Abstract
Peroxisome proliferator-activated receptor-binding protein (PBP), also known as thyroid hormone receptor-associated protein 220/vitamin D receptor-interacting protein 205/mediator 1, an anchor for multisubunit mediator transcription complex, functions as a transcription coactivator for nuclear receptors. Disruption of the PBP gene results in embryonic lethality around embryonic day 11.5 by affecting placental and multiorgan development. Here, we report that targeted deletion of PBP in liver parenchymal cells (PBPLiv-/-) results in the abrogation of hypertrophic and hyperplastic influences in liver mediated by constitutive androstane receptor (CAR) ligands phenobarbital (PB) and 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene, and of acetaminophen-induced hepatotoxicity. CAR interacts with the two nuclear receptor-interacting LXXLL (L, leucine; X, any amino acid) motifs in PBP in a ligand-dependent manner. We also show that PBP interacts with the C-terminal portion of CAR, suggesting that PBP is involved in the regulation of CAR function. Although the full-length PBP only minimally increased CAR transcriptional activity, a truncated form of PBP (amino acids 487-735) functioned as a dominant negative repressor, establishing that PBP functions as a coactivator for CAR. A reduction in CAR mRNA and protein level observed in PBPLiv-/- mouse liver suggests that PBP may regulate hepatic CAR expression. PBP-deficient hepatocytes in liver failed to reveal PB-dependent translocation of CAR to the nucleus. Adenoviral reconstitution of PBP in PBPLiv-/- mouse livers restored PB-mediated nuclear translocation of CAR as well as inducibility of CYP1A2, CYP2B10, CYP3A11, and CYP7A1 expression. We conclude that transcription coactivator PBP/TRAP220/MED1 is involved in the regulation of hepatic CAR function and that PBP deficiency in liver abrogates acetaminophen hepatotoxicity.
Keywords: constitutive androstane receptor
Nuclear receptors constitute a large superfamily of transcription factors that regulate a diverse array of biological process, including development, differentiation, and neoplasia as well as energy and xenobiotic metabolism (1-3). The binding of specific ligands to nuclear receptors influences the recruitment of initial complex of coactivator proteins with histone acetyl-transferase activity necessary for remodeling chromatin (1-3). Docking of several other cofactors results in the formation of a multiprotein coactivator complex, variously called thyroid hormone receptor-associated protein (TRAP)/vitamin D receptor-interacting protein/activator-recruited cofactor/mediator complex, that facilitates interaction with RNA polymerase II and the general basal transcription machinery (1-3). This mediator complex is anchored by peroxisome proliferator-activated receptor (PPAR)-binding protein (PBP) (4), which also is known as TRAP220/vitamin D receptor-interacting protein 205/mediator 1 (MED1) (3, 5, 6). Identification in recent years of many nuclear-receptor coactivators and coactivator-binding proteins raises questions about their functional role in gene, cell, and stage-specific transcription (2, 3, 7-11). Accordingly, delineation of the in vivo functional roles of these transcriptional coactivators becomes an important challenge.
Evidence obtained from gene knockout studies in mice established that the null mutation of coactivators PBP and peroxisome proliferator-activated receptor-interacting protein (PRIP) lead to embryonic lethality between embryonic days 11.5 and 12.5, indicating that these proteins are essential and non-redundant coactivators (12, 13). To explore the cell- and gene-specific function of PBP, we generated conditional-null mice by using the Cre-loxP strategy (14). PBP liver conditional-null mice (PBPLiv-/-) were used recently to determine the role of this coactivator in PPARα-regulated response to peroxisome proliferators (14). Targeted deletion of PBP in liver parenchymal cells resulted in the abrogation of peroxisome proliferation as well as the induction of PPARα-regulated genes in mouse liver in response to peroxisome proliferators (14). In essence, the absence of PBP in hepatocytes in vivo mimics the absence of PPARα (15), indicating that both PPARα and PBP are required for PPARα-regulated gene expression in hepatocytes. Here, we report the role of PBP in the action of constitutive androstane (active) receptor (CAR) and in acetaminophen-induced liver injury (16). Acetaminophen (APAP; also known as paracetamol, N-acetyl-p-aminophenol, and 4′-hydroxyacetanilide) overdose is the leading cause of acute liver failure in the United States (17). The hepatotoxic effects of APAP, manifested as centrilobular necrosis, are attributed to the formation of cytotoxic N-acetyl-p-benzoquinone imine by cytochrome P450 (CYP) isoforms CYP2E1, CYP1A 2, and CYP3A4 and the N-acetyl-p-benzoquinone imine-mediated depletion of cellular glutathione (GSH) (17-21). Hepatotoxicity of APAP is increased in both humans and rodents by pretreatment with inducers of CYP gene expression such as phenobarbital (PB) and the pesticide contaminant 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP), two well known inducers of CYP2B and CYP3A, and ethanol, an inducer of CYP2E and CYP3A isoforms (19-21). Because some of these inducers serve as ligands for CAR to transcriptionally up-regulate CYP isoforms, mice deficient in CAR failed to exhibit the inducibility of APAP-metabolizing enzymes in liver and thus are resistant to APAP hepatotoxicity (20, 21). In the present studies, we examined the hypothesis that expression of PBP is essential to the inducibility of genes regulated by this xenobiotic receptor CAR and for the APAP hepatotoxicity. We demonstrate that PBPLiv-/- mice are resistant to hypertrophic and hyperplastic influences in liver mediated by CAR ligands and to APAP-induced hepatotoxicity.
