Abstract
Cold hyperalgesia is a well-documented symptom of inflammatory and neuropathic pain; however, the underlying mechanisms of this enhanced sensitivity to cold are poorly understood. A subset of transient receptor potential (TRP) channels mediates thermosensation and is expressed in sensory tissues, such as nociceptors and skin. Here we report that the pharmacological blockade of TRPA1 in primary sensory neurons reversed cold hyperalgesia caused by inflammation and nerve injury. Inflammation and nerve injury increased TRPA1, but not TRPM8, expression in tyrosine kinase A–expressing dorsal root ganglion (DRG) neurons. Intrathecal administration of anti–nerve growth factor (anti-NGF), p38 MAPK inhibitor, or TRPA1 antisense oligodeoxynucleotide decreased the induction of TRPA1 and suppressed inflammation- and nerve injury–induced cold hyperalgesia. Conversely, intrathecal injection of NGF, but not glial cell line–derived neurotrophic factor, increased TRPA1 in DRG neurons through the p38 MAPK pathway. Together, these results demonstrate that an NGF-induced TRPA1 increase in sensory neurons via p38 activation is necessary for cold hyperalgesia. Thus, blocking TRPA1 in sensory neurons might provide a fruitful strategy for treating cold hyperalgesia caused by inflammation and nerve damage.
Introduction
Hyperalgesia to cold stimulation is a well-documented symptom of inflammatory and neuropathic pain both in the clinical setting and in experimental animal models. Cold hyperalgesia appears to be mediated by the activation of unmyelinated or thinly myelinated fibers (1, 2). Indeed, the behavioral signs of cold, but not mechanical, allodynia in rats with nerve injury were mediated by capsaicin-sensitive afferents (3, 4). However, the molecular mechanisms underlying this abnormal sensitivity to cold remain unknown. Temperature is sensed by a subpopulation of peripheral primary afferents known as thermoreceptors. Recently, the existence of 6 thermosensitive ion channels has been reported, all of which belong to the transient receptor potential (TRP) superfamily, including TRPV1, TRPV2, TRPV3, TRPV4, TRPM8 (also known as CMR1), and TRPA1 (previously known as ANKTM1) (5–8). Among them, TRPA1 and TRPM8 have been identified as cold-sensitive ion channels. TRPM8 is activated by menthol and cooling, with an activation temperature of approximately 25–28°C (9, 10) whereas TRPA1 is activated at approximately 17°C, a temperature that is reported as painfully cold by humans (11, 12).
Neurotrophic factors, which support neuronal survival and growth during the development of the nervous system, have attracted attention because of their important roles in pathological pain (13, 14). They include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and glial-derived NGF (GDNF). Among them, NGF is known to be a major contributor to inflammatory pain (15, 16). Levels of NGF increase dramatically in inflamed tissue, and the enhanced retrograde transport of NGF increases the expression of substance P (SP), calcitonin gene-related peptide (CGRP), BDNF, and TRPV1 in tyrosine kinase A–expressing (trkA-expressing) dorsal root ganglion (DRG) neurons (13–16). All of the neuropathic pain models are created by partial nerve injury in which some primary afferents are axotomized and others are spared. There is compelling evidence indicating that not only injured primary afferents, but also their uninjured neighbors, show an alteration of excitability and gene expression and that these changes have functional roles in neuropathic pain (17, 18). For example, SP, CGRP, BDNF, and TRPV1 also increase in spared DRG neurons after partial nerve injury (19, 20).
The activation of p38 MAPK in nociceptive neurons participates in generating pain hypersensitivity through transcription-dependent and transcription-independent means (21, 22). We now show that NGF-induced p38 activation in primary afferent neurons regulates TRPA1 expression after inflammation and nerve injury, and this induction of TRPA1 in sensory neurons contributes to the development of cold hyperalgesia. Our findings point to the potential blockade of TRPA1 in sensory neurons as a new therapeutic strategy for inflammatory and neuropathic pain.
Results
TRPA1, but not TRPM8, expression increases in trkA-expressing DRG neurons following peripheral inflammation.
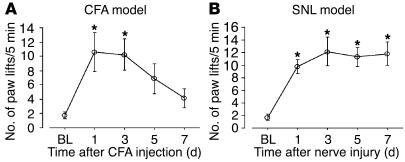
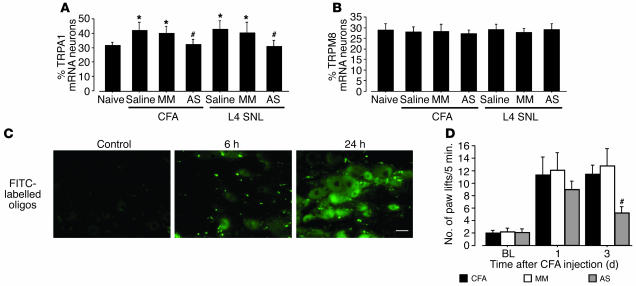
We first investigated the role of TRPA1 and TRPM8 in cold hyperalgesia after inflammation induced by CFA. The CFA injection clearly increased the number of paw lifts on a cold plate at 5°C for the 5-minute testing period (Figure 1A). The number of paw lifts increased from 1.8 ± 0.5 before CFA injection to 10.6 ± 2.7 at day 1 and 10.3 ± 2.2 at day 3; cold hyperalgesia gradually resolved by day 7. In contrast, contralateral paw lifts were remarkably few (data not shown).
Figure 1.
Time course of the exaggerated response to cold after peripheral inflammation and nerve injury. (A and B) The number of paw lifts on a cold plate at 5°C for the 5-minute testing period was examined at days 1, 3, 5, and 7 after CFA injection (A) and L5 SNL (B). Data represent mean ± SEM; n = 8 per group. BL, baseline. *P < 0.05 compared with the naive control.
