Abstract
We describe a molecular resistance biomarker to gefitinib, epithelial membrane protein-1 (EMP-1). Gefitinib is a small-molecule inhibitor that competes for the ATP-binding site on EGF receptor (EGFR) and has been approved for patients with advanced lung cancers. Treatment with gefitinib has resulted in clinical benefit in patients, and, recently, heterozygous somatic mutations within the EGFR catalytic domain have been identified as a clinical correlate to objective response to gefitinib. However, clinical resistance to gefitinib limits the utility of this therapeutic to a fraction of patients, and objective clinical responses are rare. We aimed to assess the molecular phenotype and mechanism of in vivo gefitinib resistance in xenograft models and in patient samples. We generated in vivo gefitinib-resistance models in an adenocarcinoma xenograft model by serially passaging tumors in nude mice in presence of gefitinib until resistance was acquired. EMP-1 was identified as a surface biomarker whose expression correlated with acquisition of gefitinib resistance. EMP-1 expression was further correlated with lack of complete or partial response to gefitinib in lung cancer patient samples as well as clinical progression to secondary gefitinib resistance. EMP-1 expression and acquisition of gefitinib clinical resistance was independent of gefitinib-sensitizing EGFR somatic mutations. This report suggests the role of the adhesion molecule, EMP-1, as a biomarker of gefitinib clinical resistance, and further suggests a probable cross-talk between this molecule and the EGFR signaling pathway.
Keywords: EGF receptor, HER-kinase axis, tyrosine kinase inhibitor, non-small cell lung cancer
An orally active quinazoline, gefitinib, directly inhibits tyrosine kinase (TK) phosphorylation on the EGF receptor (EGFR) molecule by competing for the ATP-binding site, which leads to suppression of the EGFR pathway (HER-kinase axis) (1, 2). Concomitantly, gefitinib can also block the pathway by inhibiting tyrosine phosphorylation on another important family member, HER2, but only at a significantly higher concentration (2). Gefitinib has an IC50 of 1.2–3.7 μM for HER2, whereas the IC50 for EGFR is 0.04–0.079 μM. The EGFR signaling pathway is well documented for its involvement in cancer development and progression (3). Gefitinib has proven to be a potent, selective inhibitor of EGFR-TK, showing substantial inhibition of tumor cell growth. Treatment has resulted in clinically meaningful disease stabilization in phase I safety, pharmacokinetic trials on five solid tumor types, namely, advanced stage non-small cell lung cancer (NSCLC), ovarian, head and neck, prostate, and colorectal carcinomas (4). Subsequently, it was successful in phase II clinical trials for stage III and IV NSCLC, which led to its conditional approval by the U.S. Food and Drug Administration as a third line monotherapy treatment of this disease (5). Overall, gefitinib is a well tolerated, orally effective antitumor agent with side effects such as acne-like skin rash and diarrhea.
Despite the accomplishments with gefitinib administration in patients with significantly advanced disease, it achieved only limited success in objective tumor response in a minority of patients. Two studies (6, 7) have provided the first correlation with gefitinib clinical response by identifying gain-of-function somatic mutations in the EGFR-TK domain. Another study has correlated the occurrence of these mutations with clinical response of a distinct ATP-competitive anilinoquinazoline EGFR TK inhibitor (TKI), erlotinib (8). These mutations mediate oncogenic effects by altering downstream signaling and antiapoptotic mechanisms (9) without altering the affinity of the EGFRTKIs to the ATP-binding site on EGFR (10). However, these mutations correlate only with partial or complete objective response to gefitinib in NSCLC patients, whereas 30% or more patients receiving gefitinib treatment have reported stable disease (11, 12). The somatic mutations have been identified only in NSCLC patients, whereas they are absent in other solid tumors such as glioblastoma and colorectal cancer (13). Other studies suggest that the presence of EGFR may be a necessary requisite for gefitinib action (14); however, it is clear from both preclinical and clinical studies that efficacy of gefitinib is independent of EGFR expression levels (4, 15–17). Furthermore, gefitinib showed objective responses in only 11% of patients (17), the remaining patients categorized as de novo resistant or nonresponders. In addition, of the patients who initially demonstrate a significant drug response, most eventually acquire resistance. These observations pose vast imprecision in predicting which patients would benefit from gefitinib treatment and emphasize the need for understanding mechanisms responsible for gefitinib resistance.
