Abstract
Extracellular vesicles (EVs) are derived from outer membranes (OMs) in Gram-negative bacteria and have diverse physiological functions. EV-mediated secretion of monovinyl protochlorophyllide (MV-Pchlide), the chlorophyll a (Chl) biosynthetic intermediate, was previously reported in a mutant lacking dark-operative Pchlide reductase in the cyanobacterium Leptolyngbya boryana. This study showed a detailed characterization of EVs from wild-type (WT) strain of L. boryana grown under photoautotrophic and dark heterotrophic conditions, focusing on the accumulation of Chl intermediates. WT L. boryana cells produce two types of EVs, low-density EVs (L-EVs) and high-density EVs (H-EVs), both under light and dark conditions. L-EVs and H-EVs showed distinct morphological features and protein compositions. L-EVs from cells grown under both light and dark conditions commonly contained carotenoids, ketomyxol glycoside and zeaxanthin as major pigments. Based on the protein compositions of EVs and other cellular membrane fractions, L-EVs and H-EVs are probably derived from low-density OMs and high-density OMs interacting with cell walls, respectively. Fluorescence detection of pigments was applied to EVs, and the two Chl intermediates, protoporphyrin IX and protoporphyrin IX monomethyl ester, were commonly detected in both L-EVs from light- and dark-grown cells, whereas L-EVs from dark-grown cells contained additional MV-Pchlide, MV-protopheophorbide and pheophorbide. The pigment ratios of L-EVs to the total culture medium of the Chl intermediates were much higher than those of carotenoids, suggesting an active transport of the Chl intermediates from the thylakoid membrane to L-EVs. Cyanobacterial EVs may play a novel role in alleviating the accumulation of Chl intermediates in cells.
Keywords: Carotenoids, Chlorophyll biosynthesis, Cyanobacteria, Extracellular vesicles, Leptolyngbya boryana, Membranes
Introduction
Chlorophyll a (Chl) that absorbs light energy from sunlight and initiates photosynthetic electron transfer is an essential tetrapyrrole pigment for photosynthesis. Oxygenic photosynthetic organisms produce Chl from glutamate in a 16-step enzymatic reaction (Tanaka and Tanaka 2007, Masuda and Fujita 2008, Fujita and Yamakawa 2017). However, free Chl and its biosynthetic intermediates are potent and strong photosensitizers that generate reactive oxygen species harmful to cells upon light irradiation. Consequently, the Chl biosynthetic pathway is strictly regulated by a multilayer regulatory mechanism at transcriptional, translational and posttranslational levels to minimize the accumulation of free Chl and its intermediates (Czarnecki and Grimm 2012, Wang et al. 2022). However, the full picture of the complex regulatory system still remains elusive. In particular, it is not fully understood how cyanobacteria, which do not have any Chl-degrading systems, unlike plants (Obata et al. 2019), efficiently remove free Chl and its intermediates generated in cells.
In the Chl biosynthetic pathway, the first half, nine reactions from glutamate to protoporphyrin IX (PPN), is shared with the heme biosynthesis pathway. The latter seven reactions specific to Chl biosynthesis (Mg branch; Fig. 1) begin with the ATP-dependent insertion of Mg2+ ion into PPN, forming Mg-PPN by the action of Mg-chelatase. Subsequently, four successive reactions occur: Mg-PPN methyltransferase, Mg-PPN monomethyl ester (MPE) cyclase, 8-vinyl reductase and protochlorophyllide (Pchlide) reductase to form chlorophyllide (Chlide). Finally, the action of Chl synthase catalyzes the attachment of phytol provided by geranylgeranyl reductase to Chl. In the cyanobacterial Chl biosynthesis pathway, two enzymes with different properties are involved in the four reactions: HemF and HemN for the oxidative decarboxylation of coproporphyrinogen III (Goto et al. 2010); HemJ, HemY and HemG for protoporphyrinogen IX oxidation (Kobayashi et al. 2014; some species such as Leptolyngbya boryana and Gloeobacter violaceus PCC 7421 carry hemJ and hemY, Kohara et al. 2023, while most species have only one gene); ChlAI and ChlAII for the oxygen-dependent MPE cyclase (Minamizaki et al. 2008; some species have another oxygen-independent MPE cyclase ChlE; Yamanashi et al. 2015) and light-dependent Pchlide reductase (LPOR) and dark-operative Pchlide reductase (DPOR) for the reduction of C17=C18 of Pchlide (Fujita 1996, Fujita et al. 1998, Yamazaki et al. 2006, Reinbothe et al. 2010). Utilizing analogous enzymes or isoforms with different properties and affinities for substrates, cyanobacteria can supply Chl without delay even in environments where light (high light, low light and darkness) and oxygen levels (aerobic, hypoxic and anaerobic) fluctuate (Fujita et al. 2015).
Fig. 1.

Chl biosynthesis pathway in cyanobacteria. Intermediates in the Mg branch of cyanobacterial Chl biosynthesis are shown in bold. The steps at which two distinct enzymes differentially operate are shown with two arrows. Demetalated derivatives from intermediates of the Mg-branch are shown by thin arrows on the right. Pheophytin a (in PSII) is produced from Chl and could be another precursor for the production of Pheobide (indicated by a dashed arrow). GGPP and Phytyl-PP are geranylgeranyl pyrophosphate and phytyl pyrophosphate, respectively.
In particular, the functional differentiation between DPOR and LPOR has been analyzed in detail in the cyanobacterium L. boryana based on the phenotype analysis of mutants lacking DPOR or LPOR. Under high light conditions, LPOR is almost solely responsible for Pchlide reduction (Fujita et al. 1998), whereas, under very low light or dark conditions, DPOR is the main enzyme for Pchlide reduction (Fujita et al. 1992, 1993, 1996, Kada et al. 2003). A mutant (∆DPOR) lacking the DPOR subunit ChlL accumulates monovinyl (MV)–Pchlide in cells during dark heterotrophic growth, and furthermore, secretes a large amount of MV-Pchlide, causing the culture medium to turn yellow. According to a previous study, ∆DPOR secretes MV-Pchlide into the culture medium via extracellular vesicles (EVs) (Usui et al. 2022). EV analysis revealed that ∆DPOR forms two EVs with different densities and that most of the MV-Pchlide is secreted via high-density EVs (H-EVs) (Fraction 2). However, it is still unclear whether the EV-mediated secretion of MV-Pchlide is a special phenomenon caused only under unusual conditions where MV-Pchlide abnormally accumulates in cells or whether wild-type (WT) L. boryana cells intrinsically produce EVs under normal growth conditions.
Most bacteria are believed to have the ability to secrete EVs, and EVs are derived from the plasma membrane (PM) (Schwechheimer and Kuehn 2015). Gram-negative bacteria, which include cyanobacteria, are characterized by a three-layered cell membrane structure comprising the outer membrane (OM), cell wall (CW; peptidoglycan layer) and inner membrane (IM). EVs secreted by Gram-negative bacteria are derived from the OM (Kim et al. 2015). EVs contain various biological substances, such as nucleic acids and proteins (Gonçalves et al. 2021). Furthermore, EVs have various physiological functions, including intercellular communication, biofilm formation, nutrient secretion and acquisition, toxin secretion, horizontal gene transfer, metabolite secretion and response to environmental stress (Schwechheimer and Kuehn 2015). The EV-mediated secretion of MV-Pchlide accumulated in cells can be regarded as a novel function of EVs (Usui et al. 2022).
In this study, EVs from WT L. boryana (dg5; Fujita et al. 1996, Hiraide et al. 2015) were examined and characterized. The WT strain also secretes two EVs with different densities under photoautotrophic and dark heterotrophic conditions. Unlike EVs from ∆DPOR, the WT strain secreted Chl intermediates mainly via low-density EVs (L-EVs). Two carotenoids, ketomyxol glycoside (KMG) and zeaxanthin (Zea), were the major pigments in L-EVs. It is of interest that Chl intermediates—PPN and PPN monomethyl ester (PPN-Me; demetalated MPE)—were commonly detected in both L-EVs from cells grown under light and dark culture conditions. L-EVs from cells grown under dark heterotrophic conditions contained MV-Pchlide and MV-protopheophorbide (MV-Protopheobide; demetalated MV-Pchlide) and pheophorbide (Pheobide) in addition to the two common pigments. These results suggest that L. boryana secretes Chl biosynthesis intermediates by L-EVs even under normal growth conditions and that EVs may play a novel regulatory role in Chl biosynthesis in cyanobacteria.
Results
EVs from the WT strain grown under photoautotrophic and dark heterotrophic conditions
To examine whether the WT strain of L. boryana produces EVs under photoautotrophic (light) and dark heterotrophic (dark) conditions, the same method for the isolation of EV fractions from ∆DPOR (Usui et al. 2022) was applied to prepare supernatants of WT cultures grown under light and dark conditions. The precipitates of the culture supernatants obtained by ultracentrifugation were separated by stepwise sucrose density gradient centrifugation (Fig. 2). Orange bands appeared at the upper interfaces (0.6–1.6 M) from WT cells grown under both conditions (Fig. 2, top panels). No clearly visible bands were observed at the lower interfaces (1.6–2.5 M). However, when the upper and lower interfaces were collected and concentrated by ultracentrifugation, not only did fractions from upper bands give rise to dark orange precipitates but also those from lower interfaces gave rise to small amounts of dark orange precipitates (Fig. 2, bottom panels), suggesting that WT L. boryana grown under both light and dark conditions produced two EVs with different densities. These EV fractions at the upper (0.6–1.6 M) and lower (1.6–2.5 M) interfaces were called L-EVs and H-EVs, respectively.
