Abstract
Activity-regulated cytoskeleton-associated protein (Arc), the product of an immediate early gene, plays critical roles in synaptic plasticity and memory. Evidence suggests that Arc function is determined by its oligomeric state; however, methods for localization of native Arc oligomers are lacking. Here, we developed a nanobody-based proximity ligation assay (PLA) for detection, localization, and quantification of Arc-Arc complexes in primary rat hippocampal neuronal cultures. We used nanobodies with single, structurally defined epitopes in the bilobar Arc capsid domain. Nanobody H11 binds inside the N-lobe ligand pocket, while nanobody C11 binds to the C-lobe surface. For each nanobody, ALFA- and FLAG-epitope tags created a platform for antibody binding and PLA. Surprisingly, PLA puncta in neuronal dendrites revealed widespread constitutive Arc-Arc complexes. Treatment of cultures with tetrodotoxin or cycloheximide had no effect, suggesting stable complexes that are independent of recent neuronal activity and protein synthesis. To assess detection of oligomers, cultures were exposed to a cell-penetrating peptide inhibitor of the Arc oligomerization motif (OligoOFF). Arc-Arc complexes detected by H11 PLA were inhibited by OligoOff but not by control peptide. Notably, Arc complexes detected by C11 were unaffected by OligoOFF. Furthermore, we evaluated Arc complex formation after chemical stimuli that increase Arc synthesis. Brain-derived neurotrophic factor increased Arc-Arc signal detected by C11, but not H11. Conversely, dihydroxyphenylglycine (DHPG) treatment selectively enhanced H11 PLA signals. In sum, nanobody-based PLA reveals constitutive and stimulus-regulated Arc oligomers in hippocampal neuronal dendrites. A model is proposed based on detection of Arc dimer by C11 and higher-order oligomer by H11 nanobody.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12035-024-04508-7.
Keywords: Arc protein, Nanobody, Proximity ligation assay, Oligomerization, Synaptic plasticity hippocampus
Introduction
Understanding how the brain encodes information for learning and memory is one of the greatest challenges in neuroscience. Representation of information in neural networks depends on synaptic plasticity mechanisms; the ability of synapses to undergo changes in the efficacy of synaptic transmission [1–3]. For long-term modifications (hours to days), de novo gene transcription is required [4]. Activity-regulated cytoskeleton-associated protein (Arc; or activity-regulated gene, Arg3.1) regulates several forms of plasticity at excitatory synapses, such as long-term potentiation (LTP), long-term depression (LTD), and synaptic scaling, and functions in postnatal cortical development and memory formation [5]. Following stimulus-evoked transcription, Arc mRNA is found in the cell body and dendrites and accumulates at stimulated post-synaptic sites for local translation. Depending on the synaptic stimulation pattern, newly synthesized Arc acts transiently to support the formation of LTP or LTD [6–10], with an estimated protein half-life of 30 to 60 min [11–13]. Exactly how Arc is targeted toward specific types of plasticity and cellular functions is not fully understood. However, mounting evidence implicates Arc oligomeric state as a major determinant of its function [14–17].
Arc evolved from ancient Ty3/Gypsy retrotransposon elements and has conserved homology with Group-specific antigen (Gag) polyprotein in modern retroviruses [18, 19]. A salient feature of purified mammalian Arc protein is its ability to self-assemble into stable low and high-order oligomeric species (dimer to 32mer) in vitro [20–23]. Mice harboring point mutations in residues important for Arc oligomerization exhibit altered synaptic plasticity and memory [22]. The largest oligomeric form of Arc is a retrovirus-like capsid containing Arc mRNA [24, 25]. In neuronal cultures, Arc capsids are released from neurons in extracellular vesicles, which transmit the capsid and release the RNA for expression of Arc in neighboring cells [24, 26, 27]. Using in situ gene labeling, evidence was obtained for transfer of tagged Arc protein between neurons in adult mouse hippocampus [28]. Recent advances in imaging Arc oligomers are based on ectopic expression of tagged protein in non-neuronal cells [29–31]. However, little is known about the formation and regulation of native Arc oligomers. In situ protein cross-linking of rat brain regions identified an Arc dimer with low constitutive expression and enhanced expression after in vivo LTP induction or treatment of hippocampal neuronal cultures with brain-derived neurotrophic factor (BDNF) [32]. Thus, to elucidate Arc function, new strategies are needed for detection, localization, and quantification of native oligomers.
Mammalian Arc has as N-terminal coiled-coil domain and a C-terminal capsid (CA) domain separated by a flexible linker [14]. The CA has two lobes, the N-lobe and C-lobe, with 3D structural homology to the retroviral Gag CA domain which mediates virion capsid assembly [19, 33]. Uniquely, the mammalian Arc N-lobe harbors a binding pocket for several synaptic protein ligands [19]. Single-domain antibodies, also known as nanobodies, are 10 times smaller than conventional antibodies with unique binding properties [34, 35]. Recently, we developed six anti-Arc nanobodies (clones B5, B12, C11, D4, E5, and H11) from immunized Alpaca and further validated application of the recombinant ALFA epitope-tagged nanobodies for immunocytochemistry and immunoprecipitation [36, 37]. Epitope mapping and isothermal calorimetry assays showed that all six nanobodies bind to Arc CA with nanomolar affinity [36, 37]. Furthermore, crystal structure analysis showed that nanobody H11 binds specifically to the N-lobe ligand pocket [37, 38].
Here, we exploited the specific epitope binding anti-Arc nanobodies to develop a novel proximity ligation assay (PLA) for detection of native Arc-Arc complexes. PLA detects protein–protein proximity in situ, amplifying the signal to give cellular localization of complexes that might be present in low levels [39–41]. Using H11 fused to ALFA and FLAG epitope tags, we identify constitutive Arc-Arc complexes in neuronal dendrites of unstimulated hippocampal neuronal cultures. We provide evidence that these complexes contain stable oligomers that do not require recent neuronal activity or de novo Arc expression. Constitutive Arc-Arc complexes are similarly detected by PLA using nanobody C11, which binds to an exposed surface of the capsid domain C-lobe [37]. Comparing chemical stimuli that enhance Arc protein expression, we demonstrate stimulus-evoked changes in Arc-Arc PLA consistent with formation of dimers and higher-order oligomers.
Material and Methods
Primary Hippocampal Neuronal Culture Preparation
All animal procedures used for the primary culture preparation were performed accordingly to the Norwegian animal care committee established regulations. This research is approved by the Norwegian National Research Ethics Committee in compliance with EU Directive 2010/63/EU, ARRIVE guidelines. The neuronal primary culture preparation was based on the protocols published for neuronal culture preparation and cryopreservation by Ishizuka et al. [42]. Before the embryonic dissection for the hippocampus isolation, coverslips (Marienfeld-Superior, Lauda-Königshofen, Germany) of 12 or 18 mm were treated with a 1 N HNO3 solution (Sigma-Aldrich, St. Louis, MO), followed by washing step with Mili-Q® sterile water and coverslip coating with 1 mg/mL poly-l-lysine (PLL; Sigma-Aldrich) in borate buffer (Thermo Fisher Scientific, Waltham, MA).
Briefly, for the animal dissection and cell culture preparation, pregnant Wistar rats were deeply anesthetized and submitted to euthanasia by CO2. Embryos from embryonic days 18 (E18) were removed from the uterus and microdissected for hippocampal collection, performed in cold Hank’s Balanced Salt Solution (HBSS), containing 10 mM Hepes (Thermo Fisher Scientific) and 100 U/mL penicillin–streptomycin under a laminar air-flow hood. After isolation, the hippocampi were kept in cold HEPES buffer, followed by tissue and cellular dissociation (37 °C), using 0.05% Trypsin–EDTA solution (Thermo Fisher Scientific) containing 10 mM HEPES and 100 U/mL penicillin–streptomycin (Thermo Fisher Scientific). After trypsin treatment, the tissue was mechanically dissociated by repetitive pipetting with a flame-polished glass Pasteur pipette. The cells were counted in a Neubauer chamber and were either cryopreserved in freezing media (80% FBS + 20% DMSO) or seeded in culture plates.
Fresh or cryopreserved neurons were seeded in a cell density of 30,000 or 60,000 cells/well (24-well plate and 12-well plate, respectively) in cell-culture plates containing pre-coated coverslips in Minimum Essential Medium (MEM, Thermo Fisher Scientific), supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich), 0.6% glucose (Sigma-Aldrich), and 1 mM sodium pyruvate (Thermo Fisher Scientific), and 100 U/mL penicillin–streptomycin). After the cells attached, the platting medium was replaced by Neurobasal™ Medium (Thermo Fisher Scientific), supplemented with 2% B-27™ (Thermo Fisher Scientific) and 0.25% GlutaMAX™-I (Thermo Fisher Scientific). On day in vitro (DIV) 4, the neurons were treated with 1 μM cytosine arabinoside (AraC, Sigma-Aldrich) to prevent a high glial proliferation.
Treatment of Primary Hippocampal Neuronal Cultures
To evaluate the stability and neuronal activity-dependent origin of putative Arc oligomers, cultures were treated with cycloheximide (CHX) to inhibit protein synthesis and tetrodotoxin (TTX) to inhibit neuronal action potentials. CHX (50 µg/mL in Milli-Q® water) was applied for 70 min (37 °C), with additional long duration treatments of 3 and 6 h. Neurons were treated with TTX (2 µM in Milli-Q® water) for 16 h (37 °C), with additional experiments performed with application of 24–72-h TTX treatment. Neuronal cultures were fixed for imaging after TTX application or following a 30-min washout period with TTX-free medium.
