Abstract
The sterol regulatory element-binding protein (SREBP) family of transcription factors controls the biosynthesis of cholesterol and other lipids, and lipid synthesis is critical for cell growth and proliferation. We were, therefore, interested in the expression and activity of SREBPs during the cell cycle. We found that the expression of a number of SREBP-responsive promoter-reporter genes were induced in a SREBP-dependent manner in cells arrested in G2/M. In addition, the mature forms of SREBP1a and SREBP1c were hyperphosphorylated in mitotic cells, giving rise to a phosphoepitope recognized by the mitotic protein monoclonal-2 (MPM-2) antibody. In contrast, SREBP2 was not hyperphosphorylated in mitotic cells and was not recognized by the MPM-2 antibody. The MPM-2 epitope was mapped to the C terminus of mature SREBP1, and the mitosis-specific hyperphosphorylation of SREBP1 depended on this domain of the protein. The transcriptional and DNA-binding activity of SREBP1 was enhanced in cells arrested in G2/M, and these effects depended on the C-terminal domain of the protein. In part, these effects could be explained by our observation that mature SREBP1 was stabilized in G2/M. In agreement with these observations, we found that the synthesis of cholesterol was increased in G2/M-arrested cells. Thus, our results demonstrate that the activity of mature SREBP1 is regulated by phosphorylation during the cell cycle, suggesting that SREBP1 may provide a link between lipid synthesis, proliferation, and cell growth.
Keywords: cell cycle, cholesterol, phosphorylation, proliferation
Members of the sterol regulatory element-binding protein (SREBP) family of transcription factors control cholesterol and lipid metabolism and play critical roles in insulin signaling and during adipocyte differentiation (1). Two genes, srebf1 and srebf2, encode three different SREBPs (SREBP1a, SREBP1c, and SREBP2) (2). SREBPs are synthesized as large precursor proteins that are inserted into the nuclear and endoplasmic reticulum (ER) membranes and are transcriptionally inactive. In sterol-depleted cells, SREBP cleavage-activating protein (SCAP) escorts SREBP to the Golgi apparatus where SREBP is processed sequentially by two membrane-associated proteases, resulting in the release of the mature form of the protein (3, 4). This transcriptionally active fragment of SREBP is translocated to the nucleus and binds to the promoters of SREBP target genes. Most of these genes are involved in the control of lipid biosynthesis and metabolism (5). In sterol-loaded cells, the SCAP/SREBP complex is trapped in the ER as a result of sterol-induced binding of SCAP to Insigs, which are resident proteins of the ER membrane (6, 7).
It is well established that the synthesis of membrane lipids is critical for cell growth and proliferation (8, 9). Here, we report that the expression of a number of SREBP-responsive promoter-reporter genes are induced in a SREBP-dependent manner in cells arrested in G2/M. In addition, the mature forms of SREBP1a and SREBP1c are hyperphosphorylated in mitotic cells, whereas mature SREBP2 is not. The phosphorylated residues were mapped to the C terminus of mature SREBP1. The transcriptional potency of mature SREBP1 was enhanced in cells arrested in G2/M, and these effects depended on the C-terminal domain of the protein. In agreement with these observations, we found that the synthesis of cholesterol was enhanced in G2/M-arrested cells. Thus, our results demonstrate that the activity of mature SREBP1 is regulated by phosphorylation during the cell cycle, suggesting that SREBP1 may provide a link between lipid synthesis, proliferation, and cell growth.
Materials and Methods
Cell Culture and Treatments. All tissue culture media and antibiotics were obtained from Sigma. HEK293T, HepG2, and HeLa cells were from the American Type Culture Collection. To arrest cells in G2/M, cells were treated with nocodazole (100 ng/ml), taxol (1 μM), or monastrol (100 μM) for 16 h. To arrest cells in G1/S and S, cells were treated with hydroxyurea (5 mM) and aphidicolin (2 μg/ml), respectively, for 16 h.
Reagents and Antibodies. Horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG and protein G Sepharose were from Amersham Pharmacia Biosciences. Anti-Flag antibody, nocodazole, taxol, and standard chemicals were from Sigma. Monastrol was from Alexis, San Diego. Monoclonal anti-SREBP1 (2A4), anti-tubulin (TU-02), anti-cyclin B1 (GNS1), anti-cyclin A (BF683), and rabbit anti-SREBP1 (H-160) antibodies were from Santa Cruz Biotechnology. Anti-phospho-Cdk1 (Thr-161) and anti-Cdc25C were from Cell Signaling Technology, Beverly, MA. Anti-mitotic protein monoclonal-2 (MPM-2) was from Upstate Biotechnology, Lake Placid, NY. The anti-phospho-serine antibody was from Zymed.
Plasmids and DNA Transfections. The expression vectors for Flag-tagged SREBP1a (amino acid residues 2–490), SREBP1a-ΔC (amino acid residues 2–417), SREBP1a-ΔTAD (TAD, transactivation domain; amino acids 90–490), SREBP1c (amino acid residues 2–466), and SREBP2 (amino acid residues 2–485) have been described (10). The 3-hydroxy-3-methylglutaryl (HMG)-CoA synthase (SYNSRE-luc), farnesyldiphosphate synthase (FPPS-luc), fatty acid synthase (FAS-luc), and low-density lipoprotein receptor (LDLr) (LDLr-luc and LDLrΔSRE-luc) promoter-reporter constructs have been described (10–12). Transient transfections were performed by using the MBS transfection kit (Stratagene). Labeling of transfected 293T cells with 32P, phosphoamino acid analysis on immunoprecipitated material and Edman degradation were performed as described (13).
