Abstract
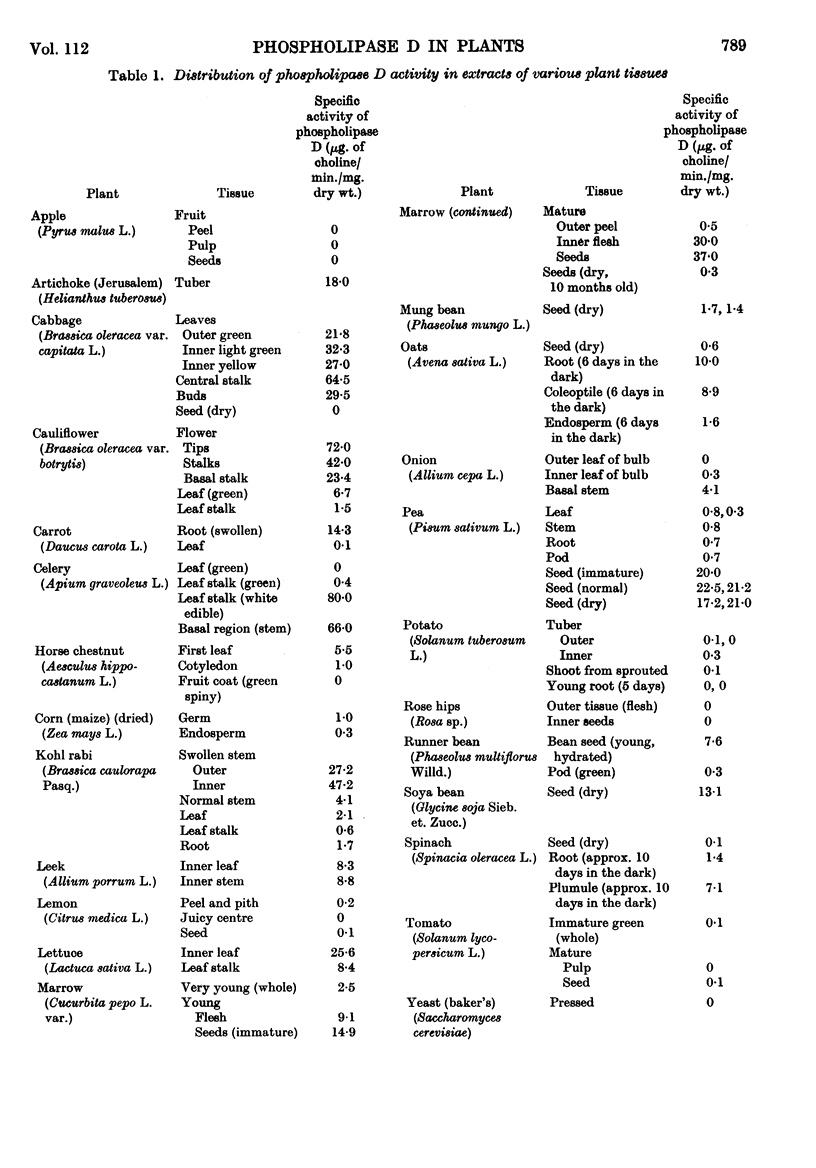
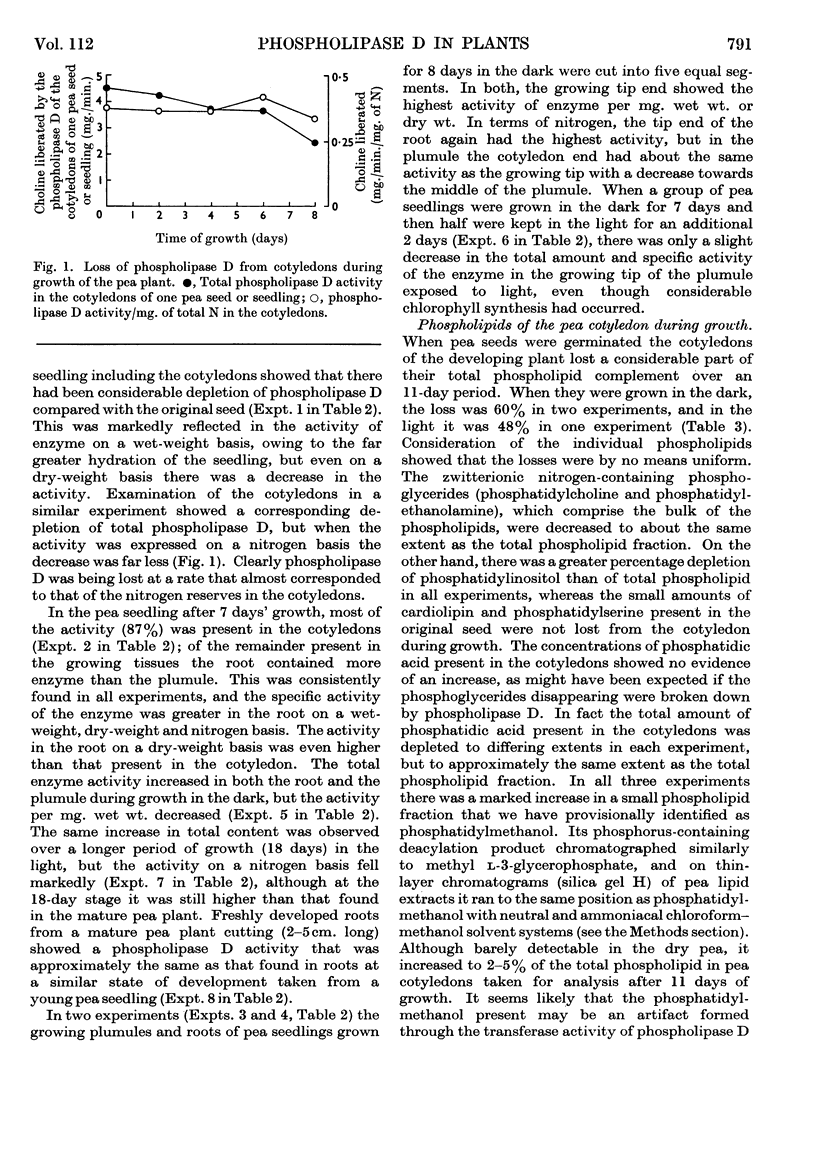
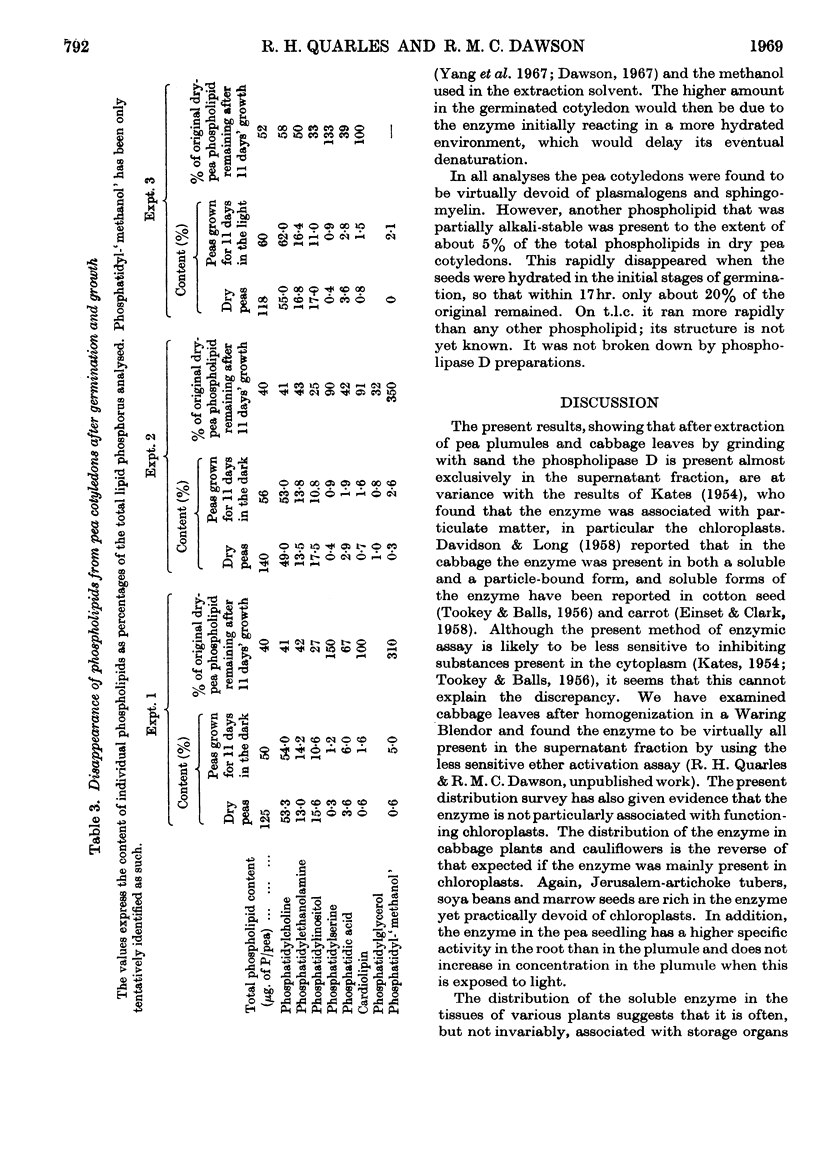
1. The distribution of phospholipase D (phosphatidylcholine phosphatido-hydrolase, EC 3.1.4.4) was examined in the tissues of a number of plants and seeds. 2. The highest activities were found in various swollen storage tissues of certain plants: cabbage, central stalk; cauliflower, flower; celery, swollen leaf stalk; Kohl rabi, swollen stem; carrot, root; pea and marrow, seed. 3. Appreciable activity was retained in pea seeds for at least 1 year after drying. After germination and growth in the dark the total activity present in the cotyledons and also in the whole seedling decreased. 4. In the growing pea seedling (7 days old), about 3% of the total activity was in the plumule, 9% in the root and the remainder in the cotyledons. However, the activity in the root on a dry-weight basis was higher than that in the cotyledons. In both the root and the plumule the activity on a wet- or a dry-weight basis was highest in the growing tip. 5. The activity per dry weight in the roots and aerial parts of pea plants declined to low values as growth continued, but roots struck from cuttings of mature plants showed the same high activity as found in roots from young seedlings with cotyledons attached. 6. The total phospholipids present in the cotyledons of pea seeds were depleted on germination and growth. Of the individual phospholipids, phosphatidylcholine and phosphatidylethanolamine showed the same loss in 11 days as the whole phospholipid