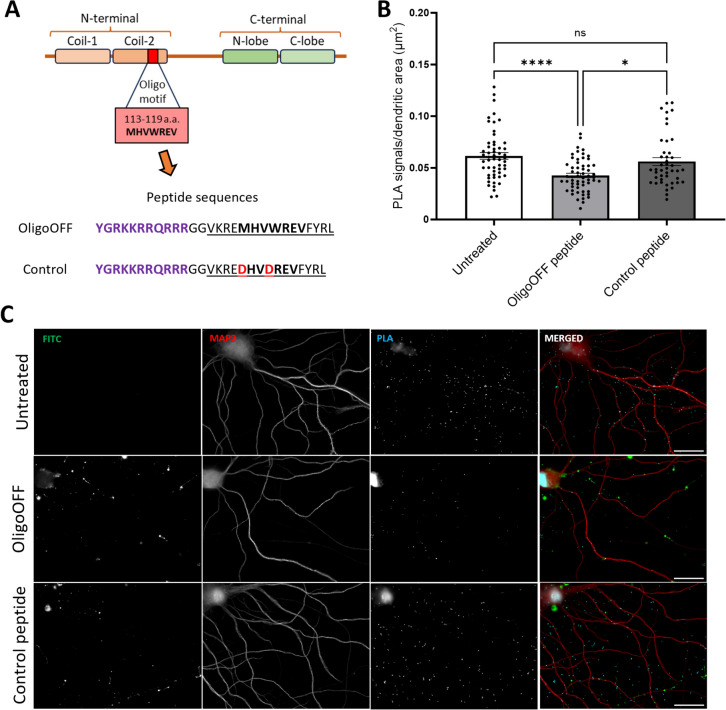
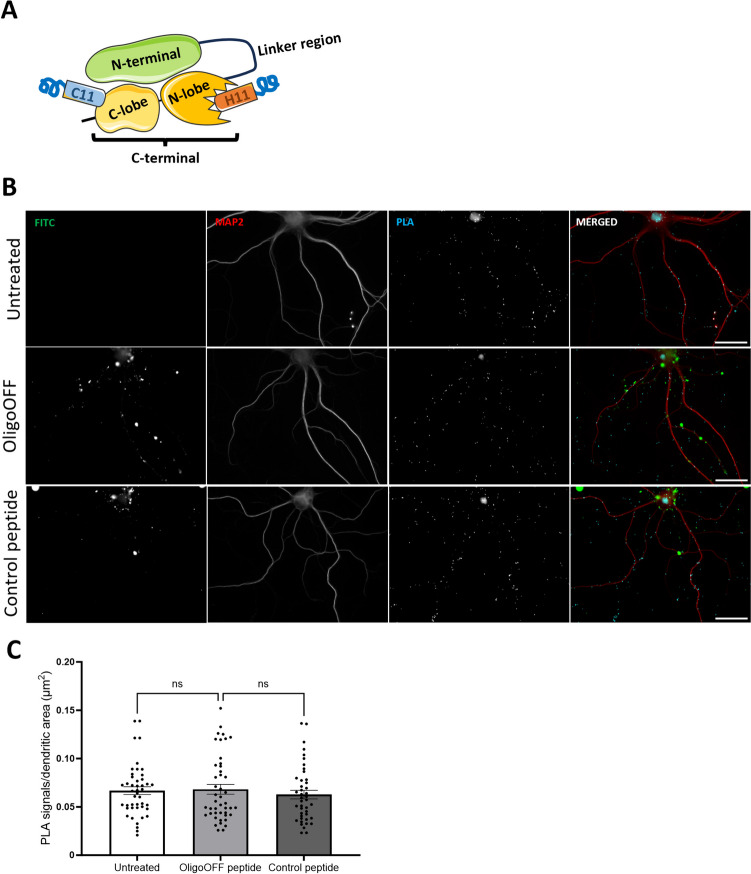
To assess detection of Arc oligomers by PLA, we designed a cell-penetrating, dominant-negative peptide inhibitor of Arc oligomerization, termed OligoOFF. The fluorescein isothiocyanate (FITC)-conjugated synthetic peptide has an HIV trans-activator of transcription (TAT) transduction domain and a double glycine linker, followed by 14 amino acids (109 VKREMHVWREVFYRL 123) from the oligomerization region of Arc’s N-terminal coiled-coil domain [23]. As a control for comparison with the wild-type sequence, we made a TAT peptide with aspartate substitutions in critical residues (M113D and W116D) within the 7-residue oligomerization motif (GenScript Biotech Corporation, Piscataway, NJ) (Fig. 6A). Cultures were exposed to 1 µM in Milli-Q® water of OligoOFF wild-type or mutant peptide control for 1 h (37 °C) followed by a 30-min washout to remove peptides that did not enter neurons before fixation and visualization of FITC.
Fig. 6.
Arc-Arc complexes detected by H11 PLA are inhibited by a cell-penetrating peptide inhibitor of Arc oligomerization. A Cell penetrating peptide dominant-negative inhibitor of Arc oligomerization motif. Top panel. Arc domain structure illustrating position of 7-residue oligomerization motif. Bottom panel. Amino acid sequence of cell-penetrating peptides. TAT sequence in purple, double glycine linker, followed by wild-type sequence of oligomerization peptide (underlined) and oligomerization motif (in bold). In control peptide, critical residues M113/W116 were replaced with aspartate (in red). B Quantification of comparison between OligoOFF group and untreated or control peptide group, expressed in PLA signals/dendritic area (μm2) ± SEM (45–60 images/group) (Kruskal–Wallis test followed by Dunn’s multiple comparisons test showing ****p ≤ 0.0001 and *p ≤ 0.05) detection. Scale bar of 25 µm. C Representative images of untreated and groups treated OligoOFF and control peptide, respectively
Additionally, on DIV 21, hippocampal neuronal cultures were treated with brain-derived neurotrophic factor (BDNF, Alomone Labs, Jerusalem, Israel, 50 ng/mL in Milli-Q® water, 37 °C) for 2 h. Moreover, DIV 21 neurons were treated with dihydroxyphenylglycine (DHPG, Tocris/Bio-techne, Minneapolis, MN, 100 µM in Milli-Q® water, 37 °C, for 10 min).
HEK293FT Cell Preparation and Transfection
The human embryonic kidney 293FT (HEK293FT) cells used in this study were acquired from Thermo Fisher Scientific and were maintained in Dulbecco’s modified Eagle medium (DMEM high-glucose, Sigma-Aldrich) containing 10% FBS and 100 U/mL penicillin–streptomycin. When confluent, HEK293FT cells were seeded in coverslips of 12 mm for further performance of the proximity ligation assay (PLA). The cells were transfected with Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific) in a concentration of 1 µL/well and 0.5 µg/well of plasmid containing either an empty vector with mTurquoise2 (mTq2) or mTq2-tagged Arc construct. Before fixation of the cells, green fluorescence was checked to confirm transfection of the constructs mentioned above.
Immunostaining Procedures
Prior to the immunostaining protocols for both immunocytochemistry (ICC) and PLA, treated and untreated cells were fixed with 4% paraformaldehyde, 4% sucrose solution in 0.1 M phosphate buffer, pH 7.4, for 20 min at room temperature.
For the immunocytochemistry protocol, fixed samples were permeabilized with Triton X-100 0.1% in phosphate-buffered saline (PBS) for 5 min at room temperature. Following the permeabilization, the samples were washed three times with PBS and blocked with 5% goat serum in PBS (blocking solution) for 1 h at room temperature. After blocking, samples were incubated with anti-drebrin (rabbit polyclonal, Bethyl Laboratories, Inc., Montgomery, TX, 1:1000 dilution) as a neuronal marker, combined with the H11-FLAG tagged nanobody (100 µg/mL) for overnight at 4 °C. On the second day, the samples were washed again, three times with PBS and incubated with an anti-FLAG antibody (Mouse monoclonal, Sigma-Aldrich F1804, 1:500 dilution) for 1 h at 37 °C. Then, the appropriate secondary antibodies: goat anti-mouse IgG (H + L) cross-adsorbed secondary antibody, Alexa Fluor 488 (Thermo Fisher Scientific, dilution 1:500) goat anti-rabbit IgG (H + L) cross-adsorbed secondary antibody, Alexa Fluor 647 (Thermo Fisher Scientific, dilution 1:500), for 1 h at room temperature, protected from the light. That was followed by another PBS wash and the cover slips were mounted with Prolong Glass Antifade mounting media (Thermo Fisher Scientific) in a microscope glass slide for imaging (Borosilicate glass D 263™ M of hydrolytic class 1, Marienfeld).
For Arc-Arc PLA, both in HEK293FT cells and hippocampal neurons, the Duolink PLA kit (Sigma-Aldrich) was used following the manufacturer’s instructions, with minor changes for the introduction of anti-Arc nanobodies. Briefly, samples were fixed and permeabilized as mentioned above, then blocked with Duolink Blocking Solution for 1 h at 37 °C. Starting the modified Duolink protocol, coverslips were incubated for overnight at 4 °C with an anti-Arc nanobody H11-FLAG and H11-ALFA or C11-FLAG/C11-ALFA. Concentrations used were 25 µg/mL for H11 and 15 µg/mL for C11 diluted in Duolink Antibody Diluent solution. This was combined with incubation with a primary anti-MAP2 antibody (chicken polyclonal, Encor Biotechnology Inc., Gainesville, dilution 1:3000), used as neuronal marker and to quantify the dendritic area in the image analysis. On the following day, the samples were incubated with anti-ALFA (rabbit polyclonal, NanoTag Biotechnologies GmbH, Göttingen, Germany, N1581, dilution 1:1000) and/or anti-FLAG antibodies (mouse monoclonal, Sigma-Aldrich F1804, dilution 1:500) for 1 h at 37 °C. Next, the samples were washed and incubated with Duolink In Situ PLA Probes Anti-Rabbit (PLUS) and Anti-Mouse (MINUS) + a secondary antibody Donkey Anti-Chicken IgY (IgG) (H + L) Antibody, CF594A Conjugated (Biotium, Inc., Fremont, CA, dilution 1:500), for 1 h at 37 °C. Following the probes incubation, the samples were washed and incubated for the ligation step with ligase and a buffer containing oligonucleotides (30 min at 37 °C). The samples were washed after the ligation step and incubated with polymerase and buffer containing fluorophore-labeled oligos (Duolink In Situ Detection Reagents FarRed) for the amplification step (100 min at 37 °C). A final washing step was done before mounting the cover slips with Prolong Glass Antifade mounting media in a microscope glass slide for imaging (Borosilicate glass D 263™ M of hydrolytic class 1, Marienfeld). For the positive (PSD95-Stg) and negative (TIP60-Stg), the conventional Duolink manufacturer protocol was used, where the nanobodies were absent.
