Abstract
A 7-year-old Labrador retriever had nonspecific clinical signs that included lethargy, malaise, and difficult ambulation. The dog was native to Vancouver Island, British Columbia, and had never left this area. Morulae were identified in polymorphonuclear cells. Serologic studies and polymerase chain reaction (PCR) testing confirmed canine anaplasmosis caused by Anaplasma phagocytophilum. The dog recovered after treatment with tetracycline.
Résumé
Infection à Anaplasma phagocytophilum (anaplasmose granulocytaire) chez un chien de l’île de Vancouver. Un Labrador retriever âgé de sept ans montrait des signes cliniques non spécifiques comprenant de la léthargie, du malaise et de la difficulté à se déplacer. Le chien était natif de l’île de Vancouver en Colombie-Britanique et n’avait jamais quitté les lieux. Des morulas ont été identifiés dans les granulocytes. Les études sérologiques et une amplification en chaîne par polymérase ont confirmé une anaplasmose canine causée par Anaplasma phagocytophilum. Le chien s’est rétabli après un traitement à la tétracycline.
(Traduit par Docteur André Blouin)
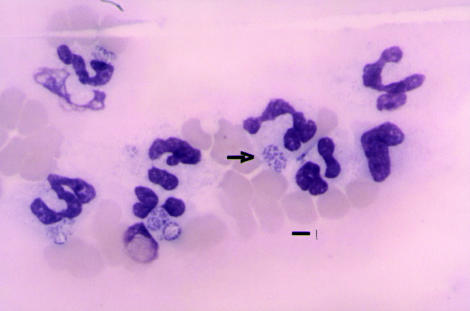
A 7-year-old, 30.6 kg, male neutered, Labrador retriever was examined for difficult ambulation and an unsteady gait, combined with anorexia and lethargy, that had developed over the preceding week. The dog was thin, had a modestly increased heart rate, panting respirations, and an elevated rectal temperature (40.3ºC). Specific abnormalities were not detected on the physical examination. Blood was collected for a complete blood cell count and serum biochemical panel. A voided urine sample was submitted for urinalysis. Hematological abnormalities included, a lymphopenia 0 × 109 cells/L (reference range, 0.980 to 4.500 × 109 cells/L) and thrombocytopenia 53 × 109 cells/L (reference range, 170 to 400 × 109 cells/L). Approximately 5% of the neutrophils on the blood smear contained discrete intra-cytoplasmic bodies consistent with morulae (Figure 1). The serum alkaline phosphatase (ALP) was elevated, 257 U/L (reference range, 4 to 113 U/L), and the serum albumin was at the low end of the reference range, 31 g/L (reference range, 31 to 42 g/L). The free catch urine sample had a specific gravity of 1.020, with hematuria and numerous coccoid bacteria observed on the microscopic evaluation.
Figure 1.
The feather edge of a peripheral blood smear from the initial blood sample demonstrating the distinctive granulocytic morulae. Bar = 6 μm.
On further questioning, the owner described removing ticks from the dog during the preceding 6 wk and confirmed that the dog had resided on Vancouver Island, British Columbia, for its entire life. No ticks were available for identification.
The dog was treated with tetracycline (Apotex, Weston, Ontario) 750 mg, PO, q8h for 14 d. The clinical signs resolved over the next month and the dog’s recovery was complete and uneventful.
Based upon the results of the blood smear examination, the ethylenediamine tetra-acetic acid (EDTA)- anticoagulated blood and serum samples obtained at the time of the original examination and 3 wk later following treatment with tetracycline were submitted for polymerase chain reaction (PCR) and serological testing for Anaplasma phagocytophilum. Following diagnostic procedures in the Vector Borne Disease Diagnostic Laboratory, North Carolina State University — College of Veterinary Medicine, DNA was amplified first by using broad primers for the genera Ehrlichia and Anaplasma (1). Samples in which DNA is detected are then processed in a nested PCR system with variable primer sets to identify the species (2,3). In this case, A. phagocytophilum DNA was amplified from the initial EDTA-treated blood. Ehrlichia canis, E. ewingii, E. chaffensis, and A. platys DNA were not detected. Further confirmation of the species identity was obtained by gene sequence analysis of the 400 base pair amplicon (Davis sequencing, Davis, California, USA) showing 100% compatibility of 317 base pairs with A. phagocytophilum. No DNA was detected in the 2nd (convalescent) blood sample when broad primers were used. Seroconversion to A. phagocytophilum antigens was documented by indirect immunofluorescent antibody testing, using a previously described procedure (4). The acute phase titer was < 1:16 and the convalescent titer was 1:4096.
