Abstract
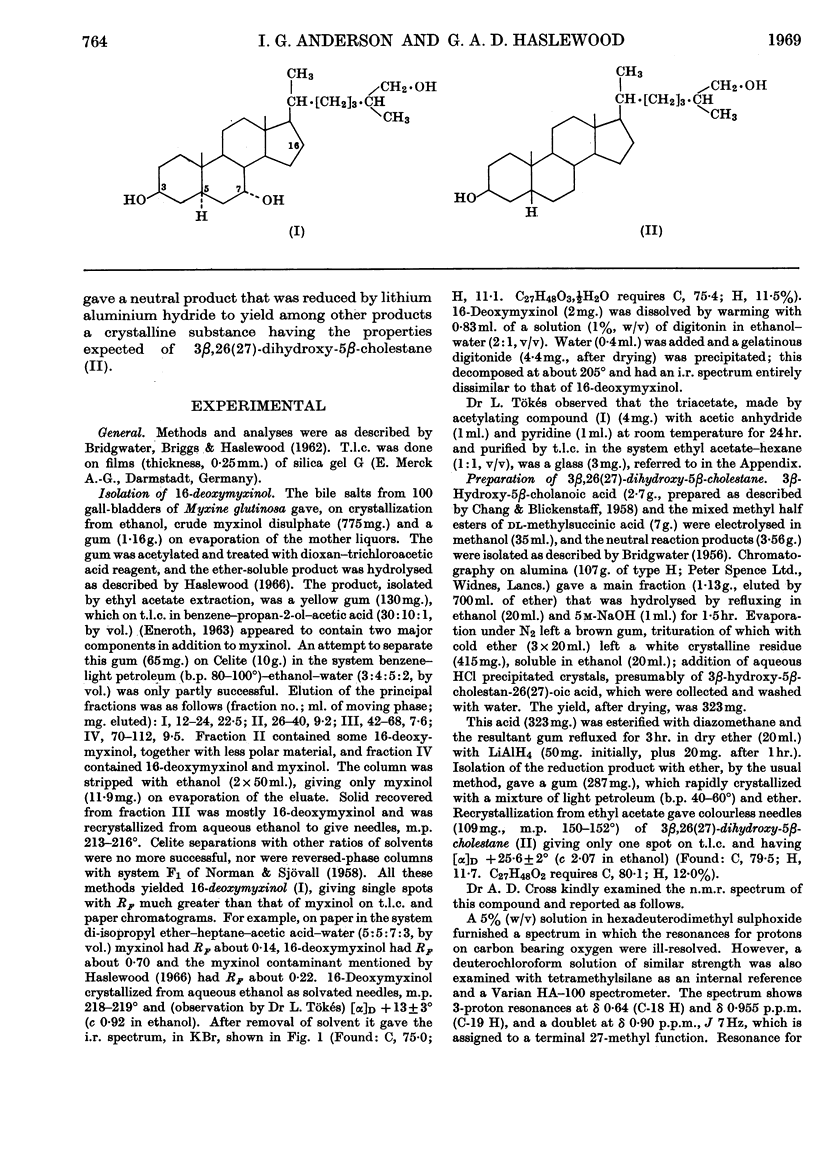
1. Material containing the less polar sulphate previously noticed in hagfish bile salts gave, after dioxan–trichloroacetic acid cleavage, 16-deoxymyxinol [3β,7α,-26(27)-trihydroxy-5α-cholestane]. 2. Anodic coupling of 3β-hydroxy-5β-cholanoic acid and the mixed half esters of dl-methylsuccinic acid, followed by lithium aluminium hydride reduction, yielded 3β,26(27)-dihydroxy-5β-cholestane. 3. 16-Deoxymyxinol, the third known bile alcohol having the 3β-hydroxy-5α-hydrogen configuration, poses again the question of how the 3β-hydroxyl group of cholesterol can be `retained' in biosynthesis of primitive bile salts.