Although there are a growing number of studies addressing the mechanism(s) of gefitinib resistance (18–21), they are all based on in vitro models, distant from the clinical setting. Recent studies have identified a somatic mutation within the EGFR tyrosine kinase domain that correlates with the acquisition of resistance to gefitinib or erlotinib (22, 23). However, this mutation has not been demonstrated to be present in patients who have primary or de novo resistance to gefitinib. A biomarker that would be able to identify primary resistance in addition to acquired resistance to gefitinib would be very useful in patient selection. In this study, we describe the generation and characterization of an in vivo gefitinib-resistance model created in an adenocarcinoma xenograft model. This model has been generated in such a way that it closely mimics gefitinib clinical acquired resistance. We identify a biomarker for gefitinib resistance in this model that is validated in 39 advanced lung cancer patient samples with known gefitinib treatment outcomes and 103 unselected lung cancer patients. The patient cohort included patients with de novo and acquired resistance to gefitinib.
Materials and Methods
Generation of Gefitinib-Resistant (GR) in Vivo Model. Female mice were injected with minced CWR22R tumor (ref. 24; an androgen-independent prostate cancer xenograft model chosen because of its well described biology, its proven utility in drug development studies of HER-kinase axis targeted therapeutics, and because it recapitulates human cancer accurately) and treated 5 days/week oral gavage with 100 mg/kg gefitinib (gift from AstraZeneca, Chesire, United Kingdom) for 3 weeks before the tumors were removed and passaged to new female mice. Tumor passaging was continued for a total of 12 generations until a GR line was generated. Two independently derived GR lines were generated. The CWR22R-GR line was continually maintained in the presence of 100 mg/kg daily gefitinib.
EMP-1 Expression Analysis in Clinical Samples. Briefly, archived paraffin tissue blocks were collected from NSCLC patients that had consented to participate in the Iressa Dose Evaluation in Advanced Lung Cancer (IDEAL) study at Cedars–Sinai Comprehensive Cancer Center. Tissue samples were removed of all patient identifiers. A total of 39 samples with known gefitinib treatment outcomes (where biopsies were performed before gefitinib treatment) were evaluated for EMP-1 mRNA expression by using a multianalyte TaqMan RT-PCR assay. Of these, seven patients (18%) demonstrated partial response, whereas the rest who demonstrated either stable disease or clinical progression were classified as nonresponders to gefitinib. Clinical outcome was evaluated by an independent clinical review panel by using response evaluation criteria in solid tumors (RECIST) (25). The RNA isolation and RT-PCR methodology used has been described (26).
Statistical Analysis. For xenograft experiments, tumor volume measurements were expressed as mean ± standard error. Group differences in tumor volumes were compared on the last day of each study. The Kruksal–Wallis test was used to assess group differences for three-group comparisons. The Wilcoxon two-sample test (with exact P value) was used to assess group differences for two-group comparisons. P values were reported for all significance tests. Calculations were made by using the statistical software package sas (version 8.2, SAS Institute, Cary, NC).
For clinical specimens, the probability of gefitinib clinical response was modeled as a function of EMP-1 expression by using a modification of logistic regression. Logistic regression estimates the probability of response as a smooth function of the expression level. A robust version of logistic regression involving weighting and M estimation was used to down-weight influential observations that overly influence parameter estimates.
Supporting Information. For details on ex vivo cell culture, RNA extraction and real-time quantitative RT-PCR, automated sequencing, microarray protocols, and data analysis, see Supporting Text, which is published as supporting information on the PNAS web site.
Results
Development of an in Vivo GR Model. We generated a GR model in the CWR22R tumors (24). This androgen-independent prostate cancer xenograft model was chosen because of its well described biology, its proven utility in drug development studies of HER-kinase axis targeted therapeutics, and because it recapitulates human cancer accurately. Furthermore, CWR22R xenograft line has been validated as gefitinib-sensitive in previous drug studies (27). As previously demonstrated, gefitinib treatment of athymic nude mice bearing subcutaneous androgen-independent (CWR22R) xenograft tumors resulted in significant reduction in tumor growth (63% growth inhibition, compared with control, P < 0.0010, Fig. 1A) validating the utility of gefitinib in this adenocarcinoma model. CWR22R parental tumors were termed as generation F0 or gefitinib-parental (GP). By generation F4, CWR22R tumors stopped responding to gefitinib and demonstrated growth in the presence of gefitinib. At generation F8, the tumors were characterized as resistant after growth comparison with control animals showed gefitinib to be ineffective in inhibiting tumor growth on two independently derived GR lines (Fig. 1B).