Fig. 2.

EVs isolated from the WT grown under light and dark conditions. EV fractions from cells grown under light (A) and dark (B) conditions were isolated with stepwise sucrose density gradient centrifugation. EVs at 0.6–1.6 M and 1.6–2.5 M interfaces were assigned as L-EVs and H-EVs, respectively. Precipitates of the EV fractions by ultracentrifugation are shown at the bottom. L-EVs (tubes 1 and 3) and H-EVs (tubes 2 and 4) were isolated from light-grown (tubes 1 and 2) and dark-grown (tubes 3 and 4) WT cells.
All four EV fractions were observed by transmission electron microscopy (TEM; Fig. 3). L-EVs and H-EVs exhibited respective common morphological characteristics irrespective of the growth conditions. L-EVs predominantly showed spherical structures (Fig. 3A and B), whereas H-EVs displayed bean-like unique structures with multiple vesicles interconnected (Fig. 3C and D), rather than a single spherical shape.
Fig. 3.

TEM images of the four EV fractions. The EV fractions were observed by TEM with negative staining. L-EVs (A and B) and H-EVs (C and D) were isolated from cells grown under light (A and C) and dark (B and D) conditions. Bar, 300 nm.
SDS-PAGE was performed to analyze the protein composition of these EV fractions (Fig. 4). The absence of thylakoid membrane (TM) or cell debris in all EVs fractions was confirmed by immunodetection using an antiserum against PsbA, photosystem II (PSII) reaction center protein D1. L-EVs and H-EVs obtained under both culture conditions showed distinct profiles. In both L-EV fractions from light and dark conditions, proteins with apparent molecular masses of ∼70 kDa and 50 kDa were predominant. In both H-EV fractions, multiple bands with apparent molecular masses of ∼50 kDa were observed as major proteins. The major proteins in the EV fractions were identified by mass spectrometry (MS; Supplementary Table S1). The 70 and 50 kDa proteins in L-EV fractions were identified to be FG-GAP (phenyl-glycyl and glycyl-alanyl-prolyl consensus sequence; Springer 1997) repeat-containing protein (FGP; LBDG_07530: bands a and f) and porin isoforms (LBDG_25860 and LBDG_41910: bands b and g), respectively. The iron complex OM receptor proteins (LBDG_X2190 and LBDG_49870) were also detected in the 70 kDa band (band a) of L-EVs from the light-grown cells. The 50 kDa proteins in the H-EV fractions were identified as porin isoforms (LBDG_41910, LBDG_25860, LBDG_34630 and LBDG_40860: bands c–e; LBDG_40860, LBDG_25860 and LBDG_41910: bands h–j). The identification of the major proteins of EVs from the WT was consistent with those of EVs from ∆DPOR in the previous study (Usui et al. 2022), indicating that under both culture conditions, WT and ∆DPOR produce two types of EVs that exhibit distinct densities and protein compositions.
Fig. 4.

SDS-PAGE of L-EVs and H-EVs from WT cells grown under light and dark conditions. SDS-PAGE (CBB stain; top) of EV fractions from WT cells grown under light (L-EVs, lane 1; H-EVs, lane 2) and dark (L-EVs, lane 3; H-EVs, lane 4) conditions. An aliquot (2.5 µg protein) was loaded into each lane. Protein bands shown with (a–j) were excised and analyzed by mass spectrometry (Supplementary Table S1). Aliquots of CL were loaded into lanes 5–8. As a marker protein for TM, D1 was detected with the anti-PsbA (D1) antiserum in the CL from WT cells grown under light (2.5 and 0.5 µg protein for lanes 5 and 6, respectively) and dark (2.5 and 0.5 µg protein for lanes 7 and 8, respectively) conditions (immunodetection; bottom).
Comparison of EVs to other membrane fractions
Cyanobacteria possess two types of membranes (TM and PM) and the CW composed of peptidoglycan. Because cyanobacteria are Gram-negative bacteria, PM consists of two distinct membranes: OM and IM.
Three membrane fractions (TM, PM and CW) were prepared from the WT cells grown under light photoautotrophic (Fig. 5A) and dark heterotrophic (Fig. 5B) conditions using differential centrifugation and sucrose density gradient centrifugation (Omata and Murata 1984) (Supplementary Fig. S1A). PM fractions were precipitated by high-speed centrifugation from the supernatants of low-speed centrifugation of crude cell lysates (CL) and separated as orange bands at the interfaces of 0.6–1.6 M (Supplementary Fig. S1B and E). TM fractions were precipitated by low-speed centrifugation and separated by sucrose density gradient centrifugation to form dark green bands in the 1.6 M sucrose layers (Supplementary Fig. S1C and F). CW fractions were separated as orange bands at the interfaces of 1.6–2.5 M sucrose density gradient centrifugation after collecting orange bands that appeared just below the dark green TM bands (Supplementary Fig. S1D and G). To validate the characteristics of these fractions, protein compositions were compared using SDS-PAGE (Fig. 5) and immunodetection with the anti-PsbA (D1) antiserum (Fig. 5, lower panels). Protein profiles stained by Coomassie brilliant blue (CBB) revealed distinct protein compositions in all three fractions, and the three membrane fractions isolated from dark-grown cells showed nearly the same protein composition as that isolated from light-grown cells. The D1 protein of PSII was exclusively detected in the CL and TM fractions, whereas it was absent in the PM and CW fractions. To identify the major proteins in the PM and CW fractions, the main protein bands were excised from the gels and subjected to MS (Supplementary Tables S2 and S3).
Fig. 5.

Membrane fractions from the WT cells grown under photoautotrophic (A) and dark-heterotrophic (B) conditions. SDS-PAGE (CBB stain; middle) and immunodetection (bottom) using the anti-PsbA (D1) antiserum for CL (lane 1), TM (lane 2), PM (lane 3), and CW (lane 4) isolated from WT cells grown under light (A) and dark conditions (B), respectively. Top, photos of suspensions of the isolated fractions. Protein bands shown with (a–h) were excised and analyzed by MS (Supplementary Tables S2 and S3). An aliquot (2.5 µg protein) was loaded into each lane.
In the PM fraction from light-grown cells (Supplementary Table S2), FGP (LBDG_07530: band a), iron complex OM receptor protein (LBDG_X2190: band a) and porin isoforms (LBDG_25860, LBDG_06050 and LBDG_40860: bands b and c) were identified. ABC transporter substrate binding proteins (LBDG_35200, LBDG_34900, LBDG_20220 and LBDG_05960: bands d and e), which were localized to IM (Norling et al. 1997), and phage shock protein A/IM 30 (LBDG_55170: band e), which was a homolog of the VIPP1 protein [56% similarity to VIPP1 from Synechocystis sp. PCC 6803 (PCC6803)] involved in TM biogenesis (Westphal et al. 2001), were also detected in the PM fraction. The protein composition suggested that the prepared PM fraction contained IM and OM. Comparing the protein composition between PM and L-EVs (Fig. 4), those of the two fractions were largely similar, although some were detected only in PM, such as ABC transporter substrate binding proteins and the VIPP1 homolog, suggesting that L-EVs are derived from the OM of the PM fraction with a lighter density.
The major proteins of the PM fraction from the dark-grown cells (Fig. 5B, Supplementary Table S3), FGP (band a), porin isoforms (bands b and c) and the VIPP1 homolog (band e), as well as those from the light-grown cells, were also identified. In contrast, some proteins, such as glycogen/starch synthase (band b) and NrtA (band d), were detected only in the PM fractions from dark-grown cells. This difference would be interesting because it reflects the different growth modes between chemoheterotroph and photoautotroph, although this is not a proteomic analysis, as only the major proteins were excised for MS.
The SDS-PAGE profile of CW was highly similar to that of H-EVs. MS identified several porin isoforms [LBDG_06050, LBDG_17810, LBDG_25860, LBDG_40860 and LBDG_41910: bands f–h (light-grown cells; Fig. 5A, Supplementary Table S2), LBDG_25860, LBDG_40860 and LBDG_41910: bands f–h (dark-grown cells; Fig. 5B, Supplementary Table S3)] in the CW fraction. In PCC6803, porin isoforms (Slr1841, Slr1908 and Slr0042) were the major proteins of OM and also interacted with peptidoglycans (Kojima et al. 2016, Qiu et al. 2021). These porins possessed S-layer homology (SLH) domains interacting with peptidoglycans (Witwinowski et al. 2022). Based on these observations, it was suggested that the CW fraction predominantly comprised OM interacting with the CW showing higher density, and H-EVs were derived from the OM region interacting with the CW.
