Abstract
Whatever the cause, it is extraordinary that dozens of genera of large mammals became extinct during the late Quaternary throughout the Western Hemisphere, including 90% of the genera of the xenarthran suborder Phyllophaga (sloths). Radiocarbon dates directly on dung, bones, or other tissue of extinct sloths place their “last appearance” datum at ≈11,000 radiocarbon years before present (yr BP) or slightly less in North America, ≈10,500 yr BP in South America, and ≈4,400 yr BP on West Indian islands. This asynchronous situation is not compatible with glacial–interglacial climate change forcing these extinctions, especially given the great elevational, latitudinal, and longitudinal variation of the sloth-bearing continental sites. Instead, the chronology of last appearance of extinct sloths, whether on continents or islands, more closely tracks the first arrival of people.
Despite the wide distribution that sloths (Phyllophaga, Xenarthra) (1) once enjoyed in the New World, almost all of them are now extinct. At the generic level, sloths have the lowest “near-time survival index” of any extant mammalian order or suborder of equivalent size (extant genera/late Quaternary extinct genera = 2/24 = 8.3%). In the late Pleistocene, at least 19 genera in the families Nothrotheriidae, Mylodontidae, Megatheriidae, and Megalonychidae lived on the American continents (2). Most of these species were large to very large [up to at least 3,000 kg for the largest species, Megatherium americanum (3)], and adults of all extinct continental genera were facultatively “ground” sloths rather than arboreal. The five Quaternary genera of megalonychid sloths that lived in the West Indies ranged in size from the Cuban graviportal Megalocnus rodens (at ≈200 kg, the largest Greater Antillean land mammal) to the smallest known sloth, the tree-dwelling Neocnus toupiti (≈4 kg) from Hispaniola (4, 5). The only surviving sloths are Choloepus (two extant species) and Bradypus (three extant species), which are small (4–8 kg) arboreal inhabitants of tropical forests (6, 7).
Whether on islands or continents, the extinction of Quaternary sloths took place within the effective range (<50,000 years) of radiocarbon (14C) dating. If anthropogenic effects, such as predation, were the most important factor contributing to their extinction, as some would argue (8–12), then “last appearance dates” (LADs) for ground sloths should coincide with human arrival. Alternatively, if climate change was responsible for these losses, we would expect youngest 14C dates to fall within the last glacial–interglacial transition, which took place from 15,000 to 9,000 years ago. On the American continents, the cause–effect situation is obscured by the coincidence of the times of human arrival and large-scale climate change (13). In this context, the West Indian situation becomes a clear-cut case: according to the archeological record and other proxy indicators, people did not colonize these islands until the mid-Holocene (14–16), long after any likely climatic effect produced by the glacial–interglacial transition. If climatic forcing were involved in sloth extinctions, as is inferred by some authors (e.g., ref. 17), then both insular and continental sloths would be expected to have disappeared concurrently. If human colonization were crucial, the extinctions would be expected to have accompanied the first arrival of people, initially on the mainland, and later on Caribbean islands.
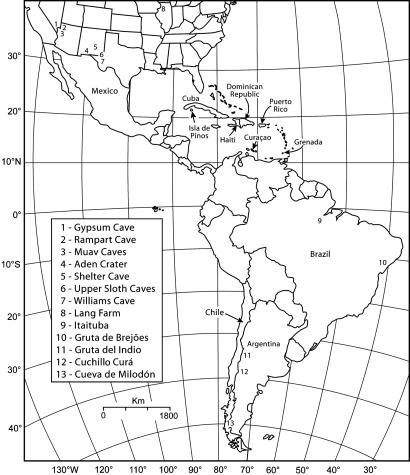
Here, we compare the 14C chronology based on sloth bones (purified collagen) from sites in the West Indies (Cuba and Hispaniola) with that from well-preserved sloth dung (also highly reliable for 14C dates) from sites in arid parts of North and South America (Fig. 1). No fossil sloth dung has been recognized in the West Indies, where the climate generally is too humid to preserve perishable organic material. We summarize existing 14C dates on sloth bones or dung for the continental Americas, and compare these to a series of previously unreported accelerator-mass spectrometer (AMS) 14C dates on bones of West Indian species. Dates given as “yr BP” are radiocarbon ages, corrected for 13C/12C as indicated. Dates given as “cal BP” are calibrated to calendar ages by using the program oxcal 3.9 (18).
Fig. 1.
The Americas, showing continental fossil sites mentioned in the text.
Last Appearance Radiocarbon Dates on Extinct Continental Sloths
LADs and true extinction dates are not necessarily the same thing, even though the former is used as a proxy for the latter. Much depends on the number and reliability of the radiometric determinations being reviewed. A thin record that inadequately covers a taxon's geographic range may miss pockets of late survival, resulting in an erroneous assessment of the time of extinction (e.g., mid-Holocene survival of giant Irish deer Megaloceros in southern Urals) (19). Even more problematic are apparently anomalous dates that lie far outside the range of previously accepted LADs. Such dates should be regarded with skepticism (20). Reanalysis of the material originally submitted for dating may help to identify and reject false dates if contamination or laboratory procedures were the source of error. Until that is done, the significance of an anomalous date cannot be properly assessed. We identify several such dates (see Supporting Text, which is published as supporting information on the PNAS web site) whose validity has not yet been demonstrated by means of replication or other critical evaluation.
