Abstract
Natural killer T cells (NKT cells) expressing a semiinvariant CD1d-reactive T cell receptor (invariant NKT, iNKT) can be rapidly activated by monocytes or immature dendritic cells (iDCs) bearing a CD1d-presented glycolipid antigen and can in turn stimulate these myeloid cells to mature and produce IL-12. Previous studies have shown that iNKT-produced IFNγ and CD40 ligand contribute to this dendritic cell maturation. This study demonstrates that CD1d ligation alone, in the absence of iNKT, could rapidly (within 24 h) stimulate production of bioactive IL-12p70 by CD1d+ human peripheral blood monocytes as well as iDCs. IFNγ alone had no effect, but it markedly enhanced CD1d-stimulated IL-12 production. Monocyte differentiation, as assessed by CD40 and CD1a up-regulation, was also accelerated by CD1d stimulation, consistent with this representing a physiological response. CD1d ligation on the human monocytic cell line THP-1 similarly specifically stimulated IL-12 production. Biochemical studies showed that IL-12 release correlated with rapid phosphorylation of IκB, a critical step in NF-κB activation. Selective NF-κB inhibition blocked this CD1d-stimulated IL-12 production. Finally, CD1d ligation could also enhance IL-12 production in the presence of suboptimal LPS or CD40 stimulation. These findings demonstrate an innate immune signaling function for CD1d and provide a mechanism for the rapid activation of monocytes and iDCs by CD1d-reactive T cells.
Keywords: antigen-presenting cell, innate immunity, natural killer T cell
CD1d is a nonpolymorphic MHC class I-like protein constitutively expressed by antigen-presenting cells (APC) and by some epithelia, whereas CD1a-c are induced upon dendritic cell (DC) maturation. CD1d is recognized by a subpopulation of T cells, many of which express markers typical of natural killer cells and have been termed natural killer T cells (NKT cells) (1-3). A major fraction of CD1d-restricted T cells recognize CD1d through an invariant T cell receptor (TCR)α chain (Vα24-Jα18 in humans). CD1d-reactive NKT cells using this invariant TCR (iNKT) are largely primed in vivo for the rapid production of T helper 1 and T helper 2 cytokines (IFNγ and IL-4) and have regulatory functions in innate and adaptive immune responses (3, 4). A glycolipid antigen isolated from marine sponge (α-galactosylceramide, αgalcer) is recognized by iNKT in the context of CD1d (5-8). In vivo, αgalcer causes activation of iNKT, rapid cytokine production, and subsequent systemic activation of innate and adaptive immune cells (9, 10).
Data from many groups indicate that iNKT functions can be mediated through interactions with CD1d+ APC including DCs and B cells (11-20). Murine studies have shown that iNKT activation can be enhanced by B7 ligation of CD28 and that IL-12 produced by DCs can further enhance iNKT IFNγ production (11, 12, 20). Conversely, murine and human studies have shown that iNKT can stimulate the maturation of αgalcer-pulsed DCs (11, 12, 18, 20). This DC stimulation appears to be mediated by CD40 ligand on the iNKT through ligation of CD40 on the DCs, with IFNγ produced by the iNKT subsequently enhancing DC IL-12 production. Overall, these observations indicate that cooperative interactions between iNKT and DCs generate a positive feedback loop that can rapidly amplify IL-12-dependent immune responses. However, it remains unclear whether these iNKT-DC interactions are initiated by CD1d-presented endogenous or exogenous antigens or by other mechanisms (19). The objective of this study was to identify molecular interactions mediating activation of human monocytes and DCs by iNKT.
Materials and Methods
Cell Culture. iNKT lines (≈90% pure) were obtained by purification from healthy donor leukapak peripheral blood mononuclear cells by using a biotinylated mAb against the invariant TCR (6B11) (21) and magnetic bead sorting with anti-biotin IgG beads (Miltenyi Biotec, Auburn, CA). The purified iNKT were expanded in vitro by culturing on anti-CD3-coated plates. Briefly, 0.5-1 × 105 purified iNKT were mixed with an equal number of irradiated (4,000 rads) autologous peripheral blood mononuclear cells in RPMI medium 1640 with 10% FBS (HyClone) and 100 units/ml IL-2 (National Biological Response Modifier Program, National Cancer Institute, Frederick, MD) (21). Each 0.2 ml of cells was added to a well of a flat-bottomed 96-well plate precoated with OKT3 mAb (American Type Culture Collection). The cells were rested in medium with 10 units/ml IL-2 overnight before use.
Human monocytes were obtained from buffy coat-derived and Ficoll-Hypaque-purified peripheral blood mononuclear cells by adhesion on tissue culture flasks in RPMI medium 1640 with 10% FBS. After extensive washing, adherent cells were released by using PBS with 1 mM EDTA for use directly as monocytes. CD14+ cells represented the great majority. To generate immature DCs (iDCs), adherent monocytes were cultured for 3 further days with GM-CSF (100 ng/ml, Immunex; or 1,000 units/ml, Berlex Biosciences, Richmond, CA) and IL-4 (25 ng/ml, Endogen, Rockford, IL; or 110 units/ml, Cell Sciences, Canton, MA) in RPMI medium 1640 with 10% FBS. THP-1 cells were cultured in RPMI medium 1640 with 10% FBS (HyClone, Logan, UT)/50 μM 2-mercaptoethanol (Sigma).