To determine the concentration used for the nanobody-based PLA, a concentration curve (15 to 300 µg/mL) was performed (same protocol as described above, using Duolink PLA Kit) to optimize the use of nanobody H11 pair. The concentration of 25 µg/mL was selected for further testing and validation, considering the best signal/noise ratio observed in the Supplementary Fig. 1. The concentration of 15 µg/mL was selected for the nanobody C11, due to a higher noise at 25 µg/mL concentration.
Imaging and Image Analysis
Except for the images from Arc-Arc PLA in HEK293FT cells, all the other samples (hippocampal neurons) were imaged on a AxioImager Z1 microscope (Carl Zeiss AG, Oberkochen, Germany). The HEK293FT cells were imaged on a Leica TCS SP8 confocal microscope (Leica microsystems GmbH, Wetzlar, Germany). The images acquired were all analyzed and processed in the software ImageJ (National Institutes of Health, Bethesda, MD).
In both ICC and PLA imaging, random, healthy, and non-overlapping neurons (to distinguish individual dendrites) were selected throughout the coverslip to attain a good representation of each sample. Arc imaging was performed after selection of neurons, thus avoiding selection bias due to Arc immunostaining or PLA. For ICC, the neuronal marker drebrin was used to visualize neuronal spines and dendritic morphology. For selection of neurons in PLA experiments, MAP2 immunostaining was used to identify neuronal dendrites, as the mouse anti-drebrin antibody cannot be combined with use of anti-mouse PLA probes.
For the Arc ICC analysis, random non-overlapping secondary dendrites from the selected neurons were used. To set the threshold, the background was measured individually in each image and multiplied by a common factor for each experimental replicate to better exclude noise. Puncta counts were normalized by the dendritic length using the formula: (number of puncta × 100 μm)/dendritic length (μm), expressed as mean number of Arc puncta/100 μm of dendritic length in the graphs. For all the PLA analysis, MAP2 staining was used to label neurons and select the dendritic area of analysis. First, the neuronal soma was manually removed and then the whole dendritic area was selected using MAP2 staining as reference. We focused our analysis on dendritic branches given long-standing evidence for enrichment of Arc in synaptic and cytoskeletal fractions [43, 44]. The detection threshold was set manually to exclude noise and count PLA signals in the dendrites and peri-dendritic area to include spines. The results were normalized by dendritic area, expressed in number of PLA puncta/dendritic area in μm2.
Statistical Analysis
All the data was processed and analyzed using the software GraphPad Prism 10.0.3. The D’Agostino & Pearson test was used to assess data distribution normality for selection of the post hoc test. Outlier detection was performed using the ROUT method (Q = 1%). A minimum of three experimental replicates were performed, where a minimum of 20 (ICC) or 45 (PLA) images of individual neurons per group were used for analysis.
One-way ANOVA was used for the analysis of Arc puncta, followed by Dunnett’s multiple comparison test for analysis containing three groups. Mann–Whitney or unpaired t-test was applied for the comparison between two groups, based on normality of the data distribution. For the PLA analysis, for experiments containing two groups, Mann–Whitney was applied, and for those containing three or more groups, one-way ANOVA followed by Dunnett’s multiple comparison test was applied for normal distribution and Kruskal–Wallis test followed by Dunn’s multiple comparison test for non-parametric tests when suitable.
Results
Anti-Arc Nanobody H11-FLAG Detects Endogenous Arc Protein via Immunocytochemistry
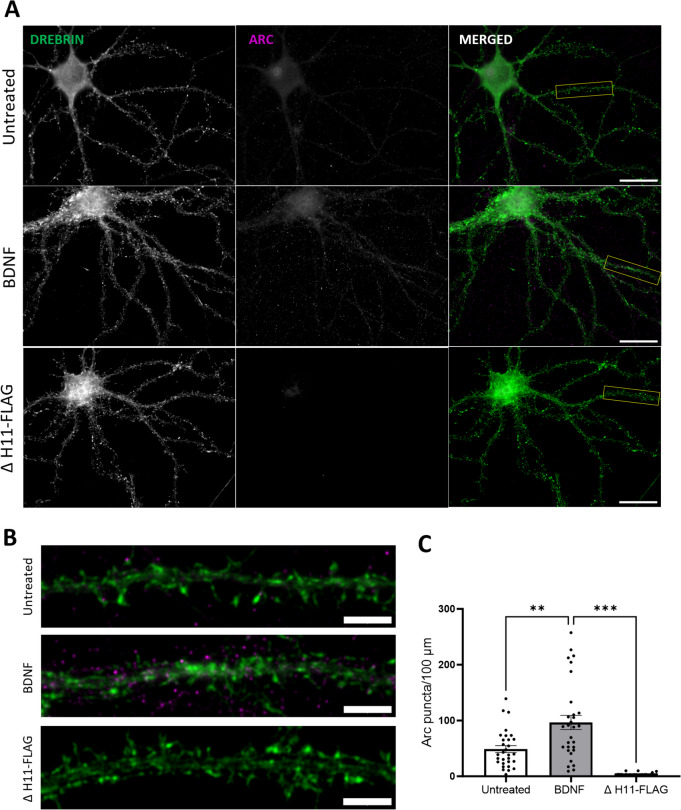
First, we sought to apply H11-FLAG for detection of Arc protein via immunocytochemistry. In untreated DIV 21 hippocampal primary neuronal cultures (Fig. 1A, row 1), sparsely distributed Arc immunoreactive somatodendritic puncta were observed. BDNF treatment significantly increased the density of Arc puncta in dendrites and spines (Fig. 1A, 2nd row), and non-specific binding of secondary antibodies was evaluated by omitting H11-FLAG from the protocol in BDNF-treated neurons. In the absence of H11-FLAG, only negligible background fluorescence was observed (Fig. 1A, 3rd row). The quantitative comparison between the groups is presented in Fig. 1C. Thus, immunocytochemical staining with H11-FLAG detects basal and BDNF-induced Arc as discrete puncta in neuronal dendrites and spines, consistent with enhanced Arc expression detected by antibody-based ICC [7, 45–47].
Fig. 1.
Use of anti-Arc H11-FLAG nanobody for immunocytochemistry in primary hippocampal neuronal cultures. A H11-FLAG immunocytochemistry detects an increase in Arc puncta in BDNF-treated neuronal cultures compared to unstimulated cultures (untreated), with a low noise signal detected in the absence of H11-FLAG (ΔH11-FLAG). 63 × magnification, 25-µm scale bar. B Digital zoom of secondary dendrites indicated by a white box in panel A (5-µm scale bar). Drebrin immunocytochemical staining in green and Arc in magenta. C Quantification of Arc puncta/100-μm dendritic length ± SEM (30 images/group). One-way-ANOVA followed by Dunnett’s multiple comparisons test; **p ≤ 0.01; ***p ≤ 0.001
Nanobody-Based PLA Detects Arc-Arc Complexes in Dendrites of Unstimulated Neuronal Cultures
We considered that specific binding of H11 to the Arc N-lobe pocket could be used to develop a PLA method for detection of Arc-Arc interaction complexes and possible oligomers. For this purpose, H11-FLAG and H11-ALFA were used to target the same epitope in two different Arc molecules. In classical PLA, detection of proximity between two proteins is based on the use of primary antibodies from different species, which are recognized by species-specific secondary antibodies conjugated to DNA oligos. If the distance between the two antibody binding sites is less than 40 nm, oligonucleotide strands can undergo ligation, allowing rolling circle DNA synthesis and detection of the amplicon by fluorescent protein conjugated to complementary oligonucleotide probes. Here, epitope-tagged nanobodies were used to bind Arc, followed by a standard PLA protocol using primary antibodies against the ALFA and FLAG epitopes (Fig. 2).
Fig. 2.
Schematic illustration of conventional and nanobody-based PLA. In conventional PLA, the proteins of interest (A, B) are labeled with a 1° antibody (ab). In nanobody-based PLA for detection of Arc-Arc complexes, we used nanobodies that bind to a single, structurally defined epitope on Arc. Nanobody H11 (depicted here) binds inside the Arc N-lobe ligand binding pocket (dashed red box). The nanobodies are C-terminally fused to ALFA or FLAG tags, which are recognized by rabbit and mouse antibodies, respectively. Subsequent steps for conventional and nanobody-based PLA are the same: Labeling with 2° ab/probes conjugated with oligonucleotides, ligation of oligonucleotides when target proteins are in close proximity (max. 40 nm), addition of DNA polymerase for rolling cycle amplification of ligated DNA, followed by the binding of multiple fluorescent detection probes to create a bright spot
As positive and negative controls for detection of protein interactions in hippocampal neurons, we used standard antibody-based PLA. For a positive control, we used the interaction between post-synaptic density protein-95 (PSD-95) and stargazin (Stg) which is an auxiliary subunit of AMPAR-type glutamate receptors. As a mechanism for tethering AMPARs to postsynaptic membranes in dendritic spines, the PSD95-Stg interaction is highly specific and abundant [48, 49]. As a negative control, we paired Stg with the nuclear histone acetylase, Tat-interactive protein 60 (TIP60). As expected, a widespread PLA signal was observed along dendrites for PSD95/Stg, whereas almost no signal was detected in the TIP60-Stg PLA (Fig. 3A and B).