North America. A number of localities in the southwestern United States, ranging in elevation (elev.) from 400 m (Muav Caves) to 2,000 m (Upper Sloth Caves), have yielded closely concordant LADs for the extinct sloth Nothrotheriops shastense (Table 1). Particularly informative is the large deposit of N. shastense dung in Rampart Cave (36°06′N, 113°56′W, elev. 535 m), which lies 200 m above the Colorado River on the southern wall of the Grand Canyon in Mojave County, AZ (8, 21–23). The 14C dates on surface sloth dung range from 10,400 ± 275 to 11,480 ± 200 radiocarbon years before present (yr BP). The oldest date is >40,000 yr BP at a depth of 130 cm.
Table 1. Radiocarbon dates (yr BP and lab numbers) on dung of Shasta ground sloth (Nothrotheriops shastense) from North America.
| Gypsum Cave, NV (610 m) | Rampart Cave, AZ (535 m) | Muav Caves, AZ (400 m) | Shelter Cave, NM (1475 m) | Aden Crater, NM (1200 m) | Upper Sloth Caves, TX (2000 m) | Williams Cave, TX (1,500 m) |
|---|---|---|---|---|---|---|
| 11,360 ± 260 A-1202 | 10,400 ± 275 I-442 | 11,140 ± 160 A-1212 | 11,330 ± 370 A-1878 | 11,080 ± 200 Y-1163B | 10,750 ± 140 A-1583 | 11,140 ± 320 A-1589 |
| 11,690 ± 250 LJ-452 | 10,780 ± 200 A-1067 | 11,290 ± 170 A-1213 | 12,330 ± 190 A-1879 | 10,780 ± 140 A-1534 | 11,930 ± 170 A-1588 | |
| 10,940 ± 60 CAMS-19997 | 12,430 ± 250 A-1880 | 11,060 ± 180 A-1584 | 12,100 ± 210 A-1563 | |||
| 11,000 ± 140 A-1066 | 11,590 ± 230 A-1519 | |||||
| 11,020 ± 200 A-1068 | ||||||
| 11,140 ± 250 A-1453 | ||||||
| 11,370 ± 300 A-1392 | ||||||
| 11,480 ± 200 A-1041 | ||||||
| 12,050 ± 400 L-437C | ||||||
| 12,440 ± 300 A-1070 |
Elevations are given for each site. For Rampart Cave, only the 10 youngest dates are given. Data are compiled from refs. 8, 22, 55, 58, 65, and 66, where additional details can be found. A, University of Arizona Radiocarbon Laboratory; CAMS, Center for Accelerator Mass Spectrometry, Lawrence Livermore National Laboratory; I, Isotopes, Inc.; L, Lamont Geological Observatory; LJ, La Jolla Radiocarbon Laboratory; Y, Yale Radiocarbon Laboratory.
Of the 25 14C dates on dung of N. shastense in Table 1 with means <13,000 yr BP, only four overlap 10,500 yr BP at their 2σ low range. All but one of these dates is >11,000 yr BP at its 2σ high range. If vegetational changes associated with glacial–interglacial warming drove the sloth extinction, we would expect the montane populations in woodlands to have lasted longer than those in lowland deserts as the elevational limit of various plants increased. However, we detect no elevational trend in the LADs from North American sites.
Among the three other late Quaternary North American megafaunal sloths (Megalonyx jeffersonii, Paramylodon harlani, and Eremotherium laurillardi), only Megalonyx has been 14C dated directly, with four very similar determinations on bone collagen from Lang Farm, IL (41°33′N, 89°40′W, elev. 198 m) that range from 11,430 ± 60 [Center for Accelerator Mass Spectrometry, Lawrence Livermore National Laboratory (CAMS)-33974] to 11,710 ± 80 (CAMS-13033) yr BP (24).
South America. The South American dung-bearing sites (Table 2) vary in location from Gruta de Brejões (≈11°S, ≈41°W, elev. ≈600 m) in the tropical, arid caatinga vegetation of Bahia, Brazil (25) to Cueva del Milodón (51°35′S, 72°38′W, elev. 200 m) in the cool temperate Nothofagus forest of southern Chile (26, 27). Gruta del Indio (34°45′S, 68°22′W, elev. 660 m), Mendoza Province, Argentina, is a basalt rock-shelter surrounded by dryland shrubs resembling in their structure, height, density, and spacing those found near Rampart Cave in the Mojave Desert. Like Rampart Cave, Gruta del Indio harbors middens made by a plant-hoarding rodent, perhaps the vizchaca (Lagidium sp.; Chinchillidae). Unlike Rampart Cave, Gruta del Indio is also an archaeological site, with charcoal as old as 10,530 ± 140 yr BP deposited immediately above the strata with the youngest dung balls (9, 28). No charcoal was found with or beneath the sloth dung. Gruta del Indio has yielded few remains of sloths other than dung. A single megatheriid tooth, and some dermal ossicles embedded in patches of hide (diagnostic of a mylodontid), suggest that two species of sloths had been present.
