Abstract
Age-related macular degeneration (AMD) is a late-onset, multifactorial, neurodegenerative disease of the retina and the leading cause of irreversible vision loss in the elderly in the Western world. We describe here a murine model that combines three known AMD risk factors: advanced age, high fat cholesterol-rich (HF-C) diet, and apolipoprotein E (apoE) genotype. Eyes of aged, targeted replacement mice expressing human apoE2, apoE3, or apoE4 and maintained on a HF-C diet show apoE isoform-dependent pathologies of differential severity. ApoE4 mice are the most severely affected. They develop a constellation of changes that mimic the pathology associated with human AMD. These alterations include diffuse sub-retinal pigment epithelial deposits, drusenoid deposits, thickened Bruch's membrane, and atrophy, hypopigmentation, and hyperpigmentation of the retinal pigment epithelium. In extreme cases, apoE4 mice also develop marked choroidal neovascularization, a hallmark of exudative AMD. Neither age nor HF-C diet alone is sufficient to elicit these changes. We document choroidal neovascularization and other AMD-like ocular pathologies in an animal model that exploits known AMD risk factors. The model is additionally attractive because it is not complicated by invasive experimental intervention. Our findings in this model implicate the human apoE E4 allele as a susceptibility gene for AMD and support the hypothesis that common pathogenic mechanisms may underlie AMD and Alzheimer's disease.
Keywords: amyloid, choroidal neovascularization, macula, retinal pigment epithelium, cholesterol
Age-related macular degeneration (AMD) is the leading cause of irreversible vision loss in people over the age of 65, accounting for the majority of registered blindness in Western Europe and North America (1). A family of disorders, AMD is characterized by progressive loss of central, high-acuity vision due to dysfunction and death of photoreceptors (PRs) in the center of the retina, the macula. AMD pathology also impacts the retinal pigment epithelium (RPE, the cells responsible for support of PRs and maintenance of the choroidal blood-eye barrier), the choriocapillaris (CC, the primary capillary bed of the choroid), and Bruch's membrane (BrM, a stratified extracellular matrix between the RPE and the CC). Early AMD is characterized by moderate vision loss associated with characteristic extracellular lesions that form between the RPE and BrM. These lesions can be focal (drusen) or diffuse (basal deposits) (2-4). Late AMD is subdivided into two forms, dry or wet. Dry (geographic atrophy) is characterized by PR loss causing severe visual impairment concomitant with extensive RPE atrophy, whereas wet (exudative) features the sequela of choroidal neovascularization (CNV, i.e., in growth of the CC through BrM and under the RPE in the plane of drusen and basal deposits) (5).
AMD is a complex disease in which the contributions of many genetic and environmental factors are confounding. The strongest known risk factor for AMD is advanced age, with the risk of developing AMD increasing from ≈2% in persons in their 50s to almost 30% in the population >75 years of age (6). Other identified risk factors for AMD include gender, ethnicity, smoking, hypertension, hypercholesterolemia, and diet (7). Genes implicated as risk factors include fibulin 5 (8), ABCA4 (9), CFH/HF1 (10-13), and apolipoprotein E [apoE (protein); human APOE (gene); human APOE* (allele); murine apoE (gene)] (14). ApoE is an amphipathic glycoprotein that mediates the distribution of lipids and cholesterol among cells and is expressed at highest levels in the brain and liver (15). In the eye, apoE immunoreactivity has been localized to Müller cells, BrM, RPE, basal deposits, and drusen (16-18). ApoE mRNAs are synthesized by Müller cells in the neural retina and by the RPE (17). The human APOE gene is polymorphic, encoding one of three common alleles (APOE*2, APOE*3, and APOE*4), with the APOE*3 allele occurring most frequently (78%) in the Caucasian population (15, 19). The association between APOE and AMD has been addressed in epidemiological studies (14, 16, 20, 21), in animal studies of apoE knockout mice (22), and in APOE3-Leiden mice (23).
To date, association studies have reported a slight protective effect for AMD in APOE*4 carriers and a detrimental effect in APOE*2 carriers (14, 16, 20, 21). This finding is in stark contrast to other major complex diseases such as Alzheimer's disease, atherosclerosis, multiple sclerosis, and stroke, which show a strong positive association of APOE*4 allele with disease (24-26).
Although AMD disease mechanisms are still poorly understood, several pathogenic pathways have been proposed, each associated with manifestations of AMD, including: oxidative damage (27), RPE cell dysfunction with accumulation of lipofuscin and impairment of lysosomal functions (28), and inflammatory processes with complement activation (29). In fact, four separate studies (10-13) have recently reported that a variation in the complement factor H gene (CFH/HF1) significantly increases the risk for AMD.
Cholesterol and its transporter, apoE, are major constituents of the lipid-rich, sub-RPE deposits in AMD eyes and have been implicated in the pathogenesis of other systemic and neurodegenerative pathologies including atherosclerosis and Alzheimer's disease (30). It seems that the combination of a number of factors can lead to the development of AMD. We describe an animal model that tests whether the combination of three established AMD risk factors [advanced age; a high fat cholesterol-rich (HF-C) diet; and APOE genotype, using human apoE-targeted replacement (TR) or “knockin” mice] is sufficient to produce an age-related retinal degeneration with the hallmarks of AMD. The retinal pathologies exhibited by these aged apoE TR mice fed a HF-C diet correlate not only with characteristic pathologic changes found in human AMD eyes, but also with molecular processes identified in other neurodegenerative and systemic diseases in which apoE has a demonstrated role.
Methods
Animals. Mice in this study were maintained and bred in accordance with the Institutional Animal Care and Use Committee at Duke University and the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research. ApoE TR mice expressing one of the three human apoE isoforms under control of the endogenous mouse apoE regulatory sequences were generated as described (31).