Canine anaplasmosis is an emerging disease with conflicting historical descriptions. The conflict in part reflects the previous lack of definitive speciation of the organism. Only recently has accurate and precise species identification been possible through the advent of PCR targeting specifically the 16S ribosomal RNA gene (1). This advance in molecular biology has allowed for diagnostic differentiation and confirmation of the separate genera and species involved in the disease processes (5,6).
Recently developed classification schemes have reclassified E. equi as A. phagocytophilum. Historically, E. ewingii and E. equi (now A. phagocytophilum) were considered the primary agents of “granulocytic ehrlichiosis” in dogs (4,7). Anaplasma phagocytophilum is also the cause of equine anaplasmosis. Other ehrlichia organisms, E. canis and E. chaffeensis, cause separate and distinct disease processes and are usually found as morulae in mononuclear cells. Ehrlichia spp. and Anaplasma sp. may also induce chronic and presumably concurrent or overlapping infections in dogs.
The disease descriptions and pathogenesis for the various clinical and pathological conditions are further complicated by the ability of a single tick to harbor and transmit multiple pathogens, and by the fact that dogs can be infected with multiple tick species. It is not uncommon to have serologic positive test results to multiple Ehrlichia spp., as well as Anaplasma sp. and Neorickettsia sp. (4,5,7).
Anaplasma phagocytophilum can infect humans and numerous animal species, including horses, cats, dogs, ruminants, and wildlife. The seroprevalence of ehrlichiosis or anaplasmosis is related to organism prevalence in the tick populations, animal density, and the species of Anaplasma and Ehrlichia found within the geographic area. The organisms are transmitted by ticks and their life cycle varies with the types of tick species, tick population density, and the wildlife that are indigenous to a specific climate and geographic location. Wildlife such as deer, rodents, and other small mammals maintain A. phagocytophilum and serve as the reservoir hosts, with transmission to domestic animals and man as a result of tick bites (4,5,7,8). Ehrlichia ewingii infection appears more commonly throughout the U.S. eastern seaboard and southern states (4). Anaplasmosis caused by A. phagocytophilum has been described mainly in the northeastern states, Minnesota, Wisconsin, and California in the United States, and in Sweden (5,7). The principle vector for A. phagocytophilum in the western United States is Ixodes pacificus, whereas in the eastern and north central United States, Ixodes scapularis is the vector (7).
Canine anaplasmosis caused by A. phagocytophilum varies from a subclinical infection to an acute febrile condition accompanied by anorexia and lethargy. Central nervous system dysfunction and lameness have also been recorded in dogs. Based on reports to date, anaplasmosis appears to cause a less severe disease in dogs than does infection with E. canis, E. chaffeensis, or E. ewingii (5,7). Lethargy, accompanied by fever, appears to be documented consistently, and the lethargy is often disproportionately severe in comparison with the general lack of abnormalities documented on physical examination. Although most dogs have a mild thrombocytopenia, anemia is uncommon and changes in the leukocyte numbers are variable. Elevated ALP activities have been reported in dogs with anaplasmosis (5,7). Recent serologic assessments in California indicate that many clinically normal dogs have titers to A. phagocytophilum; this suggests that cases may be subclinical or that the disease may be underdiagnosed (8).
The pathogenesis of anaplasmosis may involve endothelial as well as myeloid infection and has been best investigated in regards to equine anaplasmosis, but, to date, the clinical manifestations of the disease and the ability to diagnose the condition have taken precedence over information regarding the disease pathogenesis (5,7,9).
In this report, the fortuitous discovery of neutrophilic morulae enabled the diagnosis of canine anaplasmosis. Following tick transmission, morulae are found within neutrophils for only a short period of time in the acute phase of the disease and can easily go unrecognized (6,10, David Gribble, personal communication). Indeed, in this dog, the morulae were not easily detected in a blood sample drawn 48 h after the initial sample.
The thrombocytopenia in this dog was mild and partially explained by platelet clumping. Since the dog did not have petechiae or evidence of blood loss, it is unlikely that causes of thrombocytopenia would have been investigated in substantial detail. A marginal decrease in serum albumin and mildly elevated serum ALP activity have been reported with both anaplasmosis and ehrlichiosis, but these are nonspecific and could lead to evaluation for primary liver disease. Despite lameness, there was no joint distention or evidence of severe polyarthritis. Polyarthritis with joint distention is considered a more common manifestation of infection with E. ewingii than with A. phagocytophilum (5,6). The weight loss and general malaise observed in this dog were more severe clinically than could be explained by the hematologic or biochemical abnormalities or by the findings on physical examination. Without the detection of the morulae and given the geographic location, anaplasmosis or ehrlichiosis would not have been considered in the differential diagnoses. Molecular confirmation of the identity of the morulae was necessary to confirm that the inclusions were due to A. phagocytophilum and not E. ewingii or another infectious or noninfectious cause.
