Abstract
Impaired endothelial nitric oxide synthase (eNOS) function is associated with erectile dysfunction in diabetes mellitus, but the exact molecular basis for the eNOS defect in the diabetic penis remains unclear. We investigated whether hyperglycemia increases O-GlcNAc modification of eNOS in the penis, preventing phosphorylation at the primary positive regulatory site on the enzyme and hampering mechanisms of the erectile response. Type I diabetes mellitus was induced in male rats by alloxan (140 mg/kg, i.p.). After 5 wk, the diabetic rat penis exhibited increased O-GlcNAc modification of eNOS and decreased eNOS phosphorylation at Ser-1177 at baseline compared with the control rat penis; eNOS phosphorylation at Thr-495, Ser-615, and Ser-633 was not affected. In addition, eNOS phosphorylation at Ser-1177 was impaired in the diabetic rat penis in response to penile blood flow (shear stress) elicited by electrical stimulation of the cavernous nerve (ES) and to recombinant human VEGF165. Phosphorylation of Akt, a mediator of shear stress-induced eNOS phosphorylation at Ser-1177, was decreased in the diabetic penis at baseline, but it was restored by ES. Erectile response to shear stress elicited by ES and to VEGF was decreased in diabetic compared with control rats. This work demonstrates that eNOS inactivation occurs in the diabetic penis by a glycosylation mechanism specifically at Ser-1177, by which the enzyme is rendered incapable of activation by fluid shear stress stimuli and VEGF signaling. In vivo penile erection paradigm supports the physiologic relevance of O-GlcNAc modification in vascular disorders associated with diabetes.
Keywords: penis, penile erection, Akt, VEGF
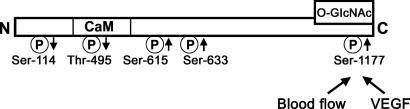
The role of endothelial nitric oxide synthase (eNOS) in penile erection is becoming increasingly recognized. Although initiation of erection is mediated by rapid, short-term calcium-dependent release of NO from neuronal NOS-containing nerve terminals in the penis, full erection and maintenance of erection is achieved by the blood flow-induced phosphatidylinositol 3-kinase/Akt/eNOS (Ser-1177) phosphorylation cascade, resulting in reduced calcium dependence and sustained release of endothelial NO and continued cavernous smooth muscle relaxation (1). In addition, VEGF induces eNOS phosphorylation at Ser-1177 in the penis and promotes penile erection, but this action appears to be independent of Akt activation (2). In contrast to Ser-1177, Thr-495 acts on eNOS as a negative regulatory site, i.e., phosphorylation of this site is associated with a decrease in eNOS activity by increasing calcium/calmodulin dependence of the enzyme (3-5). The physiological significance of phosphorylation of other sites on eNOS is less known and somewhat controversial. The phosphorylation of Ser-615 and Ser-633 causes activation of eNOS function (6, 7), whereas the phosphorylation of Ser-114 reduces eNOS function (8) (Fig. 1). Moreover, the phosphorylation of a site on eNOS may be affected by the state of phosphorylation of other residues on eNOS (9).
Fig. 1.
A model of eNOS phosphorylation sites, a proposed site of O-GlcNAc, and the site of action of blood flow-induced shear stress and VEGF on eNOS. Arrows indicate increase or decrease of the activity of the enzyme upon phosphorylation.
Impairment of penile vascular function is associated with erectile dysfunction in a variety of vascular disorders including diabetes mellitus and aging (10). In diabetic patients and experimentally induced diabetic animals, erectile dysfunction due to the inability of the cavernosal smooth muscle to undergo complete relaxation is accompanied by a decrease in NOS activity and NO availability (11-14), while the expression of eNOS is reportedly decreased (11, 13) or unchanged (12) in the penis. Furthermore, cGMP production (15, 16) and cGMP-dependent PK-1 (17) are down-regulated in the diabetic penis, whereas RhoA and Rho-kinase (13, 18), responsible substantially for cavernosal contractile activity, are increased in the diabetic penis. The exact mechanism of erectile dysfunction and, specifically, the molecular basis for the eNOS defect in the diabetic penis are, however, unclear and require further investigation.
A new line of investigation has pointed to the significance of hyperglycemia-induced O-GlcNAc modification of eNOS in the inactivation of the enzyme (19, 20). This monosaccharide modification often competes in ying-yang fashion with phosphorylation in the regulatory pathways of the cell (21). Ying-yang prediction server identifies several Ser and Thr O-GlcNAcylation sites on eNOS, including several sites around the Ser-1177 residue (22). Basal eNOS activity in bovine aortic endothelial cells and human coronary endothelial cells in vitro was found to be inhibited by hyperglycemia through O-linked GlcNAc modification of eNOS and by reciprocally decreased phosphorylation of Ser-1177 (19, 20). The same authors demonstrated that, in aortas isolated from diabetic animals and carotid plaques from diabetic patients, GlcNAc modification of eNOS was increased and eNOS (Ser-1177) phosphorylation was decreased. However, the physiologic relevance of O-GlcNAc modification of eNOS in diabetic vascular tissues is unclear.