Table 2. Radiocarbon dates (yr BP and lab numbers) on sloth dung from South America.
| Nothrotherium maquinense Gruta de Brejões, Brazil (600 m) | Sloth, species uncertain Gruta del Indio, Argentina (660 m) | Sloth, species uncertain Cuchillo Curá, Argentina (1050 m) | Mylodon darwinii Cueva del Milodón, Chile (200 m) |
|---|---|---|---|
| 12,200 ± 120 NZA-6984 | 10,200 ± 300 A-1636 | 14,665 ± 150 Ua-13871 | 10,200 ± 400 SA-49 |
| 10,285 ± 240 A-9494 | 10,400 ± 330* A-1391 | ||
| 10,610 ± 210 A-1351 | 10,575 ± 400 GX-6248 | ||
| 10,900 ± 185 A-9493 | 10,832 ± 400 C-484 | ||
| 10,950 ± 60 GrN-5558 | 10,880 ± 300 GX-6243 | ||
| 11,040 ± 130 A-9570 | 11,775 ± 480 GX-6246 | ||
| 11,820 ± 180 A-1371 | 11,810 ± 299 BM-1210 | ||
| 12,375 ± 115 A-9571 | 11,905 ± 335 GX-6247 | ||
| 24,140 ± 510 LP-1075 | 12,020 ± 460 GX-6244 | ||
| 24,730 ± 860 A-9571 | 12,270 ± 350 A-2445 |
For Gruta del Indio and Cueva del Milodón, only the 10 youngest dates are given. Data are compiled from refs. 9, 22, 25–29, where additional details can be found. A, University of Arizona Radiocarbon Laboratory; BM, British Museum Radiocarbon Laboratory; GrN, Groningen Radiocarbon Laboratory; GX, Geochronology Laboratories, Inc.; LP, Tritium and Radiocarbon Laboratory, Universidad de la Plata; NZA, Rafter Radiocarbon Laboratory; SA, Gif sur Yvette, Saclay; Ua, Uppsala Accelerator.
Body tissue rather than dung
Cuchillo Curá (38°36'S, 70°18′W, elev. 1050 m) is a limestone cave at the foot of the Andes in Neuquén Province, Argentina. An AMS 14C date on the dung of an unknown species of sloth, preserved in a late glacial vizcacha midden, is 14,665 ± 150 yr BP (29). Cueva del Milodón, a large cave in quartz conglomerate bedrock in southern Chile (26, 27), has yielded six 14C dates on dung of Mylodon darwinii that are <11,000 yr BP at their 2σ low range; three of these 14C dates are <10,000 yr BP at their 2σ low range. Two of these three dates are not older than 11,100 yr BP at their 2σ high range, just as at Gruta del Indio.
The other extinct South American sloth with a direct age determination is Eremotherium laurillardi from the Itaituba Quarry alluvial site on the lower Rio Tapajos, Pará State, Brazil, a humid tropical lowland setting (30). The single AMS 14C date available for purified collagen of E. laurillardi bone is 11,340 ± 50 yr BP.
To summarize the South American chronology, the youngest reliable dates on dung or tissue of extinct sloths have means of ≈10,600 to 10,200 yr BP (Table 2). They are slightly younger than the surface dates on Shasta sloth dung from North America. Based on this chronology, if the first Americans triggered sloth extinctions, then these people must have spread rapidly from North America through South America. Some archaeologists (e.g., ref. 31) question whether the archaeological evidence supports such a rapid human colonization and believe that both American continents sustained people earlier than 11,000 yr BP. We are at a loss to explain why these hypothesized earlier Americans would be so cryptic archaeologically, especially in well-explored North America.
Last Appearance Radiocarbon Dates on Extinct West Indian Sloths
The late Quaternary sloths of the West Indies feature five endemic genera and at least 13 species (5), with five species in Cuba and Isla de Pinos (Acratocnus antillensis, Megalocnus rodens, Neocnus gliriformis, N. major, and Parocnus browni), six in Hispaniola and its nearby small islands of Gonāve and Ile de la Tortue (Acratocnus ye, Megalocnus zile, Neocnus comes, N. dousman, N. toupiti, and Parocnus serus), one in Puerto Rico (Acratocnus odontrigonus), and one in Curaçao (Paulocnus petrifactus). In the absence of 14C dates it is not certain that Paulocnus persisted into the late Quaternary (see Supporting Text), although we regard this hypothesis as more probable than not. Associated K/Ar dates for the unnamed Grenadian sloth place this taxon in the late Pliocene (32); in the absence of late Quaternary dates, there is no basis for including it in this survey.
Before the radiometric chronology of West Indian fossil sites began to be determined, it often was assumed that humans and sloths overlapped in time because some cave localities had evidence of human occupation in addition to the remains of the extinct fauna (33, 34). As is often the case on tropical islands (35, 36), these assumed stratigraphic associations have not been, and probably cannot be, verified because of inadequate field notes and subsequent damage to the sites. Nevertheless, the larger question of the timing and significance of the West Indian losses in relation to the extinction chronology on the American continents now can be reexamined, thanks to a previously unreported series of AMS 14C dates for sloth bones from Cuba and Hispaniola.
