Abstract
How small numbers of CD4+CD25+ regulatory T cells suppress autoimmune responses in vivo is unclear. In this report we analyze the immunomodulatory activity of CD4+CD25+ T cells that are antigen-specifically redirected against myelin basic protein (MBP)89-101-specific autoreactive T cells by a MBP89-101-IAs-ζ chimeric receptor. We have previously shown that these redirected regulatory T cells are highly potent in treating a model autoimmune disease, experimental allergic encephalomyelitis. We show here that they have only limited effect in vivo on autoreactive T cell proliferation and therefore do not act by deleting or suppressing the expansion of pathologic effector cells. Rather, the redirected CD4+CD25+ T cells divert the pathologic T helper 1 self-specific T cell response to one characterized by high IL-10 and lower IL-4 production. Significantly, when isolated from the inducing CD4+CD25+ regulatory T cells, these self-specific T cells can independently suppress the autoreactive T cell response and experimental allergic encephalomyelitis development in an IL-10-dependent manner. These results provide evidence that CD4+CD25+ regulatory T cells can manipulate the adaptive immune response in vivo through the infectious induction of tolerance, specifically by promoting the formation of antigen-specific, IL-10-secreting regulatory T cells.
Keywords: immune tolerance, interleukin-10
CD4+CD25+ T cells include a lineage of anergic regulatory cells that are characterized by expression of the FoxP3 transcription factor (1, 2). How these regulatory cells modulate immune responses is not well characterized. In vitro, they can both directly target responding T cells and down-modulate antigen-presenting cells (3-5). CD4+CD25+ T cells may also convert CD4+ helper T cells into regulatory cells expressing IL-10 and/or TGF-β in culture systems (6-8). In vivo, IL-10 and/or TGF-β seem critical for regulatory T cell activity (9, 10).
Because only down-modulatory functions have been attributed to CD4+CD25+ T cells, they represent a promising candidate for the cellular immunotherapy of autoimmune disease. In several studies, adoptively transferred antigen-nonspecific CD4+CD25+ T cells prevented or treated autoimmune, alloimmune, or even inflammatory diseases (9-16). These findings suggest that merely increasing the numbers of these cells may alter the balance between tolerance and immunity. Yet, infusions of nonspecific regulatory T cells would be expected to have poor specificity and low potency, and specifically redirecting regulatory T cells against target T cells presents a significant challenge.
We developed an approach to redirect CD4+CD25+ T cells using transgenically expressed chimeric receptors that include an antigen-MHC extracellular domain and an intracellular immunoreceptor tyrosine-based activation motif (ITAM)-containing signaling domain (17-19). The extracellular antigen-MHC serves as a bait, engaging the T cell receptor of potentially pathologic antigen-specific T cells. This engagement generates an ITAM-mediated signal through the chimeric receptors' activation domain, which induces effector functions in the modified T cell.
We previously showed that myelin basic protein (MBP)89-101-IAs-ζ receptor-modified CD4+CD25+ T cells [receptor-modified T cells (RMTC)], but not unmodified CD4+CD25+ T cells or CD4+CD25- RMTC, potently treated experimental allergic encephalomyelitis (EAE) even after epitope spread had diversified the pathological T cell response to include additional nontargeted epitopes (20). In this report, we describe that the redirected regulatory T cells induce the formation of MBP-specific regulatory T cells that, when isolated from the therapeutic RMTC and transferred into naïve mice, suppress EAE in an IL-10-dependent manner. These findings provide evidence that CD4+CD25+ regulatory T cells can induce infectious tolerance in vivo, catalyzing the formation of IL-10-producing regulatory cells.
Materials and Methods
Mice. Female SJL/J mice obtained from The Jackson Laboratory were age-matched within each experiment. 25S89P [MBP89-101-IAs-ζ transgenic (Tg)] mice (18, 21) were backcrossed at least seven generations with SJL/J mice before analysis.
Peptides. MBP89-101 (VHFFKNIVTPRTP) and proteolipid protein (PLP)139-151 (HSLGKWLGHPDKF) were synthesized and purified to >90% purity by the St. Jude Hartwell Center for Biotechnology.
Antibodies and Flow Cytometry. Anti-CD4 (RM4-5) and anti-CD25 (7D4) antibodies were obtained from Pharmingen. Anti-class II MHC antibody was obtained from Devaron (Dayton, NJ; IA.B2) or Pharmingen (KH74). Flow cytometry was performed on a FACSCalibur (Becton Dickinson), and flow cytometric sorting was performed on a MoFlo high-speed cell sorter (Dako).
EAE Induction and Clinical Evaluation. EAE to MBP89-101 was induced as described in ref. 22. EAE to PLP139-151 was induced by immunizing SJL/J mice with 100 μg of peptide emulsified in complete Freund's adjuvant (Difco) subcutanteously followed by 200 ng of pertussis toxin (List Biological Laboratories, Campbell, CA) i.v. on days 0 and 2. Clinical scoring was as follows: 1, limp tail; 2, hind limb paresis or partial paralysis; 3, total hind limb paralysis; 4, hind limb paralysis and body/front limb paresis/paralysis; 5, moribund.
Adoptive Transfer and Purging. Adoptively transferred cells, described in the text, were washed three times with saline before i.v. administration in a 100-μl volume. For purging, draining lymph node (LN) cells from immunized and treated mice were stained with chimeric receptor-specific anti-class II MHC antibody. Class II+ cells were depleted by flow cytometric sorting, and 25 × 106 purged cells were washed and transferred into naïve SJL/J recipients.