Our aim in the present study was to investigate whether hyperglycemia in diabetic rats increases O-GlcNAc modification of eNOS in the penis, thus preventing phosphorylation of the enzyme on Ser-1177 and hampering a major regulatory physiologic mechanism of the erectile response (Fig. 1). We now show that eNOS function in the penis is impaired in diabetes by O-GlcNAc modification affecting specifically the Ser-1177 residue, by which the enzyme is rendered incapable of activation by normal fluid shear stress stimuli and VEGF signaling. Such eNOS impairment may contribute to erectile dysfunction and compromise long-term penile health in diabetes.
Materials and Methods
Diabetic Rat Model. Chemical diabetogenesis was induced in adult male Sprague-Dawley rats (Charles River Breeding Laboratories) by a single i.p. injection of alloxan (140 mg/kg in saline, pH 4; Sigma) as described in refs. 11 and 23. By day 5 after injection, ≈50% of rats developed diabetes. The remaining rats received a second injection, and ≈90% of them became diabetic within 3 d. Control rats were injected with an equal volume of vehicle. The onset of diabetes was confirmed by urine glucose levels of >500 mg/dl, by using chemstrips (Roche Diagnostics). Rats were weighed and used for studies 5 wk after confirmed hyperglycemia (≈6 wk after alloxan or vehicle treatment) to assess early stage diabetes, without the confounding factors associated with late diabetes such as tissue degeneration. The initial weight of rats was 342.1 ± 9.1 g. The body weights of diabetic rats 5 wk after the induction of diabetes (364.6 ± 9.9 g) were significantly (P < 0.05) less than those of vehicle-treated control rats (515.7 ± 15.2 g). All experiments were conducted in accordance with The Johns Hopkins University School of Medicine Guidelines for the Care and Use of Animals.
Western Blot Analysis. Minced penile tissue was homogenized and partially purified for NOS as described in ref. 1. Purified eNOS [for phosphoralated (P)-eNOS and O-GlcNAc analyses] or 50 μg of proteins in crude homogenate [for phosphoralated (P)-Akt and total eNOS] were resolved on 7.5% or 4-20% Tris gels and transferred to poly(vinylidene difluoride) membrane. The membranes were probed with polyclonal rabbit anti-P-eNOS (Thr-495, Ser-615, and Ser-633) Abs at 1:450 dilutions, rabbit anti-P-Akt (Ser-473) Ab at 1:1,000 dilution (Cell Signaling Technology, Beverly, MA), or O-GlcNAc mAb (Affinity BioReagents, Neshanic Station, NJ) at 1:500 dilution. Membranes used for O-GlcNAc analysis were stripped and probed with rabbit polyclonal anti-P-eNOS (Ser-1177) Ab (Cell Signaling Technology) at 1:450 dilution. Membranes used for P-protein analysis then were stripped and probed with rabbit polyclonal anti-eNOS (BD Transduction Laboratories) or rabbit polyclonal anti-Akt (Cell Signaling Technology) Abs at 1:1,000 dilutions. P-Akt densities were normalized relative to those of total Akt, and P-eNOS and O-GlcNAc densities were normalized relative to those of total eNOS. The ratio was determined in terms of arbitrary units and expressed relative to the ratio for control animals at baseline. For analysis of total eNOS expression by Western immunoblotting, a separate set of penile homogenates (50 μg) was used without purification and standardized per β-actin.
Physiologic Erection Studies. Animals were anesthetized with 40 mg/kg pentobarbital (Abbott). To monitor intracavernosal pressure (ICP), the shaft of the penis was denuded of skin and fascia, and the proximal left corpus cavernosum at the perineum was perforated with a 27-gauge needle connected via polyethylene tubing 50 to a pressure transducer (DI-190; Dataq Instruments, Akron, OH). Systemic mean arterial pressure was monitored continuously via the right carotid artery (1, 24).