Materials and Methods
Generation of Liver-Specific PBP Gene Targeted (PBPLiv-/-) Mice and Treatment with CAR Agonists. Groups of (n = 3-8) PBPLiv-/- mice (14), aged 6-8 weeks, were given PB i.p at a daily dose of 100 mg/kg for 3 days. TCPOBOP was administered i.p at a dose of 3 mg/kg for 3 days. Livers were fixed in either 10% formalin or 4% paraformaldehyde, and 4-μm-thick paraffin sections were cut and stained with either hematoxylin and eosin or immunohistochemically with antibodies against PBP [TRAP220 (C-19); Lot B230] and CAR (M-150, SC-13065; Lot I2404), both from Santa Cruz Biotechnology). Mice given BrdUrd (0.5 mg/ml) in drinking water for 4 days were analyzed immunohistochemically for liver nuclear-labeling indices (14, 22). The Northwestern University Animal Care and Use Committee approved all animal studies.
Northern and Immunoblot Analyses. Total RNA isolated by TRIzol reagent (Invitrogen) was glyoxylated, separated on 0.8% agarose gel, transferred to nylon membrane, and probed with selected cDNAs. Liver extracts were used for immunoblotting using antibodies against CAR (Santa Cruz Biotechnology and M. Negishi, National Institute of Environmental Health Sciences, Research Triangle Park, NC), GSTπ (Dako), and catalase.
Zoxazolamine Paralysis Test. Mice pretreated for 3 days with either corn oil or PB as above were administered a single i.p. injection of zoxazolamine (300 mg/kg) after the last dose of PB, and paralysis time was monitored as described in refs. 21 and 23.
APAP Treatment and GSH and Alanine Aminotransferase (ALT) Assays. Mice pretreated with corn oil, PB, or TCPOBOP as described above were given a single i.p. injection of APAP (250 mg/kg) 24 h before killing. Blood collected from inferior vena cava was used for determination of serum ALT activity by using an ALT assay kit (Sigma). Liver homogenates were used for GSH assay.
PBP-CAR Interactions. [35S]Methionine-labeled proteins were synthesized by in vitro translation using the TnT-coupled transcription-translation system (Promega). Immobilized GST fusion protein and 35S-labeled proteins were used in GST pull-down assays in the presence or absence of TCPOBOP (4, 24). Bound proteins were resolved on SDS/PAGE.
Electrophoretic gel mobility-shift assays were performed by using in vitro-translated proteins and [γ-32P]ATP end-labeled double-stranded oligonucleotides encompassing the NR1 box (TCTGTACTTTCCTGACCTTG) (25). Liver chromatin immunoprecipitation assays were performed as described in ref. 14. Purified liver nuclei were fixed for 30 min in 1% formaldehyde to cross-link the DNA-binding proteins to cognate cis-acting elements. Nuclear homogenates were sonicated to shear the chromosomal DNA to an average length of ≈1,000 bp (14). The extracted DNA samples were amplified by using primers [5′-CTCCAGTGACTTAGGAGGAAG-3′ and 5′-AAGTATTGTGCCAGTTGCTG-3′ that encompass the PB response unit of the mouse CYP2B10 promoter region (25).
Adenovirus (Ad)-Mediated Expression of PBP. Ad-His-PBP (Ad/PBP) was generated by using the full-length PBP coding region generated by PCR from plasmid pCMX-PBP (4, 9). Recombinant adenoviruses expressing PBP and LacZ (Ad/LacZ) (a gift from W. El-Deiry, University of Pennsylvania, Philadelphia) were injected intravenously through the tail vein, and the mice were killed 4 days later to assess the PB-dependent nuclear translocation of CAR.
Results and Discussion
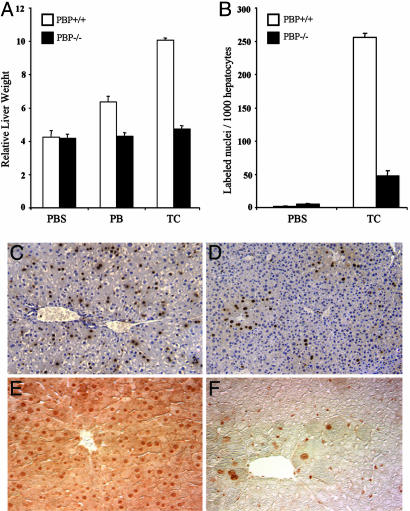
The Deficiency of Liver-Specific PBP Abrogates the CAR Ligand-Induced Hepatic Hypertrophy and Hyperplastic Responses and Gene Expression. CAR mediates the response evoked by a class of xenobiotics referred to as the “PB-like inducers” (16, 26). Short-term treatment with either PB or TCPOBOP increased liver mass in wild-type PBP+/+ mice but not in PBPLiv-/- mice (Fig. 1A). The hepatomegalic effect of 3-day treatment with PB is less pronounced than that exerted by the potent mitogen TCPOBOP in PBP+/+ livers (20, 21, 26). DNA synthesis in liver is markedly increased in wild-type mice after TCPOBOP treatment (Fig. 1 B and C). PBPLiv-/- mice have smaller livers, in part due to diminished liver cell size, displaying only a modest increase in the number of BrdUrd-labeled hepatocytes that were confined to the centrilobular areas (Fig. 1 B and D). Immunohistochemical staining revealed nuclear localization of PBP in all hepatocytes of wild-type livers (Fig. 1E). The smaller size of PBP-null liver cells may be due to the possible global effects of this coactivator in liver cell function or due to reduced liver cell proliferation. Few centrilobular hepatocytes in PBP-null livers escaped gene deletion (Fig. 1F) and thus appeared to respond to the cell-proliferative effect of TCPOBOP (Fig. 1D). PBPLiv-/- mice also failed to respond to the hyperplastic effects on liver of PPARα ligands (14). Many of the known nuclear-receptor ligands such as peroxisome proliferators, thyroid hormone, retinoic acids, and TCPOBOP exert direct hyperplastic effects in liver (26). Our findings implicate a role for the coactivator PBP in mediating the cell-proliferative effects of liganded nuclear receptors. Additional studies are needed to determine whether PBP-deficient livers regenerate in response to other stimuli such as two-thirds partial hepatectomy and chemical injury.
