ABSTRACT
Signaling between rhizosphere microorganisms is crucial in bacteria interaction and communication, shaping the rhizomicrobiome. Plant growth-promoting bacterium Pseudomonas produces a spectrum of important antibiotics to inhibit plant pathogens, albeit with an associated metabolic burden. Antibiotics could function as intra- and interspecies signals at subinhibitory concentrations to coordinate gene expression and microbial behaviors. In this work, we characterized pyoluteorin as an interspecies signal that modulates the biosynthesis of 2,4-diacetylphloroglucinol (2,4-DAPG), a broad-spectrum biocontrol agent, in non-pyoluteorin-producing Pseudomonas fluorescens 2P24. We demonstrated that the key transcriptional repressor PhlF from the 2,4-DAPG biosynthetic gene cluster spontaneously senses pyoluteorin, enhancing repression of the phlA promoter activity and inhibiting 2,4-DAPG synthesis in P. fluorescens 2P24. Pyoluteorin also binds to another transcriptional repressor, PhlH, from the 2,4-DAPG biosynthetic gene cluster, subsequently releasing the transcription of phlG, which facilitates the hydrolysis of 2,4-DAPG. Both PhlF and PhlH are simultaneously involved in sensing exogenous pyoluteorin to regulate the 2,4-DAPG biosynthetic operon, playing a crucial role in controlling antibiotic metabolites in response to environmental changes. Further phylogenetic and structural analyses demonstrated that PhlH and PhlF are widely distributed across Pseudomonas spp. with conserved ligand-binding domains. The findings shed new light on the regulatory mechanism of 2,4-DAPG biosynthesis underlying interspecies signaling by pyoluteorin and provide invaluable clues for the rational design of co-inhabiting Pseudomonas spp. as biocontrol agents.
IMPORTANCE
Rhizosphere microorganisms release vital signals that shape microbial communities, with antibiotics at low concentrations acting as intra- and interspecies signals. However, the mechanisms of these signals in coordinating gene expression are unclear. In non-pyoluteorin-producing Pseudomonas fluorescens 2P24, pyoluteorin was identified as an interspecies signal that regulates the phl biosynthesis gene cluster for 2,4-DAPG production. TetR family repressors PhlH and PhlF were found to positively regulate 2,4-DAPG hydrolysis and negatively regulate its synthesis in response to pyoluteorin. Structural modeling and docking analyses revealed the interactions between pyoluteorin and both PhlH and PhlF, modulating gene expression. Phylogenetic analyses showed a wide distribution of PhlH and PhlF across Pseudomonas spp. with conserved ligand-binding domains. These findings deepen our understanding of interspecies signaling mechanisms and highlight the potential for designing co-inhabiting Pseudomonas spp. as effective biocontrol agents.
KEYWORDS: Pseudomonas; pyoluteorin; interspecies signaling; transcriptional repressor; 2,4-diacetylphloroglucinol
INTRODUCTION
Rhizosphere microorganisms, known as plant-associated microbial communities, are composed of diverse bacteria affecting plant development (1). Microorganisms in the rhizosphere interact and communicate with one another, playing a pivotal role in the ecological fitness of host plants (2). Signaling among microorganisms in the rhizosphere involves a common class of molecular signals, including quorum-sensing signals acyl-homoserine lactone and diffusible signal factors (3), volatile organic compounds (4), diketopiperazines (5), and antibiotics at subinhibitory concentrations (6). These signaling molecules are essential for modifying and shaping microbial communities (2). Certain antibiotics can function as intra- and interspecies signaling molecules at low and non-inhibitory concentrations, most often associated with coordinating gene expression and microbial behaviors (7).
Pseudomonas is a predominant group of plant growth-promoting bacteria that produce a spectrum of important agricultural antibiotics with antagonistic activities to suppress plant pathogens (8). Pyoluteorin (PLT) and 2,4-diacetylphloroglucinol (2,4-DAPG), the two polyketide metabolites produced by Pseudomonas spp., are broad-spectrum antibiotics with antibacterial and antifungal activities (9). PLT is synthesized by a hybrid non-ribosomal peptide synthetase and polyketide synthase gene cluster and exhibits excellent antagonistic activity against the oomycete, including the plant pathogen Pythium ultimum (10). A type III polyketide synthase gene cluster is responsible for synthesizing 2,4-DAPG that contributes to the inhibition of fungal pathogens. Within the typical phl gene cluster of 2,4-DAPG, two pairs of oppositely transcribed operons, namely, phlF–phlACBD and phlG–phlH, are integral to the biosynthesis of 2,4-DAPG (11). The phlD gene encodes type III polyketide synthase responsible for converting malonyl-CoA into PG, which is further transformed into monoacetylphloroglucinol (MAPG) and 2,4-DAPG by acetyltransferase PhlACB through a series of enzymatic steps (12). Meanwhile, phlG encodes a hydrolase enzyme that degrades 2,4-DAPG into MAPG (13). Many Pseudomonas protegens strains possess both PLT and 2,4-DAPG biosynthetic gene clusters, such as Pseudomonas protegens Pf-5, CHA0, H78, FD6, and MP12, while certain Pseudomonas strains produce either PLT or 2,4-DAPG exclusively (14–17). PLT-producing Pseudomonas strains, while encompassing some strains of Pseudomonas aeruginosa and Pseudomonas sp. M18, typically do not produce 2,4-DAPG (18, 19). On the other hand, certain strains of Pseudomonas spp., such as Pseudomonas aureofaciens Q2-87, Pseudomonas fluorescens F113, and Pseudomonas fluorescens 2P24, synthesize 2,4-DAPG but not PLT (15, 20).
PLT acts as an endogenous signal and autoinduces the activation of the LysR-type regulator PltR and TetR-type regulator PltZ, which positively and negatively regulate the plt gene cluster, respectively (21). Similarly, 2,4-DAPG is recognized by TetR family repressors and PhlF of the 2,4-DAPG biosynthetic operon. 2,4-DAPG dissociates the repressor PhlF from the phlACBD operon to activate transcription (22). PhlH recognizes 2,4-DAPG to derepress the expression of PhlG and decrease the production of 2,4-DAPG (23). In addition to the autoregulation of PLT and 2,4-DAPG, there is evidence of cross-regulation between these compounds in the signaling pathway. Phloroglucinol (PG), an intermediate for the synthesis of 2,4-DAPG, is converted into chlorinated derivative PG-Cl and PG-Cl2 by halogenases encoded by pltM in the plt gene cluster from P. protegen Pf-5. These chlorinated derivatives function as an intraspecies signal regulating PltR to induce the transcription plt gene cluster (14, 24). Recent studies indicate that PLT negatively regulates the biosynthesis of 2,4-DAPG via PhlH in P. protegen Pf-5 (25). It elucidates that PLT as an intraspecies signal is crucial for the cross-regulation between the biosynthesis pathways of PLT and 2,4-DAPG within a single Pseudomonas strain. The interspecies competition between P. protegens and Pseudomonas capeferrum reveals that PLT produced by P. protegens can be part of intraspecies signaling, resulting in the subsequent repression of 2,4-DAPG biosynthetic gene clusters and cell lysis of P. capeferrum (26).