To induce erection by electrical stimulation of the cavernous nerve (ES), bipolar electrode attached to a Grass Instruments S48 stimulator (Quincy, MA) was placed around the cavernous nerve as described in ref. 24. Stimulation parameters were 3 or 6 V (as specified below) at a frequency of 16 Hz with squarewave duration of 5 ms. Response parameters were calculated by using matlab software (Mathworks, Natick, MA) and expressed per mean arterial pressure. Statistical analysis was performed on (i) maximal ICP, corresponding to the magnitude of erectile response; (ii) ICP area above baseline pressure, defined as the area under the curve which corresponds to the duration of ES; and (iii) rise time, defined as the time needed to reach 50% of maximal ICP from the start of ES.
Shear Stress Stimulation. The protocol for causing biomechanical shear stress in vivo involved induction of blood flow by ES. The neurogenic application initiates an increase in penile blood flow and thereby mechanical shear stress on the corpora cavernosa (1). Groups of diabetic and control rats underwent 6 V ES for 1 min, and erectile responses were measured. Separate groups of rats were used for collection of penes both at baseline (flaccid, unstimulated) and after ES at 6 V (erect, stimulated for 1 min) for Western blot analyses (1).
VEGF Administration. Recombinant human VEGF (rhVEGF)165 (0.5 μg/PBS/0.1% BSA; Calbiochem) or vehicle was injected into the right corpus cavernosum. After 25 min, groups of diabetic and control rats underwent erection physiologic testing at submaximal voltage (3 V). Separate groups of rats were used for collection of penes at baseline without ES for Western blot analyses, to eliminate the effect of blood flow on eNOS. We recently showed that rhVEGF165 at this time point and at this dose is an effective inducer of constitutive eNOS activation in the penis by stimulating the phosphorylation of the enzyme at Ser-1177 (25). Our protocol is supported by the kinetics of initial calcium-dependent (several minutes) vs. phosphorylation-(Ser-1177)-dependent (10-30 min), NO production in response to VEGF-stimulation (26), and the lack of the proliferative effect of VEGF at this dose (27). The submaximal stimulation parameter (3 V) was chosen based on our previous studies showing that maximal neurostimulation obscures measurements of VEGF-induced changes in eNOS (Ser-1177) phosphorylation in penes of mice treated intracavernosally with VEGF (2).
Statistical Evaluation. Statistical analysis was performed by using one-way ANOVA followed by Newman-Keuls multiple comparison test or by t test when appropriate. The data are expressed as the mean ± SEM. A value of P < 0.05 was considered to be significant.
Results
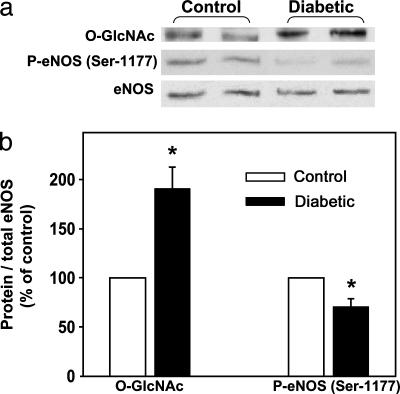
O-GlcNAc Modification of eNOS Is Increased and P-eNOS (Ser-1177) Is Decreased in the Diabetic Penis. The levels of eNOS linked O-GlcNAc were significantly (P < 0.05) increased, whereas the levels of P-eNOS (Ser-1177) were significantly (P < 0.05) reduced in penes of diabetic rats relative to control rat penes at baseline (Fig. 2). However, total eNOS protein expression, normalized to β-actin, was not significantly different between control (100%) and diabetic (82.8 ± 46.3%) rat penes.
Fig. 2.
O-GlcNAc modification of eNOS is increased, and P-eNOS (Ser-1177) is decreased in the diabetic rat penis. Flaccid, nonerect penes (at baseline) were excised 5 wk after the induction of diabetes by alloxan (140 mg/kg). O-GlcNAc and P-eNOS were examined in partially purified penile homogenates by Western blotting. (a) Representative Western immunoblot of O-GlcNAc, P-eNOS (Ser-1177), and total eNOS in penes of control and diabetic rats at baseline. (b) Quantitative densitometry of O-GlcNAc and P-eNOS (Ser-1177) in penes of control and diabetic rats at baseline. Results are expressed as the ratio of P-eNOS (Ser-1177) to total eNOS and O-GlcNAc to total eNOS. Values are normalized to controls. n = 6; *, P < 0.05 compared with controls.
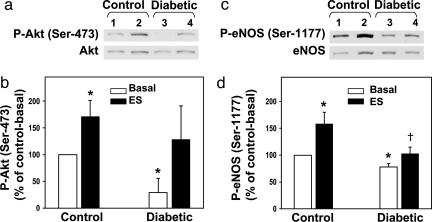
To evaluate whether the decreased P-eNOS (Ser-1177) levels in the diabetic penis arise from altered activation by P-Akt, the upstream mediator of eNOS phosphorylation at Ser-1177, we measured P-Akt levels in penes. Fig. 3 a and b shows that basal levels of P-Akt were significantly (P < 0.05) reduced in penes of diabetic rats by ≈60% relative to basal levels in the control rat penis.