Fig. 1.
Liver weight and hepatocellular proliferation. (A) Liver weight/body weight ratios in PBP+/+ and PBPLiv-/- mice treated i.p. with PB (100 mg/kg), or TCPOBOP (TC) (3 mg/kg) daily for 3 days, compared with controls. PBP+/+ mice treated with PB (P = 0.003) or TC (P = 0.0001) had significantly larger livers, compared with similarly treated PBPLiv-/- mice. (B) Liver cell proliferation. Control and TC-treated mice (as above) were given BrdUrd in drinking water (0.5 mg/ml). Liver nuclear labeling in PBP+/+ mice treated with TC was significantly higher, compared with PBPLiv-/- mice (P = 0.0001). (C and D) Representative photomicrographs illustrate random distribution of BrdUrd-labeled nuclei in the TC-treated wild-type liver (C) but mostly restricted to centrilobular regions in PBPLiv-/- livers (D). (E and F) Liver sections immunohistochemically stained for PBP reveal nuclear staining in all hepatocytes in wild-type mouse liver (E) but staining was limited to a few hepatocytes in PBPLiv-/- mouse liver that represent cells escaping gene deletion (F).
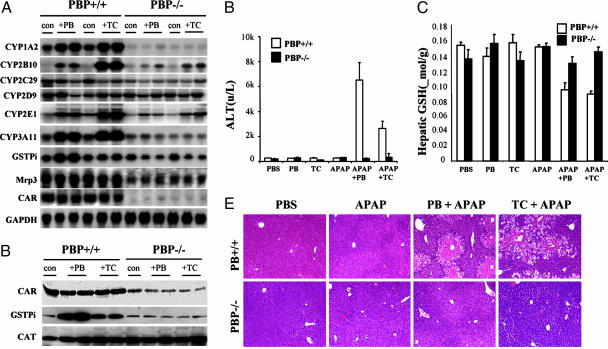
We examined the effect of PB and TCPOBOP on the inducibility of expression of certain selected mRNAs in liver in PBPLiv-/- mice (Fig. 2A). Deletion of the PBP gene significantly attenuated CAR-mediated induction of its target genes after PB or TCPOBOP treatment (Fig. 2 A). Treatment with either PB or TCPOBOP for 3 days resulted in the induction of hepatic CYP3A11, CYP1A2, and CYP2B10 mRNAs in wild-type mice (16, 27) but not in PBPLiv-/- animals. In CAR-null mice, CYP2B10 induction is abolished because of the deletion of this gene in all cells (20, 21). CYP1A2 induction did not significantly change in CAR-null livers (20), whereas PBP absence diminished basal and inducible CYP1A2 levels in liver (Fig. 2 A). Modest induction of GSTπ mRNA was discerned in the livers of PB-treated wild-type mice but not in PBPLiv-/- mice (Fig. 2 A). CYP2E1 basal expression was diminished in PBPLiv-/- livers, and the degree of induction of this gene by CAR activators also was reduced (Fig. 2 A). Finally, CYP2C29 and multidrug resistance protein 3 (Mrp3) mRNA levels revealed a decrease in basal and inducible expression in PBPLiv-/- livers. PBP-null mice have reduced hepatic Mrp3 mRNA levels, and these levels did not change after exposure to CAR ligands (Fig. 2 A). A moderate reduction in CAR mRNA level and protein content was seen in PBPLiv-/- livers, raising the possibility that PBP may play a regulatory role in CAR expression (Fig. 2 A and B). Basal levels of GSTπ in PBPLiv-/- livers appeared somewhat reduced, compared with that in wild-type livers. Unlike in wild-type mice, this protein was not induced in PBPLiv-/- livers after PB and TCPOBOP administration (Fig. 2B).
Fig. 2.
The role of PBP in APAP hepatotoxicity. (A) Northern blot analysis of total liver RNA prepared from control, PBP+/+, and PBPLiv-/- mice and those treated with either PB or TC for 3 days. cDNA probes used for analysis are indicated; GAPDH serves as RNA loading control. (B) Immunoblot analysis of liver proteins of control, PB-, and TC-treated PBP+/+ and PBPLiv-/- mice for CAR, GSTπ, and catalase (CAT). (C and D) Serum ALT and hepatic GSH levels are shown. Mice were pretreated for 3 days with PB or PBS and given APAP (250 mg/kg i.p) 24 h before being killed. Serum ALT levels (C) and hepatic GSH levels (D) were determined at the time of being killed. ALT levels in PB + APAP and TC + APAP-treated PBP+/+ mice were significantly high, compared with PBPLiv-/- mice (P = 0.0021 and P = 0.0222, respectively). (E) Histological examination of livers from different groups. Note the centrilobular hepatic necrosis in PBP+/+ mice pretreated with PB or TC before APAP administration and abrogation of necrosis in the livers of PBPLiv-/- mice. PBS and APAP alone showed no hepatic necrosis.