Full text
PDF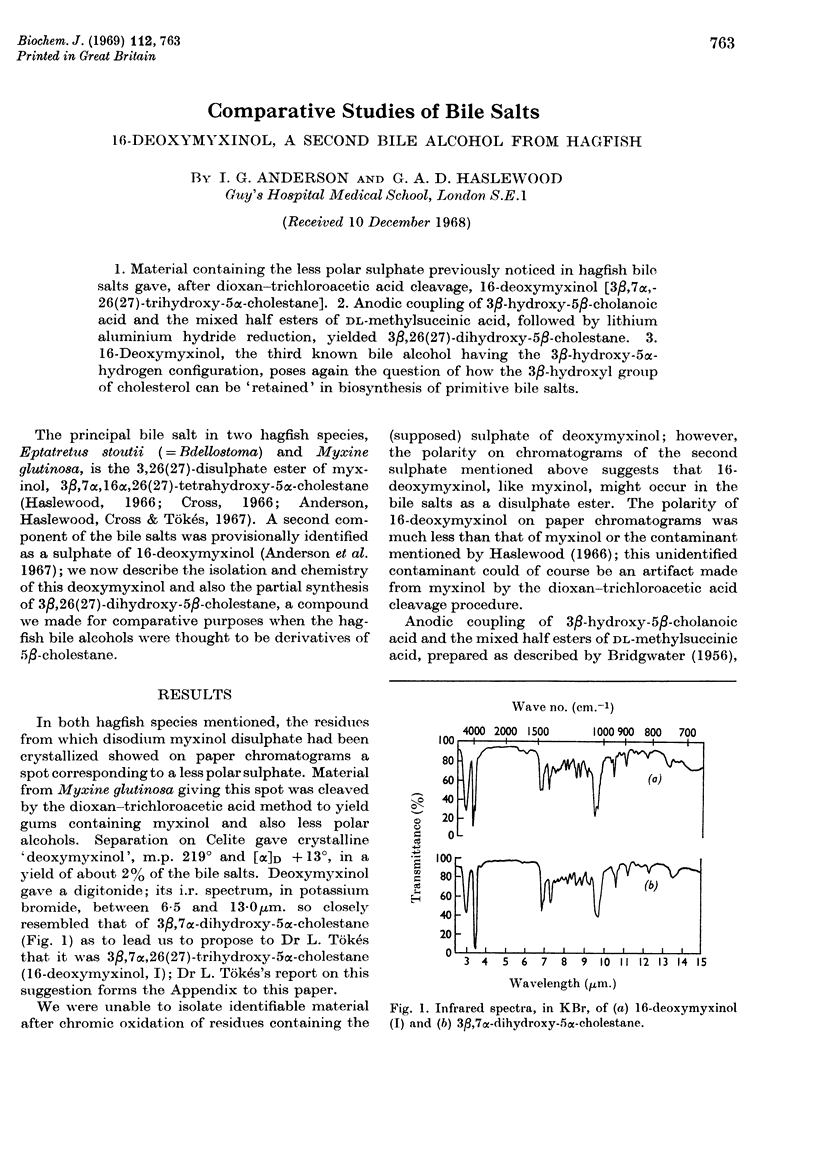

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. G., Haslewood G. A., Cross A. D., Tökés L. New evidence for the structure of myxinol. Biochem J. 1967 Sep;104(3):1061–1063. doi: 10.1042/bj1041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIDGWATER R. J., BRIGGS T., HASLEWOOD G. A. Comparative studies of 'bile salts'. 14. Isolation from shark bile and partial synthesis of scymnol. Biochem J. 1962 Feb;82:285–290. doi: 10.1042/bj0820285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIDGWATER R. J. Partial synthesis of the two 3alpha:7alpha:12alpha-trihydroxycoprostanic acids and of similar bile acids with extended chains. Biochem J. 1956 Dec;64(4):593–599. doi: 10.1042/bj0640593a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. D. Nuclear-magnetic-resonance and mass-spectral study of myxinol tetra-acetate. Biochem J. 1966 Jul;100(1):238–241. doi: 10.1042/bj1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENEROTH P. THIN-LAYER CHROMATOGRAPHY OF BILE ACIDS. J Lipid Res. 1963 Jan;4:11–16. [PubMed] [Google Scholar]
- Haslewood G. A. Comparative studies of bile salts, myxinol disulphate, the principal bile salt of hagfish (Myxinidae). Biochem J. 1966 Jul;100(1):233–237. doi: 10.1042/bj1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulos K. A., Myant N. B. The formation of lithocholic acid, chenodeoxycholic acid and alpha- and beta-muricholic acids from cholesterol incubated with rat-liver mitochondria. Biochem J. 1967 May;103(2):472–479. doi: 10.1042/bj1030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORMAN A., SJOVALL J. On the transformation and enterohepatic circulation of cholic acid in the rat: bile acids and steroids 68. J Biol Chem. 1958 Oct;233(4):872–885. [PubMed] [Google Scholar]
- Wachtel N., Emerman S., Javitt N. B. Metabolism of cholest-5-ene-3 beta, 26-diol in the rat and hamster. J Biol Chem. 1968 Oct 10;243(19):5207–5212. [PubMed] [Google Scholar]