Rhizosphere microorganisms release vital signals that shape microbial communities, with antibiotics at low concentrations acting as intra- and interspecies signals. However, the mechanisms of these signals in coordinating gene expression are largely unclear. Investigating the potential of PLT as an interspecies signal is important for understanding the interspecies communications in Pseudomonas and its impact on biocontrol activity. Our current work demonstrates that non-PLT-producing P. fluorescens 2P24 senses subinhibitory concentrations of exogenous PLT to down-regulate the production of 2,4-DAPG via the TetR family repressors PhlH and PhlF. Pyoluteorin was identified as an interspecies signal in P. fluorescens 2P24, regulating the phl biosynthetic gene cluster for 2,4-DAPG production. TetR repressors PhlH and PhlF positively regulate 2,4-DAPG hydrolysis and negatively regulate its synthesis in response to pyoluteorin. Docking and structural analyses revealed the interaction of pyoluteorin with PhlH and PhlF, modulating gene expression. Phylogenetic analysis showed a wide distribution of PhlH and PhlF across Pseudomonas spp. with conserved ligand-binding domains (LBDs). These findings enhance understanding of the PLT-mediated interspecies signaling pathway, with implications for designing co-inhabiting Pseudomonas spp. as effective biocontrol agents.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions
The bacterial strains, plasmids, and primers used in this study are listed in Tables S1 and S2, respectively. P. fluorescens 2P24 and its derivatives were grown at 28°C at 200 rpm in liquid King’s B (KB) medium or agar plates. Escherichia coli and its derivatives were grown at 37°C at 200 rpm in Luria–Bertani (LB) broth or agar plates. When necessary, the bacterial growth media were supplemented with ampicillin (50 mg/L), kanamycin sulfate (30 mg/L), chloramphenicol (100 mg/L), sucrose (10%) (wt/vol), and isopropyl β-D-1-thiogalactopyranoside (0.2 mM).
Cell growth assay
All strains were grown to stationary phase in LB medium and then diluted in KB medium. The initial turbidity of each Pseudomonas fluorescens 2P24 culture was adjusted to an OD600 of 0.1. Each culture was grown in 100 mL of KB medium at 28°C. The cultures were treated with either 0 µM (control) or 20 or 40 µM of PLT. All cultures were incubated at 28°C for 50 h, and growth was monitored by measuring OD600 at regular intervals. The growth curves were recorded at 600 nm by a multimode reader. Three independent experiments were performed, and the error bars were calculated based on the data.
Construction of P. fluorescens 2P24 mutants and derivative strains
A two-step homologous recombination method was used to construct an in-frame deletion mutant of P. fluorescens 2P24. The DNA fragments containing both flanking sequences (~1 kb) of target genes were amplified by PCR using primers in Table S1. The DNA fragments were cloned into the suicide vector pK18mobsacB-Km. The resulting plasmids were conjugated into the 2P24 strain with the help of the donor E. coli S17-1 strain on the KB plate. After two homologous recombination and selection with high concentrations of sucrose, the wild-type copy was replaced by the deleted version. The deleted version replaced the wild type after two recombination events under high-sucrose stress. All the deletion mutants were confirmed by PCR amplification and sequencing.
Quantification of 2,4-DAPG
The production of 2,4-DAPG was quantified using high-performance liquid chromatography (HPLC) following the method described by Bonsall et al. (27). P. fluorescens 2P24 was inoculated at a starting OD600 of 0.02 in 50 mL King’s B medium. After 20, 28, and 34 h of the fermentation, with and without 20 μM PLT, 1 mL of culture broth was collected and centrifuged at 12,000 rpm for 10 min. The supernatant was filtered through a 0.22 µm mixed cellulose ester syringe filter and further analyzed by high-performance liquid chromatography (LC-2030plus, SHIMADZU, Japan) equipped with WondaSil C18-WR column (5 µm, 4.6 × 150 mm). Quantification of 2,4-DAPG was performed using a water/acetonitrile gradient (5%–100% acetonitrile with 0.1% trifluoroacetic acid) over 15 min at a wavelength of 270 nm, with a flow rate of 1 mL/min. The column was pre-equilibrated with 5% acetonitrile for 1 h. The composition of the mobile phase was changed by a linear gradient mode. The gradient was performed from 5% to 40% acetonitrile in 4.5 min, from 40% to 100% acetonitrile in 4.5 min, and then from 100% to 0% acetonitrile in 4 min, at a flow rate of 1.0 mL/min. The retention time was 8.9 min for 2,4-DAPG. Quantitation was based on a standard curve prepared using a chemical standard of 2,4-DAPG (Sigma-Aldrich).
Total RNA extraction, cDNA preparation, and quantitative real-time PCR assay
Total RNA was extracted by the TRIzol method. Samples were harvested by centrifugation at low temperatures, and then total RNA was extracted by TRIzol and chloroform. The cDNAs were synthesized by using RT-PCR Kit (HiScript III RT SuperMix for qPCR, Vazyme). For real-time PCR experiments, each PCR reaction (20 μL) contained 10 μL of AceQ Universal SYBR qPCR Master Mix (Vazyme), 2 μL of cDNA samples, and 0.2 μM primers. The reactions were performed in a QuantStudio 6 Flex Real-Time PCR System. Quantitative analysis of the initial template was achieved by real-time monitoring of the fluorescence signal changes during the PCR reaction. The 16S rRNA gene was set as an internal reference to normalize the data, and The −ΔΔCT method was used for relative quantification. Three independent experiments were performed, and the error bars were calculated.
Protein expression and purification
The recombinant protein of PhlH was expressed and purified, as described in our previous study (28). The phlF gene was amplified by PCR from P. fluorescens 2P24 (GenBank accession number DQ083928) and cloned into the modified pET28a vector (Novagene), which contains an N-terminal 6× His tag. The cloning junctions were confirmed by DNA sequencing. Each recombinant plasmid was transformed into the E. coli Rosetta (DE3) strain (Novagen). Cells were grown at 37°C in LB medium containing 100 mg/L kanamycin and 40 mg/L chloramphenicol. Expression of PhlF was induced at an OD600 of 0.6 by adding 0.2 mM isopropyl-β-D-1-thiogalactopyranoside followed by incubation at 16°C for 20 h. The cells were harvested and sonicated in 20 mM Tris–HCl buffer, pH 8.0, containing 100 mM NaCl in an ice-water bath. After centrifugation, the His-tagged fusion proteins were isolated with Ni-NTA affinity column (GE Healthcare) and purified by gel filtration (Superdex 75, GE Healthcare) in a buffer containing 20 mM Tris–HCl, pH 8.0, with 100 mM NaCl. The eluted proteins were collected and concentrated using centrifugal ultrafiltration for further study.
Electrophoretic mobility shift assay
6-Carboxyfluoresce in-labeled DNA fragments were obtained by annealing primers. The DNA fragments were added at a concentration of 0.2 µM and incubated at room temperature for 10 min with protein PhlH and PhlF in a buffer containing 0 mM Tris–HCl at pH 8.0, 0.5 mM KCl, 6 mM MgCl2, 0.1% (vol/vol) glycerol, 2 mM EDTA, and 1 mM dithiothreitol and 3 µM of human serum albumin in a total volume of 20 µL. Reactions were prepared by adding PLT to a final concentration of 0–500 µM or 0–1 mM. After incubation for 30 min at 25°C, the mixtures were directly subjected to 6% native PAGE with 1× Tris–acetate–EDTA buffer. Electrophoresis was performed at 80 V, 4°C, in an ice-cold bath. For 6-carboxyfluorescein-labeled probes, the images were collected and analyzed on a Typhoon FLA-9500 imaging system (GE Healthcare).