Fig. 1.
Gefitinib treatment of prostate cancer xenograft tumors. The response of androgen-independent CWR22R GP (A) and GR (B) xenograft tumor to gefitinib (open diamonds) administered at a dosage of 100 mg/kg QD, oral gavage 5 days/week, or vehicle-treated control (filled diamonds). Arrow indicates initiation of therapy. Results are presented as mean tumor volume (n = 10) ± SE, and P values are indicated.
Recent studies have correlated specific EGFR tyrosine kinase domain somatic mutations with gefitinib sensitivity (6, 7) as well as acquired resistance (22, 23). Analysis of the EGFR and HER2 tyrosine kinase domains (28, 29) of the CWR22R xenograft model did not reveal any gain-of-function catalytic domain mutations that may correlate with gefitinib sensitivity. Moreover, analysis of tumor generations F0–F9 did not demonstrate the recently identified T790M EGFR mutation that correlates with acquired resistance to gefitinib (22, 23), thereby eliminating the contribution of this mutation toward the resistance mechanism in this in vivo generated model. Amplification of target receptor genes, i.e., EGFR and HER2 or HER-kinase ligands (30), EGFRvIII (31), or MDR1 (32), as possible reasons for gefitinib resistance in this model were also ruled out (data not shown).
Gene Expression Profiles of GR Tumors Demonstrate Increased EMP-1 Expression. We compared the total gene expression profiles of the GR and GP tumors using whole genome cDNA microarrays. The comparison included the GP tumors (generation F0), GP tumors treated with gefitinib for 12, 24, and 48 h (to identify gefitinib-inducible genes) and generation F8 and F9 tumors from both the independently generated GR lines. After statistical analyses, five distinct genes were identified whose expression levels changed significantly in the GR tumors, compared with GP tumors, but were not gefitinib-inducible. Two genes whose expression was significantly up-regulated in the GR tumor were EMP-1 (Unigene ID Hs.306692; ≈34-fold) and integrin-α1 (≈10-fold). Studies were pursued with EMP-1, and it was determined that up-regulation of EMP-1 was not gefitinib-inducible (Fig. 2A). This finding was independently confirmed by a real-time quantitative RT-PCR analysis over the entire spectrum of tumor generations from F0 to F9. As shown in Fig. 2B, EMP-1 expression is up-regulated 9- to 11-fold in GR generation F6, compared with the GP tumor, F0. This difference becomes even more pronounced when EMP-1 mRNA expression is analyzed within the ex vivo cells derived from the GP and GR xenograft tumors (Fig. 2C). The same ex vivo cells were also analyzed for EGFR and HER2 expression and, in contrast to EMP-1 expression, EGFR and HER2 showed a similar level of mRNA expression between the two lines (data not shown).
Fig. 2.
EMP-1 is up-regulated in the GR model. (A) Representation of EMP-1 mRNA expression as determined in the gene chip microarray analysis. The white bars represent the gefitinib-sensitive (GP or F0) tumors, light gray bars are the gefitinib-sensitive tumors treated with vehicle alone for 12 h, dark gray bars are the gefitinib-sensitive tumors treated with gefitinib for 12 h, and the black bars represent two different generations of the GR tumors. The y axis represents the absolute EMP-1 mRNA expression. (B) Representative real-time quantitative RT-PCR analysis of EMP-1 mRNA expression in tumor generations F0–F9. The absolute EMP-1 mRNA expression was normalized to β-actin mRNA expression, and the data are presented as relative receptor expression to that observed in the F0 generation. (C) Representative real-time quantitative RT-PCR analysis of EMP-1 mRNA expression in ex vivo cells derived from the gefitinib-sensitive and -resistant tumors. The absolute EMP-1 mRNA expression was normalized to β-actin mRNA expression, and the data are presented as relative receptor expression to that observed in sensitive ex vivo cells.