Growth of MBP89-101-Specific T Cell Lines. T cells isolated from mice immunized with MBP89-101/complete Freund's adjuvant were cultured with 15 μg/ml MBP89-101 peptide, 1 ng/ml recombinant murine IL-2 (R & D Systems), and irradiated syngeneic splenocytes for 2 days, purified over Lymphocyte Separation Medium (Cambrex, East Rutherford, NJ), and recultured in IL-2 for an additional 5-7 days before restimulation as above.
T Cell Proliferation. Draining LN cells from immunized mice were cultured at 2-3 × 105 per well in 96-well plates without antigenic peptide or with the designated peptide concentration, pulsed with 1 μCi (1 Ci = 37 GBq) of [3H]thymidine after 72 h of culture, and then harvested for scintillation counting. Samples were analyzed in triplicate.
Cytokine Analysis of Draining LN Cells and Splenocytes. LN cells and splenocytes were harvested and washed, and 2-3 × 105 cells were cultured in 96-well plates without antigenic peptide or with the designated peptide concentration. Culture supernatants were assayed at 48 h for IL-10, IFN-γ, and IL-4 by sandwich ELISA (Pharmingen) or Bio-Plex (Bio-Rad) assays according to the manufacturers' protocols. TGF-β1 was measured by using the Duoset ELISA development kit (R & D Systems). Alternatively, antigenically stimulated or unstimulated LN cells were analyzed by RNase protection assay by using the mCK-1 mouse cytokine probe template (Pharmingen) and [32P]dUTP. Expression of TGF-β was confirmed by quantitative competitive PCR (Maxim Biotech, South San Francisco, CA).
Evaluation of the Effect of Anti-IL-4 and Anti-IL-10R Antibodies on EAE and Proliferation. Mice were immunized to induce EAE disease or draining LN proliferative responses. Therapeutic or control cells were administered i.v. at the time of immunization (day 0). The mice were further treated i.p. with 1 mg of purified blocking anti-mouse IL-4 (11B.11, National Cancer Institute Biological Resources Branch, Rockville, MD) or/and 0.5 mg of anti-mouse IL-10R (1B1.3a, Pharmingen) antibodies or with control antibody (rat IgG) on days 0 and 7.
Statistics. Statistical significance in EAE studies was determined by calculating the mean daily score for individual animals during the time of observation. Experimental and control groups were compared by using two-sided t tests at P < 0.05 with excel spreadsheet software (Microsoft). Error bars show ± 1 SD.
Results
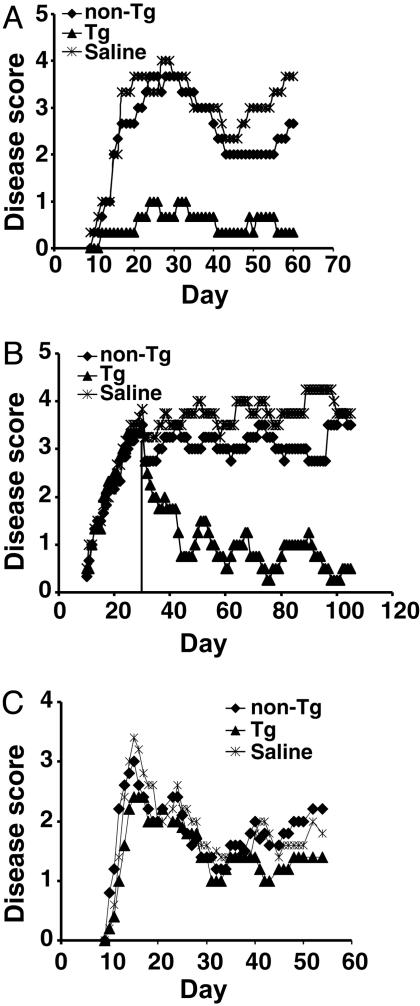
Characterization of Receptor-Modified CD4+CD25+ T Cells. 25S89P Tg mice express a MBP89-101-IAs-ζ chimeric receptor selectively on T cells (18, 21). Tg CD4+CD25+ T cells are anergic and mediate bystander suppression like non-Tg regulatory cells. They secrete regulatory cytokines such as IL-4 and IL-10 when stimulated through their chimeric receptor by MBP89-101-specific T cells in a pattern identical to that produced when Tg or non-Tg CD4+CD25+ T cells are mitogenically stimulated (20). Tg cells at low doses are further able to antigen-specifically prevent EAE induced with MBP89-101 peptide and treat it even 1 month after induction, when epitope spreading has diversified the T cell response to include specificities not directly targeted by the RMTC (Fig. 1 A and B and ref. 20). The potency of the Tg regulatory cells was significantly greater than that of non-Tg CD4+CD25+ T cells, which have been shown to down-modulate EAE in other models (10, 11). We found limited and inconsistent effects of these cells in our system at doses of up to 3 × 106 cells, contrasting with the consistently strong suppression mediated by the Tg CD4+CD25+ cells at doses as low as 5 × 105 cells (Fig. 1 and ref. 20). In contrast to regulatory T cells, Tg CD4+CD25- T cells showed no disease-inhibitory activity at any dose tested (20). The Tg regulatory cells acted in an antigen-specific manner and were unable to suppress disease mediated by an alternative encephalitic antigen, PLP139-151 (Fig. 1C).