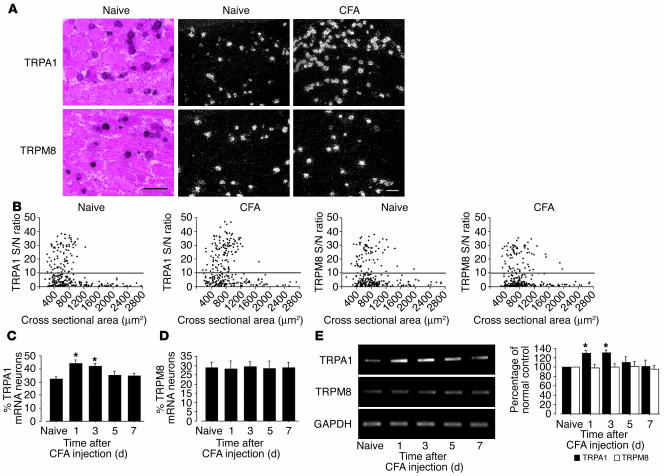
In situ hybridization histochemistry (ISHH) revealed that most of the TRPA1 and TRPM8 mRNA-labeled neurons in the DRG were small or medium in size (Figure 2A), consistent with previous studies (9–11). Using a computerized image analysis, we found that 32.4% ± 1.8% and 29.1% ± 2.7% of DRG neurons were positively labeled for TRPA1 and TRPM8 mRNA in naive control rats, respectively (Figure 2B). There was no change in the percentage of TRPA1 mRNA–positive neurons on the contralateral side (data not shown); however, inflammation induced a significant increase in the percentage of TRPA1 mRNA–positive neurons in the ipsilateral DRG at days 1 and 3 (44.1% ± 2.9% and 42.0% ± 2.2%, respectively); the levels gradually declined, returning to normal by day 7 (Figure 2C). This upregulation corresponded well with the development and maintenance of CFA-induced cold hyperalgesia. In contrast, there was no significant difference in the percentages of TRPM8 mRNA–positive neurons over 7 days (Figure 2D). These changes in TRPA1 and TRPM8 mRNA were also confirmed by RT-PCR (Figure 2E). In the spinal cord, neither TRPA1 nor TRPM8 mRNA were detected (data not shown).
Figure 2.
Marked upregulation of TRPA1, but not TRPM8, mRNA in DRG neurons after peripheral inflammation induced by CFA. (A) Bright- and dark-field photomicrographs of ISHH showing expression of TRPA1 and TRPM8 mRNA in the naive DRG and the ipsilateral DRG at day 1 after inflammation. Scale bars: 100 μm. (B) Scatterplot diagrams made by plotting the individual cell profiles at day 1 after CFA injection. The gray lines represent the borderlines between the negatively and positively labeled neurons (signal/noise [S/N] ratio = 10). (C and D) Time course of the mean percentages of TRPA1 (C) and TRPM8 (D) mRNA–positive neurons after CFA injection. (E) mRNA expression of TRPA1 and TRPM8 in the DRG after inflammation, as detected by RT-PCR. Quantification of RT-PCR data is shown at right. Data represent mean ± SD; n = 4 per group. *P < 0.05 compared with the naive control.
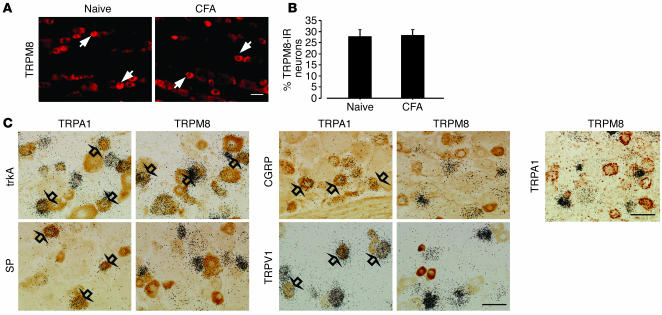
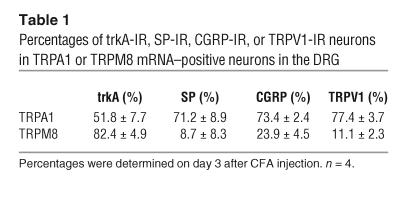
Several studies have demonstrated an increase in levels of TRPV1 protein, but not in levels of mRNA, after peripheral inflammation (23, 24). Therefore, we examined the changes of TRPM8 expression in the DRG at the protein level using immunohistochemistry (Figure 3A). Consistent with the data obtained from ISHH, there was no difference in the percentages of TRPM8-immunoreactive neurons at day 3 after inflammation (Figure 3B). We found that inflammation increased TRPA1 expression in small- and medium-size neurons (Figure 2, A and B). Cutaneous nociceptors can be divided into 2 broad groups: NGF-responsive/trkA-expressing neurons and GDNF-responsive/c-ret–expressing neurons (25). Double labeling showed that both TRPA1 and TRPM8 heavily colocalized with trkA after inflammation (Figure 3C). However, only TRPA1-expressing neurons coexpressed SP, CGRP, and TRPV1 (Table 1). Furthermore, approximately 10% of TRPA1-labeled neurons were also labeled for TRPM8, indicating that inflammation increased TRPA1 in TRPM8-negative peptidergic neurons.
Figure 3.
No change of TRPM8 protein in the DRG and no overlap between TRPM8- and TRPA1-expressing neurons after inflammation. (A) Protein expression of TRPM8 in the naive DRG and the ipsilateral DRG at day 3 after inflammation, as detected by immunohistochemistry. TRPM8-immunoreactive (TRPM8-IR) neurons were invariably small or medium in size (arrows). (B) Quantification of the percentage of TRPM8-IR neurons at day 3 after CFA injection. (C) Double labeling by a combined method of ISHH and immunohistochemistry for TRPA1 or TRPM8 mRNA and trkA-IR, SP-IR, CGRP-IR, and TRPV1-IR in the DRG at day 3 after CFA injection. Double labeling for TRPA1 and TRPM8 mRNA by dual ISHH is shown at right. TRPA1- and TRPM8-expressing neurons were clearly distinguishable. Open arrows indicate double-labeled neurons. Scale bars: 50 μm.
Table 1.
Percentages of trkA-IR, SP-IR, CGRP-IR, or TRPV1-IR neurons in TRPA1 or TRPM8 mRNA–positive neurons in the DRG
Anti-NGF and a p38 MAPK inhibitor alleviate inflammation-induced cold hyperalgesia and TRPA1 upregulation.