Further information on materials and certain methods used in this study [immunoprecipitations and immunoblotting, analysis of lipid synthesis, luciferase and β-gal assays, RT-PCR and chromatin immunoprecipitation (ChIP) assays, double-thymidine block, and FACS analysis] can be found in Supporting Methods, which is published as supporting information on the PNAS web site.
Results
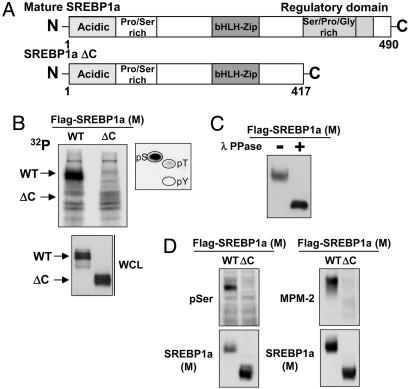
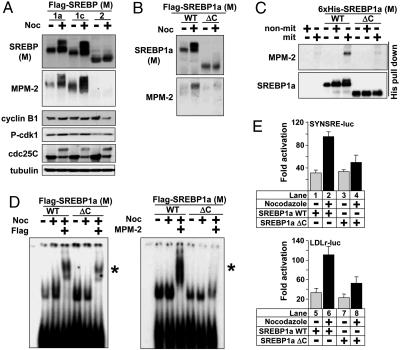
Mature SREBP1 Is Highly Phosphorylated. Early observations indicated that SREBP1a is phosphorylated in vivo (14). Subsequently, it has been suggested that both SREBP1 and SREBP2 are phosphorylated by various protein kinases, both in vivo and in vitro (15–17). We were interested in mapping the major phosphorylated residues in SREBP1, especially those found in the Ser/Thr/Gly-rich C terminus of the mature protein (Fig. 1A). To address this issue, 293T cells were transfected with plasmids expressing either full-length mature SREBP1a or a protein lacking the C-terminal domain (ΔC). After metabolic labeling with 32P, the proteins were immunoprecipitated and separated by SDS/PAGE. WT SREBP1a was highly phosphorylated, whereas only little radioactivity was incorporated in the ΔC protein (Fig. 1B), indicating that the C terminus of mature SREBP1a contains major phosphorylation sites. Phospho-amino acid analysis revealed that phosphorylation of serine residues was the dominating modification (Fig. 1B Right). To confirm that nuclear SREBP1a was phosphorylated, transfected mature SREBP1a was immunoprecipited from 293T cells and treated with λ-phosphatase. Phosphatase-treated mature SREBP1a migrated faster in the SDS/PAGE gel (Fig. 1C), indicating that mature SREBP1a is highly phosphorylated in vivo. These results were confirmed when the phosphorylation of SREBP1a was monitored with an antibody directed against phosphorylated serine residues. Mature SREBP1a was recognized by this antibody, whereas the antibody failed to detect the protein lacking the C-terminal domain (Fig. 1D Left). In addition, transfected mature SREBP1a was recognized by the MPM-2 phospho-epitope-specific antibody, whereas it failed to detect the ΔC protein (Fig. 1D Right).
Fig. 1.
Mature SREBP1 is highly phosphorylated. (A) Schematic illustration of mature SREBP1a and the ΔC mutant, lacking the C-terminal domain. bHLH, basic helix–loop–helix. (B) 293T cells were transfected with Flag-tagged mature (M) SREBP1a, either WT or ΔC, followed by metabolic labeling with 32P and immunoprecipitation. The migration of the WT and ΔC proteins is indicated. The band corresponding to WT SREBP1a was excised and used for phosphoamino acid analysis (Right). The levels of SREBP1a in whole-cell lysates (WCL) were detected by Western blotting. (C) Mature SREBP1a was immunoprecipitated from transfected 293T cells and incubated in the absence or presence of λ-phosphatase, separated on SDS/PAGE, and visualized by Western blotting. (D) 293T cells were transfected with mature SREBP1a, either WT or ΔC, followed by immunoprecipitation of the Flag-tagged proteins and separation on SDS/PAGE. The phosphorylation of SREBP1a was monitored with phospho-serine (pSer, Left) or MPM-2 (Right) antibodies.