Cuba. Sloths survived into the Holocene in Cuba, as evidenced by an AMS 14C date of 6,250 ± 50 yr (Table 3) on a humerus of the largest West Indian sloth, Megalocnus rodens, from a limestone cave (Cueva Beruvides) in Matanzas Province (37). An even younger date (4,960 ± 280 yr BP) is available for a smaller species, Parocnus brownii, from Las Breas de San Felipe, a small tar pit in Matanzas Province (38, 39). The associated fauna at Cueva Beruvides has not yet been published by its discoverers but is known to include several species of sloths as well as endemic rodents and insectivores. Las Breas de San Felipe has yielded a great variety of fossils in addition to vertebrates, including plants, insects, decapods, and molluscs. The oldest reliable 14C date for a cultural site on Cuba is 5,270 ± 20 yr BP (39).
Table 3. AMS radiocarbon dates on sloth bones from Cuba.
| 14C Lab-no. | Museum catalog no. or collector | Species | Locality and elevation | Skeletal element | d13C | 14C age, yr BP | cal BP |
|---|---|---|---|---|---|---|---|
| AA-35290 | MNHNCu V626 | Parocnus brownii | Las Breas de San Felipe (15–20 m) | Proximal humerus | –26.9 | 4,960 ± 280 | 6,350–4,950 |
| AA-35291 | MNHNCu V627 | P. brownii | Las Breas de San Felipe (15–20 m) | Distal humerus | nd | 10,520 ± 440 | 13,450–11,050 |
| AA-35292 | MNHNCu V649 | P. brownii | Las Breas de San Felipe (15–20 m) | Proximal humerus | –27.9 | 11,880 ± 420 | 15,450–12,950 |
| B-115697 | E. Abreu | Megalocnus rodens | Cueva Beruvides (25–50 m) | Humerus | –19.6 | 6,250 ± 50 | 7,270–6,010 |
AA-no. is the sample number for the University of Arizona Acceleration Radiocarbon Laboratory. B-no. is the sample number for Beta Analytic Inc. cal BP dates are reported at 95% confidence. MNHNCu, Museo Nacional de Historia Natural (Cuba); nd, no data (d13C correction made using –25). Binomial nomenclature follows refs. 5 and 32. Information is from refs. 35, 37, and 39.
Hispaniola. Although several Holocene “whole bone” 14C dates have been reported for Haitian cave sites (40–42), these dates were not necessarily tied to individual taxa. For sloths, this task would have been difficult in do in any event, because Hispaniolan sloths were not revised systematically until recently (5). Nevertheless, a single “whole-bone” date (8,120 ± 216 yr BP) on an assortment of sloth bones (42) provided the first evidence that phyllophagans had persisted into the Holocene on that island.
Here, we report nine previously undescribed AMS 14C dates (Table 4) on sloth bones identified to species, all from limestone caves and sinkholes on the Haitian side of Hispaniola. Three of the seven sites are in the Massif de la Selle in southeastern Haiti; another three are on the Plain Formon of the Tiburon Peninsula (41). The seventh site, Trouing Gallery, is on Ile de la Tortue, 7.5 km off Haiti's northern coast. As in Cuba, none of the dated Haitian sloth bones is associated with cultural features or artifacts.
Table 4. New AMS radiocarbon dates on sloth bones from Haiti.
| AA no. | UF no. | Species | Locality and elevation | Skeletal element | d13C | 14C age (yr BP) | cal BP |
|---|---|---|---|---|---|---|---|
| 58439 | 170123 | Neocnus comes | Trouing Ismays (970 m) | Right humerus, left ulna | –26.83 | 4,391 ± 42 | 5,260–5,180, 5,060–4,840 |
| 58430 | 170210 | N. comes | Trouing Deron 1 (1,000 m) | Right pelvis | –24.39 | 4,486 ± 39 | 5,300–5,030, 5,020–4,970 |
| 58435 | 170429 | N. comes | Trouing Attie (1,770 m) | Right and left tibia | –25.32 | 6,161 ± 45 | 7,210–6,900 |
| 58432 | 170308 | N. comes | Trouing Jeremie 5 (1,275 m) | Left tibia | –23.918 | 6,875 ± 47 | 7,790–7,610 |
| 58433 | 170311 | N. comes | Trouing Jeremie 5 (1,275 m) | Right tibia | –24.55 | 7,411 ± 51 | 8,350–8,150, 8,140–8,110, 8,090–8,040 |
| 58431 | 170046 | N. comes | Trouing Jeremie 5 (1,275 m) | Right radius, right ulna | –24.16 | 8,326 ± 57 | 9,490–9,240, 9,220–9,190, 9,180–9,130 |
| 58434 | 75469 | N. dousman | Trouing Marassa/Trujin Bridge (1,875 m) | Right tibia | –24.268 | 9,897 ± 65 | 11,560–11,170 |
| 58436 | 169942 | Parocnus serus | Trouing Gallery (40 m) | Right femur | –23.32 | >14,200 | — |
| 58438 | 170195 | N. comes | Trouing de la Scierie (1,815 m) | Left tibia | –22.40 | 27,720 ± 560 | — |
All specimens are from the Haitian mainland except the single bone of Parocnus serus from Ile de la Tortue, 7.5 km north of the Haitian mainland. AA no. is the sample number for the University of Arizona Acceleration Radiocarbon Laboratory. UF no. is the catalog number in the Vertebrate Paleontology Collection, Florida Museum of Natural History. cal BP dates are reported at 95% confidence. Binomial nomenclature follows refs. 5 and 32.