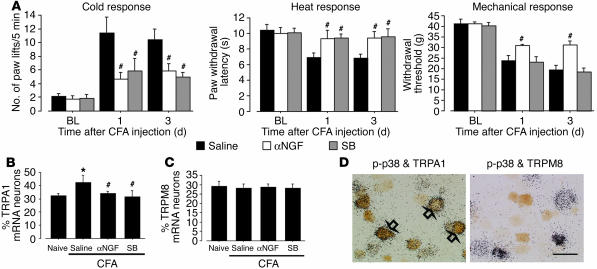
NGF injection in a peripheral target induces p38 MAPK activation in trkA-expressing DRG neurons; activation of p38 MAPK in DRG neurons contributes to persistent inflammatory pain via the transcriptional regulation of key gene products (23). To determine whether alterations of the endogenous NGF and p38 MAPK pathway might be involved in inflammation-induced cold hyperalgesia, anti-NGF or a p38 MAPK inhibitor, SB203580, was delivered intrathecally before CFA injection and maintained for 3 days via a catheter whose tip was positioned close to the L4/5 DRG. Intrathecal administration of anti-NGF or SB203580 into naive rats produced no significant changes in basal pain sensitivity (data not shown). We found that both anti-NGF (1 μg/μl–1/h–1) and SB203580 (0.5 μg/μl–1/h–1) infusion significantly inhibited inflammation-induced cold hyperalgesia at days 1 and 3 (Figure 4A). Furthermore, anti-NGF reduced inflammation-induced heat hyperalgesia and mechanical allodynia whereas SB203580 attenuated heat hyperalgesia but not mechanical allodynia at days 1 and 3, consistent with previous reports (23).
Figure 4.
Anti-NGF and a p38 MAPK inhibitor reverse cold hyperalgesia and TRPA1 upregulation induced by inflammation. (A) Cold and heat hyperalgesia were tested using the cold plate test and the plantar test, respectively, at days 1 and 3 after CFA injection. Mechanical allodynia was determined with a Dynamic Plantar Aesthesiometer. Data represent mean ± SEM; n = 8 per group. (B and C) Quantification of the percentages of TRPA1 (B) and TRPM8 (C) mRNA–positive neurons at day 3 after CFA injection. Data represent mean ± SD; n = 4 per group. (D) Double labeling for TRPA1 or TRPM8 mRNA and p-p38–IR at day 3 after CFA injection. Open arrows indicate double-labeled neurons. SB, SB203580. *P < 0.05 compared with the naive control; #P < 0.05 compared with the vehicle group. Scale bar: 50 μm.
We then assessed the effects of anti-NGF and SB203580 on inflammation-induced TRPA1 upregulation in the DRG. CFA induced a substantial increase in the percentage of TRPA1-positive neurons in the vehicle group at day 3, but pretreatment with either anti-NGF or SB203580 prevented this increase (Figure 4B). However, there was no apparent change in the expression of TRPM8 (Figure 4C). Furthermore, double labeling showed that phosphorylated p38 (p-p38) was predominantly expressed in TRPA1- but not TRPM8-positive neurons at day 3 after inflammation (Figure 4D).
NGF, but not GDNF, increases TRPA1 expression in DRG neurons through the p38 MAPK pathway.
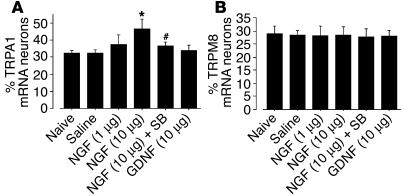
To further investigate the effects of NGF and the p38 MAPK pathway on TRPA1 expression in the DRG neurons, we injected NGF (1 μg or 10 μg in 10 μl saline) intrathecally into naive rats. Three days after the 10-μg NGF injections, 46.8% ± 5.7% of the neurons were TRPA1 positive; this percentage was higher than that of TRPA1-positive neurons in the saline group (32.6% ± 1.5%). This increase, however, was significantly reversed by cotreatment with SB203580 (Figure 5A). Additionally, TRPA1 levels did not change after the 10-μg GDNF injection, and neither NGF nor GDNF significantly altered TRPM8 expression (Figure 5B).
Figure 5.
NGF, but not GDNF, induces an increase of TRPA1 expression in DRG neurons via p38 MAPK activation. Quantification of the percentage of TRPA1 (A) and TRPM8 (B) mRNA–positive neurons at day 3 after the injection. Data represent mean ± SD; n = 4 per group. *P < 0.05 compared with the naive control. #P < 0.05 compared with the NGF 10-μg group.
TRPA1 induced in spared DRG neurons contributes to cold hyperalgesia following nerve injury.
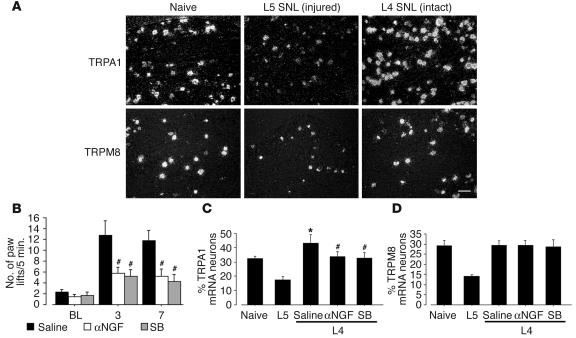
The L5 spinal nerve ligation (SNL) model (26), which is one of the most popular models of neuropathic pain, is unique because the L4 DRG neurons are clearly separated from the axotomized L5 neurons (20). Recent studies have shown increased expressions of SP, CGRP, BDNF, and TRPV1 in the spared L4 DRG following L5 SNL, which indicates that the molecular phenotypic changes in the spared L4 DRG are just like those produced by peripheral inflammation (18–20). Therefore, we investigated the role of TRPA1 and TRPM8 in nerve injury–induced cold hyperalgesia using the L5 SNL model. The L5 SNL clearly increased the number of paw lifts on a cold plate, and cold hyperalgesia lasted for more than 7 days after surgery (Figure 1B). The contralateral side of nerve-ligated rats and both sides of sham-operated rats did not show cold hyperalgesia (data not shown). We then examined the changes of TRPA1 and TRPM8 expression in the L4 and L5 DRG after L5 SNL. Seven days after surgery, the L5 SNL decreased both TRPA1 and TRPM8 mRNA expression in the L5 DRG (Figure 6A). In contrast, the percentage of TRPA1 mRNA–positive neurons significantly increased in the L4 DRG at day 7 after L5 SNL (43.1% ± 5.9%), mainly in small- to medium-diameter neurons. However, there was no change in the expression of TRPM8 in the L4 DRG.