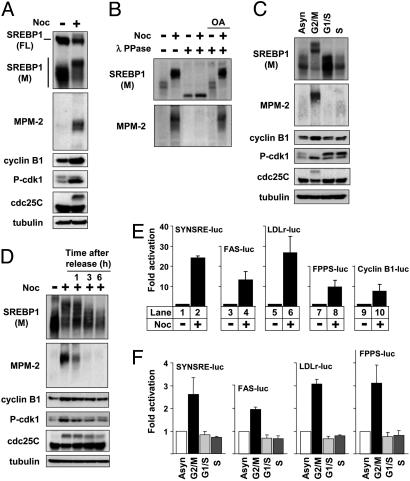
Mature SREBP1 Is Hyperphosphorylated in G2/M. The MPM-2 antibody recognizes phophorylated Ser/Thr residues in a subset of mitotic phosphoproteins. We wanted, therefore, to determine whether SREBP1 was phosphorylated during mitosis. To address this issue, we analyzed the electrophoretic mobility of endogenous SREBP1 in asynchronous HeLa cells and cells arrested in G2/Mby nocodazole treatment. To monitor cell-cycle arrest, the levels of cyclin B1, phospho-Cdk1, and Cdc25C in the samples were also determined. The migration of mature SREBP1 was dramatically shifted to higher molecular species in G2/M-arrested cells (Fig. 2A). Mature SREBP1 was also recognized by the MPM-2 antibody in G2/M-arrested cells, and the MPM-2 signal was associated with the shifted form of the protein. The precursor form of SREBP1 was not shifted in response to nocodazole treatment and was not recognized by the MPM-2 antibody, indicating that this modification is specific for mature SREBP1. Similar results were also obtained in HepG2 and U2OS cells (data not shown). The shift in apparent molecular weight and the SREBP1-specific MPM-2 signal were lost by λ-phosphatase treatment (Fig. 2B), indicating that mature SREBP1 is hyperphosphorylated in cells arrested in G2/M. Interestingly, endogenous SREBP2 was not phosphorylated in arrested cells and was not recognized by the MPM-2 antibody (Fig. 7, which is published as supporting information on the PNAS web site). The hyperphosphorylation of SREBP1 and the MPM-2 signal were specific for cells arrested in G2/M and were not observed in cells arrested in other phases of the cell cycle (Fig. 2C; FACS profiles are provided in Fig. 8, which is published as supporting information on the PNAS web site). The hyperphosphorylation of SREBP1 and the SREBP1-specific MPM-2 signal were rapidly lost after mitotic exit and reentry into G1 after release from nocodazole arrest (Fig. 2D), suggesting that the hyperphosphorylation of SREBP1 is a dynamic process.
Fig. 2.
Mature SREBP1 is hyperphosphorylated in cells arrested in G2/M. (A) HeLa cells were left untreated or treated with nocodazole (Noc) to induce G2/M arrest. After immunoprecipitation of SREBP1, the levels and phosphorylation (MPM-2) of SREBP1 were determined by Western blotting. The migration of the precursor (FL) and mature (M) forms of SREBP1 is indicated. (B) HeLa cells were treated as in A, incubated in the absence or presence of λ-phosphatase in the absence or presence of the phosphatase inhibitor okadaic acid (OA), and separated by SDS/PAGE, and the levels and phosphorylation (MPM-2) of SREBP1 were determined by Western blotting. (C) HeLa cells were left untreated or arrested in G2/M, G1/S, or S as described in Materials and Methods. After immunoprecipitation of SREBP1, the levels and phosphorylation (MPM-2) of SREBP1 were determined by Western blotting. Asyn, asynchronous. (D) HeLa cells were left untreated or treated with nocodazole to induce G2/M arrest. Where indicated, the nocodazole-treated cells were washed with PBS and released from the G2/M arrest in normal media for the indicated times. After immunoprecipitation of SREBP1, the levels and phosphorylation (MPM-2) of SREBP1 were determined by Western blotting. (E) HepG2 cells were transfected with SYNSRE-luc, LDLr-luc, FAS-luc, FPPS-luc, and CyclinB1-luc. Twenty-four hours after transfection, cells were treated with nocodazole, and luciferase activity was measured. (F) HeLa cells were transfected with SYNSRE-luc, FAS-luc, LDLr-luc, and FPPS-luc. Twenty-four hours after transfection, cells were treated as in C, and luciferase activity was measured.
Our data suggest that SREBP1 is highly phosphorylated in G2/M. Thus, the transcriptional activity of SREBP1 could potentially be regulated during this phase of the cell cycle. To test this idea, HepG2 cells were transfected with various SREBP-responsive promoter-reporter genes. After transfection, the cells were either left untreated or treated with nocodazole to induce G2/M arrest. The expression of all of the reporter genes was significantly induced in response to nocodazole (Fig. 2E), indicating that the SREBP pathway is activated in G2/M. The induction was specific for cells arrested in G2/M and was not observed in cells arrested in G1/Sor S phase (Fig. 2F; FACS profiles are provided in Fig. 8). The SYNSRE-luc, FAS-luc, and FPPS-luc promoter-reporter genes were also induced in G2/M in HeLa cells stably expressing these reporters (Fig. 9, which is published as supporting information on the PNAS web site).
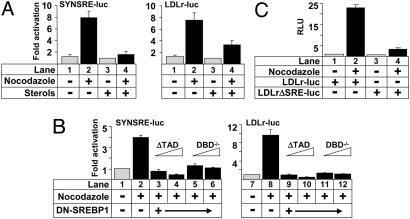
Transcriptional Activation of SREBP-Responsive Promoter-Reporter Genes in G2/M Depends on Functional SREBP. To test whether the activation of SREBP-responsive promoter-reporter genes in G2/M required SREBP, HeLa cells were transfected with two separate SREBP-responsive promoter-reporter genes and either left untreated or treated with nocodazole to induce G2/M arrest. The nocodazole-dependent induction of the reporter genes was reduced in cells treated with sterols to suppress the activation of SREBP (Fig. 3A), indicating that the induction, in part, depends on mature SREBP. This hypothesis was strengthened when the experiment was repeated in the absence or presence of two dominant-negative versions of SREBP1, either lacking a TAD (ΔTAD) or having a mutation in its DNA-binding domain (DBD) that prevents DNA binding (DBD–/–). Expression of the dominant-negative versions of SREBP1 decreased the induction of the promoter-reporter genes in response to nocodazole treatment (Fig. 3B), suggesting that the induction of the reporter genes depends on the transcriptional activity of SREBP. Similar results were obtained when these experiments were repeated in HeLa cells (data not shown). Transcriptional activation of SREBP target genes depends on the binding of SREBP to specific sterol-responsive elements (SREs) within the promoters of these genes. Mutation of the SRE in the LDLr promoter-reporter gene attenuated the nocodazole-dependent induction of the promoter gene (Fig. 3C), confirming that SREBP is required for the enhanced expression of this reporter in G2/M. This hypothesis was strengthened by our observation that the nocodazole-dependent induction of the LDLr and HMG-CoA synthase promoter-reporter genes was blocked when cotransfected with a vector expressing SREBP1-directed short hairpin RNA (data not shown).