Experimental Protocol and Diet Paradigm. Aged male (n = 84; 65-123 weeks), female (n = 40; 75-127 weeks), and young male (n = 28; 12-30 weeks) C57BL/6 apoE TR mice (32) were bred and housed conventionally, under ambient conditions (12 h dark, 12 h light) and fed a high fat diet rich in cholesterol (T.D. 88051; 75% Purina mouse chow no. 5015, 7.5% protein (casein), 2.5% monohydrate dextrose, 1.625% sucrose, 7.5% cocoa butter, 1.25% cholesterol, 0.5% sodium cholate, 1.25% fiber, 0.125% choline chloride; Harlan Teklad, Indianapolis) and water ad libitum for the 8 weeks immediately before analysis. Age-matched control apoE TR littermates were fed standard rodent chow (Isopurina no. 5001; Prolab, Dewitt, NY) and water ad libitum. ApoE TR mice of each isoform (n = 33-50 apoE2, apoE3, and apoE4) at an average age of 80 weeks were switched from normal mouse chow to the HF-C diet for 8 weeks. At the end of the diet exposure, mice were euthanized with an overdose of avertin and flushed with a 20-ml bolus of saline. Blood samples were taken before diet and at the time of euthanasia.
Fluorescein Angiography (FA). FA was performed on conscious mice at two time points, 4 and 8 weeks on diet, and recorded by using a Kowa RC-2 camera with an external 66-diopter condensing lens mounted between the camera and the eye, as described by Hawes et al. (33). The pupil was dilated with 1-2 drops of atropine sulfate, and mice were injected i.p. with 10% sodium fluorescein (AKFLUOR, Akorn, Decatur, IL) at a dose of 0.03 ml/5-6 gram weight.
Histology and EM. The right eye was fixed in 4% paraformaldehyde for 2 h, transferred to 1% paraformaldehyde, and stored at 4°C until processing for light microscopy. Fixed eyes were embedded in acrylamide (34) and cryoprotected in OCT compound. Specimens were cryosectioned from the superior cup through the optic nerve to the inferior cup in 10-μm increments. Left eyes were fixed in 2% gluteraldehyde, embedded in Spurrs resin, and processed for EM. Ocular distribution of neutral lipid was evaluated by using oil red O. Morphology of the retina/RPE/choroid was visualized in Mayers hematoxylin-stained (MHS-16, Sigma-Aldrich) cryosections and toluidine blue-stained plastic sections. Patterns and origins of neovascularization (NV) were determined in serial 1-μm sections. Each eye was evaluated for the presence of RPE changes, including atrophy, hyperpigmentation, hypopigmentation, sub-RPE basal deposits, and soft or hard drusenoid deposits (one or more, greater than half the RPE height), NV including CNV, and PR degeneration.
Human CNV Membranes. CNV membranes (CNVM) were surgically excised from patients with exudative AMD, during macular translocation surgery with 360° peripheral retinectomy. These were obtained with Institutional Review Board exemption in accordance with Duke University Medical Center regulations. Isolated CNVM were fresh frozen in OCT compound or fixed in 1% paraformaldehyde, embedded in OCT compound, cryosectioned at 10 μm, mounted onto slides, and stored at -20°C.
Immunohistochemistry. Growth factors [VEGF (SC-507, Santa Cruz Biotechnology), TGF-β (SC-90, Santa Cruz Biotechnology), basic FGF (Sc-79, Santa Cruz Biotechnology), insulin-like growth factor-1 (IGF-1) (MN no. AF-291-NA, R & D Systems), IGF-1 receptor (IGF-1R) (no. AF-305-NA, R & D Systems), and amyloid beta (Aβ) peptide (amino acids 17-24, Clone 4G8, Senetek PLC, Napa, CA)] were immunolocalized in mouse and human cryosections as described (35). Nonspecific immunostaining was blocked with normal serum appropriate to the secondary antibody species diluted in 0.5% Triton X-100/PBS with CaCl2 and MgCl2. Signal amplification was obtained by using a Vectastain ABC kit, followed by color development with a peroxidase substrate-diaminobenzidine kit (DAB; Vector Laboratories). Control slides containing sequential sections were probed with nonimmune serum, buffer without primary antibody, or an irrelevant primary antibody and secondary antibody.
Plasma Cholesterol Levels. Total plasma cholesterol levels were measured by collecting 750 μl of whole blood from mice fasted for 5 h, through retroorbital bleed before and after administration of an 8-week HF-C diet. Plasma was removed after centrifugation at 8,000 × g for 10 min at 4°C, and total cholesterol was measured by using diagnostic CII kits (Wako, Richmond, VA).
Protein Biochemistry. After removal of the anterior segment and sclera, the remaining retina/RPE/choroid (n = 3-7/isoform/diet) were homogenized in 2% SDS. ApoE and VEGF protein levels were measured in the extract by using a sandwich ELISA (R & D Systems). Protein levels were normalized against total protein in the extract (Micro BCA protein assay kit, Pierce). Student's t test was used to determine the statistical significance of the results.