Fig. 1.
Prevention and treatment of EAE with CD4+CD25+ RMTC. (A) Prophylactic treatment. A total of 1 × 106 flow cytometrically purified CD4+CD25+ RMTC, control CD4+CD25+ non-Tg T cells, or saline was i.v. administered into SJL/J mice at the time of disease induction, and clinical disease was monitored. (B) Treatment of EAE after epitope spread. Treatment with 1 × 106 Tg or non-Tg cells or saline was delayed until 31 days after disease induction (vertical line). At this time T cell response was detected not only against the initiating MBP89-101 epitope but also against pathologic PLP139-151 and PLP178-191 epitopes (18). Disease curves were recalculated at the time of therapy to exclude animals that were not treated because of death or a moribund state. (C) Treatment with 1 × 106 Tg or non-Tg CD4+CD25+ cells or saline at the time of induction of EAE with PLP139-151. Plots show mean clinical score from four to six animals per treatment group. Representative experiments are shown.
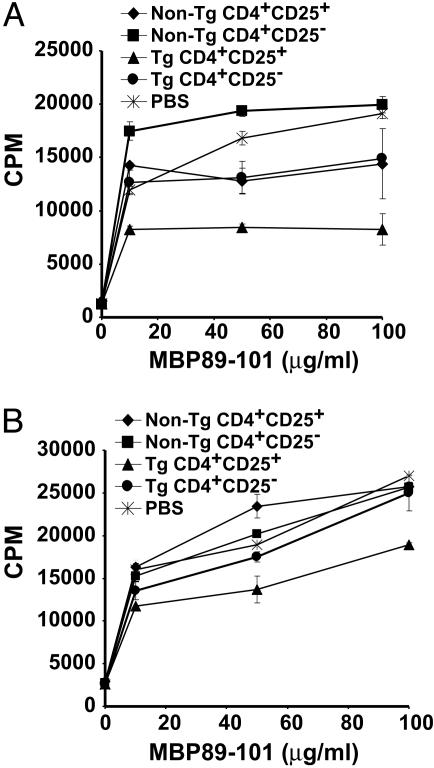
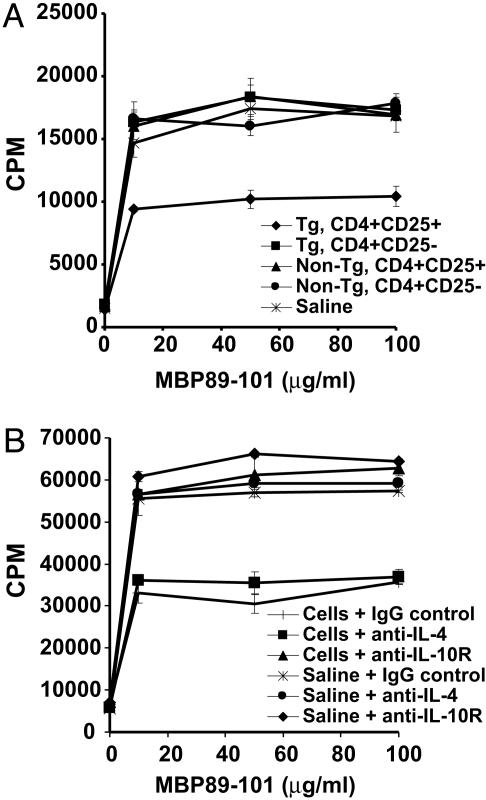
CD4+CD25+ RMTC Induce a Cytokine Shift in MBP89-101-Reactive T Cells. To better define the mechanism of action of the CD4+CD25+ RMTC, we monitored MBP89-101-specific proliferative response in the draining LN cells of mice immunized with MBP89-101 and treated with CD4+CD25+ RMTC or controls. In contrast to the strong bystander inhibition of T cell proliferation characteristic of CD4+CD25+ T cells in vitro (20), incomplete proliferation inhibition was observed in vivo (Fig. 2A). The implication that the CD4+CD25+ RMTC did not suppress disease by eliminating or restricting proliferation of autoantigen-specific T cells was verified in studies of T cell responses from mice treated with RMTC 31 days after immunization. In these mice, analysis of splenocytes from 10 days after RMTC treatment (Fig. 2B) to as long as 70 days after treatment (data not shown) showed MBP-specific T cell proliferative responses that were only subtly decreased relative to controls. Despite disease resolution at these time points (Fig. 1B), CD4+CD25+ RMTC therefore had only limited effects on autoantigen-mediated T cell proliferative responses.
Fig. 2.
Ex vivo proliferation of MBP89-101-specific T cells after RMTC treatment. (A) SJL mice received 1 × 106 flow cytometrically purified CD4+CD25+ RMTC, designated control cells, or saline i.v. at the time of s.c. immunization with MBP89-101 in complete Freund's adjuvant. On day 10, draining LN cells were isolated and cultured for 3 days in the presence of graded doses of MBP89-101 peptide. Peptide-specific proliferation was measured at 72 h by [3H]thymidine incorporation. (B) EAE was induced in SJL mice with MBP89-101 peptide. Thirty-one days later, the mice were treated with 1 × 106 RMTC, control cells, or saline. Ten days after treatment, splenocytes were isolated, and MBP89-101-specific proliferative response was measured as in A. Essentially identical results were obtained in analyses of LN cells from the same mice (data not shown). Mean cpm incorporation from three animals per group, each independently assayed in triplicate, ±1 SD is plotted.