Loss of PBP Expression on Zoxazolamine Paralysis and APAP Hepatotoxicity. The essentiality of coactivator PBP in CAR-mediated transcription was further confirmed by the assessment of muscle relaxant zoxazolamine-induced paralysis and APAP hepatotoxicity (21, 23). CYP enzymes CYP1A2 and CYP2E1 metabolically inactivate zoxazolamine, and induction of CYP1A2, in particular, by certain xenobiotics, including CAR ligands, leads to rapid metabolic inactivation of zoxazolamine, resulting in decreased duration of paralysis (18, 23). Because mice lacking PBP in liver failed to show induction of CYP1A2 mRNA levels by CAR ligands, it was expected that these mice would metabolize zoxazolamine more slowly and therefore remain paralyzed for a longer period, as did CAR-null mice (21). A zoxazolamine paralysis test revealed that most wild-type mice treated with zoxazolamine recovered after 6-12 h of paralysis, whereas animals pretreated with PB were not paralyzed (Table 1). All of the PBPLiv-/- mice treated with zoxazolamine died between 5 and 12 h of paralysis. Likewise, 7/8 PBPLiv-/- mice pretreated with PB also died after zoxazolamine-induced paralysis. Paralytic death of PBPLiv-/- mice indicates that loss of PBP significantly enhances zoxazolamine sensitivity, and this response is correlated with lack of induction of CYP enzymes. These responses are similar to those observed in mice deficient in nuclear receptor CAR (21) or CYP1A2 (18).
Table 1. Zoxazolamine paralysis test.
| Mice | PBP+/+ | PBPLiv −/− |
|---|---|---|
| Control | 2/7 < 6-h paralysis; recovery | 2/8 < 5-h paralysis; dead |
| 1/7 < 6-h paralysis; dead | 1/8 < 10-h paralysis; dead | |
| 4/7 < 12-h paralysis; recovery | 5/8 > 12-h paralysis; dead | |
| PB | 8/8 not paralyzed | 2/8 < 5-h paralysis; dead |
| 5/8 > 12-h paralysis; dead | ||
| 1/8 > 12-h paralysis; recovery |
Control and 3-day PB-treated male mice (5-6 weeks old) were given a single i.p. injection of zoxazolamine (300 mg/kg of body weight), and paralysis duration was recorded (21).
The hepatotoxic effects of APAP are influenced by many factors that culminate in the conversion of APAP to its toxic intermediate metabolite, N-acetyl-p-benzoquinone imine (15, 19, 20). Induction of CYP isoforms by activators of nuclear receptors CAR and pregnane X receptor (PXR) enhances the hepatotoxic effects of APAP, whereas disruption of the CAR or PXR gene abrogates APAP hepatotoxicity (20, 28). Pretreatment of wild-type mice with CAR agonists PB and TCPOBOP or PXR agonist pregnenolone 16α-carbonitrile markedly enhances APAP-induced hepatic injury, as revealed by increased serum levels of the liver enzyme ALT and centrilobular hepatic necrosis (20, 28). These observations suggest that CAR and PXR play a critical role in APAP-induced hepatotoxicity, probably by inducing CYP2B10 and CYP3A11 expression (20, 28). To assess the functional implication of PBP gene disruption in the liver, we studied the effect of pretreatment with PB or TCPOBOP on acute APAP hepatotoxicity in wild-type and PBPLiv-/- mice. Administration of neither inducers alone nor APAP alone induced hepatotoxicity in wild-type mice, but APAP hepatotoxicity was prominent when these mice were pretreated with PB or TCPOBOP, as evidenced by increase in serum ALT (Fig. 2C). Pretreatment of PBP+/+ mice with PB or TCPOBOP resulted in enhanced APAP-induced hepatocellular necrosis (Fig. 2E). Of interest is that PBPLiv-/- mice pretreated with inducers revealed no such APAP hepatotoxicity (Fig. 2 C and E). These results suggest that failure to induce CYP2B10 and CYP3A11 expression in the liver of PBPLiv-/- mice inhibits APAP bioactivation similar to that noted in CAR- and PXR-null mice (20, 28). Decrease in hepatic GSH to about 50% was observed in PBP+/+ mouse liver with hepatic necrosis (Fig. 2D), whereas GSH levels remained unaltered in APAP-treated PBPLiv-/- mice. These observations show that the absence of coactivator PBP possibly affects the function of several nuclear receptors. Further studies with other nuclear receptor ligands, such as ligands for PXR or glucocorticoid receptor (GR), would be of interest to establish PBP as a global regulator of nuclear receptor functions. In this context, it is worth noting that GR signaling appears central to the expression of CAR mRNA (29). It is thus possible that PBP may regulate GR function and hepatic homeostasis.