Fig. 3.
Transcriptional activation of SREBP-responsive promoter-reporter genes in G2/M. (A) HeLa cells were transfected with SYNSRE-luc and LDLr-luc. Twenty-four hours after transfection, cells were treated with nocodazole in the absence or presence of sterols (cholesterol and 25-hydroxycholesterol; 50 and 5.0 μg/ml, respectively) to suppress the activation of endogenous SREBPs, and luciferase activity was measured. (B) HepG2 cells were transfected with SYNSRE-luc and LDLr-luc in the absence or presence of empty expression vector or dominant-negative SREBP1 (ΔTAD and DBD–/–). Twenty-four hours after transfection, cells were treated with nocodazole, and luciferase activity was measured. (C) HepG2 cells were transfected with LDLr-luc or LDLrΔSRE-luc. Twenty-four hours after transfection, cells were treated with nocodazole, and luciferase activity was measured. RLU, relative light units.
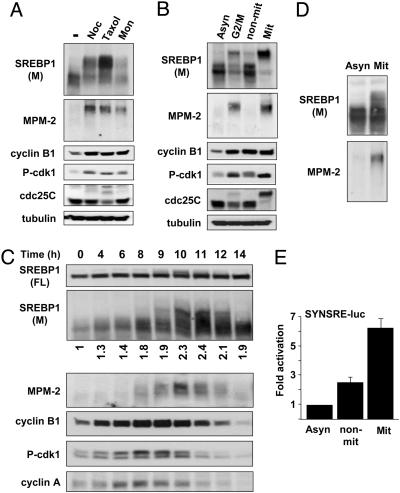
The Hyperphosphorylation and Activation of Mature SREBP1 Is Specific for Mitotic Cells. Nocodazole arrests cells in G2/M by preventing tubulin polymerization. To make sure that the hyperphosphorylation of SREBP1 was specific for G2/M arrest, we tested the effect of two other drugs that are known to induce G2/M arrest, taxol and monastrol. Taxol binds and stabilizes microtubules, whereas monastrol is a specific inhibitor of Eg5, a microtubule-based motor protein. The mature form of endogenous SREBP1 was hyperphosphorylated and recognized by the MPM-2 antibody in HeLa cells treated with all of these drugs (Fig. 4A), demonstrating that the hyperphosphorylation of SREBP1 is specific for G2/M arrest. When HeLa cells arrested in G2/M were fractionated by mitotic shake-off, hyperphosphorylated SREBP1 and the SREBP1-specific MPM-2 signal were recovered in the mitotic cells (Fig. 4B), demonstrating that the hyperphosphorylation of SREBP1 is specific for M phase. To confirm that the hyperphosphorylation of mature SREBP1 was specific for mitosis, we analyzed the expression and phosphorylation of SREBP1 at different intervals after release from a double-thymidine G1/S-phase block. The hyperphosphorylation of mature SREBP1 and the SREBP1-specific MPM-2 signal reached their highest points close to the mitotic index peak (Fig. 4C), as measured by FACS analysis (Fig. 10, which is published as supporting information on the PNAS web site). Interestingly, the steady-state levels of mature SREBP1 were enhanced in mitotic cells and peaked at the same time as the hyperphosphorylation of SREBP1 (Fig. 4C), suggesting that phosphorylation of mature SREBP1 could stabilize the protein during mitosis. The increase in steady-state levels of mature SREBP1 was even more pronounced when cells were released in nocodazole-containing media, when a majority of cells were in the M phase of the cell cycle (Fig. 11, which is published as supporting information on the PNAS web site). The hyperphosphorylated form of SREBP1 and the SREBP1-specific MPM-2 signal were also associated with the mitotic fraction when mitotic cells were isolated by mitotic shake-off from asynchronous HeLa cells in the absence of drug treatment (Fig. 4D). To determine whether SREBP was transcriptionally activated in mitotic cells, HeLa cells were transfected with SYNSRE-luc and treated with nocodazole to induce G2/M arrest, and the cells were fractionated by mitotic shake-off. The activity of the promoter-reporter gene was enhanced in both the nonmitotic and mitotic populations (Fig. 4E). However, the relative increase was more pronounced in M phase, indicating that the transcriptional potency of SREBP1 is enhanced in mitotic cells. Thus, our data demonstrate that mature SREBP1 is specifically hyperphosphorylated in mitotic cells, possibly involving residues in the C terminus of the mature protein.
Fig. 4.