Cell Stimulations and Cytokine Measurement. Monocytes or iDCs (1 × 105) were cocultured in 96-well flat-bottomed plates with iNKT. Triplicate wells were supplemented with αgalcer (100 ng/ml, Kirin Brewery, Gunma, Japan), LPS (25-250 ng/ml for monocytes, up to 20 μg/ml for iDCs, Sigma-Aldrich), or phytohemagglutinin (5 μg/ml, Invitrogen), and supernatants were analyzed by ELISA. For stimulations by plate-bound Abs, 96-well flat-bottomed plates were coated with protein G for 4 h (20 μg/ml in PBS, Sigma), washed, and blocked with 2% BSA in PBS. Wells were then washed and incubated for 4 h with 10 μg/ml CD1d mAb 51.1 (IgG2b) or isotype control, except where indicated. Additional CD1d mAbs were 27.1 and 42.1 (both IgG1), 75.1 (IgG2a), or 59.1 (IgM) (22-24), or anti-CD40 (BD Pharmingen) in PBS. After washing, monocytes, iDC, or THP-1 cells (1 × 105 per well) in RPMI medium 1640 with 10% FBS were added, with or without IFNγ at 20 ng/ml (R & D Systems), except where indicated, and culture supernatants were analyzed for cytokines by ELISA after 1-8 days. ELISA IFNγ- and IL-4-matched Ab pairs and standards (Endogen) were used. Matched Ab pairs and standards for IL-12p70 ELISA were from Endogen, and matched Ab pairs and standards for IL-12p40 ELISA were from R & D Systems.
Parthenolide and N-acetyl-l-leucinyl-l-leucinyl-methional (LLM) were from Sigma. Neutralizing IFNγ mAb (Chemicon) and CD40 mAb were used where indicated. Results show the mean of at least triplicates ± SD, representative of at least three experiments with monocytes derived from multiple healthy donors.
Flow Cytometry and Immunoblotting. FACS was performed with 1 × 106 cells after blocking with 10% human serum. iNKT lines were analyzed with anti-Vα24-phycoerythrin (PE) (Coulter) and 6B11-FITC (anti-invariant TCR) (21) or anti-Vβ11-FITC (Coulter). Expression of APC CD1 molecules and CD40 were assessed by flow cytometry. For single-color FACS, primary mAb were used at 10 μg/ml followed by anti-mouse IgG-FITC (Kirkegaard & Perry Laboratories). The primary mAb for CD1a-c were Vit6, 4A76, and M241, respectively (22), anti-CD40, and isotypes (BD Pharmingen), trace lymphocytes being gated out for CD1d FACS. Two-color FACS used lineage markers CD1a-PE, CD1d 42.1-PE, CD14-FITC, and CD40-FITC (BD Pharmingen). The CD1d and mock-transfected C1R cells were described in ref. 22.
For immunoblotting, cells were lysed in 1% SDS and equivalent amounts of protein were run on SDS/PAGE and transferred to nitrocellulose. CD1d was detected with C3D5, a mouse mAb generated against a CD1d-GST fusion protein (23). Phospho-IκB (pIκB) was detected with a pIκB-specific mAb (Cell Signaling Technology, Beverly, MA). Secondary anti-mouse IgG-horseradish peroxidase and chemiluminescence substrate were from PerkinElmer Life Sciences.
Results
Reciprocal CD1d-Mediated Activation of Human iNKT, Monocytes, and iDCs. Previous studies have shown that CD1d is constitutively expressed by human monocytes as well as at somewhat elevated levels by DCs (23, 24). We confirmed this pattern of CD1d expression (Fig. 1A). Interaction of CD1d+ APC pulsed with αgalcer and iNKT stimulates them to release IL-12 and IFNγ, respectively (18, 23-25). Murine iNKT similarly induce APC to produce IL-12 through IFNγ- and CD40L-dependent mechanisms (11, 12, 20). To determine whether there were functional differences between CD1d+ monocytes versus iDCs in their stimulation of iNKT, iNKT lines were prepared from healthy donors with a mAb against complementarity-determining region 3 of the invariant TCR (21). More than 90% of these iNKT lines from multiple donors expressed the invariant CD1d-reactive Vα24-Jα18 TCR and Vβ11 and were a mixture of CD4+CD8- and CD4-CD8- cells (data not shown).
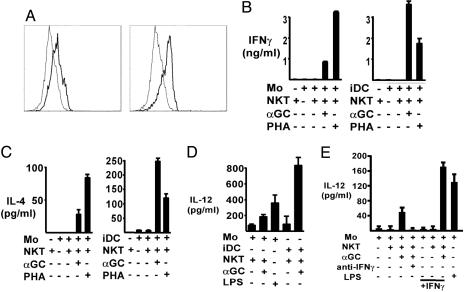
Fig. 1.