EMP-1 Is a Clinical Biomarker of both de Novo and Acquired Gefitinib Resistance, but Its Expression Does Not Correlate with the Presence of Somatic Mutations in the EGFR TK Domain. In an attempt to validate the clinical utility of the xenograft model data, specimens from 39 patients who received gefitinib monotherapy with advanced NSCLC were studied. Correlation of EMP-1 expression to gefitinib response/nonresponse was determined by using a graph with threshold of 10%. Using logistic regression, the estimated probability of response is <10% for expression values greater than the threshold. Results of this analysis showed a significant (P = 0.04) relationship between probability of response and expression level for the gene. Specifically, for low expression levels of EMP-1, the probability of response was high (≈70%). However, as expression of EMP-1 increased, the estimated probability of response decreased to <5%. For analysis purposes, this information was used to set an arbitrary threshold of 10%. As shown in Fig. 3A, it was observed that the probability of gefitinib response decreased as the expression of EMP-1 increased. When looking at EMP-1 expression in individual samples (Fig. 3B), none of the clinical responders to gefitinib expressed EMP-1, with one exception. EMP-1 is deemed as expressed when the expression level relative to the reference genes is above the threshold. Of the 39 NSCLC patient samples analyzed, 14 patients who expressed EMP-1 demonstrated either stable disease or clinical disease progression with one exception and were classified as gefitinib nonresponders. It is of note that the tissue biopsy studied, from the one patient who is an exception, was acquired 16 months before initiation of gefitinib therapy. Expression levels of EGFR did not display any correlation with drug response in our study (data not shown) as has been demonstrated (4, 17).
Fig. 3.
EMP-1 expression in tumor samples from NSCLC patients receiving gefitinib monotherapy. (A) Probability of clinical response to gefitinib decreases as EMP-1 expression increases. The x axis represents the reference normalized cycle threshold for EMP-1 expression, and the y axis represents the probability of response. The red dashed curves indicate the 95% confidence interval. (B) EMP-1 expression in individual samples. The red dashed line represents an arbitrary threshold of 10%. The red circles represent the samples from patients who responded to gefitinib therapy, whereas the blue diamonds represent the nonresponders. The yellow circle highlights the gefitinib responder who eventually acquired gefitinib resistance, and the sample was further characterized. (C) Normal human organ distribution of EMP-1 mRNA expression. A representative graph showing the EMP-1 mRNA expression in normal body tissues, compared with that in the GP and GR tumors.
EMP-1 mRNA expression was further analyzed in 103 unselected lung cancer patient samples, which included both adenocarcinoma as well as squamous cell carcinoma samples. EMP-1 was expressed in 66% of the squamous cell carcinomas and 40.9% of the adenocarcinomas (data not shown). EMP-1 expression was also analyzed in a panel of normal human tissues, and it was found that the expression was most prevalent in tissues of the gastrointestinal tract, i.e., esophagus, larynx, lip, oral mucosa, pharyngeal mucosa, colonic epithelium, and tongue (Fig. 3C).
Gefitinib clinical response has recently been correlated (6, 7) with the presence of somatic mutations in exons 18, 19, or 21 of the EGFR TK domain. In light of these studies, we looked for these documented mutations in the NSCLC patient samples with reported gefitinib outcomes to correlate them with EMP-1 expression. Interestingly, all samples (even the one exception who was a partial responder) that expressed EMP-1 lacked the reported EGFR mutations (Table 1).
Table 1. EMP-1–positive NSCLC tumors.
| Patient ID | Gender | Clinical outcome | Diagnosis | Reported EGFR mutations* |
|---|---|---|---|---|
| 1 | F | Progressive | Large cell carcinoma | Absent |
| 2 | F | Progressive | Adeno/bronchioloalveolar carcinoma | Absent |
| 3 | M | Progressive | Adenocarcinoma | Absent |
| 4 | M | Progressive | Large cell carcinoma | Absent |
| 5 | F | Progressive | Adenocarcinoma | Absent |
| 6 | F | Progressive | Adenocarcinoma | Absent |
| 7 | M | Progressive | Adenocarcinoma | Absent |
| 8 | F | Progressive | Adenocarcinoma | Ex 18 was NE |
| 9 | F | Progressive | Large cell carcinoma | Ex 19 and 21 were NE |
| 10 | F | Progressive | Adenocarcinoma | Absent |
| 11 | F | Stable | Adenocarcinoma | Absent |
| 12 | F | Progressive | Adenocarcinoma | Absent |
| 13 | F | Stable | Adenocarcinoma | Absent |
| 14 | M | Partial response | Adenocarcinoma | Absent |