Results
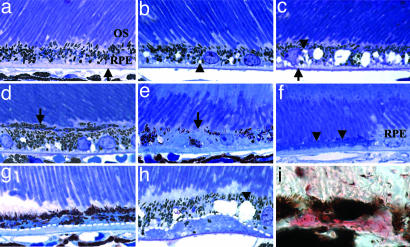
Light Microscopic Evaluation of apoE TR Mice. Eyes from apoE TR mice in two age groups were evaluated in this study: young (12-30 weeks) and aged (65-127 weeks) mice. Histological examination of eyes from apoE TR mice fed a HF-C diet revealed striking age-and APOE allele-dependent pathologies. No remarkable ocular abnormalities were found in eyes from wild-type C57BL/6J mice, young apoE TR mice exposed to a HF-C diet, or from apoE TR mice maintained on a normal mouse chow diet (Fig. 1a). Eyes of aged apoE3 TR mice fed a HF-C diet exhibited only minor RPE changes (e.g., RPE vacuolization, Fig. 1b and Table 1). Aged apoE2 TR mice fed the HF-C diet showed a slightly more severe RPE phenotype in which RPE vacuolization was accompanied by RPE mottling, including hyperpigmentation, hypopigmentation, and BrM thickening (Fig. 1c and Table 1). The most extensive degenerative changes were observed in eyes from aged apoE4 TR mice fed a HF-C diet (Fig. 1 d-h and Table 1). These mice showed RPE hyperpigmentation (Fig. 1d), hypopigmentation (Fig. 1e), and atrophy (Fig. 1f). There was BrM thickening, thick sub-RPE basal deposits (Fig. 1g), and “soft” drusenoid deposits (Fig. 1h) rich in neutral lipids as demonstrated by positive oil red O staining (Fig. 1i). The most striking change, detected exclusively in aged apoE4 TR mice fed the HF-C diet (19% of males, n = 7/37 and 18% of females, n = 2/11), was NV. This result ranged from mild NV confined to an area adjacent to the RPE and BrM (Fig. 2a) to more extensive NV that extended through the RPE and subretinal space and into the neural retina (Fig. 2 b-d). More extensive neovascular lesions may involve the outer retinal vasculature as well (Fig. 2d), as seen in human end-stage disciform disease (5). Evidence indicating RPE migration (Fig. 2c) and proliferation (Fig. 2d) was also noted in areas of vascular invasion of the retina.
Fig. 1.
Morphology of the RPE of aged apoE TR mice maintained on a normal or HF-C diet. (a-h) Shown are 1-μm plastic sections stained with toluidine blue. (a) Example of normal retinal, RPE, Bruch's membrane (arrow) morphology in apoE TR mice on normal chow (apoE3 isoform shown). (b-i) HF-C diet fed mice. (b) Some vacuolization (arrowhead) was seen in the RPE of apoE3 TR mice, but otherwise the eye seemed normal. (c) ApoE2 TR mice show more extensive vacuolization in the RPE (arrowhead) and some BrM thickening (arrow). Changes observed in the eyes of apoE4 TR mice (d-i) included RPE hyperpigmentation (d, arrow), RPE hypopigmentation (e, arrow), RPE atrophy (f, arrowheads), thick basal deposits (g, below dotted line), vacuolization (h, arrowhead), and soft drusenoid deposits (below dotted line). (i) Oil red O histochemistry (pink) of soft drusenoid deposit in 10-μm-thick cryosection from apoE4 TR mouse. (Magnification: ×60. OS, outer segments.)
Table 1. Incidence of structural changes in eyes (1-μm plastic sections) of apoE TR mice fed a HF-C diet.
| RPE mottling
|
Sub-RPE deposit
|
Neovascularization
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ApoE isoform | No. of eyes* | Hyperpigmentation | Hypopigmentation | Atrophy | Basal deposit | Drusenoid | Choroidal | Retinal | Both† |
| E2 | 14 | 12 | 1 | 2 | 7 | 1 | 1 | 1 | 1 |
| E3 | 17 | 5 | 1 | 0 | 6 | 0 | 0 | 0 | 0 |
| E4 | 21 | 16 | 2 | 4 | 16 | 4 | 5 | 3 | 3 |
Number of eyes graded
Both retinal and choroidal neovascularization present
Fig. 2.
Neovascularization in eyes of aged apoE4 TR mice maintained on a HF-C diet. Shown are 1-μm plastic sections stained with toluidine blue. (a) Mild NV (arrow) located between BrM and RPE adjacent to OS disruption and thinning of ONL (double headed arrow). (b and c) More extensive NV with vessels and RPE extending into the retina. (d) Widespread NV invading the RPE, ONL, and inner retina. (Magnification: ×20. OS, outer segments.)
Human CNV is described as NV that originates in choroidal blood vessels and grows through BrM, usually at multiple sites, into the sub-RPE space (36). CNV extending through the RPE into the subretinal space is often associated with subretinal fluid, blood accumulation, and RPE proliferation (36). To distinguish between retinal and choroidal NV in the aged apoE4 TR animals, FA was performed on living animals after 4 and 8 weeks on the HF-C diet, followed by analysis of serial sections through the NV lesions in fixed postmortem tissue. FA revealed early hyperfluorescent spots separate from the retinal vasculature. These sites increased in intensity and leaked in late frames of the angiogram consistent with neovascularization. On FA, retinal vascular connections to the leaky sites were not seen (Fig. 3 a-c). Histological evaluation of 72 1-μm-thick sections through this lesion showed that it was confined between the RPE and CC without anastomosis with retinal vasculature (Fig. 3 d-m). The serial sections encompassed, and extended beyond, the area of the hyperfluorescent, leaking spot shown by FA, supporting a choroidal rather than retinal source for the NV lesion. In the sections at the beginning and end of this sequential series, RPE hyperpigmentation, and sub-RPE deposits, including diffuse basal and “drusenoid” deposits, were evident (Fig. 3 d and m). Deeper in the series, where the lesion was larger, the overlying RPE cell layer was thinner, with regions of atrophy (Fig. 3k). There was also thinning of the outer nuclear layer (ONL) (Fig. 3 e versus i). Near the center of the NV lesion, breaks in BrM concomitant with cellular migration across BrM (Fig. 3 g and h), as well as disruption of the structural integrity of BrM (Fig. 3 i and j), were seen.
Fig. 3.