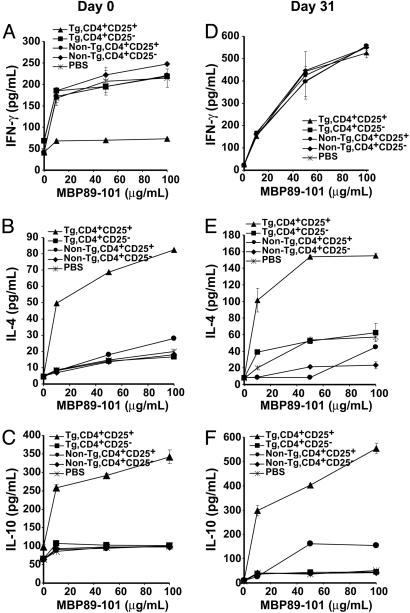
The continued responsiveness of autoreactive T cells after treatment with CD4+CD25+ RMTC despite the regression of disease symptoms suggested that the RMTC blocked the pathogenicity of autoreactive lymphocytes. Pathologic MBP-specific T cells must be T helper 1 (Th1)-polarized to orchestrate the CNS inflammation that causes EAE. To determine whether this polarization persisted after RMTC treatment, we analyzed autoantigen-induced cytokine production in draining LN cells from MBP89-101 immunized mice. Cells from untreated animals, as well as animals treated with control non-Tg CD4+CD25+ T cells or CD4+CD25- RMTC, produced significant amounts of the Th1 cytokine IFN-γ (Fig. 3A). In contrast, cells derived from mice treated with CD4+CD25+ RMTC produced little of this cytokine. This finding suggests that the CD4+CD25+ RMTC did not eliminate the autoreactive T cells, but rather acted to reduce their pathogenicity.
Fig. 3.
Ex vivo cytokine production by MBP89-101-specific T cells after RMTC treatment. Mice were treated at the time of immunization (A-C) or 31 days after EAE induction (D-F) as in Fig. 1. Draining LN cells (A-C) or splenocytes (D-F) were isolated 10 days later and stimulated with the designated concentration of MBP89-101 peptide for 48 h before analyzing cytokine secretion in the supernatant. Splenocytes from day-0-treated mice and LN cells from day-31-treated mice showed essentially identical results (data not shown). The mean cytokine production from three animals per group, each analyzed in triplicate, ±1 SD is plotted.
IL-4 and IL-10 are protective T cell-derived cytokines in EAE (23-26); therefore, we analyzed MBP89-101-induced IL-4 and IL-10 production by draining LN autoreactive T cells (Fig. 3 B and C). Autoantigen-induced IL-4 production was increased in T cells treated with the CD4+CD25+ RMTC but not other cell types. The amount of IL-4 produced was low. Indeed, we have also used Th2-differentiated RMTC to similarly target MBP89-101-specific T cells in vivo, and, when this is done, the MBP89-101-induced IL-4 production is ≈5-fold higher than after the use of CD4+CD25+ RMTC here (27).
In contrast to the relatively weak IL-4 production, autoantigen-induced IL-10 production was strongly increased, implying a role for this cytokine in the immunotherapeutic effect. This increased IL-10 production was confirmed by RNase protection assay, with a 67-fold increase in IL-10 mRNA in MBP89-101-stimulated draining LN cells from mice receiving CD4+CD25+ RMTC compared with control non-Tg CD4+CD25+ T cells (data not shown).
TGF-β may also down-modulate inflammatory T cell functions. However, little TGF-β production was detected in draining LN cells cultured from mice treated with CD4+CD25+ RMTC, and this TGF-β was not produced in an autoantigen-dependent manner. Furthermore, levels of TGF-β mRNA, determined by quantitative competitive PCR, were identical in MBP89-101-stimulated LN cells from mice treated with either Tg or non-Tg CD4+CD25+ lymphocytes (data not shown). Our results demonstrate that treatment with CD4+CD25+ RMTC shifts the autoreactive T cell response from a Th1 response to one characterized by high IL-10 and more limited IL-4 production. Consistent with the results in Fig. 1C, treatment with the CD4+CD25+ RMTC was antigen-specific and the RMTC did not influence the PLP-specific draining LN proliferation or Th1-skewed pattern of cytokine production after immunization with PLP139-151 (data not shown).
Cytokine production in MBP-specific T cells from mice treated with RMTC 31 days after disease induction differed from that of cells from mice treated at the time of immunization. Whereas treatment on day 0 substantially reduced IFN-γ production by autoreactive T cells, day-31-treated cells showed undiminished production of this Th1 cytokine (Fig. 3D) despite disease resolution (Fig. 1B).
In contrast to the unchanged IFN-γ response, day-31 treatment induced MBP89-101-specific T cells producing significant amounts of IL-10 and lower levels of IL-4 (Fig. 3 E and F). These results imply that, although the CD4+CD25+ RMTC were not capable of eliminating Th1-deviated autoreactive T cells at this late time point, they could induce the development or expansion of IL-4- and IL-10-producing T cells capable of counteracting their pathologic effects.