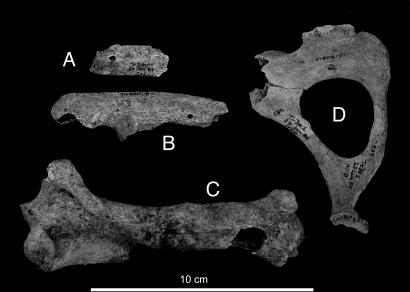
Seven of the nine AMS 14C dates are for bones of the ubiquitous small sloth Neocnus comes (Fig. 2). The two youngest dates (≈4,500 yr BP = ≈5,000 cal BP) lie within the period of demonstrated human presence on Hispaniola (see Discussion and Conclusions). Four of the remaining five AMS 14C dates on N. comes also lie within the Holocene, which reinforces the evidence that at least one species of Hispaniolan sloth survived the glacial–interglacial transition. These localities also yielded remains of other species of extinct mammals, including the endemic Hispaniolan pitheciid platyrrhine monkey Antillothrix bernensis, which may have survived even later (42).
Fig. 2.
AMS 14C-dated bones of Neocnus comes, an extinct sloth from Haiti. (A–C) Associated left distal (A) and proximal (B) ulna and right humerus (C), UF 170123, dated to 4,391 ± 42 yr BP. (D) Right pelvis, UF 170210, dated to 4,486 ± 39 yr BP.
Puerto Rico. No bones of Acratocnus odontrigonus, Puerto Rico's only species of sloth, have been successfully 14C dated, apparently because available samples have suffered total diagenetic loss of collagen (R.D.E.M,, unpublished observations). Charcoal from a dated sediment sequence in Laguna Tortuguero indicates a major shift in the Puerto Rican fire regime at ≈5,000 yr BP, which may indicate the arrival of people (14). If organically well-preserved bones of A. odontrigonus can be found, we suspect that at least some of the resulting 14C dates would fall within the Holocene.
Discussion and Conclusions
When examined critically, the youngest sloth dung deposits in South America are only centuries later than the youngest from North America. Although younger age estimates appear from time to time (see Supporting Text), no remains of megafaunal sloths or any other large, extinct mammal in either North or South America have been reliably dated to within the last 10,000 14C years (≈11,600 cal BP). Sloth remains are absent from abundant Holocene fossil deposits on both continents. The entire pattern is in step with conventional views of human colonization in the Americas, with Clovis arrival in western North America no more than 1,000 yr before people arrived in South America (43).
In Cuba and Haiti, conversely, eight of the 13 AMS 14C dates are Holocene rather than Pleistocene, with two of them <5,000 yr BP. The early (>2,000 yr BP) archaeology of these islands has not been studied intensively, although several sites have produced lithic artifacts (especially large chert blades) in probable association with 14C dates as old as ≈5,500 yr BP (15, 16, 44–46). Sloth bones have not been found with certainty in any West Indian archaeological locality. Nevertheless, as on the continents, the extinction of West Indian sloths followed the peopling of these islands, which took place in the Holocene rather than the Pleistocene.
If the extinction of West Indian sloths was forced by the Upper Dryas or some other late Quaternary cold stage, it is hard to understand how final loss could have been delayed into the mid-Holocene. That insular sloths persisted 5,000–6,000 yr longer than their continental relatives agrees with the global pattern of late Quaternary vertebrate extinction following human dispersal, whether on continents or islands (12, 47, 48). Deferred extinction of insular sloths resembles the situation with mammoths (Mammuthus), where isolated populations survived on Wrangel Island (Chukchi Sea, northeastern Asia) and St. Paul Island (Bering Sea) into the early to mid-Holocene, which is several millennia later than on the Eurasian or American continents (49, 50).
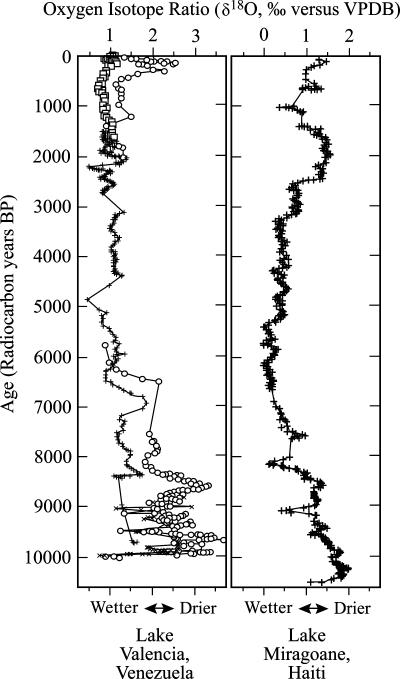
By themselves, the AMS 14C dates do not demonstrate that people caused the extinction of N. comes. In yielding 14C ages ranging from ≈27,000 to 4,500 yr BP, however, the seven dated bones of Neocnus comes do show that this sloth survived the glacial-interglacial (Pleistocene–Holocene) transition when the West Indian climate in general and the Hispaniolan climate in particular became warmer and wetter (≈11,000 to 8,000 yr BP) (51), only to perish in the mid-Holocene, during a period of relatively stable local climate (Fig. 3). There is both archaeological and environmental evidence of considerable mid-Holocene human activity in the West Indies, including burning (14, 52, 53). From sediments in Lake Miragoāne in Haiti, for example, charcoal influx increases dramatically and pollen of forest taxa (such as Palmae and Podocarpus) decline to near absence at ≈5,730 yr BP, accompanied by a major increase in pollen of Ambrosia, a disturbance indicator (52).