Isothermal titration calorimetry
Isothermal titration calorimetry (ITC) experiments were performed at 25°C, employing a MicroCal iTC200 instrument (GE Healthcare). The proteins (PhlH and PhlF) and ligands (2,4-DAPG and PLT) were dissolved in a buffer containing 20 mM Tris–HCl, pH 8.0, 100 mM NaCl, and 0.5% (vol/vol) dimethyl sulfoxide (DMSO) and degassed before use. The sample cell was loaded with 220 µL protein samples PhlH at 20 μM or PhlF at 50 μM, whereas the injection syringe was loaded with 40 µL PLT at 0.3 or 0.75 mM. An initial 0.4 µL injection, which was subsequently removed during data analysis, was followed by 19 injections of 2.0 µL each. These injections were spaced at 2 min intervals. Additionally, heats of dilution were determined by titrating the PLT into a solution buffer (20 mM Tris–HCl, pH 8.0, 100 mM NaCl, 0.5% (vol/vol) DMSO and subtracted from the raw titration data. Data analysis was performed with the MicroCal Origin software accompanying the ITC instrument.
Construction of transcriptional enhanced green fluorescent protein (EGFP) fusion and luminescence dose–response microplate assay
The upstream phlG gene promoter PphlG (147 bp) and the upstream phlA gene promoter PphlA (417 bp) were amplified from P. fluorescens 2P24 genomic DNA by PCR. Purified PCR products were digested with appropriate restriction enzymes and cloned ahead of a promoterless EGFP gene at the pSEVA225T-derived vector pSEVA225T-EGFP, respectively. The derived vector pSEVA225T-EGFP contains a promoter-free EGFP gene, which can be used to characterize the expression level of the target gene. After verification by sequencing, the constructed plasmid was transferred to the original P. fluorescens 2P24 and the corresponding deletion strain by shuttling strain E. coli S17-1, and the fluorescent reporter strain fused with EGFP was obtained (2P24::pSEVA225T-EGFP-PphlG, 2P24::pSEVA225T-EGFP-PphlA, ∆phlH::pSEVA225T-EGFP-PphlG, ∆phlF::pSEVA225T-EGFP-PphlA). The fused strains transformed into plasmids were grown to stationary phase at 28°C at 200 rpm in sterilized LB broth, then inoculated into sterilized KB medium in the presence or absence of 20 μM PLT and incubated for 7 h to a cell turbidity of 0.8 at 600 nm. Cultured bacterial solution (200 μL) was taken as the sample, and the luminescence of the sample was measured by a Varioskan Flash Spectral Scanning Multimode Reader (Thermo Scientific) at excitation and emission wavelengths of 485 and 510 nm and normalized to cell density. The software GraphPad Prism version 8 was used for data analysis and drawing. Assays were performed in at least three duplicates.
Phylogenetic tree construction
The phylogenetic tree was constructed using the bootstrap neighbor-joining (NJ) method with a confidence level of 1,000 replicates in MEGA version 11.0 (29). Bootstrap values were indicated at the nodes for robustness. Homologous PhlH and PhlF protein sequences from six Pseudomona strains producing 2,4-DAPG were retrieved from the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov) and the Pseudomonas Genome Database (https://www.pseudomonas.com/).
Statistics
Statistical analyses were performed using GraphPad Prism version 10.2.1. A two-way analysis of variance with multiple comparison test was used to assess significance among all groups. For comparisons between two means, a two-tailed Student’s t-test was applied. Unless otherwise stated, data are presented as mean ± standard deviation.
RESULTS
PLT contributes to the delayed growth of non-PLT-producing P. fluorescens 2P24 and inhibits the 2,4-DAPG production
Considering the limited research on the effects of environmental factors and nutrients on PLT production, it is difficult to fully determine the exact concentration of PLT synthesized in the environment. Pseudomonas PA1201 produces PLT at its highest concentration, reaching 24.5 mg/(OD600·L) at 24 h post-inoculation (hpi) and 48.3 mg/(OD600·L) at 48 hpi in nutrient-poor minimal medium, equivalent to 90 µM/OD600 and 177 µM/OD600, respectively (30) In comparison, the well-studied PLT-producing strain Pseudomonas protegens Pf-5 can produce PLT at concentrations as high as 0.635 mg/(OD600·L) in NBGly medium, equivalent to 2.3 µM/OD600 (24). Although the levels of PLT vary significantly between different strains, it still suggests that PLT-producing Pseudomonas strains may synthesize high levels of PLT in close proximity to other bacteria, with production influenced by environmental conditions and nutrient availability.
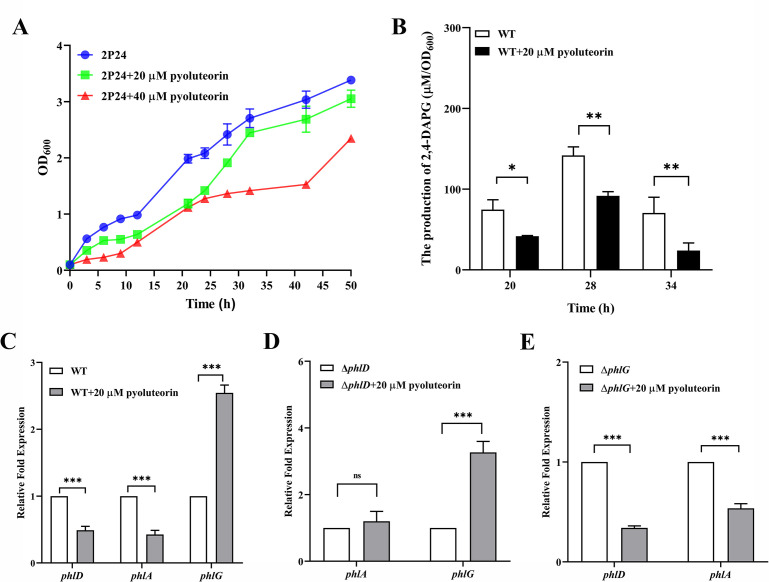
To assess the effects of PLT on bacterial physiology, we compared the growth characteristics of non-PLT-producing P. fluorescens 2P24 with and without 20 or 40 µM PLT. Our findings indicated that the presence of 20 µM PLT caused an initial suppression of the growth of P. fluorescens 2P24 during the lag phase. Furthermore, increasing the concentration of PLT to 40 µM resulted in a marked reduction in the growth starting from the exponential phase (Fig. 1A). The growth of P. fluorescens 2P24 exhibited a negative correlation with PLT, suggesting that subinhibitory levels of PLT suppressed bacterial growth and potentially affected its metabolic function. In PLT- and 2,4-DAPG-producing strain P. protegens Pf-5, transcriptional repressor PhlH from P. protegens Pf-5 (Pf-5_PhlH) binds to PLT to negatively regulate the biosynthesis of 2,4-DAPG (25). We further investigated the impact of PLT on 2,4-DAPG production by P. fluorescens 2P24. HPLC analysis revealed that treatment with 20 µM PLT consistently resulted in a significant reduction in 2,4-DAPG production, with up to a 50% decrease compared to P. fluorescens 2P24 without PLT treatment (Fig. 1B). These results suggest that even at low concentrations, PLT could substantially impede the biosynthesis of 2,4-DAPG in P. fluorescens 2P24.
Fig 1.
