Abstract
Neural hyperexcitability of the central auditory system is a key pathological characteristic of tinnitus, but its underlying molecular mechanisms remain elusive. The large-conductance Ca2+-activated K+ channel (BK) plays a crucial role in down- or upregulating neuronal activity. This study aims to investigate the role of BK channels in mediating tinnitus-associated neural hyperexcitability and elucidate the mechanisms behind it. Immunofluorescent staining revealed extensive expression of the BK channels on neurons within the central auditory system of rats. After long-term systemic administration of salicylate, a stable tinnitus inducer, we observed a significant change in the expression levels of BKα and β4 subunits in the rat central auditory system. In addition, salicylate was found to enhance the outward potassium currents mediated by the BK channel when exogenously expressed in HEK293 cells. Interestingly, this effect could be blocked by ryanodine, a potent inhibitor of ryanodine receptors (RyRs). Molecular docking identified Gln4020 within the central domain of RyR as a key residue in RyR-salicylate interactions. The results indicated that salicylate might directly activate RyRs leading to Ca2+ release from endoplasmic reticulum, and increased BK currents subsequently. Systemic treatment with paxilline, a potent blocker of BK channel, selectively reversed the increased P4/P1 amplitude ratios in the frequency region of tinnitus perception induced by single-dose salicylate administration. These results suggest that BK channels and ryanodine receptors may play a selective role in salicylate-induced tinnitus.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12035-024-04533-6.
Keywords: Tinnitus, Salicylate, Hyperexcitability, BK channel, Ryanodine receptor
Introduction
Tinnitus is a psycho-physiological disorder characterized by the perception of ringing or buzzing in the ears in the absence of outside sounds, and it occurs in approximately 10 to 15% of people worldwide. Despite its prevalence, there is currently no targeted medical cure for tinnitus, and the underlying mechanisms that cause the condition continue to be a subject of intrigue [1, 2]. Clinical evidence suggests that hearing loss due to noise exposure, ototoxic drugs, aging, injury, or diseases in cochlea is one of the main risk factors for tinnitus. The initial source of tinnitus is thought to be a decrease in neural output from the cochlea. However, the maintenance of tinnitus is thought to involve a cascade of neural changes within the central auditory system [3]. The dorsal cochlear nucleus (DCN), which is the immediate recipient of auditory nerve fiber input in the brain, has been deemed to be a site of tinnitus generation. Notably, the ablation of the DCN before traumatic noise exposure can prevent tinnitus, but if the DCN is ablated after noise exposure, tinnitus still persists [4, 5]. In addition, increased spontaneous firing rate and neural synchrony of glutamatergic fusiform neurons in the DCN are characteristic restricted to animals that exhibit tinnitus following noise exposure [6]. Salicylate can reduce the spontaneous firing of glycinergic cartwheel neurons in the DCN, which are the primary inhibitory sources for fusiform neurons [7, 8]. The inferior colliculus (IC) is an integration site, which receives ascending input from the DCN and sends projections to the auditory thalamus, the medial geniculate body (MGB). Several studies have found that hyperactivities in the IC and MGB are linked to the generation and maintenance of tinnitus, primarily due to reduced spontaneous activity of GABAergic and glycinergic neurons [9–14]. Auditory cortex serves as the core brain area for receiving ascending information and forming auditory perception. Electroencephalography and magnetoencephalography studies of tinnitus patients have shown altered spontaneous neural activity in the auditory cortex [15, 16], which has also been confirmed in animal studies. Increased neuronal spontaneous firing rate (SFR) and neural synchrony in the primary auditory (A1) cortex of animals after noise exposure are considered major pathological features of tinnitus [17, 18]. In recent years, neuronal hyperexcitability in the central auditory system, primarily attributed to inhibitory-excitability imbalance, has been recognized as the neural basis of tinnitus [19]. The majority of studies have attempted to restore this balance and alleviate tinnitus by manipulating GABA and NMDA receptors using compounds that enhance inhibitory GABAergic neurotransmission and reduce excitatory glutamatergic neurotransmission [20, 21]. However, until now, few if any pharmacological treatments have reliably reduced or eliminated tinnitus in most patients so far.
Potassium channels are expressed in almost all living cells and play a key role in maintaining intracellular homeostasis, regulating membrane potential, and modulating action potential. As a result, they have been identified as valuable therapeutic targets for related diseases [22]. The decreased function of Kv7.2/7.3 potassium channels is associated with hyperexcitability of DCN and the development of noise-induced tinnitus. Activators of Kv7.2/7.3 are able to prevent the development of tinnitus in mice, providing a potential therapeutic target [23, 24]. Large conductance calcium-gated and voltage-gated potassium (BK, also known as Maxi-K, Slo1 or KCa1.1) channels consisting of pore-forming α subunits and regulatory β (β1-4) and γ (γ1-4) subunits are widely expressed in a variety of excitable and non-excitable cells, and represent potential molecular targets for drug development in diseases characterized by aberrant cellular and network excitability, such as seizures, pain, and movement disorders [25–28]. Notably, the pharmacology and functional characteristics of BK channels, such as their sensitivity to voltage and intracellular Ca2+, are significantly influenced by auxiliary subunits, co-assembling with diverse auxiliary subunits to form distinct BK channel phenotypes with varied functionality. BKβ4 is commonly referred as the “neuronal auxiliary subunit” since it is the most abundant of the four β subunits in the central nervous system, distributed mostly overlap with α subunits. The brain-specific BK channel regulatory β4 subunit increases and decreases channel activity in high and low concentrations of intracellular Ca2+, respectively. It can also slow down the activation and deactivation kinetics of BK channels at all Ca2+ concentrations [29].
Typically, the activation of BK channels leads to a rapid and massive efflux of K+ ions that hyperpolarizes cellular membrane potential to downregulate neuronal excitability. However, it seems paradoxical that gain-of-function mutation in the BKα subunit can actually also give rise to neuronal hyperexcitability by inducing rapid repolarization of action potentials and high-frequency firing, resulting in epilepsy and paroxysmal dyskinesia [30, 31]. There are growing evidence supporting that upregulation or downregulation of BK channels under different pathological conditions are consistent with the fact that BK currents can speed up or slow down neuronal excitability depending on the cellular context. Thus, both activators and blockers of BK channels have been tentatively employed to suppress neuronal hyperexcitability in related diseases [29, 32].
Salicylate is an active metabolite of aspirin, which can induce tinnitus in both humans and animals [33, 34], and has been widely employed to investigate the pathogenesis of tinnitus. Our previous studies have demonstrated that both single-dose and chronic administrations of salicylate can induce reversible tinnitus in rats [35, 36]. Recently, two studies have originally explored the effects of BK channel modulators in suppressing salicylate-induced tinnitus in animals [37, 38]. Given their double-faced and inscrutability roles in controlling neuronal excitability, the precise performance of BK channels in tinnitus pathophysiology needs to be further attention, and its potential as a drug target deserves to be comprehensively attempted. The aim of the current study was to investigate the involvement of neuronal-type BK (α + β4) channel in tinnitus and evaluate the therapeutic efficacy of BK channel modulator in suppressing salicylate-induced acute and chronic tinnitus.
Materials and Methods
Animals Care
Male Sprague–Dawley rats (200–220 g) were obtained from SPF Biotechnology Co., Ltd. (Beijing, China), and were raised under 12-h light/dark cycle with ad libitum access to food and water. The experimental procedures were carried out in accordance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals and were approved by the Ethics Committee of Hebei University. All efforts have been made to minimize the number of animals used in this study.
Immunofluorescent Staining
Rats deeply anesthetized with intraperitoneal injection of pentobarbital (75 mg/kg) underwent perfusion with saline and 4% paraformaldehyde (PFA). The brains were removed, immobilized with PFA, and then subjected to dehydration in sucrose. Coronal sections were collected using a Cryostat Microtome (HM525; Thermo Fisher Microm, Walldorf, Germany). Frozen sections were permeabilized with 1% Triton X-100 and blocked in Immunol-staining Blocking Buffer (P0102; Beyotime, China) for 1 h at room temperature. The tissues were subsequently incubated overnight at 4 °C with the following primary antibodies: polyclonal rabbit anti-sloα1 (also named kcnma1, ab3586, Abcam) at a 1:300 dilution, polyclonal rabbit anti-sloβ4 (also named kcnmb1, APC-061, alomone) at a 1:200 dilution, monoclonal mouse anti-NeuN (ab104224, Abcam, 1:800), monoclonal mouse anti-GFAP (3670, Cell Signaling Technology, 1:400), and polyclonal goat anti-Iba1 (ab5076, Abcam, 1:1000), each respectively diluted in 5% serum. After three washes in PBS, the sections were incubated in the corresponding secondary antibodies for 1 to 2 h at room temperature in the dark. Immunolabeled images were captured using a laser scan confocal fluorescent microscope (LSM710; Carl Zeiss, Jena, Germany).
Quantitative PCR
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Quantitative PCR (qPCR) was performed using PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Dalian, China) and SYBR Green Realtime PCR Master Mix (Toyobo, Osaka, Japan). Primer sequences are shown in Table S1. Expression levels were calculated using the 2−ΔΔCT formula, with β-actin and GAPDH as internal controls. The “n” value represents the number of normalized values. Three groups (7C, control group with 7 consecutive days of saline administration; 7 T, tinnitus group with consecutive days of salicylate administration; 7 T + 7R, recovery group) in chronic experiments.
Electrophysiological Recordings
Plasmid and Cell Line
The plasmid carrying hSloα (U23767) was gifted by J.D. Lippiat (Leeds university) and N.W. Davies (University of Leicester) [39]. The experimental schemes referred to previous studies [40, 41]. Experiments were conducted using human embryonic kidney (HEK) 293 T cell lines. The cells were cultured in DMEM (Life Technologies, Grand Island, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, USA). Culture dishes were incubated at 37 °C in a humidified atmosphere containing 5% CO2 and subcultured every 2–3 days. One day before transfection, cells were transferred to 24-well plates. When got suitable cell density, cells were transiently transfected using Lipofectamine™ 3000 (Invitrogen, Carlsbad, CA, USA) at a ratio of 2 µL reagent with 1 µg total plasmid per well. Electrophysiological recordings were performed at 2 days after transfection.
Solution and Drug
The standard bath solution contained the following components (in mM): NaCl 135, KCl 5, MgCl2·6H2O 1, CaCl2 1.8, HEPES 10, glucose 10 (pH = 7.4). The pipette solution contained the following components (in mM): NaCl 10, KCl 117, MgSO4 4, CaCl2 0.628, HEPES 10, EGTA 1 (pH = 7.2). The total Ca2+ concentration to achieve a free concentration of 300 nM was calculated using the program WEBMAXC STANDARD (https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator/webmaxc/webmaxcS.htm). The liquid junction potential between the pipette and the bath solutions was estimated to be zero, using the Calculate Junction Potentials module in Clampex. After pipette offset, the voltage values of recorded cells ranged from − 1 to − 3 mV. Sodium salicylate (S3007; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in bath solution at the desired working concentration before use. Ryanodine (ab120083; Abcam, Cambridge, MA, USA) was dissolved in ethanol at a stock concentration of 10 mM before being added to the incubation solution at a final concentration of 10 μM.
Voltage-clamp Recording
Whole cell voltage-clamp experiments were conducted using an Axon Multiclamp 700B (Molecular Devices, USA) at room temperature (23–25 °C). Patch pipettes were fabricated from glass capillary tubes by PC-10 Puller (Narishige, Japan) with a resistance of 2–3 MΩ. Data acquisition and stimulation protocols were controlled by a computer equipped with pCLAMP10.3. Capacitance transients were cancelled, and cells with a seal resistance < 1 GΩ were omitted. Series resistance (Rs) was compensated (80–90%) to minimize voltage errors, and the cells whose value of series resistance higher than 10 MΩ (without compensation) were omitted. Leak subtraction was performed using the P/N protocol. Data were sampled at 50 kHz and low-pass filtered at 10 kHz. BK channel currents were elicited by 200-ms depolarizing pulses ranging from − 50 to + 100 mV with 10-mV increments; the holding potential was − 80 mV. To ensure sufficient exchange between intracellular fluid and pipette solution, the recording protocols were carried out 10 min after the establishment of the whole-cell mode. Current density calculation used the formula pA/pF, where pA represents the current of BK channel and pF represents the membrane area of measured cell. For determining the voltage dependence of activation, the conductance was calculated using the formula G = I/(V − Vr), where I is the current at the command voltage (V), and Vr is the reversal potential. The conductance was normalized to the maximal value and the voltage dependence for activation was fitted to a Boltzmann equation f(x) = − 1/(1 + exp((x-V1/2)/k)) + 1, where V1/2 is the voltage at which half-maximal activation occurs, and k describes the slope of the fit.