Fig. 3.
eNOS (Ser-1177), but not Akt (Ser-473), phosphorylation is impaired in the diabetic penis in response to shear stress elicited by ES. (a and c) Representative Western immunoblots. (b and d) A quantitative densitometry of P-Akt (Ser-473) and P-eNOS (Ser-1177), respectively, in penes of control and diabetic rats at baseline (lanes 1 and 3, respectively) and in penes of control and diabetic rats after ES (lanes 2 and 4, respectively). Results are expressed as the ratio of P-Akt to total Akt and P-eNOS to total eNOS. Values are normalized to controls; n = 5-7; *, P < 0.05 vs. control-basal; †, P < 0.05 vs. control-ES.
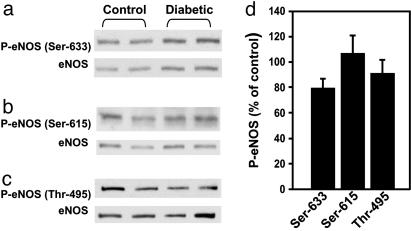
Although it has been shown that the glucosamine modification on eNOS is rather specific to sites around Ser-1177 (19), it is possible that reciprocal interactions could occur involving other eNOS phosphorylation sites. However, as shown in Fig. 4, the levels of eNOS phosphorylated at Thr-495, which inactivates eNOS, and Ser-615 and Ser-633, which supposedly activate eNOS, did not differ in the diabetic compared with the control rat penis. Thus, of the examined phosphorylation sites on eNOS in the penis, Ser-1177 is the only site affected by diabetes. Because the function of Ser-116 phopshorylation on eNOS is not well characterized, and it does not appear to be affected by shear stress and VEGF (7), we did not examine this phosphorylation site.
Fig. 4.
Phosphorylation of eNOS at Ser-633, Ser-615, and Thr-495 in penes is not affected by diabetes. (a-c) Representative Western immunoblots of eNOS phosphorylation sites and total eNOS. (d) A quantitative densitometry of eNOS phosphorylated at Ser-633, Ser-615, and Thr-495 in penes of control and diabetic rats at baseline. Results are expressed as the ratio of P-eNOS to total eNOS. Values are normalized to controls; n = 4-6.
eNOS Phosphorylation at Ser-1177 by Shear Stress and VEGF Is Impaired in the Diabetic Penis. Because eNOS activity/phosphorylation is dynamically regulated by diverse extracellular stimuli, we next wanted to determine whether the phosphorylation of Ser-1177 residue is altered in the diabetic penis in response to characteristic stimuli for eNOS activation, shear stress, and VEGF, which specifically target this phosphorylation site on eNOS in the penis (1, 2). Although ES-induced increase in blood flow significantly (P < 0.05) increased the levels of P-eNOS (Ser-1177) in penes of control rats by ≈2-fold, it did not significantly increase levels of P-eNOS (Ser-1177) in penes of diabetic rats (Fig. 3 c and d). ES significantly (P < 0.05) increased P-Akt in penes of control rats, and it produced a similar, although not significant, increase in diabetic rat penes (Fig. 3 a and b).
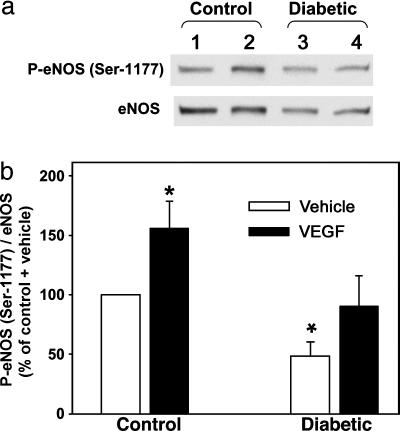
After rhVEGF165 administration, P-eNOS (Ser-1177) levels were significantly (P < 0.05) increased by ≈60% above basal levels in penes of control rats (Fig. 5). rhVEGF165 administration did not significantly affect measurements of P-eNOS (Ser-1177) in diabetic rat penes from basal levels (Fig. 5).
Fig. 5.
eNOS (Ser-1177) phosphorylation by VEGF is impaired in the diabetic penis. rhVEGF165 (0.5 μg) or vehicle was injected into the corpus cavernosum 25 min before penes collection at baseline. (a) Representative Western immunoblot of P-eNOS (Ser-1177) and total eNOS. (b) Quantitative densitometry of P-eNOS (Ser-1177) in penes of control and diabetic rats treated with vehicle (lanes 1 and 3, respectively) and in penes of control and diabetic rats treated with VEGF (lanes 2 and 4, respectively). Results are expressed as the ratio of P-eNOS (Ser-1177) to total eNOS. Values are normalized to controls; n = 6-7; *, P < 0.05 vs. control-vehicle.