Fig. 3.
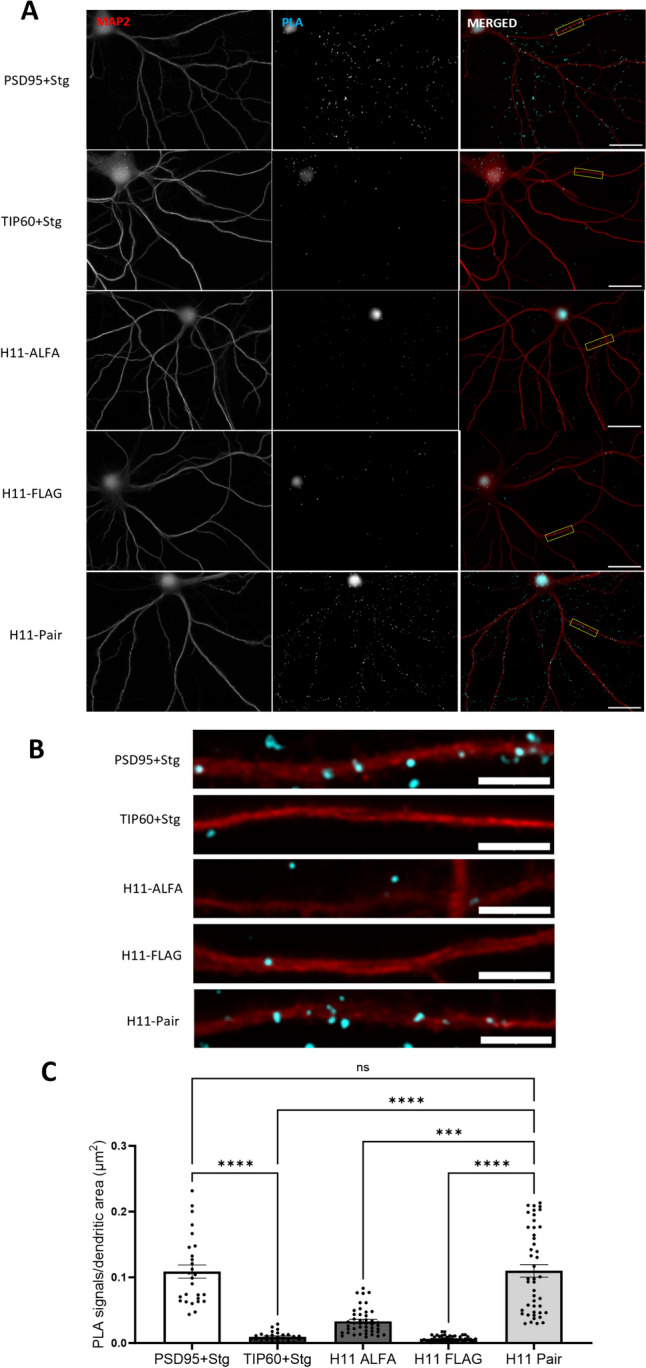
H11 nanobody-based PLA detects Arc-Arc complexes in hippocampal neuronal dendrites of unstimulated cultures. A Representative image from positive control (PSD95 + Stg) and negative control (TIP60 + Stg) antibody-based PLA; nanobody PLA using only ALFA-H11 or FLAG-H11 FLAG (rows 3 and 4, respectively), and nanobody PLA using the ALFA-H11/FLAG-H11 pair. Scale bar is 25 µm. B Digital zoom of secondary dendrites indicated by a white box in panel (A) (5-µm scale bar). C Quantification of PLA signals/dendritic area (μm2) ± SEM (30–45 images/group). One-way-ANOVA followed by Dunnett’s multiple comparisons test showing ***p ≤ 0.001 and ****p ≤ 0.0001
In the H11 nanobody-based PLA, we observed a widespread signal in dendrites like that observed in PSD95-Stg positive control, suggesting ubiquitous detection of Arc-Arc complexes. When either H11-ALFA or H11-FLAG was omitted from the protocol, only sparse PLA signals were detected, similar to the TIP60-Stg negative control. To quantify the difference observed in the images, the neuronal marker, MAP2, was used to randomly select neurons. PLA puncta were counted using ImageJ software and normalized by dendritic area determined using MAP2 staining, focusing only on the dendritic and synaptic signals, expressed as PLA puncta/dendritic area (μm2). PLA signal density in the H11 ALFA/FLAG pair protocol was equivalent to that of the PSD95-Stg positive control and significantly higher than the TIP60-Stg negative control and the H11-ALFA or H11-FLAG alone controls. (Fig. 3C; one-way-ANOVA followed by Dunnett’s multiple comparisons test showing p > 0.999). These results show that Arc-Arc interactions detected by H11 PLA are similar in density to PSD95-Stg interactions in hippocampal neuronal cultures.
To further validate H11-specific labeling of Arc-Arc interaction, we used HEK293FT cell line (Supplementary Fig. 2). HEK293FT cells do not express endogenous Arc, but ectopically expressed mTq2-tagged Arc in these cells is detected as monomers and oligomers by in situ protein crosslinking analysis [23]. Using the H11 nanobody pair, cells transfected with mTq2-tagged Arc exhibited clear and abundant PLA signals, while non-transfected cells or cells transfected with empty vector (mTq2) show sparse signals, equivalent to background noise. Similarly, in cells expressing mTq2-tagged Arc, only sparse signal was detected when either of the epitope-tagged H11 nanobodies was omitted.
Basal Arc-Arc Complexes Are Independent of Recent Neuronal Activity and Protein Synthesis
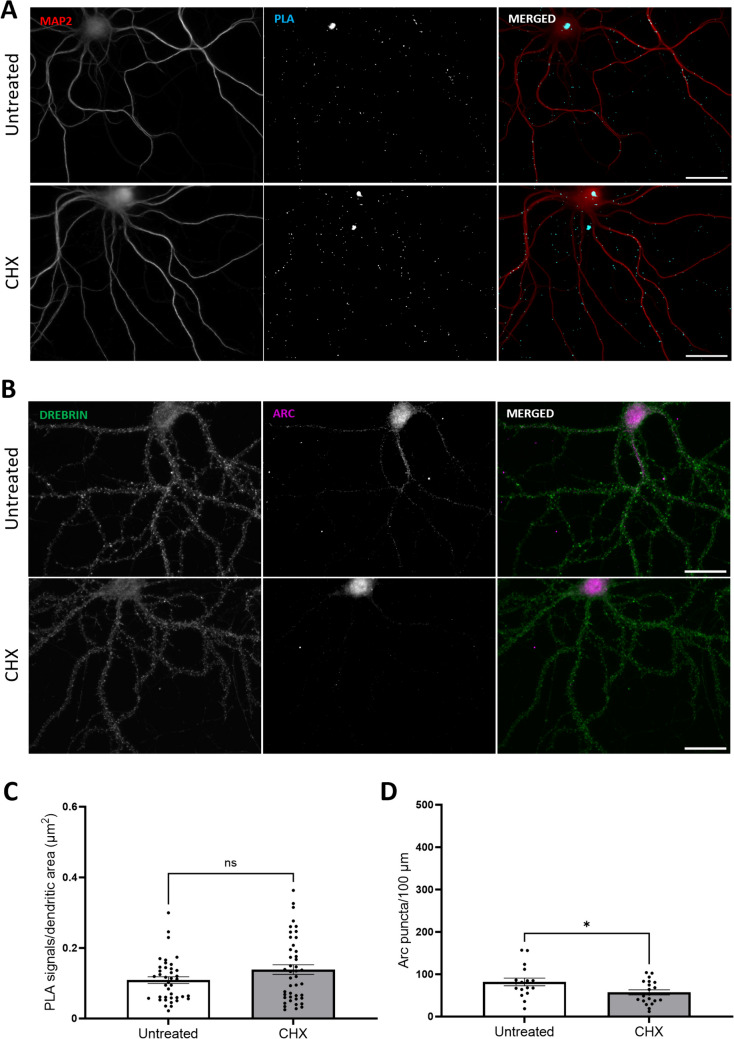
Arc is known for its rapid activity-dependent transcription, translation, and rapid protein degradation, with protein half-life estimates of 0.5 to 1 h [11–13, 50]. In unstimulated hippocampal neuronal cultures, expression of Arc mRNA and other immediate early genes occurs in a subfraction of neurons [51–53]. The ubiquitous Arc-Arc PLA signal found in unstimulated neuronal cultures is therefore surprising and suggests the existence of constitutive Arc complexes. To assess the origin and stability of Arc complexes, tetrodotoxin (TTX) was used to block action potentials (Fig. 4), and cycloheximide (CHX) was used to inhibit protein synthesis (Fig. 5).
Fig. 4.