Calcium Imaging
HEK293T cells without exogenous expression were washed three times with Mg2+-free solution. Subsequently, cells were loaded with the same solution containing 5 μM Fluo-4 AM (F14217, Invitrogen, Carlsbad, CA, USA) for 30 min in the dark at 37 ℃. Ryanodine (10 μM) was added accompanied by fluorescent dye in the working solution for part of the experiment. To remove the extracellular Fluo-4 AM, the loaded cells were rinsed three times with the Mg2+-free solution, and then the cells were incubated with Ringer’s solution which contained the following components (in mM): NaCl 145, KCl 5, MgSO4 1, HEPES 10, glucose 16, EGTA 1 (pH = 7.4). Intracellular free Ca2+ was measured with Fluo-4/AM using a confocal laser scanning microscope (LSM 880 NLO, ZESSS, Germany). Real-time calcium imaging was typically collected at 2.5-s intervals and recorded at least 6 min. The regions of interest (ROIs) were selected in individual HEK293T cells by Zeiss LSM Image Browser (Zeiss, Germany) to track the changes in the ratio of fluorescence intensity. The ratio (F/F0) of fluorescence intensity was calculated by dividing fluorescence intensity at time (F) with the initial fluorescence intensity (F0) of the experiment.
Homology Modeling and Molecular Docking
Ligand and Protein Preparation
The chemical structures of salicylate (CID_54675850) and 4-chloro-m-cresolwere (4-CmC) (CID_1732) downloaded from the PubChem database. The primary structure of salicylate was hydroxy-based; after ion structure optimization using Discovery Studio’s Prepare Ligands program, the carboxylate form was stable with lower energy, which aligns with the anionic structure of sodium salicylate. 4-CmC, a potent and specific activator of ryanodine receptors (RyRs), was employed here as a positive control for the molecular docking of salicylate with RyRs due to similar chemical structure. We demonstrated the hydrogenation treatment of the small molecules and prepared the ligand processing protocol. Due to the unavailability of 3D structures of human RyRs (hRyR1 and hRyR3) in the PDB database, we performed homology modeling based on known crystal structures of RyRs. The 3D models were generated using the Build homology models (MODELER) in Discovery Studio 4.0 (Accelrys, San Diego, CA, USA).
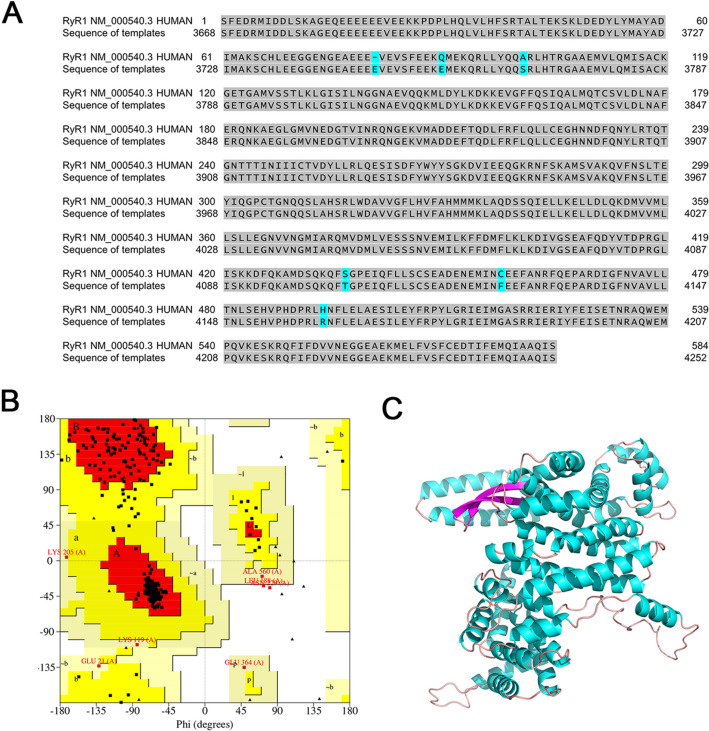
For homology modeling of hRyR1, it has been found that the central region of rabbit RyR1 plays a crucial role in 4-CmC-RyR1 interaction and RyR1 activation [42–44]. Therefore, the central region (residues: 3668–4251) of hRyR1 (NM_000540.3) from the NCBI database was selected to accurately demonstrated the binding mode of salicylate with hRyR1. BLAST algorithm was used to search the protein sequences similar to the central region. Proteins with a similarity greater than 30% and high precision were chosen as templates. In order to ensure continuity in homology modeling, the multi-template modeling method is adopted in this study. A total of five protein templates from rabbit RyR1 (originating from Oryctolagus cuniculus) based on the highest sequence identity score (98.97%) are selected, and their cryo-EM structures were downloaded from the PDB database: (1) 7T64 chain A [45]; (2) 5GKY chain A [46]; (3) 6WOT chain A [47]; (4) 7K0S chain A; (5) 3J8H chain A [48]. We used the central regions of these five templates as main structure and modeled it using the Modeller homology modeling program. The amino acid sequence of the hRyR1 central region corresponds to residues 1–583 in our homology modeling sequence.
For homology modeling, ERRAT and Ramachandran plots were used to verify the validity and reliability of the model. Ramachandran plots describe the dihedral angle distributions of the protein and distinguish permissible and disallowed conformations. If the percentage of residues in the core and allowed regions exceeds 90%, the model’s conformation is considered reasonable. ERRAT was used to evaluate the stereochemical quality of protein models, with higher scores indicating greater reliability. Unreasonable residues were optimized using loop optimization to enhance model’s credibility and accuracy. After multi-template modeling, we conducted hydrogenation treatment, cleaned the protein, and applied Chemistry at HARvard Macromolecular Mechanics (CHARMm) electrostatic field forces to achieve energy minimization.
The crystal structure of hRyR2 in the closed state was recently resolved (PDB: 7u9q)[49], and was directly used for molecular docking with small molecules. 3D model of hRyR3 was generated using homology modeling based on multi-templates from PDB database: 7U9Q [49], 7U9X [49], 6WOV [47], 6WOU [47], and 5GO9 [50]. The processing procedures were similar to those of hRyR1.
Protein–Ligand Binding Prediction
Once the 3D models of hRyRs were generated, the chemical structures of small molecules (4-CmC and salicylate) were used for docking analysis using the Libdock docking module in Discovery Studio 4.0 (Accelrys Software Inc., San Diego, CA, USA). In this study, 4-CmC, a potent and specific agonist of RyRs, serves as a positive control for salicylate-RyR molecular docking. Semi-flexible docking approach known for its speed and accuracy was employed. The docking pocket was positioned with a 10-Å radius centered around the Gln4020 residue. The best salicylate-RyR complex was selected based on two principles: the docking score met a threshold determined by the Libdock score of 4-CmC, and small molecule interactions with at least three residues generated major non-bonded interaction. Discovery Studio Visualizer was used to observe the two-dimensional plots of interactions between a protein and a ligand and to visualize the three-dimensional binding patterns.
Auditory Brainstem Responses Testing and Analysis
Human and animal studies have shown that changes in the auditory brainstem responses (ABRs) may be linked to tinnitus [51, 52]. ABRs are acoustically stimulated signals that represent the synchronized neural activation along the auditory pathways. The first wave (I) of ABR is associated with activity of the peripheral auditory nerve, while waves II–V reflect activity in auditory brainstem structures from the cochlear nucleus (CN) to the inferior colliculus (IC) [53, 54]. Increased sound-evoked activity near the tinnitus percept in brainstem auditory structures is considered an objective indicator of salicylate-induced tinnitus in rats [38, 55–57]. Our previous studies have confirmed that single-dose acute and seven consecutive days chronic administration of salicylate were both able to induce reversible tinnitus-like behaviors in rats [35, 36]. These two paradigms were used to examine the effect of paxilline (an antagonist of BK channel) on salicylate-induced tinnitus in the current study. Sodium salicylate (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in saline with 350 mg/kg or 200 mg/kg for intraperitoneal injection. The applied concentration of paxilline refers previous studies [58, 59]. Paxilline (Cayman Chemical, Ann Arbor, MI, USA) was resuspended to 10 mM in DMSO and then diluted 1:2000 in saline. Paxilline (3 μg/kg) or vehicle (0.05% (v/v) DMSO) was injected intraperitoneally 3 h before ABR tests.
In the acute experiment, ABR recordings were initially performed as baseline; after 7 days, the same rats were administrated with 350 mg/kg salicylate, and ABR recordings were performed 2 h post salicylate administration. After another 7 days, the same rats were orderly administrated with paxilline (3 h before ABR recording) and salicylate (2 h before ABR recording). In the chronic experiment, the rats were randomly divided into three groups: control, salicylate, salicylate plus paxilline. At first, the rats from three groups underwent ABR tests as baseline. Then, the rats from salicylate group and salicylate plus paxilline group were injected with 200 mg/kg salicylate twice daily (8:00 and 20:00) for seven consecutive days. The rats from control group were treated with the same volume of saline. ABR was recorded 12 h after last injection. In salicylate plus paxilline group, paxilline was injected intraperitoneally 3 h before ABR tests.
ABR measurements were conducted in a sound-attenuating chamber (Guangdong Shengzuo Acoustics Co., Ltd.); the recording procedures referred to previous studies [60, 61]. Rats were anesthetized with intraperitoneal injection of ketamine (100 mg/kg) and xylazine (25 mg/kg) (Medchem, New Jersey, USA). The body temperature was maintained at 37 °C with an isothermal pad (Homeothermic Monitoring System, Harvard Apparatus) placed under abdomen. Three subdermal needle electrodes (Rhythmlink International LLC, USA) were positioned at the vertex of the skull (active), the mastoid region of right ear (reference), and the left shoulder (ground). The impedances of both the input channel and the reference ranged from 0.6 to 0.9 kΩ. Stimulus sounds were presented free-field via MF1 speaker (Tucker–Davis Technologies, Alachua, FL, USA) placed 10 cm away from the vertex. A short tone burst of 3-ms duration with 1-ms rise and fall time was generated by the RZ6 workstation and BioSigRZ software. Stimulus frequencies of 32 to 4 kHz were presented in half-octave step (32, 22.6, 16, 11.3, 8, 5.6, 4 kHz). The sound level was decreased from 90 to 0 dB SPL in 5 dB steps. The calibration of these frequencies was conducted according to the standard configuration files provided by BioSigRZ. Stimulus presentation rate is 20 per second, and 400 trials were averaged at each frequency each level to acquire stable ABR traces. Hearing thresholds were defined as minimal stimulus level that evoked any one of the initial four peaks. All latencies and amplitudes of ABR peaks I–IV were measured and analyzed by using BioSigRZ software. Latency referred to the time from the onset of the stimulus signal to the peak, while amplitude was determined by averaging the △V of both sides of the peak. The ABR thresholds and the latencies and amplitudes of wave I were analyzed to assess the impact of salicylate on cochlear output. The P2/P1 and P4/P1 amplitude ratios were used to evaluate the salicylate-induced enhancement in central gain within brainstem auditory structures, serving as a neurophysiological marker of tinnitus [38, 57].
Statistical Analysis
Data were expressed as mean ± SEM and statistically analyzed using GraphPad Prism software (GraphPad Software Inc., San Diego, USA). The investigators who performed the data acquisition and quantification were blind to the experimental conditions. Student’s t-test and analysis of variance (ANOVA) followed by Bonferroni’s post hoc comparisons were used for data analysis. p < 0.05 was considered to be statistically significant.