Regulatory Activity of RMTC-Induced, IL-10-Producing, Self-Specific T Cells. IL-10 down-regulates effector macrophage as well as other cells needed for the development and sustenance of autoimmunity. Considering the efficacy of small numbers of CD4+CD25+ RMTC, it was possible that they acted in part by catalyzing the development of additional regulatory T cells. We therefore wanted to determine whether the IL-10-producing, MBP-specific T cells induced by the CD4+CD25+ RMTC had regulatory function independent of the CD4+CD25+ RMTC. To test for this function, we immunized mice with MBP89-101 and treated them with 106 RMTC or controls. Draining LN cells were isolated 10 days after immunization and purged of RMTC by staining with a chimeric receptor (class II MHC)-specific antibody and flow cytometric negative selection. A total of 2.5 × 107 of the RMTC-purged cells, which contain the MBP-specific, IL-10-producing T cells, were then transferred into naïve recipients. These recipients were immunized with MBP89-101, and the development of clinical EAE was monitored.
In preliminary studies we determined the efficiency of our purging regimen. Because of the small number of RMTC transferred in our immunotherapy system, the CD4+CD25+ chimeric receptor-positive (class II MHC+) T cells were not detectable from background staining (≈0.8% of total draining LN cells) before purging. Thus, a dose of 2.5 × 107 draining LN cells would contain at most 2 × 105 class II+ RMTC. To test purging efficiency of these cells, non-Tg draining LN cells were mixed with an equal number of Tg cells, purged, and restained and analyzed for residual RMTC. These cells contained only ≈1% of the original CD4+ class II+ population. Thus, after purging, a dose of 2.5 × 107 cells would be expected to include <2 × 103 class II+ CD4+CD25+ RMTC, well less than the minimal 2.5 × 105 dose we previously found to be required for a partial therapeutic effect (20).
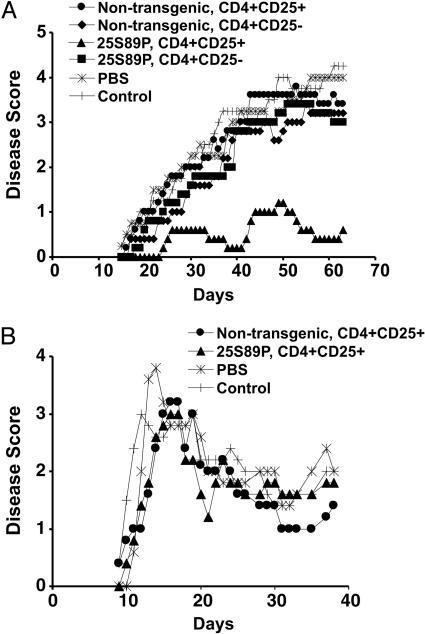
EAE severity in mice treated with purged cells derived from each of the control animals was similar to that of saline-treated mice. In contrast, mice treated with purged cells derived from CD4+CD25+ RMTC-treated animals were protected (Fig. 4A). The MBP-specific cells acted antigen-specifically and were not effective in preventing disease induced with PLP139-151 (Fig. 4B). To verify that the induced cells were indeed MBP-specific, we expanded the purged cells with MBP89-101 in culture for three generations. The culture conditions lacked the high-dose IL-2 required for the maintenance/growth of CD4+CD25+ regulatory cells and thus provided an additional mechanism to exclude low numbers of residual unpurged RMTC. The cultured cells from RMTC-treated mice, but not controls, protected naïve mice from the development of EAE similarly to the freshly isolated cells (data not shown). Therefore, the CD4+CD25+ RMTC catalyze the development of regulatory T cells that independently and antigen-specifically suppress EAE development.
Fig. 4.
Induction of regulatory T cells by CD4+CD25+ RMTC. (A) A total of 1 × 106 Tg CD4+CD25+ RMTC, the indicated control populations, or saline (PBS) was i.v. administered into SJL mice at the time of s.c. immunization with MBP89-101. Draining LN cells were isolated 10 days later and purged of the RMTC by staining with chimeric receptor-specific antibody (anti-IAs) and flow cytometric sorting. A total of 25 × 106 of the purged cells or saline (control) was directly transferred into naïve SJL mice before EAE induction. Clinical disease was monitored. (B) Treatment with the purged draining LN cells of EAE mediated by an alternative autoantigen, PLP139-151, is shown. The purged cells were obtained as in A. Means of five mice per group are plotted.
To better understand the mechanism of action of the purged RMTC-induced regulatory T cells, we treated immunized mice with them and analyzed the proliferative response of draining LN cells. Whereas adoptively transferred RMTC-purged cells isolated from control cell-treated mice did not influence MBP-specific T cell proliferative responses when compared with saline-treated mice, cells derived from CD4+CD25+ RMTC-treated animals showed significantly diminished response (Fig. 5A). This finding demonstrates that this induced population can suppress the development of MBP-specific T cell responses.
Fig. 5.
Impact of induced regulatory T cells on MBP-specific T cell response. (A) A total of 25 × 106 LN cells from purged mice, acquired as in Fig. 4, were adoptively transferred into naïve mice, which were then immunized with MBP89-101. The MBP89-101-specific proliferative response of draining LN cells was measured 10 days later. Three independently assayed mice per group were each analyzed in triplicate. Mean ± 1 SD is plotted. (B) Analyses were performed as in A except the mice were treated with blocking anti-IL-4 or IL-10R antibody or control antibody.