Pigment compositions of EVs and other membrane fractions: carotenoids
To compare the pigment compositions in the two EVs and other membrane fractions from light- and dark-grown cells, pigments were extracted from each fraction with 90% methanol and subjected to high-performance liquid chromatography (HPLC) analysis, and pigments were detected at an absorbance of 440 nm to assess the overall pigment composition (Fig. 6A and B). Chl (peak 5) was the predominant pigment in CL and TM, whereas almost no Chl peaks were detected in PM, CW, L-EVs and H-EVs (Fig. 6A and B). In PM and L-EVs, carotenoids, specifically KMG (peak 3) and Zea (peak 4), were the common major pigments (Supplementary Fig. S2; Hida et al. 2024), whereas CW and H-EVs contained only trace levels of these carotenoids.
Fig. 6.

Pigment analysis of the L-EVs, H-EVs, and other fractions from the WT cells grown under light (A and C) and dark conditions (B and D). Pigments in CL, TM, PM, CW, L-EVs and H-EVs fractions were detected at an absorbance of 440 nm (A and B). Chl intermediates were detected by fluorescence emission (dashed lines, λem/λex 660/430 nm; solid lines, λem/λex 630/400 nm) (C and D). The protein concentration in each fraction was adjusted to 0.1 mg ml-1, and an aliquot (20 µl) of each extracted pigment was injected. Peak assignment is as follows: 1, MV-Pchlide; 2, MV-Protopheobide; 3, KMG; 4, Zea; 5, Chl (A and B); a, Chlide; b, MV-Pchlide; c, MV-Protopheobide; d, PPN; e, Pheobide and f, PPN-Me (C and D). PDA absorption spectra of KMG (peak 3) and Zea (peak 4) are shown in Supplementary Fig. S2, and that of MV-Protopheobide [peak 2 (and peak c)] in Supplementary Fig. S4. Peaks with a single asterisk and double asterisks were not identified, but the pigment of peak with a single asterisk would be a Chlide derivative (Supplementary Fig. S6).
The KMG and Zea contents in the pigment-rich fractions (TM, PM and L-EVs) were quantified (Supplementary Fig. S3). Although determining the KMG and Zea contents per protein (nmol/mgprotein) was difficult given that they tended to fluctuate widely across samples, a consistent trend was observed: both KMG and Zea contents per protein were higher in PM and L-EVs than in TM, with the highest values (KMG, 84 nmol/mg; Zea, 9.7 nmol/mg) observed in L-EVs from the light samples (Supplementary Fig. S3A). This trend was also observed in samples from dark-grown cells (the highest values: KMG, 340 nmol/mg; Zea, 170 nmol/mg; Supplementary Fig. S3B), suggesting that both pigments are enriched in PM and L-EVs. Furthermore, the Zea content was increased in the dark samples, which is reflected in the much lower KMG/Zea ratios of the dark samples compared with those of the light samples (Supplementary Fig. S3C and D). Carotenoid contents and the KMG/Zea ratios of L-EVs were more similar to PM than to TM. This feature appears to be consistent with the OM origin of L-EVs based on their similarity in protein composition. Protein composition and carotenoid contents suggested that L-EVs are derived from low-density OM.
Pigment compositions of EVs and other membrane fractions: Chl biosynthetic intermediates
Interestingly, under dark conditions, besides carotenoids, Chl biosynthetic intermediates, MV-Pchlide (peak 1) and its demetalated derivative MV-Protopheobide (peak 2; Supplementary Fig. S4), were detected in L-EVs (Fig. 6B). To explore the presence of Chl biosynthetic intermediates in the membrane fractions with higher sensitivity, fluorescence spectroscopic detection at two different sets of wavelength (dashed line, λem/λex 660/430 nm; solid line, λem/λex 630/400 nm) was conducted in the HPLC analysis for the samples from cells grown under light and dark conditions (Fig. 6C and D). In the HPLC protocol, Chl intermediates before phytol attachment are eluted from 5 min to 15 min (Aoki et al. 2014).
In the samples from light-grown cells (Fig. 6C), Chlide (peak a) and its derivative (peak *) were detected as major Chl intermediates in TM (Supplementary Figs. S5 and S6). Pheobide (peak e; Supplementary Fig. S5), besides Chlide, was mainly detected in PM. Interestingly, PPN (peak d) and PPN-Me (peak f) were detected in L-EVs (Supplementary Fig. S7) besides the occasional detection of Pheobide. PPN and PPN-Me were specific to L-EVs and not detected in other membrane fractions.
In the samples from dark-grown cells, MV-Pchlide was detected as an obvious peak (peak b) in the L-EVs and as a minor peak in CL, TM and PM (Fig. 6D). Furthermore, in L-EVs, MV-Protopheobide (peak c) was significantly accumulated. PPN, PPN-Me and Pheobide were also detected in L-EVs from dark-grown cells. Conversely, no Chl intermediate was detected in the light-grown H-EV samples, except for the MV-Pchlide, which was detected only as a tiny peak in the dark-grown H-EV sample.
The content per protein of each Chl intermediate in L-EVs was determined (Supplementary Fig. S8). In the L-EVs from the light-grown culture, the amounts of PPN and PPN-Me were 0.058–0.25 and 0.058–0.19 nmol/mg, respectively, which were lower than those of the carotenoids (Supplementary Fig. S3A). Additionally, no Pheobide was detected in three of the five L-EV samples examined. For the L-EVs from dark-grown culture, while PPN was not quantified because a minor peak for PPN largely overlapped by the major peak for MV-Protopheobide (Fig. 6D), the amounts of PPN-Me, MV-Pchlide, MV-Protopheobide and Pheobide were determined. As expected from the HPLC profile with detection at 440 nm absorbance, MV-Pchlide (0.81–4.3 nmol/mg) and MV-Protopheobide (7.2–38 nmol/mg) were detected as the major Chl intermediates in the dark L-EV samples (Supplementary Fig. S8B). The amount of PPN-Me (0.075–0.16 nmol/mg) was comparable to that of the light L-EV samples. In contrast to light conditions, Pheobide was detected in all samples at concentrations of 0.64–0.91 nmol/mg. To detect Mg-PPN and MPE, which could be the direct precursor of PPN-Me (Fig. 1), CL and L-EV fractions were analyzed at fluorescence wavelengths (λem/λex 600/417 nm) using HPLC (Supplementary Fig. S9, dotted line). MPE was detected as a very tiny peak in the CL fractions of dark-grown cells and L-EVs under both conditions. However, Mg-PPN (expected to be eluted at ∼7 min; Aoki et al. 2014) was not detected in any fraction.
These quantitative analyses indicated that although carotenoids are the major pigments in L-EVs, various Chl biosynthetic intermediates and their demetalated derivatives accumulate in L-EVs, and their composition and contents vary significantly depending on the culture conditions (i.e. light/dark and photoautotroph/heterotroph).
Ratios of EV-localized pigments to the total pigments in the cultures
How much of the total amount of pigments in cells is localized to EVs? The EV localization ratios could provide insights into how pigments are transported to EVs. We determined the ratio of each pigment in the EVs to the total amount in the culture (Fig. 7). For this analysis, the crude EV fractions before sucrose density gradient centrifugation were used to minimize loss of EVs during preparation. Since the pigment contents of H-EVs were very low (Figs. 2 and 6), the values obtained from the crude EV analysis were regarded to be closely approximate to those of L-EVs.
Fig. 7.
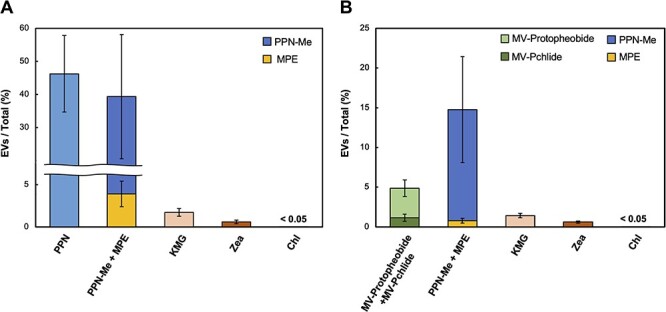
The ratios of pigment content in EVs to the total cultures. From the relative contents of pigments in EVs and cells, the ratio (%) of each pigment in EVs to those of the total culture content was calculated for samples prepared from cultures grown under both light (A) and dark (B) conditions. All samples were analyzed in six replicates.
For carotenoids, the contents of the EV fractions of KMG and Zea relative to those of the total cultures were 1.7% ± 0.47% and 0.60% ± 0.21%, respectively, in the light-grown cultures (Fig. 7A). In the dark-grown cultures, the ratios were quite similar, 1.4% ± 0.28% and 0.61% ± 0.14%, respectively (Fig. 7B). Conversely, for Chl biosynthetic intermediates and their derivatives, 46% ± 12% of PPN and 39% ± 20% of MPE and its demetalated derivative (MPE; 3.9% ± 1.5 and PPN-Me; 35% ± 19%) were present in EVs in the light-grown cultures. In the dark-grown cultures, 4.9% ± 1.5% of Pchlide and its demetalated derivative (MV-Pchlide; 1.2% ± 0.45% and MV-Protopheobide; 3.7% ± 1.0%) and 15% ± 6.6% of MPE and its demetalated derivative (MPE; 0.78% ± 0.30% and PPN-Me; 14% ± 6.7%) were present in EVs. The EV-localized ratios of the Chl intermediates were significantly higher than those of KMG and Zea. These results suggest that Chl intermediates and their derivatives are secreted via EVs at higher efficiency than carotenoids.