Results
Changes in BK Channel Expression in the Rat Central Auditory System After Salicylate Administration
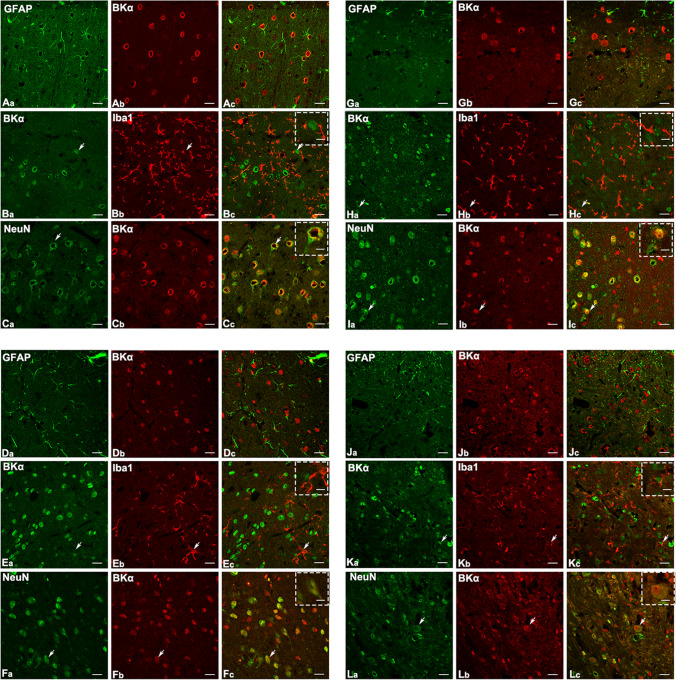
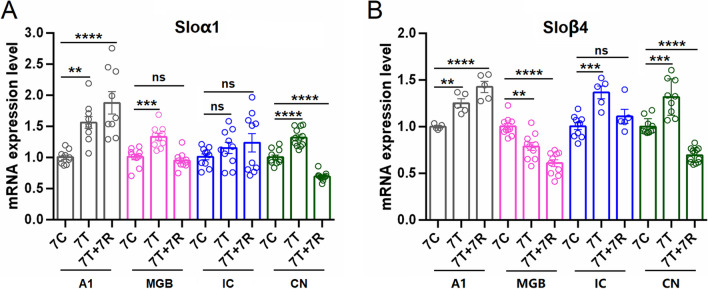
BK channels in the central system are primarily comprised of α subunit and β4 subunit [62]. Immunofluorescent staining experiments revealed that BKα and β4 subunits were extensively expressed in the NeuN-immunoreactive neurons of the central auditory system (A1, MGB, IC, and CN). Additionally, a few IBA1-immunoreactive microglia in the A1 cortex, MGB, IC, and CN were found to express α and β4 subunits (Fig. 1, Fig. 2). To explore the involvement of the BK channels in tinnitus, the expression patterns of neuronal-type BK channel (α + β4) were evaluated. Quantitative PCR showed a significant increase in BKα subunit expression in the A1 cortex (p < 0.01), MGB (p < 0.001), and CN (p < 0.0001) after chronic systematic salicylate administration compared to the control group (Fig. 3A). BKβ4 subunit expression also increased significantly in the A1 cortex (p < 0.01), IC (p < 0.001), and CN (p < 0.001) following salicylate administration, but decreased in the MGB (p < 0.01) compared to the control group (Fig. 3B). Our previous studies have shown that both acute single-dose and chronic salicylate administration can induce reversible tinnitus-like behaviors in rats [35, 36]. These findings suggest that upregulation of BK(α + β4) expression in the central auditory system may be involved in salicylate-induced tinnitus. Notably, the altered expression patterns of BKα and β4 subunits did not return to control levels in the recovery group when tinnitus disappeared [35, 36], indicating a possible rebalancing of cellular molecular events essential for maintaining homeostasis in the central auditory system.
Fig. 1.
Distribution of BKα subunit in the central auditory system. Representative images show the co-localization of the BKα subunit with three specific markers: GFAP (labeling astrocytes), IBA1 (labeling microglia), and NeuN (labeling neurons). A–C The A1 cortex, D–F MGB, G–I IC, and J–L CN. GFAP-immunoreactivity (ir) is shown in green (Aa, Da, Ga and Ja), IBA1-ir is shown in red (Bb, Eb, Hb and Kb), NeuN-ir is shown in green (Ca, Ga, Ia, and La), BKα subunit-ir is shown in red (Ab, Cb, Db, Fb, Gb, Ib, Jb, Lb) and in green (Ba, Ea, Ha, Ka). Merged signal are shown in yellow. White arrows indicate representative cells that are co-labeled. Scale bars, 20 μm; scale bar in the dotted frame, 10 μm
Fig. 2.
Distribution of BKβ4 subunit in the central auditory system. Representative images show the co-localization of the BKβ4 subunit with three specific markers: GFAP (labeling astrocytes), IBA1 (labeling microglia), and NeuN (labeling neurons). A–C The A1 cortex, D–F MGB, G–I IC, and J–L CN. GFAP-ir is shown in green (Aa, Da, Ga and Ja), IBA1-ir is shown in red (Bb, Eb, Hb) and in green (Ka), NeuN-ir is shown in green (Ca, Fa, Ia, and La), BKβ4 subunit-ir is shown in red (Ab, Cb, Db, Fb, Gb, Ib, Jb, Kb, Lb) and in green (Ba, Ea, Ha). Merged signal are shown in yellow. White arrows indicate cells that are co-labeled. Scale bars, 20 μm; scale bar in the dotted frame, 10 μm
Fig. 3.
Altered expression of BKa and BKβ4 subunits in the central auditory system following salicylate administration. A Quantitative analysis of mRNA of BKα subunit in the A1 cortex, MGB, IC, and CN from three groups (7C, control group receiving saline for 7 consecutive days; 7 T, tinnitus group receiving salicylate for 7 consecutive days; 7 T + 7R, recovery group) in chronic experiments. B Quantitative analysis of BKβ4 subunit mRNA expression in the same brain regions and groups as in panel A. One-way analysis of variance (ANOVA) followed by post hoc Scheffé test was used for multiple comparisons. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the control group
Salicylate Increases BK Current Heterologously Expressed in HEK293T Cells
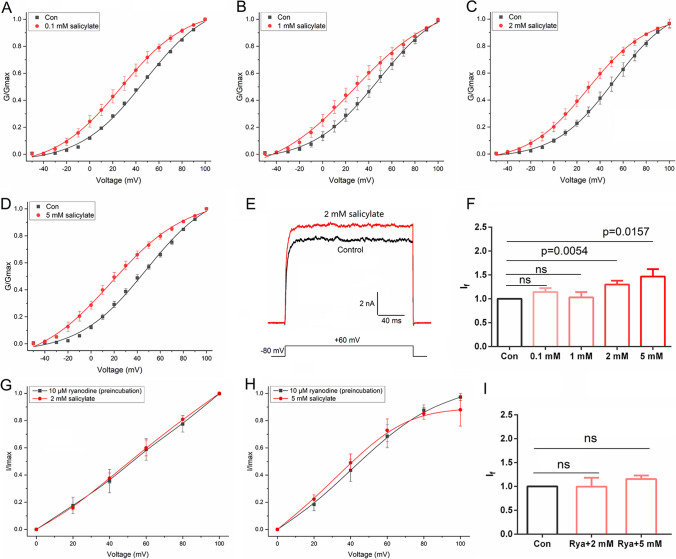
Acute single-dose salicylate administration is known to induce tinnitus-like behavior in rodents, and the direct pharmacological action is thought to be the primary mechanism [63, 64]. In this study, we aimed to determine whether salicylate perfusion affects BK channel currents. We measured the outward currents of HEK293T cells expressing BK channels in response to incremental voltage pulses (ranging from − 50 to + 100 mV), while treating them with increasing concentrations of salicylate. As shown in Fig. 4A–D, salicylate at all concentrations (0.1, 1, 2, 5 mM) significantly altered the voltage-dependent activation of the BK channel. In the presence of salicylate, the activation curve of BK channels as well as the half-maximal voltage (V1/2) of activation was significantly shifted leftward compared to the control state (absence of salicylate), while the slope factor (k) was not changed (Table 1). Salicylate at the concentrations of 2 mM and 5 mM significantly enhanced BK channel conductance, increasing it by approximately 30% and 47%, respectively, during depolarization at + 60 mV (If = 1.298 ± 0.082, p < 0.01, n = 10; If = 1.468 ± 0.153, p < 0.05, n = 9) (Fig. 4E, F). Additionally, current density values were also significantly increased in the presence of 2 mM (129.52 ± 14.71 compared to 101.79 ± 14.56, p < 0.01, n = 10) and 5 mM (146.91 ± 30.23 compared to 106.59 ± 24.82, p < 0.05, n = 9) salicylate (Table 1).
Fig. 4.
Effect of salicylate on BK channel expressed in HEK293T cells. A–D Normalized conductance plots fit well with the Boltzmann function in the absence and presence of salicylate at various concentrations (0.1 mM, n = 3; 1 mM, n = 8; 2 mM, n = 10; 5 mM, n = 9). The holding voltage was − 80 mV, and the currents were elicited by step pulses ranging from − 50 to + 100 mV for 200 ms with 10-mV increments. Salicylate significantly shifted the voltage-dependent activation curves. E Representative whole-cell current traces from HEK 293 T cells expressing BK channels before and after the application of 2 mM salicylate. The holding voltage was − 80 mV and the currents were elicited by a + 60 mV pulse. F Statistical analysis of the currents evoked by + 60 mV pulse before and after salicylate application. Data are presented as mean ± SEM. The significant difference between control and salicylate application was assessed using a paired Student’s t-test, with p < 0.05 considered significant. G–H Normalized currents of BK channels preincubated with 10 μM ryanodine, before and after application of 2 mM (n = 5) and 5 mM (n = 5) salicylate. I Statistical analysis of currents evoked by + 60 mV pulse, before and after application of 2 mM and 5 mM salicylate. Data are presented as mean ± SEM, with significant difference assessed by paired Student’s t-test
Table 1.
Activation of BK channel in the absence and presence of salicylate. Note: Values are expressed as mean ± SEM. Statistical analysis was performed using a paired Student’s two-tailed t-test. Statistical significance was considered at a p-value < 0.05 relative to the control condition
| Group | V1/2(mV) | k (mV) | Current density (pA/pF) | n |
|---|---|---|---|---|
| 0.1 mM Group | ||||
| Control | 47.6018 ± 2.0942 | 27.9952 ± 1.9519 | 104.3238 ± 2.7445 | 3 |
|
0.1 mM salicylate |
26.9561 ± 0.7872 (p < 0.001) |
28.6439 ± 1.0181 (p > 0.05) |
119.5390 ± 11.4971 (p > 0.05) |
3 |
| 1 mM Group | ||||
| Control | 48.7885 ± 1.7075 | 28.8379 ± 1.5589 | 114.69931 ± 32.98034 | 8 |
|
1 mM salicylate |
27.0317 ± 2.4028 (p < 0.0001) |
35.3475 ± 3.6087 (p > 0.05) |
111.05554 ± 30.41373 (p > 0.05) |
8 |
| 2 mM Group | ||||
| Control | 52.5654 ± 1.0983 | 25.8303 ± 0.90777 | 101.78705 ± 14.56017 | 10 |
|
2 mM salicylate |
29.6104 ± 0.6535 (p < 0.0001) |
26.1201 ± 0.7874 (p > 0.05) |
129.52468 ± 14.70878 (p = 0.0025) |
10 |
| 5 mM Group | ||||
| Control | 47.6405 ± 2.6521 | 29.1208 ± 2.4915 | 106.59488 ± 24.82392 | 9 |
|
5 mM salicylate |
17.6620 ± 1.7584 (p < 0.0001) |
32.6632 ± 2.3687 (p > 0.05) |
146.91408 ± 30.23363 (p = 0.0131) |
9 |
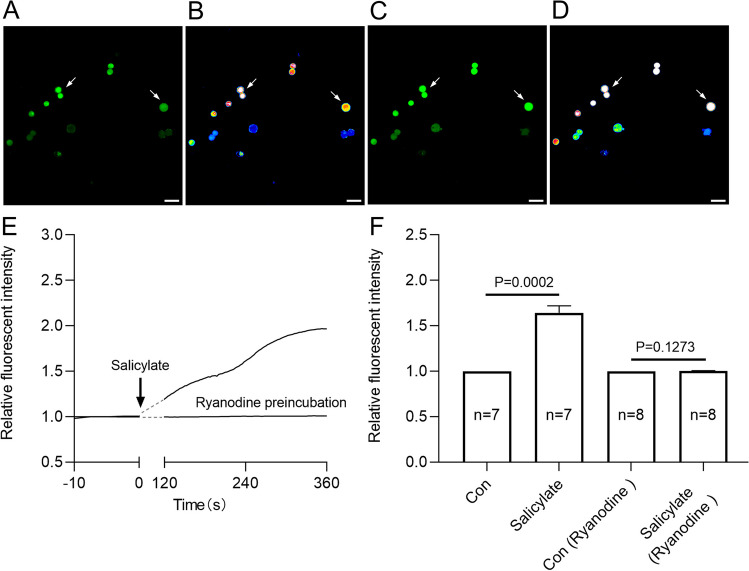
Elevated intracellular Ca2+ concentration is known to increase BK channel conductance [62]. RyR1 and RyR2 have been confirmed to be endogenously expressed in HEK293 cells [65]. To investigate whether the enhancement of BK channel conductance in the presence of salicylate is due to increased intracellular Ca2+ concentration, ryanodine (an irreversible inhibitor of the RyRs) was applied. Pretreatment of HEK293T cells with 10 μM ryanodine prevented salicylate from increasing BK channel currents (Fig. 4G–I). Calcium imaging in HEK293 cells revealed a noticeable rise in cytoplasmic Ca2+ levels upon exposure to a solution containing 2 mM salicylate (p < 0.001, n = 7) (Fig. 5). Similarly, calcium imaging of brain slices confirmed that 2 mM salicylate perfusion elevated cytoplasmic Ca2+ levels in neurons of the A1 cortex (p < 0.0001, n = 9) (Figure S1). However, pre-incubation with 10 μM ryanodine abolished the salicylate-induced cytoplasmic Ca2+ elevation in HEK293 cells (Fig. 5). These results indicated that salicylate elevates cytoplasmic Ca2+ concentration by activating RyRs in the endoplasmic reticulum, thereby increasing BK channel activity.