Choroidal origin of neovascularization in eyes of aged, HF-C diet-fed apoE4 TR mice. (a-c) Sequential fluorescein angiograms of the retina of a 90-week-old apoE4 TR mouse fed the HF-C diet for 8 weeks. (a) At 1 min post dye injection, one hyperfluorescent spot (arrow) appears below the filled major retinal vessels. (b) At 4 min, the hyperfluorescent spot (arrow) has increased in size. (c) At 6 min, the size of the hyperfluorescent spot (arrow) has enlarged even more as the choroidal vessels fill. (d-n) Sequential 1-μm sections stained with toluidine blue through the hyperfluorescent spot (shown in a-c) revealed the appearance and disappearance of a NV lesion confined between the RPE and BrM. Sections depicted are separated by ≈8 μm. (d) Lesion first appears as a thick deposit with focal drusenoid deposits (arrow) located between the RPE and BrM. (e-l) The vascular lesion thickens and proliferates between the RPE and BrM. Double headed arrow in e shows thinning of the adjacent outer nuclear layer. (g) A capillary adjacent to a disruption in BrM (arrow). (h) Higher magnification of Inset in g showing apparent cellular migration at this break in BrM (arrow). (i) Double headed arrow shows the thinning of the ONL. (j) Higher magnification of Inset in i showing the splitting of BrM. (k-m) Continued sections through NV lesion showing RPE atrophy (k, arrow), decreasing extent of NV lesion, and drusenoid deposits (l and m, arrows) underlying relatively normal appearing neural retina. [Magnifications: ×20 (d-g, i, and k-m) and ×100 (h and j).]
Electron Microscopic Evaluation of Aged apoE TR Mice Fed a HF-C Diet. EM was used to further evaluate the histological abnormalities documented at the light microscopic level. EM analysis of RPE vacuoles observed by light microscopy in the apoE TR mice (Fig. 1b, for example) revealed that they contained membranous material (Fig. 4a). In the retinas of apoE2 (data not shown) and apoE4 TR mice RPE basal infoldings were disorganized or absent (Fig. 4b). There were regions of BrM thickening, compared with apoE3 TR mice (Fig. 4 c-e), and accumulation of deposits between RPE and BrM of varying thickness (Fig. 4 d and e). Material of increased electron density was also associated with disorganized and thickened basal infoldings of RPE (Fig. 4c). Membranous and vesicular material similar to that detected in sub-RPE deposits in human AMD was also seen (Fig. 4g). In apoE4 TR mice fed the HF-C diet that developed NV, there were regions of thinning or loss of BrM (Fig. 4 h and i), and capillaries present on both the RPE and choroidal sides of BrM (Fig. 4j).
Fig. 4.
Electron micrographs of RPE/BrM/CC interface of eyes from apoE4 TR mice on HF-C diet. (a) Vacuoles (V) containing membranous material are present on the basal side of RPE cells; the RPE basal infoldings are normal (arrow); elastic layer of BrM is normal (arrowhead). (b) Misaligned and disorganized RPE basal infoldings (white arrow); elastic layer of BrM appears thin and discontinuous (white arrowheads). (c) Thickened elastic layer of BrM (arrowheads) and electron dense material associated with the basal infoldings of the RPE (white double-headed arrow). (d and e) Thick basal deposits with electron dense material (black double-headed arrows); disrupted basal infoldings of RPE (white arrow in e). (f) Thickened elastic layer of BrM, which seems to split (arrowheads) underlying RPE basal infolding with electron dense material (white double head arrow). (g) Basal deposit with membranous vesicular material (arrow). (h) Breaks in and thinning of BrM (arrow and arrowhead, respectively) with extensive lipid droplets in the overlying RPE cell. (i and j) Capillary vessels (c) present on both sides of BrM. Black arrowheads in all figures point to elastic layer of BrM. [Magnifications: ×2,500 (a, e, and g-j), ×4,000 (b, c, and f), and ×4,500 (d).]
Detection of Proteins Associated with Human CNV in NV Lesions of Aged apoE4 TR Mice Fed a HF-C Diet. We examined NV lesions in aged HF-C-fed apoE4 TR mouse retinas for localization of the proangiogenic factor, VEGF, and compared them with human CNVM from patients with exudative AMD. NV in the mice was confined to an area adjacent to the RPE/BrM, with some extending through the ONL (Fig. 5a). Both the mouse and human NV were VEGF-positive (Fig. 5 c and d). Aβ deposition, a neurological hallmark of Alzheimer's disease, has also been localized to drusen in AMD eyes (37). It accumulates and colocalizes with VEGF in amyloid plaques in the brains of patients with Alzheimer's disease (38). Human CNVM and NV tissue from aged apoE4 TR mice fed the HF-C diet showed perivascular Aβ immunoreactivity (Fig. 5 e and g). Aβ immunoreactivity was also observed in the nonneovascular deposits (Fig. 5f). Our findings are consistent with cerebrovascular Aβ deposition in aged apoE4 mice (39).
Fig. 5.
Immunohistology of NV in HF-C diet-fed apoE4 TR mouse eyes (a, c, e, f, and h) and human CNVM (b, d, g, and i). Shown are 10-μm-thick cryosections. Mouse retina with NV (a) and excised (b) human CNVM stained with Mayer's hematoxylin (fuchsia color). (c) VEGF immunolocalization (pink) to mouse NV (arrow). (d) Diffuse VEGF immunolocalization within human CNVM (arrowheads; brown color), compared with adjacent CNVM negative control section (i). (e and g) Aβ immunoreactivity (purple color) is seen associated with neovascular vessels in mouse and human NV (arrowheads). (f) Aβ immunoreactivity is also associated with nonneovascular deposits located between the RPE and Bruch's membrane in mouse NV (arrow). (h and i) Negative control sections labeled with secondary antibody alone. (b, d, g, and i) Thin arrow points to RPE cells in human CNVM (brown). [Magnifications: ×20 (a-d and g-i) and ×40 (e).]
Other growth factors that have been detected in human CNV include, IGF-1 (40), IGF-1 receptor (IGF-1R) (35), basic FGF (41), and TGF-β (40). Both the mouse and human NV were IGF-1R positive (data not shown). In addition, basic FGF, TGF-β, and IGF-1 immunoreactivities were abundant throughout the mouse and human NV (data not shown).