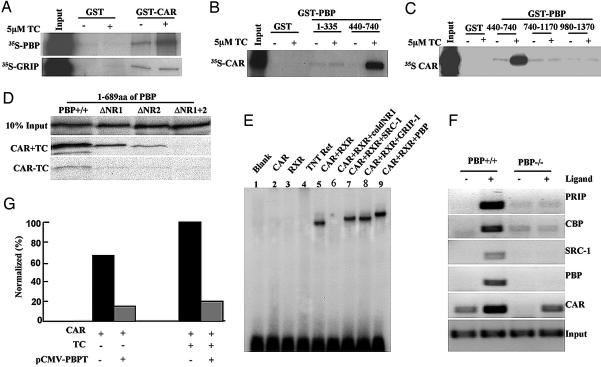
PBP-CAR Interactions. PBP was initially cloned and characterized as a nuclear receptor coactivator by using PPARγ as bait in the yeast two-hybrid system (4). It has since been shown to interact with several nuclear hormone receptors (1, 4-7, 30, 31). We now examined in detail the interaction of PBP with nuclear receptor CAR. Binding of [35S]PBP to GST-CAR increased in the presence of CAR ligand TCPOBOP (Fig. 3A). PBP bound to the C-terminal CAR fragment (amino acids 206-358), which contains AF-2 domain, but failed to bind to the N-terminal region of CAR (data not shown). The binding of [35S]CAR to PBP fragments, with and without the nuclear receptor-binding LXXLL (L, leucine, X, any amino acid) signature motif(s) (1), was examined by using GST-PBP-1-335 (amino acids 1-335 containing no LXXLL motifs), GST-PBP-440-740 (amino acids 440-740, containing two LXXLL motifs, NR1 and NR2) (Fig. 3B), and GST-PBP-740-1170 and GST-PBP-980-1370 containing no LXXLL motifs (Fig. 3C). In the presence of TCPOBOP, 35S-CAR bound avidly to GST-PBP440-740, containing two LXXLL motifs (Fig. 3 B and C), but not to other PBP fragments that lack LXXLL motifs. We further evaluated the role of two LXXLL motifs in PBP in its interaction with nuclear receptor CAR by using deletion constructs (Fig. 3D). 35S-labeled in vitro-translated PBP (amino acids 1-689) containing both LXXLL motifs binds to GST-CAR in the presence of TCPOBOP. PBP mutants that are devoid of LXXLL motifs NR1 or NR2 also bound to CAR, but a PBP mutant lacking both LXXLLs did not bind to CAR (Fig. 3D). We also used NR1 (GST-PBPmut NR1, amino acids 527-924) and NR2 (GST-PBPmut NR2, amino acids 527-924) PBP mutants in which LXXLL motif is changed to LXXAA (30) to determine PBP-CAR interaction. We noted that an intact distal LXXLL motif in PBP is slightly more efficient in binding to CAR (data not shown), somewhat similar to that described for PBP interactions with estrogen receptor, PPAR, and farnesoid X receptor (6, 30).
Fig. 3.
CAR-PBP interactions. (A-D) GST pull-down assays. (A) Interaction of in vitro-translated 35S-labeled PBP with bacterially expressed GST-CAR in the presence (+) and absence (-) of TCPOBOP (TC). (B and C) Binding of in vitro-translated 35S-labeled CAR to bacterially expressed GST-PBP. CAR does not bind to PBP fragments consisting of amino acids 1-335, 740-1170, and 980-1370 (no LXXLL motifs) but does bind to amino acids 440-740 (contains both LXXLL motifs). Binding of CAR to PBP fragments with two LXXLL motifs is enhanced in the presence of ligand TC. (D) 35S-labeled in vitro-translated PBP (amino acids 1-689) binds to GST-CAR in the presence of ligand. PBP mutants devoid of LXXLL motif NR1 or NR2 bound to CAR but PBP lacking both LXXLLs did not bind to CAR. (E) Electrophoretic gel mobility-shift analysis. In vitro-translated CAR, RXR, truncated mouse SRC-1 (amino acids 576-775), glucocorticoid receptor-interacting protein 1 (amino acids 617-769), and PBP (amino acids 487-751) proteins were used in the assay. CAR and RXR were allowed to bind radiolabeled oligonucleotide as the probe in the presence of ligand (TC). To this complex, coactivator proteins containing the LXXLL motifs were added as shown. The complex was separated on nondenaturing 5% PAGE. (F) Chromatin immunoprecipitation assays to show recruitment of PBP, peroxisome proliferator-activated receptor-interacting protein, SRC-1, and CREB-binding protein to the PB response unit in PBP+/+ mouse liver. No detectable coactivator recruitment was found in PBPLiv-/- mouse liver. (G) Repression of CAR-mediated transactivation by truncated PBP (PBPT). PB-responsive element-TK-LUC was cotransfected with pCMV-CAR along with pCMV-PBPT (amino acids 487-739) and pCMV into CV-1 cells in the presence or absence of TC. For control transfections, pCMV-FLAG2 plasmid was used instead of pCMV-PBPT. Luciferase activity is presented as percent, where induced CAR activity in the presence of TC is arbitrarily set at 100% (4).
Electrophoretic gel mobility-shift assays performed with in vitro-translated/transcribed CAR and retinoid X receptor (RXR) revealed that PBP, steroid receptor coactivator 1 (SRC-1) and glucocorticoid receptor-interacting protein 1 caused a definite supershift of the CAR/RXR heterodimer on CYP2B gene PB response unit (Fig. 3E). The addition of excess of unlabeled specific oligonucleotide (NR1 probe) essentially abolished CAR/RXR complex formation (Fig. 3E). Chromatin immunoprecipitation assays using liver chromatin from wild-type PBP+/+ mice demonstrated PBP recruitment to the CAR-target gene promoter region along with coactivators peroxisome proliferator-activated receptor-interacting protein, SRC-1, and CREB-binding protein in response to CAR ligand (Fig. 3F). No appreciable recruitment of these coactivators was noted in the PBPLiv-/- mice, implying that, under in vivo conditions, PBP is necessary for the recruitment of other coactivators (Fig. 3F). To determine the functional consequences of PBP-CAR interactions, CAR-mediated transactivation was examined by using HepG2 cells. No significant enhancement of CAR-mediated transactivation of CYP2B10 PB response unit was observed when full-length PBP was used (data not shown). However, a dominant-negative truncated PBP plasmid (pCMV-PBPT; amino acids 487-739) caused a significant repression of transactivation activity in CV-1 cells (Fig. 3G). It should be noted that PBP only modestly enhanced PPARγ transcriptional activity in the in vitro transactivation assays, despite its essential role in PPARγ- and PPARα-regulated gene expression (4, 24).