Figure 6.
Anti-NGF and a p38 MAPK inhibitor suppress cold hyperalgesia and TRPA1 upregulation in the spared L4 DRG neurons caused by injury to the L5 spinal nerve. (A) Dark-field photomicrograph of ISHH showing the expression of TRPA1 and TRPM8 mRNA in the naive DRG, the injured L5 DRG, and the intact L4 DRG at day 7 after L5 SNL. (B) Cold hyperalgesia was determined using the cold plate test at days 3 and 7 after L5 SNL. Data represent mean ± SEM; n = 8 per group. (C and D) Quantification of the percentage of TRPA1 (C) and TRPM8 (D) mRNA–positive neurons at day 7 after surgery. Data represent mean ± SD; n = 4 per group. *P < 0.05 compared with the naive control; #P < 0.05 compared with the vehicle group. Scale bar: 100 μm.
To ascertain whether NGF-induced p38 activation in the uninjured L4 DRG regulates TRPA1 expression and contributes to cold hyperalgesia after L5 SNL, we administered anti-NGF or SB203580 into the intrathecal space. The treatment of anti-NGF and SB203580 diminished L5 SNL-induced cold hyperalgesia at days 3 and 7 (Figure 6B). Furthermore, we found that both anti-NGF and SB203580 application blocked the L5 SNL–induced increase in TRPA1 but not TRPM8 expression in the spared L4 DRG at day 7 (Figure 6, C and D).
Knockdown of the TRPA1 gene prevents and reverses inflammation- and nerve injury–induced cold hyperalgesia.
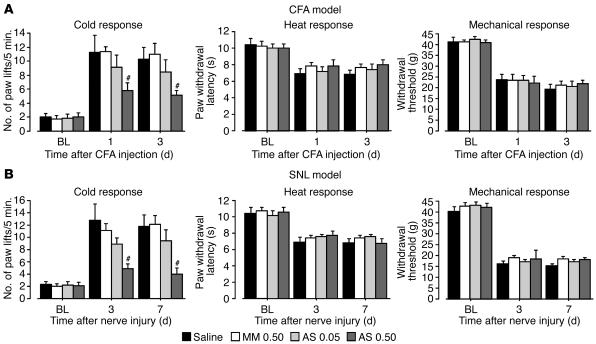
Our results suggest that cold hyperalgesia after inflammation and nerve injury is critically dependent on functional TRPA1 in DRG neurons. We therefore predicted that a selective knockdown of TRPA1 expression should prevent inflammation- and nerve injury–induced cold hyperalgesia. To test this, rats with peripheral inflammation or nerve injury were intrathecally treated with either an antisense oligodeoxynucleotide (AS-ODN) targeting TRPA1 or a mismatch ODN (MM-ODN) beginning 12 hours before CFA injection or L5 SNL. We found that the CFA- and SNL-induced increase in the number of paw lifts on a cold plate was significantly less in the TRPA1 AS-ODN group (0.5 nmol/μl–1/h–1) than in the MM-ODN group (0.5 nmol/μl–1/h–1) (Figure 7, A and B). The number of paw lifts in the MM-ODN and AS-ODN groups (0.05 nmol/μl–1/h–1) groups was not different from that of the vehicle control rats. However, AS-ODN had no effect on CFA- and SNL-induced mechanical allodynia and heat hyperalgesia.
Figure 7.
Reversal of hyperalgesia to cold by a selective blockade of TRPA1 expression. (A and B) Pretreatment with TRPA1 AS-ODN (0.5 nmol/μl–1/h–1) (AS 0.50) prevents cold hyperalgesia, but not heat hyperalgesia or mechanical allodynia, induced by peripheral inflammation (A) and injury to the L5 spinal nerve (B). Cold and heat hyperalgesia were tested using the cold plate test and the plantar test, respectively, at days 1 and 3 after CFA injection or at days 3 and 7 after L5 SNL. Mechanical allodynia was examined using a Dynamic Plantar Aesthesiometer. Data represent mean ± SEM; n = 8 per group. #P < 0.05 compared with the MM-ODN (0.5 nmol/μl–1/h–1) (MM 0.50) group.
We then confirmed that the level of TRPA1 mRNA in the DRG neurons of the AS-ODN–treated rats was significantly lower than that in the MM-ODN–treated rats (Figure 8A) whereas there was no difference in TRPM8 expression between TRPA1 AS-ODN–treated rats and control rats both in the CFA model and in the L5 SNL model (Figure 8B). The distribution of FITC-labeled ODN showed the progressive increase in the fluorescence associated with DRG cell bodies, indicating that the uptake of the ODN occurred in a time-dependent manner (Figure 8C). Furthermore, the treatment with AS-ODN, beginning 12 hours after CFA injection, reversed the inflammation-induced cold hyperalgesia at day 3 but not at day 1 (Figure 8D).
Figure 8.
Confirmation of a selective blockade of TRPA1 expression in a time-dependent manner. (A and B) TRPA1 AS-ODN (0.5 nmol/μl–1/h–1) attenuates the induction of TRPA1, but not TRPM8, mRNA induced by peripheral inflammation and injury to the L5 spinal nerve. Quantification of the percentage of TRPA1 (A) and TRPM8 (B) mRNA–positive neurons at day 3 after CFA injection or in the L4 DRG at day 7 after L5 SNL. Data represent mean ± SD; n = 4 per group. (C) Fluorescence in the naive DRG and the DRG at 6 and 24 hours after intrathecal injection of FITC-labeled ODN. Scale bar: 50 μm. (D) Effect of treatment with TRPA1 AS-ODN (0.5 nmol/μl–1/h–1) following CFA injection on CFA-induced cold hyperalgesia at days 1 and 3. Data represent mean ± SEM; n = 8 per group. *P < 0.05 compared with naive control. #P < 0.05 compared with MM-ODN (0.5 nmol/μl–1/h–1) group.
Noxious cold stimulation induces very rapid phosphorylation of p38 MAPK through the TRPA1 channel.