Measurement of Cholesterol Levels in apoE TR Mice. Plasma cholesterol levels of aged apoE TR mice, maintained for their last 8 weeks on a HF-C diet, were found to be 1.5- to 2.5-fold higher than in age-matched apoE TR animals on a normal diet (apoE2 P < 0.0009; apoE3, P < 5.22 × 10-14; apoE4, P < 1.36 × 10-11; Fig. 6a).
Fig. 6.
Plasma cholesterol levels in retina/RPE/choroid of apoE TR mice. (a) Plasma cholesterol levels in aged, HF-C-fed, apoE TR mice (age 65-127 weeks) was greater for all three genotypes versus animals on normal diet (apoE2 P < 0.0009; apoE3, P < 5.22 × 10-14; apoE4, P < 1.36 × 10-11). (b) In apoE4 TR mice, plasma cholesterol levels in young (12-30 weeks) and aged (65-127 weeks) animals were higher than in age-matched controls (P < 1 × 10-4 and P < 1 × 10-6, respectively). The relative increase in the cholesterol levels after HF-C diet was greater in the young versus old apoE4 TR mice (P < 1.35 × 10-11). Error bars represent the standard deviation of fasted mouse plasma levels. n = 10 animals per isoform. APOE genotypes are represented by numerical designation (e.g., 2/2 = apoE2 homozygote).
Plasma cholesterol levels of young apoE4 animals after administration of the 8-week HF-C diet increased by 4.3-fold compared with age-matched apoE4 mice on normal chow (P < 1 × 10-6;Fig. 6b). The overall increase of cholesterol levels after administration of the diet was greater in young versus old apoE4 mice (4.3-versus 2.5-fold increase; P < 1.36 × 10-11; Fig. 6b).
ApoE Protein Levels in Retina/RPE/Choroid of apoE TR Mice. ApoE TR mice maintained on normal rodent chow express human apoE isoforms at physiological levels and in tissue distributions similar to wild-type mice and humans (42). The concentrations of apoE protein in the retinal/RPE/choroidal complex of old animals were measured and compared with age-matched controls. ApoE levels decreased slightly in all of the aged HF-C fed apoE TR mice compared with age-matched mice on normal chow (apoE2, P < 0.08; apoE3, P < 0.12; apoE4, P < 0.10, not statistically significant; Fig. 7a, which is published as supporting information on the PNAS web site). Interestingly, the concentration of apoE in retina/RPE/choroid of young apoE4 animals fed normal chow was 1.5-fold higher (P < 0.001) than old apoE4 animals on normal chow (Fig. 7b). The HF-C diet caused a statistically significant decrease in apoE levels in young apoE4 TR mice (P < 0.03; Fig. 7b).
VEGF in Retina/RPE/Choroid of apoE TR Mice. VEGF protein levels in retina/RPE/choroid tissue homogenates were determined by ELISA and showed a slight, but statistically insignificant, decrease in aged apoE TR mice on the HF-C diet compared with age-matched apoE TR mice on normal diet (apoE2, P < 0.23; apoE3, P < 0.20; apoE4, P < 0.24; Fig. 8a, which is published as supporting information on the PNAS web site). A slight HF-C diet-associated decrease was also seen in young apoE4s. In contrast, there was an age-related increase (1.4- to 1.7-fold) in VEGF levels in the retina/RPE/choroid of old apoE4 TR mice irrespective of diet (P < 0.0005; Fig. 8b).
Discussion
AMD is one of the most common eye diseases in the elderly and is likely to be both multifactorial and polygenic in nature (43). Development of an AMD animal model is particularly challenging because the causative agent(s), genetic associations, and pathomechanisms of AMD are not fully understood. We hypothesized that combining an environmental risk factor (HF-C diet) and a known genetic risk (APOE) with the established risk of advanced age could cause retinal changes analogous to those seen in human AMD. Unlike previously studied APOE transgenic mouse models of AMD (23, 44), the apoE TR mice used in these studies express human apoE at physiological levels in the same temporal and spatial pattern observed in humans (31). In addition, apoE TR mice not only are able to respond to external stimuli (i.e., diet) appropriately, but also allow comparison of all three apoE isoforms in vivo, with no concern for founder effects or differences in protein expression. Importantly, the effects of invasive techniques do not complicate the resulting animal model. For example, one of the most popular models of CNV, the laser injury model, has contributed significantly to our understanding of CNV, but it is complicated by its limited reproducibility and technical artifacts, including nonspecific, local inflammatory reactions secondary to laser treatment (45).
We show that specific apoE isoform expression alone results in only mild retinal changes with aging. However, in the apoE2, and more so the apoE4 expressing animals, the combination of advanced age and HF-C diet results in more extensive degenerative changes in the retina/RPE/choroid. Specifically, in the eyes of HF-C fed, aged apoE4 TR mice, these changes were comparable with the pathology associated with human AMD. Hallmarks of AMD, including diffuse sub-RPE deposits (Figs. 1g and 4 d and e), “soft drusen-like” deposits (Fig. 1 h and i), thickened BrM (Fig. 4 c and f), patchy regions of RPE atrophy (Figs. 1f and 3k), and retinal and choroidal NV (Figs. 2 and 3) were documented in these animals. The presence of growth factors associated with human CNV, including VEGF, further supports the validity of this animal model (36)
Previous models of AMD produced by phototoxicity (46), senescence acceleration (47), high fat diets (22, 23, 44, 46, 48, 49), injections of basic FGF (50), and laser photocoagulation of BrM (51, 52) do not manifest the range of human AMD-like pathologies documented here. Dietary fat and hyperlipidemia have been implicated in the pathogenesis of AMD (53), because plasma lipids, including apolipoproteins, cholesterol, triglycerides, and other lipid-rich substances, accumulate within BrM and create a hydrophobic barrier (46). Although these studies support the deleterious effects of high fat intake on the retina, the animals do not exhibit many of the hallmarks of AMD. For example, studies using aged transgenic mice expressing mutant forms of APOE (APOE3-Leiden mice) and maintained on a high fat diet documented mild to moderate accumulation of basal deposits in these animals (23), but did not show other AMD-associated changes.