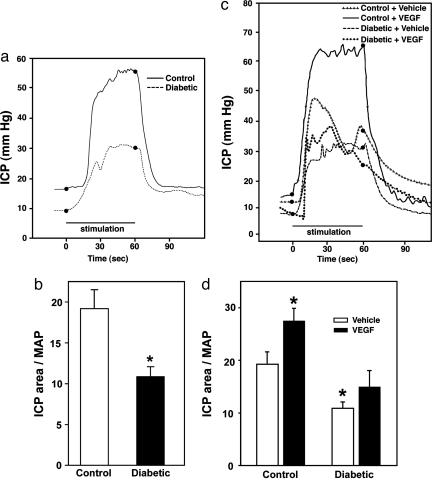
Erectile Response to Shear Stress and VEGF Is Impaired in Diabetic Rats. We assessed the physiologic effects of shear stress stimulation and VEGF administration. In response to shear stress stimuli, ICP area (full erectile status) was decreased by ≈40% (P < 0.05, Fig. 6 a and b), and maximal ICP (magnitude of erectile response) was decreased by ≈30% (P < 0.05) in diabetic (0.42 ± 0.03 units) compared with control (0.59 ± 0.01 units) rats. Furthermore, tumescence rise time was increased by ≈70% (P < 0.05) in diabetic (21.9 ± 2.2 units) compared with control (12.2 ± 2.2 units) rats.
Fig. 6.
Erectile response to shear stress and VEGF is impaired in diabetic rats. (a) Representative ICP responses to 6 V ES. (b) Quantitative analysis of the erectile response to shear stress elicited by ES (6 V, 1 min) in control and diabetic rats. (c) Representative ICP responses to VEGF during 3 V ES. (d) Quantitative analysis of the erectile response to VEGF during submaximal ES (3 V, 1 min) in control and diabetic rats. The stimulus interval is indicated by a solid bar. ICP area, expressed per mean arterial pressure, is defined as the area under the curve, which corresponds to the duration of ES (full erectile status). n = 6; *, P < 0.05 compared with controls.
Erectile response (ICP area) in rhVEGF165-treated control rats was significantly (P < 0.05) increased by ≈45% compared with vehicle-treated control rats. In contrast, erectile response in rhVEGF165-treated diabetic rats was not significantly increased compared with vehicle-treated diabetic rats (Fig. 6 c and d).
Discussion
The results of the present study demonstrate that diabetes-related erectile dysfunction is associated with hyperglycemia-induced increase in O-GlcNAc modification of eNOS and decreased phosphorylation of eNOS at Ser-1177 in the penis, both at baseline and in response to fluid shear stress stimuli and VEGF signaling. This modification of eNOS appears to be specific for Ser-1177 because other examined eNOS phosphorylation sites (Thr-495, Ser-615, and Ser-633) were not affected by hyperglycemia. Whereas increased eNOS phosphorylation (Ser-1177) in the penis increases erectile response, as we have shown (1, 2, 25) and in this study, the converse, that is, decreased phosphorylation of this site in the penis, decreases erectile response. In combination with our physiologic results showing impairment of eNOS-dependent attainment (tumescence rate) and maintenance (magnitude) of full erection response in diabetic rats, these findings further establish that eNOS regulation at Ser-1177 is critically important for penile erection.
The levels of P-Akt, an upstream mediator of eNOS phosphorylation at Ser-1177 (28-30), were markedly decreased in the diabetic compared to the control rat penis at baseline. These results suggest that the diabetes-related deficit in basal eNOS activity may be due to both O-GlcNAc modification of the enzyme and a loss of Akt-mediated phosphorylation of eNOS at Ser-1177. Reduced levels of the phosphorylated, active form of Akt, which is also an antiapoptotic factor, may indicate reduced antiapoptotic capability of the diabetic penis, as previously shown in type 2 diabetes (31). Enhanced apoptotic and decreased antiapoptotic activity has been demonstrated in the corpora cavernosa of diabetic rats (31-34). Shear stress was, however, capable of restoring P-Akt to some extent, although not P-eNOS (Ser-1177), in penes of diabetic rats to similar levels found in penes of nondiabetic rats. Thus, it appears that the reduction of Akt-mediated eNOS phosphorylation at Ser-1177 by shear stress in the diabetic penis is primarily due to O-GlcNAc modification of eNOS.