Fig. 5.
Salicylate elevates intracellular Ca2+ through activating ryanodine receptors. A–D Representative calcium images of HEK293T cells before and after salicylate treatment. E Normalized traces of the Ca2+ transient in HEK293T cells induced by the treatment of salicylate. F Bar graph quantifies the effect of 2 mM salicylate on the intracellular Ca2+ concentration, without or with preincubation of 10 µM ryanodine before calcium imaging. White arrows mark representative recorded cells. Data are presented as the mean ± SEM, significant differences between control and salicylate (n = 7 recorded cells), or control and salicylate in the present of ryanodine preincubation (n = 8 recorded cells) were tested by paired Student’s t-test, p value is compared with the control group
Modelling Reveals the Interaction Between Salicylate and Ryanodine Receptors
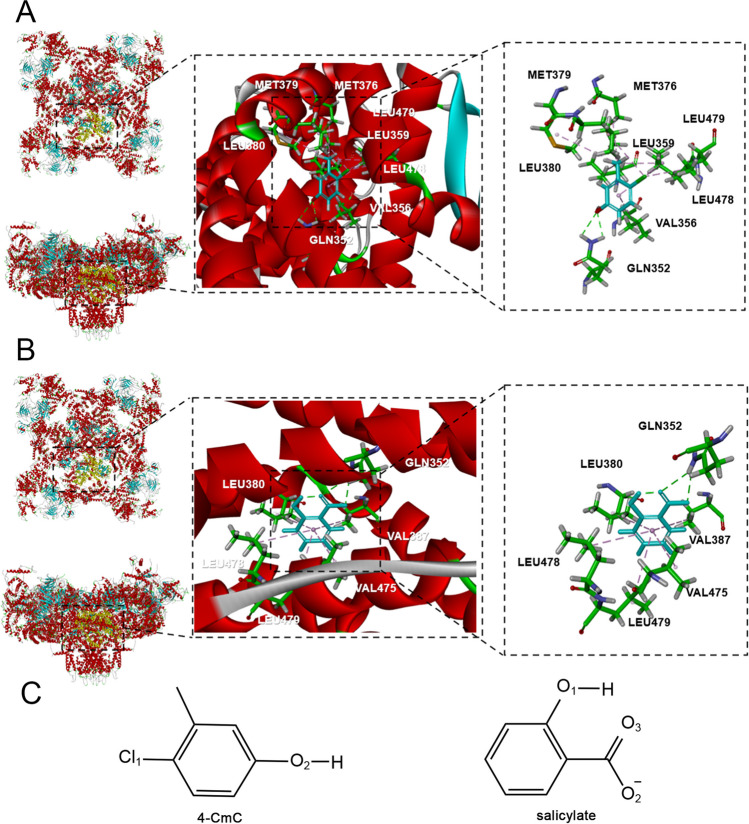
The central domain of RyR is a transducer for long-range allosteric gating of channel opening, which serves as a signaling hub that controls the activity of RyR [42, 66]. The key residue Gln4020 of RyR1 activated by 4-CmC (a potent and specific agonist of RyR1) is distributed in the central domain (residues 3668 − 4251) [42]. Based on the similar chemical structure between 4-CMC and salicylate (Fig. 6C), as well as intracellular Ca2+ elevation in the presence of salicylate, it was hypothesized that salicylate could interact with the central domain of RyRs. A homology model of hRyR1 central domain was constructed using the crystal structure of the rRyR1 as the template (Fig. 6). The small molecule-hRyR1 complex was obtained through small molecule-protein docking. The model revealed that 4-CmC forms hydrogen bond with the critical residue Gln4020 in the central domain of hRyR1 and engages in hydrophobic interaction with seven amino acid residues (Leu4147, Leu4048, Leu4146, Met4047, Leu4027, Met4044, Val4024). Notably, salicylate can also form hydrogen bond with Gln4020 of hRyR1, similar to 4-CmC (Fig. 7A, Figure S2A1). Additionally, it can engage in hydrophobic interaction with other five amino acid residues (Leu4147, Leu4048, Leu4146, Val4055, Leu4143) (Fig. 7B, Figure S2A2, Table 2).
Fig. 6.
Crystal structure map of the central domain of hRyR1. A Homologous amino acid sequence of the central domain from human RyR1 (top), and amino acid sequence of the central domain from rabbit RyR1 (bottom). B Ramachandran image of hRyR1. C 3D structure of the central domain of hRyR1
Fig. 7.
Docking results for the interaction of 4-CmC and salicylate with the central domain of hRyR1. A 4-CmC forms a hydrogen bond with Gln352 (4020), and engage in hydrophobic interaction with other seven residues (Leu380 (4048), Leu478 (4146), Met379 (4047), Leu479 (4147), Leu359 (4027), Met376 (4044), Val356 (4024)) in the central domain of hRyR1. B Salicylate forms two hydrogen bonds with Gln352 (4020), and engage in hydrophobic interaction with other five residues (Leu479 (4147), Leu380 (4048), Val387 (4055), Leu478 (4146), Leu475 (4143)) in the central domain of hRyR1. The numbers listed in brackets represent the location of residues in full-length amino acid sequence of RyR1. C The chemical structures of 4-CmC and salicylate obtained from PubChem database
Table 2.
Docking results for the interaction of salicylate and 4-CmC with ryanodine receptors. The docking scores and the ligand-receptor interaction details are summarized herein
| Receptor-ligand hydrogen bonds | |||||||
|---|---|---|---|---|---|---|---|
| Ligands | Receptor | Unfavorable effect | Libdock score | Atom in ligand | Atom in amino acid | Bond length/Å | Hydrophobic group |
| 4-CmC | RyR1 | / | 37.0596 | O2 | Gln4020 HE22 HE21 | 2.59 2.42 | Leu4048, Leu4146, Met4047, Leu4147, Leu4027, Met4044, Val4024 |
| Salicylate | RyR1 | / | 38.2048 | O2 O3 | Gln4020 HE22 HE21 | 2.67 2.67 | Leu4147, Leu4048, Val4055, Leu4146, Leu4143 |
| 4-CmC | RyR2 | / | 41.6891 | Cl1 | Gln3975 NE2 | 3.01 | Ile4013, Val4010, Leu4014, Val4098, Val3979, Leu3948 |
| Salicylate | RyR2 | / | 46.9879 | O3 | Gln3975 NE2 | 2.76 | Val4098, Met3978, Leu3948, Val3979 |
| 4-CmC | RyR3 | Gln3872 | 52.4159 | O2 | / | / | Trp3786 |
| Salicylate | RyR3 | Asp3873, Asp3869, Glu3779 | 48.0453 | O2 | Gln3872 | 2.34 | Trp3786 |
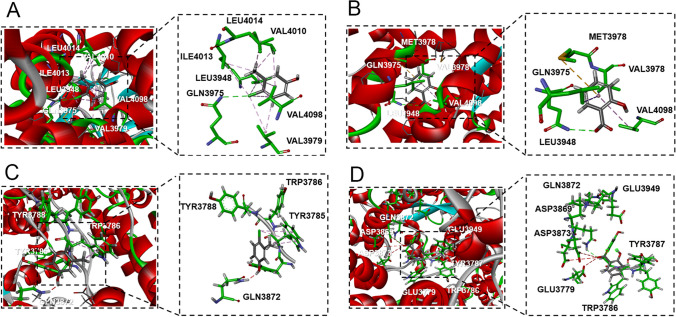
Apart from RyR1, RyR2 can also be efficiently activated by 4-CmC [42, 66]. The structure of hRyR2 has been recently resolved (PDB: 7u9q) [49], and it was used for molecular docking with small molecules. 4-CmC forms a hydrogen bond interaction with the critical residue Gln3975 of hRyR2 (corresponding to Gln4020 in RyR1), and hydrophobic interactions with Ile4013, Val4010, Leu4014, Val4098, Val3979, and Leu3948 (Fig. 8A and Figure S2B1). On the other hand, salicylate can form a hydrogen bond with Gln3975, and engage in hydrophobic interactions with Val4098, Met3978, Leu3948, and Val3979 of hRyR2 (Fig. 8B and Figure S2B2). The docking scores and ligand-receptor interaction details are summarized in Table 2. Highly similar interacting patterns between the ligand-RyR1/2 and higher docking score confirm that salicylate can efficiently activate RyR1 and RyR2 in the same way as 4-CmC.
Fig. 8.
Docking results for the interaction of 4-CmC and salicylate with the central domain of hRyR2 and hRyR3. A 4-CmC forms a hydrogen bond with Gln3975 residue, and engages in hydrophobic interaction with other six residues (Ile4013, Val4010, Leu4014, Val4098, Val3979, Leu3948) in the central domain of hRyR2. B Salicylate forms a hydrogen bond with Gln3975 residue, and engage in hydrophobic interaction with other four residues (Val4098, Met3978, Leu3948, Val3979) in the central domain of hRyR2. C 4-CmC forms an adverse interaction with Gln3872 of RyR3, and just engage in hydrophobic interaction with Trp3786. D Salicylate forms a hydrogen bond with Gln3872 and engage in hydrophobic interaction with Trp3786 in the central domain of hRyR3
RyR3 can be activated by 4-CmC [67], albeit with lower potency compared to the other two isoforms, possibly due to sequence divergence within RyR3 [66]. 3D model of hRyR3 was generated based on multiple templates, and small molecule-RyR3 complexes were obtained by small molecule-protein docking. The model revealed no stable interaction between 4-CmC and the critical residue Gln3872 (corresponding to Gln4020 in RyR1) in the central domain of RyR3. Instead, 4-CmC only formed a hydrophobic interaction with Trp3786 (Fig. 8C and Figure S2C1). In contrast, salicylate could form a hydrogen bond with Gln3872 and engage in a hydrophobic interaction with Trp3786 in the central domain of hRyR3 (Fig. 8D, Figure S2C2, Table 2).
Paxilline Reverses the Biomarkers of Salicylate-induced Tinnitus
We employed ABRs to assess the impact of salicylate on sound-evoked activity in cochlea and auditory brainstem structures, and the anti-tinnitus effects of paxilline, a specific and potent blocker of BKCa channel. ABRs were recorded before and after salicylate administration to observe changes in cochlear and brainstem auditory processing. In acute experiments, salicylate administration caused a broad-spectrum reduction in cochlear responses, as indicated by increased ABR thresholds of approximately 20 dB across the frequency spectrum (from 4 to 32 kHz) (Fig. 9A). Reduced cochlear sensitivity following acute salicylate administration was further indicated by longer P1 latencies, especially at high frequencies, and reduced P1 amplitudes (16 kHz, 22.6 kHz, and 32 kHz) relative to baseline (Fig. 9B, C). When paxilline and salicylate were administered concurrently before ABR recordings, paxilline treatment did not significantly alter salicylate-induced changes in ABR thresholds, P1 latencies, and P1 amplitudes (Figs. 9). Thus, paxilline treatment did not significantly impact the salicylate-induced decrease in cochlear thresholds.
Fig. 9.