The hyperphosphorylation and activation of SREBP1 is specific for mitotic cells. (A) HeLa cells were left untreated or treated with nocodazole (Noc), taxol, or monastrol (Mon) to induce G2/M arrest. After immunoprecipitation of SREBP1, the levels and phosphorylation (MPM-2) of mature (M) SREBP1 were determined by Western blotting. (B) HeLa cells were left untreated or treated with nocodazole to induce G2/M arrest (lanes 1 and 2), and M-phase cells were separated by mitotic shake-off (lanes 3 and 4). After immunoprecipitation of SREBP1, the levels and phosphorylation (MPM-2) of mature SREBP1 were determined by Western blotting. Asyn, asynchronous; non-mit, non-mitotic; Mit, mitotic. (C) HeLa cells were synchronized at the G1/S transition by a double-thymidine treatment. Cells were collected at the indicated time points after release from the second thymidine block. Cell lysates were generated and analyzed by Western blotting with antibodies to the indicated proteins. The migration of the precursor (FL) and mature (M) forms of SREBP1 is indicated. The relative expression of mature SREBP1 is indicated at the bottom of Upper. After immunoprecipitation of SREBP1, the phosphorylation (MPM-2) of mature SREBP1 was determined by Western blotting. (D) Asynchronous HeLa cells were left untreated, and M-phase cells were separated by mitotic shake-off. After immunoprecipitation of SREBP1, the levels and phosphorylation (MPM-2) of mature SREBP1 were determined by Western blotting. (E) HeLa cells were transfected with the SYNSRE-luc promoter-reporter gene and treated with nocodazole to induce G2/M arrest, and M-phase cells were separated by mitotic shake-off, and luciferase activity was measured.
Phosphorylation of the C Terminus of Mature SREBP1 in Mitotic Cells. In vivo, mature SREBP1 is produced through proteolytic cleavage of a membrane-associated precursor protein. To determine whether the mature form of SREBP1 in isolation was hyperphosphorylated in mitotic cells, 293T cells were transfected with expression vectors for mature SREBP1a, SREBP1c, and SREBP2, and the cells were left untreated or treated with nocodazole. Mature SREBP1a and SREBP1c were hyperphosphorylated and recognized by the MPM-2 antibody in G2/M-arrested cells (Fig. 5A), demonstrating that the mature forms of these proteins are targeted by phosphorylation. In agreement with our earlier results, mature SREBP2 was not hyperphosphorylated under these conditions, indicating that the M-phase phosphorylation is specific for the SREBP1 isoforms. Mature SREBP1a and SREBP1c were also hyperphosphorylated and recognized by the MPM-2 antibody in cells arrested in G2/M by taxol treatment (Fig. 12, which is published as supporting information on the PNAS web site). Interestingly, the levels of SREBP1a and SREBP1c were enhanced in response to nocodazole treatment, whereas the expression of SREBP2 was unaffected, suggesting that phosphorylation of mature SREBP1 could stabilize the protein during mitosis. This hypothesis is in agreement with our results on endogenous SREBP1 (Fig. 4C). In agreement with our previous results, the hyperphosphorylation of transfected SREBP1a was specific for G2/M, because we observed no change in the migration of the protein in 293T cells arrested in G1/S or S (Fig. 12). Our initial results indicated that the MPM-2 epitope in SREBP1a resides in the C-terminal domain. To test this hypothesis, 293T cells were transfected with mature SREBP1a or the ΔC mutant and treated with nocodazole to induce G2/M arrest. The WT protein was hyperphosphorylated in response to nocodazole treatment, whereas the mutant protein was not (Fig. 5B). In addition, the ΔC protein was not recognized by the MPM-2 antibody in G2/M-arrested cells. Similar results were obtained in cells arrested in G2/M by taxol treatment (Fig. 12). The levels of WT SREBP1a were enhanced in cells arrested in G2/M, whereas the levels of the nonphosphorylated mutant were slightly reduced (Figs. 5B and 12), indicating that phosphorylation of the C-terminal domain could influence the stability of SREBP1. Thus, our data indicate that the C-terminal domain in SREBP1 is targeted by phosphorylation during mitosis. To test whether mitotic kinases could phosphorylate SREBP1, whole-cell lysates were prepared from asynchronous or G2/M-arrested HeLa cells and used in kinase assays with recombinant mature SREBP1a. Phosphorylation of SREBP1a was monitored with the MPM-2 antibody. Incubation of SREBP1a with extracts from asynchronous cells failed to generate an SREBP1-specific MPM-2 epitope, whereas mitotic extracts induced a robust MPM-2 signal (Fig. 5C). The ΔC protein was not phosphorylated under the same conditions, indicating that mitotic kinases target the C-terminal domain in SREBP1. Transcriptional activation of SREBP target genes depends on the DNA-binding activity of SREBP. To test whether phosphorylation of SREBP1 could affect its DNA binding, mature SREBP1a and the ΔC mutant were expressed in 293T cells, and the cells were arrested in G2/M and whole-cell lysates from the transfected cells were used in EMSAs. Interestingly, nocodazole treatment enhanced the DNA-binding activity of the full-length protein 2-fold, whereas the DNA-binding activity of the nonphosphorylated ΔC mutant was slightly reduced (Fig. 5D Left). In extracts from G2/M-arrested cells expressing mature SREBP1a, the SREBP1:DNA complex was supershifted with the MPM-2 antibody, whereas the complex containing the ΔC protein was not (Fig. 5D Right). Interestingly, a major portion of the SREBP1:DNA complex in extracts from cells expressing WT SREBP1a was shifted by the MPM-2 antibody, indicating that a majority of the DNA-associated SREBP1 is phosphorylated on MPM-2 epitopes. To test whether phosphorylation of the C terminus could affect the transcriptional activity of SREBP1, cells were transfected with WT SREBP1a or the ΔC mutant together with the SYNSRE-luc promoter-reporter gene and the cells were treated with nocodazole. The transcriptional activity of WT SREBP1a was enhanced in nocodazole-treated cells, whereas the activity of the ΔC protein was unaffected (Fig. 5E), indicating that phosphorylation of the C terminus of mature SREBP1 contributes to its enhanced transcriptional potency in mitotic cells.
Fig. 5.