Discussion
In this study, we found that the WT of the filamentous cyanobacterium L. boryana secreted two types of EVs (L-EVs and H-EVs) with different densities under light and dark conditions. L-EVs and H-EVs commonly contain porin isoforms, but exhibit distinct protein compositions and carotenoid contents, which suggested that L-EVs and H-EVs are derived from low-density OM and high-density OM interacting with CW, respectively. Furthermore, we found that L-EVs contain Chl biosynthetic intermediates and their derivatives, depending on the culture conditions.
EV formation
Based on these results, we propose a model for EV formation in WT L. boryana (Fig. 8A and B). The similarity between L-EVs and PM in density, pigment content and protein composition was consistent with that of L-EVs derived from PM. The PM fraction contained both proteins localized in IM and OM (Supplementary Tables S2 and S3), whereas L-EVs contained mainly proteins localized in OM (Supplementary Table S1) and the content of FGP was higher than that of PM (Figs. 4 and 5). This feature suggested that L-EVs originate from the OM region, which interacts less with the CW than with other regions. It also indicates that FGP is predominantly localized in L-EVs (Fig. 8 A and B), consistent with the observation that L-EVs have a lower content of porin isoforms compared to the CW fraction, which contains OM interacting with the CW (Figs. 4 and 5).
Fig. 8.
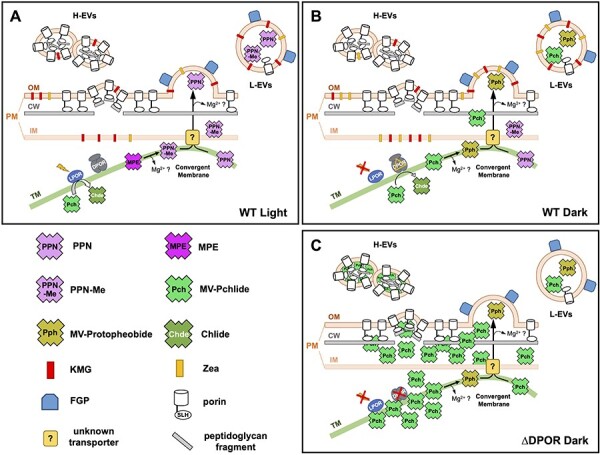
Models for the formation of two types of EVs in L. boryana cells. (A and B) L. boryana WT secretes two types of EVs under light (A) and dark (B) conditions: L-EVs, whose major protein and pigments are FGP and carotenoids, respectively; and H-EVs, whose major protein is porin isoforms and less pigment per protein than L-EVs. In WT, free Chl intermediates accumulated intracellularly are loaded into only L-EVs and secreted out from the cells. Under light conditions, PPN and PPN-Me are mainly secreted out of the cell (A). Whereas, in the dark, MV-Pchlide accumulates in the TM due to the rate-limiting step of the DPOR reaction step or a partial inhibition of DPOR, and it is secreted mainly as MV-Protopheobide via L-EVs (B). An unknown transporter of Chl intermediates from TM to IM is shown. This transporter could be present in the convergent membrane. L-EV-mediated secretion could alleviate the accumulation of Chl intermediates within the cell. (C) L. boryana ∆DPOR also secretes two types of EVs. During the dark growth of ∆DPOR, a large amount of MV-Pchlide accumulates in the cells. Such abnormally accumulated MV-Pchlide could stimulate the formation of H-EVs, and MV-Pchlide, and MV-Protopheobide secreted by H-EVs (Usui et al. 2022). Since the carotenoid contents of ∆DPOR under dark conditions have not been determined, the notation of carotenoids is not shown.
H-EVs differed significantly from L-EVs in terms of higher density, less pigment contents, protein composition and shape. H-EVs had a unique bean-like shape, and some vesicles were linked together (Fig. 3C and D), which were different from the spherical shape of L-EVs. The pigment content and protein composition of H-EVs were more similar to the CW fraction than those of the PM fraction (Figs. 4 and 5). Porin isoforms were major proteins in H-EVs. Porin contains the N-terminal SLH domain that interacts specifically with peptidoglycans (Kojima et al. 2016, Witwinowski et al. 2022). These features suggest that H-EVs originate from the OM regions where porin isoforms are present at higher densities and interact extensively with the CW (Fig. 8A and B). In the other words, we hypothesized that L-EVs and H-EVs are generated through a budding process from the distinct OM regions with low- and high-density, respectively.
FGP: the main protein of L-EVs
FGP was identified as the major protein in L-EVs and was present in the PM fraction (Figs. 4 and 5; Supplementary Tables S1–S3). The FG-GAP motif of FGP is conserved in extracellular proteins, such as adhesins and integrins, which play roles in cell adhesion and biofilm formation (Velling et al. 1999, Absalon et al. 2011). In a Gram-negative bacterium Vibrio cholerae, the Bap1 protein containing the FG-GAP motif is involved in biofilm formation and is localized in EVs, however, as a minor protein (Duperthuy et al. 2013). Thus, it is implied that FGP is localized at the outer surface of OM and is involved in cell adhesion and biofilm formation in L. boryana. Interestingly, PCC6803 does not contain FGP, and FGP is largely distributed only among some filamentous cyanobacterial species, such as Anabaena sp. PCC 7120, Phormidium and Pseudoanabaena. Thus, the presence of FGP as the major protein is one of the unique features of EVs of L. boryana.
Carotenoids in EVs
L-EVs contained two carotenoids (KMG and Zea) as the major pigments (Fig. 6A and B and Supplementary Fig. S3A and B). The EVs from PCC6803 were recovered as orange precipitates via ultracentrifugation (Biller et al. 2022b), suggesting the presence of carotenoids as the major pigments. Zea and α- and β-carotenes were also identified as the major pigments in EVs from the marine cyanobacterium Prochlorococcus (Biller et al. 2022a). These observations suggest that carotenoids, especially Zea, are the major common pigments in cyanobacterial EVs.
The higher Zea content in L-EVs from cells grown in the dark compared to those grown in light likely reflects the increased intracellular Zea levels (Fig. 6B and Supplementary Fig. S3B). In Synechococcus elongatus PCC 7942, it has been reported that the Zea content in PM increases by 2.7-fold under high light conditions compared with that under low light conditions (Masamoto et al. 1999). Considering that xanthophylls, including Zea, protect against oxidative stress in cyanobacteria (Zhu et al. 2010), the increase in the Zea content may be due to the oxidative stress under the dark heterotrophic conditions. This could be consistent with the partial inhibition of DPOR, an oxygen-sensitive enzyme (Yamazaki et al. 2006, Yamamoto et al. 2011), causing the MV-Pchlide accumulation in the dark. However, the detailed mechanism behind Zea accumulation in the dark remains unclear.
The carotenoid ratios (KMG/Zea) of the L-EVs were similar to those of the PM (Supplementary Fig. S3C and D). Furthermore, the ratio of EV-localization ratio of each carotenoid to the total culture (KMG ≈ 1.5%, Zea ≈ 0.6%) was nearly identical under the two conditions (light and dark) (Fig. 7). These observations appear consistent with the model in which KMG and Zea are passively transported from the OM to EVs via the budding process (Fig. 8).
Chl biosynthetic intermediates in EVs
In the previous paper (Usui et al. 2022), we reported EV-mediated MV-Pchlide secretion when the mutant ∆DPOR grew in the dark, which could represent an unusual cellular response to the abnormal massive accumulation of MV-Pchlide. In this study, we detected the Chl biosynthetic intermediates in L-EVs from WT cells grown under light and dark conditions for the first time (Fig. 6C and D). The Chl intermediate content per protein was much lower than that of carotenoids (KMG and Zea) (Supplementary Figs. S3 and S8). However, their EV-localization ratios to the total cultures were very high (4.9–46%) compared with those of KMG and Zea (0.6–1.7%) (Fig. 7). These different ratios probably reflect the different transport systems for carotenoids and Chl intermediates to EVs. Chl intermediates were specifically detected in EVs, especially L-EVs, but not in PM or CW, suggesting that there is an efficient transport system to L-EVs from TM where Chl intermediates are mainly produced. Interestingly, the EV-localization ratios to the total cultures of Chl intermediates were significantly higher under light condition (39–46%) than in dark (4.9–15%). Contrarily, the EV localization ratios of the carotenoids were constant under light and dark conditions. This suggests that the activity of the transport system from the TM to L-EVs for Chl intermediates is stimulated under light conditions to relieve intermediate-induced photosensitizing.