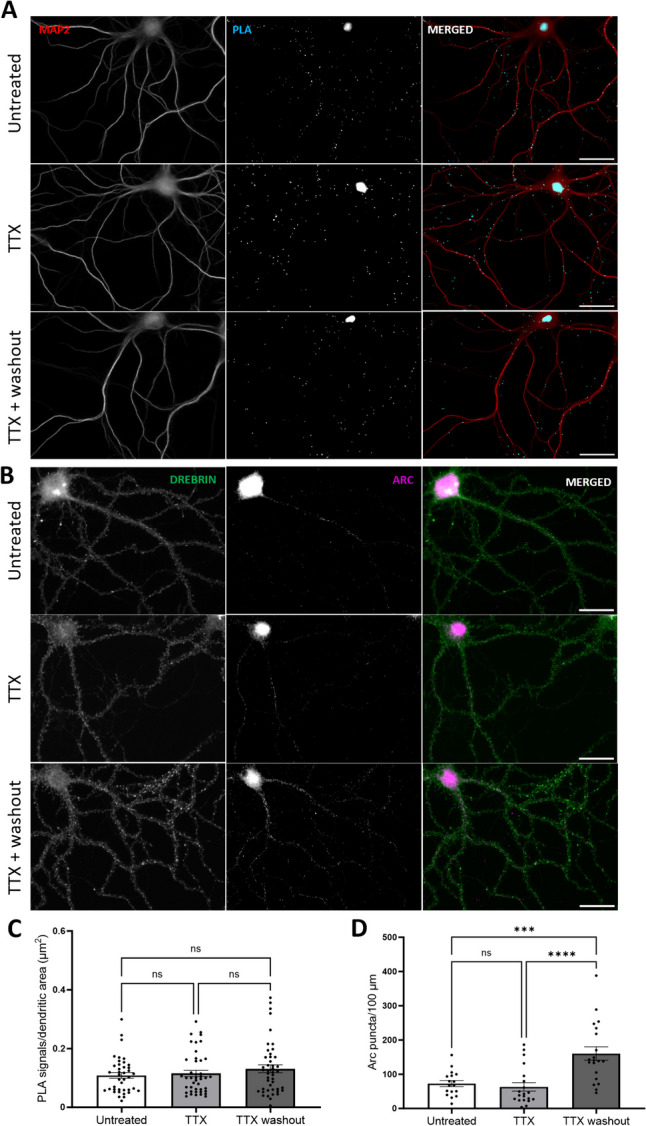
Inhibition of neuronal firing by TTX does not affect basal Arc-Arc complexes. A Representative Arc-Arc H11 PLA images from hippocampal neuronal cultures treated with TTX for 16 h, TTX followed by 30-min washout, or no treatment. B Representative immunocytochemical staining using anti-Arc H11-FLAG nanobody. C Quantification of the comparison between treated (TTX and TTX + washout, 2nd and 3rd rows, respectively) groups and untreated groups, expressed in PLA signals/dendritic area (μm2) ± SEM (45 images/group). Kruskal–Wallis test followed by Dunn’s multiple comparisons test showing p ≥ 0.999 for both groups. D Quantification of the ICC comparing treated groups (TTX and TTX + washout, 2nd and 3rd rows, respectively) and untreated groups, expressed in Arc puncta/100-μm dendritic length ± SEM (20 images/group). One-way-ANOVA followed by Dunnett’s multiple comparisons test showing ***p ≤ 0.001 and ****p ≤ 0.0001. Scale bar of 25 µm
Fig. 5.
Inhibition of protein synthesis does not affect basal Arc-Arc complexes. A Representative image of treated and untreated groups for PLA detection. B Representative images of treated and untreated groups for ICC. C Quantification of the comparison between treated and untreated groups, expressed in PLA signals/dendritic area (μm2) ± SEM (45 images/group). Mann–Whitney test showing p = 0.21. D Quantification of the ICC comparison between treated and untreated groups, expressed in Arc puncta/100-μm dendritic length ± SEM (20 images/group). Unpaired t test showing *p ≤ 0.05. Scale bar of 25 µm
Silencing of neuronal firing with TTX is known to inhibit Arc transcription, while washout of TTX results in rebound of neuronal activity and enhanced transcription [51]. We found that TTX treatment (2 µM) for 16 h did not affect the abundance of Arc-Arc PLA signal (Fig. 5C). Washout for 30 min post-TTX also did not alter PLA signal (Fig. 4A and C), although ICC analysis (Fig. 4B and D) with H11 nanobody demonstrated a robust increase in Arc puncta density in hippocampal neuronal dendrites. Longer TTX treatments for 24, 48, and 72 h also had no effect on the Arc-Arc PLA signal abundance (Supplementary Fig. 3).
CHX 50 µg/mL treatment for 70 min also did not alter Arc-Arc PLA signal abundance in neuronal dendrites (Fig. 5C), even though CHX significantly reduced Arc expression (Fig. 5D) as detected by ICC staining. Even extended periods of CHX treatment of 3 and 6 h did not significantly alter the PLA (Supplementary Fig. 4). Thus, inhibition of recent neuronal activity and protein synthesis affects expression of Arc but does not alter the abundance of the Arc/Arc PLA signal, suggesting the presence of stable Arc complexes in neuronal dendrites.
Arc-Arc PLA Signal Is Inhibited by a Cell-Penetrating Peptide Inhibitor of Arc Oligomerization
Next, we asked whether stable Arc complexes reflect the detection of oligomeric Arc. The Arc N-terminal coiled-coil domain plays a critical role in Arc self-association and oligomerization. Mutation of the oligomerization motif in coil-2 inhibits the assembly of dimers into higher-order oligomers [23]. We therefore designed a cell-penetrating TAT peptide (OligoOFF) as a dominant-negative inhibitor of Arc oligomerization. OligoOFF has 14 residues from the coil-2 oligomerization region harboring the 7-residue oligomerization motif (a.a. 113–119, MHVWREV) (Fig. 6A). M113 and W116 are critical residues in the Arc-Arc atomic interface, and aspartate mutation of these residues inhibits oligomerization [23]. As a control TAT peptide, we therefore substituted M113 and W116 with aspartate.
PLA detection was performed with H11 after incubation of cultures with fluorescent FITC-conjugated TAT peptide for 1 h. Both OligoOFF and control peptide penetrated the neuronal cell body and dendrites, as shown by FITC fluorescence in Fig. 6C. Application of OligoOFF strongly reduced the PLA signal relative to untreated neurons and cultures treated with control peptide (Fig. 6B). In addition, there was no difference in PLA signal between the control peptide-treated cultures and untreated cultures, demonstrating the importance of the intact oligomerization motif for inhibition of Arc-Arc interaction (Fig. 6B). These results suggest that H11 PLA signal reflects the detection of constitutive, stably expressed oligomeric Arc.
Nanobody PLA Detects Arc-Arc Complexes Induced by BDNF and DHPG
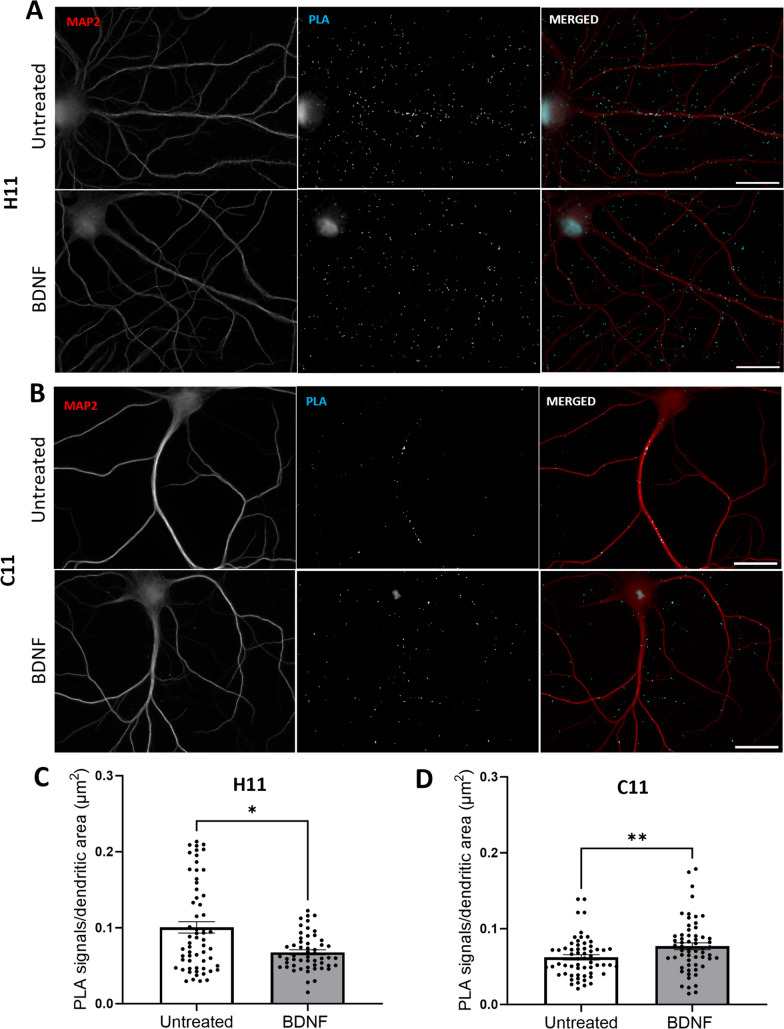
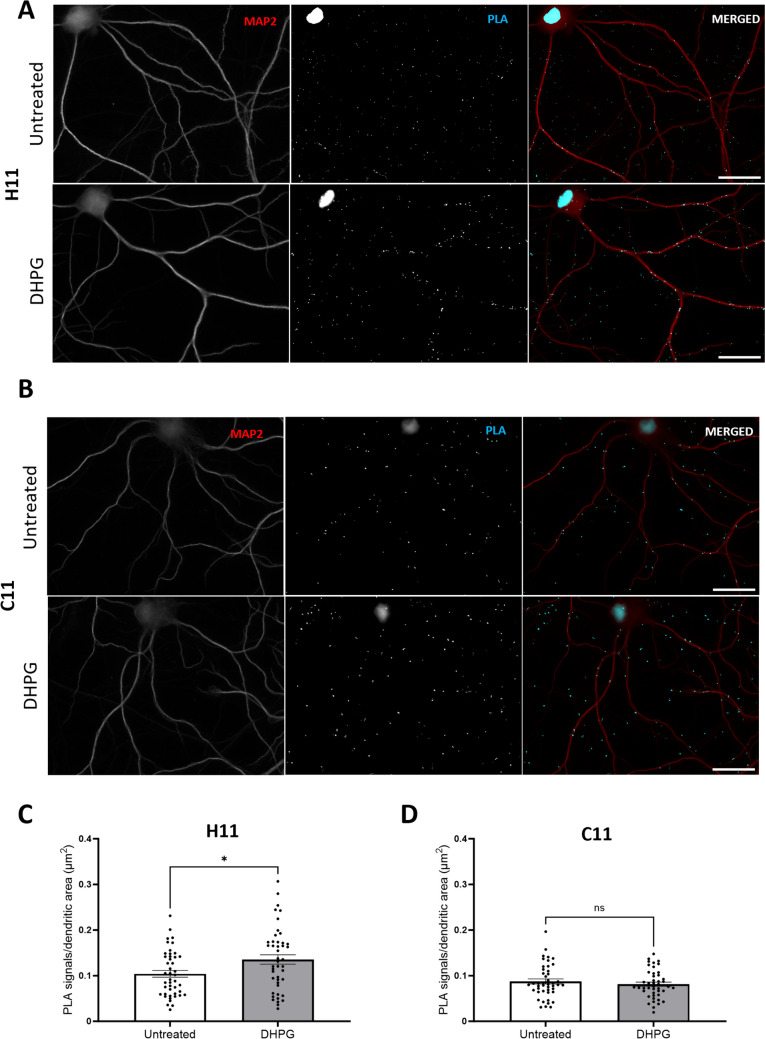
Next, we asked whether Arc complexes are regulated by stimuli that are known to induce Arc expression. As BDNF enhances Arc expression in hippocampal neuronal dendrites (Fig. 1), we expected to observe enhanced Arc-Arc PLA following BDNF treatment. Surprisingly, BDNF treatment significantly reduced signal abundance detected by H11 PLA (Fig. 7C). Although H11 detects constitutive Arc complexes, it might not detect all oligomeric forms. We therefore employed another anti-Arc nanobody, clone C11, which binds to an exposed surface of the capsid domain C-lobe [37]. First, we show that PLA performed with C11-ALFA and C11-FLAG detects constitutive Arc-Arc complexes in hippocampal neuronal dendrites, as observed with H11 PLA (Supplementary Fig. 5). Again, treatment with TTX and CHX did not affect the constitutive Arc-Arc PLA signal density (Supplementary Fig. 6). However, in contrast to H11, the C11-based PLA detected enhanced Arc-Arc interaction in BDNF treatment neurons (Fig. 7).