Effect of PLT on growth and 2,4-DAPG production of P. fluorescens 2P24 by regulating the transcription of 2,4-DAPG-related genes. (A) Effects of 20 and 50 µM PLT on the cell growth of P. fluorescens 2P24. The initial cell turbidity was 0.1 at 600 nm. (B) HPLC quantitative analysis of 2,4-DAPG in P. fluorescens 2P24 after the addition of 20 µM PLT. (C) Relative expression levels of phlA, phlD, and phlG were quantified by RT-qPCR using RNA extracted from P. fluorescens 2P24 in the absence or presence of 20 µM PLT, respectively, at 18 h post-inoculation. (D) Relative expression levels of phlA and phlG were quantified by RT-qPCR using RNA extracted from 2P24ΔphlD in the absence or presence of 20 µM PLT, respectively, at 18 h post-inoculation. (E) Relative expression levels of phlA and phlD were quantified by RT-qPCR using RNA extracted from 2P24ΔphlG in the absence or presence of 20 µM PLT, respectively, at 18 h post-inoculation. Error bars denote standard deviations of three independent replicates (n = 3). Statistical analyses were performed using t-test and two-way analysis of variance. *P < 0.05; **P < 0.01; ***P < 0.001. ns, non-significant; RT-qPCR, reverse transcription quantitative PCR; WT, wild type.
PLT alters 2,4-DAPG autoinduced signaling pathways by regulating the transcription of 2,4-DAPG-related genes
We treated P. fluorescens 2P24 with 20 µM PLT and subsequently utilized RT-qPCR to quantify the relative expression levels of phlA, phlD, and phlG. Compared to untreated P. fluorescens 2P24, the transcription levels of phlA and phlD decreased by more than half, while the transcription level of the phlG showed a onefold increase following 20 µM PLT treatment (Fig. 1C). Since PhlH typically derepresses the expression of PhlG under high levels of 2,4-DAPG, we speculated that the regulation of phlG expression by PLT may depend on PhlH, and PLT may induce the negative transcriptional regulation of phlA and phlD through alternative mechanisms distinct from the 2,4-DAPG autoinduction mediated by binding transcriptional repressor PhlF.
Considering the potential simultaneous effects of 2,4-DAPG and PLT on the transcription of phl genes, we then utilized the ΔphlD gene deletion mutant of P. fluorescens 2P24 (2P24ΔphlD), rendering the bacteria deficient in 2,4-DAPG synthesis. The 2P24ΔphlD mutant was confirmed to be deficient in 2,4-DAPG production. The transcriptional analysis of phlG was performed by RT-qPCR in the 2P24ΔphlD mutant with or without PLT. These results showed that the transcript levels of the phlG were significantly increased in 20 µM PLT compared to the control without PLT (Fig. 1D). This demonstrated that the regulatory mechanism governing phlG gene expression is mediated by PLT.
To further investigate the potential influence of the low levels of 2,4-DAPG on decreased transcription of phlA and phlD by affecting PhlF, we generated the ΔphlG gene deletion mutant of P. fluorescens 2P24 (2P24ΔphlG) with the increased production of 2,4-DAPG identified by HPLC. Further analysis of phlA and phlD transcription in 2P24ΔphlG mutant treated with or without 20 µM PLT showed that PLT significantly inhibited phlA and phlD in the 2P24ΔphlG mutant (Fig. 1E). This suggests that PLT is involved in the down-regulation of the phlACB operon. We also examined phlA and phlD expression in a phlF deletion mutant (2P24ΔphlF), with or without PLT treatment. The results indicated that deletion of phlF did not affect the expression levels of phlA and phlD under these conditions (Fig. S1). Overall, these findings imply that PLT affects the expression of phl genes in P. fluorescens 2P24 through both 2,4-DAPG-independent signaling pathways, highlighting the exogenous PLT signaling regulatory network governing 2,4-DAPG production in this bacterium.
Transcription regulators PhlF and PhlH can bind PLT to regulate the phl biosynthesis gene cluster
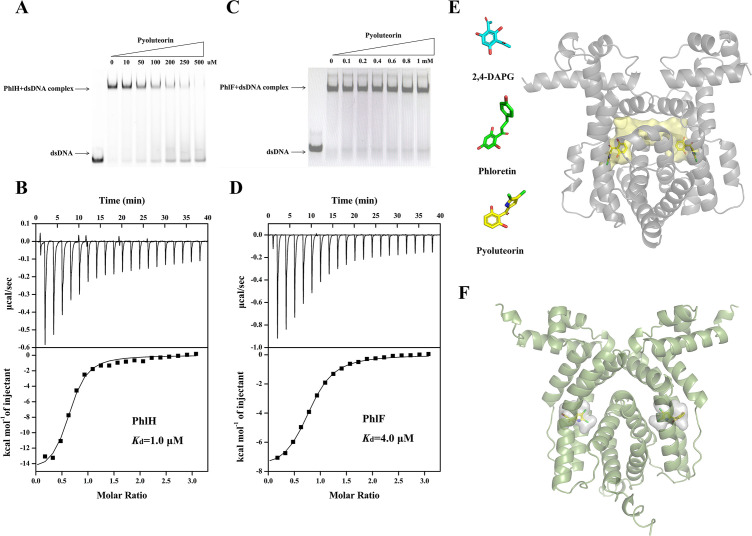
The PhlH from P. fluorescens 2P24 (2P24_PhlH) shows 69% sequence identity with Pf-5_PhlH. We deduced that 2P24_PhlH could recognize heterologous PLT to induce the expression of phlG. Electrophoretic mobility shift assays (EMSAs) were performed to monitor if PLT induces the dissociation of purified His6-tagged PhlH and the 30 bp DNA fragment of the promoter region of phlG (PphlG). PhlH binds to the phlG operator sequence between phlH and phlG, which is released from DNA with an increased PLT concentration (Fig. 2A). The structure of 2P24_PhlH in its 2,4-DAPG-bound form reveals a hydrophobic tunnel-like cavity that confers promiscuity for binding hydrophobic ligands such as 2,4-DAPG and plant flavonoid phloretin (28). PLT shares a similar phloroglucinol moiety with 2,4-DAPG and phloretin, which can form a similar mode of interaction with PhlH by engaging in π–π stacking with the aromatic amino acid Phe. Molecular docking analysis revealed that PLT occupies a similar binding site as 2,4-DAPG in 2P24_PhlH (Fig. 2E). ITC confirmed that 2P24_PhlH has a high binding affinity for PLT, with a dissociation constant (Kd) of 1 µM, which is much smaller than the dissociation constant for the 2P24_PhlH–2,4-DAPG interaction (Table 1; Fig. 2B). This implies that 2,4-DAPG and PLT compete for binding to 2P24_PhlH, with PLT exhibiting higher affinity.
Fig 2.
Transcription regulators PhlF and PhlH can bind PLT for regulating the phl biosynthesis gene cluster. (A and C) Electrophoretic mobility shift assays of 2P24_PhlH (A) or 2P24_PhlF (C) with the upstream region of phlG (free DNA) in the increasing concentrations (0–500 µM) of PLT or with the upstream region of phlG (free DNA) in the rising concentrations (0–1 mM) of PLT, respectively. (B and D). The binding affinities of 2P24_PhlH (B) or 2P24_PhlF (D) with PLT were evaluated using ITC analysis. The binding curves corrected for the dilution effects were fit to a one-site binding model, and the Kd values were calculated by the NanoAnalyze software. (E) Cartoon representation of 2P24_PhlH in complex with the docked PLT. 2P24_PhlH is in light gray. The ligand-binding tunnel is the hydrophobic tunnel in ligand recognition and is yellow as a transparent surface. 2,4-DAPG, phloretin, and PLT are shown as sticks in cyan, green, and yellow, respectively. (F) 2P24_PhlF model calculated using AlphaFold and cartoon representation of model 2P24_PhlF in complex with the docked PLT. Model 2P24_PhlF is in light green. The ligand-binding pocket is predicted ligand recognition and is light gray as a transparent surface. The bound docked PLT is shown as sticks in green. All structure figures were prepared with PyMOL.