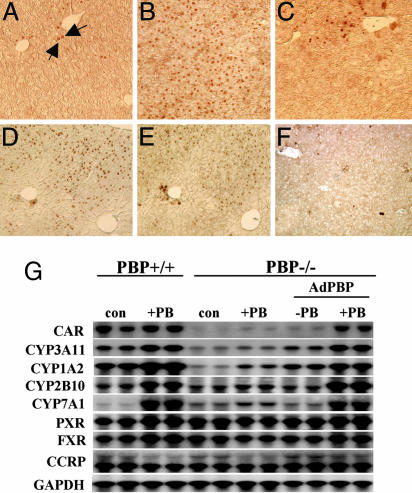
PBP Is Necessary for PB-Mediated Nuclear Translocation of CAR in Mouse Liver. CAR is normally localized to the hepatocyte cytoplasm, but treatment with PB causes a dramatic shift to the nucleus (16, 32). Truncated CAR lacking the C-terminal 10 residues (AF-2 domain) translocates to the nucleus in response to PB, implying that the PB-inducible translocation is AF-2 independent (16). To examine the relevance of PBP binding to CAR, we immunohistochemically localized CAR in the livers of wild-type and PBPLiv-/- mice after 3-day exposure to PB (Fig. 4A). In wild-type mouse liver, CAR was found in the nuclei of an occasional hepatocyte (Fig. 4A, arrows), whereas PB treatment resulted in a dramatic translocation of CAR into the nucleus of all hepatocytes (Fig. 4B). In the PBP-deficient liver cells of PBPLiv-/- mice, PB treatment failed to induce wide-spread nuclear translocation of CAR except for a few scattered cells located in centrilobular areas that apparently escaped gene deletion (Fig. 4C). Failure to induce CAR-mediated genes in PBPLiv-/- mouse liver may reflect reduced translocation of CAR to the nucleus for the formation of CAR-RXR heterodimers for binding to a PB-responsive promoter element in the target gene promoter (16). The failure to assemble coactivator complexes in the absence of PBP may also adversely affect the linkage of responsive element bound CAR-RXR heterodimer to the basal transcription machinery (1-3, 24). Recently, we showed that the absence or reduction of PBP affects the formation of coactivator subcomplex pulled down with PRIP-interacting protein with methyltransferase domain (PIMT) (9, 14). Further studies will be necessary to establish the integrity in the livers of PBPLiv-/- mice of TRAP complex (5, 24). The diversity of nuclear receptor coactivators raises an important issue as to which of the many coactivators is/are essential for the transcriptional efficiency of a given nuclear receptor. Recent work suggests that coactivators SRC-1, glucocorticoid receptor-interacting protein 1 (GRIP-1) and ASC-2/PRIP may be necessary for CAR function (25, 33-35). The availability of genetically altered SRC-1 (22), GRIP-1 (36), and PRIP (10) mice will enable studies on the in vivo role of these coactivators in CAR function. Our previous studies have shown that SRC-1 is redundant for PPARα action (22), whereas PBP deficiency abrogates PPARα function (14).
Fig. 4.
The role of PBP in nuclear translocation of CAR by PB in mouse liver. (A-C) Immunohistochemical staining of liver sections for CAR of PBP+/+ control (A), PBP+/+ mouse treated with PB (WT + PB) (B), and PBPLiv-/- mouse treated with PB (PBPLiv-/- + PB) (C). Few hepatocytes in wild-type control liver show nuclear localization of CAR, whereas CAR is nuclear in almost all hepatocytes after PB treatment in wild-type mice. A PBPLiv-/- mouse treated with PB for 3 days revealed only a few cells with nuclear CAR. (D-F) Adenoviral expression of PBP. Adjacent liver sections of adenovirally reexpressed PBP in PBPLiv-/- mice were immunostained for PBP (D) and CAR (E). Note that reexpression of PBP leads to PB-induced nuclear translocation of CAR. (F) CAR translocation to nucleus was not seen in the absence of PB treatment in Ad/PBP-injected PBPLiv-/- mouse liver. (G) Northern blot of liver RNA obtained from wild-type (PBP+/+) and PBP liver-null (PBP-/-) mice on control or PB-treated mice and from PBP liver-conditional mice infected with adenoviral vector expressing PBP (Ad/PBP). Reexpression of PBP in the liver of PBP-null mice restores PBP inducibility of CYP1A2 and CYP2B10 and, to a certain extent, increases the CAR mRNA level.