We suspected that the immunomodulatory activity of the RMTC-induced regulatory cells depended on the IL-10 and/or IL-4 cytokines that they produced. To analyze this cytokine dependence, we performed similar experiments; however, recipient mice were also treated with blocking anti-IL-4 (11B.11) or anti-IL-10R (1B1.3A) antibodies (Fig. 5B). Blockade of the IL-4 signaling pathway did not alter the proliferation inhibition of the induced regulatory T cells. In contrast, blockade of IL-10 signaling abolished this effect. This finding demonstrates that the CD4+CD25+ RMTC induce a regulatory population that inhibits autoantigen-specific responses in an IL-10-dependent manner.
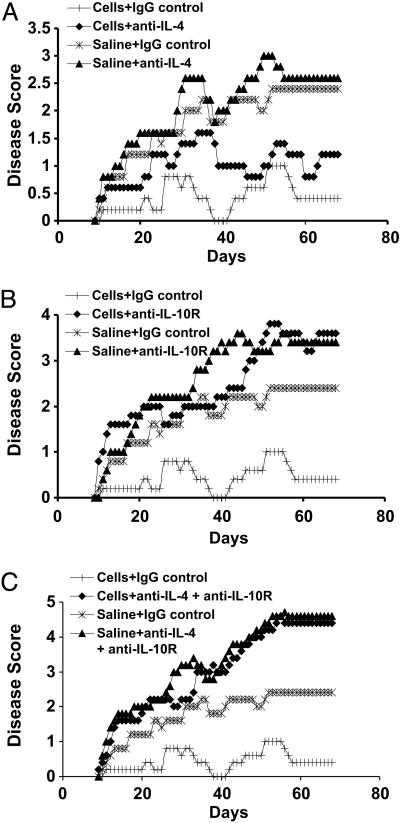
To determine whether IL-10 production by the induced MBP-specific regulatory T cells played a similar role in their protective effect in EAE, we adoptively transferred the RMTC-purged regulatory cells into naïve mice and induced EAE. As in Fig. 3, animals treated with the induced regulatory cells showed significantly decreased disease symptoms when compared with saline-treated controls (Fig. 6A). Treatment with anti-IL-4 antibody resulted in a trend toward more severe disease in both control saline-treated and regulatory cell-treated mice. However, in both cases, this trend failed to achieve statistical significance (P > 0.05). In contrast, treatment with anti-IL-10R antibody significantly impacted the immunomodulatory function of the transferred regulatory cells (Fig. 6B). Clinical disease in animals treated with both regulatory cells and anti-IL-10R was not significantly different from that of control saline-treated animals that received either control antibody or anti-IL-10R (P > 0.05). Their disease was significantly worse than that of animals receiving both regulatory cells and control antibodies (P < 0.001). A similar inhibition of the induced regulatory cell activity was observed in mice treated with both anti-IL-4 and anti-IL-10R (Fig. 6C; P < 0.01). These results show that the CD4+CD25+ RMTC promote the generation of a population of MBP-specific T cells that can independently suppress both autoantigen-specific responses and EAE in an IL-10-dependent manner.
Fig. 6.
Induced regulatory cell suppression of EAE is IL-10-dependent. (A) The experiment was performed as in Fig. 4, except that the mice receiving the regulatory T cells were also treated with blocking anti-IL-4 or control antibodies as indicated. (B) The experiment was performed as in A except for the use of blocking anti-IL-10R antibody. (C) Treatment with combined blocking anti-IL-4 and anti-IL-10R antibodies.
Discussion
CD4+CD25+ T cells can down-modulate immune responses through multiple regulated mechanisms, only some of which may operate in any specific setting (9, 28-31). Yet how small numbers of regulatory cells suppress immune responses in vivo remains uncertain. CD4+CD25+ T cells may induce the formation of either IL-10- or TGF-β-producing antigen-specific regulatory T cells in vitro. These findings have led to the proposal that small numbers of antigen-nonspecific CD4+CD25+ T cells may increase their effectiveness by catalyzing the formation of additional antigen-specific regulatory T cells (6-8). We show here that this conversion occurs in vivo. Specifically, we demonstrate the formation of regulatory T cells that, when purged of the inducing CD4+CD25+ cells, are capable of independently inhibiting the development of EAE. We further demonstrate that IL-10, but not IL-4, is required for their suppressive function. Our results support the concept that CD4+CD25+ T cells promote the formation of additional antigen-specific regulatory T cells, thereby amplifying their effect.
IL-10 has several down-modulatory functions that may diminish EAE immunopathology. IL-10 down-regulates p40 synthesis and thus the IL-12 and IL-23 production needed for the formation of the inflammatory T cells responsible for EAE (32, 33). IL-10 suppresses NO production and other functions of activated macrophage that damage neural tissue (34, 35). IL-10-deficient mice develop more severe EAE symptoms than wild-type mice, and some IL-10 promoter polymorphisms are associated with progression of multiple sclerosis, suggesting a natural role for IL-10 in disease activity (35, 36). IL-10 has further been implicated in several immunotherapeutic models, and administration of IL-10 can inhibit EAE symptoms (37-43).
A role for IL-10 in the down-modulation of EAE by CD4+CD25+ regulatory T cells has also been shown by Zhang et al. (10). Adoptive transfer of antigen-nonspecific CD4+CD25+ T cells can suppress EAE in C57BL6 mice through an IL-10-dependent, but not TGF-β-dependent, mechanism. One source of IL-10 is the CD4+CD25+ T cells themselves. Thus, CD4+CD25+ T cells from IL-10-/- mice manifest diminished activity in a colitis model system (44). Our results suggest that CD4+CD25+ cells are not the only source. IL-10 is crucial for the down-modulatory function of CD4+CD25+-induced, antigen-specific regulatory T cells, which may serve as an additional source.