Only two demetalated porphyrins, PPN and PPN-Me, of the various Chl intermediates and their demetalated derivatives in the Mg branch, were commonly detected in L-EVs from light- and dark-grown cultures. The selective accumulation of PPN and PPN-Me in L-EVs suggests that excess amounts of PPN (or Mg-PPN) and MPE are generated intracellularly, and L-EVs secrete some portions out of the cell. There are three possible causes of PPN accumulation: (1) decreased activity of Mg-chelatase and/or ferrochelatase; (2) autoxidation of the substrate protoporphyrinogen IX of protoporphyrinogen IX oxidase due to decreased activity of the enzyme; and (3) decreased activity of Mg-PPN methyltransferase causing Mg-PPN accumulation, followed by demetalation. At this stage, it is unclear which processes are primarily responsible for the PPN. A possible cause of MPE accumulation could be the decreased activity of MPE cyclase. Alternatively, MPE cyclase may intrinsically be a rate-limiting step of the Mg branch under light and dark conditions, resulting in the constant accumulation of small amounts of MPE. It has been reported that PPN is methylated by MgPPN methyltransferase BchM to produce PPN-Me in an in vivo expression system in Escherichia coli (Johnson and Schmidt-Dannert 2008). Therefore, the possibility that PPN-Me is also produced by ChlM (MgPPN methyltransferase) in L. boryana cells cannot be completely excluded. However, the fact that PPN-Me was not detected in the cells under both light and dark conditions (Supplementary Fig. S9) suggests that the direct production of PPN-Me from PPN within cells is unlikely to occur.
MV-Pchlide and MV-Protopheobide were also detected in L-EVs from dark-grown cultures. Specifically under dark conditions, a small amount of MV-Pchlide accumulated in the TM (Fig. 6D). In Chl biosynthesis, LPOR, which requires light for reaction, does not work in the dark. The sole Pchlide reductase, DPOR, in the dark conditions, is an oxygen-sensitive enzyme (Yamazaki et al. 2006, Yamamoto et al. 2011). Thus, the DPOR activity could be partially inhibited by oxygen due to the aerobic incubation in the dark, resulting in a slight accumulation of Pchlide.
We determined that approximately 5% of the total MV-Pchlide in the culture was secreted via L-EVs in the dark, which was unexpectedly low compared with the ratios of PPN and PPN-Me in EVs under light conditions (Fig. 7B). The LPOR may serve as a Pchlide accumulation pool in the dark, contributing to the lower EV-localization ratio of MV-Pchlide. In a previous study, it was reported that the overexpression of LPOR formed intracellular prolamellar body-like structures, similar to etioplasts in angiosperms, in the ∆DPOR transformant expressing LPOR (Yamamoto et al. 2020). In other words, a portion of the MV-Pchlide may be present as an LPOR-bound form in dark-grown cells keeping the EV-localization ratio low.
Pheobide behaved differently from other Chl intermediates. Under dark conditions, where the Pchlide reduction step is considered the rate-limiting step in the Chl biosynthetic pathway, a constant amount of Pheobide was always detected (Supplementary Fig. S8). However, under light conditions, Pheobide was detected in some L-EV samples, despite Chlide and its derivatives always being detected in the TM fraction under light conditions. These counterintuitive results suggest that Pheobide secretion to EV does not occur exclusively through Chlide accumulation. Pheobide could be produced in the PSII repair cycle operating continuously in the light conditions. In this case Pheobide is produced from pheophytin in the PSII reaction centers using pheophytin dephytylase (Takatani et al. 2022). Thus, the PSII repair cycle could be another source of Pheobide in L-EV in the light. However, the reason why Pheobide was also detected in L-EVs from dark-grown cells is still unknown.
EV secretion system for Chl intermediates
A novel secretion system for the Chl intermediates is shown in the model of EV formation mechanism (Fig. 8). The Chl biosynthesis system of L. boryana inevitably causes the accumulation of small amounts of intermediates depending on its culture conditions and growth modes. Under light conditions, PPN and PPN-Me are primarily transported to L-EVs (Fig. 8A). Under dark heterotrophic conditions, MV-Pchlide accumulated due to the partial inhibition of DPOR is transported to EVs in addition to PPN and PPN-Me (Fig. 8B). In this model, the intermediates are transported from TM to IM, presumably via ‘convergent membrane’ (Rast et al. 2019), followed by efflux to the periplasm by an unknown transporter localized in the IM. This transporter remains unidentified; however, one example of a transporter that exports PPN outside of the cell could be BCRP (ABCG2) in animal cells (Jonker et al. 2005). This transporter can also transport Pheobide (Robey et al. 2004). The genome of L. boryana encodes a protein (LBDG_41990) that shows significant similarity with ABCG2 (≈30%) and is a candidate for the unknown transporter. Finally, Chl intermediates in the periplasm are loaded into L-EVs and secreted out of the cell. Additionally, Chl intermediates detected in L-EVs are commonly depleted of the central metal Mg. L-EVs from dark-grown cells also showed a high ratio of MV-Protopheobide to MV-Pchlide, suggesting that demetalated intermediates may be selectively loaded into L-EVs or that the environment in the L-EVs may facilitate the demetalation.
Loading of MV-Pchlide to H-EVs in ∆DPOR
The two EVs (L-EVs and H-EVs) from the WT showed almost the same protein compositions as the two EVs (Fractions 1 and 2) in ∆DPOR, respectively (Usui et al. 2022). In other words, Fractions 1 and 2 in ∆DPOR correspond to WT L-EVs and H-EVs, respectively. This suggested that EV formation containing large amounts of MV-Pchlide found in ∆DPOR is not a special process caused by the abnormal MV-Pchlide accumulation, but rather that the ∆DPOR cells use EVs (H-EVs) operating in WT cells to exclude MV-Pchlide accumulated in cells (Fig. 8C). The abnormal accumulation of MV-Pchlide in high-density OM regions may promote the EV formation via budding and the loading of MV-Pchlide into H-EVs. This is inferred from the MV-Pchlide concentration extracted from the H-EV fraction and the significant redshift (40 nm) of the Qy peak of MV-Pchlide in the absorption spectrum of H-EVs (Usui et al. 2022). Because the accumulation of misfolded proteins and peptidoglycan fragments in the periplasm promotes EV formation (Schwechheimer and Kuehn 2015), MV-Pchlide accumulation in the periplasm may be a novel factor that promotes EV formation in cyanobacteria.
EV-mediated secretion as a novel regulatory system for Chl biosynthesis
Because Chl and its intermediates are potent strong photosensitizers, the Chl biosynthetic process is strictly regulated to minimize the accumulation of intermediates and free Chl. The EV-mediated Chl intermediate secretion system may act as a novel regulatory mechanism in Chl biosynthesis. Divinyl-Chl a/b are contained in EVs in Prochlorococcus (Biller et al. 2022a), and mutants in which genes for bacteriochlorophyll biosynthesis are disrupted excrete the biosynthetic intermediates accumulated in cells to the culture medium in photosynthetic bacteria (Bollivar and Bauer 1992). Unlike plants with an efficient degradation pathway for Chls, photosynthetic prokaryotes, such as cyanobacteria do not have such a Chl degradation system (Obata et al. 2019). Thus, EV-mediated efflux of Chl intermediates may play an important role as a novel regulatory mechanism for Chl biosynthesis contributing to alleviating the accumulation of Chl intermediates in cells to minimize their intracellular concentrations.
Materials and Methods
Cyanobacterial strains and culture conditions
The filamentous cyanobacterium L. boryana IAM M-101 strain dg5 (Fujita et al. 1996, Hiraide et al. 2015) was used as the WT in this work. For cell cultivation, dg5 was grown in BG-11 medium supplemented with 20 mM HEPES-KOH (pH 7.5) and solidified with BactoTM Agar (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) at a final concentration of 1.5% (w/v). Agar plates inoculated with dg5 were incubated under continuous light (20 µmol/m2/s, fluorescent lamp, FL15EX-N-A, Hitachi, Tokyo, Japan) for 7 d at 30ºC. This culture condition was used as a pre-culture for liquid cultivation under photoautotrophic conditions.
The pH of the BG-11 liquid medium was adjusted to 8.2 with 20 mM HEPES-KOH for higher reproducibility of EV production under photoautotrophic conditions. The pre-cultured cells were suspended in BG-11 liquid medium (50 ml) to an initial OD730 of 0.1, and bubbled with air containing 2% (v/v) CO2 at 30ºC under continuous light for 6 d [50 µmol/m2/s; model SCS-2 L-LED (W) Microalgae culture lighting unit, Nippon Medical and Chemical Instruments, Osaka, Japan].
For liquid cultures under dark heterotrophic conditions, dg5 was grown on BG-11 agar plates (pH 8.2) supplemented with glucose (30 mM; BG-11 + Glc) under continuous light (10 µmol/m2/s, fluorescent lamp FL 15EX-N-A) at 30ºC for 7 d. For the main liquid culture, cells grown initially on agar plates were suspended in 100 ml BG-11 + Glc liquid medium (a 500 ml Sakaguchi flask) to an OD730 of 0.5 and incubated at 30ºC in the dark with reciprocal shaking (106 rpm; Bio-Shaker BR-3000LF, TITEC, Koshigaya, Japan) for 5 d.
Preparation of the EV fractions
EVs were prepared as previously described in Usui et al. 2022. The culture supernatant was collected via centrifugation (6000 rpm, 10 min, 4ºC; R10A2 rotor, Himac CR21F; Eppendorf Himac Technologies, Hitachinaka, Japan). For cultures grown under photoautotrophic conditions, the supernatant was filtered through a 0.22 µm filter (150 ml vacuum filter system; Corning, NY, USA) to remove cells and debris. For cultures grown under dark heterotrophic conditions, the culture supernatant was prepared via centrifugation (6000 rpm, 10 min, 4ºC; R10A2 rotor, Himac CR21F) because the culture medium tended to clog a 0.22 µm filter. The crude EV fractions were prepared via ultracentrifugation (45,000 rpm, 1 h, 4ºC; S50A rotor, Himac Ultracentrifuge CS100GXII; Eppendorf Himac Technologies) of the prepared supernatants.