Several studies have recently described diabetes-related changes in eNOS phosphorylation. In aortas isolated from diabetic animals and carotid plaques from diabetic patients, O-GlcNAc modification of eNOS was increased and eNOS (Ser-1177) phosphorylation was decreased (19, 20). Here, we show that P-eNOS (Ser-1177) in penes of diabetic rats is reduced because of O-GlcNAc modification of the enzyme at the basal state, and it is not influenced by characteristic up-regulatory stimuli such as shear stress and VEGF, which target this residue. Thus, our in vivo penile erection paradigm advances the idea that O-GlcNAc modification of eNOS is a physiologically relevant factor in vascular disorders associated with diabetes.
VEGF is known to have important trophic and tonic roles in the penile vasculature and on the erectile response. We previously found that the erectogenic action of VEGF involves constitutive activation of eNOS by its phosphorylation at Ser-1177, which may not involve the mediatory role of P-Akt (2). In the present study we show that, although VEGF increased erectile response and penile P-eNOS (Ser-1177) in control rats, it had no effect on erectile response in diabetic rats. The lack of improvement of erectile function in diabetic rats by VEGF is apparently due to down-regulated eNOS (Ser-1177) phosphorylation in the diabetic penis because of its increased O-GlcNAc modification interfering with VEGF signaling at this site. We contrast our findings with those of Yamanaka et al. (33), who demonstrated that 6 wk of intracavernosal VEGF164 treatment restored erectile function in diabetic rats evidently by inhibition of apoptosis in the penis. This disparity may pertain to a different manner of VEGF treatment in the two different studies. It is possible that long-term VEGF treatment may overcome the residual activity of eNOS in the diabetic penis and have effects on other components of the erectile tissue unrelated to eNOS.
We acknowledge that other mechanisms of decreased NO availability may contribute to decreased NO-mediated erectile function in diabetes. Superoxide anion may directly inactivate NO (35). Advanced glycation end products, formed between high levels of glucose, proteins, and nucleic acids, quench NO and impair extracellular matrix and tissue remodeling (36-38). Hyperglycemia also modulates PKC activity with eventual disordered NO production and abnormalities in blood flow and permeability (39). Reactive nitrogen species, such as peroxynitrite, derived from NO and superoxide anion, oxidize eNOS resulting in the dissociation of eNOS dimers into monomers and eNOS uncoupling, oxidation of cellular proteins and lipids, release of vasoconstrictors, increased apoptosis, and tissue injury (40-42).
In this study, we did not evaluate whether insulin treatment could reverse the effect of O-GlcNAc on eNOS phosphorylation. It is known that insulin has a direct effect on eNOS phosphorylation (43), which could have confounded our results. Further studies are needed to evaluate in vivo insulin effect on eNOS phosphorylation in the diabetic penis. In addition, the role of O-GlcNAc modification of eNOS in the pathogenesis of erectile dysfunction associated with type 2 diabetes mellitus, a predominant form of diabetes, awaits further evaluation.
In conclusion, eNOS function in the penis is impaired in diabetes mellitus by a specific glycosylation mechanism, by which the enzyme is rendered incapable of activation by normal fluid shear stress stimuli and VEGF signaling. Such eNOS impairment may contribute to erectile dysfunction and compromise long-term penile health in diabetes.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grants DK 02568 and DK 067223 (to A.L.B.) and National Kidney Foundation-Maryland Professional Development Award (to B.M.).
Author contributions: B.M. and A.L.B. designed research; B.M., M.F.K., and R.E.B. performed research; B.M. analyzed data; and B.M. and A.L.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ES, electrical stimulation of the cavernous nerve; eNOS, endothelial nitric oxide synthase; ICP, intracavernosal pressure; rhVEGF, recombinant human VEGF.