We were able to analyze EMP-1 expression in a frozen pleural effusion sample obtained from an NSCLC patient after the patient had acquired resistance to gefitinib. This patient who was diagnosed with bronchioloalveolar adenocarcinoma was originally an objective partial responder to gefitinib. The clinical response to gefitinib correlated with the presence of the EGFR exon 21, L858R mutation (6, 7) in the pretherapy sample of this patient. EMP-1 expression in this pre-gefitinib therapy sample was below the threshold of 10% (pretherapy sample depicted with the yellow circle in Fig. 3B), which is considered as null EMP-1 expression based on our analysis. Eventually, the patient acquired resistance to the drug after receiving 160 days of gefitinib treatment (posttherapy sample). Interestingly, the posttherapy sample was demonstrated to harbor the recently identified threonine-to-methionine somatic mutation at position 790 of EGFR (22, 23) that has been correlated with acquired resistance to gefitinib treatment. This mutation was absent in the pretherapy sample. Therefore, even though we could only analyze one such clinical sample, it is a bona fide example of acquired resistance as it displays the molecular correlate for it. The posttherapy sample also had the gain-of-function EGFR exon 21 mutation, demonstrating that the patient had acquired resistance to gefitinib despite the presence of EGFR somatic mutation. We analyzed EMP-1 mRNA expression in the posttherapy sample and compared it to the EMP-1 expression in the tumor tissues obtained from the GP (gefitinib responder) and GR (gefitinib nonresponder) models. As shown in Fig. 4, GAPDH normalized EMP-1 expression levels in the posttherapy sample are comparable to those in the GR tumors, thus confirming that EMP-1 expression in this gefitinib acquired resistance clinical sample is up-regulated as is the case in the GR model. We were unable to compare the EMP-1 expression in the pretherapy and posttherapy sample within the same assay (even though the same set of EMP-1 primer/probes and RT-PCR reaction conditions were used in the two assays) because of the limited amounts of the clinical material and the timing when these samples became available to us. However, given the fact that the pretherapy sample expressed EMP-1 below the threshold limit and the EMP-1 expression in the posttherapy sample was comparable to the GR tumors is a strong indication that EMP-1 is a biomarker of gefitinib resistance in this clinical setting.
Fig. 4.
EMP-1 expression in NSCLC clinical samples. Real-time quantitative RT-PCR analysis of EMP-1 mRNA expression in NSCLC patient sample who acquired gefitinib resistance (gray bar) is shown. The expression in the NSCLC sample is compared to that in three GP tumors (white bars) and three GR tumors (black bars). One of the GR tumors was assigned as a calibrator sample (expression value = 1), and the relative EMP-1 mRNA expression was normalized to GAPDH mRNA expression.
Discussion
We have identified EMP-1 as a potential biomarker of gefitinib resistance, both de novo and acquired. EMP-1 expression correlates with gefitinib nonresponse, but is independent of the EGFR kinase domain somatic mutations.
Results from our clinical analysis demonstrate that (i) objective gefitinib responders acquired resistance to gefitinib despite the presence of the gefitinib-sensitizing EGFR kinase domain mutation (L858R), indicating that these gain-of-function somatic mutations cannot prevent gefitinib resistance but are only predictors of gefitinib response in a subset of patients. This observation has also been reported by other groups (8). (ii) EMP-1 expression increased in the posttherapy sample described above, which still had the EGFR L858R mutation, indicating that the EMP-1 expression mechanism is distinct from the EGFR somatic mutation-dependent gefitinib response mechanism. (iii) The posttherapy sample also displayed the EGFR T790M somatic mutation associated with acquired resistance to gefitinib (22, 23). It is unlikely that EMP-1 expression correlates with or depends on the T790M mutation, as we did not find the T790M mutation in our acquired resistance adenocarcinoma model; however, further correlative studies are required.
Lack of EMP-1 expression does not predict gefitinib clinical response. It is important to note that not all patients who were gefitinib nonresponders expressed EMP-1, suggesting that there are other mechanisms of gefitinib resistance. An important observed correlation is that many tissues in the body that express EMP-1 have significant EGFR expression, but rarely is EGFR-TK inhibitor toxicity observed in these tissues. Even though the patient cohort that we analyzed in this study was small, it was notable that we did achieve statistical significance for the correlation of EMP-1 expression and objective response (P = 0.04). Prospective clinical trials are ongoing at several institutions to expand this clinical evaluation.