The resulting precipitate was suspended in 1 ml phosphate-buffered saline (PBS) containing 1 mM EDTA (all buffer solutions referred to as PBS hereafter contains 1 mM EDTA). This suspension was used as the crude EV fraction for EVs from cultures grown under photoautotrophic conditions. For EVs from cultures grown under dark heterotrophic conditions, the precipitate obtained via ultracentrifugation was suspended and centrifuged again at low speed (6000 rpm, 2 min, 4ºC; ARO 15–24 rotor, centrifuge MX-301; Tommy Seiko, Tokyo, Japan) to remove any remaining cells. The resulting supernatant was then used as the crude EV fraction. Stepwise sucrose density gradient centrifugation was performed for the two EV fractions obtained as previously described in Usui et al. (2022).
Preparation of membrane fractions
Supplementary Fig. S1 illustrates the preparation of the membrane fractions. Cells grown under photoautotrophic and dark heterotrophic conditions were collected via centrifugation (6000 rpm, 10 min, 4ºC; R10A2 rotor, Himac CR21F, Eppendorf Himac Technologies) and washed with a small amount of PBS. After washing, the suspended cells were dispensed into 1.5 ml tubes at a volume of 500 µl. Approximately 0.1 g of glass beads (150–212 µm; Sigma-Aldrich, St. Louis, MO, USA) were added to the samples, and cells were disrupted using a bead beater (an LA intensity, 1 h, 4ºC; BugCrasher GM-01; TITEC, Koshigaya, Japan). To remove cell debris and glass beads, samples were centrifuged (3000 rpm, 3 min, 4ºC; ARO 15–24 rotor, MX-301), and phenylmethylsulfonyl fluoride (PMSF) was added to the supernatants to a final concentration of 0.2 mM. The resulting CLs were stored at −80ºC.
TM, PM and CW fractionation from cyanobacteria was carried out using sucrose density gradient centrifugation, with minor modifications to the methods described in Omata and Murata (1984) and Kowata et al. (2017) (Supplementary Fig. S1). First, CL was ultracentrifuged (19,000 rpm, 15 min, 4ºC; S50A rotor, Himac CS100GXII) to obtain a precipitate of the high-density membrane fraction [low-speed precipitate; Supplementary Fig. S1A (2)]. The supernatant was further ultracentrifuged (40,000 rpm, 30 min, 4ºC; S50A rotor; Himac CS100GXII) to obtain precipitates of the low-density membrane fraction [high-speed precipitate; Supplementary Fig. S1A (1)]. These two precipitates were suspended in small volumes of PBS, and 1-ml aliquots of these suspensions were subjected to stepwise sucrose density gradient centrifugation (2.5, 1.6 and 0.6 M; 25,000 rpm, 20 h, 4ºC; SRP28SA rotor, Himac CP65β, Eppendorf Himac Technologies; Supplementary Fig. S1B, C, E, F). From the low-density membrane fraction, the orange band at the 0.6- to 1.6-M interface was collected as the crude PM fraction, and PBS was added three to five times the volume of the collected fraction to wash it. To remove any other membrane fractions remaining, ultracentrifugation (19,000 rpm, 10 min, 4ºC; S50A rotor; Himac CS100GXII) was performed, and the supernatant was ultracentrifuged again (40,000 rpm, 1 h, 4ºC; S50A rotor; Himac CS100GXII). The resulting precipitate was suspended in PBS to obtain the final PM fraction. For the high-density membrane fractions, green and orange bands were collected as the TM and CW fractions, respectively. After the fractions were collected, PBS was added three to five times the volume of each fraction, and the precipitate obtained via ultracentrifugation (27,000 rpm, 30 min, 4ºC; S50A rotor; Himac CS100GXII) was again suspended in a small volume of PBS. To further purify the CW fraction, the same sucrose density gradient centrifugation method was repeated (Supplementary Fig. S1D and G). Each membrane fraction obtained was stored at −80ºC in the dark.
Determination of the protein concentration
Protein concentrations were determined using the BCA method (TaKaRa BCA Protein Assay Kit; Takara, Shiga, Japan) after adding 1% SDS to solubilize the samples. Bovine serum albumin was used as the standard.
Chlide and Pheobide preparation
Chlide was prepared using a ∆bchZ/bchF mutant of Rhodobacter capsulatus (CB1200; Bollivar et al. 1994), as previously described in Nomata et al. (2006). Tween-20 (final concentration of 0.2%, v/v) was added to the culture (RCV-2/3PY; Young et al. 1989) to promote pigment production. The prepared Chlide was dissolved in dimethyl sulfoxide (DMSO). The final Chlide sample was prepared by diluting 5 µl of Chlide in DMSO with 95 µl of water. To prepare Pheobide, the central Mg2+ was removed through acid treatment with a drop of hydrochloric acid (35− 37%) to the final Chlide sample.
MPE and PPN-Me preparation
MPE and PPN-Me were prepared using a mutant ∆operon of PCC6803 (Aoki et al. 2014). The ∆operon mutant was isolated by replacing the chlAII-ho2-hemN operon with a kanamycin-resistance cartridge (Aoki et al. 2014). ∆operon cannot grow under microoxic conditions due to the removal of the three genes essential for Chl and heme biosynthesis under low-oxygen conditions. When ∆operon cells were exposed to microoxic conditions, they accumulated various Chl biosynthetic intermediates; the main pigments were MPE, PPN-Me and PPN (Supplementary Fig. S7). First, ∆operon was cultivated on a BG-11 agar plate containing kanamycin (15 µg/ml) under continuous light conditions (20 µmol/m2/s, fluorescent lamp, FL15EX-N-A) for 7 d at 30°C. The agar plate was transferred to an anaerobic jar (AnaeroPack square jar; Mitsubishi Gas Chemical, Tokyo, Japan) containing an oxygen removal pouch (AnaeroPack-Anaero, Mitsubishi Gas Chemical) for 5 d. The maintenance of anaerobic conditions was confirmed using a strip of methylene blue–containing indicator (Dry Anaerobic Indicator; BD BBL, Franklin Lake, NJ, USA) in the jar. After anaerobic incubation, cells were suspended in 100 µl of water and used for HPLC, as described further.
Pigment analysis
Each sample was mixed with methanol (to a final concentration of 90%, v/v) and kept on ice for 20 min in the dark, followed by centrifugation at 15,000 rpm at 4ºC for 10 min (ARO15-24 rotor, MX-301). Subsequently, a 20-µl aliquot of the supernatant was analyzed using HPLC (Zapata et al. 2000) with a column (4.6 × 150 mm Symmetry C8 3.5 µm; Waters, Milford, MA, USA) coupled with a Shimadzu LC series HPLC system (Shimadzu, Kyoto, Japan). Pigments were detected at an absorption wavelength of 440 nm (SPD-20AV; Shimadzu), and the absorption spectrum of the eluted pigments was continuously monitored using a photodiode array detector (SPD-M20A; Shimadzu). The fluorescence of Chl intermediates and their derivatives was detected using a fluorescence detector (RF-20AXS; Shimadzu). Three sets of different fluorescence emission and excitation wavelengths were employed for pigment detection: λem/λex 660/430, 630/400 and 600/417 nm.
For pigment quantification, calibration curves were made from the area values of the HPLC peaks using standards for each pigment, and the pigment concentration in each sample was calculated. Given that there was no standard for KMG, it was estimated from a Zea standard (DHI Institute of Water and Environment, Hørsholm, Denmark), assuming that their molar absorbance coefficients for KMG and Zea were largely identical. From the ratio of absorbance at 440 nm and the maximum absorption peak (KMG, 483 nm; Zea, 453 nm), it was estimated that the ratio of area values (of 440 nm peak) for equal amounts of KMG and Zea was approximately 1:1.66 (Supplementary Fig. S10). Given that PPN-Me showed the same absorption spectrum as PPN, its concentration was calculated using a PPN standard (Sigma-Aldrich, St. Louis, MO, USA). MV-Pchlide was purified from the supernatant of the ∆DPOR culture medium according to our previous study (Usui et al. 2022) and used as a standard. MV-Protopheobide was obtained via acid treatment of the MV-Pchlide standard. By comparing the increase in the area value of MV-Protopheobide and the decrease in the area value of MV-Pchlide at 440 nm before and after acid treatment, the ratio of the area values of equal amounts of MV-Protopheobide to MV-Pchlide was estimated to be approximately 1:2.09, allowing the MV-Protopheobide concentration to be determined (Supplementary Fig. S11A, B). Pheobide standards were obtained using the method described earlier.