Fig. 7.
BDNF increases Arc-Arc PLA signal detected by the C-lobe binding nanobody C11 but not by H11. A Representative image of untreated and BDNF-treated groups detected by H11 pair. B Representative images of untreated and BDNF-treated groups detected by H11 pair. C Quantification of the comparison between control and BDNF groups, expressed in PLA signals/dendritic area (μm2) ± SEM (60 images/group) detected by H11 nanobody (Mann–Whitney test showing *p < 0.05). D Quantification of the comparison between control and BDNF groups, expressed in PLA signals/dendritic area (μm2) ± SEM (60 images/group) detected by C11 nanobody (Mann–Whitney test showing **p < 0.01). Scale bar of 25 µm
We considered that epitopes for H11 (N-lobe ligand pocket) and C11 (C-lobe surface) are sensitive to Arc oligomeric state (Fig. 8A). Previous work using in situ protein cross-linking to trap Arc-Arc interactions showed that BDNF treatment of hippocampal neurons dramatically enhances expression of Arc monomers and dimers, with no evidence of tetramers [32]. The present results could be explained by preferential detection of dimer by C11 and higher-order oligomer by H11. To test this hypothesis, we repeated the OligoOFF peptide experiment in non-stimulated neuronal cultures using C11 for PLA (Fig. 8B). In contrast to H11, the C11 PLA showed no difference in signal density between OligoOFF treated and untreated cultures (Fig. 8C). Notably, the oligomerization motif mediates dimer assembly into higher-order oligomers, but not dimer formation itself [23]. Therefore, the results with C11 are consistent with detection of BDNF-induced dimers and persistence of dimers following disruption of constitutive Arc-Arc complexes by OligoOFF.
Fig. 8.
Arc-Arc complexes detected by C11 are not inhibited by OligoOFF-peptide. A Cartoon illustrating anti-Arc nanobody H11-ALFA binding to the N-lobe ligand pocket and C11-ALFA binding to the C-lobe surface of the Arc’s C-terminal capsid domain. C11-ALFA and C11-FLAG were used for the PLA data shown here. B Representative image of untreated and groups treated OligoOFF and control peptide. C Quantification of comparison between OligoOFF group and untreated or control peptide group, expressed in PLA signals/dendritic area (μm2) ± SEM (45–60 images/group), for C11 detection (Kruskal–Wallis test followed by Dunn’s multiple comparisons test showing no significance in the comparisons). Scale bar of 25 µm
To compare with BDNF, hippocampal neuronal cultures were treated with the metabotropic glutamate receptors agonist DPHG. DHPG-induced Arc synthesis is critical for synaptic depression, and this mechanism is attenuated by mutations that prevent Arc higher-order assembly from tetramers to 32-mers [8, 22, 26]. We investigated whether DHPG stimulation would induce changes in Arc-Arc PLA detected via H11 or C11 (Fig. 9A and B, respectively). Quantification of PLA signals for nanobody H11 demonstrated a significant increase in signal abundance in the DHPG treatment group relative to untreated control (Fig. 9C). In contrast, PLA signals for nanobody C11 did not differ between DHPG-treated and untreated control (Fig. 9D). These results again show differential detection of Arc-Arc complexes by H11 and C11 nanobodies, consistent with preferential detection of higher-order oligomers by H11.
Fig. 9.
DHPG increases Arc-Arc PLA signal detected by H11 but not C11 nanobody. A Representative image of untreated and DHPG-treated groups detected by H11 pair. B Representative images of untreated and DHPG-treated groups detected by C11 pair. C Quantification of the comparison between control and DHPG groups, expressed in PLA signals/dendritic area (μm2) ± SEM (45 images/group) detected by H11 nanobody (unpaired t test showing *p < 0.05). D Quantification of the comparison between control and DHPG groups, expressed in PLA signals/dendritic area (μm2) ± SEM (45 images/group) detected by C11 nanobody (unpaired Student’s t-test showing p = 0.3906). Scale bar of 25 µm
Discussion
The novel nanobody-based PLA provides a method for detection, localization, and quantification of native Arc-Arc complexes. Surprisingly, PLA revealed widespread Arc-Arc complexes in neuronal dendrites under basal conditions in unstimulated hippocampal neuronal cultures. The pool of Arc detected by PLA is not reliably detected by ICC staining, indicating the higher sensitivity of the PLA method [39, 40, 54]. The Arc-Arc PLA signal density and distribution is similar to PLA for the Stg-PSD95 interaction in dendritic spines. The basal Arc-Arc PLA signal is also unaffected by inhibition of neuronal activity (TTX treatment for up to 72 h) and inhibition of protein synthesis (CHX treatment for up to 6 h), demonstrating constitutive expression of stable Arc-Arc complexes in dendrites or dendritic spines. These complexes can be attributed to oligomeric Arc based on the strong inhibition of H11 PLA signal by OligoOFF peptide, but not control peptide in which two residues critical for Arc-Arc interaction (methionine 113 and tryptophan 116) were changed to aspartate.
In principle, Arc-Arc PLA signal could reflect Arc molecules bound to partner proteins within crowded environments such as the PSD. Localization of Arc to postsynaptic sites depends on PSD95 and it is estimated that 5% of biochemically identified PSD95 supercomplexes contain Arc and 58% of Arc supercomplexes contain PSD95 [55]. Arc binds through its N-lobe with several PSD proteins including NMDA receptor subunit 2B, stargazin (TARPγ2), and guanylate kinase anchoring protein (GKAP) [19, 56]. In addition, Arc purifies with the crude F-actin fraction [43] and interacts with the actin-binding protein, drebrin A, within the spine core [44]. Under basal conditions, in non-stimulated hippocampal dentate gyrus, subcellular fractionation and co-immunoprecipitation with immunoblot analysis show that Arc is highly enriched in the synaptic, cytoskeletal fraction in complex with drebrin A [44]. It is therefore possible that constitutive Arc-Arc PLA reflects oligomeric Arc in complex with partner proteins in the spine core and PSD.
Arc oligomeric state has emerged as a determinant of Arc function. However, current functional data in neuronal cultures, brain slices, and live rodents is based on mutations that impair oligomerization of purified Arc protein, while regulation of endogenous Arc oligomers is largely unknown. The present study provides evidence for stimulus-evoked increases in Arc-Arc complexes in response to both BDNF and DHPG. Moreover, differential detection of stimulus-induced complexes by H11 and C11 indicates recognition of distinct oligomeric forms or altered access of nanobodies to binding sites. We show that BDNF treatment induces an increase in C11 but not H11 PLA signal abundance, whereas DHPG-induced Arc-Arc complexes are detected by H11 but not C11 PLA. Using in situ protein cross-linking of hippocampal cultures to capture weak non-covalent interactions, Mergiya and collaborators (2023) found several fold increases in Arc monomer and dimer following BDNF treatment, with no apparent increase in tetramer or other larger species [32]. BDNF induces Arc-dependent LTP [6, 7], whereas DHPG induces Arc synthesis-dependent LTD. Zhang and colleagues (2019) showed reduction of DHPG-induced LTD in mice harboring mutations that impair oligomerization from tetramer to 32mers in the recombinant Arc protein. Together these data are consistent with a working hypothesis presented in Fig. 10, in which C11 PLA detects Arc dimer and H11 higher-order oligomers. Basal Arc complexes are detected in dendrites at equal density by both H11 and C11 PLA, but only H11 PLA is disrupted by OligoOFF peptide. OligoOFF targets the N-terminal motif which is critical for dimer interactions toward higher-order oligomers and capsids but does not mediate dimer formation itself [23]. Accordingly, the differential effect of OligoOFF also supports preferential detection of dimers by C11 and higher-order oligomers by H11 PLA.