The mechanism of EMP-1 up-regulation, such as in our acquired resistance GR model or in the described patient who acquired resistance to gefitinib, remains to be determined. A “preexisting” EMP-1 expressing cell population may be selected under gefitinib pressure, thereby shifting the focus of the signal transduction pathway to amino acid residues outside the core kinase domain or alternative adaptor molecules where gefitinib may not act with a high affinity. EMP-1 expression was analyzed in three different paired clinical samples where the two samples were collected at two different times before gefitinib therapy. In each pair, the sample that was closer to the time of gefitinib treatment had a greater EMP-1 expression than the one that was collected earlier (data not shown). This interesting correlation further suggests that EMP-1 is a biomarker of gefitinib resistance and that EMP-1 in association with the epithelial interactions of the tumor microenvironment may play a role in tumor growth and gefitinib resistance. We hypothesize that (i) the “EMP-1-positive” tumor cell population has a survival advantage as in the case of de novo resistance or (ii) the presence of gefitinib gradually enriches for the “EMP-1-positive” cell population as in the case of acquired resistance.
We propose a model for gefitinib in vivo resistance in Fig. 5. EMP-1 (33) and integrin-α1, the two genes that are significantly up-regulated in the GR model, are both cell surface proteins that are involved in cell–cell adhesions and interactions with the extracellular matrix. We hypothesize that the up-regulation of cell adhesion molecules, i.e., EMP-1 and integrin-α1, during EGFR-TK inhibitor resistance leads to a shift in signaling such that there is increased involvement of the tumor microenvironment, thus leading to activation of alternative downstream signaling pathways (34) that may not be optimally targeted by small molecule inhibitors of EGFR kinase.
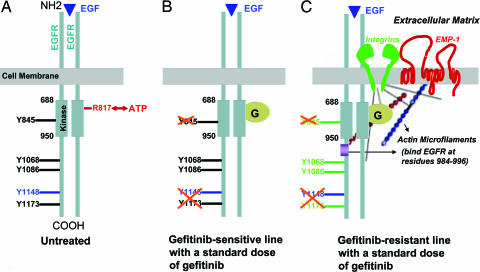
Fig. 5.
A model for in vivo resistance to gefitinib therapy. (A) In untreated cells, EGF stimulation (shown by a blue triangle) causes EGFR dimerization followed by phosphorylation events that leads to productive downstream signaling events. The core kinase domain extends from amino acid residues 688 to 950, and the ATP-binding site, R817, is shown in red. Positions of certain key tyrosine residues (Y845, Y1068, Y1086, Y1148, and Y1173) are shown. (B) When tumors are treated with a standard dose of gefitinib, gefitinib preferentially competes for its high-affinity binding site on the kinase domain, i.e., the ATP-binding site (gefitinib is shown as “G” in the yellow circle). We hypothesize that the major dephosphorylation events may occur only at a few key tyrosine residues such as Y845 within the kinase domain, Y1148, which is an EGF-stimulated phosphorylation site, and Y1173, which is the major autophosphorylation site. The other tyrosine residues may continue to signal under these conditions, albeit less robustly, and therefore, a reduction in tumor growth, and not an inhibition in tumor growth, is observed upon gefitinib treatment. (C) Cells that acquire resistance to gefitinib or the GR cells that are enriched by selection express high levels of integrin family members (shown in green) and EMP-1 (shown in red). Treating these cells with a standard dose of gefitinib can efficiently block probably only the high-affinity ATP-binding site and the related autophosphorylation sites on EGFR. The activation of integrins and adhesion molecules such as EMP-1 may enhance increased interactions with the extracellular matrix and constitutive signaling of the EGFR signaling pathway despite the presence of gefitinib. Actin (shown as red and blue beaded filaments) can increase EGFR signaling by binding to EGFR between residues 984 and 996, and EMP-1 may also enhance this process.
In summary, by studying the in vivo resistance mechanism to gefitinib, the EMP-1/integrin pathway has been identified as an alternative pathway in cancer cells, and thus is an important therapeutic target and an important patient selective marker for resistance to EGFR-targeted therapies that is independent of the EGFR somatic mutations.
Supplementary Material
Acknowledgments
This work was supported by a Prostate Cancer Foundation grant and National Institutes of Health Prostate Specialized Program of Research Excellence (SPORE) P50 CA92131 (to D.B.A.), American Association for Cancer Research–California Department of Health Services Career Development Award in Prostate Cancer Research 97-12013 (to A.J.), and a Prostate Cancer Foundation Young Investigator Award (to I.L.).