TABLE 1.
Thermodynamic parameters obtained by isothermal titration calorimetry for TetR-type repressors PhlH and PhlF binding to PLT or 2,4-DAPG
| Protein | Ligand | Ka (Μ−1) | Kd (μΜ) | ΔG (kcal/mol) | ΔH (kcal/mol) | TΔS (kcal/mol) |
|---|---|---|---|---|---|---|
| PhlH | PLT | 9.75 × 105 | 1.0 | −8.5 | −15.3 | −7.2 |
| PhlHa | 2,4-DAPGa | 1.17 × 105a | 8.5a | −6.8a | −12.2a | −5.4a |
| PhlF | PLT | 2.47 × 105 | 4.0 | −7.3 | −8.0 | −0.7 |
Data from reference (26).
The transcription levels of phlA and phlD were decreased by heterologous PLT, and PhlF as repressor down-regulate expression of phlA and phlD in P. fluorescens 2P24. EMSAs were conducted to check for the effect of PLT on the dissociation of purified His6-tagged PhlF from the operator DNA sequence between phlF and phlA (Fig. 2C). Although 2P24_PhlF shares only 20% of sequence identity with 2P24_PhlH, it has a high similarity with the ligand-binding site, especially conserved residue Phe for binding phloroglucinol moiety of ligands via π–π stacking. Structural modeling and docking of 2P24_PhlF, predicted using AlphaFold (31) and AutoDock Vina (32), revealed a similar hydrophobic pocket with conserved residue Phe to bind PLT (Fig. 2F). ITC experiments showed that 2P24_PhlF can bind to PLT with a Kd of 4 μM (Table 1; Fig. 2D). Interestingly, PLT does not dissociate PhlF from the DNA but exhibits a significantly high affinity for PhlF, indicating that PLT acts as an inhibitor targeting PhlF, potentially preventing PhlF dissociation from DNA and enhancing the repression of phlD and phlA transcription.
PLT is required for transcription regulators PhlF and PhlH to impact the phl biosynthesis gene expression in vivo
To confirm the roles of PhlH and PhlF in regulating the in vivo expression of phlG and phlA in response to exogenous PLT, we constructed PhlH or PhlF deletion mutants using P. fluorescens 2P24 as the parental strain for subsequent promoter-EGFP analysis. We then measured the EGFP fluorescence of the mutants and their parental strain in the presence or absence of PLT. Upon the addition of 20 µM PLT, the phlG-EGFP reporter activity was increased in the wild-type strain, but no significant changes were observed in the ΔphlH strain (Fig. 3A). This indicates that the induction of phlG expression by PLT was dependent on PhlH. Conversely, the phlA-EGFP reporter activity was decreased in the wild-type strain, but no significant changes were observed in the ΔphlF strain (Fig. 3B), indicating that the repression of phlA expression by PLT was dependent on PhlF.
Fig 3.
In response to PLT, PhlH regulates the expression of phlG, while PhlF regulates the expression of phlA. (A) The fluorescent activity of the pSEVA225T-EGFP-PphlG plasmid was evaluated in WT and ΔphlH strains to test the expression of phlG. The bars illustrate the relative fluorescent units (RFU) normalized to a 1 mL culture with OD600 = 1. (B) The fluorescent activity of the pSEVA225T-EGFP-PphlA plasmid was evaluated in WT and ΔphlF strains to test the expression of phlA. (C–E) 2,4-DAPG production of 2P24ΔphlH (C), 2P24ΔphlF (D), and 2P24ΔphlHΔphlF (E) strains grown in KB medium with or without 20 µM PLT. Error bars denote standard deviations of three independent replicates (n = 3). Statistical analyses were performed using t-test and two-way analysis of variance. *P < 0.05, **P < 0.01, ***P < 0.001. ns, non-significant.
Further analysis using HPLC confirmed a significant reduction in 2,4-DAPG production in the 2P24ΔphlH mutant, nearing 30 µM, with even lower levels observed after treatment with 20 µM PLT. This suggests that PhlF continues to repress phl biosynthesis gene transcription by sensing PLT (Fig. 3C). Similarly, HPLC analysis showed a significant increase in 2,4-DAPG in the 2P24ΔphlF mutant, reaching approximately 600 µM. Upon treatment with 20 µM PLT, the levels of 2,4-DAPG decreased further in the 2P24ΔphlF mutant, indicating that PhlH derepresses phlG transcriptions by sensing PLT (Fig. 3D). Further investigation using HPLC analysis to determine 2,4-DAPG production in the 2P24ΔphlHΔphlF double mutant treated with or without 20 µM PLT revealed no significant changes (Fig. 3E). This demonstrates that PhlH and PhlF are the main regulators impacting phl biosynthesis gene expression by sensing exogenous PLT.
Phylogenetic analysis of 2,4-DAPG gene clusters and homolog identification of its transcriptional factor in signaling
The metabolic co-regulation between 2,4-DAPG and PLT biosynthetic pathways has been identified in strains of P. protegens Pf-5 (14, 25). Besides acting as biocontrol agents against different pathogens, 2,4-DAPG and PLT are significant for intraspecies signaling and cross-regulation in P. protegens Pf-5. TetR-type repressors PhlH and PhlF play a crucial role in recognizing signals to regulate 2,4-DAPG biosynthesis. To understand the conservation of phl gene clusters, we compared PhlH and PhlF complete genomes available on the NCBI database. We used a phl gene cluster (phlHGFACBD) of P. protegens Pf-5 as a query to search genomic databases and classified them into two major groups. One includes both PLT and 2,4-DAPG-producing Pseudomonas protegens strains like P. protegens Pf-5, CHA0, H78, and FD6, while the other comprises only 2,4-DAPG-producing strains like P. fluorescens 2P24 and F113 (Fig. 4A).
Fig 4.
The PhlF and PhlH share similarities with the ligand-binding domain (LBD). (A) Phylogenetic tree of TetR-type repressors PhlH and PhlF in representative strains form Pseudomonas spp. constructed using the neighbor-joining method. (B) Multiple sequence alignment of PhlH and PhlF proteins from representative Pseudomonas spp. Residues involved in binding 2,4-DAPG are shown in green boxes. The alignment is performed using MultAlin (33) and ESPript (34). The secondary structural elements of 2P24_PhlH are displayed at the top of the alignment. (C) The superposition of 2,4-DAPG-bound PhlH (PDB code 7E1N) (gray) and model PLT-bound PhlF (green) dimers is shown in the cartoon representation (top). The N-terminal DNA-binding domain (DBD) comprises three helices, with helices α2 and α3 forming the conventional helix-turn-helix-DNA-binding motif. The LBD is composed of the remaining helices. 2,4-DAPG and the bound docked PLT are shown as sticks in gray and green, respectively. A close-up view of the interaction mode between the AlphaFold-predicted PhlF (shown in transparent green) and the docked PLT (shown in dark green) is displayed at the bottom. The PLT-binding pocket, along with aromatic residues in the vicinity of PLT (highlighted in green), is shown as a stick, illustrating the potential interactions involved in the ligand-binding process. All structure figures were prepared with PyMOL.