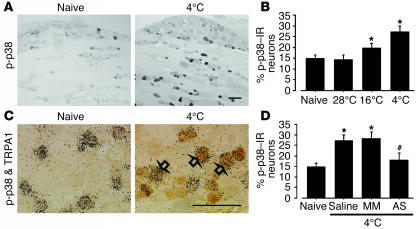
Recent work has failed to reproduce cold responsiveness in TRPA1 in vitro (27). To investigate how TRPA1 can be activated by noxious cold stimulation in vivo, we examined the phosphorylation of p38 MAPK in the DRG 2 minutes after noxious cold stimulation. We did this because activation of p38 in primary afferents is involved in acute nociceptive processing by a nontranscriptional mechanism and examination of p-p38 is very useful as an indicator of the activated DRG neurons after noxious stimulation in vivo (28). We applied thermal stimulation by immersion of the hind paw of naive rats into cool to cold water (28°C, 16°C, and 4°C) and found that at 28°C, there was no increase in p38 phosphorylation in the DRG (Figure 9, A and B). However, noxious cold stimulation at lower temperatures (16°C and 4°C) increased the phosphorylation of p38 in the DRG, mainly in small-size neurons 2 minutes after stimulation. Double labeling showed that at 4°C, the majority of the p-p38–labeled neurons also expressed TRPA1 (Figure 9C). Furthermore, we found that pretreatment with AS-ODN but not MM-ODN inhibited noxious cold stimulation–induced p38 activation (Figure 9D), which suggests that the TRPA1 channel is required for p38 activation after noxious cold stimulation.
Figure 9.
Activation of p38 MAPK in TRPA1-containing neurons by noxious cold stimulation. (A) p-p38 labeling in naive DRG and ipsilateral DRG 2 minutes after cold stimulation at 4°C. (B) Quantification of the percentage of p-p38–IR neurons after innocuous and noxious cold stimulation. (C) Double labeling for TRPA1 mRNA and p-p38–IR in naive DRG and ipsilateral DRG 2 minutes after cold stimulation at 4°C. Open arrows indicate double-labeled neurons. (D) Effect of pretreatment with TRPA1 AS-ODN (0.5 nmol/μl–1/h–1) on the percentage of p-p38–IR neurons after cold stimulation at 4°C. Data represent mean ± SD; n = 4 per group. *P < 0.05 compared with naive control. #P < 0.05 compared with MM-ODN (0.5 nmol/μl–1/h–1) group. Scale bars: 100 μm.
Discussion
The present study demonstrates the following new findings: (a) CFA and SNL increased TRPA1, but not TRPM8, expression in trkA-expressing DRG neurons; (b) anti-NGF and SB203580 decreased the induction of TRPA1 and alleviated cold hyperalgesia after injection of CFA and SNL; (c) TRPA1 AS-ODN prevented and reversed only cold hyperalgesia after injection of CFA and SNL; (d) NGF, but not GDNF, increased TRPA1 expression in DRG neurons via p38 MAPK activation; (e) noxious cold stimulation induced p38 activation in TRPA1-containing neurons, but p38 activation was prevented by administration of TRPA1 AS-ODN.
Because of beneficial effects of cooling on pain, there have been few studies examining the underlying mechanisms of cold hyperalgesia after peripheral inflammation. However, cold hyperalgesia is often observed in patients suffering from rheumatoid arthritis (29), and noxious, but not innocuous, cold (< 10°C) induces an exaggerated response after CFA induces inflammation in rats (30, 31). We found that TRPA1, but not TRPM8, expression increased in trkA-expressing DRG neurons after inflammation and that the time course of the change in TRPA1 level in the DRG matched the emergence of enhanced sensitivity to cold (5°C). Furthermore, anti-NGF and SB203580 diminished this inflammation-induced cold hyperalgesia and TRPA1 upregulation. Because TRPA1 is activated by cold-range temperatures with a threshold of approximately 17°C, which is close to the reported noxious cold threshold (11, 12), these findings suggest that inflammation increases TRPA1 expression in DRG neurons via an NGF-induced p38 activation and that this induction of TRPA1 contributes to hyperalgesia to noxious, but not innocuous, cold stimulation.
We found that after inflammation, TRPA1-expressing neurons expressed SP and CGRP whereas TRPM8 did not colocalize with these nociceptive markers. Further, there was no overlap between TRPM8- and TRPA1-expressing neurons. Because our histological characterization does not address whether the number of active receptors changed, we cannot deny the possibility that TRPM8 may participate in inflammation-induced cold hyperalgesia. However, it seems likely that TRPA1 and TRPM8 are expressed in sensory neurons in 2 clearly distinct populations and might have different functional roles in pathological pain conditions. An unexpected finding in the present study was that neither inflammation nor NGF injection increased TRPM8 expression, although TRPM8 heavily colocalizes with trkA. Furthermore, TRPM8-expressing neurons did not coexpress TRPV1 after inflammation. In primary cultures, approximately 50% of the menthol-sensitive neurons also respond to capsaicin in the presence of NGF (11), and sensitivity to cold and capsaicin in these neurons was increased by NGF (32, 33). This discrepancy may be due to the high levels of NGF, which sensitize and upregulate TRPV1 levels, that are present in these cultures (6, 11). On the other hand, the percentages of TRPA1 and TRPM8 mRNA–positive neurons in the present study are much larger than those previously obtained in mouse DRG (3.6% for TRPA1 and 5–10% for TRPM8) (9–11). This discrepancy may be due to the species difference in ion channel expression between rat and mouse DRGs. Alternatively, another explanation is that there were differences in the criterion used to distinguish between positively and negatively labeled neurons (34).
Cold hyperalgesia is also a common feature of neuropathic pain, for which there is currently no effective therapy. In previous studies, much attention was focused on the directly damaged primary afferents and their influence on the activity of the dorsal horn neurons (35, 36). However, it is evident that the spared L4 DRG neurons and their fibers are also functionally important in the maintenance of neuropathic pain in the L5 SNL model (17, 18). For example, L4, but not L5, dorsal rhizotomy can reverse mechanical allodynia (37), and spontaneous activity in C fibers from the L4 spinal nerve has been recorded (38). A recent report of an in vitro study showed that after L5 SNL, the percentage of cold-responsive neurons increased in the uninjured neighbors of injured neurons, with no significant changes in the response properties (39). These observations are consistent with our data showing that L5 SNL increased TRPA1 expression in the uninjured L4 DRG.