Author contributions: A.J. and D.B.A. designed research; A.J., C.A.T., I.L., J.B.H., J.C., A.G., D.E.A., N.A., S.S., and R.B.N. performed research; A.J., C.A.T., I.L., D.E.A., S.S., R.B.N., and D.B.A. analyzed data; A.J. wrote the paper; and N.A. was the clinical sample coordinator.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TK, tyrosine kinase; EGFR, EGF receptor; NSCLC, non-small cell lung cancer; GR, gefitinib-resistant; GP, gefitinib-parental.
References
- 1.Barker, A. J., Gibson, K. H., Grundy, W., Godfrey, A. A., Barlow, J. J., Healy, M. P., Woodburn, J. R., Ashton, S. E., Curry, B. J., Scarlett, L., et al. (2001) Bioorg. Med. Chem. Lett. 11, 1911-1914. [DOI] [PubMed] [Google Scholar]
- 2.Wakeling, A. E., Guy, S. P., Woodburn, J. R., Ashton, S. E., Curry, B. J., Barker, A. J. & Gibson, K. H. (2002) Cancer Res. 62, 5749-5754. [PubMed] [Google Scholar]
- 3.Yarden, Y. & Sliwkowski, M. X. (2001) Nat. Rev. Mol. Cell. Biol. 2, 127-137. [DOI] [PubMed] [Google Scholar]
- 4.Baselga, J., Rischin, D., Ranson, M., Calvert, H., Raymond, E., Kieback, D. G., Kaye, S. B., Gianni, L., Harris, A., Bjork, T., et al. (2002) J. Clin. Oncol. 20, 4292-4302. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, M. H., Williams, G. A., Sridhara, R., Chen, G. & Pazdur, R. (2003) Oncologist 8, 303-306. [DOI] [PubMed] [Google Scholar]
- 6.Lynch, T. J., Bell, D. W., Sordella, R., Gurubhagavatula, S., Okimoto, R. A., Brannigan, B. W., Harris, P. L., Haserlat, S. M., Supko, J. G., Haluska, F. G., et al. (2004) N. Engl. J. Med. 350, 2129-2139. [DOI] [PubMed] [Google Scholar]
- 7.Paez, J. G., Janne, P. A., Lee, J. C., Tracy, S., Greulich, H., Gabriel, S., Herman, P., Kaye, F. J., Lindeman, N., Boggon, T. J., et al. (2004) Science 304, 1497-1500. [DOI] [PubMed] [Google Scholar]
- 8.Pao, W., Miller, V., Zakowski, M., Doherty, J., Politi, K., Sarkaria, I., Singh, B., Heelan, R., Rusch, V., Fulton, L., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 13306-13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sordella, R., Bell, D. W., Haber, D. A. & Settleman, J. (2004) Science 305, 1163-1167. [DOI] [PubMed] [Google Scholar]
- 10.Fabian, M. A., Biggs, W. H., III, Treiber, D. K., Atteridge, C. E., Azimioara, M. D., Benedetti, M. G., Carter, T. A., Ciceri, P., Edeen, P. T., Floyd, M., et al. (2005) Nat. Biotechnol. 23, 329-336. [DOI] [PubMed] [Google Scholar]
- 11.Kris, M. G., Natale, R. B., Herbst, R. S., Lynch, T. J., Jr., Prager, D., Belani, C. P., Schiller, J. H., Kelly, K., Spiridonidis, H., Sandler, A., et al. (2003) J. Am. Med. Assoc. 290, 2149-2158. [DOI] [PubMed] [Google Scholar]
- 12.Fukuoka, M., Yano, S., Giaccone, G., Tamura, T., Nakagawa, K., Douillard, J. Y., Nishiwaki, Y., Vansteenkiste, J., Kudoh, S., Rischin, D., et al. (2003) J. Clin. Oncol. 21, 2237-2246. [DOI] [PubMed] [Google Scholar]
- 13.Barber, T. D., Vogelstein, B., Kinzler, K. W. & Velculescu, V. E. (2004) N. Engl. J. Med. 351, 2883. [DOI] [PubMed] [Google Scholar]
- 14.Anido, J., Matar, P., Albanell, J., Guzman, M., Rojo, F., Arribas, J., Averbuch, S. & Baselga, J. (2003) Clin. Cancer Res. 9, 1274-1283. [PubMed] [Google Scholar]