Fig. 3.
Oxygen isotopic composition of carbonate shell material (ostracods or gastropods) for Lake Valencia (Venezuela) and Lake Miragoāne (Haiti). Image was redrawn from ref. 51.
All extinct continental species of Quaternary sloths were exclusively ground-dwelling, but those of the West Indies were more varied. Morphometric analyses suggest that the largest (Megalocnus, Parocnus) were entirely terrestrial or nearly so, whereas the small ones such as Neocnus comes had skeletal features consistent with arboreality (4). It is easy to conceive unsustainable levels of direct predation by skilled human hunters on any species of ground-dwelling sloth, which moved slowly and had not been exposed previously to human predators, whether on islands or continents. The survival of small tree-dwelling sloths on continents is probably because they are so cryptic (live high in trees, are silent, relatively immobile, and have algally camouflaged fur; see ref. 54). The extent to which N. comes had these characteristics is unknown, although spending time on the ground would be safer for a small sloth on an island lacking placental carnivores (such as any of the West Indies) than on the continents, where felids and canids patrol. The arrival of people may have rendered the small insular sloths more vulnerable than their arboreal counterparts on continents.
Should further work on the chronology of sloth extinction support what has been learned so far, it will be difficult to explain how a change in climate or habitat could have eliminated this durable group from North and South America thousands of years before they became extinct on Caribbean islands. Because all surviving species of sloths and other xenarthrans are tropical or subtropical, we see no reason why the glacial–interglacial global warming that took place at the Pleistocene–Holocene transition should have had a negative effect on sloths or their relatives, whether on islands or continents. The plants identified in the ancient sloth dung in North or South America are dominated by species still found near the fossil sites (refs. 23, 26, 29, and 55–59; see also Supporting Text). If they were alive today, megafaunal sloths could eat the same kind of plants that they ingested at the end of their existence. This finding argues further against a climate-driven change in habitat as causing the extinction of sloths by affecting their food supply.
Many other endemic West Indian mammals became extinct in the late Quaternary along with the sloths, namely 75% of the species of insectivores, 80% of the rodents, and 100% of the primates (39, 59–64). A fruitful topic for new research would be to compare the extinction chronology of insular sloths with that of the other endemic mammals (e.g., 37). With a more intensive program of AMS 14C dating on islands, we predict that more species of West Indian sloths will be found to have existed during the period of human occupation. We also encourage a renewed effort to locate and study early archaeological sites across the West Indies.
Supplementary Material
Acknowledgments
We thank R. C. Hulbert, Jr., and B. J. MacFadden for access to the Florida Museum of Natural History Vertebrate Paleontology collection. This work was supported by National Science Foundation Grants EAR-9714819 (to D.W.S.) and EAR-0115488 (to A.J.T.J.) and the Adler Fund (R.D.E.M.).
Author contributions: D.W.S. and P.S.M. designed research; D.W.S., P.S.M., A.J.T.J., C.A.W., M.I.-V., and G.W.L.H. performed research; A.J.T.J. and H.G.M. analyzed data; and D.W.S., P.S.M., and R.D.E.M. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AMS, accelerator-mass spectrometer; yr BP, radiocarbon years before present; 14C, radiocarbon; LAD, last appearance date; elev., elevation.
References
- 1.McKenna, M. C. & Bell, S. K. (1997) Classification of Mammals Above the Species Level (Columbia Univ. Press, New York).
- 2.Martin, P. S., Twilight of the Mammoth: Extinction and Resurrection of Ice Age Megafauna (Univ. of California Press, Berkeley, CA), in press.
- 3.Christiansen, P. & Fariña, R. A. (2003) Senckenbergiana Biol. 83, 95-101. [Google Scholar]
- 4.White, J. L. (1993) Ph.D. thesis (State Univ. of New York, Stony Brook, NY).
- 5.White, J. L. & MacPhee, R. D. E. (2001) in Biogeography of the West Indies: Patterns and Perspectives, eds. Woods, C. A. & Sergile, F. E. (CRC, Boca Raton, FL), pp. 201-235.
- 6.Silva, M. & Downing, J. A. (1995) CRC Handbook of Mammalian Body Masses (CRC, Boca Raton, FL).
- 7.Eisenberg, J. F. & Redford, K. H. (1999) Mammals of the Neotropics (Univ. of Chicago Press, Chicago), Vol. 3.
- 8.Long, A., Martin, P. S. & Hansen, R. M. (1974) Geol. Soc. Am. Bull. 85, 1843-1848. [Google Scholar]
- 9.Long, A., Martin, P. S. & Lagiglia, H. A. (1998) Radiocarbon 40, 693-700. [Google Scholar]
- 10.Martin, P. S. (1984) in Quaternary Extinctions, eds. Martin, P. S. & Klein, R. G. (Univ. of Arizona Press, Tucson), pp. 354-403.