Reciprocal αgalcer-dependent iNKT and monocyte/iDC activation. (A) CD1d (thick lines) and isotype mAb control (thin lines) staining of monocytes (Left) and iDCs (Right). Viable cells were gated on CD3- and CD19-negative cells. Modest CD1d expression by both cell types is shown, with iDC expressing consistently higher levels. (B and C) IFNγ and IL-4 release by iNKT cultured with monocytes (Left) or iDCs (Right) in the absence or presence of the indicated stimuli. Results shown are for day 1. (D) iNKT were cocultured with monocytes or iDCs with the indicated stimuli. Culture supernatants were assayed for IL-12p70. Results shown are for day 3. (E) iNKT were cocultured with monocytes in the presence of neutralizing anti-IFNγ or isotype mAb or with exogenous IFNγ as shown. Day 3 cultures were assayed for IL-12p70.
Rested iNKT lines were stimulated with CD1d+ peripheral blood monocytes or iDCs in the presence or absence of αgalcer and then assessed for cytokine production as shown in Fig. 1B. There was no significant production of IFNγ when iNKT were cultured with monocytes or iDCs in the absence of αgalcer, but addition of αgalcer resulted in substantial IFNγ production after 1 day in both the monocyte and iDC cultures (Fig. 1B). IFNγ production was ≈4-fold higher in the iDC cultures (Fig. 1B), consistent with the greater amount of IL-12 produced by the iDCs (see below). IL-4 production was also stimulated by αgalcer-pulsed monocytes and iDCs and was greater in the iDC cultures (Fig. 1C). These results demonstrated that both monocytes and iDC could stimulate iNKT to produce IFNγ and IL-4, although iDCs were more potent inducers of both cytokines.
Previous studies showed that iDCs were stimulated to produce IL-12 when cultured with iNKT and αgalcer (11, 12, 18). IL-12 production was next assessed to determine whether iNKT could stimulate IL-12 production from human monocytes as well as iDCs. Significantly, both monocytes and iDC were rapidly (day 1) stimulated to produce bioactive IL-12p70 when cultured with iNKT and αgalcer (Fig. 1D). The higher levels of IL-12 produced by the iDCs were consistent with relative CD1d levels (Fig. 1 A) as well as with the increased potency of iDC as APC in general and in stimulating iNKT cytokine production (Fig. 1 B and C). Finally, experiments were performed to determine whether iNKT-induced human monocyte and iDC IL-12 responses were dependent on iNKT IFNγ as previously shown in mice (11, 12, 20). Similar coincubations of iNKT with αgalcer and monocytes were performed in the presence or absence of neutralizing mAb to IFNγ. The results showed that anti-IFNγ could substantially inhibit monocyte IL-12 production (Fig. 1E). Furthermore, additional exogenous IFNγ could enhance iNKT-dependent monocyte IL-12 production, whereas IFNγ without αgalcer had no effect (Fig. 1E).
Based on these data showing that CD1d+ human monocytes (as well as iDCs) could rapidly stimulate and be activated by iNKT, we initiated studies to further define the molecular mechanisms mediating monocyte activation by iNKT.
CD1d Ligation Induces IL-12 Production and Maturation of Monocytes. Murine studies have shown that CD40L expressed by iNKT can stimulate DCs through CD40 ligation and that IL-12 production by the activated DCs can be enhanced by iNKT-produced IFNγ (11, 12, 20). Stimulation of human iDCs by iNKT was similarly found to be CD40L-dependent (18). Significantly, DC activation by iNKT in these studies was also αgalcer-dependent, suggesting that ligation of CD1d by the invariant TCR might directly contribute to DC activation (in addition to the clear role of TCR ligation by CD1d in activating the iNKT). The data above (Fig. 1E) further supported a similar IFNγ-dependent mechanism. Therefore, the potential roles of CD1d and CD40 ligation and of IFNγ in stimulating IL-12 production by monocytes and iDC were directly assessed.
CD40 and CD1d ligation by iNKT were modeled by using plate-bound CD40 and CD1d mAb, and iNKT-produced IFNγ was replaced by exogenous IFNγ. To minimize potential inhibitory effects mediated by Fc receptors and to optimize mAb orientation, plates were first coated with protein G followed by specific or control mAb. Peripheral blood monocytes cultured on these plates for up to 8 days in the absence of IFNγ did not produce significant amounts of IL-12p40, but in the presence of IFNγ CD1d ligation resulted in IL-12p40 production (Fig. 2A). In contrast, IFNγ alone had no effect, and monocyte IL-12p40 production was not stimulated by CD40 mAb.
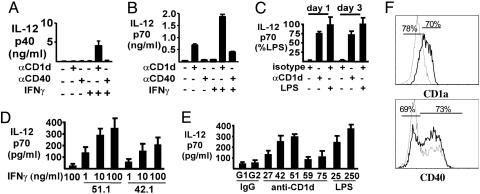
Fig. 2.