- 15.Moasser, M. M., Basso, A., Averbuch, S. D. & Rosen, N. (2001) Cancer Res. 61, 7184-7188. [PubMed] [Google Scholar]
- 16.Janmaat, M. L., Kruyt, F. A., Rodriguez, J. A. & Giaccone, G. (2003) Clin. Cancer Res. 9, 2316-2326. [PubMed] [Google Scholar]
- 17.Cappuzzo, F., Gregorc, V., Rossi, E., Cancellieri, A., Magrini, E., Paties, C. T., Ceresoli, G., Lombardo, L., Bartolini, S., Calandri, C., et al. (2003) J. Clin. Oncol. 21, 2658-2663. [DOI] [PubMed] [Google Scholar]
- 18.Li, B., Chang, C. M., Yuan, M., McKenna, W. G. & Shu, H. K. (2003) Cancer Res. 63, 7443-7450. [PubMed] [Google Scholar]
- 19.She, Q. B., Solit, D., Basso, A. & Moasser, M. M. (2003) Clin. Cancer Res. 9, 4340-4346. [PubMed] [Google Scholar]
- 20.Bianco, R., Shin, I., Ritter, C. A., Yakes, F. M., Basso, A., Rosen, N., Tsurutani, J., Dennis, P. A., Mills, G. B. & Arteaga, C. L. (2003) Oncogene 22, 2812-2822. [DOI] [PubMed] [Google Scholar]
- 21.Blencke, S., Ullrich, A. & Daub, H. (2003) J. Biol. Chem. 278, 15435-15440. [DOI] [PubMed] [Google Scholar]
- 22.Pao, W., Miller, V. A., Politi, K. A., Riely, G. J., Somwar, R., Zakowski, M. F., Kris, M. G. & Varmus, H. (2005) PLoS. Med. 2, 1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi, S., Boggon, T. J., Dayaram, T., Janne, P. A., Kocher, O., Meyerson, M., Johnson, B. E., Eck, M. J., Tenen, D. G. & Halmos, B. (2005) N. Engl. J. Med. 352, 786-792. [DOI] [PubMed] [Google Scholar]
- 24.Nagabhushan, M., Miller, C. M., Pretlow, T. P., Giaconia, J. M., Edgehouse, N. L., Schwartz, S., Kung, H. J., de Vere White, R. W., Gumerlock, P. H., Resnick, M. I., et al. (1996) Cancer Res. 56, 3042-3046. [PubMed] [Google Scholar]
- 25.Therasse, P., Arbuck, S. G., Eisenhauer, E. A., Wanders, J., Kaplan, R. S., Rubinstein, L., Verweij, J., Van Glabbeke, M., van Oosterom, A. T., Christian, M. C. & Gwyther, S. G. (2000) J. Natl. Cancer Inst. 92, 205-216. [DOI] [PubMed] [Google Scholar]
- 26.Cronin, M., Pho, M., Dutta, D., Stephans, J. C., Shak, S., Kiefer, M. C., Esteban, J. M. & Baker, J. B. (2004) Am. J. Pathol. 164, 35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sirotnak, F. M., She, Y., Lee, F., Chen, J. & Scher, H. I. (2002) Clin. Cancer Res. 8, 3870-3876. [PubMed] [Google Scholar]
- 28.Boonstra, J., Rijken, P., Humbel, B., Cremers, F., Verkleij, A. & van Bergen en Henegouwen, P. (1995) Cell Biol. Int. 19, 413-430. [DOI] [PubMed] [Google Scholar]
- 29.Callaghan, T., Antczak, M., Flickinger, T., Raines, M., Myers, M. & Kung, H. J. (1993) Oncogene 8, 2939-2948. [PubMed] [Google Scholar]
- 30.Goker, E., Waltham, M., Kheradpour, A., Trippett, T., Mazumdar, M., Elisseyeff, Y., Schnieders, B., Steinherz, P., Tan, C., Berman, E., et al. (1995) Blood 86, 677-684. [PubMed] [Google Scholar]
- 31.Pedersen, M. W., Meltorn, M., Damstrup, L. & Poulsen, H. S. (2001) Ann. Oncol. 12, 745-760. [DOI] [PubMed] [Google Scholar]
- 32.Bodo, A., Bakos, E., Szeri, F., Varadi, A. & Sarkadi, B. (2003) Toxicol. Lett. 140–141, 133-143. [DOI] [PubMed] [Google Scholar]
- 33.Ben-Porath, I. & Benvenisty, N. (1996) Gene 183, 69-75. [DOI] [PubMed] [Google Scholar]
- 34.Marcoux, N. & Vuori, K. (2003) Oncogene 22, 6100-6106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.