To explore the functions of these regulators in sensing signals, we analyzed the homology of PhlH and PhlF from representative strains P. protegens (Pf-5, CHA0, H78, and FD6) and P. fluorescens (2P24 and F113) from two groups. Using the identified PhlH and PhlF proteins, we constructed a phylogenetic tree with a bootstrap value with 1,000 replicates through NJ. These analyses revealed a common consensus for PhlH and PhlF, consistent with the classification group from phl gene cluster comparison. Although PhlH had low sequence similarity to PhlF, both belong to the TetR-family, featuring N-terminal DNA-binding domains (DBDs) with a helix-turn-helix motif and C-terminal LBDs. Multiple alignments of PhlH and PhlF from the two groups revealed that its ligand-binding domains are more conserved than their DNA-binding domains, indicating that the ligand-binding domains from PhlH and PhlF are evolutionarily related (Fig. 4B). Interestingly, both PhlH and PhlF from P. fluorescens 2P24 can sense 2,4-DAPG, suggesting similar ligand-binding sites. Superposition of 2,4-DAPG-bound PhlH and model PLT-bound PhlF dimers from P. fluorescens 2P24 revealed that the LBD showed a similar interior pocket for ligand binding, surrounded by helices. The Phe165 residue from PhlF is conserved with Phe173 in PhlH from P. fluorescens 2P24, which plays a crucial role in facilitating the binding of the phloroglucinol moiety of 2,4-DAPG through π–π stacking interactions. Additionally, aromatic residues adjacent to the PLT-binding pocket, such as Tyr69, Trp100, Phe109, and Trp169 in PhlF, may also contribute significantly to binding, as predicted by the AlphaFold model (Fig. 4C). This suggests that both PhlH and PhlF in Pseudomonas spp. may share a similar mechanism for the recognition and binding of PLT to sense the signal molecule. However, while PLT binds directly to PhlF, it does not induce dissociation of PhlF from DNA. This suggests that PLT binding does not trigger the typical structural rearrangements in molecular switches of PhlF that are generally involved in the allosteric regulation of transcription for the phl gene cluster. These findings imply that PhlF may employ an atypical allosteric switching mechanism.
DISCUSSION
Rhizosphere microorganisms have evolved the capability to produce and release signals that play crucial roles in shaping microbial communities, influencing both microbial competition and cooperation (2). Among these signals, antibiotics at low and non-inhibitory concentrations serve as intra- and interspecies signals, modulating microbial growth and activity (7). However, the precise mechanisms by which these antibiotics act as interspecies signals to coordinate gene expression remain largely unexplored. In our study, we elucidate the role of exogenous PLT as a key component in an interspecies signaling pathway that regulates the expression of phl biosynthesis genes for 2,4-DAPG production, which are integral to biological activity. Interestingly, although PLT and 2,4-DAPG have been identified as biological agents against plant pathogens, there appears to be cross-regulation between these compounds in Pseudomonas strains, indicating a complex interplay among rhizosphere microbial species with implications for plant health and disease resistance.
Antibiotics are effective against competitors only above a certain threshold concentration, requiring a sufficiently high density of cells (35). Moreover, the production of antibiotics, which can impose metabolic costs on cooperation, is closely related to bacterial community stability within environmental conditions (36). Polyketide antibiotics, 2,4-DAPG and PLT, are produced by a wide range of Pseudomonas spp. in soil and rhizosphere environments (10). 2,4-DAPG is biosynthesized through the decarboxylative condensation of malonyl-CoA-derived extender units in a process similar to fatty acid synthesis (37). On the other hand, the hybrid polyketide-non-ribosomal peptide molecule PLT involved condensation of malonyl-CoA to generate a resorcinol ring with chlorinated pyrrole moiety (38). Interestingly, these two biosynthetic pathways may compete for utilizing malonyl-CoA, potentially impacting the production of these polyketide antibiotics. It presents extensive cross-regulation between the synthesis of 2,4-DAPG and PLT in P. protegens Pf-5. The 2,4-DAPG biosynthetic intermediate chlorinated phloroglucinol can function as a signal regulating PLT production (14). Moreover, in response to 2,4-DAPG, the PLT biosynthetic regulator PltZ homolog from Pseudomonas aeruginosa American Type Culture Collection 27853 can induce a putative ABC transporter and enhance antibiotic resistance (39). The biosynthesis of 2,4-DAPG is negatively regulated by PhlH sensing to PLT in P. protegen Pf-5 (25). The sequential signaling pathway between P. protegens and P. capeferrum shows that a high level of PLT induced by P. capeferrum results in the lysis of P. capeferrum and subsequently inhibits 2,4-DAPG production of P. protegens (26). Taken together, these works show that interspecies signaling between 2,4-DAPG and PLT influences biological control activity and bacterial communication in soil and rhizosphere environments.
Considering the perspective of these antibiotics as intraspecies signaling, we propose that PLT can act as an interspecies signal to manipulate non-PLT but 2,4-DAPG-producing Pseudomonas strains, thereby facilitating communication among microorganisms in the rhizosphere. P. fluorescens 2P24, isolated from wheat rhizosphere in take-all decline soil, produces antimicrobial metabolite 2,4-DAPG (40). This compound exhibits broad-spectrum antifungal activities, effectively suppressing soil-borne pathogens such as Fusarium oxysporum, Septoria tritici, Thielaviopsis basicola, and Rhizoctonia solani (41). PhlH and PhlF belong to the TetR family, and both act as transcriptional repressors of the 2,4-DAPG biosynthetic genes. PhlF repression is relieved by 2,4-DAPG, leading to positive feedback regulation of 2,4-DAPG biosynthesis (22). Conversely, PhlH, in response to 2,4-DAPG, provides negative-feedback regulation of 2,4-DAPG biosynthesis (23).
The DBD of the TetR family repressor is well known for its role in interacting with the DNA major groove, and its LBD is responsible for binding a wide range of diverse ligands (42). In our previous study, crystal structures of PhlH in both its apo form and 2,4-DAPG-bound form revealed its ligand-recognizing and allosteric switching mechanisms. PhlH harbors a long, hydrophobic ligand-binding tunnel that is essential for ligand-induced DNA dissociation from PhlH, suggesting promiscuity binding modes for diverse ligands such as 2,4-DAPG or several plant-derived flavonoids phloretin (28). Interestingly, the LBD between PhlH and PhlF shows higher sequence similarity than its DBD, implying PhlH and PhlF possibly share a similar ligand-binding pocket. Subsequent analysis shows that PhlF can bind to PLT at the micromolar range and repress the transcription of phlA. Structural predictions showed that PhlF presents a hydrophobic inner pocket in its LBD, where PLT molecules can be docked, reminiscent of the crystal structure of 2,4-DAPG-bound PhlH. In addition to PLT, the production of 2,4-DAPG can be inhibited by the mycotoxin fusaric acid from Fusarium spp. through an unknown mechanism (43). Moreover, plant-derived flavonoids, including phloretin, participate in the repression of 2,4-DAPG production (44). These studies imply that a wide range of interspecies signals play a crucial role in bacterial metabolic co-regulation of 2,4-DAPG synthesis.
In summary, our study provides new insights into interspecies signaling, demonstrating how PLT inhibits 2,4-DAPG production in P. fluorescens 2P24 by finely tuning the repressors PhlH and PhlF (Fig. 5). This regulatory mechanism effectively manages the trade-off between the costs and benefits of antibiotic production, especially in complex and dynamic environmental conditions. Specifically, the binding of PLT to PhlH leads to the dissociation of PhlH from the promotor region, enabling RNA polymerase to initiate the transcription of the phlG gene, which promotes the hydrolysis of 2,4-DAPG. Conversely, PLT binding to PhlF does not induce protein–DNA dissociation from the phlA promoter, confirming its role in maintaining a repressed state of PhlF, thereby inhibiting the synthesis of 2,4-DAPG. These findings not only unveil a novel function of the PhlH and PhlF repressors in P. fluorescens 2P24, which coordinates secondary metabolic pathways by sensing the interspecies signal PLT, but also suggest a potentially widespread and conserved mechanism among Pseudomonas spp. This mechanism, mediated by TetR family regulators, could play a crucial role in modulating microbial competition, survival strategies, and cell–cell communication.