Adenoviral Reconstitution of PBP in PBPLiv-/- Restores PB-Mediated Translocation of CAR. To resolve the issue of whether reduction in CAR expression plays a role in PBPLiv-/- mouse response or reduction of CAR function is due to the loss of PBP, a crucial CAR coactivator, we decided to reexpress PBP in PBPLiv-/- mouse by using an adenoviral expression approach. Reexpression of PBP followed by PB treatment resulted in CAR nuclear translocation in PBP-reexpressing liver cells (Fig. 4 D and E). In the absence of PB treatment, PBP staining was evident after Ad/PBP injection, but nuclear staining of CAR was not observed (Fig. 4F). Ad/PBP-mediated reexpression of PBP in PBP-deficient livers also resulted in the restoration of CYP1A2, CYP3A11, CYP2B10, and CYP7A1 induction in PBPLiv-/- livers by PB (Fig. 4G). PXR and farnesoid X receptor mRNA levels were similar in all groups (Fig. 4G), suggesting that PBP deficiency does not affect the levels of these two nuclear receptors. There was no difference in the mRNA levels of cytoplasmic CAR retention protein (Fig. 4G) (37). It also is of interest that the addition of PBP along with PB treatment resulted in a modest increase in the CAR mRNA level in PBP-null mouse liver (Fig. 4G), suggesting that PBP may play a regulatory role in CAR gene transcription or mRNA stability and that this effect appears to require PB. In addition to the conclusion that PBP is needed to mediate the PB-induced translocation of the existing CAR in PBPLiv-/- liver cells to the nucleus, there also is an indication that PBP, along with PB, plays a role in the regulation of hepatic levels of CAR (Fig. 4G). It is conceivable that PBP may regulate the level of CAR in liver cells, but irrespective of the regulatory events, the observation that PBP is essential coactivator for CAR function is of great interest.
Conclusion
These results demonstrate that transcription coactivator PBP/TRAP220/MED1 is involved in the constitutive expression of CAR and certain CYP proteins in liver cells. It also is required for the response to PB-like inducers of xenobiotic metabolism that mediate their action through the nuclear receptor CAR (16). A central role of PBP in xenobiotic-induced transcriptional activation of certain nuclear receptors in liver is exemplified by its requirement in PPARα-regulated gene transcription (14). It is of interest to note that the responses of PBPLiv-/- mice to CAR or PPARα ligands are similar to those of mice lacking the respective receptor (14, 15, 20, 21). The results described here provide evidence for an additional level of complexity, namely at the level of specific coactivators in xenobiotic sensing and responsiveness. These observations implicate the involvement of a transcription coactivator in APAP hepatotoxicity and provide an opportunity to explore the role of agents that modulate PBP interaction with nuclear receptors such as CAR.
Acknowledgments
We thank Drs. David Moore, Masahiko Negishi, Balachandra Diwan, Stephen Safe, Amedeo Columbano, Michael Garabedian, and Robert Costa for valuable reagents. This work was supported by National Institutes of Health Grants GM23750 (to J.K.R.) and CA104578 (to J.K.R.).
Author contributions: Y.J., G.L.G., M.S.R., B.K., K.G., F.J.G., and J.K.R. designed research; Y.J., G.L.G., S.S., J.S., C.Q., D.G., J.X., P.K., S.Y., Y.-W.C., and K.G. performed research; Y.J., G.L.G., S.S., J.S., J.X., M.S.R., B.K., K.G., F.J.G., and J.K.R. analyzed data; and J.K.R. wrote the paper.
Abbreviations: PPAR, peroxisome proliferator-activated receptor; PBP, PPAR-binding protein; PBPLiv-/-, PBP liver conditional-null; CAR, constitutive androstane receptor; PXR, pregnane X receptor; GR, glucocorticoid receptor; APAP, acetaminophen; CYP, cytochrome P450; PB, phenobarbital; TCPOBOP, 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene; Ad, adenovirus; RXR, retinoid X receptor; TRAP, thyroid hormone receptor-associated protein; SRC-1, steroid receptor coactivator 1; ALT, alanine aminotransferase.
References
- 1.Glass C. K. & Rosenfeld, M. G. (2000) Genes Dev. 14, 121-141. [PubMed] [Google Scholar]
- 2.McKenna, N. J. & O'Malley, B. W. (2002) Cell 108, 465-474. [DOI] [PubMed] [Google Scholar]
- 3.Lewis, B. A. & Reinberg, D. (2003) J. Cell Sci. 116, 3667-3675. [DOI] [PubMed] [Google Scholar]
- 4.Zhu, Y., Qi, C., Jain, S., Rao, M. S. & Reddy, J. K. (1997) J. Biol. Chem. 272, 25500-25506. [DOI] [PubMed] [Google Scholar]
- 5.Yuan, C. X., Ito, M., Fondell, J. D., Fu, Z. Y. & Roeder, R. G. (1998) Proc. Natl. Acad. Sci. USA 95, 7939-7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rachez, C., Lemon, B. D., Suldan, Z., Bromleigh, V., Gamble, M., Naar, A. M., Erdjument-Bromage, H., Tempst, P. & Freedman, L. P. (1999) Nature 398, 824-828. [DOI] [PubMed] [Google Scholar]