We also found that both anti-NGF and SB203580 prevented L5 SNL–induced cold hyperalgesia. Wallerian degeneration following nerve lesion leads to an increase in cytokines and growth factors (40, 41). NGF content increases as early as 1 day after L5 SNL in the sciatic nerve, which contains both axotomized L5 nerves and spared L4 nerves (19).The MAPK family includes ERK, p38 MAPK, and JNK, and we recently demonstrated that L5 SNL induces activation of p38, but not ERK or JNK, in the L4 DRG through alterations in the target-derived NGF (20). Some reports have shown that deprivation of NGF does not attenuate cold hyperalgesia in other neuropathic pain models (42, 43). Nevertheless, our observations suggest that after L5 SNL, NGF is synthesized and released in the degenerative nerve fibers and acts upon nearby sensory fibers, inducing TRPA1 upregulation in the intact L4 DRG through the p38 MAPK pathway and thus increasing cold hyperalgesia.
The role of TRPA1 in cold transduction is still controversial; a recent report showed that TRPA1 was not activated by cooling to 4°C in vitro (27). Indeed, TRPA1 can be activated by stimulation other than temperature, such as mustard oils and a cannabinoid compound by bath application (12, 27). However, we found that TRPA1 AS-ODN reversed only cold hyperalgesia induced by inflammation and nerve injury. In addition, noxious cold stimulation induced a stimulus intensity–dependent p38 MAPK activation, predominantly in TRPA1-expressing neurons, which was prevented by administration of TRPA1 AS-ODN. Although it is possible that the phosphorylation of MAPK may not be an accurate reflection of activity (28, 44), our findings indicate that noxious cold stimulation is one of the most likely physiological activators of TRPA1 in vivo. Alternatively, other membrane proteins, such as K+ channels, Na+/K+ ATPases, and degenerin/epithelial sodium channels, may also be involved in transduction of signals mediating cold sensing (45).
We also cannot exclude the possibility that the results of the cold plate test may reflect a number of pathophysiological alternations in the spinal cord and the higher central nervous system, especially in the neuropathic pain model (46). For example, the effects of the p38 MAPK inhibitor on pathological pain can be mediated by the inhibition of p38 activation in the spinal cord (47, 48). However, several reports showed that p38 inhibition in the DRG occurred after intrathecal administration of SB203580, using the same dosage in the present study (20, 23). Furthermore, TRPA1 is specifically expressed in primary sensory neurons but not in the spinal cord or brain (6, 11). In fact, we demonstrated that pharmacologically preventing the induction of TRPA1 in DRG neurons attenuated cold hyperalgesia. Taken together, these results indicate that increased TRPA1 in primary afferent neurons probably has a crucial role in the pathogenesis of cold hyperalgesia after inflammation and nerve injury. Considering that TRPV1 upregulation in sensory neurons contributes to inflammation and nerve injury–induced heat hyperalgesia (8, 20), blocking TRPA1 and TRPV1 simultaneously may represent a new approach to effectively treating clinical inflammatory and neuropathic pain.
Methods
Animals.
Male Sprague-Dawley rats weighing 200–250 g were used. All procedures were approved by the Hyogo College of Medicine Committee on Animal Research and were performed in accordance with National Institutes of Health guidelines on animal care. Rats that did not receive surgery (n = 8) were used as naive controls for immunohistochemistry, ISHH, and RT-PCR.
Surgical procedures.
All procedures were performed with the rats under pentobarbital anesthesia (50 mg/kg, i.p.). For peripheral inflammation, the rats received a subcutaneous injection of 100 μl of CFA diluted 1:1 with saline into the plantar surface of the left hind paw. For partial nerve injury, we used the SNL model (26) with the following modification: the left L5 spinal nerve was tightly ligated and cut just distal to the ligature. In sham-operated rats, the nerve was exposed without ligation. Intrathecal delivery of 10 μl of NGF (1 μg or 10 μg; R & D Systems), GDNF (10 μg; R & D Systems), and the p38 inhibitor SB203580 (10 μg; Calbiochem) was performed as previously described (19). To obtain a sustained drug infusion, an ALZET osmotic pump (7 day pump, 1 μl/hr; DURECT) was filled with SB203580 (0.5 μg/μl) in 50% DMSO or anti-NGF antibody (1 μg/μl; Chemicon International) in saline, and the associated catheter was implanted intrathecally 12 hours before CFA injection or L5 SNL. The lack of effects of DMSO was determined in preliminary experiments, consistent with previous reports (49, 50). AS-ODN (5′-TCTATGCGGTTATGTTGG-3′), MM-ODN (5′-ACTACTACACTAGACTAC-3′), and FITC-labeled ODN directed to TRPA1 were designed and manufactured by BIOGNOSTIK. Although it has frequently been questioned whether ODN can reach DRG with sufficient concentration by intrathecal delivery, several reports have demonstrated that intrathecal ODN accumulates in DRG cells (51, 52). Therefore, AS-ODN (0.05 or 0.5 nmol/μl–1/h–1) and MM-ODN (0.5 nmol/μl–1/h–1) were delivered intrathecally by an osmotic pump either 12 hours before CFA injection or L5 SNL or 12 hours after CFA injection.
Stimulation.
Cold (28°C, 16°C, and 4°C) stimulation was produced by immersion of the hind paw into a water bath (6 times in 10 seconds, interval 10 seconds, total 2 minutes). The survival time after stimulation in all experiments was 2 minutes. In an additional group of rats, either AS-ODN (0.05 or 0.5 nmol/μl–1/h–1) or MM-ODN (0.5 nmol/μl–1/h–1) was delivered intrathecally by an osmotic pump 24 hours before stimulation.
Behavioral analysis.