References
- 1.Hurt, K. J., Musicki, B., Palese, M. A., Crone, J. K., Becker, R. E., Moriarity, J. L., Snyder, S. H. & Burnett, A. L. (2002) Proc. Natl. Acad. Sci. USA 99, 4061-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musicki, B., Palese, M. A., Crone, J. K. & Burnett, A. L. (2004) Biol. Reprod. 70, 282-289. [DOI] [PubMed] [Google Scholar]
- 3.Harris, M. B., Ju, H., Venema, V. J., Liang, H., Zou. R., Michell, B. J., Chen, Z. P., Kemp, B. E. & Venema, R. C. (2001) J. Biol. Chem. 276, 16587-16591. [DOI] [PubMed] [Google Scholar]
- 4.Michell, B. J., Chen, Z., Tiganis, T., Stapleton, D., Katsis, F., Power, D. A., Sim, A. T. & Kemp, B. E. (2001) J. Biol. Chem. 276, 17625-17628. [DOI] [PubMed] [Google Scholar]
- 5.Fleming, I., Fisslthaler, B., Dimmeler, S., Kemp, B. E. & Busse, R. (2001) Circ. Res. 88, E68-E75. [DOI] [PubMed] [Google Scholar]
- 6.Michell, B. J., Harris, M. B., Chen, Z. P., Ju, H., Venema, V. J., Blackstone, M. A., Huang, W., Venema, R. C. & Kemp, B. E. (2002) J. Biol. Chem. 277, 42344-42351. [DOI] [PubMed] [Google Scholar]
- 7.Boo, Y. C., Hwang, J., Sykes, M., Michell, B. J., Kemp, B. E., Lum, H. & Jo, H. (2002) Am. J. Physiol. 283, H1819-H1828. [DOI] [PubMed] [Google Scholar]
- 8.Kou, R., Greif, D. & Michel, T. (2002) J. Biol. Chem. 277, 29669-29673. [DOI] [PubMed] [Google Scholar]
- 9.Bauer, P. M., Fulton, D., Boo, Y. C., Sorescu, G. P., Kemp, B. E., Jo, H. & Sessa, W. C. (2003) J. Biol. Chem. 278, 14841-14849. [DOI] [PubMed] [Google Scholar]
- 10.Bivalacqua, T. J., Usta, M. F., Champion, H. C., Kadowitz, P. J. & Hellstrom, J. G. (2003) J. Androl. 24, Suppl. 6, S17-S37. [DOI] [PubMed] [Google Scholar]
- 11.Akingba, A. G. & Burnett, A. L. (2001) Mol. Urol. 5, 189-197. [DOI] [PubMed] [Google Scholar]
- 12.Escrig, A., Marin, R., Abreu, P., Gonzalez-Mora, J. L. & Mas, M. (2002) Biol. Reprod. 66, 185-189. [DOI] [PubMed] [Google Scholar]
- 13.Bivalacqua, T. J., Champion, H. C., Usta, M. F., Cellek, S., Chitaley, K., Webb, R. C., Lewis, R. L., Mills, T. M., Hellstrom, W. J. & Kadowitz, P. J. (2004) Proc. Natl. Acad. Sci. USA 101, 9121-9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bivalacqua, T. J., Usta, M. F., Champion, H. C., Adams, D., Namara, D. B., Abdel-Mageed, A. B., Kadowitz, P. J. & Hellstrom, W. J. (2003) J. Urol. 169, 1911-1917. [DOI] [PubMed] [Google Scholar]
- 15.Thompson, C. S., Mumtaz, F. H., Khan, M. A., Wallis, R. M., Mikhailidis, D. P., Morgan, R. J., Angelini, G. D. & Jeremy, J. Y. (2001) Eur. J. Pharmacol. 425, 57-64. [DOI] [PubMed] [Google Scholar]
- 16.Bivalacqua, T. J., Usta, M. F., Champion, H. C., Leungwattanakij, S., Dabisch, P. A., McNamara, D. B., Kadowitz, P. J. & Hellstrom, W. J. (2004) Int. J. Impot. Res. 16, 21-29. [DOI] [PubMed] [Google Scholar]
- 17.Chang, S., Hypolite, J. A., Velez, M., Changolkar, A., Wein, A. J., Chacko, S. & DiSanto, M. E. (2004) Am. J. Physiol. 287, R950-R960. [DOI] [PubMed] [Google Scholar]
- 18.Chang, S., Hypolite, J. A., Changolkar, A., Wein, A. J., Chacko, S. & DiSanto, M. E. (2003) Int. J. Impot. Res. 15, 53-62. [DOI] [PubMed] [Google Scholar]
- 19.Du, X. L., Edelstein, D., Dimmeler, S., Ju, Q., Sui, C. & Brownlee, M. (2001) J. Clin. Invest. 108, 1341-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Federici, M., Menghini, R., Mauriello, A., Hribal, M. L., Ferrelli, F., Lauro, D., Sbraccia, P., Spagnoli, L. G., Sesti, G. & Lauro, R. (2002) Circulation 106, 466-472. [DOI] [PubMed] [Google Scholar]