Paxilline treatment does not affect the suppressed auditory nerve output caused by acute salicylate administration. A ABR stimulus frequency plotted as a function of threshold shift relative to baseline for post-salicylate and post-salicylate plus paxilline. A two-way ANOVA of threshold shifts showed a main effect of treatment (F(2,168) = 543.0, p < 0.0001) and frequency (F(6,168) = 197.8, p < 0.0001) when salicylate (red) and salicylate plus paxilline (blue) groups were included. Post hoc analyses showed that thresholds increased relative to baseline following salicylate administration, but were not further altered by paxilline treatment. B The latencies of ABR wave I of different frequency in salicylate and salicylate plus paxilline groups are prolonged compared to those in baseline. Two-way ANOVAs indicated a main effect of treatment for all frequencies (11.3 kHz: F(2, 103) = 18.43, p < 0.0001; 16 kHz: F(2,183) = 243.6, p < 0.0001; 22.6 kHz: F(2,127) = 122.3, p < 0.0001; 32 kHz: F(2,88) = 68.19, p < 0.0001). Post hoc analyses show the delayed latencies following salicylate administration compared with baseline, paxilline has no further impaction. C The amplitudes of ABR wave I plotted as a function of intensity for 11.3, 16, 22.6, and 32 kHz tones. Two-way ANOVAs indicated a main effect of treatment for all slightly higher frequencies (11.3 kHz: F(2,103) = 4.988, p = 0.0086; 16 kHz: F(2,183) = 25.48, p < 0.0001; 22.6 kHz: F(2,128) = 25.83, p < 0.0001; 32 kHz: F(2,76) = 38.81, p < 0.0001). Post hoc analyses indicate that salicylate reduces P1 amplitudes relative to baseline when administered alone or in the presence of paxilline. Red asterisks indicate a significant difference between baseline and salicylate, blue asterisks indicate a significant difference between baseline and salicylate plus paxilline. Data are presented as mean ± SEM and evaluated with two-way ANOVA followed by Bonferroni post-test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; n = 8)
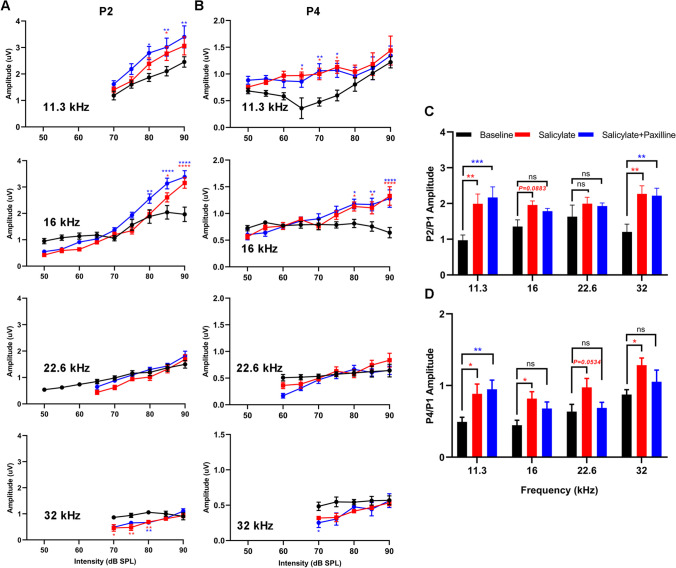
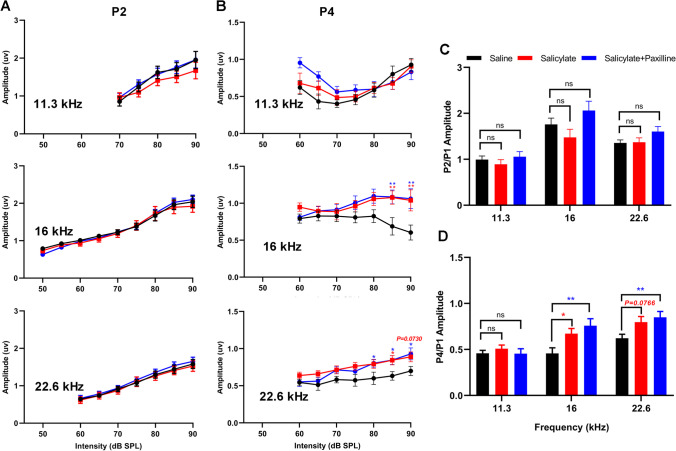
Despite reduced cochlear sound sensitivity, tinnitus is associated with amplified sound-induced activity in brainstem auditory structures. The amplitudes and latencies of P2 and P4 were concurrently measured and compared with P1. After acute salicylate administration, P2 amplitudes for 11.3 kHz and 16 kHz stimuli at high intensity (85–90 dB) increased, but at lower intensity (70–80 dB), P2 amplitudes at 32 kHz were less than baseline (Fig. 10A). P4 amplitudes were not significantly different from baseline for 22.6- to 32-kHz stimuli (Fig. 10B). In contrast, for 11.3- and 16-kHz stimuli, P4 amplitudes remained constant or increased with intensity (Fig. 10B). The ratios of P2/P1 amplitude were substantially increased for three frequencies (11.3 kHz, 16 kHz, 32 kHz) (p < 0.01, p = 0.0883, and p < 0.01, respectively), but not for 22.6-kHz stimuli (Fig. 10C). The P4/P1 amplitude ratios for 11.3 kHz, 16 kHz, and 32 kHz (p < 0.05, p < 0.05, and p < 0.05, respectively) significantly increased after acute salicylate administration, and the upward trend of P4/P1 amplitude ratio for 22.6 kHz was maintained without significance (p = 0.0534) (Fig. 10D). Paxilline treatment did not substantially alter or reverse the increases in P2/P1 amplitude ratios but could suppress the upward momentum of P4/P1 amplitudes caused by acute salicylate administration, especially for 16 kHz, 22.6 kHz, and 32 kHz (Fig. 10C, D). These results indicate that blockade of BK channel using paxilline partially suppresses the enhancement in central gain in higher brainstem auditory structures after acute systemic administration of salicylate.
Fig. 10.
Paxilline treatment suppresses the increased gain caused by acute salicylate administration. A Changes in the amplitude of ABR wave II, compared to the baseline, Two-way ANOVAs indicated a main effect of treatment for all frequencies (11.3 kHz: F(2,105) = 13.18, p < 0.0001; 16 kHz: F(2,183) = 10.99, p < 0.0001; 22.6 kHz: F(2,144) = 4.465, p < 0.0001; 32 kHz: F(2,91) = 13.93, p < 0.0001). There was also a main effect of intensity (11.3 kHz: F(4,105) = 21.40, p < 0.0001; 16 kHz: F(8,183) = 81.96, p < 0.0001; 22.6 kHz: F(5,144) = 21.59, p < 0.0001; 32 kHz: F(4,91) = 7.447, p < 0.0001). Post hoc analyses show the amplitudes increase in salicylate group and salicylate plus paxilline group at 11.3 kHz and 16 kHz (ranging from 80 to 90 dB), the amplitudes decrease at 32 kHz (ranging from 70 to 80 dB). B Changes in the amplitude of ABR wave IV compared to baseline. Two-way ANOVAs indicated a main effect of treatment for all frequencies (11.3 kHz: F(2, 170) = 14.93, p < 0.0001; 16 kHz: F(2, 182) = 9.879, p < 0.0001; 22.6 kHz: F(2,128) = 2.257, p = 0.1089; 32 kHz: F(2,89) = 6.445, p = 0.0024). There was also a main effect of intensity (11.3 kHz: F(8,170) = 6.079, p < 0.0001; 16 kHz: F(8,182) = 8.597, p < 0.0001; 22.6 kHz: F(6,128) = 7.386, p < 0.0001; 32 kHz: F(4,89) = 3.764, p = 0.0071). Post hoc analyses show the amplitudes increase in salicylate group and salicylate plus paxilline group at 11.3 kHz across 65 to 75 dB and 16 kHz across 80 to 90 dB. C Mean amplitude of P2 relative to P1 for 90 dB tones. A two-way ANOVA showed a main effect of treatment (F(2, 84) = 17.16; p < 0.0001), but not frequency (F(3, 84) = 0.6797). Post hoc analyses indicated that salicylate administration alone (red) and salicylate plus paxilline treatment (blue) both increase the P2/P1 ratio relative to baseline (black), at 11.3 kHz, 16 kHz, and 32 kHz. D Mean amplitude of P4 relative to P1 for 90 dB tones. The P4/P1 ratios increase following salicylate treatment (F(2, 84) = 12.87; p < 0.0001), paxilline treatment reserves the increased P4/P1 ratios at 16 kHz, 22.6 kHz, and 32 kHz. Data are presented as mean ± SEM and evaluated with two-way ANOVA followed by Bonferroni post-test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; n = 8). Red and blue asterisks indicate significant difference of salicylate vs baseline and salicylate plus paxilline vs baseline, respectively
In chronic experiments, compared to the control group, seven consecutive days systemic administration of salicylate had no substantial effect on cochlear responses, as indicated by no obvious changes in ABR thresholds, as well as the amplitudes and latencies of P1 (Fig. 11A–C). The lack of effect could be attributed to the extensive metabolic elimination of salicylate during at least 12-h recovery period after the last administration. Additionally, chronic administration of salicylate had no obvious effects on the P2 amplitudes, but it increased the P4 amplitude for 16 kHz (85 dB, p < 0.01; 90 dB, p < 0.01) and 22.6 kHz (85 dB, p < 0.05; 90 dB, p = 0.073), especially at high-intensity stimuli (Fig. 12B). Therefore, the P2/P1 amplitude for different frequencies were not changed following chronic salicylate administration (Fig. 12C). However, the P4/P1 amplitude ratio for 16 kHz significantly increased (p < 0.05), and that for 22.6 kHz also showed an increased trend (p = 0.0766) (Fig. 12D). When paxilline treatment was performed before ABR recordings, there was only a reduction in ABR threshold for 32 kHz (p < 0.001), without any significant changes in the thresholds of other frequencies, the amplitudes, and latencies of P1, compared to the control group (Fig. 11A–C). In addition, paxilline treatment did not alter salicylate-induced changes in P2/P1 amplitude ratios (Fig. 12A, C), and even further increased the P4/P1 amplitude ratios for 16 kHz (p < 0.01) and 22.6 kHz (p < 0.01), on the basis of chronic salicylate administration (Fig. 12B, D). Thus, paxilline treatment did not significantly impact the cochlear responses and was insufficient to suppress the enhancement in central gain in higher brainstem auditory regions after chronic systemic administration of salicylate. Overall, these data support the ability of paxilline treatment to counteract tinnitus-associated neurophysiological changes in central auditory system, particularly in the case of acute tinnitus induced by single-dose salicylate.
Fig. 11.
Paxilline treatment and chronic salicylate administration do not affect auditory nerve output. A A two-way ANOVA of threshold showed no effect of treatment (salicylate vs. saline, F(1, 140) = 0.05227, p = 0.8195; salicylate plus paxilline vs. saline, F(1, 132) = 0.03440, p = 0.8531). Post hoc analyses showed that thresholds are not changed relative to saline following salicylate administration, salicylate plus paxilline treatment decreases the thresholds at 32 kHz. B The plot showed the latencies of ABR wave I at 11.3 kHz, 16 kHz, 22.6 kHz, and 32 kHz. Two-way ANOVAs indicated a main effect of treatment for all frequencies (11.3 kHz: F(2,151) = 6.756, p = 0.0015; 16 kHz: F(2,272) = 11.46, p < 0.0001; 22.6 kHz: F(2,242) = 9.606, p < 0.0001; 32 kHz: F(2,201 = 8.800, p = 0.0002). There was also a main effect of intensity (11.3 kHz: F(4,151) = 29.70, p < 0.0001; 16 kHz: F(8,272) = 131.1, p < 0.0001; 22.6 kHz: F(7,242) = 78.78, p < 0.0001; 32 kHz: F(6,201) = 46.35, p < 0.0001). Post hoc analyses show no significant difference in latency values compared with saline group. C The plot showed the amplitudes of ABR wave I at 11.3 kHz, 16 kHz, 22.6 kHz, and 32 kHz. Two-way ANOVAs indicated a main effect of treatment for all frequencies (11.3 kHz: F(2,155) = 3.107, p = 0.0475; 16 kHz: F(2,273) = 5.019, p = 0.0072; 22.6 kHz: F(2,245) = 1.218, p = 0.2976; 32 kHz: F(2,209) = 4.108, p = 0.0178). There was also a main effect of intensity (11.3 kHz: F(4,155) = 47.23, p < 0.0001; 16 kHz: F(8,273) = 39.65, p < 0.0001;22.6 kHz: F(7,245) = 86.57, p < 0.0001; 32 kHz: F(6,209) = 33.28, p < 0.0001). Post hoc analyses show no significant difference in amplitude values compared with saline group. Red and blue asterisks indicate significant difference of salicylate vs saline and salicylate plus paxilline vs saline, respectively. Data are presented as mean ± SEM and evaluated with two-way ANOVA followed by Bonferroni post-test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; n = 10–14)
Fig. 12.