Since the detection of morulae is problematic, the diagnosis of anaplasmosis is most often based on serologic testing. Anaplasma phagocytophilum serologic testing would be uncommonly requested in this region, as previously the organism has only been detected in this area in 1 horse (10).
Immunoflourescent (IFA) procedures are used to detect antibodies to surface antigens of closely related organisms, and although E. canis and E. ewingii may have cross reacting antigenic epitopes, A. phagocytophilum antibody does not consistently cross react with E.canis antigens. A single acute phase serologic test may be negative, as seroreactivity may not develop for 7 to 21 d post infection (5,7). This fact was well illustrated by this case, where antibodies were not detected in the acute phase serum sample but were subsequently detected at a reciprocal A. phagocytophilum IFA titer of 4096. It is prudent, therefore, to obtain paired, acute and convalescent, serum samples in dogs with short duration illness and to be aware of the species of Anaplasma or Ehrlichia that can be transmitted by ticks within the specific geographic region. Detection of transmission of disease from ticks to cats, dogs, or horses also raises the possibility of tick transmission of organisms to humans within the same geographic locale.
The PCR detection of A. phagocytophilum DNA is both sensitive and specific for the acute phase diagnosis of anaplasmosis (1,2). With the advent of real-time PCR and with the increased availability of molecular diagnostic testing, veterinarians should be able to obtain rapid confirmation of the diagnosis of anaplasmosis.
Since the disease prevalence is unknown in this geographic location, it would be prudent to consider anaplasmosis in dogs, cats, or horses with a history of either tick exposure or residing in areas where tick exposure is probable that are presenting for nonspecific malaise, fever, and lethargy, without significant biochemical and only minimal hematologic abnormalities. CVJ
References
- 1.Hancock SI, Breitschwerdt EB, Pitulle CW. Differentiation of Ehrlichia platys and Ehrlichia equi infections in dogs by using 16S rDNA based PCR. J Clin Microbiol. 2001;39:4577–4578. doi: 10.1128/JCM.39.12.4577-4578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lappin MR, Breitschwerdt EB, Jensen WA, et al. Molecular and serologic evidence of Anaplasma phagocytophilum infection in cats in North America. J Am Vet Med Assoc. 2004;225:893–896. doi: 10.2460/javma.2004.225.893. [DOI] [PubMed] [Google Scholar]
- 3.Kordick SK, Breitschwerdt EB, Hegarty BC, et al. Coinfection with multiple tick borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–2638. doi: 10.1128/jcm.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Susksawt J, Hegarty BC, Breitschwerdt EB. Seroprevalence of Ehrlichia canis, Ehrlichia equi and Ehrlichia risticii in sick dogs from North Carolina and Virginia. J Vet Intern Med. 2000;14:50–55. doi: 10.1892/0891-6640(2000)014<0050:socear>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 5.McQuiston JH, McCall CL, Nicholson WL. Ehrlichiosis and related infections. J Am Vet Med Assoc. 2003;223:1750–1756. doi: 10.2460/javma.2003.223.1750. [DOI] [PubMed] [Google Scholar]
- 6.Goodman RA, Hawkins EC, Olby NJ, et al. Molecular identification of Ehrlichia ewingii infection in dogs: 15 cases (1997–2001) J Am Vet Med Assoc. 2003;222:1102–1107. doi: 10.2460/javma.2003.222.1102. [DOI] [PubMed] [Google Scholar]
- 7.Preziosi DE, Cohn LA. The increasingly complicated story of Ehrlichia. Compend Contin Educ Pract Vet. 2002;24:277–288. [Google Scholar]
- 8.Foley JE, Foley P, Madigan JE. Spatial distribution of seropositivity to the causative agent of granulocytic ehrlichiosis in dogs in California. Am J Vet Res. 2001;62:1599–1605. doi: 10.2460/ajvr.2001.62.1599. [DOI] [PubMed] [Google Scholar]
- 9.Munderloh UG, Lymch MJ, Herron MJ, et al. Infection of endothelial cells with Anaplasma marginale and A. phagocytophilum. Vet Microbiol. 2004;101:53–64. doi: 10.1016/j.vetmic.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Berrington AB, Moats R, Lester SJ. A case of Ehrlichia equi in an adult horse in British Columbia. Can Vet J. 1996;37:174–175. [PMC free article] [PubMed] [Google Scholar]