Rats were habituated and basal pain hypersensitivity was tested before drug administration or surgery. Room temperature and humidity remained stable for all experiments. To estimate cold sensitivity of the paw, the cold plate test was performed as previously described (31, 46). Briefly, each rat was placed in a plastic cage with a Peltier plate (Neuroscience) kept at a cold temperature (5 ± 0.5°C). After 5 minutes of adaptation, the number of paw lifts in the next 5 minutes was recorded. Paw withdrawal latency to a noxious heat stimulus was measured using the Hargreaves radiant heat apparatus. Mechanical withdrawal thresholds on the plantar surface of the hind paw were measured with a Dynamic Plantar Aesthesiometer (Ugo Basile), which is an automated von Frey–type system. The threshold was taken as the force applied to elicit a reflex removal of the hind paw. Both latencies and threshold were determined as the mean of 3 measurements per paw (20).
Immunohistochemistry.
The left L4/5 DRGs (16 μm) were cut and processed for trkA, SP, CGRP, TRPV1, TRPM8, and p-p38 immunohistochemistry according to previously described methods (53). The polyclonal primary antibodies were used in the following dilutions: trkA, 1:400 (Chemicon International); SP, 1:400 (DiaSorin); CGRP, 1:400 (Amersham Biosciences); TRPV1, 1:400 (Oncogene Science); TRPM8, 1:100 (developed by M. Tominaga, Okazaki Institute for Integrative Bioscience); and p-p38, 1:400 (Cell Signaling Technology).
ISHH.
The procedure for ISHH was performed according to previously described methods (54). The rat TRPA1 and TRPM8 cRNA probes corresponding to nucleotides 302–788 and 264–837, respectively, were prepared. For the double labeling of TRPA1 or TRPM8 mRNA and trkA, SP, CGRP, TRPV1, or p-p38 protein, immunohistochemical staining of trkA, SP, CGRP, TRPV1 or p-p38 was performed using an avidinbiotinylated peroxidase complex (ABC) kit (Vector). Afterwards, TRPA1 and TRPM8 mRNA were detected using ISHH with a radioisotope-labeled probe as previously described (55). For the double labeling of TRPA1 and TRPM8 mRNA, we used dual ISHH with digoxigenin-labeled and 35S-labeled probes in the same sections as previously described (55). At least 1200 neurons from 4 rats were quantified for each AS probe in the single ISHH and the combined immunohistochemistry/ISHH study according to previously described methods (55).
RT-PCR.
RT-PCR analyses were carried out according to previously described methods (20). PCR amplification was performed using Taq DNA polymerase (PerkinElmer) and primers specific for the following: Trpa1, 5′-CCCCACTACATTGGGCTGCA-3′ and 5′-CCGCTGTCCAGGCACATCTT-3′; Trpm8, 5′-GCCCAGAGCCAGCATATCGA-3′ and 5′-CCACAAGCAGCAGGTGGGTA-3′; and Gapd (GAPDH), 5′-TGCTGGTGCTGAGTATGTCG-3′ and 5′-GCATGTCAGATCCACAACGG-3′. The ratio of TRPA1 or TRPM8 to GAPDH mRNAs was considered to indicate the level of each transcript. The mRNA level was expressed as a percentage of the mRNA level in the normal control ganglia.
Statistical analyses.
Differences of values between treatment groups were tested using 1-way ANOVA followed by individual post hoc comparisons (Fisher exact test) or pairwise comparisons (2-tailed Student’s t test). P < 0.05 was accepted as significant.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research and the Open Research Center grant, Hyogo College of Medicine, both from the Japanese Ministry of Education, Science, and Culture. This work was also supported by Grants-in-Aid for Researchers in Hyogo College of Medicine. We thank Yuki Obata and Nobumasa Ushio for technical assistance. We thank D.A. Thomas for correcting the English usage.
Footnotes
Nonstandard abbreviations used: AS, antisense; BDNF, brain-derived neurotrophic factor; CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglion; GDNF, glial-derived NGF; ISHH, in situ hybridization histochemistry; MM, mismatch; NGF, nerve growth factor; ODN, oligodeoxynucleotide; p-p38, phosphorylated p38; SNL, spinal nerve ligation; SP, substance P; trkA, tyrosine kinase A; TRP, transient receptor potential.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.LaMotte RH, Thalhammer JG. Response properties of high-threshold cutaneous cold receptors in the primate. Brain Res. 1982;244:279–287. doi: 10.1016/0006-8993(82)90086-5. [DOI] [PubMed] [Google Scholar]
- 2.Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J. Neurophysiol. 1992;68:581–595. doi: 10.1152/jn.1992.68.2.581. [DOI] [PubMed] [Google Scholar]
- 3.Hao JX, Yu W, Xu XJ, Wiesenfeld-Hallin Z. Capsaicin-sensitive afferents mediate chronic cold, but not mechanical, allodynia-like behavior in spinally injured rats. Brain Res. 1996;722:177–180. doi: 10.1016/0006-8993(96)00216-8. [DOI] [PubMed] [Google Scholar]
- 4.Hama AT. Capsaicin-sensitive primary afferents mediate responses to cold in rats with a peripheral mononeuropathy. Neuroreport. 2002;13:461–464. doi: 10.1097/00001756-200203250-00020. [DOI] [PubMed] [Google Scholar]
- 5.Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr. Opin. Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 6.Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation [review] Nat. Rev. Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- 7.Moran MM, Xu H, Clapham DE. TRP ion channels in the nervous system. Curr. Opin. Neurobiol. 2004;14:362–369. doi: 10.1016/j.conb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Tominaga M, Caterina MJ. Thermosensation and pain. J. Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- 9.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 10.Peier AM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 11.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 12.Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 13.Pezet S, Malcangio M, McMahon SB. BDNF: a neuromodulator in nociceptive pathways [review]? Brain Res. Brain Res. Rev. 2002;40:240–249. doi: 10.1016/s0165-0173(02)00206-0. [DOI] [PubMed] [Google Scholar]
- 14.Sah DW, Ossipov MH, Porreca F. Neurotrophic factors as novel therapeutics for neuropathic pain. Nat. Rev. Drug Discov. 2003;2:460–472. doi: 10.1038/nrd1107. [DOI] [PubMed] [Google Scholar]
- 15.Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci. 1993;16:353–359. doi: 10.1016/0166-2236(93)90092-z. [DOI] [PubMed] [Google Scholar]
- 16.Woolf CJ, Ma QP, Allchorne A, Poole S. Peripheral cell types contributing to the hyperalgesic action of nerve growth factor in inflammation. J. Neurosci. 1996;16:2716–2723. doi: 10.1523/JNEUROSCI.16-08-02716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold MS. Spinal nerve ligation: what to blame for the pain and why [review] Pain. 2000;84:117–120. doi: 10.1016/s0304-3959(99)00309-7. [DOI] [PubMed] [Google Scholar]