High IL-10 production is characteristic of the Tr1 class of regulatory T cells. However, Tr1 cells frequently produce significant amounts of TGF-β, which we failed to observe in the RMTC-induced regulatory population. The induced regulatory T cells do produce detectable levels of IL-4, suggesting that these may include Th2 cells. Indeed, in a study by Kohm et al. (11), suppression of EAE by antigen-nonspecific CD4+CD25+ regulatory T cells was associated with a Th2 shift among autoreactive lymphocytes. However, in analyses similar to those performed here, we have found that Th2-differentiated RMTC can Th2-deviate the response of autoreactive MBP89-101-specific T lymphocytes (27). This deviation results in greater than four to five times the level of IL-4 production and less than one-third the IL-10 production observed with the use of CD4+CD25+ RMTC, suggesting that the regulatory cells that we observe here are not exclusively Th2. Indeed, the induced regulatory cells here appear more similar to regulatory populations characterized by several groups that primarily produce IL-10 (45). Their cloning and further characterization will be important to more precisely define their biological potential. In summary, we demonstrate that the function of CD4+CD25+ T cells can be focused in vivo on autoreactive T cells through the use of chimeric MHC-ζ receptors. Furthermore, these redirected regulatory cells act in part through the induction of infectious tolerance and the generation of additional antigen-specific regulatory T cells that can independently down-modulate EAE in an IL-10-dependent manner.
Acknowledgments
We thank Richard Cross, Jennifer Hoffrage, and Dick Ashmun for assistance with flow cytometric sorting and Janet Gatewood for assistance with Bio-Plex cytokine analysis. This work was supported by National Institutes of Health Grants R21 AI49872 and R01 AI056153 (to T.L.G.) and by the American Lebanese Syrian Associated Charities/St. Jude Children's Research Hospital (D.J.M., R.S.A., and T.L.G.).
Author contributions: D.J.M., R.S.A., and T.L.G. designed research; D.J.M. and R.S.A. performed research; D.J.M., R.S.A., and T.L.G. analyzed data; and D.J.M. and T.L.G. wrote the paper.
Abbreviations: Tg, transgenic; EAE, experimental allergic encephalomyelitis; RMTC, receptor-modified T cell; MBP, myelin basic protein; PLP, proteolipid protein; LN, lymph node; Th, T helper.
References
- 1.Shevach, E. M. (2002) Nat. Rev. Immunol. 2, 389-400. [DOI] [PubMed] [Google Scholar]
- 2.Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. (2003) Nat. Immunol. 4, 330-336. [DOI] [PubMed] [Google Scholar]
- 3.Cederbom, L., Hall, H. & Ivars, F. (2000) Eur. J. Immunol. 30, 1538-1543. [DOI] [PubMed] [Google Scholar]
- 4.Thornton, A. M. & Shevach, E. M. (1998) J. Exp. Med. 188, 287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonuleit, H., Schmitt, E., Stassen, M., Tuettenberg, A., Knop, J. & Enk, A. H. (2001) J. Exp. Med. 193, 1285-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng, S. G., Wang, J. H., Gray, J. D., Soucier, H. & Horwitz, D. A. (2004) J. Immunol. 172, 5213-5221. [DOI] [PubMed] [Google Scholar]
- 7.Jonuleit, H., Schmitt, E., Kakirman, H., Stassen, M., Knop, J. & Enk, A. H. (2002) J. Exp. Med. 196, 255-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieckmann, D., Bruett, C. H., Ploettner, H., Lutz, M. B. & Schuler, G. (2002) J. Exp. Med. 196, 247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maloy, K. J., Salaun, L., Cahill, R., Dougan, G., Saunders, N. J. & Powrie, F. (2003) J. Exp. Med. 197, 111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang, X., Koldzic, D. N., Izikson, L., Reddy, J., Nazareno, R. F., Sakaguchi, S., Kuchroo, V. K. & Weiner, H. L. (2004) Int. Immunol. 16, 249-256. [DOI] [PubMed] [Google Scholar]
- 11.Kohm, A. P., Carpentier, P. A., Anger, H. A. & Miller, S. D. (2002) J. Immunol. 169, 4712-4716. [DOI] [PubMed] [Google Scholar]
- 12.Hori, S., Nomura, T. & Sakaguchi, S. (2003) Science 299, 1057-1061.12522256 [Google Scholar]
- 13.Mottet, C., Uhlig, H. H. & Powrie, F. (2003) J. Immunol. 170, 3939-3943. [DOI] [PubMed] [Google Scholar]
- 14.van Maurik, A., Herber, M., Wood, K. J. & Jones, N. D. (2002) J. Immunol. 169, 5401-5404. [DOI] [PubMed] [Google Scholar]
