Abstract
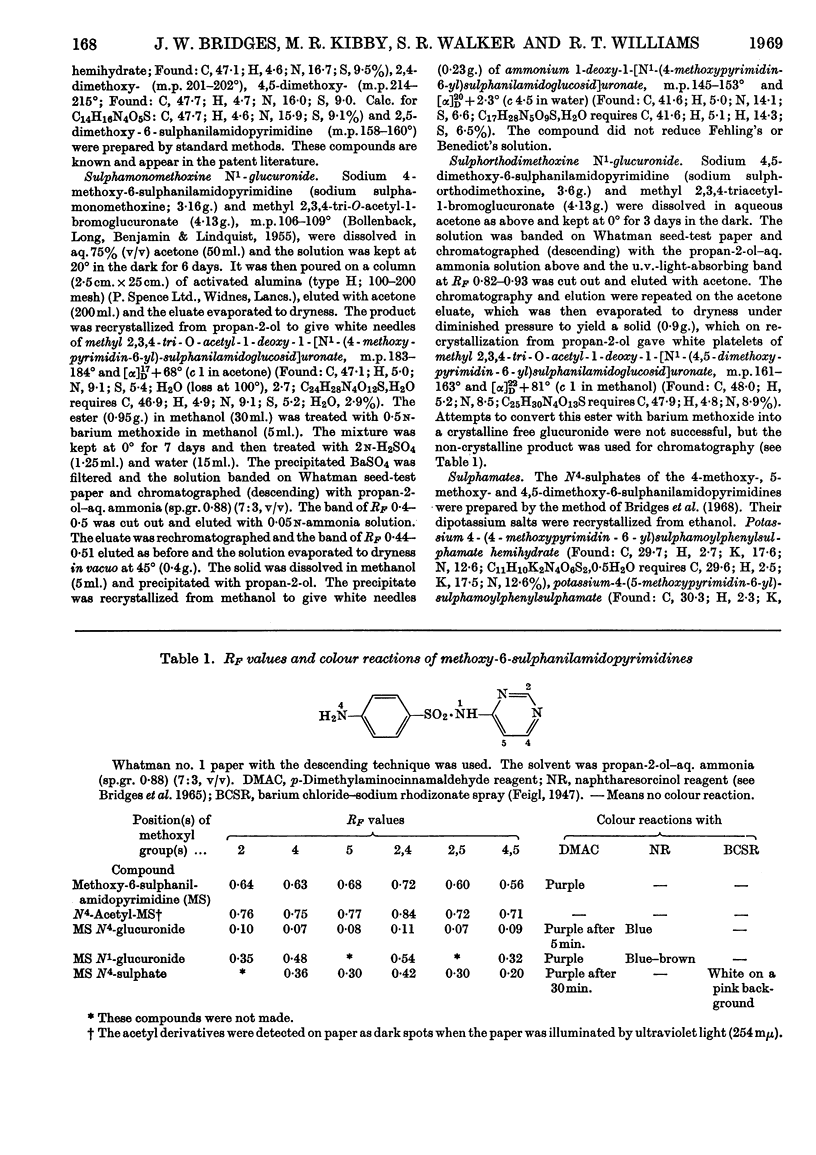
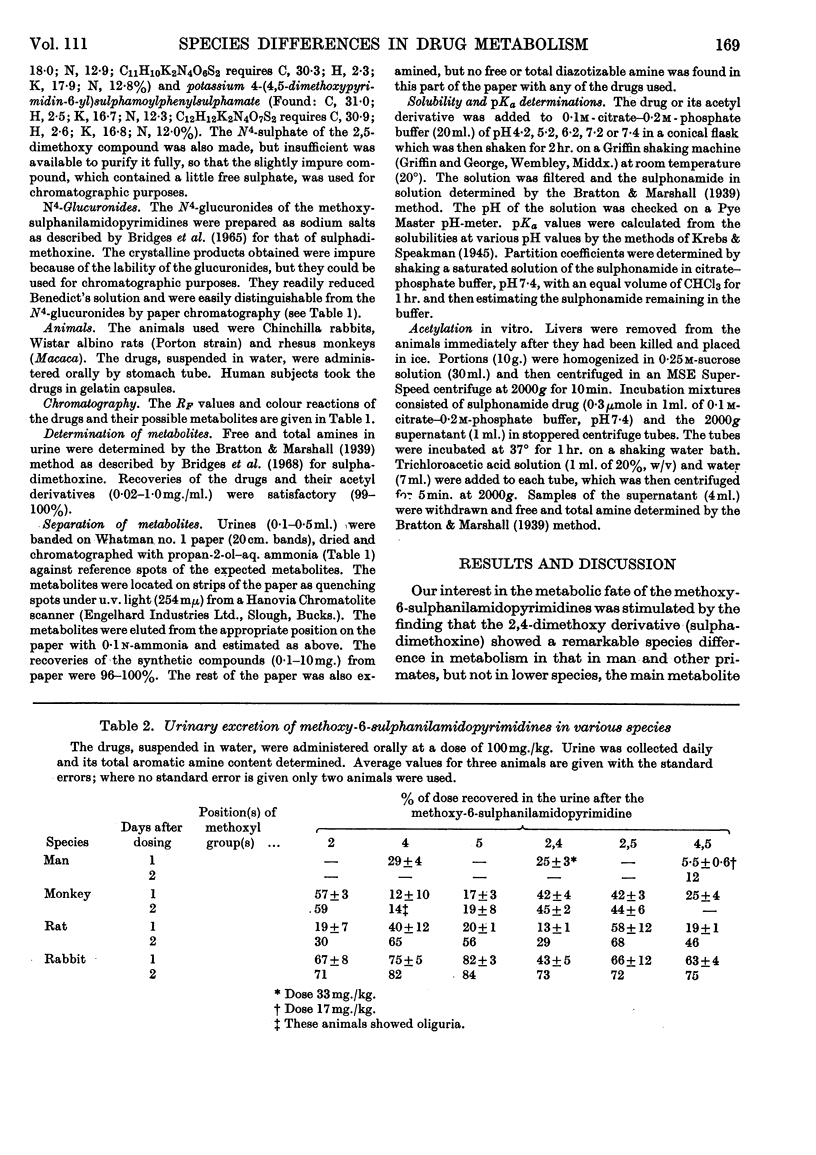
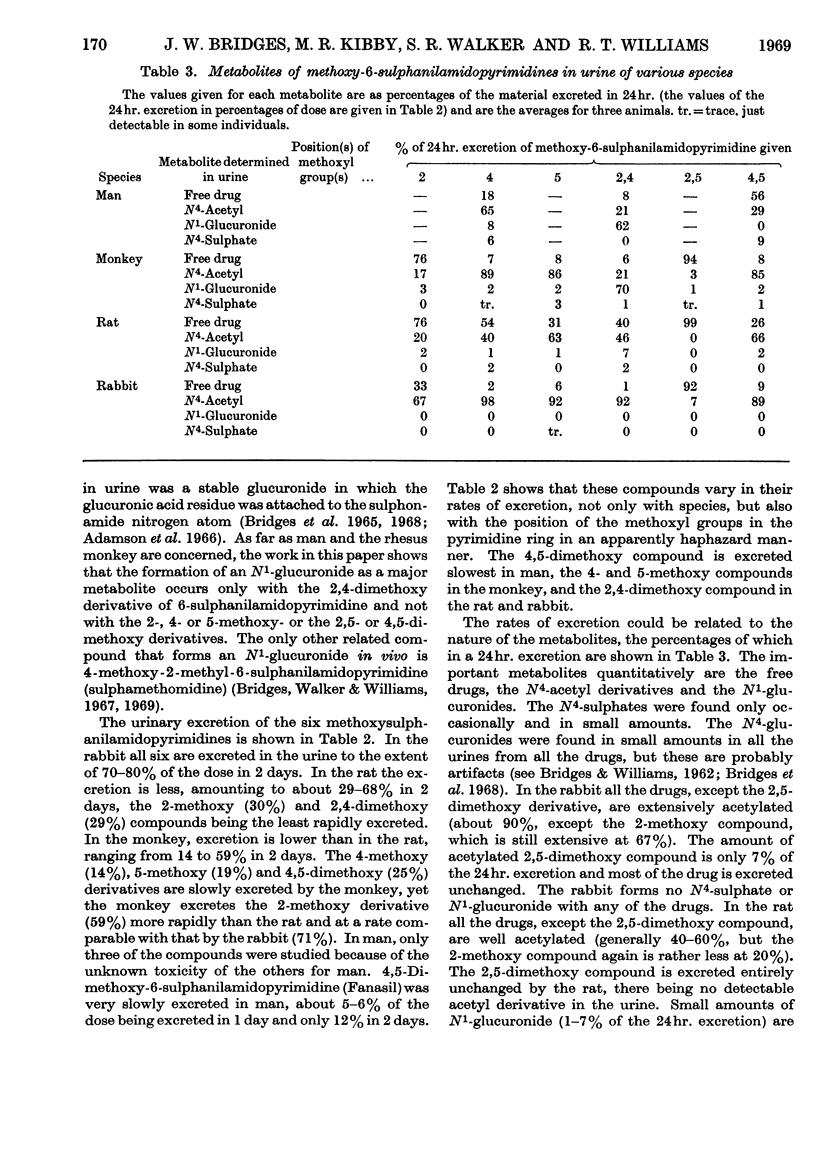
1. A comparative study was made in man, rhesus monkey, rat and rabbit of the urinary excretion of 2-, 4- and 5-methoxy- and 2,4-, 2,5- and 4,5-dimethoxy-6-sulphanilamidopyrimidines given orally. 2. In the rabbit, 70–80% of the dose of each drug was excreted in 2 days, mainly as N4-acetyl derivatives, except 2,5-dimethoxy-6-sulphanilamidopyrimidine, which was mainly excreted unchanged. 3. In the rat, 50–70% of the dose of each drug was excreted in 2 days, except the 2-methoxy and 2,4-dimethoxy compounds, whose excretion was about 30%. The N4-acetyl derivatives accounted for 20–70% of the drugs excreted, except the 2,5-dimethoxy derivative, which was excreted unchanged. 4. In the rhesus monkey, some 40–60% of the dose of the 2-methoxy, 2,4-dimethoxy and 2,5-dimethoxy compounds was excreted in 2 days, but the 4-methoxy, 5-methoxy and 4,5-dimethoxy compounds were excreted at less than half this rate. The 4-methoxy, 5-methoxy and 4,5-dimethoxy