- 7.Surapureddi, S., Yu, S., Bu, Y., Hashimoto, T., Yeldandi, A. V., Kashireddy, P., Cherkaoui-Malki, M., Qi, C., Zhu, Y.-J., Rao, M. S. & Reddy, J. K. (2002) Proc. Natl. Acad. Sci. USA 99, 11836-11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu, Y., Qi, C., Cao, W.-Q., Yeldandi, A. V., Rao, M. S. & Reddy, J. K. (2001) Proc. Natl. Acad. Sci. USA 98, 10380-10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Misra, P., Qi, C., Yu, S., Shah, S. H., Cao, Q.-W., Rao, M. S., Thimmapaya, B., Zhu, Y. & Reddy, J. K. (2002) J. Biol. Chem. 277, 20011-20019. [DOI] [PubMed] [Google Scholar]
- 10.Zhu, Y.-J., Crawford, S. E., Stellmach, V., Dwivedi, R. S., Rao, M. S., Gonzalez, F. J. & Reddy, J. K. (2003) J. Biol. Chem. 278, 1986-1990. [DOI] [PubMed] [Google Scholar]
- 11.Mahajan, M. A. & Samuels, H. H. (2005) Endocr. Rev. 26, 583-597. [DOI] [PubMed] [Google Scholar]
- 12.Ito, M., Yuan, C. X., Okano, H. J., Carnell, R. B. & Roeder, R. G. (2000) Mol. Cell 5, 683-693. [DOI] [PubMed] [Google Scholar]
- 13.Zhu, Y., Qi, C., Jia, Y., Nye, J. S., Rao, M. S. & Reddy, J. K. (2000) J. Biol. Chem. 275, 14779-14782. [DOI] [PubMed] [Google Scholar]
- 14.Jia, Y., Qi, C., Kashireddi, P., Surapureddi, S., Zhu, Y.-J., Rao, M. S., Le Roith, D., Chambon, P., Gonzalez, F. J. & Reddy, J. K. (2004) J. Biol. Chem. 279, 24427-24434. [DOI] [PubMed] [Google Scholar]
- 15.Lee, S. S., Pineau, T., Drago, J., Lee, E. J., Owens, J. W., Kroetz, D. L., Fernandez-Salguero, P. M., Westphal, H. & Gonzalez, F. J. (1995) Mol. Cell. Biol. 15, 3012-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swales, K. & Negishi, M. (2004) Mol. Endocrinol. 18, 1589-1598. [DOI] [PubMed] [Google Scholar]
- 17.Henderson, C. J., Wolf, C. R., Kitteringjham, N., Powell, H., Otto, D. & Park, B. K. (2000) Proc. Natl. Acad. Sci. USA 97, 12741-12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang, H. C., Li, H., McKinnon, R. A., Duffy, J. J., Potter, S. S., Puga, A. & Nebert, D. W. (1996) Proc. Natl. Acad. Sci. USA 93, 1671-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaher, H., Buters, J. T., Ward, J. M., Bruno, M. K., Lucas, A. M., Stern, S. T., Cohen, S. D. & Gonzalez, F. J. (1998) Toxicol. Appl. Pharmacol. 152, 193-199. [DOI] [PubMed] [Google Scholar]
- 20.Zhang, J., Huang, W., Chua, S. S., Wei, P. & Moore, D. D. (2002) Science 298, 422-424. [DOI] [PubMed] [Google Scholar]
- 21.Wei, P., Zhang, J., Egan-Hafley, M., Liang, S. & Moore, D. D. (2000) Nature 407, 920-923. [DOI] [PubMed] [Google Scholar]
- 22.Qi, C., Zhu, Y., Pan, J., Yeldandi, A. V., Rao, M. S., Maeda, N., Subbarao, V., Pulikuri, S., Hashimoto, T. & Reddy, J. K. (1999) Proc. Natl. Acad. Sci. USA 96, 1585-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson, J. R. & Nebert, D. W. (1974) Mol. Pharmacol. 10, 484-493. [PubMed] [Google Scholar]
- 24.Ge, K., Guermah, M., Yuan, C.-X., Ito, M., Walberg, E., Spiegelman, B. M. & Roeder, R. G. (2002) Nature 417, 563-566. [DOI] [PubMed] [Google Scholar]
- 25.Min, G., Kemper, J. K. & Kemper, B. (2002) J. Biol. Chem. 277, 26356-26363. [DOI] [PubMed] [Google Scholar]
- 26.Columbano, A. & Ledda-Columbano, G. M. (2003) Cell Death Differ. 10, S19-S21. [DOI] [PubMed] [Google Scholar]
- 27.Honkakoski, P. & Negishi, M. (2000) Biochem. J. 347, 321-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo, G. L., Moffit, J. S., Nicol, C. J., Ward, J. M., Aleksunas, L. A., Slitt, A. L., Kliewer, S. A., Manauto, J. E. & Gonzalez, F. J. (2004) Toxicol. Sci. 82, 374-380. [DOI] [PubMed] [Google Scholar]
- 29.Pascussi, J.-M., Gerbal-Chalon, S., Fabre, J.-M., Maurel, P. & Vilarem, M.-J. (2000) Mol. Pharmacol. 58, 1441-1450. [DOI] [PubMed] [Google Scholar]
- 30.Torra, I. P., Freedman, L. P. & Garabedian, M. J. (2004) J. Biol. Chem. 279, 36184-36191. [DOI] [PubMed] [Google Scholar]
- 31.Arnold, K. A., Eichelbaum, M. & Burk, O. (2004) Nucl. Recept. 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamoto, T., Sueyoshi, T., Zelko, I., Moore, R., Washburn, K. Y. & Negishi, M. (1999) Mol. Cell. Biol. 19, 6318-6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muangmoonchai, R., Smirlis, D., Wong, S.-C., Edwards, M., Phillips, I. R. & Shephard, E. A. (2001) Biochem. J. 355, 71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia, J. & Kemper, B. (2005) J. Biol. Chem. 280, 7285-7293. [DOI] [PubMed] [Google Scholar]
- 35.Choi, E., Lee, S., Yeom, S.-Y., Lee, J. W. & Kim, S.-W. (2005) Mol. Endocrinol. 19, 1711-1719. [DOI] [PubMed] [Google Scholar]
- 36.Gehin, M., Mark, M., Dennefeld, C., Dierich, A., Gronemeyer, H. & Chambon, P. (2002) Mol. Cell. Biol. 22, 5923-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi, K., Suehoshi, T., Inoue, K., Moore, R. & Negishi, M. (2003) Mol. Pharmacol. 64, 1069-1075. [DOI] [PubMed] [Google Scholar]