The AMD-like pathologies we observe in the apoE4 TR mice exposed to a HF-C diet could be due in part to an inability of apoE4, to regulate cholesterol homeostasis when challenged with a high cholesterol diet (32, 54, 55). Interestingly, even though plasma cholesterol levels were substantially higher in the apoE2 mice compared with apoE4 mice, the severity of retinal pathologies was greater in the apoE4 mice. This finding suggests that high plasma cholesterol levels alone are not sufficient to elicit retinal pathology; rather, expression of the apoE4 isoform is required. ApoE4 TR mice have been shown to clear cholesterol by means of the liver very efficiently under normal diet conditions. However, when dietary cholesterol levels are high, apoE4 becomes less efficient at clearing total cholesterol compared with apoE3 (32, 54, 55). Excess cellular cholesterol can then lead to an inflammatory cascade and chronic degeneration of peripheral tissue. Finally, apoE4 has the lowest antioxidant activity of the three apoE isoforms, which may promote increased lipid peroxidation (56), a pathogenic mechanism implicated in AMD (57).
Currently, a prominent hypothesis for apoE linkage to Alzheimer's disease is that apoE4 accelerates the pathogenic Aβ deposition in neurons (30). Aβ is also deposited in cerebral vessels in Alzheimer's disease brains with cerebral amyloid angiopathy (25) and colocalizes with VEGF in amyloid plaques (38). Immunolocalization of Aβ within the NV and sub-RPE deposits of the aged apoE4 TR animals fed a HF-C diet, and within drusen (37) and CNV (Fig. 5g) in human AMD, supports the hypothesis that apoE may also promote amyloid deposition in AMD, in an APOE*4 allele-dependent manner.
Our findings support an association of APOE with AMD. Specifically, they implicate the APOE*4 genotype as a susceptibility gene that confers an increased risk for AMD. This finding, however, is in direct contrast with human epidemiological studies that find the APOE*4 allele to be slightly protective against AMD (14, 16, 20, 21). This apparent discrepancy could be due to one or a combination of the following factors. First, in this study, all of the animals expressing the human apoE were APOE homozygotes whereas, in the human studies, the subjects were predominantly, if not exclusively, heterozygous for the APOE*2 or APOE*4 alleles. Second, in the human studies, the effect of diet as a modifier of the APOE allele effect on AMD risk was not investigated. Finally, the difference could arise from differences in mouse and human physiology or to other, yet-to-be-determined factors that influence the incidence of AMD in the human population. Regardless, we have identified an experimental model that can be used to further elucidate the role of APOE in development of basal deposits and CNV. Currently, virtually all CNV-related therapies are antiangiogenic in their mechanism of action. Association of lipid transport/dysregulation and amyloid deposition in the pathogenesis of the retinal changes observed in our model provides a molecular mechanism for AMD that requires further investigation and parallels current findings in Alzheimer's disease (38). In light of recent studies linking genetic polymorphisms in the complement regulator factor H and AMD (10-13), this animal model may be of additional value in elucidating molecular mechanisms underlying this relationship. For example, the model will allow characterization of complex formation between factor H and Aβ assemblies present in sub-RPE deposits in AMD and in apoE4 TR mice, and that may have local proinflammatory consequences important in AMD pathogenesis (58). Characterization of molecular pathways that precede CNV in the pathogenesis of neovascular AMD will provide novel targets for therapies to treat, and eventually prevent loss of, vision and blindness. These animals should also serve as excellent models to study basal deposit formation and PR/RPE dysfunction in dry AMD.
Supplementary Material
Acknowledgments
We gratefully acknowledge Drs. Glenn Jaffe and Michael Boulton for scientific discussions, the technical assistance of Christy Sweet and Michelle Staples, and the following funding agencies: National Eye Institute (NEI) Grant R01 EY11286 (to C.B.R.), NEI Grant R01 EY11527 (to L.V.J.), NEI Core Grant P30EY0054722, National Institute on Aging Grant P50 AG05128-20 (to D.E.S. and G.M.), a Research to Prevent Blindness (RPB) Career Development Award (to C.B.R.), an RPB core grant, and an American Health Assistance Foundation Macular Degeneration Research 2004 grant (to C.B.R.).
Author contributions: G.M. and C.B.R. designed research; G.M., B.E.M., P.S., and C.B.R. performed research; G.M., L.V.J., D.W.R., C.A.T., P.M.S., and C.B.R. analyzed data; D.E.S., C.A.T., and P.M.S. contributed new reagents/analytic tools; and G.M., L.V.J., and C.B.R. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AMD, age-related macular degeneration; apoE, apolipoprotein E; PR, photoreceptor; RPE, retinal pigment epithelium; BrM, Bruch's membrane; CC, choriocapillaris; NV, neovascularization; CNV, choroidal NV; CNVM, CNV membrane(s); TR, targeted replacement; HF-C, high fat cholesterol-rich; FA, fluorescein angiography; IGF, insulin-like growth factor; ONL, outer nuclear layer; Aβ, amyloid beta.