compounds were highly acetylated (80–90%) whereas the 2-methoxy compound was poorly acetylated (17%) and the 2,5-dimethoxy compound hardly at all. The major metabolite of the 2,4-dimethoxy compound in the monkey was the N1-glucuronide. 5. In man, 30% of the dose of the 4-methoxy and 2,4-dimethoxy compounds was excreted in 24 hr., whereas the 4,5-dimethoxy compound (Fanasil) was very slowly excreted (12% in 2 days). The 4-methoxy compound was well acetylated (65%), but the 2,4- and 4,5-dimethoxy compounds were not (20–30%). The main metabolite of the 2,4-dimethoxy compound in man was the N1-glucuronide. 6. N1-Glucuronide formation occurred extensively only with the 2,4-dimethoxy compound and only in man and the rhesus monkey. It did not occur in the rabbit and only to a minor extent in the rat. 7. The 2,5-dimethoxy compound was not significantly acetylated in vivo in the rabbit, rat or monkey, but acetylation occurred in vitro in rabbit or monkey liver homogenates. 8. These findings are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridges J. W., Kibby M. R., Walker S. R., Williams R. T. Species differences in the metabolism of sulphadimethoxine. Biochem J. 1968 Oct;109(5):851–856. doi: 10.1042/bj1090851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges J. W., Kibby M. R., Williams R. T. The structure of the glucuronide of sulphadimethoxine formed in man. Biochem J. 1965 Sep;96(3):829–836. doi: 10.1042/bj0960829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges J. W., Walker S. R., Williams R. T. Species differences in the metabolism and excretion of sulphasomidine and sulphamethomidine. Biochem J. 1969 Jan;111(2):173–179. doi: 10.1042/bj1110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger-Thiemer E., Hansen R. Die Lösung pharmakologischer Probleme durch Rechenautomaten. 7. Ein ALGOL 60-Programm für die quantenchemische Methode der Molekülzustände nack Hückel (HMO-Metode) Arzneimittelforschung. 1966 Nov;16(11):1453–1466. [PubMed] [Google Scholar]
- Milne M. D. Influence of acid-base balance on efficacy and toxicity of drugs. Proc R Soc Med. 1965 Nov;58(11 Pt 2):961–963. [PMC free article] [PubMed] [Google Scholar]