Calculation of the pigment ratios in EVs to the total cultures
The ratio of pigment content in EVs to the total culture was calculated based on the total amount of pigment (Cell + EVs) in a 50-ml culture medium. First, a 10-ml aliquot of the culture medium was used to quantify the pigments in the cells, and the remaining culture medium supernatant (40 ml) was used to quantify the EV-localized pigments. The crude EV fractions were obtained from the culture medium supernatant via centrifugation (6000 rpm, 10 min, 4ºC; R10A2 rotor, Himac CR21F) using the method described earlier and then suspended in 100 µl of PBS. The pigments were extracted by adding 900 µl of methanol to 100 µl of cell suspension and 100 µl of the crude EV fractions. The area value of each pigment was determined via HPLC analysis. The ratio of EV-localized pigment to the total pigment in the culture was calculated using the following formula:
% ratio of EVs = 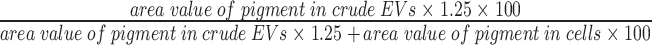
The Chl biosynthetic intermediates detected in the cells were PPN, MPE and MV-Pchlide. PPN-Me and MV-Protopheobide found in EVs needed to be quantified with the amounts of their respective precursors, MPE and MV-Pchlide. MPE was treated with acid, given that it was extracted from the ∆operon (Aoki et al. 2014). From the ratio of the decreased area value of MPE at λem/λex 600/417 nm to the increased area value of PPN-Me at λem/λex 630/400 nm before and after acid treatment, we estimated the ratio of the area values of equal amounts of MPE to PPN-Me to be approximately 2.80:1. This ratio was used to calculate the relative amount of each pigment (Supplementary Fig. S11C, D).
SDS-PAGE and immunodetection
SDS-PAGE and immunodetection were performed as previously described in Usui et al. (2022). The anti-PsbA (D1) antiserum (Agrisera, Vännäs, Sweden) was used at a dilution of 1:1000.
MS analysis
MS analysis was performed as previously described in Usui et al. (2022) using MS (QTRA5500, Sciex, Tokyo, Japan) and nanoLC chromatography (Eksigent LC425, Sciex) with 0.1% formic acid and 2–35% acetonitrile. An 8-µl aliquot of the sample was injected into a nano HPLC capillary column (NTCC-360/75-3-125; Nikkyo Technos, Tokyo, Japan) and inserted into the MS. For qualitative analysis, information-dependent acquisition was performed using an MS scan range of 400–1000 Da and an enhanced product-ion scan range of 100–1000 Da. Results were analyzed using ProteinPilotTM software 5.0 (Sciex), and proteins with a total ProtScore of >10 are listed in each table (Supplementary Tables S1–S3).
Transmission electron microscopy
For negative staining, a 5-µl aliquot of the purified EV fraction was placed on a copper grid (200 mesh; Nissin EM, Tokyo, Japan) coated with formvar (Nissin EM) and incubated for 5 min. Excess solution was absorbed by filter paper, and 4 µl of 2% uranium acetate solution was placed on the grid and stained for 2 min. After staining, the excess staining solution was absorbed using a filter paper and dried for a few minutes. The stained grids were observed using TEM (H-7500, Hitachi), and images were captured using a CCD camera (Advanced Microscopy Technique, Woburn, MA, USA).
Supplementary Material
Acknowledgments
We thank Takao Oi and Mitsutaka Taniguchi (the Laboratory of Plant Physiology and Morphology, Graduate School of Bioagricultural Sciences, Nagoya University) for support of TEM observation. We also thank Takahiro Arase for the preparation of Chlide, Ji Won Kim for preparing MV-Pchlide standard and proofreading of the manuscript, and Asako Segawa for technical help. Additionally, we appreciate Takafumi Yamashino, Mari Banba and all members of the Laboratory of Molecular and Functional Genomics for discussion and technical help.
Contributor Information
Kentaro Usui, Graduate School of Bioagricultural Sciences, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8601, Japan.
Haruki Yamamoto, Graduate School of Bioagricultural Sciences, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8601, Japan.
Hitoshi Mori, Graduate School of Bioagricultural Sciences, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8601, Japan; Institute for Glyco-core Research, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8601, Japan.
Yuichi Fujita, Graduate School of Bioagricultural Sciences, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8601, Japan.
Supplementary Data
Supplementary data are available at PCP online.
Data Availability
Data are contained within the article or Supplementary materials. All raw data have been deposited into the data management system of Nagoya University.
Funding
Grants-in-Aid for Scientific Research (nos 18K19173, 22K19146 and 24H02075) from the Japan Society for the Promotion of Science (JSPS to Y.F.); and Grant-in-Aid for JSPS Fellows (No. 23KJ1118 to K.U.).
Author Contributions
K.U. and Y.F. conceived the study and designed the experiments. K.U. performed all experiments. K.U. conducted TEM observation. H.M. and K.U. conducted MS analysis. K.U. and Y.F. wrote the manuscript. All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Disclosures
The authors have no conflicts of interest to declare.
References
- Absalon C., Van Dellen K. and Watnick P.I. (2011) A communal bacterial adhesin anchors biofilm and bystander cells to surfaces. PLoS Pathog. 7: e1002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki R., Hiraide Y., Yamakawa H. and Fujita Y. (2014) A novel “oxygen-induced” greening process in a cyanobacterial mutant lacking the transcriptional activator ChlR involved in low-oxygen adaptation of tetrapyrrole biosynthesis. J. Biol. Chem. 289: 1841–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller S.J., Lundeen R.A., Hmelo L.R., Becker K.W., Arellano A.A., Dooley K., et al. (2022a) Prochlorococcus extracellular vesicles: molecular composition and adsorption to diverse microbes. Environ. Microbiol. 24: 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller S.J., Muñoz-Marín M.C., Lima S., Matinha-Cardoso J., Tamagnini P. and Oliveira P. (2022b) Isolation and characterization of cyanobacterial extracellular vesicles. J. Vis. Exp. 180: e63481. [DOI] [PubMed] [Google Scholar]
- Bollivar D., Suzuki J., Beatty J., Dobrowolski J. and Bauer C. (1994) Directed mutational analysis of bacteriochlorophyll a biosynthesis in Rhodobacter capsulatus. J. Mol. Biol. 237: 622–640. [DOI] [PubMed] [Google Scholar]
- Bollivar D.W. and Bauer C.E. (1992) Association of tetrapyrrole intermediates in the bacteriochlorophyll a biosynthetic pathway with the major outer-membrane porin protein of Rhodobacter capsulatus. Biochem. J. 282: 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki O. and Grimm B. (2012) Post-translational control of tetrapyrrole biosynthesis in plants, algae, and cyanobacteria. J. Exp. Bot. 63: 1675–1687. [DOI] [PubMed] [Google Scholar]
- Duperthuy M., Sjöström A.E., Sabharwal D., Damghani F., Uhlin B.E. and Wai S.N. (2013) Role of the Vibrio cholerae matrix protein Bap1 in cross-resistance to antimicrobial peptides. PLoS Pathog. 9: e1003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y. (1996) Protochlorophyllide reduction: a key step in the greening of plants. Plant Cell Physiol. 37: 411–421. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Matsumoto H., Takahashi Y. and Matsubara H. (1993) Identification of a nifDK-like gene (ORF467) involved in the biosynthesis of chlorophyll in the cyanobacterium Plectonema boryanum. Plant Cell Physiol. 34: 305–314. [PubMed] [Google Scholar]
- Fujita Y., Takagi H. and Hase T. (1996) Identification of the chlB gene and the gene product essential for the light-independent chlorophyll biosynthesis in the cyanobacterium Plectonema boryanum. Plant Cell Physiol. 37: 313–323. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Takagi H. and Hase T. (1998) Cloning of the gene encoding a protochlorophyllide reductase: the physiological significance of the co-existence of light-dependent and -independent protochlorophyllide reduction systems in the cyanobacterium Plectonema boryanum. Plant Cell Physiol. 39: 177–185. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Takahashi Y., Chuganji M. and Matsubara H. (1992) The nifH-like (frxC) gene is involved in the biosynthesis of chlorophyll in the filamentous cyanobacterium Plectonema boryanum. Plant Cell Physiol. 81: 81–92. [PubMed] [Google Scholar]
- Fujita Y., Tsujimoto R. and Aoki R. (2015) Evolutionary aspects and regulation of tetrapyrrole biosynthesis in cyanobacteria under aerobic and anaerobic environments. Life (Basel) 5: 1172–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Yamakawa H. (2017) Biochemistry of chlorophyll biosynthesis in photosynthetic prokaryotes. In Modern Topics in the Phototrophic Prokaryotes – Metabolism, Bioenergetics, and Omics. Edited by Hallenbeck, P. pp. 67–122. Cham, Switzerland: Springer. [Google Scholar]
- Gonçalves C.F., Lima S., Oliveira P. (2021) Product export in cyanobacteria. In Cyanobacteria Biotechnology. Edited by Hudson, P. pp. 369–406. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- Goto T., Aoki R., Minamizaki K. and Fujita Y. (2010) Functional differentiation of two analogous coproporphyrinogen III oxidases for heme and chlorophyll biosynthesis pathways in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 51: 650–663. [DOI] [PubMed] [Google Scholar]