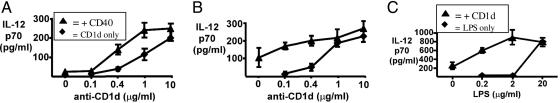
CD1d-dependent IL-12 production by peripheral blood monocytes. (A-E) Peripheral blood monocytes were cultured on plates coated with protein G followed by CD1d (51.1), CD40, or isotype control mAb (IgG2b or IgG1, respectively), with or without IFNγ (20 ng/ml), as indicated. (A) Supernatants were collected and assayed for IL-12p40 at day 8. (B) Supernatants were collected at day 3 and assayed for IL-12p70. (C) Monocytes were stimulated by culturing with CD1d mAb as above or by LPS, and IL-12p70 was measured at 1 or 3 days. (D) IFNγ dose dependence of monocyte IL-12p70 induction by plate-bound CD1d mAb 51.1 (IgG2b) and 42.1 (IgG1). Results shown are for day 3. (E) Monocytes were cultured on a panel of plate-bound CD1d or isotype control mAb plus IFNγ as above with LPS at 25 or 250 ng/ml as indicated. Results shown are for day 3; supernatants were assayed for IL-12p70. (F) Monocytes were treated with plate-bound CD1d mAb or control and cultured for 3 days with IFNγ as above. CD1a and CD40 (thick lines) or isotype mAb control (thin lines) staining. Viable cells were gated as CD3- and CD19-negative. CD1a and CD40 up-regulation was specifically accelerated by CD1d ligation.
Although the transcriptional control of IL-12 is primarily through IL-12p40, this chain associates with IL-12p35 to generate the bioactive IL-12p70. Therefore, IL-12p70 production by CD40- and CD1d-stimulated monocytes was also assessed. Monocytes cultured with CD1d mAb, even in the absence of IFNγ, produced substantial IL-12p70 (Fig. 2B). This CD1d-stimulated IL-12p70 production was further enhanced by the addition of IFNγ. CD40 mAb also stimulated IL-12p70 production, but only in the presence of IFNγ, and it was less potent than CD1d (Fig. 2B).
Based on these results, we compared monocyte production of IL-12 in response to CD1d ligation versus LPS stimulation, the latter being a well established mediator of myeloid cell maturation and IL-12 production that functions through Toll-like receptor 4. LPS rapidly stimulated the production of IL-12p70. Significantly, CD1d mAb stimulated comparable levels of IL-12p70 production after 1 or 3 days (Fig. 2C).
We also compared the activity of CD1d mAb 51.1, as used above, to several distinct mAb recognizing at least three CD1d epitopes (22-24). Both 51.1 and 42.1 recognizing distinct but overlapping CD1d epitopes showed marked induction of IL-12 with a dose-dependent enhancement by IFNγ, whereas IFNγ alone had no effect (Fig. 2D). As shown in Fig. 2E, the other CD1d mAb were also able to stimulate some monocyte IL-12p70 production. These results showed that CD1d ligation with any of several mAb directed at multiple epitopes could alone provide a rapid and potent stimulus for monocyte production of bioactive IL-12p70, which could be further enhanced by IFNγ.
Next, we addressed whether CD1d ligation could also influence monocyte differentiation. CD40 is minimally expressed by monocytes but is markedly up-regulated on iDC, and CD1a is expressed only by iDC. As shown in Fig. 2F, CD40 and CD1a up-regulation by cultured monocytes was specifically enhanced by CD1d ligation. This effect was most pronounced at day 3. By the seventh day of culture, CD40 and CD1a were up-regulated even without CD1d ligation. CD80 up-regulation was so rapid that CD1d ligation had little augmenting effect (data not shown).
CD1d Ligation Stimulates IL-12 Production by the THP-1 Monocytic Cell Line. To investigate the mechanism of CD1d-stimulated IL-12 production, we screened human cell lines and found that THP-1, a human monocytic leukemia cell line, expressed CD1d at levels similar to those observed on peripheral blood monocytes (≈1% of the level of C1R.CD1d cells), confirmed by immunoblotting (data not shown). In addition to CD1d, the THP-1 cells expressed low levels of CD1a but did not express detectable CD1b, CD1c, or CD40 (data not shown).
We next assessed IL-12 production in response to CD1d ligation on the THP-1 cells. There was no detectable production of the bioactive IL-12p70 under any conditions, including LPS, and no significant stimulation of IL-12p40 in the absence of exogenous IFNγ (data not shown). However, with the addition of IFNγ, the plate-bound CD1d mAb specifically stimulated IL-12p40 production at levels that were comparable to LPS stimulation (Fig. 3A). Consistent with the lack of detectable CD40 expression on the THP-1 cells, there was no stimulation by CD40 mAb. To further determine whether the IL-12 stimulation was specific to CD1d, the effect of CD1a ligation on THP-1 cells was also assessed. In contrast to CD1d, the plate-bound CD1a mAb did not stimulate IL-12p40 release (Fig. 3A). Plate-bound CD1b and CD1c mAb similarly had no effect.
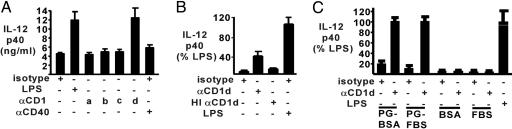
Fig. 3.
CD1d ligation-induced IL-12 production by THP-1 cells. THP-1 cells were cultured on plates coated with protein G and the indicated mAb or LPS, plus IFNγ (20 ng/ml). Supernatants were collected on day 3 and assayed for IL-12p40 (ng/ml). (A) mAb against CD1a-d or CD40 were used as stimuli. (B) CD1d mAb with or without heat inactivation (HI) or LPS were used as stimuli. (C) CD1d mAb were used either bound via protein G (PG) or directly applied to plates blocked with BSA or FBS as indicated.
