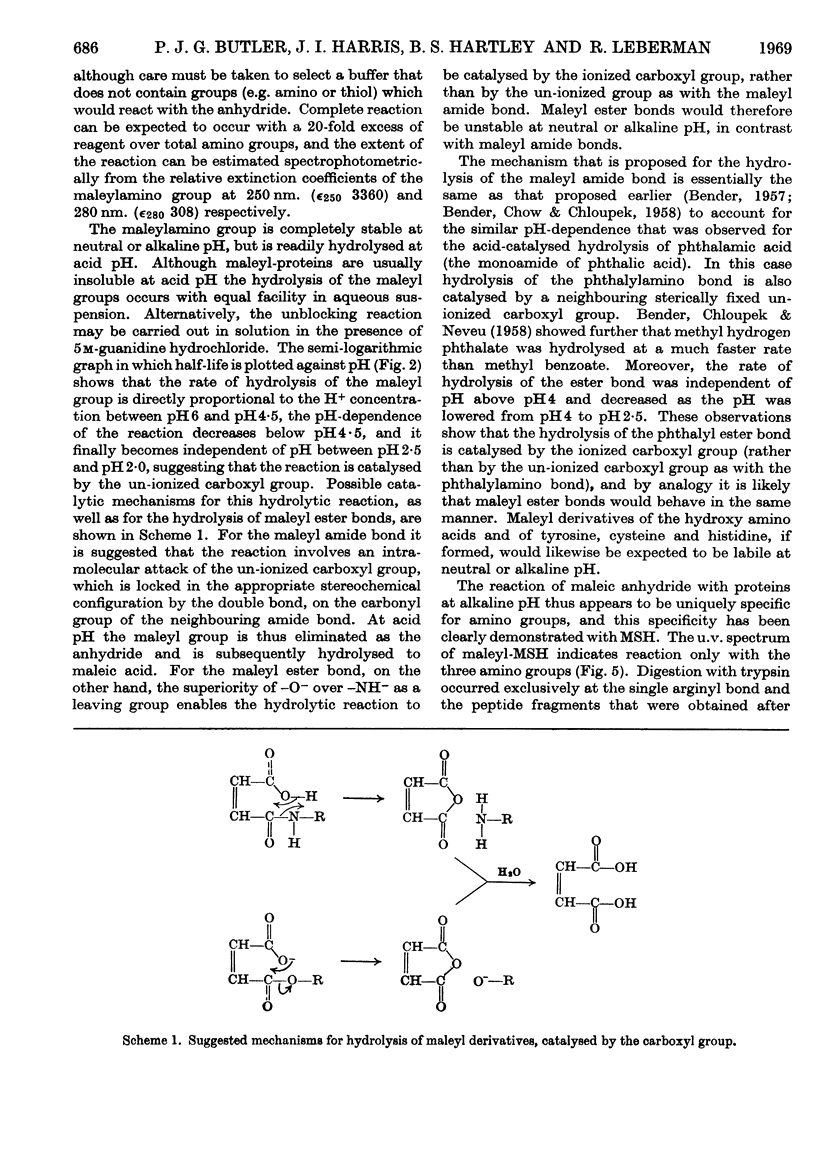
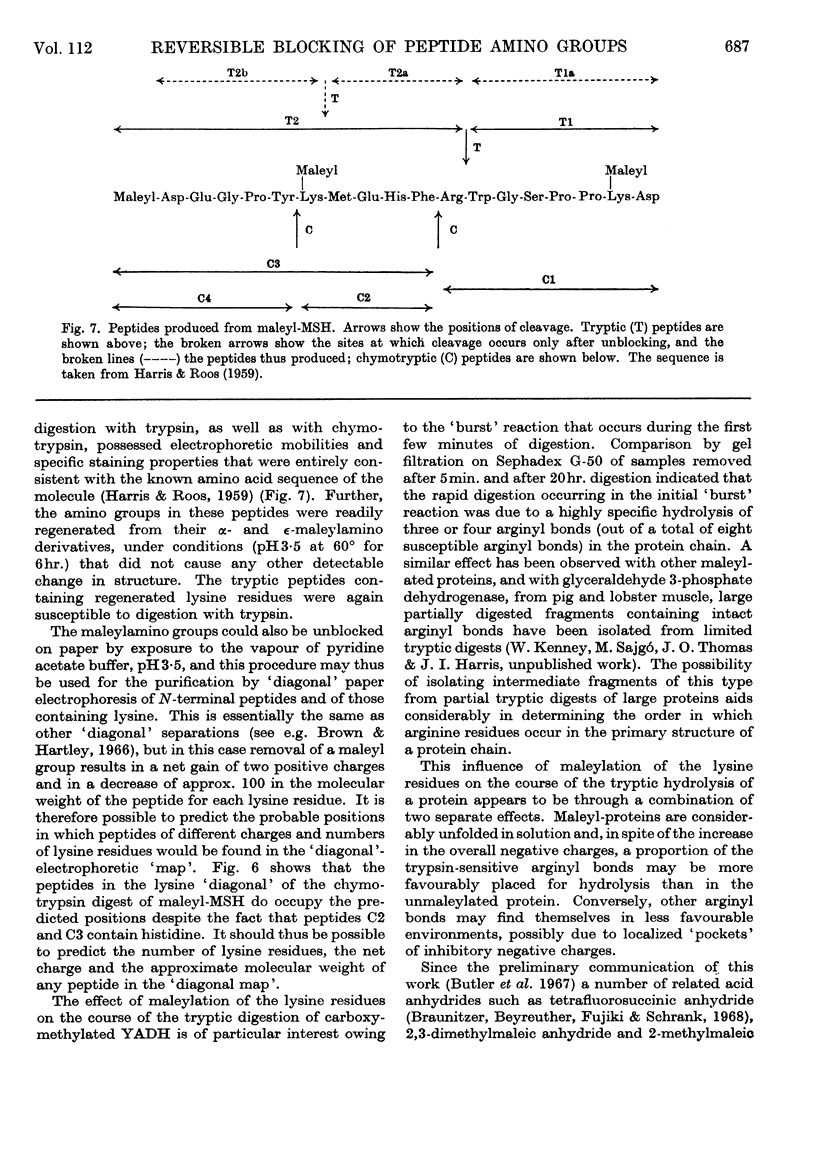
1. Maleic anhydride was shown to react rapidly and specifically with amino groups of proteins and peptides. Complete substitution of chymotrypsinogen was achieved under mild conditions and the extent of reaction could be readily determined from the spectrum of the maleyl-protein. 2. Maleyl-proteins are generally soluble and disaggregated at neutral pH. Trypsin splits the blocked proteins only at arginine residues and there is frequently selectivity in this cleavage, e.g. in yeast alcohol dehydrogenase and pig glyceraldehyde 3-phosphate dehydrogenase. 3. The group is removed by intramolecular catalysis at acid pH. The half-time was 11–12hr. at 37° at pH3·5 in ∈-maleyl-lysine or in maleyl-chymotrypsinogen. 4. The unblocking reaction can be used as the basis for a `diagonal'-electrophoretic separation of lysine peptides and N-terminal peptides, as shown by studies with β-melanocyte-stimulating hormone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHER R., CROCKER C. Réactions colorées spécifiques de l'arginine et de la tyrosine réalisées après chromatographie sur papier. Biochim Biophys Acta. 1952 Dec;9(6):704–705. doi: 10.1016/0006-3002(52)90236-9. [DOI] [PubMed] [Google Scholar]
- Braunitzer G., Beyreuther K., Fujiki H., Schrank B. Tetrafluorbernsteinsäure-anhydrid, ein neues Reagens zur spezifischen und reversiblen Maskierung der Aminogruppen in Proteinen. Hoppe Seylers Z Physiol Chem. 1968 Feb;349(2):265–265. [PubMed] [Google Scholar]
- Brown J. R., Hartley B. S. Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J. 1966 Oct;101(1):214–228. doi: 10.1042/bj1010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton C. J., Hartley B. S. Sub-unit structure and specificity of methionyl-transfer-ribonucleic acid synthetase from Escherichia coli. Biochem J. 1968 Jun;108(2):281–288. doi: 10.1042/bj1080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P. J., Harris J. I., Hartley B. S., Leberman R. Reversible blocking of peptide amino groups by maleic anhydride. Biochem J. 1967 Jun;103(3):78P–79P. [PMC free article] [PubMed] [Google Scholar]
- DALGLIESH C. E. The relation between pyridoxin and tryptophan metabolism, studied in the rat. Biochem J. 1952 Sep;52(1):3–14. doi: 10.1042/bj0520003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B. E., Sajgò M., Noller H. F., Harris J. I. Amino-acid sequence of glyceraldehyde 3-phosphate dehydrogenase from lobster muscle. Nature. 1967 Dec 23;216(5121):1181–1185. doi: 10.1038/2161181a0. [DOI] [PubMed] [Google Scholar]
- Dixon H. B., Perham R. N. Reversible blocking of amino groups with citraconic anhydride. Biochem J. 1968 Sep;109(2):312–314. doi: 10.1042/bj1090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. H., Grossberg A. L., Pressman D. The effects of complete modification of amino groups on the antibody activity of antihapten antibodies. Reversible inactivation with maleic anhydride. Biochemistry. 1968 May;7(5):1941–1950. doi: 10.1021/bi00845a044. [DOI] [PubMed] [Google Scholar]
- Frist R. H., Bendet I. J., Smith K. M., Lauffer M. A. The protein subunit of cucumber virus 4; degradation of viruses by succinylation. Virology. 1965 Aug;26(4):558–566. doi: 10.1016/0042-6822(65)90318-1. [DOI] [PubMed] [Google Scholar]
- HABEEB A. F., CASSIDY H. G., SINGER S. J. Molecular structural effects produced in proteins by reaction with succinic anhydride. Biochim Biophys Acta. 1958 Sep;29(3):587–593. doi: 10.1016/0006-3002(58)90016-7. [DOI] [PubMed] [Google Scholar]