References
- 1.Tomany, S. C., Wang, J. J., Van Leeuwen, R., Klein, R., Mitchell, P., Vingerling, J. R., Klein, B. E., Smith, W. & De Jong, P. T. (2004) Ophthalmology 111, 1280-1287. [DOI] [PubMed] [Google Scholar]
- 2.Sarks, S. H. (1976) Br. J. Ophthalmol. 60, 324-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curcio, C. A. & Millican, C. L. (1999) Arch. Ophthalmol. 117, 329-339. [DOI] [PubMed] [Google Scholar]
- 4.Green, W. R. (1999) Mol. Vis. 5, 27. [PubMed] [Google Scholar]
- 5.Grossniklaus, H. E. & Green, W. R. (2004) Am. J. Ophthalmol. 137, 496-503. [DOI] [PubMed] [Google Scholar]
- 6.Wang, J. J., Foran, S. & Mitchell, P. (2000) Clin. Exp. Ophthalmol. 28, 268-273. [DOI] [PubMed] [Google Scholar]
- 7.van Leeuwen, R., Klaver, C. C., Vingerling, J. R., Hofman, A. & de Jong, P. T. (2003) Eur. J. Epidemiol. 18, 845-854. [DOI] [PubMed] [Google Scholar]
- 8.Stone, E. M., Braun, T. A., Russell, S. R., Kuehn, M. H., Lotery, A. J., Moore, P. A., Eastman, C. G., Casavant, T. L. & Sheffield, V. C. (2004) N. Engl. J. Med. 351, 346-353. [DOI] [PubMed] [Google Scholar]
- 9.Allikmets, R. (2000) Am. J. Hum. Genet. 67, 487-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards, A. O., Ritter, R., 3rd, Abel, K. J., Manning, A., Panhuysen, C. & Farrer, L. A. (2005) Science 308, 421-424. [DOI] [PubMed] [Google Scholar]
- 11.Hageman, G. S., Anderson, D. H., Johnson, L. V., Hancox, L. S., Taiber, A. J., Hardisty, L. I., Hageman, J. L., Stockman, H. A., Borchardt, J. D., Gehrs, K. M., et al. (2005) Proc. Natl. Acad. Sci. USA 102, 7227-7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haines, J. L., Hauser, M. A., Schmidt, S., Scott, W. K., Olson, L. M., Gallins, P., Spencer, K. L., Kwan, S. Y., Noureddine, M., Gilbert, J. R., et al. (2005) Science 308, 419-421. [DOI] [PubMed] [Google Scholar]
- 13.Klein, R. J., Zeiss, C., Chew, E. Y., Tsai, J. Y., Sackler, R. S., Haynes, C., Henning, A. K., Sangiovanni, J. P., Mane, S. M., Mayne, S. T., et al. (2005) Science 308, 385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baird, P. N., Guida, E., Chu, D. T., Vu, H. T. & Guymer, R. H. (2004) Invest. Ophthalmol Vis. Sci. 45, 1311-1315. [DOI] [PubMed] [Google Scholar]
- 15.Mahley, R. W. & Rall, S. C., Jr. (2000) Annu. Rev. Genomics Hum. Genet. 1, 507-537. [DOI] [PubMed] [Google Scholar]
- 16.Klaver, C. C., Kliffen, M., van Duijn, C. M., Hofman, A., Cruts, M., Grobbee, D. E., van Broeckhoven, C. & de Jong, P. T. (1998) Am. J. Hum. Genet. 63, 200-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson, D. H., Ozaki, S., Nealon, M., Neitz, J., Mullins, R. F., Hageman, G. S. & Johnson, L. V. (2001) Am. J. Ophthalmol. 131, 767-781. [DOI] [PubMed] [Google Scholar]
- 18.Malek, G., Li, C. M., Guidry, C., Medeiros, N. E. & Curcio, C. A. (2003) Am. J. Pathol. 162, 413-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallman, D. M., Boerwinkle, E., Saha, N., Sandholzer, C., Menzel, H. J., Csazar, A. & Utermann, G. (1991) Am. J. Hum. Genet. 49, 338-349. [PMC free article] [PubMed] [Google Scholar]
- 20.Souied, E. H., Benlian, P., Amouyel, P., Feingold, J., Lagarde, J. P., Munnich, A., Kaplan, J., Coscas, G. & Soubrane, G. (1998) Am. J. Ophthalmol. 125, 353-359. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt, S., Klaver, C., Saunders, A., Postel, E., De La Paz, M., Agarwal, A., Small, K., Udar, N., Ong, J., Chalukya, M., et al. (2002) Ophthalmic Genet. 23, 209-223. [DOI] [PubMed] [Google Scholar]
- 22.Dithmar, S., Curcio, C. A., Le, N. A., Brown, S. & Grossniklaus, H. E. (2000) Invest. Ophthalmol. Vis. Sci. 41, 2035-2042. [PubMed] [Google Scholar]
- 23.Kliffen, M., Lutgens, E., Daemen, M. J., de Muinck, E. D., Mooy, C. M. & de Jong, P. T. (2000) Br. J. Ophthalmol. 84, 1415-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalaria, R. N. (1997) Lancet 349, 1174. [DOI] [PubMed] [Google Scholar]
- 25.Weller, R. O. & Nicoll, J. A. (2003) Neurol. Res. 25, 611-616. [DOI] [PubMed] [Google Scholar]
- 26.Enzinger, C., Ropele, S., Smith, S., Strasser-Fuchs, S., Poltrum, B., Schmidt, H., Matthews, P. M. & Fazekas, F. (2004) Ann. Neurol. 55, 563-569. [DOI] [PubMed] [Google Scholar]
- 27.Winkler, B. S., Boulton, M. E., Gottsch, J. D. & Sternberg, P. (1999) Mol. Vis. 5, 32. [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, J., Itagaki, Y., Ben-Shabat, S., Nakanishi, K. & Sparrow, J. R. (2000) J. Biol. Chem. 275, 29354-29360. [DOI] [PubMed] [Google Scholar]
- 29.Anderson, D. H., Mullins, R. F., Hageman, G. S. & Johnson, L. V. (2002) Am. J. Ophthalmol. 134, 411-431. [DOI] [PubMed] [Google Scholar]