Additional controls confirming that crosslinking by the plate-bound CD1d mAb was mediating the THP-1 cell stimulation included heat inactivation, which abrogated the ability of the CD1d mAb to stimulate IL-12 production (Fig. 3B). CD1d stimulation also depended on protein G coating (Fig. 3C). These results confirmed that THP-1 stimulation was directly mediated by CD1d mAb.
THP-1 Stimulation by CD1d Ligation Is Mediated Through NF-κB. THP-1 cells were next used to investigate the mechanism of CD1d-mediated activation. Because NF-κB plays a major role in regulating myeloid expression of IL-12p40 in response to activation by LPS and other Toll-like receptors, we determined whether the NF-κB pathway was activated in response to CD1d ligation on THP-1 cells. An obligate initial step in NF-κB activation is phosphorylation of IκB by IκB kinase, with subsequent ubiquitination and proteosome-mediated degradation of pIκB and release of NF-κB. To assess this activation step, we carried out immunoblotting for pIκB. THP-1 cells were preincubated with LLM (10 μM), a proteosome inhibitor to block pIκB degradation, and then added to CD1d mAb or control-coated wells. An increase in pIκB was observed within 15 min specifically in wells coated with protein G and CD1d mAb (Fig. 4A). A further increase was seen at 45 min, comparable to the activation observed with LPS at 15 min.
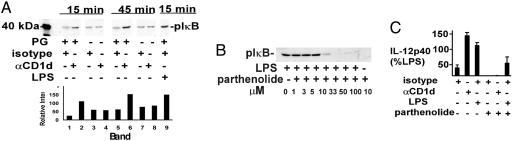
Fig. 4.
CD1d-mediated activation of NF-κB in THP-1 cells. (A) THP-1 cells were preincubated for 15 min with LLM (10 μM) and then cultured on wells coated with protein G (PG) and 51.1 CD1d or control mAb, as indicated. Cells were lysed at 15 or 45 min, and lysates were immunoblotted with anti-pIκB Ab. Densitometry was as shown. (B) THP-1 cells were pretreated for 1 h with LLM (10 μM) and parthenolide at the indicated concentrations and then stimulated with LPS for 15 min. Cell lysates were then immunoblotted with anti-pIκB. (C) THP-1 cells were cultured on wells coated with protein G and the indicated mAb in medium with IFNγ (20 ng/ml), without or with parthenolide (10 μM, preincubated for 1 h before adding to wells). Supernatants were collected on day 3 and assayed for IL-12p40. The data are presented as % LPS control (without parthenolide).
We next carried out inhibitor studies to determine whether the production of IL-12p40 in response to CD1d ligation depended on NF-κB activation. CD1d-stimulated production of IL-12 could be blocked by a number of relatively nonspecific antagonists of NF-κB, including proteosome inhibitors and pyrrolidine dithiocarbamate (not shown). Parthenolide is a relatively specific NF-κB antagonist that inhibits activity of the IκB kinase complex and phosphorylation of IκB (26). To enhance specificity, we first carried out parthenolide dose-response studies to determine the minimal concentration of drug needed to suppress LPS-mediated phosphorylation of IκB in THP-1 cells. As shown in Fig. 4B, parthenolide at a concentration of 10 μM (in the presence of LLM) was required to substantially inhibit the response to LPS.
Based on this dose-response, THP-1 cells were pretreated with 10 μM parthenolide (in the absence of LLM) and then stimulated with CD1d or isotype control mAb. Parthenolide completely blocked the basal and CD1d mAb-stimulated production of IL-12p40 (Fig. 4C). LPS-stimulated IL-12 production was only partially blocked, either reflecting more robust NF-κB activation by LPS or an NF-κB independent pathway to IL-12 production. Taken together, these data indicated that CD1d ligation stimulated IL-12 production through an NF-κB-mediated pathway.
Synergistic Effects of CD1d with Either LPS or CD40 Ligation on APC Activation. The studies above indicate that CD1d ligation can provide a rapid signal that is presumably integrated with other physiological stimuli for DC activation. To test this hypothesis, costimulation by CD1d and either CD40 ligation or LPS was assessed. As shown in Fig. 5A, CD40 mAb (1 μg/ml) alone was a very weak early stimulus of iDC at 1 day, but it significantly enhanced the stimulation by CD1d. Synergy was again observed at day 3, with CD40 enhancing stimulation by lower concentrations of CD1d mAb (Fig. 5B). At lower concentrations of CD40 mAb, similar synergy was seen, whereas at 10 μg/ml CD1d (Fig. 5 A and B) or CD40 mAb (data not shown) the stronger iDC responses precluded detection of CD1d synergy.
Fig. 5.
CD1d-dependent IL-12 production by iDCs. Peripheral blood-derived iDC were incubated on plates coated with protein G followed by CD1d (51.1) and CD40, isotype control mAb (IgG2b and IgG1, respectively), or LPS (0-20 μg/ml) plus IFNγ. Supernatants were assayed for IL-12p70. (A) Day 1 iDC dose-response curves for CD1d mAb with or without 1 μg/ml CD40 mAb. (B) Day 3 iDC dose-response curves for CD1d mAb with or without 1 μg/ml CD40 mAb. (C) Day 3 iDC dose-response curve for LPS with or without 1 μg/ml CD1d mAb.