- HARRIS J. I., ROOS P. Studies on pituitary polypeptide hormones. I. The structure of beta-melanocyte-stimulating hormone from pig pituitary glands. Biochem J. 1959 Mar;71(3):434–445. doi: 10.1042/bj0710434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Harris J. I., Perham R. N. Glyceraldehyde 3-phosphate dehydrogenase from pig muscle. Nature. 1968 Sep 7;219(5158):1025–1028. doi: 10.1038/2191025a0. [DOI] [PubMed] [Google Scholar]
- Irreverre F. A modified Sakaguchi spray. Biochim Biophys Acta. 1965 Dec 16;111(2):551–552. doi: 10.1016/0304-4165(65)90069-3. [DOI] [PubMed] [Google Scholar]
- KLOTZ I. M., KERESZTES-NAGY S. HEMERYTHRIN: MOLECULAR WEIGHT AND DISSOCIATION INTO SUBUNITS. Biochemistry. 1963 May-Jun;2:445–452. doi: 10.1021/bi00903a008. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Perham R. N., Jones G. M. The determination of the order of lysine-containing tryptic peptides of proteins by diagonal paper electrophoresis. A carboxyl-terminal sequence for pepsin. Eur J Biochem. 1967 Jul;2(1):84–89. doi: 10.1111/j.1432-1033.1967.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Porter R. R., Sanger F. The free amino groups of haemoglobins. Biochem J. 1948;42(2):287–294. doi: 10.1042/bj0420287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYLE A. P., SANGER F., SMITH L. F., KITAI R. The disulphide bonds of insulin. Biochem J. 1955 Aug;60(4):541–556. doi: 10.1042/bj0600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi H., Anfinsen C. B., Sodja A. The amino acid sequence of an extracellular nuclease of Staphylococcus aureus. 3. Complete amino acid sequence. J Biol Chem. 1967 Oct 25;242(20):4752–4758. [PubMed] [Google Scholar]
- WOFSY L., SINGER S. J. Effects of the amidination reaction on antibody activity and on the physical properties of some proteins. Biochemistry. 1963 Jan-Feb;2:104–116. doi: 10.1021/bi00901a019. [DOI] [PubMed] [Google Scholar]