Phosphorylation of the C terminus of mature SREBP1 in mitotic cells. (A) 293T cells were transfected with mature (M) SREBP1a, SREBP1c, or SREBP2 and left untreated or treated with nocodazole (Noc). After immunoprecipitation, the levels and phosphorylation (MPM-2) of the SREBPs were determined by Western blotting. (B) 293T cells were transfected with mature SREBP1a, either WT or ΔC, and left untreated or treated with nocodazole. After immunoprecipitation, the levels and phosphorylation (MPM-2) of SREBP1 were determined by Western blotting. (C) Recombinant 6×His-SREBP1a, either WT or ΔC, was incubated with whole-cell lysates from asynchronous HeLa cells or cells arrested in G2/M in kinase buffer (10 mM Hepes, pH 7.4/3.5 mM MgCl2/0.2 mM DTT/1 mM ATP/10 mM β-glycerophosphate). The 6×His-tagged proteins were captured on NiTA-agarose, washed, and resolved by SDS/PAGE. The phosphorylation of SREBP1a was monitored with the MPM-2 antibody. non-mit, non-mitotic; mit, mitotic. (D) 293T cells were transfected with mature SREBP1a, either WT or ΔC, and left untreated or treated with nocodazole. Whole-cell lysates were used in EMSAs with a 32P-labeled probe containing the SRE-1 sequence from the LDLr promoter. Where indicated, anti-Flag (Left) or MPM-2 (Right) antibodies were included in the assay. *, Supershifted complexes. (E) HepG2 cells were transfected with SYNSRE-luc or LDLr-luc in the presence of mature SREBP1a, either WT or ΔC. Twenty-four hours after transfection, cells were treated with nocodazole, and luciferase activity was measured.
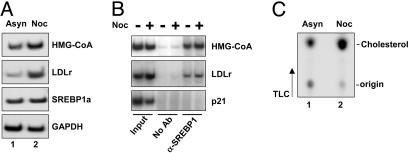
The Expression of SREBP Target Genes and Cholesterol Synthesis Are Enhanced in G2/M. Our results indicate that the expression of SREBP-responsive promoter-reporter genes is enhanced in G2/M. To test whether endogenous SREBP target genes were induced in G2/M, we used semiquantitative RT-PCR to analyze the expression of the HMG-CoA synthase and LDLr genes in asynchronous HepG2 cells or cells arrested in G2/M by nocodazole treatment. The expression of both genes was enhanced in nocodazole-treated cells (Fig. 6A), demonstrating that SREBP target genes are induced in G2/M. During mitosis, chromosomes are highly condensed, making DNA less accessible to transcription factors. However, recent data indicate that mitotic chromosomes are accessible to transcription factors and chromatin proteins. Using chromatin immunoprecipitation, we found that endogenous SREBP1 was associated with the promoters of the LDLr and HMG-CoA synthase genes in G2/M (Fig. 6B). The SREBP family of transcription factors regulates genes involved in cholesterol and lipid metabolism, and our data indicate that the transcriptional potency of SREBP1 is enhanced in mitosis. Thus, we speculated that lipid synthesis should be enhanced during this phase of the cell cycle. To test this hypothesis, HeLa cells were treated with nocodazole and the synthesis of cholesterol was monitored in untreated and treated cells. In agreement with our hypothesis, the synthesis of cholesterol was enhanced up to 3-fold in cells arrested in G2/M (Fig. 6C). Thus, our results demonstrate that the function of mature SREBP1 is regulated by phosphorylation during the cell cycle.
Fig. 6.
The expression of SREBP target genes and cholesterol synthesis are enhanced in G2/M. (A) RNA was isolated from asynchronous (Asyn, lane 1) or nocodazole (Noc)-treated (lane 2) HepG2 cells. Total RNA was used to determine the expression of the LDLr, HMG-CoA synthase (HMG-CoA), SREBP1a, and GAPDH genes by semiquantitative RT-PCR. (B) HeLa cells were left untreated or treated with nocodazole to induce G2/M arrest. Cells were processed for chromatin immunoprecipitation analysis of the LDLr, HMG-CoA synthase, and p21 (negative control) genes, using anti-SREBP1 antibodies for immunoprecipitation. (C) HeLa cells were left untreated or treated with nocodazole to induce G2/M arrest. Two hours before the end of the experiment, cells were placed in fresh media supplemented with [14C]acetate. Lipids were extracted and resolved by TLC. Radioactive products were visualized by PhosphorImage analysis.
Discussion
In the current study we demonstrate that mature SREBP1 is hyperphosphorylated in cells arrested in G2/M and show that the hyperphosphorylated form of the protein is associated with the mitotic population. In mitotic cells, mature SREBP1 is recognized by the MPM-2 antibody, an antibody that recognizes a subset of M-phase phosphoproteins. These effects are specific for SREBP1a and SREBP1c, because SREBP2 fails to be hyperphosphorylated in G2/M. In addition, we demonstrate that the transcriptional potency of mature SREBP1 is enhanced when cells are arrested in G2/M and that the HMG-CoA synthase and LDLr genes, both of which are SREBP target genes, are enhanced in response to nocodazole treatment. The hyperphosphorylation and transcriptional activation of SREBP1 depend on its Ser/Thr-rich C-terminal domain that also contains the MPM-2 epitope. Thus, our results suggest that phosphorylation of mature SREBP1 could represent a novel mechanism to regulate SREBP-dependent transcription during the cell cycle.