Similarly, low concentrations of LPS produced significantly less monocyte IL-12, which could be specifically enhanced in the presence of CD1d mAb (Fig. 5C). A second CD1d mAb, 42.1, which alone could also induce IL-12 (Fig. 2 D and E), could also act synergistically with CD40 mAb (data not shown). Therefore, two distinct known physiologically important stimuli of APC IL-12 production (LPS and CD40) could act in synergy with ligation by different CD1d mAb.
Discussion
This study addressed the molecular interactions mediating activation of human CD1d+ monocytic lineage cells by CD1d-reactive NKT cells. We initially showed that cultured human iNKT could stimulate the rapid production of IL-12 from both αgalcer-pulsed CD1d+ monocytes and iDCs. Ab blocking implicated IFNγ in the IL-12 response of monocytes, consistent with murine data (11, 12). Possible mechanisms mediating this stimulation, including CD40L ligation of APC CD40, TCR ligation of the CD1d-αgalcer complex, and iNKT-produced IFNγ, were then further dissected. Remarkably, CD1d ligation alone, through any of multiple independent mAb against at least three CD1d epitopes, could rapidly stimulate CD1d+ monocytes as well as iDC to produce bioactive IL-12p70. Furthermore, CD1d ligation enhanced monocyte differentiation, consistent with this representing a physiological response. Notably, IFNγ by itself had no effect, but it markedly increased the CD1d-mediated stimulation of IL-12 production. CD1d-mediated stimulation of IL-12 production was confirmed in the human THP-1 monocytic leukemia cell line and was found to be mediated by rapid activation of the NF-κB signal transduction pathway. Finally, we found that both CD40 and LPS, two known physiological stimuli of iDC and monocytes, respectively, could act synergistically with CD1d to stimulate IL-12p70 production. Taken together, these studies support a direct role for CD1d ligation in the rapid activation of the innate immune response by CD1d-reactive NKT cells.
A major function of conventional activated CD4+ T cells is to interact with iDCs bearing cognate MHC class II-presented antigens and stimulate their further maturation through CD40L ligation of CD40. Autoreactive T cell clones specific for group 1 CD1 proteins (CD1a, b, or c) were similarly found to stimulate the maturation of human iDCs, but distinct mechanisms including TNFα appeared to be involved and IL-12 production was observed only when iDCs were also stimulated with LPS (18). In contrast to previous studies, the data reported here demonstrate that CD1d ligation can directly mediate monocytic cell activation. The ability to directly stimulate monocytes through CD1d ligation may be a unique property of CD1d-reactive NKT cells and reflect an important physiological role for iNKT-monocyte interactions in the initiation of innate immune responses. The hypothesis that monocytes are physiologically activated by iNKT through CD1d ligation is consistent with the constitutive expression of CD1d on monocytes as well as DCs (23, 24), versus the regulated expression of proteins mediating interactions with conventional T cells (MHC class II, CD1a-c, CD40, CD80, and CD86).
CD1d has not been shown to associate with adaptor proteins that might link it to NF-κB, and previous biochemical studies of CD1d have focused on endosomal targeting and its role in antigen presentation (27, 28). However, there are data suggesting that the CD1d cytoplasmic tail may mediate other interactions. An early study found that human T cell lines could be stimulated to release intracellular Ca2+ by Abs to CD1b or CD1c but not by Abs to CD1a (29), which uniquely lacks a cytoplasmic tail tyrosine. More recently it was found that crosslinking of CD1d, but not a cytoplasmic tail deletion mutant, on intestinal epithelial cells could stimulate IL-10 production (30).
In other studies CD1d was shown to bind to the MHC class II-invariant chain complex in the endoplasmic reticulum and associate with this complex on the cell surface, interactions that were independent of the CD1d cytoplasmic tail (31). This MHC class II association is intriguing, because TCR-mediated ligation of MHC class II proteins can provide a coactivation signal in B cells (32). However, this B cell signal is transduced through MHC class II association with the B cell receptor and its signal transduction complex, which are not present in monocytes. One study has shown that human monocytes can be activated by MHC class II crosslinking through mitogen-activated protein kinase pathways (33). Although we have not observed consistent mitogen-activated protein kinase pathway activation in response to CD1d ligation (data not shown), a link between MHC class II and CD1d signaling in monocytic cells remains possible. The established role of NF-κB in activating IL-12p40 transcription in response to other stimuli suggests that CD1d ligation functions similarly upstream of NF-κB. Further studies are underway to determine whether CD1d is linked to NF-κB by AP-2 or MHC class II proteins or by a distinct mechanism.