- Hida S., Nishio M., Uesaka K., Banba M., Takatani N., Takaichi S., et al. (2024) Microevolution toward loss of photosynthesis: mutations promoting dark-heterotrophic growth and suppressing photosynthetic growth in cyanobacteria. BioRxiv. [Google Scholar]
- Hiraide Y., Oshima K., Fujisawa T., Uesaka K., Hirose Y., Tsujimoto R., et al. (2015) Loss of cytochrome cM stimulates cyanobacterial heterotrophic growth in the dark. Plant Cell Physiol. 56: 334–345. [DOI] [PubMed] [Google Scholar]
- Johnson E.T. and Schmidt-Dannert C. (2008) Characterization of three homologs of the large subunit of the magnesium chelatase from Chlorobaculum tepidum and interaction with the magnesium protoporphyrin IX methyltransferase. J. Biol. Chem. 283: 27776–27784. [DOI] [PubMed] [Google Scholar]
- Jonker J.W., Merino G., Musters S., van Herwaarden A.E., Bolscher E., Wagenaar E., et al. (2005) The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat. Med. 11: 127–129. [DOI] [PubMed] [Google Scholar]
- Kada S., Koike H., Satoh K., Hase T. and Fujita Y. (2003) Arrest of chlorophyll synthesis and differential decrease of photosystems I and II in a cyanobacterial mutant lacking light-independent protochlorophyllide reductase. Plant Mol. Biol. 51: 225–235. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Lee J., Park J. and Gho Y.S. (2015) Gram-negative and Gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 40: 97–104. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Masuda T., Tajima N., Wada H. and Sato N. (2014) Molecular phylogeny and intricate evolutionary history of the three isofunctional enzymes involved in the oxidation of protoporphyrinogen IX. Genome Biol. Evol. 6: 2141–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara R., Lim H.-S., Kanamoto Y., Murakami A., Fujita Y., Tanaka A., et al. (2023) Heterologous complementation systems verify the mosaic distribution of three distinct protoporphyrinogen IX oxidase in the cyanobacterial phylum. J. Plant Res. 136: 107–115. [DOI] [PubMed] [Google Scholar]
- Kojima S., Muramoto K. and Kusano T. (2016) Outer membrane proteins derived from non-cyanobacterial lineage cover the peptidoglycan of Cyanophora paradoxa cyanelles and serve as a cyanelle diffusion channel. J. Biol. Chem. 291: 20198–20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowata H., Tochigi S., Takahashi H. and Kojima S. (2017) Outer membrane permeability of cyanobacterium Synechocystis sp. strain PCC 6803: studies of passive diffusion of small organic nutrients reveal the absence of classical porins and intrinsically low permeability. J. Bacteriol. 199: e00371–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K., Zsiros O. and Gombos Z. (1999) Accumulation of zeaxanthin in cytoplasmic membranes of the cyanobacteirum Synechococcus sp. Strain PCC 7942 grown under high light condition. J. Plant Physiol. 155: 136–138. [Google Scholar]
- Masuda T. and Fujita Y. (2008) Regulation and evolution of chlorophyll metabolism. Photochem. Photobiol. Sci. 7: 1131–1149. [DOI] [PubMed] [Google Scholar]
- Minamizaki K., Mizoguchi T., Goto T., Tamiaki H. and Fujita Y. (2008) Identification of two homologous genes, chlAI and chlAII, that are differentially involved in isocyclic ring formation of chlorophyll a in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 283: 2684–2692. [DOI] [PubMed] [Google Scholar]
- Nomata J., Mizoguchi T., Tamiaki H. and Fujita Y. (2006) A second nitrogenase-like enzyme for bacteriochlorophyll biosynthesis: reconstitution of chlorophyllide a reductase with purified X-protein (BchX) and YZ-protein (BchY-BchZ) from Rhodobacter capsulatus. J. Biol. Chem. 281: 15021–15028. [DOI] [PubMed] [Google Scholar]
- Norling B., Zarka A. and Boussiba S. (1997) Isolation and characterization of plasma membranes from cyanobacteria. Physiol. Plant. 99: 495–504. [Google Scholar]
- Obata D., Takabayashi A., Tanaka R., Tanaka A. and Ito H. (2019) Horizontal transfer of promiscuous activity from nonphotosynthetic bacteria contributed to evolution of chlorophyll degradation pathway. Mol. Biol. Evol. 36: 2830–2841. [DOI] [PubMed] [Google Scholar]
- Omata T. and Murata N. (1984) Isolation and characterization of 3 types of membranes from the cyanobacterium (blue-green algae) Synechocystis PCC6714. Arch. Microbiol. 139: 113–116. [Google Scholar]
- Qiu G.W., Jiang H.B., Lis H., Li Z.K., Deng B., Shang J.L., et al. (2021) A unique porin meditates iron-selective transport through cyanobacterial outer membranes. Environ. Microbiol. 23: 376–390. [DOI] [PubMed] [Google Scholar]
- Rast A., Schaffer M., Albert S., Wan W., Pfeffer S., Beck F., et al. (2019) Biogenic regions of cyanobacterial thylakoids form contact sites with the plasma membrane. Nat. Plants 5: 436–446. [DOI] [PubMed] [Google Scholar]
- Reinbothe C., El Bakkouri M., Buhr F., Muraki N., Nomata J., Kurisu G., et al. (2010) Chlorophyll biosynthesis: spotlight on protochlorophyllide reduction. Trends Plant Sci. 15: 614–622. [DOI] [PubMed] [Google Scholar]
- Robey R.W., Steadman K., Polgar O., Morisaki K., Blayney M., Mistry P., et al. (2004) Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 64: 1242–1246. [DOI] [PubMed] [Google Scholar]
- Schwechheimer C. and Kuehn M.J. (2015) Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13: 605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T.A. (1997) Folding of the N-terminal, ligand-binding region of integrin a-subunits into a b-propeller domain. Proc. Natl. Acad. Sci. U.S.A. 94: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatani N., Uenosono M., Hara Y., Yamakawa H., Fujita Y. and Omata T. (2022) Chlorophyll and pheophytin dephytylating enzymes required for efficient repair of PSII in Synechococcus elongatus PCC 7942. Plant Cell Physiol. 63: 410–420. [DOI] [PubMed] [Google Scholar]
- Tanaka R. and Tanaka A. (2007) Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 58: 321–346. [DOI] [PubMed] [Google Scholar]
- Usui K., Yamamoto H., Oi T., Taniguchi M., Mori H. and Fujita Y. (2022) Extracellular vesicle-mediated secretion of protochlorophyllide in the cyanobacterium. Plants 11: 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velling T., Kusche-Gullberg M., Sejersen T. and Gullberg D. (1999) cDNA cloning and chromosomal localization of human α11 integrin. A collagen-binding, I domain-containing, β1-associated integrin α-chain present in muscle tissues. J. Biol. Chem. 274: 25735–25742. [DOI] [PubMed] [Google Scholar]
- Wang P., Ji S. and Grimm B. (2022) Post-translational regulation of metabolic checkpoints in plant tetrapyrrole biosynthesis. J. Exp. Bot. 73: 4624–4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal S., Heins L., Soll J. and Vothknecht U.C. (2001) Vipp1 deletion mutant of Synechocystis: a connection between bacterial phage shock and thylakoid biogenesis? Proc. Natl. Acad. Sci. U.S.A. 98: 4243–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwinowski J., Sartori-Rupp A., Taib N., Pende N., Tham T.N., Poppleton D., et al. (2022) An ancient divide in outer membrane tethering systems in bacteria suggests a mechanism for the diderm-to-monoderm transition. Nat. Microbiol. 7: 411–422. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Kojima-Ando H., Ohki K. and Fujita Y. (2020) Formation of prolamellar-body-like ultrastructures in etiolated cyanobacterial cells overexpressing light-dependent protochlorophyllide oxidoreductase in Leptolyngbya boryana. J. Gen. Appl. Microbiol. 66: 129–139. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Kurumiya S., Ohashi R. and Fujita Y. (2011) Functional evaluation of a nitrogenase-like protochlorophyllide reductase encoded by the chloroplast DNA of Physcomitrella patens in the cyanobacterium Leptolyngbya boryana. Plant Cell Physiol. 52: 1983–1993. [DOI] [PubMed] [Google Scholar]
- Yamanashi K., Minamizaki K. and Fujita Y. (2015) Identification of the chlE gene encoding oxygen-independent Mg-protoporphyrin IX monomethyl ester cyclase in cyanobacteria. Biochem. Biophys. Res. Commun. 463: 1328–1333. [DOI] [PubMed] [Google Scholar]
- Yamazaki S., Nomata J. and Fujita Y. (2006) Differential operation of dual protochlorophyllide reductases for chlorophyll biosynthesis in response to environmental oxygen levels in the cyanobacterium Leptolyngbya boryana. Plant Physiol. 142: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D.A., Bauer C.E., Williams J.C. and Marrs B.L. (1989) Genetic evidence for superoperonal organization of genes for photosynthetic pigments and pigment-binding proteins in Rhodobacter capsulatus. Mol. Gen. Genet. 218: 1–12. [DOI] [PubMed] [Google Scholar]
- Zapata M., Rodoriguez R. and Garrido J.L. (2000) Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 195: 29–45. [Google Scholar]
- Zhu Y., Graham J.E., Ludwig M., Xiong W., Alvey R.M., Shen G., et al. (2010) Roles of xanthophyll carotenoids in protection against photoinhibition and oxidative stress in the cyanobacterium Synechococcus sp. strain PCC 7002. Arch. Biochem. Biophys. 504: 86–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article or Supplementary materials. All raw data have been deposited into the data management system of Nagoya University.