Fig. 10.
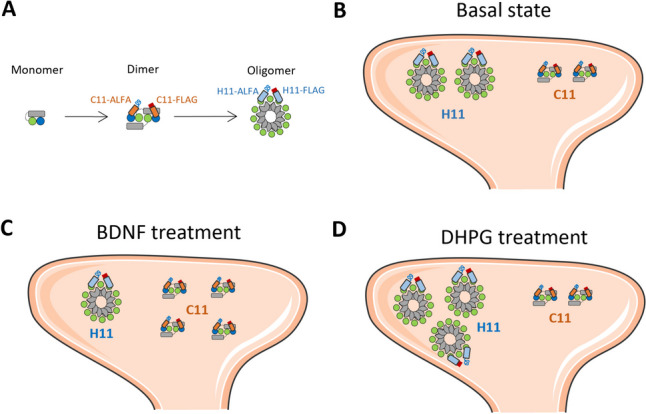
Working hypothesis of constitutive and stimulus-induced Arc oligomers detected by H11 and C11 PLA. A Schematic cartoon of Arc protein domains in monomer, dimer, and higher-order oligomer. Arc NTD (gray rectangle), CTD capsid domain with N-lobe (green circle), and C-lobe (blue circle). The dimer is the building block for higher-order assembly. The figure depicts a putative domain-swapped stable dimer and a higher-order oligomer. We propose that H11 PLA preferentially detects higher-order oligomer with multiple, accessible N-lobe ligand binding sites, while C11 PLA preferentially detects dimer. B Basal state showing constitute expression of dimer and high-order oligomer in a dendritic spine. Both H11 and C11 PLA detect Arc-Arc complexes in the neuronal basal state. C BDNF treatment increases Arc-Arc C11 PLA signal while decreasing H11 signal, consistent with enhanced de novo synthesis of dimer and either disassembly of higher-order oligomer or blocked access of H11 to the N-lobe pocket. D DHPG treatment increases H11, but not C11, PLA signal, consistent with enhanced selective formation of higher-order oligomers
H11 and C11 bind to Arc with low nanomolar affinity and crystal structures of the nanobodies in complex with the capsid domain have been solved. The H11 complementarity determining region 3 binds inside the ligand pocket through a conserved N-lobe binding motif. C11 binds to an exposed surface of the C-lobe formed by helix-6 and helix-7 which includes conserved sites of C-lobe/C-lobe inter-subunit binding important for retroviral capsid formation [37]. Clearly, the regulation of H11 and C11 PLA signals indicates major difference in the availability of epitopes for binding in native Arc-Arc complexes. The H11 PLA results indicate enhanced access to N-lobe (green circles) hydrophobic pockets in higher-order oligomers (Fig. 10) that is lost or dispersed upon disruption by OligoOFF. In turn, the C11 results suggest that C-lobe (blue circles) surfaces are not exposed for PLA interaction in the larger oligomers nor are they created after disruption by OligoOFF. Clearly, complex interaction between exposure of vacant binding sites and occlusion of sites by endogenous ligands may occur. For instance, the observed reduction in H11 PLA following BDNF treatment could reflect enhanced binding of constitutive oligomers to N-lobe binding partners at synapses or release of capsids from the neuron, in parallel with the observed enhanced synthesis of Arc and increased abundance of C11 PLA signal.
Conclusion
The present study introduces nanobody-PLA as a method for detecting native Arc-Arc complexes, including oligomers, in neuronal cultures in situ. Using nanobodies that recognize structurally defined epitopes in the Arc capsid domain, we identified widespread constitutive Arc-Arc oligomers in neuronal dendrites that are stably expressed and not dependent on recent neuronal firing activity or protein synthesis. We also uncover regulation of Arc-Arc complexes specific to BDNF and DPHG stimuli. This work thus places focus on the role of constitutive oligomeric Arc and dynamic regulation oligomers in the context of neuronal signaling and plasticity.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Dr. Hongyu Zhang for providing TAT-peptides for this study.
Abbreviations
- AMPAR
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors
- ANOVA
Analysis of variance
- AraC
Cytosine arabinoside
- Arg3.1
Activity-regulated gene 3.1
- Arc
Activity-regulated cytoskeleton-associated protein
- BDNF
Brain-derived neurotrophic factor
- CHX
Cycloheximide
- CDR3
Complementarity determining region 3
- CA
C-terminal capsid domain
- DHPG
Dihydroxyphenylglycine
- DIV
Day in vitro
- DMEM
Dulbecco’s modified Eagle medium
- FBS
Fetal bovine serum
- Gag
Group-specific antigen
- HBSS
Hank’s balanced salt solution
- HEK
Human embryonic kidney cells
- ICC
Immunocytochemistry
- LTD
Long-term depression
- LTP
Long-term potentiation
- MAP2
Microtubule-associated protein 2
- MEM
Minimum essential medium
- PBS
Phosphate-buffered saline
- PLA
Proximity ligation assay
- PLL
Poly-l-lysine
- PSD
Post-synaptic density
- Stg
Stargazin
- TAT
Trans-activator of transcription
- TIP60
Tat-interactive protein 60
- TTX
Tetrodotoxin
Author Contribution
Rodolfo Baldinotti, Francois Pauzin, and Clive Bramham designed and supervised the study. Rodolfo Baldinotti developed the nanobody-based PLA and performed the PLA and ICC work with nanobodies H11 and C11. Hauk Fevang performed experiments and data analysis for C11 nanobody PLA for Figs. 7 and 8 and Supplementary Fig. 5. Rodolfo Baldinotti and Francois Pauzin performed image data analysis and statistics. Rodolfo Baldinotti prepared the figures. Rodolfo Baldinotti prepared the hippocampal neuronal cultures, with contribution of cryopreserved neurons from Yuta Ishizuka. Rodolfo Baldinotti and Clive Bramham wrote the manuscript, with contributions from all authors. All authors have read and approved the final manuscript.
Funding
Open access funding provided by University of Bergen (incl Haukeland University Hospital). This was work was funded by grants from the Research Council of Norway (249951) and the Trond Mohn Foundation (grant TMS2021TMT04) to CRB. RB was recipient of a PhD fellowship from the Faculty of Medicine, University of Bergen. FP was supported by a postdoctoral fellowship through Norway-Poland EAA grant to CRB. HF was supported by a scholarship from the Medical Student Research Program at the Faculty of Medicine, University of Bergen.
Data Availability
All data used in this study are available from the corresponding author upon reasonable request.
Declarations
Ethics Approval
All animal procedures and handling were performed accordingly with the Norwegian National Research Ethics Committee in compliance with EU Directive 2010/63/EU, ARRIVE guidelines.
Consent to Participate
No human subjects nor samples were used in the present study.