Recently, physiologically relevant endogenous and pathogen-derived relatively high-affinity CD1d-presented antigens recognized by the invariant TCR have been defined (34-36). This also is significant with respect to CD1d-mediated signal transduction, because the invariant TCR may require a high-affinity antigen to achieve a threshold level of CD1d crosslinking. However, an alternative to a high-affinity antigen is a lower-affinity multivalent glycolipid antigen. The invariant TCR could function to stabilize or enhance the clustering of CD1d that is bound to low-affinity multivalent antigen on the monocyte cell surface. In this context, CD1d is known to accumulate in lipid rafts, and their disruption can inhibit iNKT recognition (37). An interaction between CD1d and MHC class II on the cell surface may provide a further mechanism for CD1d clustering through CD4 binding of MHC class II/CD1d complexes in the case of CD4+ iNKT. In any case, even with a relatively high-affinity CD1d ligand it is not clear whether the invariant TCR can mediate the level of CD1d crosslinking achieved with CD1d Abs. Indeed, it seems likely that the signal generated in vivo by CD1d ligation will in many cases not be strong enough to by itself trigger monocyte activation and will instead be integrated with other signals. In addition to CD40 ligation, this includes at least one Toll-like receptor-mediated signal (LPS) functioning through NF-κB, which would provide a molecular mechanism for iNKT modulation of innate immune responses.
Acknowledgments
We thank colleagues, especially Dr. S. Porcelli (Albert Einstein College of Medicine, Bronx, NY) for CD1d mAb; Drs. M. Brenner, S. Porcelli, and S. B. Wilson for advice; and Kirin Brewery for αgalcer. This work was supported by the National Institutes of Health (Grant R01 DK066917 to M.A.E. and Grant R01 AI42955 to S.P.B.), the Dana Farber/Harvard Cancer Center Melanoma Specialized Program of Research Excellence (Grant P50 CA93683), and the Hershey Family Prostate Cancer Research Fund.
Author contributions: S.P.B. and M.A.E. designed research; S.C.Y., A.S., and R.W. performed research; S.C.Y., A.S., R.W., and M.A.E. analyzed data; and M.A.E. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NKT cell, natural killer T cell; iNKT, invariant NKT cell; αgalcer, α-galactosylceramide; DC, dendritic cell; iDC, immature DC; LLM, N-acetyl-l-leucinyl-l-leucinyl-methional; APC, antigen-presenting cell; TCR, T cell receptor; pIκB, phospho-IκB.
References
- 1.Bendelac, A., Rivera, M. N., Park, S. H. & Roark, J. H. (1997) Annu. Rev. Immunol. 15, 535-562. [DOI] [PubMed] [Google Scholar]
- 2.Porcelli, S. A. & Modlin, R. L. (1999) Annu. Rev. Immunol. 17, 297-329. [DOI] [PubMed] [Google Scholar]
- 3.Benlagha, K. & Bendelac, A. (2000) Semin. Immunol. 12, 537-542. [DOI] [PubMed] [Google Scholar]
- 4.Stein-Streilein, J. (2003) J. Exp. Med. 198, 1779-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawano, T., Cui, J., Koezuka, Y., Toura, I., Kaneko, Y., Motoki, Ueno, H., Nakagawa, R., Sato, H., Kondo, E., et al. (1997) Science 278, 1626-1629. [DOI] [PubMed] [Google Scholar]
- 6.Brossay, L., Chioda, M., Burdin, N., Koezuka, Y., Casorati, G., Dellabona, P. & Kronenberg, M. (1998) J. Exp. Med. 188, 1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burdin, N., Brossay, L., Koezuka, Y., Smiley, S. T., Grusby, M. J., Gui, M., Taniguchi, M., Hayakawa, K. & Kronenberg, M. (1998) J. Immunol. 161, 3271-3281. [PubMed] [Google Scholar]
- 8.Spada, F. M., Koezuka, Y. & Porcelli, S. A. (1998) J. Exp. Med. 188, 1529-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carnaud, C., Lee, D., Donnars, O., Park, S. H., Beavis, A., Koezuka, Y. & Bendelac, A. (1999) J. Immunol. 163, 4647-4650. [PubMed] [Google Scholar]
- 10.Eberl, G. & MacDonald, H. R. (2000) Eur. J. Immunol. 30, 985-992. [DOI] [PubMed] [Google Scholar]
- 11.Tomura, M., Yu, W. G., Ahn, H. J., Yamashita, M., Yang, Y. F., Ono, S., Hamaoka, T., Kawano, T., Taniguchi, M., Koezuka, Y. & Fujiwara, H. (1999) J. Immunol. 163, 93-101. [PubMed] [Google Scholar]