- 11.Martin, P. S. (1990) Palaeogeog. Paleaoclim. Palaecol. 82, 187-201. [Google Scholar]
- 12.Martin, P. S. & Steadman, D. W. (1999) in Extinctions in Near Time: Causes, Contexts, and Consequences, ed. MacPhee, R. D. E. (Kluwer Academic/Plenum, New York), pp. 17-55.
- 13.Barnosky, A. D., Koch, P. L., Feranec, R. S., Wing, S. L. & Shabel, A. B. (2004) Science 306, 70-75. [DOI] [PubMed] [Google Scholar]
- 14.Burney, D. A., Burney, L. P. & MacPhee, R. D. E. (1994) J. Archaeol. Sci. 21, 273-281. [Google Scholar]
- 15.Keegan, W. F. (1994) J. Archaeol. Res. 2, 255-284. [Google Scholar]
- 16.Wilson, S. M. (2001) in Biogeography of the West Indies: Patterns and Perspectives, eds. Woods, C. A. & F. E. Sergile, F. E. (CRC, Boca Raton, FL), pp. 519-527.
- 17.Graham, R. W., Lundelius, E. L., Jr., Graham, M. A., Schroeder, E. K., Toomey, R. S., III, Anderson, E., Barnosky, A. D., Burns, J. A., Churcher, C. S., Grayson, D. K., et al. (1996) Science 272, 1601-1606. [DOI] [PubMed] [Google Scholar]
- 18.Stuiver, M. P., Reimer, P. J., Bard, E., Beck, J. W., Burr, G. S., Hughen, K. A., Kromer, B., McCormac, G., van der Plicht, J. & Spurk, M. (1998) Radiocarbon 40, 1041-1083. [Google Scholar]
- 19.Stuart, A. J., Kosintsev, P. A., Higham, T. F. G. & Lister, A. M. (2004) Nature 431, 684-689. [DOI] [PubMed] [Google Scholar]
- 20.Araujo, A. G. M., Neves, W. A. & Piló, L. B. (2004) J. Biogeogr. 31, 2039-2040. [Google Scholar]
- 21.Martin, P. S., Sabels, B. E. & Shutler, R., Jr. (1961) Am. J. Sci. 259, 102-127. [Google Scholar]
- 22.Long, A. & Martin, P. S. (1974) Science 186, 638-640. [DOI] [PubMed] [Google Scholar]
- 23.Hansen, R. M. (1978) Paleobiology 4, 302-319. [Google Scholar]
- 24.Schubert, B. W., Graham, R. W., McDonald, H. G., Grimm, E. C. & Stafford, T. W., Jr. (2004) Quaternary Res. 61, 231-240. [Google Scholar]
- 25.Czaplewski, N. J. & Cartelle, C. (1998) J. Mamm. 79, 784-803. [Google Scholar]
- 26.Markgraf, V. (1985) Science 228, 1110-1112. [DOI] [PubMed] [Google Scholar]
- 27.Borrero, L. (1997) Ann. Inst. Patagonia Ser. Cien. Hum. 25, 89-102. [Google Scholar]
- 28.García, A. (2003) Radiocarbon 45, 33-39. [Google Scholar]
- 29.Hofreiter, M., Betancourt, J. L., Sbriller, A. P., Markgraf, V. & McDonald, H. G. (2003) Quaternary Res. 59, 364-378. [Google Scholar]
- 30.Rossetti, D. F., de Toledoa, P. M., Moraes-Santosa, H. M. & de Araújo Santos, A. E., Jr. (2004) Quaternary Res. 61, 289-300. [Google Scholar]
- 31.Meltzer, D. J. (2004) in The Quaternary Period in the United States, eds. Gillespie, A. R., Porter, S. C. & Atwater, B. F. (Elsevier, Amsterdam), pp. 539-563.
- 32.MacPhee, R. D. E., Singer, R. & Diamond, M. (2000) Am. Museum Novitates 3302, 1-20. [Google Scholar]
- 33.Harrington, M. R. (1921) Indian Notes and Monographs (Museum of the American Indian/Heye Foundation, New York).
- 34.Miller, G. S. (1929) Smithsonian Miscellaneous Collections 82, 1-30. [Google Scholar]
- 35.MacPhee, R. D. E. & Marx, P. A. (1997) in Natural Change and Human Impact in Madagascar, eds. Goodman, S. M. & Patterson, B. D. (Smithsonian Inst. Press, Washington, DC), pp. 169-217.
- 36.Steadman, D. W., Extinction and Biogeography of Tropical Pacific Birds (Univ. of Chicago Press, Chicago), in press.