Fig 5.
Schematic representation of TetR repressors PhlH and PhlF from the 2,4-DAPG biosynthetic gene cluster positively regulated the hydrolysis of 2,4-DAPG and negatively regulated 2,4-DAPG synthesis in response to interspecies signal PLT.
ACKNOWLEDGMENTS
We thank the staff for providing technical support by using the facility of the School of Life Sciences and the Institute of Health Sciences and Technology of Anhui University.
This work was supported by grants from the National Natural Science Foundation of China (grant nos. 31970103, 31971363, 32370196, and 32470120), Major Research Project of Scientific Research in Higher Education Institutions of Anhui Province (2024AH040006), the Gansu Research Program (22C × 8NA001), and the Gansu Association for Science and Technology (GSHZSF2023-06).
N.Z., Y.-X.H., and H.G.: conceptualization and supervision; N.Z., X.Z., J.L., Y.-X.H., H.G., and L.-Q.Z.: methodology; N.Z., X.Z., X.T., J.L., Q.T., X.L., L.-M.L., Y.-X.H., and H.G.: formal analysis; N.Z. and X.Z.: writing (original draft); N.Z., X.Z., X.T., J.L., Y.-X.H., and H.G.: investigation; N.Z., Y.-X.H., and H.G.: writing (review and editing); N.Z., X.Z., X.T., J.L., and Y.-X.H.: software; N.Z., Y.-X.H., and H.G.: project administration; N.Z., Y.-X.H., and H.G.: funding acquisition; X.Z., X.T., J.L., Q.T., X.L., and L.-M.L.: data curation; X.Z., X.T., and J.L.: validation; P.Z. and L.-Q.Z.: resources.
Contributor Information
Nannan Zhang, Email: zhangnn@ahu.edu.cn.
Yong-Xing He, Email: heyx@lzu.edu.cn.
Honghua Ge, Email: hhge@ahu.edu.cn.
Erik F. Y. Hom, University of Mississippi, University, Mississippi, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.01886-24.
Tables S1 and S2; Fig. S1.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Zhalnina K, Louie KB, Hao Z, Mansoori N, da Rocha UN, Shi S, Cho H, Karaoz U, Loqué D, Bowen BP, Firestone MK, Northen TR, Brodie EL. 2018. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol 3:470–480. doi: 10.1038/s41564-018-0129-3 [DOI] [PubMed] [Google Scholar]
- 2. Venturi V, Keel C. 2016. Signaling in the rhizosphere. Trends Plant Sci 21:187–198. doi: 10.1016/j.tplants.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 3. Zhou L, Zhang L-H, Cámara M, He Y-W. 2017. The DSF family of quorum sensing signals: diversity, biosynthesis, and turnover. Trends Microbiol 25:293–303. doi: 10.1016/j.tim.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 4. Bitas V, Kim H-S, Bennett JW, Kang S. 2013. Sniffing on microbes: diverse roles of microbial volatile organic compounds in plant health. Mol Plant Microbe Interact 26:835–843. doi: 10.1094/MPMI-10-12-0249-CR [DOI] [PubMed] [Google Scholar]
- 5. Zhu J, Zhang Y, Deng J, Jiang H, Zhuang L, Ye W, Ma J, Jiang J, Feng L. 2019. Diketopiperazines synthesis gene in Shewanella baltica and roles of diketopiperazines and resveratrol in quorum sensing. J Agric Food Chem 67:12013–12025. doi: 10.1021/acs.jafc.9b04620 [DOI] [PubMed] [Google Scholar]
- 6. Raaijmakers JM, Mazzola M. 2012. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu Rev Phytopathol 50:403–424. doi: 10.1146/annurev-phyto-081211-172908 [DOI] [PubMed] [Google Scholar]
- 7. Andersson DI, Hughes D. 2014. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12:465–478. doi: 10.1038/nrmicro3270 [DOI] [PubMed] [Google Scholar]
- 8. Preston GM. 2004. Plant perceptions of plant growth-promoting Pseudomonas. Philos Trans R Soc Lond B Biol Sci 359:907–918. doi: 10.1098/rstb.2003.1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bender CL, Rangaswamy V, Loper J. 1999. Polyketide production by plant-associated pseudomonads. Annu Rev Phytopathol 37:175–196. doi: 10.1146/annurev.phyto.37.1.175 [DOI] [PubMed] [Google Scholar]
- 10. Gross H, Loper JE. 2009. Genomics of secondary metabolite production by Pseudomonas spp. Nat Prod Rep 26:1408–1446. doi: 10.1039/b817075b [DOI] [PubMed] [Google Scholar]
- 11. Biessy A, Filion M. 2021. Phloroglucinol derivatives in plant-beneficial Pseudomonas spp.: biosynthesis, regulation, and functions. Metabolites 11:182. doi: 10.3390/metabo11030182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pavkov-Keller T, Schmidt NG, Żądło-Dobrowolska A, Kroutil W, Gruber K. 2019. Structure and catalytic mechanism of a bacterial friedel-crafts acylase. Chembiochem 20:88–95. doi: 10.1002/cbic.201800462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bottiglieri M, Keel C. 2006. Characterization of PhlG, a hydrolase that specifically degrades the antifungal compound 2,4-diacetylphloroglucinol in the biocontrol agent Pseudomonas fluorescens CHA0. Appl Environ Microbiol 72:418–427. doi: 10.1128/AEM.72.1.418-427.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan Q, Philmus B, Chang JH, Loper JE. 2017. Novel mechanism of metabolic co-regulation coordinates the biosynthesis of secondary metabolites in Pseudomonas protegens. Elife 6:e22835. doi: 10.7554/eLife.22835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loper JE, Hassan KA, Mavrodi DV, Davis EW 2nd, Lim CK, Shaffer BT, Elbourne LDH, Stockwell VO, Hartney SL, Breakwell K, et al. 2012. Comparative genomics of plant-associated Pseudomonas spp.: insights into diversity and inheritance of traits involved in multitrophic interactions. PLoS Genet 8:e1002784. doi: 10.1371/journal.pgen.1002784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang X, Wang Z, Liu Y, Zhang X. 2017. Complete genome sequence of Pseudomonas protegens H78, a plant growth–promoting rhizobacterium. Genome Announc 5:e00233-17. doi: 10.1128/genomeA.00233-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Y, Shi H, Wang Z, Huang X, Zhang X. 2018. Pleiotropic control of antibiotic biosynthesis, flagellar operon expression, biofilm formation, and carbon source utilization by RpoN in Pseudomonas protegens H78. Appl Microbiol Biotechnol 102:9719–9730. doi: 10.1007/s00253-018-9282-0 [DOI] [PubMed] [Google Scholar]
- 18. Huang X, Zhu D, Ge Y, Hu H, Zhang X, Xu Y. 2004. Identification and characterization of pltZ, a gene involved in the repression of pyoluteorin biosynthesis in Pseudomonas sp. M18. FEMS Microbiol Lett 232:197–202. doi: 10.1016/S0378-1097(04)00074-6 [DOI] [PubMed] [Google Scholar]
- 19. Souza JT, Raaijmakers JM. 2003. Polymorphisms within the prnD and pltC genes from pyrrolnitrin and pyoluteorin-producing Pseudomonas and Burkholderia spp. FEMS Microbiol Ecol 43:21–34. doi: 10.1111/j.1574-6941.2003.tb01042.x [DOI] [PubMed] [Google Scholar]