Paxilline treatment does not suppress the increased gain in early auditory structures caused by chronic salicylate administration. A Compared to the saline group, there are no significant changes of the amplitude of ABR wave II in the two treatment groups. Two-way ANOVAs indicated a main effect of treatment for all frequencies (11.3 kHz: F(2, 151) = 1.788, p = 0.1708; 16 kHz: F(2, 261) = 0.3734, p = 0.6888; 22.6 kHz: F(2, 218) = 1.290, p = 0.2774). There was also a main effect of intensity (11.3 kHz: F(4,151) = 15.06, p < 0.0001; 16 kHz: F(8,261) = 64.17, p < 0.0001; 22.6 kHz: F(6,218) = 40.57, p < 0.0001). Post hoc analyses show the amplitudes not change in salicylate group and salicylate plus paxilline group. B Changes in the amplitude of ABR wave IV compared to saline. Two-way ANOVAs indicated main effect of treatment for all frequencies (11.3 kHz (F(2,188) = 2.492, p = 0.0855; 16 kHz: F(2, 223) = 12.71, p < 0.0001; 22.6 kHz: F(2, 218) = 15.11, p < 0.0001). Post hoc analyses show the amplitudes not change in salicylate group and salicylate plus paxilline group at 11.3 kHz, the amplitudes increase in salicylate group and salicylate plus paxilline group at 16 kHz (ranging from 85 to 90 dB) and 22.6 kHz (ranging from 80 to 90 dB). C P2/ P1 ratios for 90 dB tones. A two-way ANOVA showed a main effect of treatment (F(2, 90) = 5.162; p = 0.0075), and a main effect of frequencies (F(2, 90) = 28.23, p < 0.0001). Post hoc analyses indicated that salicylate administration alone (red) and salicylate plus paxilline treatment (blue) not change the P2/P1 ratio relative to saline (black), at 11.3 kHz, 16 kHz, and 22.6 kHz. D P4/ P1 ratios for 90 dB tones. A two-way ANOVA showed a main effect of treatment (F(2,115) = 7.898, p = 0.0006), and a main effect of frequencies (F(2, 115) = 18.77, p < 0.0001). Post-hoc analyses indicated that salicylate administration alone (red) and salicylate plus paxilline treatment (blue) increased the P4/P1 ratio compared to saline (black), at 16 kHz and 22.6 kHz. Data are presented as mean ± SEM and evaluated with two-way ANOVA followed by Bonferroni post-test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; n = 10–14)
Discussion
Tinnitus arise from maladaptive neuroplastic changes in the central nervous system most often provoked by cochlear pathology [3, 19]. The treatment of tinnitus remains a challenging endeavor due to its multifaceted nature and uncertain mechanism. For decades, pharmacological treatments for tinnitus have primarily focused on addressing the underlying mechanisms, such as cochlear damage and neurotransmitter imbalances in the auditory central system including CN, IC MGB, and auditory cortex, as well as in the extra-auditory network [21]. To date, pre-existing drug molecules and few novel molecules targeting proteins involved in tinnitus have been evaluated in animals and clinic. However, the efficacy of most of them is not supported by strong evidence [21, 68]; the tinnitus drug pipeline is still lean. BK channels located in the plasma membrane of neurons influence the shape, frequency, and propagation of action potentials, as well as neurotransmitter release. The α subunit and β1/β4 subunits of the BK channel in cochlear hair cells of adult animals play a role in defining the temporal fine structure and the dynamic range of auditory information [69–71]. Although there were limited clues about BK expression in central auditory system, it has been confirmed in the cartwheel cells of the DCN and IC [72, 73]. The present study detected the expression patterns of the neuronal-type BK channel (α + β4) in the rat central auditory system and revealed an extensive expression on neurons from CN, IC, MGB, and A1 cortex. Additionally, a few microglia in the central auditory system also expressed the BKα subunit. Microglia, as a housekeeper of the central nervous system, plays fundamental roles in the control of immune responses and the maintenance of homeostasis. Microglia activation accompanied with increased inflammation in the A1 cortex has been linked to salicylate-induced and noise exposure-induced tinnitus in animals recently [35, 74]. Although the involvement of microglial BK channel in pain has been preliminary disclosed [29, 75, 76], its role in auditory perception and tinnitus pathophysiology remains not at all clear. The roles of the BK channel in the central auditory system in processing auditory information and related diseases deserve more in-depth systematic exploration. The present study focuses on the potential contribution of the neuronal BK channel (α + β4) in salicylate-induced tinnitus.
Acute single-dose and chronic salicylate administration reliably produce a narrowband tinnitus percept in humans and rodents, roughly centered around 16 kHz, with evidences including tinnitus-like behaviors and an enhancement in central gain [35, 36, 77]. In the present study, the dynamic expression of BKα and/or β4 subunits was verified in the central auditory system, including IC, MGB, and A1 cortex of rats following chronic salicylate administration. The result suggests that the BK (α + β4) channel might be involved in salicylate-induced chronic tinnitus. Under physiological conditions, activation of neuronal BK channel contributes to action potential repolarization, giving rise to the fast afterhyperpolarization that follows the action potential, shapes dendritic Ca2+ spikes, and influences neurotransmitter release [62]. Thus, elevated expression and activation of the BK channels might hyperpolarize the resting membrane potential in excitatory neurons and reduce tinnitus induced by chronic salicylate. On the other hand, it is well-known that downregulation of inhibitory neurotransmission contributes to tinnitus-associated neuronal hyperexcitability [78]. Therefore, it might also be speculated that upregulation of the BK channel in inhibitory neurons (either GABAergic and/or glycinergic), resulting in disinhibition of excitatory neurons and hence augmented transmission of glutamatergic activity, might worsen chronic salicylate-induced tinnitus. More notably, both decreased and increased BK channel function are associated with network hyperexcitability, likely due to the complex interaction and varied expression patterns of ion channels [79–82]. For example, both gain- and loss-of-function of BK channels lead to epileptogenic neuronal hyperexcitability, and are regarded as potentially important molecular targets for developing drugs to prevent epileptogenesis [82]. Overall, the increased expression of BK channels observed in the current study per se might or might not suggest a positive contribution of BK channels to chronic tinnitus induced by salicylate.
Salicylate can pass through the blood–brain barrier and directly target on and multiple receptors and ion channels, and modulate neuronal activity in the central auditory system after in vivo acute administration [63], resulting in acute salicylate-induced tinnitus. In the present study, the enhancement of BK currents in HEK293 cells in the presence of millimolar salicylate suggests a potential participation of the BK channels in modulating tinnitus-associated neuronal hyperactivity and acute tinnitus following salicylate administration. Similarly, as described above for chronic tinnitus induced by salicylate, the increasing effect of acute salicylate on BK currents per se might or might not suggest a positive contribution of BK channels to acute tinnitus induced by salicylate. It is interesting that the salicylate-induced enhancement of BK currents and elevation of intracellular free Ca2+ concentration could be blocked by micromolar ryanodine in the present study. As well-known, BK channels are activated by both membrane depolarization and increased intracellular Ca2+ [62]. Ryanodine receptors (RyRs) are a family of high conductance intracellular cation channels that release Ca2+ from Ca2+ stores such as the endoplasmic reticulum and sarcoplasmic reticulum [83]. The results suggest that acute salicylate might increase BK currents through a direct activation of RyR.
Three known RyR isoforms (RyR1-3) are expressed in the brain, with RyR2 predominating [84, 85]. The central domain of RyR is the transducer that couples the conformational changes of the cytoplasmic platform to the gating of the central pore, which serves as a signaling hub that controls the activity of RyR [46]. 4-CmC is a potent and specific activator of RyRs, which can directly activate the RyR1 and RyR2 with more than tenfold higher potency compared with caffeine [43, 66]. Besides, 4-CmC activates RyR3 with tenfold lower potency compared with its ability to activate RyR1 and RyR2, which could be attributed to sequence divergence within RyR3 [67]. The residue Gln4020 (RyR1) or Gln3975 (RyR2) distributed in the central domain is essential for 4-CmC binding and activation [42]. Based on the highly similar chemical structure between 4-CMC and salicylate, as well as intracellular Ca2+ elevation in the presence of salicylate revealed in the current study, we raise a possibility that salicylate can activate RyRs located on the endoplasmic reticulum membrane, increase Ca2+ release from calcium store, and consequently increase intracellular Ca2+ and BK currents. In the present study, 4-CmC here was applied to be a positive control to evaluate the binding affinities between salicylate and hRyRs. Homology modeling and molecular docking analysis revealed 4-CmC and salicylate can both bind to the critical residue Gln4020/3975 with a hydrogen bond in the central domain of hRyR1 and hRyR2. Additionally, the residues distributed around Gln of hRyR1 and hRyR2 respectively form hydrophobic interactions with 4-CmC and salicylate which were partially overlapping. The high Libdock scores gained in this study validate that there exists a stronger binding affinity between the salicylate and hRyR1/2. Salicylate binding to the same region of hRyR1/2 as 4-CmC indicated that the bioactivity of salicylate might be similar to that of 4-CmC. For RyR3, 4-CmC is unable to form a stably interaction with the critical residue Gln3872 (corresponding to Gln4020 of RyR1), instead of a hydrophobic bond with Trp3786. The performance may account for the extreme lower potency of 4-CmC against RyR3. However, salicylate can bind to Gln3872 with a hydrogen bond and forms a hydrophobic interaction same as 4-CmC. Although the agonistic effect of salicylate on RyRs need to be further validated, the elevation of cytoplasmic Ca2+ induced by salicylate seems to be a more important factor in regulating neuronal activity. These results mentioned above draw tightly connected lines of RyRs and BK channel involvement in tinnitus-associated hyperexcitability following salicylate administration.
Paxilline can potently block BK channels consisting of the complexes α/β4 in the context of seizures, motor disturbances, and neuronal hyperexcitability [29]. For the involvement of BK channels in salicylate-induced tinnitus, the present study explored the effects of systemic paxilline treatment on neurophysiological biomarkers of tinnitus. Single high dose of salicylate has long been known to cause temporary suppression of cochlear function, mainly by reducing outer hair cell electromotility and decreasing the neural output of the cochlea [86, 87]. Furthermore, following acute systemic administration, salicylate readily crosses the blood–brain barrier and then disrupt brain activity [77, 88, 89]. On count of the comprehensive pharmacological performance in the peripheral and central systems, acute administration of salicylate can induce reversible tinnitus-like behaviors in rodents [35, 36, 77, 90], together with enhanced central gain, which has been regarded as an objective indicator of tinnitus [38, 55, 91]. The study demonstrated that acute salicylate administration caused enhanced gain in the rat central auditory system indicated by increased P2/P1 and P4/P1 amplitude ratios from 11.3 kHz to 22.6 Hz, coincide with raised cochlear thresholds, delayed latencies, and decreased amplitudes of ABR P1, which is consistent with previous studies discussed above. Neurophysiological assessment showed that paxilline treatment did not substantially influence the salicylate-induced increase in cochlear thresholds, although there is an irreplaceable role of the BK channel in cochlear hair cells [71]. BK channel knockout has shown that the lack of BK channels in cochlear hair cells has an effect on the dynamic range and signal/noise ratio of responses within the auditory system, but with normal cochlear function, as indicated by normal ABRs and distortion product otoacoustic emissions [69, 70]. Although there was no significant impact on ABR threshold and P1, paxilline treatment significantly suppressed the enhancement in central gain in the auditory brainstem, especially at the frequencies associated with tinnitus. Thus, these findings suggest that blockade of the BK channel after paxilline treatment may suppress tinnitus caused by acute salicylate administration, through shaping network activity in the central auditory system, with evident influences in the auditory midbrain.
Repetitive administration of a relatively high dose of salicylate not only induces tinnitus perception in humans [63], but also consistently elicits tinnitus-like behaviors in animals through daily administrations spanning seven to fourteen consecutive days. These observations recorded in various studies [33, 90, 92–94] may stem from different concentrations and durations of salicylate administration. In our current investigation, the salicylate dosage regimen employed to induce chronic tinnitus aligns with our prior works, demonstrating its efficacy in reliably inducing reversible tinnitus-like behaviors in rodents [35, 36]. The present study found that seven consecutive days of salicylate administration had no significant impact on cochlear function. However, it led to an enhancement in central gain, as evidenced by increased P4/P1 amplitude ratios at 16 kHz and 22.6 kHz. This contrasts with the outcomes of acute single-dose salicylate administration. In the cause of chronic tinnitus, where a direct pharmacological mechanism of salicylate may be less pronounced, and central plasticity manifested through structural and functional variation in neurons emerges as a primarily contributor for the enhanced central gain, this is particularly noteworthy in higher center than the CN, as inferred from the absence of substantial changes in ABR P2.
In addition, the dynamic regulation of BK(α + β4) expression in the IC, MGB, and A1 cortex emerges as a significant factor in mediating neural network hyperexcitability. This upregulation, especially in inhibitory neurons, suggests a potential role in the disinhibition of excitatory, thereby amplifying glutamatergic activity. However, despite these insights, neurophysiological assessment demonstrated that paxilline treatment could not effectively suppress the enhanced central gain following chronic salicylate administration. Unexpectedly, an increasing trend was observed, superimposed on the salicylate-induced effects. The distinct modes of peripheral damage and central plasticity between acute and chronic salicylate-induced tinnitus underscore profound differences. To comprehensively evaluate the potential of paxilline treatment for chronic tinnitus, a more detailed examination of the roles of the BK channel is required. Moreover, the exploration of alternative tinnitus induction models, such as noise-induced tinnitus, will be pivotal in elucidating the involvement of the BK channel.