Although there is a general repression of transcription during mitosis (18), certain genes retain transcriptionally active complexes on their promoters and are expressed during this stage of the cell cycle (19–24). Although it remains to be demonstrated that SREBP1 is actively involved in transcriptional activation of endogenous target genes during mitosis, we could demonstrate that SREBP1 is associated with target promoters in cells arrested in G2/M and that the expression of the corresponding genes was enhanced at G2/M. The possibility of an active chromatin structure at SREBP target genes was supported by our observation that the overall acetylation of histones H3 and H4 at the LDLr and HMG-CoA synthase genes was preserved in nocodazole-arrested HepG2 cells (data not shown). We could also show that a number of SREBP-responsive promoter-reporter genes were induced in an SREBP-dependent manner after arrest of cells in G2/M. Interestingly, the activation of SREBP1 in G2/M depended on the C-terminal domain of the mature protein, indicating that phosphorylation of the C terminus is required for the transcriptional activation. Taken together, our results indicate that it is important for cells to retain a pool of transcriptionally active SREBP1 during mitosis, either to activate SREBP-responsive genes during this phase of the cell cycle or at the onset of mRNA synthesis at mitotic exit.
SREBP target genes are involved in the biosynthesis of fatty acids and cholesterol. It has been suggested that cholesterol is essential for proper mitotic progression (25). This hypothesis is supported by our observation that cholesterol synthesis was enhanced in G2/M. De novo synthesis of fatty acids appears to be required for cell growth and proliferation (26). Expression of the FAS gene is elevated in a wide variety of human cancers, including prostate and breast cancer (27, 28). Although the mechanisms underlying the increased FAS expression in tumors are not fully understood, several studies indicate that overexpression of FAS is part of a more general and coordinated activation of lipogenic gene expression, mediated at least in part by activation of the SREBP pathway (27, 28). Thus, it may be important to control the expression levels and activity of SREBP1 during the cell cycle, independent of sterol-regulated cleavage of the precursor protein. Our results suggest that phosphorylation of specific Ser and/or Thr residues in the C-terminal portion of mature SREBP1 could be one such mechanism of regulation.
We found that SREBP1-dependent transcription was activated in cells arrested in G2/M through a mechanisms involving phosphorylation of the C terminus of the mature protein. In part, this activation could be explained by our observation that the level of nuclear SREBP1 was enhanced in response to nocodazole-induced cell-cycle arrest, whereas the expression of the ΔC protein was not, indicating that phosphorylation of the C terminus could stabilize mature SREBP1 during mitosis. In the case of endogenous SREBP1, this hypothesis was confirmed by our observation that the steady-state levels of mature SREBP1 were enhanced in mitotic cells after release from a double-thymidine block. Interestingly, the C terminus of mature SREBP1 contains two PEST motifs, sequences that are usually found in unstable proteins. Phosphorylation of Ser and Thr residues within PEST motifs have been shown to regulate the degradation of a number of proteins (29), including SREBPs (15). The C terminus of mature SREBP1 contains a total of 19 Ser and Thr residues, and it will be important to determine which of these are phosphorylated and how these modifications affects the stability of the protein. Stabilization of mature SREBP1 during mitosis would ensure that cells maintain a certain amount of transcriptionally potent SREBP1 as they leave mitosis and an active chromatin structure is reformed. It is also possible that phosphorylation of the C terminus of mature SREBP1 could affect interactions with coactivators, components of the basic transcriptional machinery or other proteins that could regulate its transcriptional activity. Alternatively, phosphorylation could affect the conformation of SREBP1, thereby affecting its transcriptional potential. Many proteins containing MPM-2 epitopes are substrates of the mitotic regulator Pin1, a peptidyl-prolyl isomerase that is present throughout the cell cycle and that is thought to alter its mitotic targets by changing their conformation. Pin1 has been shown to regulate the activity of a number of transcription factors, including p53 and c-Myc, in a phosphorylation-dependent manner (30, 31). Interestingly, Pin1 also regulates the dephosphorylation and degradation of c-Myc (30). Thus, the identification of proteins that interact with SREBP1 in a phosphorylation-dependent manner during mitosis will be important. Our results demonstrate that the activity of mature SREBP1 is regulated by phosphorylation during mitosis, suggesting that SREBP1 may provide a link between lipid synthesis, proliferation, and cell growth.
Supplementary Material
Acknowledgments
We thank V. Lukiyanchuk for technical support. This work was supported by grants from the Swedish Research Council and the Novo Nordisk Foundation (to J.E.). J.E. is a Research Fellow of the Royal Swedish Academy of Sciences through a grant from the Knut and Alice Wallenberg Foundation.
Author contributions: M.T.B.-A. and J.E. designed research; M.T.B.-A., T.P., and J.E. performed research; M.T.B.-A., T.P., and J.E. analyzed data; and M.T.B.-A. and J.E. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SREBP, sterol regulatory element-binding protein; MPM-2, mitotic protein monoclonal-2; FAS, fatty acid synthase; LDLr, low-density lipoprotein receptor; FPPS, farnesyl diphosphate synthase; HMG, 3-hydroxy-3-methylglutaryl; DBD, DNA-binding domain; TAD, transactivation domain.