fraction, whereas phosphatidylinositol was decreased to a greater extent and cardiolipin and phosphatidylserine were not decreased. There was no increase of phosphatidic acid, as might have been expected if the phospholipids had disappeared through phospholipase D hydrolysis. 7. It is concluded that phospholipase D in plant storage tissues and seeds may be related to the rapid growth involved in their formation rather than being necessary for the utilization of their food reserve substances.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKER L., ERNST G. Uber das Vorkommen eines phosphatidspaltenden Ferments in Cerealien. Biochem Z. 1954;325(4):253–257. [PubMed] [Google Scholar]
- Brown A. P., Wray J. L. Correlated changes of some enzyme activities and cofactor and substrate contents of pea cotyledon tissue during germination. Biochem J. 1968 Jul;108(3):437–444. doi: 10.1042/bj1080437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON F. M., LONG C. The structure of the naturally occurring phosphoglycerides. 4. Action of cabbage-leaf phospholipase D on ovolecithin and related substances. Biochem J. 1958 Jul;69(3):458–466. doi: 10.1042/bj0690458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M., HEMINGTON N., DAVENPORT J. B. Improvements in the method of determining individual phospholipids in a complex mixture by successive chemical hydrolyses. Biochem J. 1962 Sep;84:497–501. doi: 10.1042/bj0840497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Dawson R. M., Hemington N. Some properties of purified phospholipase D and especially the effect