In summary, the findings from this study implicate a selective involvement of the BK channel and RyRs in acute salicylate-induced tinnitus. Future study may explore in-depth mechanism underlying the role of BK and RyRs in salicylate-induced tinnitus following acute and chronic administration and noise-induced tinnitus.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
YZ conceived and designed the study. WYS, QZ, HWG and YZ conducted experiments and undertook data collection and analysis. CY and FJ provided the technical advice. NL and HJW contributed to material preparation. YZ and YHJ supervised the project. YZ, ZYT and YHJ wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Funding
This research was supported by the Natural Science Foundation of Hebei Province (Grant No. H2022201059), the National Natural Science Foundation of China (Grant No. 81900930), and the Medical Science Foundation of Hebei University (Grant No. 2022A04) to You Zhou.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Ethics Approval
All procedures involving animals were carried out in accordance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals, and were approved by the Ethics Committee of Hebei University, China.
Consent to Participate
Not applicable.
Consent for Publication
All authors have read and approved the final manuscript for publication.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenying Shi and Qi Zhao contributed equally to this work.
References
- 1.Baguley D, McFerran D, Hall D (2013) Tinnitus. Lancet 382(9904):1600–1607. 10.1016/S0140-6736(13)60142-7 [DOI] [PubMed] [Google Scholar]
- 2.Piccirillo JF, Rodebaugh TL, Lenze EJ (2020) Tinnitus. JAMA 323(15):1497–1498. 10.1001/jama.2020.0697 [DOI] [PubMed] [Google Scholar]
- 3.Shore SE, Roberts LE, Langguth B (2016) Maladaptive plasticity in tinnitus–triggers, mechanisms and treatment. Nat Rev Neurol 12(3):150–160. 10.1038/nrneurol.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brozoski TJ, Bauer CA (2005) The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res 206(1–2):227–236. 10.1016/j.heares.2004.12.013 [DOI] [PubMed] [Google Scholar]
- 5.Brozoski TJ, Wisner KW, Sybert LT, Bauer CA (2012) Bilateral dorsal cochlear nucleus lesions prevent acoustic-trauma induced tinnitus in an animal model. J Assoc Res Otolaryngol 13(1):55–66. 10.1007/s10162-011-0290-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C, Martel DT, Shore SE (2016) Increased synchrony and bursting of dorsal cochlear nucleus fusiform cells correlate with tinnitus. J Neurosci 36(6):2068–2073. 10.1523/JNEUROSCI.3960-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zugaib J, Leao RM (2018) Enhancement of endocannabinoid-dependent depolarization-induced suppression of excitation in glycinergic neurons by prolonged exposure to high doses of salicylate. Neuroscience 376:72–79. 10.1016/j.neuroscience.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 8.de Siqueira DVF, Strazza PS Jr, Benites NM, Leao RM (2022) Salicylate activates K(ATP) channels and reduces spontaneous firing in glycinergic cartwheel neurons in the dorsal cochlear nucleus of rats. Eur J Pharmacol 926:175026. 10.1016/j.ejphar.2022.175026 [DOI] [PubMed] [Google Scholar]
- 9.Niu Y, Kumaraguru A, Wang R, Sun W (2013) Hyperexcitability of inferior colliculus neurons caused by acute noise exposure. J Neurosci Res 91(2):292–299. 10.1002/jnr.23152 [DOI] [PubMed] [Google Scholar]
- 10.Sturm JJ, Zhang-Hooks YX, Roos H, Nguyen T, Kandler K (2017) Noise trauma-induced behavioral gap detection deficits correlate with reorganization of excitatory and inhibitory local circuits in the inferior colliculus and are prevented by acoustic enrichment. J Neurosci 37(26):6314–6330. 10.1523/JNEUROSCI.0602-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ (2005) Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci 28(6):325–333. 10.1016/j.tins.2005.04.006 [DOI] [PubMed] [Google Scholar]
- 12.Ma L, Ono M, Qin L, Kato N (2020) Acoustic trauma induced the alteration of the activity balance of excitatory and inhibitory neurons in the inferior colliculus of mice. Hear Res 391:107957. 10.1016/j.heares.2020.107957 [DOI] [PubMed] [Google Scholar]
- 13.Butt S, Ashraf F, Porter LA, Zhang H (2016) Sodium salicylate reduces the level of GABAB receptors in the rat’s inferior colliculus. Neuroscience 316:41–52. 10.1016/j.neuroscience.2015.12.021 [DOI] [PubMed] [Google Scholar]
- 14.Kalappa BI, Brozoski TJ, Turner JG, Caspary DM (2014) Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J Physiol 592(22):5065–5078. 10.1113/jphysiol.2014.278572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisz N, Muller S, Schlee W, Dohrmann K, Hartmann T, Elbert T (2007) The neural code of auditory phantom perception. J Neurosci 27(6):1479–1484. 10.1523/JNEUROSCI.3711-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adjamian P, Sereda M, Zobay O, Hall DA, Palmer AR (2012) Neuromagnetic indicators of tinnitus and tinnitus masking in patients with and without hearing loss. J Assoc Res Otolaryngol 13(5):715–731. 10.1007/s10162-012-0340-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komiya H, Eggermont JJ (2000) Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol 120(6):750–756. 10.1080/000164800750000298 [DOI] [PubMed] [Google Scholar]
- 18.Seki S, Eggermont JJ (2003) Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res 180(1–2):28–38. 10.1016/s0378-5955(03)00074-1 [DOI] [PubMed] [Google Scholar]
- 19.Shore SE, Wu C (2019) Mechanisms of noise-induced tinnitus: insights from cellular studies. Neuron 103(1):8–20. 10.1016/j.neuron.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y, McTavish J, Smith PF (2021) Pharmacological evaluation of drugs in animal models of tinnitus. Curr Top Behav Neurosci 51:51–82. 10.1007/7854_2020_212 [DOI] [PubMed] [Google Scholar]
- 21.Henton A, Tzounopoulos T (2021) What’s the buzz? The neuroscience and the treatment of tinnitus. Physiol Rev 101(4):1609–1632. 10.1152/physrev.00029.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagal SK, Brown AD, Cox PJ, Omoto K, Owen RM, Pryde DC, Sidders B, Skerratt SE et al (2013) Ion channels as therapeutic targets: a drug discovery perspective. J Med Chem 56(3):593–624. 10.1021/jm3011433 [DOI] [PubMed] [Google Scholar]
- 23.Li S, Choi V, Tzounopoulos T (2013) Pathogenic plasticity of Kv7.2/3 channel activity is essential for the induction of tinnitus. Proc Natl Acad Sci U S A 110(24):9980–9985. 10.1073/pnas.1302770110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalappa BI, Soh H, Duignan KM, Furuya T, Edwards S, Tzingounis AV, Tzounopoulos T (2015) Potent KCNQ2/3-specific channel activator suppresses in vivo epileptic activity and prevents the development of tinnitus. J Neurosci 35(23):8829–8842. 10.1523/JNEUROSCI.5176-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey CS, Moldenhauer HJ, Park SM, Keros S, Meredith AL (2019) KCNMA1-linked channelopathy. J Gen Physiol 151(10):1173–1189. 10.1085/jgp.201912457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zang K, Zhang Y, Hu J, Wang Y (2018) The large conductance calcium- and voltage-activated potassium channel (BK) and epilepsy. CNS Neurol Disord Drug Targets 17(4):248–254. 10.2174/1871527317666180404104055 [DOI] [PubMed] [Google Scholar]
- 27.Meredith AL (2023) BK channelopathies and KCNMA1-linked disease models. Annu Rev Physiol. 10.1146/annurev-physiol-030323-042845 [DOI] [PubMed] [Google Scholar]
- 28.Lu R, Lukowski R, Sausbier M, Zhang DD, Sisignano M, Schuh CD, Kuner R, Ruth P et al (2014) BKCa channels expressed in sensory neurons modulate inflammatory pain in mice. Pain 155(3):556–565. 10.1016/j.pain.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 29.Contet C, Goulding SP, Kuljis DA, Barth AL (2016) BK channels in the central nervous system. Int Rev Neurobiol 128:281–342. 10.1016/bs.irn.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, Kotagal P, Luders HO et al (2005) Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet 37(7):733–738. 10.1038/ng1585 [DOI] [PubMed] [Google Scholar]
- 31.Wang B, Rothberg BS, Brenner R (2009) Mechanism of increased BK channel activation from a channel mutation that causes epilepsy. J Gen Physiol 133(3):283–294. 10.1085/jgp.200810141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.N’Gouemo P (2014) BKCa channel dysfunction in neurological diseases. Front Physiol 5:373. 10.3389/fphys.2014.00373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jastreboff PJ, Brennan JF, Sasaki CT (1988) An animal model for tinnitus. Laryngoscope 98(3):280–286. 10.1288/00005537-198803000-00008 [DOI] [PubMed] [Google Scholar]
- 34.Bauer CA, Brozoski TJ, Rojas R, Boley J, Wyder M (1999) Behavioral model of chronic tinnitus in rats. Otolaryngol Head Neck Surg 121(4):457–462. 10.1016/S0194-5998(99)70237-8 [DOI] [PubMed] [Google Scholar]
- 35.Xia C, Yin M, Wu C, Ji Y, Zhou Y (2020) Neuroglial activation in the auditory cortex and medial geniculate body of salicylate-induced tinnitus rats. Am J Transl Res 12(10):6043–6059 [PMC free article] [PubMed] [Google Scholar]
- 36.Yin M, Xia C, Wu C, Ji Y, Zhou Y (2020) Aberrant expression of Nav1.6 in the cochlear nucleus correlates with salicylate-induced tinnitus in rats. Biochem Biophys Res Commun 526(3):786–792. 10.1016/j.bbrc.2020.03.123 [DOI] [PubMed] [Google Scholar]
- 37.Lobarinas E, Dalby-Brown W, Stolzberg D, Mirza NR, Allman BL, Salvi R (2011) Effects of the potassium ion channel modulators BMS-204352 Maxipost and its R-enantiomer on salicylate-induced tinnitus in rats. Physiol Behav 104(5):873–879. 10.1016/j.physbeh.2011.05.022 [DOI] [PubMed] [Google Scholar]
- 38.Scott LL, Lowe AS, Brecht EJ, Franco-Waite L, Walton JP (2022) Small molecule modulation of the large-conductance calcium-activated potassium channel suppresses salicylate-induced tinnitus in mice. Front Neurosci 16:763855. 10.3389/fnins.2022.763855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lippiat JD, Standen NB, Harrow ID, Phillips SC, Davies NW (2003) Properties of BK(Ca) channels formed by bicistronic expression of hSloalpha and beta1-4 subunits in HEK293 cells. J Membr Biol 192(2):141–148. 10.1007/s00232-002-1070-0 [DOI] [PubMed] [Google Scholar]
- 40.Shi J, He HQ, Zhao R, Duan YH, Chen J, Chen Y, Yang J, Zhang JW et al (2008) Inhibition of martentoxin on neuronal BK channel subtype (alpha+beta4): implications for a novel interaction model. Biophys J 94(9):3706–3713. 10.1529/biophysj.107.122150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tao J, Shi J, Yan L, Chen Y, Duan YH, Ye P, Feng Q, Zhang JW et al (2011) Enhancement effects of martentoxin on glioma BK channel and BK channel (alpha+beta1) subtypes. PLoS One 6(3):e15896. 10.1371/journal.pone.0015896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fessenden JD, Feng W, Pessah IN, Allen PD (2006) Amino acid residues Gln4020 and Lys4021 of the ryanodine receptor type 1 are required for activation by 4-chloro-m-cresol. J Biol Chem 281(30):21022–21031. 10.1074/jbc.M600670200 [DOI] [PubMed] [Google Scholar]
- 43.Herrmann-Frank A, Richter M, Sarkozi S, Mohr U, Lehmann-Horn F (1996) 4-Chloro-m-cresol, a potent and specific activator of the skeletal muscle ryanodine receptor. Biochem Biophys Acta 1289(1):31–40. 10.1016/0304-4165(95)00131-x [DOI] [PubMed] [Google Scholar]
- 44.Chen YH, Wang XH, Zhai HF, Zhang YL, Huang JM (2021) Identification of potential human ryanodine receptor 1 agonists and molecular mechanisms of natural small-molecule phenols as anxiolytics. ACS Omega 6(44):29940–29954. 10.1021/acsomega.1c04468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iyer KA, Hu Y, Klose T, Murayama T, Samso M (2022) Molecular mechanism of the severe MH/CCD mutation Y522S in skeletal ryanodine receptor (RyR1) by cryo-EM. Proc Natl Acad Sci U S A 119(30):e2122140119. 10.1073/pnas.2122140119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai XC, Yan Z, Wu JP, Li ZQ, Yan N (2016) The Central domain of RyR1 is the transducer for long-range allosteric gating of channel opening. Cell Res 26(9):995–1006. 10.1038/cr.2016.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iyer KA, Hu Y, Nayak AR, Kurebayashi N, Murayama T, Samso M (2020) Structural mechanism of two gain-of-function cardiac and skeletal RyR mutations at an equivalent site by cryo-EM. Sci Adv 6(31):eabb2964. 10.1126/sciadv.abb2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan Z, Bai XC, Yan CY, Wu JP, Li ZQ, Xie T, Peng W, Yin CC et al (2015) Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 517(7532):50-+. 10.1038/nature14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miotto MC, Weninger G, Dridi H, Yuan Q, Liu Y, Wronska A, Melville Z, Sittenfeld L et al (2022) Structural analyses of human ryanodine receptor type 2 channels reveal the mechanisms for sudden cardiac death and treatment. Sci Adv 8(29):eabo1272. 10.1126/sciadv.abo1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng W, Shen H, Wu J, Guo W, Pan X, Wang R, Chen SR, Yan N (2016) Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science 354(6310). 10.1126/science.aah5324 [DOI] [PubMed]