- 37.MacPhee, R. D. E., Flemming, C. & Lunde, D. P. (1999) Am. Museum Novitates 3264, 1-19. [Google Scholar]
- 38.Iturralde-Vinent, M., MacPhee, R. D. E., Díaz-Franco, S., Rojas-Consuegra, R., Suárez, W. & Lomba, A. (2000) Caribbean J. Sci. 36, 300-313. [Google Scholar]
- 39.Jull, A. J. T., Iturralde-Vinent, M., Malley, J. O., MacPhee, R., McDonald, H., Martin, P., Moody, J. & Rincón, A. (2004) Nuclear Instrum. Methods Phys. Res. B 223–224, 668-671. [Google Scholar]
- 40.Morgan, G. S. & Woods, C. A. (1986) Biol. J. Linn. Soc. 28, 167-203. [Google Scholar]
- 41.Woods, C. A. (1989) Sci. Ser. Nat. Hist. Museum Los Angeles County 33, 59-89. [Google Scholar]
- 42.MacPhee, R. D. E., White, J. L. & Woods, C. A. (2000) Am. Museum Novitates 3303, 1-32. [Google Scholar]
- 43.Haynes, C. V., Jr. (1992) in Radiocarbon After Four Decades: An Interdisciplinary Perspective, eds. Taylor, R. E., Long, A. & Kra, R. S. (Springer, New York).
- 44.Moore, C. (1991) Rep. Archeo-Anthropol. Inst. Netherlands Antilles 9, 92-104. [Google Scholar]
- 45.Veloz Maggiolo, M. & Vega, B. (1982) J. New World Archaeol. 5, 33-44. [Google Scholar]
- 46.Rouse, I. (1992) The Tainos: Rise and Decline of the People Who Greeted Columbus (Yale Univ. Press, New Haven, CT).
- 47.Steadman, D. W. (1995) Science 267, 1123-1131. [DOI] [PubMed] [Google Scholar]
- 48.Steadman, D. W. & Martin, P. S. (2003) Earth-Sci. Rev. 61, 133-147. [Google Scholar]
- 49.Arslanov, Kh., Cook, G. T., Gulliksen, S., Harkness, D. D., Kankainen, T., Scott, E. M., Vartanyan, S. & Zaitseva, G. (1998) Radiocarbon 40, 289-294. [Google Scholar]
- 50.Guthrie, R. D. (2004) Nature 429, 746-748. [DOI] [PubMed] [Google Scholar]
- 51.Curtis, J., Brenner, M. & Hodell, D. A. (2001) in Biogeography of the West Indies: Patterns and Perspectives, eds. Woods, C. A. & Sergile, F. E. (CRC, Boca Raton, FL), pp. 35-54.
- 52.Higuera-Gundy, A., Brenner, M., Hodell, D. A., Curtis, J. H., Leyden, B. W. & Binford, M. W. (1999) Quaternary Res. 52, 159-170. [Google Scholar]
- 53.Horn, S. P., Orvis, K. H., Kennedy, L. M. & Clark, M. (2000) Caribbean. J. Sci. 36, 10-18. [Google Scholar]
- 54.Emmons, L. H. (1997) Neotropical Rainforest Mammals (Univ. of Chicago Press, Chicago).
- 55.Laudermilk, J. D. & Munz, P. A. (1938) Carnegie Inst. Wash. Pub. 487, 271-281. [Google Scholar]
- 56.Spaulding, W. G. & Martin, P. S. (1977) Nat. Park Service Proc. Trans. Ser. 4, 259-269. [Google Scholar]
- 57.d'Antoni, H. (1983) in Quaternary of South America and Antarctic Peninsula, ed. Rabassa, G. (A. A. Balkema, Rotterdam), pp. 83-108.
- 58.Phillips, A. M., III (1984) in Quaternary Extinctions, eds. Martin, P. S. & Klein, R. G. (Univ. of Arizona Press, Tucson), pp. 148-158.
- 59.Poinar, H. N., Hofreiter, M., Spaulding, W. G., Martin, P. S., Stankiewicz, B. A., Bland, H., Evershed, R. P., Possnert, G. & Pääbo, S. (1998) Science 281, 402-406. [DOI] [PubMed] [Google Scholar]
- 60.Woods, C. A. (1989) in Biogeography of the West Indies: Past, Present, and Future, ed. Woods, C. A. (Sandhill Crane, Gainesville, FL), pp. 741-798.
- 61.Pregill, G. K., Steadman, D. W. & Watters, D. R. (1994) Bull. Carnegie Museum Nat. Hist. 30, 1-51. [Google Scholar]
- 62.MacPhee, R. D. E. & Flemming, C. (1999) in Extinctions in Near Time: Causes, Contexts, and Consequences, ed. MacPhee, R. D. E. (Kluwer Academic/Plenum, New York), pp. 333-372.
- 63.McFarlane D. A., Vale, A., Christenson, K., Lundberg, J., Atilles, G. & Lauritzen S. E. (2000) Caribbean J. Sci. 36, 163-166. [Google Scholar]
- 64.Woods, C. A., Borroto Paéz, R. & Kilpatrick, C. W. (2001) Biogeography of the West Indies: Patterns and Perspectives, eds. Woods, C. A. & Sergile, F. E. (CRC, Boca Raton, FL), pp. 335-353.
- 65.Mead, J. I., Thompson, R. S. & Long, A. (1978) Radiocarbon 20, 171-191. [Google Scholar]
- 66.Thompson, R. S., Van Devender, T. R., Martin, P. S., Foppe, T. & Long, A. (1980) Quaternary Res. 14, 360-376. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.