of amphipathic substances. Biochem J. 1967 Jan;102(1):76–86. doi: 10.1042/bj1020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M. The formation of phosphatidylglycerol and other phospholipids by the transferase activity of phospholipase D. Biochem J. 1967 Jan;102(1):205–210. doi: 10.1042/bj1020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EINSET E., CLARK W. L. The enzymatically catalyzed release of choline from lecithin. J Biol Chem. 1958 Apr;231(2):703–715. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- KATES M. Lecithinase systems in sugar beet, spinach, cabbage, and carrot. Can J Biochem Physiol. 1954 Sep;32(5):571–583. [PubMed] [Google Scholar]
- Pusztai A. General properties of a protease inhibitor from the seeds of kidney bean. Eur J Biochem. 1968 Jul;5(2):252–259. doi: 10.1111/j.1432-1033.1968.tb00365.x. [DOI] [PubMed] [Google Scholar]
- SMITH R. H. The phosphatides of the latex of Hevea brasiliensis. 1. Biochemical changes. Biochem J. 1954 Feb;56(2):240–250. doi: 10.1042/bj0560240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOOKEY H. L., BALLS A. K. Plant phospholipase D. I. Studies on cottonseed and cabbage phospholipase D. J Biol Chem. 1956 Jan;218(1):213–224. [PubMed] [Google Scholar]
- YOUNG J. L., VARNER J. E. Enzyme synthesis in the cotyledons of germinating seeds. Arch Biochem Biophys. 1959 Sep;84:71–78. doi: 10.1016/0003-9861(59)90555-7. [DOI] [PubMed] [Google Scholar]
- Yang S. F., Freer S., Benson A. A. Transphosphatidylation by phospholipase D. J Biol Chem. 1967 Feb 10;242(3):477–484. [PubMed] [Google Scholar]