- 30.Wellington, C. L. (2004) Clin. Genet. 66, 1-16. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan, P. M., Mezdour, H., Aratani, Y., Knouff, C., Najib, J., Reddick, R. L., Quarfordt, S. H. & Maeda, N. (1997) J. Biol. Chem. 272, 17972-17980. [DOI] [PubMed] [Google Scholar]
- 32.Wang, C., Wilson, W. A., Moore, S. D., Mace, B. E., Maeda, N., Schmechel, D. E. & Sullivan, P. M. (2005) Neurobiol. Dis. 18, 390-398. [DOI] [PubMed] [Google Scholar]
- 33.Hawes, N. L., Smith, R. S., Chang, B., Davisson, M., Heckenlively, J. R. & John, S. W. (1999) Mol. Vis. 5, 22. [PubMed] [Google Scholar]
- 34.Johnson, L. V. & Blanks, J. C. (1984) Curr. Eye Res. 3, 969-974. [DOI] [PubMed] [Google Scholar]
- 35.Rosenthal, R., Wohlleben, H., Malek, G., Schlichting, L., Thieme, H., Bowes Rickman, C. & Strauss, O. (2004) Biochem. Biophys. Res. Commun. 323, 1203-1208. [DOI] [PubMed] [Google Scholar]
- 36.Campochiaro, P. A. (2000) J. Cell. Physiol. 184, 301-310. [DOI] [PubMed] [Google Scholar]
- 37.Anderson, D. H., Talaga, K. C., Rivest, A. J., Barron, E., Hageman, G. S. & Johnson, L. V. (2004) Exp. Eye Res. 78, 243-256. [DOI] [PubMed] [Google Scholar]
- 38.Yang, S. P., Bae, D. G., Kang, H. J., Gwag, B. J., Gho, Y. S. & Chae, C. B. (2004) Neurobiol. Aging 25, 283-290. [DOI] [PubMed] [Google Scholar]
- 39.Fryer, J. D., Simmons, K., Parsadanian, M., Bales, K. R., Paul, S. M., Sullivan, P. M. & Holtzman, D. M. (2005) J. Neurosci. 25, 2803-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amin, R., Puklin, J. E. & Frank, R. N. (1994) Invest. Ophthalmol. Vis. Sci. 35, 3178-3188. [PubMed] [Google Scholar]
- 41.Soubrane, G., Cohen, S. Y., Delayre, T., Tassin, J., Hartmann, M. P., Coscas, G. J., Courtois, Y. & Jeanny, J. C. (1994) Curr. Eye Res. 13, 183-195. [DOI] [PubMed] [Google Scholar]
- 42.Knouff, C., Hinsdale, M. E., Mezdour, H., Altenburg, M. K., Watanabe, M., Quarfordt, S. H., Sullivan, P. M. & Maeda, N. (1999) J. Clin. Invest. 103, 1579-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seddon, J. M., Rosner, B., Sperduto, R. D., Yannuzzi, L., Haller, J. A., Blair, N. P. & Willett, W. (2001) Arch. Ophthalmol. 119, 1191-1199. [DOI] [PubMed] [Google Scholar]
- 44.Ong, J. M., Zorapapel, N. C., Rich, K. A., Wagstaff, R. E., Lambert, R. W., Rosenberg, S. E., Moghaddas, F., Pirouzmanesh, A., Aoki, A. M. & Kenney, M. C. (2001) Invest. Ophthalmol. Vis. Sci. 42, 1891-1900. [PubMed] [Google Scholar]
- 45.Schwesinger, C., Yee, C., Rohan, R. M., Joussen, A. M., Fernandez, A., Meyer, T. N., Poulaki, V., Ma, J. J., Redmond, T. M. & Liu, S. (2001) Am. J. Pathol. 158, 1161-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cousins, S. W., Espinosa-Heidmann, D. G., Alexandridou, A., Sall, J., Dubovy, S. & Csaky, K. (2002) Exp. Eye Res. 75, 543-553. [DOI] [PubMed] [Google Scholar]
- 47.Majji, A. B., Cao, J., Chang, K. Y., Hayashi, A., Aggarwal, S., Grebe, R. R. & De Juan, E., Jr. (2000) Invest. Ophthalmol. Vis. Sci. 41, 3936-3942. [PubMed] [Google Scholar]
- 48.Miceli, M. V., Newsome, D. A., Tate, D. J., Jr. & Sarphie, T. G. (2000) Curr. Eye Res. 20, 8-16. [PubMed] [Google Scholar]
- 49.Fliesler, S. J., Richards, M. J., Miller, C., Peachey, N. S. & Cenedella, R. J. (2000) Neurochem. Res. 25, 685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura, H., Sakamoto, T., Hinton, D. R., Spee, C., Ogura, Y., Tabata, Y., Ikada, Y. & Ryan, S. J. (1995) Invest. Ophthalmol. Vis. Sci. 36, 2110-2119. [PubMed] [Google Scholar]
- 51.Miller, H., Miller, B. & Ryan, S. J. (1986) Invest. Ophthalmol. Vis. Sci. 27, 1644-1652. [PubMed] [Google Scholar]
- 52.Dobi, E. T., Puliafito, C. A. & Destro, M. (1989) Arch. Ophthalmol. 107, 264-269. [DOI] [PubMed] [Google Scholar]
- 53.Seddon, J. M., Cote, J. & Rosner, B. (2003) Arch. Ophthalmol. 121, 1728-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davignon, J., Gregg, R. E. & Sing, C. F. (1988) Arteriosclerosis 8, 1-21. [DOI] [PubMed] [Google Scholar]
- 55.Malloy, S. I., Altenburg, M. K., Knouff, C., Lanningham-Foster, L., Parks, J. S. & Maeda, N. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 91-97. [DOI] [PubMed] [Google Scholar]
- 56.Smith, J. D., Miyata, M., Poulin, S. E., Neveux, L. M. & Craig, W. Y. (1998) Int. J. Clin. Lab. Res. 28, 116-121. [DOI] [PubMed] [Google Scholar]
- 57.Kopitz, J., Holz, F. G., Kaemmerer, E. & Schutt, F. (2004) Biochimie 86, 825-831. [DOI] [PubMed] [Google Scholar]
- 58.Strohmeyer, R., Ramirez, M., Cole, G. J., Mueller, K. & Rogers, J. (2002) J. Neuroimmunol. 131, 135-146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.