- 51.Milloy V, Fournier P, Benoit D, Norena A, Koravand A (2017) Auditory brainstem responses in tinnitus: a review of who, how, and what? Front Aging Neurosci 9:237. 10.3389/fnagi.2017.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Domarecka E, Olze H, Szczepek AJ (2020) Auditory brainstem responses (ABR) of rats during experimentally induced tinnitus: literature review. Brain Sci 10(12). 10.3390/brainsci10120901 [DOI] [PMC free article] [PubMed]
- 53.Melcher JR, Kiang NY (1996) Generators of the brainstem auditory evoked potential in cat. III: Identified cell populations. Hear Res 93(1–2):52–71. 10.1016/0378-5955(95)00200-6 [DOI] [PubMed] [Google Scholar]
- 54.Alvarado JC, Fuentes-Santamaria V, Jareno-Flores T, Blanco JL, Juiz JM (2012) Normal variations in the morphology of auditory brainstem response (ABR) waveforms: a study in Wistar rats. Neurosci Res 73(4):302–311. 10.1016/j.neures.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 55.Castaneda R, Natarajan S, Jeong SY, Hong BN, Kang TH (2019) Electrophysiological changes in auditory evoked potentials in rats with salicylate-induced tinnitus. Brain Res 1715:235–244. 10.1016/j.brainres.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 56.Rezapour M, Akbari M, Dargahi L, Zibaii MI, Shahbazi A (2023) The auditory brainstem response (ABR) test, supplementary to behavioral tests for evaluation of the salicylate-induced tinnitus. Indian J Otolaryngol Head Neck Surg 75(Suppl 1):6–15. 10.1007/s12070-022-03117-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farhadi M, Gorji A, Mirsalehi M, Muller M, Poletaev AB, Mahboudi F, Asadpour A, Ebrahimi M et al (2023) The human neuroprotective placental protein composition suppressing tinnitus and restoring auditory brainstem response in a rodent model of sodium salicylate-induced ototoxicity. Heliyon 9(8):e19052. 10.1016/j.heliyon.2023.e19052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi TY, Lee SH, Kim SJ, Jo Y, Park CS, Choi SY (2018) BK channel blocker paxilline attenuates thalidomide-caused synaptic and cognitive dysfunctions in mice. Sci Rep 8(1):17653. 10.1038/s41598-018-36367-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheehan JJ, Benedetti BL, Barth AL (2009) Anticonvulsant effects of the BK-channel antagonist paxilline. Epilepsia 50(4):711–720. 10.1111/j.1528-1167.2008.01888.x [DOI] [PubMed] [Google Scholar]
- 60.Zhao J, Li G, Zhao X, Lin X, Gao Y, Raimundo N, Li GL, Shang W et al (2020) Down-regulation of AMPK signaling pathway rescues hearing loss in TFB1 transgenic mice and delays age-related hearing loss. Aging (Albany NY) 12(7):5590–5611. 10.18632/aging.102977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Lin G, Xue N, Wang Y, Du T, Liu H, Xiong W, Shang W et al (2023) Differential outcomes of high-fat diet on age-related rescaling of cochlear frequency place coding. FASEB J 37(10):e23167. 10.1096/fj.202300457RR [DOI] [PubMed] [Google Scholar]
- 62.Latorre R, Castillo K, Carrasquel-Ursulaez W, Sepulveda RV, Gonzalez-Nilo F, Gonzalez C, Alvarez O (2017) Molecular determinants of BK channel functional diversity and functioning. Physiol Rev 97(1):39–87. 10.1152/physrev.00001.2016 [DOI] [PubMed] [Google Scholar]
- 63.Cazals Y (2000) Auditory sensori-neural alterations induced by salicylate. Prog Neurobiol 62(6):583–631. 10.1016/s0301-0082(00)00027-7 [DOI] [PubMed] [Google Scholar]
- 64.Wang HT, Luo B, Zhou KQ, Xu TL, Chen L (2006) Sodium salicylate reduces inhibitory postsynaptic currents in neurons of rat auditory cortex. Hear Res 215(1–2):77–83. 10.1016/j.heares.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 65.Querfurth HW, Haughey NJ, Greenway SC, Yacono PW, Golan DE, Geiger JD (1998) Expression of ryanodine receptors in human embryonic kidney (HEK293) cells. Biochem J 334(Pt 1):79–86. 10.1042/bj3340079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fessenden JD, Perez CF, Goth S, Pessah IN, Allen PD (2003) Identification of a key determinant of ryanodine receptor type 1 required for activation by 4-chloro-m-cresol. J Biol Chem 278(31):28727–28735. 10.1074/jbc.M303821200 [DOI] [PubMed] [Google Scholar]
- 67.Matyash M, Matyash V, Nolte C, Sorrentino V, Kettenmann H (2002) Requirement of functional ryanodine receptor type 3 for astrocyte migration. FASEB J 16(1):84–86. 10.1096/fj.01-0380fje [DOI] [PubMed] [Google Scholar]
- 68.Dyhrfjeld-Johnsen J, Cederroth CR (2020) Current clinical trials for tinnitus: drugs and biologics. Otolaryngol Clin North Am 53(4):651–666. 10.1016/j.otc.2020.03.012 [DOI] [PubMed] [Google Scholar]
- 69.Kurt S, Sausbier M, Ruttiger L, Brandt N, Moeller CK, Kindler J, Sausbier U, Zimmermann U et al (2012) Critical role for cochlear hair cell BK channels for coding the temporal structure and dynamic range of auditory information for central auditory processing. FASEB J 26(9):3834–3843. 10.1096/fj.11-200535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pyott SJ, Meredith AL, Fodor AA, Vazquez AE, Yamoah EN, Aldrich RW (2007) Cochlear function in mice lacking the BK channel alpha, beta1, or beta4 subunits. J Biol Chem 282(5):3312–3324. 10.1074/jbc.M608726200 [DOI] [PubMed] [Google Scholar]
- 71.Pyott SJ, Duncan RK (2016) BK channels in the vertebrate inner ear. Int Rev Neurobiol 128:369–399. 10.1016/bs.irn.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 72.Kim Y, Trussell LO (2007) Ion channels generating complex spikes in cartwheel cells of the dorsal cochlear nucleus. J Neurophysiol 97(2):1705–1725. 10.1152/jn.00536.2006 [DOI] [PubMed] [Google Scholar]
- 73.N’Gouemo P, Yasuda RP, Faingold CL (2009) Protein expression of small conductance calcium-activated potassium channels is altered in inferior colliculus neurons of the genetically epilepsy-prone rat. Brain Res 1270:107–111. 10.1016/j.brainres.2009.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang W, Zhang LS, Zinsmaier AK, Patterson G, Leptich EJ, Shoemaker SL, Yatskievych TA, Gibboni R et al (2019) Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models. PLoS Biol 17(6):e3000307. 10.1371/journal.pbio.3000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayashi Y, Morinaga S, Zhang J, Satoh Y, Meredith AL, Nakata T, Wu Z, Kohsaka S et al (2016) BK channels in microglia are required for morphine-induced hyperalgesia. Nat Commun 7:11697. 10.1038/ncomms11697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hayashi Y, Kawaji K, Sun L, Zhang X, Koyano K, Yokoyama T, Kohsaka S, Inoue K et al (2011) Microglial Ca(2+)-activated K(+) channels are possible molecular targets for the analgesic effects of S-ketamine on neuropathic pain. J Neurosci 31(48):17370–17382. 10.1523/JNEUROSCI.4152-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su YY, Luo B, Jin Y, Wu SH, Lobarinas E, Salvi RJ, Chen L (2012) Altered neuronal intrinsic properties and reduced synaptic transmission of the rat’s medial geniculate body in salicylate-induced tinnitus. PLoS One 7(10):e46969. 10.1371/journal.pone.0046969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zugaib J, Ceballos CC, Leao RM (2016) High doses of salicylate reduces glycinergic inhibition in the dorsal cochlear nucleus of the rat. Hear Res 332:188–198. 10.1016/j.heares.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 79.Kimm T, Khaliq ZM, Bean BP (2015) Differential regulation of action potential shape and burst-frequency firing by BK and Kv2 channels in substantia nigra dopaminergic neurons. J Neurosci : the official journal of the Society for Neuroscience 35(50):16404–16417. 10.1523/JNEUROSCI.5291-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ly C, Melman T, Barth AL, Ermentrout GB (2011) Phase-resetting curve determines how BK currents affect neuronal firing. J Comput Neurosci 30(2):211–223. 10.1007/s10827-010-0246-3 [DOI] [PubMed] [Google Scholar]
- 81.Niday Z, Bean BP (2021) BK channel regulation of afterpotentials and burst firing in cerebellar Purkinje neurons. J Neurosci : the official journal of the Society for Neuroscience 41(13):2854–2869. 10.1523/JNEUROSCI.0192-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.N’Gouemo P (2011) Targeting BK (big potassium) channels in epilepsy. Expert Opin Ther Targets 15(11):1283–1295. 10.1517/14728222.2011.620607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Woll KA, Van Petegem F (2022) Calcium-release channels: structure and function of IP(3) receptors and ryanodine receptors. Physiol Rev 102(1):209–268. 10.1152/physrev.00033.2020 [DOI] [PubMed] [Google Scholar]
- 84.Furuichi T, Furutama D, Hakamata Y, Nakai J, Takeshima H, Mikoshiba K (1994) Multiple types of ryanodine receptor/Ca2+ release channels are differentially expressed in rabbit brain. J Neurosci : the official journal of the Society for Neuroscience 14(8):4794–4805. 10.1523/JNEUROSCI.14-08-04794.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hertle DN, Yeckel MF (2007) Distribution of inositol-1,4,5-trisphosphate receptor isotypes and ryanodine receptor isotypes during maturation of the rat hippocampus. Neuroscience 150(3):625–638. 10.1016/j.neuroscience.2007.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen GD, Kermany MH, D’Elia A, Ralli M, Tanaka C, Bielefeld EC, Ding D, Henderson D et al (2010) Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res 265(1–2):63–69. 10.1016/j.heares.2010.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kakehata S, Santos-Sacchi J (1996) Effects of salicylate and lanthanides on outer hair cell motility and associated gating charge. J Neurosci : the official journal of the Society for Neuroscience 16(16):4881–4889. 10.1523/JNEUROSCI.16-16-04881.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen GD, Stolzberg D, Lobarinas E, Sun W, Ding D, Salvi R (2013) Salicylate-induced cochlear impairments, cortical hyperactivity and re-tuning, and tinnitus. Hear Res 295:100–113. 10.1016/j.heares.2012.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boettcher FA, Bancroft BR, Salvi RJ (1990) Concentration of salicylate in serum and perilymph of the chinchilla. Arch Otolaryngol Head Neck Surg 116(6):681–684. 10.1001/archotol.1990.01870060039005 [DOI] [PubMed] [Google Scholar]
- 90.Jastreboff PJ, Brennan JF, Coleman JK, Sasaki CT (1988) Phantom auditory sensation in rats: an animal model for tinnitus. Behav Neurosci 102(6):811–822. 10.1037/0735-7044.102.6.811 [DOI] [PubMed] [Google Scholar]
- 91.Liu XP, Chen L (2012) Auditory brainstem response as a possible objective indicator for salicylate-induced tinnitus in rats. Brain Res 1485:88–94. 10.1016/j.brainres.2012.04.048 [DOI] [PubMed] [Google Scholar]
- 92.Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL (2003) Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci : the official journal of the Society for Neuroscience 23(9):3944–3952. 10.1523/JNEUROSCI.23-09-03944.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Farhadi M, Gorji A, Mirsalehi M, Poletaev AB, Asadpour A, Mahboudi F, Jafarian M, Farrahizadeh M et al (2023) Electrophysiological and molecular changes following neuroprotective placental protein administration on tinnitus-induced rats. Laryngoscope Investigative Otolaryngol 8(5):1410–1420. 10.1002/lio2.1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yi B, Hu S, Zuo C, Jiao F, Lv J, Chen D, Ma Y, Chen J et al (2016) Effects of long-term salicylate administration on synaptic ultrastructure and metabolic activity in the rat CNS. Sci Rep 6:24428. 10.1038/srep24428 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.