- 15.Taylor, P. A., Lees, C. J. & Blazar, B. R. (2002) Blood 99, 3493-3499. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu, J., Yamazaki, S. & Sakaguchi, S. (1999) J. Immunol. 163, 5211-5218. [PubMed] [Google Scholar]
- 17.Geiger, T. L. & Jyothi, M. D. (2001) Transfus. Med. Rev. 15, 21-34. [DOI] [PubMed] [Google Scholar]
- 18.Jyothi, M. D., Flavell, R. A. & Geiger, T. L. (2002) Nat. Biotechnol. 20, 1215-1220. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen, P. & Geiger, T. L. (2003) Gene Ther. 10, 594-604. [DOI] [PubMed] [Google Scholar]
- 20.Mekala, D. J. & Geiger, T. L. (2005) Blood 105, 2090-2092. [DOI] [PubMed] [Google Scholar]
- 21.Geiger, T., Leitenberg, D. & Flavell, R. (1999) J. Immunol. 162, 5931-5939. [PubMed] [Google Scholar]
- 22.Gaur, A., Boehme, S. A., Chalmers, D., Crowe, P. D., Pahuja, A., Ling, N., Brocke, S., Steinman, L. & Conlon, P. J. (1997) J. Neuroimmunol. 74, 149-158. [DOI] [PubMed] [Google Scholar]
- 23.Anderson, A. C., Reddy, J., Nazareno, R., Sobel, R. A., Nicholson, L. B. & Kuchroo, V. K. (2004) J. Immunol. 173, 828-834. [DOI] [PubMed] [Google Scholar]
- 24.Bettelli, E., Das, M. P., Howard, E. D., Weiner, H. L., Sobel, R. A. & Kuchroo, V. K. (1998) J. Immunol. 161, 3299-3306. [PubMed] [Google Scholar]
- 25.Begolka, W. S., Vanderlugt, C. L., Rahbe, S. M. & Miller, S. D. (1998) J. Immunol. 161, 4437-4446. [PubMed] [Google Scholar]
- 26.Brocke, S., Gijbels, K., Allegretta, M., Ferber, I., Piercy, C., Blankenstein, T., Martin, R., Utz, U., Karin, N., Mitchell, D., et al. (1996) Nature 379, 343-346. [DOI] [PubMed] [Google Scholar]
- 27.Mekala, D. J., Alli, R. S. & Geiger, T. L. (2005) J. Immunol. 174, 3789-3797. [DOI] [PubMed] [Google Scholar]
- 28.Serra, P., Amrani, A., Yamanouchi, J., Han, B., Thiessen, S., Utsugi, T., Verdaguer, J. & Santamaria, P. (2003) Immunity 19, 877-889. [DOI] [PubMed] [Google Scholar]
- 29.Oldenhove, G., de Heusch, M., Urbain-Vansanten, G., Urbain, J., Maliszewski, C., Leo, O. & Moser, M. (2003) J. Exp. Med. 198, 259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fallarino, F., Grohmann, U., Hwang, K. W., Orabona, C., Vacca, C., Bianchi, R., Belladonna, M. L., Fioretti, M. C., Alegre, M. L. & Puccetti, P. (2003) Nat. Immunol. 4, 1206-1212. [DOI] [PubMed] [Google Scholar]
- 31.Pasare, C. & Medzhitov, R. (2003) Science 299, 1033-1036. [DOI] [PubMed] [Google Scholar]
- 32.Segal, B. M., Dwyer, B. K. & Shevach, E. M. (1998) J. Exp. Med. 187, 537-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trinchieri, G. (2003) Nat. Rev. Immunol. 3, 133-146. [DOI] [PubMed] [Google Scholar]
- 34.O'Farrell, A. M., Liu, Y., Moore, K. W. & Mui, A. L. (1998) EMBO J. 17, 1006-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samoilova, E. B., Horton, J. L. & Chen, Y. (1998) Cell Immunol. 188, 118-124. [DOI] [PubMed] [Google Scholar]
- 36.Almeras, L., Meresse, B., Seze, J., De Lefranc, D., Dubucquoi, S., Fajardy, I., Vermersch, P. & Prin, L. (2002) Eur. Cytokine Netw. 13, 200-206. [PubMed] [Google Scholar]
- 37.Rott, O., Fleischer, B. & Cash, E. (1994) Eur. J. Immunol. 24, 1434-1440. [DOI] [PubMed] [Google Scholar]
- 38.Faria, A. M., Maron, R., Ficker, S. M., Slavin, A. J., Spahn, T. & Weiner, H. L. (2003) J. Autoimmun. 20, 135-145. [DOI] [PubMed] [Google Scholar]
- 39.Okura, Y., Jee, Y. & Matsumoto, Y. (2003) Int. Immunol. 15, 437-446. [DOI] [PubMed] [Google Scholar]
- 40.Wildbaum, G., Netzer, N. & Karin, N. (2002) J. Clin. Invest. 110, 701-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maron, R., Slavin, A. J., Hoffmann, E., Komagata, Y. & Weiner, H. L. (2002) Int. Immunol. 14, 131-138. [DOI] [PubMed] [Google Scholar]
- 42.Adlard, K., Tsaknardis, L., Beam, A., Bebo, B. F., Jr., Vandenbark, A. A. & Offner, H. (1999) Autoimmunity 31, 237-248. [DOI] [PubMed] [Google Scholar]
- 43.Stohlman, S. A., Pei, L., Cua, D. J., Li, Z. & Hinton, D. R. (1999) J. Immunol. 163, 6338-6344. [PubMed] [Google Scholar]
- 44.Mason, D. & Powrie, F. (1998) Curr. Opin. Immunol. 10, 649-655. [DOI] [PubMed] [Google Scholar]
- 45.O'Garra, A., Vieira, P. L., Vieira, P. & Goldfeld, A. E. (2004) J. Clin. Invest. 114, 1372-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]