References
- 1.Brown, M. S. & Goldstein, J. L. (1999) Proc. Natl. Acad. Sci. USA 96, 11041–11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards, P. A., Tabor, D., Kast, H. R. & Venkateswaran, A. (2000) Biochim. Biophys. Acta 1529, 103–113. [DOI] [PubMed] [Google Scholar]
- 3.Rawson, R. B., Zelenski, N. G., Nijhawan, D., Ye, J., Sakai, J., Hasan, M. T., Chang, T. Y., Brown, M. S. & Goldstein, J. L. (1997) Mol. Cell 1, 47–57. [DOI] [PubMed] [Google Scholar]
- 4.Sakai, J., Rawson, R. B., Espenshade, P. J., Cheng, D., Seegmiller, A. C., Goldstein, J. L. & Brown, M. S. (1998) Mol. Cell 2, 505–514. [DOI] [PubMed] [Google Scholar]
- 5.Horton, J. D., Goldstein, J. L. & Brown, M. S. (2002) J. Clin. Invest. 109, 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang, T., Espenshade, P. J., Wright, M. E., Yabe, D., Gong, Y., Aebersold, R., Goldstein, J. L. & Brown, M. S. (2002) Cell 110, 489–500. [DOI] [PubMed] [Google Scholar]
- 7.Yabe, D., Brown, M. S. & Goldstein, J. L. (2002) Proc. Natl. Acad. Sci. USA 99, 12753–12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, M. S. & Goldstein, J. L. (1974) J. Biol. Chem. 249, 7306–7314. [PubMed] [Google Scholar]
- 9.Al-Feel, W., DeMar, J. C. & Wakil, S. J. (2003) Proc. Natl. Acad. Sci. USA 100, 3095–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundqvist, A. & Ericsson, J. (2003) Proc. Natl. Acad. Sci. USA 100, 13833–13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dooley, K. A., Millinder, S. & Osborne, T. F. (1998) J. Biol. Chem. 273, 1349–1356. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez, H. B., Yieh, L. & Osborne, T. F. (1995) J. Biol. Chem. 270, 1161–1169. [DOI] [PubMed] [Google Scholar]
- 13.Hansen, K., Johnell, M., Siegbahn, A., Rorsman, C., Engstrom, U., Wernstedt, C., Heldin, C. H. & Ronnstrand, L. (1996) EMBO J. 15, 5299–5313. [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, X., Sato, R., Brown, M. S., Hua, X. & Goldstein, J. L. (1994) Cell 77, 53–62. [DOI] [PubMed] [Google Scholar]
- 15.Sundqvist, A., Bengoechea, M. T., Ye, X., Lukiyanchuk, V., Jin, J., Harper, J. W. & Ericsson, J. (2005) Cell Metabolism 1, 379–391. [DOI] [PubMed] [Google Scholar]
- 16.Kotzka, J., Lehr, S., Roth, G., Avci, H., Knebel, B. & Muller-Wieland, D. (2004) J. Biol. Chem. 279, 22404–22411. [DOI] [PubMed] [Google Scholar]
- 17.Roth, G., Kotzka, J., Kremer, L., Lehr, S., Lohaus, C., Meyer, H. E., Krone, W. & Muller-Wieland, D. (2000) J. Biol. Chem. 275, 33302–33307. [DOI] [PubMed] [Google Scholar]
- 18.Gottesfeld, J. M. & Forbes, D. J. (1997) Trends Biochem. Sci. 22, 197–202. [DOI] [PubMed] [Google Scholar]
- 19.Kouskouti, A. & Talianidis, I. (2005) EMBO J. 24, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen, D., Dundr, M., Wang, C., Leung, A., Lamond, A., Misteli, T. & Huang, S. (2005) J. Cell Biol. 168, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho, R. J., Huang, M., Campbell, M. J., Dong, H., Steinmetz, L., Sapinoso, L., Hampton, G., Elledge, S. J., Davis, R. W. & Lockhart, D. J. (2001) Nat. Genet. 27, 48–54. [DOI] [PubMed] [Google Scholar]
- 22.Whitfield, M. L., Sherlock, G., Saldanha, A. J., Murray, J. I., Ball, C. A., Alexander, K. E., Matese, J. C., Perou, C. M., Hurt, M. M., Brown, P. O. & Botstein, D. (2002) Mol. Biol. Cell 13, 1977–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, H., Cuenin, C., Murr, R., Wang, Z. Q. & Herceg, Z. (2004) EMBO J. 23, 4824–4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laoukili, J., Kooistra, M. R., Bras, A., Kauw, J., Kerkhoven, R. M., Morrison, A., Clevers, H. & Medema, R. H. (2005) Nat. Cell Biol. 7, 126–136. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez, C., Lobo, M. d. V. T., Gomez-Coronado, D. & Lasuncion, M. A. (2004) Exp. Cell Res. 300, 109–120. [DOI] [PubMed] [Google Scholar]
- 26.Chirala, S. S., Chang, H., Matzuk, M., Abu-Elheiga, L., Mao, J., Mahon, K., Finegold, M. & Wakil, S. J. (2003) Proc. Natl. Acad. Sci. USA 100, 6358–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang, Y. A., Morin, P. J., Han, W. F., Chen, T., Bornman, D. M., Gabrielson, E. W. & Pizer, E. S. (2003) Exp. Cell Res. 282, 132–137. [DOI] [PubMed] [Google Scholar]
- 28.Swinnen, J. V., Heemers, H., Deboel, L., Foufelle, F., Heyns, W. & Verhoeven, G. (2000) Oncogene 19, 5173–5181. [DOI] [PubMed] [Google Scholar]
- 29.Rechsteiner, M. & Rogers, S. W. (1996) Trends Biochem. Sci. 21, 267–271. [PubMed] [Google Scholar]
- 30.Yeh, E., Cunningham, M., Arnold, H., Chasse, D., Monteith, T., Ivaldi, G., Hahn, W. C., Stukenberg, P. T., Shenolikar, S., Uchida, T., et al. (2004) Nat. Cell Biol. 6, 308–318. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani, F., Gostissa, M., Collavin, L. & Del Sal, G. (2004) Cell Cycle 3, 905–911. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.