- 18.Fukuoka T, Noguchi K. Contribution of the spared primary afferent neurons to the pathomechanisms of neuropathic pain. Mol. Neurobiol. 2002;26:57–67. doi: 10.1385/MN:26:1:057. [DOI] [PubMed] [Google Scholar]
- 19.Fukuoka T, Kondo E, Dai Y, Hashimoto N, Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J. Neurosci. 2001;21:4891–4900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obata K, et al. Role of the MAPK activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J. Neurosci. 2004;24:10211–10222. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji RR. Mitogen-activated protein kinases as potential targets for pain killers. Curr. Opin. Investig. Drugs. 2004;5:71–75. [PubMed] [Google Scholar]
- 22.Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004;74:2643–2653. doi: 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 24.Tohda C, et al. Axonal transport of VR1 capsaicin receptor mRNA in primary afferents and its participation in inflammation-induced increase in capsaicin sensitivity. J. Neurochem. 2001;76:1628–1635. doi: 10.1046/j.1471-4159.2001.00193.x. [DOI] [PubMed] [Google Scholar]
- 25.Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors [review] Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 27.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 28.Mizushima T, et al. Activation of p38 MAPK in primary afferent neurons by noxious stimulation and its involvement in the development of thermal hyperalgesia. Pain. 2005;113:51–60. doi: 10.1016/j.pain.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 29.Jahanshahi M, Pitt P, Williams I. Pain avoidance in rheumatoid arthritis. J. Psychosom. Res. 1989;33:579–589. doi: 10.1016/0022-3999(89)90065-2. [DOI] [PubMed] [Google Scholar]
- 30.Perrot S, Attal N, Ardid D, Guilbaud G. Are mechanical and cold allodynia in mononeuropathic and arthritic rats relieved by systemic treatment with calcitonin or guanethidine? Pain. 1993;52:41–47. doi: 10.1016/0304-3959(93)90111-2. [DOI] [PubMed] [Google Scholar]
- 31.Jasmin L, Kohan L, Franssen M, Janni G, Goff JR. The cold plate as a test of nociceptive behaviors: description and application to the study of chronic neuropathic and inflammatory pain models. Pain. 1998;75:367–382. doi: 10.1016/s0304-3959(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 32.Reid G, Babes A, Pluteanu F. A cold- and menthol-activated current in rat dorsal root ganglion neurones: properties and role in cold transduction. J. Physiol. 2002;545:595–614. doi: 10.1113/jphysiol.2002.024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur. J. Neurosci. 2004;20:2276–2282. doi: 10.1111/j.1460-9568.2004.03695.x. [DOI] [PubMed] [Google Scholar]
- 34.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devor, M., and Seltzer, Z. 1999. Pathophysiology of damaged nerves in reaction to chronic pain. In Textbook of pain. P.D. Wall and R. Melzack, editors. Churchill Livingstone. London, United Kingdom. 129–164.
- 36.Scholz J, Woolf CJ. Can we conquer pain [review]? Nat. Neurosci. 2002;5(Suppl.):1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Dorsi MJ, Meyer RA, Belzberg AJ. Mechanical hyperalgesia after an L5 spinal nerve lesion in the rat is not dependent on input from injured nerve fibers. Pain. 2000;85:493–502. doi: 10.1016/S0304-3959(00)00250-5. [DOI] [PubMed] [Google Scholar]
- 38.Schafers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J. Neurosci. 2003;23:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Djouhri L, Wrigley D, Thut PD, Gold MS. Spinal nerve injury increases the percentage of cold-responsive DRG neurons. Neuroreport. 2004;15:457–460. doi: 10.1097/00001756-200403010-00015. [DOI] [PubMed] [Google Scholar]
- 40.Cui JG, Holmin S, Mathiesen T, Meyerson BA, Linderoth B. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain. 2000;88:239–248. doi: 10.1016/S0304-3959(00)00331-6. [DOI] [PubMed] [Google Scholar]
- 41.Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J. Neurosci. 2002;22:3052–3060. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ro LS, Chen ST, Tang LM, Jacobs JM. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain. 1999;79:265–274. doi: 10.1016/s0304-3959(98)00164-x. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Xian CJ, Zhong JH, Zhou XF. Lumbar 5 ventral root transection-induced upregulation of nerve growth factor in sensory neurons and their target tissues: a mechanism in neuropathic pain. Mol. Cell. Neurosci. 2003;23:232–250. doi: 10.1016/s1044-7431(03)00062-9. [DOI] [PubMed] [Google Scholar]
- 44.Dai Y, et al. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J. Neurosci. 2002;22:7737–7745. doi: 10.1523/JNEUROSCI.22-17-07737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Askwith CC, Benson CJ, Welsh MJ, Snyder PM. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6459–6463. doi: 10.1073/pnas.111155398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 47.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J. Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svensson CI, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J. Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 49.Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J. Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Obata K, et al. Differential activation of extracellular signal-regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J. Neurosci. 2003;23:4117–4126. doi: 10.1523/JNEUROSCI.23-10-04117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barclay J, et al. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J. Neurosci. 2002;22:8139–8147. doi: 10.1523/JNEUROSCI.22-18-08139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai J, et al. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain. 2002;95:143–152. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- 53.Noguchi K, Kawai Y, Fukuoka T, Senba E, Miki K. Substance P induced by peripheral nerve injury in primary afferent sensory neurons and its effect on dorsal column nucleus neurons. J. Neurosci. 1995;15:7633–7643. doi: 10.1523/JNEUROSCI.15-11-07633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamanaka H, He X, Matsumoto K, Shiosaka S, Yoshida S. Protease M/neurosin mRNA is expressed in mature oligodendrocytes. Brain Res. Mol. Brain Res. 1999;71:217–224. doi: 10.1016/s0169-328x(99)00187-4. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi K, et al. Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. J. Comp. Neurol. 2005;481:377–390. doi: 10.1002/cne.20393. [DOI] [PubMed] [Google Scholar]