- 20. Wu X-G, Duan H-M, Tian T, Yao N, Zhou H-Y, Zhang L-Q. 2010. Effect of the hfq gene on 2,4-diacetylphloroglucinol production and the PcoI/PcoR quorum-sensing system in Pseudomonas fluorescens 2P24. FEMS Microbiol Lett 309:16–24. doi: 10.1111/j.1574-6968.2010.02009.x [DOI] [PubMed] [Google Scholar]
- 21. Yan Q, Liu M, Kidarsa T, Johnson CP, Loper JE. 2021. Two pathway-specific transcriptional regulators, PltR and PltZ, coordinate autoinduction of pyoluteorin in Pseudomonas protegens Pf-5. Microorganisms 9:1489. doi: 10.3390/microorganisms9071489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schnider-Keel U, Seematter A, Maurhofer M, Blumer C, Duffy B, Gigot-Bonnefoy C, Reimmann C, Notz R, Défago G, Haas D, Keel C. 2000. Autoinduction of 2,4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J Bacteriol 182:1215–1225. doi: 10.1128/JB.182.5.1215-1225.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan X, Yang R, Zhao R-X, Han J-T, Jia W-J, Li D-Y, Wang Y, Zhang N, Wu Y, Zhang L-Q, He Y-X. 2017. Transcriptional regulator PhlH modulates 2,4-diacetylphloroglucinol biosynthesis in response to the biosynthetic intermediate and end product. Appl Environ Microbiol 83:e01419-17. doi: 10.1128/AEM.01419-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kidarsa TA, Goebel NC, Zabriskie TM, Loper JE. 2011. Phloroglucinol mediates cross-talk between the pyoluteorin and 2,4-diacetylphloroglucinol biosynthetic pathways in Pseudomonas fluorescens Pf-5. Mol Microbiol 81:395–414. doi: 10.1111/j.1365-2958.2011.07697.x [DOI] [PubMed] [Google Scholar]
- 25. Luo L-M, Xu H, Zhang N, Ge H, Xiang Y, Yang H, He Y-X. 2024. Pyoluteorin regulates the biosynthesis of 2,4-DAPG through the TetR family transcription factor PhlH in Pseudomonas protegens Pf-5. Appl Environ Microbiol 90:e0174323. doi: 10.1128/aem.01743-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hansen ML, Wibowo M, Jarmusch SA, Larsen TO, Jelsbak L. 2022. Sequential interspecies interactions affect production of antimicrobial secondary metabolites in Pseudomonas protegens DTU9.1. ISME J 16:2680–2690. doi: 10.1038/s41396-022-01322-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bonsall RF, Weller DM, Thomashow LS. 1997. Quantification of 2,4-diacetylphloroglucinol produced by fluorescent Pseudomonas spp. in vitro and in the rhizosphere of wheat. Appl Environ Microbiol 63:951–955. doi: 10.1128/aem.63.3.951-955.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang N, Wu J, Zhang S, Yuan M, Xu H, Li J, Zhang P, Wang M, Kempher ML, Tao X, Zhang L-Q, Ge H, He Y-X. 2022. Molecular basis for coordinating secondary metabolite production by bacterial and plant signaling molecules. J Biol Chem 298:102027. doi: 10.1016/j.jbc.2022.102027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tamura K, Stecher G, Kumar S. 2021. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027. doi: 10.1093/molbev/msab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cui Y, Song K, Jin Z-J, Lee L-H, Thawai C, He Y-W. 2023. Fructose promotes pyoluteorin biosynthesis via the CbrAB-CrcZ-Hfq/Crc pathway in the biocontrol strain Pseudomonas PA1201. Synth Syst Biotechnol 8:618–628. doi: 10.1016/j.synbio.2023.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, et al. 2021. Highly accurate protein structure prediction with AlphaFold. Nature 596:583–589. doi: 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trott O, Olson AJ. 2010. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. doi: 10.1002/jcc.21334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Corpet F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16:10881–10890. doi: 10.1093/nar/16.22.10881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gouet P, Robert X, Courcelle E. 2003. ESPript/ENDscript: extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res 31:3320–3323. doi: 10.1093/nar/gkg556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bernier SP, Surette MG. 2013. Concentration-dependent activity of antibiotics in natural environments. Front Microbiol 4:20. doi: 10.3389/fmicb.2013.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kelsic ED, Zhao J, Vetsigian K, Kishony R. 2015. Counteraction of antibiotic production and degradation stabilizes microbial communities. Nature 521:516–519. doi: 10.1038/nature14485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Achkar J, Xian M, Zhao H, Frost JW. 2005. Biosynthesis of phloroglucinol. J Am Chem Soc 127:5332–5333. doi: 10.1021/ja042340g [DOI] [PubMed] [Google Scholar]
- 38. Dorrestein PC, Yeh E, Garneau-Tsodikova S, Kelleher NL, Walsh CT. 2005. Dichlorination of a pyrrolyl-S-carrier protein by FADH2-dependent halogenase PltA during pyoluteorin biosynthesis. Proc Natl Acad Sci U S A 102:13843–13848. doi: 10.1073/pnas.0506964102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo D-D, Luo L-M, Ma H-L, Zhang S-P, Xu H, Zhang H, Wang Y, Yuan Y, Wang Z, He Y-X. 2020. The regulator PltZ regulates a putative ABC transporter system PltIJKNOP of Pseudomonas aeruginosa ATCC 27853 in response to the antimicrobial 2,4-diacetylphloroglucinol. Front Microbiol 11:1423. doi: 10.3389/fmicb.2020.01423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wei H-L, Zhang L-Q. 2006. Quorum-sensing system influences root colonization and biological control ability in Pseudomonas fluorescens 2P24. Antonie Van Leeuwenhoek 89:267–280. doi: 10.1007/s10482-005-9028-8 [DOI] [PubMed] [Google Scholar]
- 41. Gong L, Tan H, Chen F, Li T, Zhu J, Jian Q, Yuan D, Xu L, Hu W, Jiang Y, Duan X. 2016. Novel synthesized 2, 4-DAPG analogues: antifungal activity, mechanism and toxicology. Sci Rep 6:32266. doi: 10.1038/srep32266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cuthbertson L, Nodwell JR. 2013. The TetR family of regulators. Microbiol Mol Biol Rev 77:440–475. doi: 10.1128/MMBR.00018-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Quecine MC, Kidarsa TA, Goebel NC, Shaffer BT, Henkels MD, Zabriskie TM, Loper JE. 2015. An interspecies signaling system mediated by fusaric acid has parallel effects on antifungal metabolite production by Pseudomonas protegens strain Pf-5 and antibiosis of Fusarium spp. Appl Environ Microbiol 82:1372–1382. doi: 10.1128/AEM.02574-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu X-Q, Yan X, Zhang M-Y, Zhang L-Q, He Y-X. 2020. Flavonoids repress the production of antifungal 2,4-DAPG but potentially facilitate root colonization of the rhizobacterium Pseudomonas fluorescens. Environ Microbiol 22:5073–5089. doi: 10.1111/1462-2920.15052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2; Fig. S1.