- 21.Wells, L., Vosseller, K. & Hart, G. W. (2001) Science 291, 2376-2378. [DOI] [PubMed] [Google Scholar]
- 22.Julenius, K., Mølgaard, A., Gupta, R. & Brunak, S. (2005) Glycobiology 15, 153-164. [DOI] [PubMed] [Google Scholar]
- 23.Szkudelski, T. (2001) Physiol. Res. 50, 536-546. [PubMed] [Google Scholar]
- 24.Burnett, A. L., Lowenstein, C. J., Bredt, D. S., Chang, T. S. & Snyder, S. H. (1992) Science 257, 401-403. [DOI] [PubMed] [Google Scholar]
- 25.Musicki, B., Kramer, M. F., Becker, R. E. & Burnett, A. L. (2005) J. Sex. Med. 2, 347-357. [DOI] [PubMed] [Google Scholar]
- 26.Brouet, A., Sonveaux, P., Dessy, C., Balligand, J. L. & Feron, O. (2001) J. Biol. Chem. 276, 32663-32669. [DOI] [PubMed] [Google Scholar]
- 27.Burke, P. A., Lehmannbruinsma, K. & Powell, J. S. (1995) Biochem. Biophys. Res. Commun. 207, 348-354. [DOI] [PubMed] [Google Scholar]
- 28.Dimmeler, S., Fleming, I., Fisslthaler, B., Hermann, C., Busse, R. & Zeiher, A. M. (1999) Nature 399, 601-605. [DOI] [PubMed] [Google Scholar]
- 29.Michell, B. J., Griffiths, J. E., Mitchelhill, K. I., Rodriguez-Crespo, I., Tiganis, T., Bozinovski, S., de Montellano, P. R., Kemp, B. E. & Pearson, R. B. (1999) Curr. Biol. 9, 845-848. [DOI] [PubMed] [Google Scholar]
- 30.Fulton, D., Gratton, J. P., McCabe, T. J., Fontana, J., Fujio, Y., Walsh, K., Franke, T. F., Papapetropoulos, A. & Sessa, W. C. (1999) Nature 399, 597-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jesmin, S., Sakuma, I., Salah-Eldin, A., Nonomura, K., Hattori, Y. & Kitabatake, A. (2003) Mol. Endocrinol. 31, 401-418. [DOI] [PubMed] [Google Scholar]
- 32.Yamanaka, M., Shirai, M., Shiina, H., Tanaka, Y., Tsujimura, A., Matsumiya, K., Okuyama, A. & Dahiya, R. (2003) J. Urol. 170, 291-297. [DOI] [PubMed] [Google Scholar]
- 33.Yamanaka, M., Shirai, M., Shiina, H., Tanaka, Y., Enokida, H., Tsujimura, A., Matsumiya, K., Okuyama, A. & Dahiya, R. (2005) J. Urol. 173, 318-323. [DOI] [PubMed] [Google Scholar]
- 34.Podlasek, C. A., Zelner, D. J., Harris, J. D., Meroz, C. L., Tang, Y., McKenna, K. E. & McVary, K. T. (2003) Biol. Reprod. 69, 816-827. [DOI] [PubMed] [Google Scholar]
- 35.Katusic, Z. S. (1996) Free Radical Biol. Med. 20, 443-448. [DOI] [PubMed] [Google Scholar]
- 36.Seftel, A. D., Vaziri, N. D., Ni, Z., Razmjouei, K., Fogarty, J., Hampel, N., Polak, J., Wang, R. Z., Ferguson, K., Block, C., et al. (1997) Urology 50, 1016-1026. [DOI] [PubMed] [Google Scholar]
- 37.Usta, M. F., Bivalacqua, T. J., Yang, D. Y., Ramanitharan, A., Sell, D. R., Viswanathan, A., Monnier, V. M. & Hellstrom, W. J. (2003) J. Urol. 170, 1437-1442. [DOI] [PubMed] [Google Scholar]
- 38.Cartledge, J. J., Eardley, I. & Morrison, J. F. B. (2001) BJU Int. 87, 402-407. [DOI] [PubMed] [Google Scholar]
- 39.Ganz, M. B. & Seftel, A. (2000) Am. J. Physiol. 278, E146-E152. [DOI] [PubMed] [Google Scholar]
- 40.Ryu, J. K., Kim, D. J., Lee, T., Kang, Y. S., Yoon, S. M. & Suh, J. K. (2003) Yonsei Med. J. 44, 236-241. [DOI] [PubMed] [Google Scholar]
- 41.De Young, L., Yu, D., Bateman, R. M. & Brock, G. B. (2004) J. Androl. 25, 830-836. [DOI] [PubMed] [Google Scholar]
- 42.Zou, M. H., Cohen, R. & Ullrich, V. (2004) Endothelium 11, 89-97. [DOI] [PubMed] [Google Scholar]
- 43.Montagnani, M., Chen, H., Barr, V. A. & Quon, M. J. (2001) J. Biol. Chem. 276, 30392-30398. [DOI] [PubMed] [Google Scholar]