Consent for Publication
No human subjects nor samples were used in the present study.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martin SJ, Grimwood PD, Morris RGM (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711. 10.1146/ANNUREV.NEURO.23.1.649 [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi T, Duszkiewicz AJ, Morris RGM (2014) The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Philos Trans R Soc B Biol Sci 369. 10.1098/RSTB.2013.0288 [DOI] [PMC free article] [PubMed]
- 3.Abraham WC, Jones OD, Glanzman DL (2019) Is plasticity of synapses the mechanism of long-term memory storage? NPJ Sci Learn 4(1):1–10. 10.1038/s41539-019-0048-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisaz R, Travaglia A, Alberini CM (2014) The neurobiological bases of memory formation: from physiological conditions to psychopathology. Psychopathology 47:347. 10.1159/000363702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikolaienko O, Patil S, Eriksen MS, Bramham CR (2018) Arc protein: a flexible hub for synaptic plasticity and cognition. Semin Cell Dev Biol 77:33–42 [DOI] [PubMed] [Google Scholar]
- 6.Messaoudi E, Kanhema T, Soulé J et al (2007) Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci 27:10445–10455. 10.1523/JNEUROSCI.2883-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuipers SD, Trentani A, Tiron A et al (2016) BDNF-induced LTP is associated with rapid Arc/Arg3.1-dependent enhancement in adult hippocampal neurogenesis. Sci Rep 6. 10.1038/SREP21222 [DOI] [PMC free article] [PubMed]
- 8.Waung MW, Pfeiffer BE, Nosyreva ED et al (2008) Rapid translation of Arc/Arg3.1 selectively mediates mGluR dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron 59:84. 10.1016/J.NEURON.2008.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzowski JF, Lyford GL, Stevenson GD et al (2000) Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci 20:3993–4001. 10.1523/JNEUROSCI.20-11-03993.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okuno H, Akashi K, Ishii Y et al (2012) Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIβ. Cell 149:886–898. 10.1016/J.CELL.2012.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soulé J, Alme M, Myrum C et al (2012) Balancing Arc synthesis, mRNA decay, and proteasomal degradation: maximal protein expression triggered by rapid eye movement sleep-like bursts of muscarinic cholinergic receptor stimulation. J Biol Chem 287:22354–22366. 10.1074/JBC.M112.376491 [DOI] [PMC free article] [PubMed]
- 12.Bateup HS, Denefrio CL, Johnson CA et al (2013) Temporal dynamics of a homeostatic pathway controlling neural network activity. Front Mol Neurosci 6:63678. 10.3389/FNMOL.2013.00028/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao VR, Pintchovski SA, Chin J et al (2006) AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat Neurosci 9:887–895. 10.1038/NN1708 [DOI] [PubMed] [Google Scholar]
- 14.Eriksen MS, Bramham CR (2022) Molecular physiology of Arc/Arg3.1: the oligomeric state hypothesis of synaptic plasticity. Acta Physiol 236:e13886. 10.1111/APHA.13886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Bramham CR (2020) Arc/Arg3.1 function in long-term synaptic plasticity: emerging mechanisms and unresolved issues. Eur J Neurosci 54(8):6696–6712. 10.1111/ejn.14958 [DOI] [PubMed]
- 16.Steward O, Farris S, Pirbhoy PS et al (2015) Localization and local translation of Arc/Arg3.1 mRNA at synapses: some observations and paradoxes. Front Mol Neurosci 7:1–15. 10.3389/FNMOL.2014.00101/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okuno H, Minatohara K, Bito H (2018) Inverse synaptic tagging: an inactive synapse-specific mechanism to capture activity-induced Arc/arg3.1 and to locally regulate spatial distribution of synaptic weights. Semin Cell Dev Biol 77:43–50. 10.1016/J.SEMCDB.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 18.Campillos M, Doerks T, Shah PK, Bork P (2006) Computational characterization of multiple Gag-like human proteins. Trends Genet 22:585–589. 10.1016/J.TIG.2006.09.006 [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Wu J, Ward MD et al (2015) Structural basis of Arc binding to synaptic proteins: implications for cognitive disease. Neuron 86:490–500. 10.1016/j.neuron.2015.03.030 [DOI] [PMC free article] [PubMed]
- 20.Myrum C, Baumann A, Bustad HJ et al (2015) Arc is a flexible modular protein capable of reversible self-oligomerization. Biochem J 468:145–158. 10.1042/BJ20141446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byers CE, Barylko B, Ross JA et al (2015) Enhancement of dynamin polymerization and GTPase activity by Arc/Arg3.1. Biochim Biophys Acta 1850:1310. 10.1016/J.BBAGEN.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Chuang Y-A, Na Y et al (2019) Arc oligomerization is regulated by CaMKII phosphorylation of GAG domain; an essential mechanism for plasticity and memory formation. Mol Cell 75:13–25. 10.17632/tt3hrs3r2m.1. (HHS Public Access) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksen MS, Nikolaienko O, Hallin EI et al (2021) Arc self-association and formation of virus-like capsids are mediated by an N-terminal helical coil motif. FEBS J 288:2930–2955. 10.1111/FEBS.15618 [DOI] [PubMed] [Google Scholar]
- 24.Ashley J, Cordy B, Lucia D et al (2018) Retrovirus-like Gag protein Arc1 binds RNA and traffics across synaptic boutons. Cell 172:262–274. 10.1016/J.CELL.2017.12.022 [DOI] [PMC free article] [PubMed]
- 25.Erlendsson S, Morado DR, Cullen HB et al (2020) Structures of virus-like capsids formed by the Drosophila neuronal Arc proteins. Nat Neurosci 23(2):172–175. 10.1038/s41593-019-0569-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pastuzyn ED, Day CE, Kearns RB et al (2018) The neuronal gene arc encodes a repurposed retrotransposon gag protein that mediates intercellular RNA transfer. Cell 172:275. 10.1016/J.CELL.2017.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hantak MP, Einstein J, Kearns RB, Shepherd JD (2021) Intercellular communication in the nervous system goes viral. Trends Neurosci 44:248–259. 10.1016/J.TINS.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avallone M, Pardo J, Mergiya TF et al (2023) Visualizing Arc protein dynamics and localization in the mammalian brain using AAV-mediated in situ gene labeling. Front Mol Neurosci 16. 10.3389/FNMOL.2023.1140785 [DOI] [PMC free article] [PubMed]
- 29.Hedde PN, Malacrida L, Barylko B et al (2021) Membrane remodeling by Arc/Arg3.1. Front Mol Biosci 8:20. 10.3389/fmolb.2021.630625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goo BMSS, Sanstrum BJ, Holden DZY et al (2018) Arc/Arg3.1 has an activity-regulated interaction with PICK1 that results in altered spatial dynamics. Sci Rep 8(1):1–12. 10.1038/s41598-018-32821-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volpato A, Ollech D, Alvelid J et al (2022) Extending fluorescence anisotropy to large complexes using reversibly switchable proteins. Nat Biotechnol 41(4):552–559. 10.1038/s41587-022-01489-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mergiya TF, Gundersen JET, Kanhema T et al (2023) Detection of Arc/Arg3.1 oligomers in rat brain: constitutive and synaptic activity-evoked dimer expression in vivo. Front Mol Neurosci 16:1142361. 10.3389/FNMOL.2023.1142361/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau Dalby Nielsen A, Parsbaek Pedersen C (2019) The capsid domain of Arc changes its oligomerization propensity through direct interaction with the NMDA receptor. Structure/Fold Des 27:1071-1081.e5. 10.1016/j.str.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 34.Fuller JC, Burgoyne NJ, Jackson RM (2009) Predicting druggable binding sites at the protein-protein interface. Drug Discov Today 14:155–161. 10.1016/J.DRUDIS.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 35.Hamers-Casterman C, Atarhouch T, Muyldermans S et al (1993) Naturally occurring antibodies devoid of light chains. Nature 363(6428):446–448. 10.1038/363446a0 [DOI] [PubMed] [Google Scholar]
- 36.Ishizuka Y, Mergiya TF, Baldinotti R et al (2022) Development and validation of Arc nanobodies: new tools for probing Arc dynamics and function. Neurochem Res 47:2656–2666. 10.1007/S11064-022-03573-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markússon S, Hallin EI, Bustad HJ et al (2022) High-affinity anti-Arc nanobodies provide tools for structural and functional studies. PLoS One 17. 10.1371/JOURNAL.PONE.0269281 [DOI] [PMC free article] [PubMed]
- 38.Godoy Muñoz JM, Neset L, Markússon S et al (2024) Structural characterization of two nanobodies targeting the ligand-binding pocket of human Arc. PLoS One 19:e0300453. 10.1371/JOURNAL.PONE.0300453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fredriksson S, Gullberg M, Jarvius J et al (2002) Protein detection using proximity-dependent DNA ligation assays. Nat Biotechnol 20:473–477. 10.1038/NBT0502-473 [DOI] [PubMed] [Google Scholar]
- 40.Alam MS (2018) Proximity ligation assay (PLA). Curr Protoc Immunol 123:e58. 10.1002/CPIM.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Söderberg O, Gullberg M, Jarvius M et al (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods 3:995–1000. 10.1038/nmeth947 [DOI] [PubMed] [Google Scholar]
- 42.Ishizuka Y, Bramham CR (2020) A simple DMSO-based method for cryopreservation of primary hippocampal and cortical neurons. J Neurosci Methods. 10.1016/j.jneumeth.2019.108578 [DOI] [PubMed] [Google Scholar]
- 43.Lyford GL, Yamagata K, Kaufmann WE et al (1995) Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 14:433–445. 10.1016/0896-6273(95)90299-6 [DOI] [PubMed] [Google Scholar]
- 44.Nair RR, Patil S, Tiron A et al (2017) Dynamic Arc SUMOylation and selective interaction with F-actin-binding protein drebrin a in LTP consolidation in vivo. Front Synaptic Neurosci 9:8. 10.3389/fnsyn.2017.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng F, Luo Y, Wang H (2009) Regulation of BDNF-mediated transcription of immediate early gene Arc by intracellular calcium and calmodulin. J Neurosci Res 87:380. 10.1002/JNR.21863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myrum C, Soulé J, Bittins M et al (2017) Arc interacts with the integral endoplasmic reticulum protein, calnexin. Front Cell Neurosci 11:294. 10.3389/fncel.2017.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujimoto T, Tanaka H, Kumamaru E et al (2004) Arc interacts with microtubules/microtubule-associated protein 2 and attenuates microtubule-associated protein 2 immunoreactivity in the dendrites. J Neurosci Res 76:51–63. 10.1002/JNR.20056 [DOI] [PubMed] [Google Scholar]
- 48.Opazo P, Labrecque S, Tigaret CM et al (2010) CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67:239–252. 10.1016/j.neuron.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 49.Nair D, Hosy E, Petersen JD et al (2013) Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J Neurosci 33:13204–13224. 10.1523/JNEUROSCI.2381-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mabb AM, Je HS, Wall MJ et al (2014) Triad3A regulates synaptic strength by ubiquitination of Arc. Neuron 82:1299–1316. 10.1016/J.NEURON.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das S, Lituma PJ, Castillo PE, Singer RH (2023) Maintenance of a short-lived protein required for long-term memory involves cycles of transcription and local translation. Neuron 111:2051-2064.e6. 10.1016/J.NEURON.2023.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang Y, Van Dongen AMJ (2021) Selective increase of correlated activity in Arc-positive neurons after chemically induced long-term potentiation in cultured hippocampal neurons. eNeuro 8. 10.1523/ENEURO.0540-20.2021 [DOI] [PMC free article] [PubMed]
- 53.Wibrand K, Pai B, Siripornmongcolchai T et al (2012) MicroRNA regulation of the synaptic plasticity-related gene Arc. PLoS One 7. 10.1371/JOURNAL.PONE.0041688 [DOI] [PMC free article] [PubMed]
- 54.Dore K, Pao Y, Lopez JS et al (2020) SYNPLA, a method to identify synapses displaying plasticity after learning. Proc Natl Acad Sci U S A 117:3214–3219. 10.1073/PNAS.1919911117/-/DCSUPPLEMENTAL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernández E, Collins MO, Frank RAW et al (2017) Arc requires PSD95 for assembly into postsynaptic complexes involved with neural dysfunction and intelligence. Cell Rep 21:679–691. 10.1016/J.CELREP.2017.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hallin EI, Eriksen MS, Baryshnikov S et al (2018) Structure of monomeric full-length ARC sheds light on molecular flexibility, protein interactions, and functional modalities. J Neurochem 147:323–343. 10.1111/jnc.14556 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study are available from the corresponding author upon reasonable request.