- 12.Kitamura, H., Iwakabe, K., Yahata, T., Nishimura, S., Ohta, A., Ohmi, Y., Sato, M., Takeda, K., Okumura, K., Van Kaer, L., et al. (1999) J. Exp. Med. 189, 1121-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson, S. B., Kent, S. C., Horton, H. F., Hill, A. A., Bollyky, P. L., Hafler, D. A., Strominger, J. L. & Byrne, M. C. (2000) Proc. Natl. Acad. Sci. USA 97, 7411-7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang, O. O., Racke, F. K., Nguyen, P. T., Gausling, R., Severino, M. E., Horton, H. F., Byrne, M. C., Strominger, J. L. & Wilson, S. B. (2000) J. Immunol. 165, 3756-3762. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura, T., Kitamura, H., Iwakabe, K., Yahata, T., Ohta, A., Sato, M., Takeda, K., Okumura, K., Van Kaer, L., Kawano, T., et al. (2000) Int. Immunol. 12, 987-994. [DOI] [PubMed] [Google Scholar]
- 16.Naumov, Y. N., Bahjat, K. S., Gausling, R., Abraham, R., Exley, M. A., Koezuka, Y., Balk, S. B., Strominger, J. L., Clare-Salzer, M. & Wilson, S. B. (2001) Proc. Natl. Acad. Sci. USA 98, 13838-13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faunce, D. E. & Stein-Streilein, J. (2002) J. Immunol. 169, 31-38. [DOI] [PubMed] [Google Scholar]
- 18.Vincent, M. S., Leslie, D. S., Gumperz, J. E., Xiong, X., Grant, E. P. & Brenner, M. B. (2002) Nat. Immunol. 3, 1163-1168. [DOI] [PubMed] [Google Scholar]
- 19.Brigl, M., Bry, L., Kent, S. C., Gumperz, J. E. & Brenner, M. B. (2003) Nat. Immunol. 4, 1230-1237. [DOI] [PubMed] [Google Scholar]
- 20.Hayakawa, Y., Takeda, K., Yagita, H., Van Kaer, L., Saiki, I. & Okumura, K. (2001) J. Immunol. 166, 6012-6018. [DOI] [PubMed] [Google Scholar]
- 21.Tahir, S. M., Cheng, O., Shaulov, A., Koezuka, Y., Bubley, G. J., Wilson, S. B., Balk, S. P. & Exley, M. A. (2001) J. Immunol. 167, 4046-4050. [DOI] [PubMed] [Google Scholar]
- 22.Exley, M., Garcia, J., Balk, S. P. & Porcelli, S. (1997) J. Exp. Med. 186, 109-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Exley, M., Garcia, J., Wilson, S. B., Spada, F., Gerdes, D., Tahir, S. M., Patton, K. T., Blumberg, R. S., Porcelli, S., Chott, A. & Balk, S. P. (2000) Immunology 100, 37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spada, F. M., Borriello, F., Sugita, M., Watts, G. F., Koezuka, Y. & Porcelli, S. A. (2000) Eur. J. Immunol. 30, 3468-3477. [DOI] [PubMed] [Google Scholar]
- 25.Nieda, M., Nicol, A., Koezuka, Y., Kikuchi, A., Takahashi, T., Nakamura, H., Furukawa, H., Yabe, T., Ishikawa, Y., Tadokoro, K., et al. (1999) Hum. Immunol. 60, 10-19. [DOI] [PubMed] [Google Scholar]
- 26.Hehner, S. P., Hofmann, T. G., Droge, W. & Schmitz, M. L. (1999) J. Immunol. 163, 5617-5623. [PubMed] [Google Scholar]
- 27.Roberts, T. J., Sriram, V., Spence, P. M., Gui, M., Hayakawa, K., Bacik, I., Bennink, J. R., Yewdell, J. W. & Brutkiewicz, R. R. (2002) J. Immunol. 168, 5409-5414. [DOI] [PubMed] [Google Scholar]
- 28.Cernadas, M., Sugita, M., van der, W., Cao, X., Gumperz, J. E., Maltsev, S., Besra, G. S., Behar, S. M., Peters, P. J. & Brenner, M. B. (2003) J. Immunol. 171, 4149-4155. [DOI] [PubMed] [Google Scholar]
- 29.Theodorou, I. D., Boumsell, L., Calvo, C. F., Gouy, H., Beral, H. M. & Debre, P. (1990) J. Immunol. 144, 2518-2523. [PubMed] [Google Scholar]
- 30.Colgan, S. P., Hershberg, R. M., Furuta, G. T. & Blumberg, R. S. (1999) Proc. Natl. Acad. Sci. USA 96, 13938-13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang, S. J. & Cresswell, P. (2002) EMBO J. 21, 1650-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang, P., Stolpa, J. C., Freiberg, B. A., Crawford, F., Kappler, J., Kupfer, A. & Cambier, J. C. (2001) Science 291, 1537-1540. [DOI] [PubMed] [Google Scholar]
- 33.Matsuoka, T., Tabata, H. & Matsushita, S. (2001) J. Immunol. 166, 2202-2208. [DOI] [PubMed] [Google Scholar]
- 34.Zhou, D., Mattner, J., Cantu, C., Schrantz, N., Yin, N., Gao, Y., Sagiv, Y., Hudspeth, K., Wu, Y. P., Yamashita, T., et al. (2004) Science 306, 1786-1789. [DOI] [PubMed] [Google Scholar]
- 35.Wu, D., Xing, G. W., Poles, M. A., Horowitz, A., Kinjo, Y., Sullivan, B., Bodmer-Narkevitch, V., Plettenburg, O., Kronenberg, M., Tsuji, M., et al. (2005) Proc. Natl. Acad. Sci. USA 102, 1351-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattner, J., DeBord, K. L., Ismail, N., Goff, R. D., Cantu, C., III, Zhou, D., Saint-Mezard, P., Wang, V., Gao, Y., Yin, N., et al. (2005) Nature 434, 525-529. [DOI] [PubMed] [Google Scholar]
- 37.Lang, G. A., Maltsev, S. D., Besra, G. S. & Lang, M. L. (2004) Immunology 112, 386-396. [DOI] [PMC free article] [PubMed] [Google Scholar]