Abstract
Background
Vestibular migraine (VM) is a disorder characterized by recurrent episodes of dizziness or vertigo and is often accompanied by headache. The mechanisms underlying vestibular dysfunction and pain in VM remain unclear.
Methods
Chronic migraine (CM) and VM models were induced by NTG and kainic acid, respectively. Behavioral assessments were conducted to evaluate vestibular dysfunction and pain in the VM and CM models. Transmission electron microscopy (TEM) was used to examine peripheral receptor impairment. Immunofluorescence, including staining for Cellular Proto-oncogene (c-Fos), Neuronal Nuclei (NeuN), and calcitonin gene-related peptide (CGRP), identified activated brain regions such as the cortex, midbrain, and cerebellum. Multiplex immunohistochemistry and cholera toxin subunit B (CTB) tracing were performed to analyze nuclear heterogeneity and neural communication. Additionally, RNA sequencing (RNA-Seq) and Ionized calcium-binding adapter molecule 1 (IBA1) immunostaining were used to investigate ion channel expression in the spinal trigeminal nucleus caudalis (Sp5c).
Results
CM and VM-related behaviors, such as allodynia and balance disturbance, were successfully reproduced in mouse model. TEM revealed significant damage to peripheral sensory receptors, particularly in the trigeminal ganglion and cochlear cells. Distinct activation patterns of c-Fos and CGRP were observed in VMs and CMs. CTB tracing confirmed that signals are transmitted from the vestibulocerebellum (VbC) to the Sp5c via the vestibular nuclei (VN). Furthermore, RNA-Seq combined with coimmunostaining revealed an increased expression of transient receptor potential vanilloid 2 (TRPV2) ion channels in microglia within Sp5c, indicating their potential role in VM pathology.
Conclusions
This study preliminarily explored VbC-VN-Sp5c communication and identified TRPV2 ion channels in microglia as key players in neuron-glia crosstalk in VM. These findings provide new insights into the mechanisms underlying vestibular migraine and suggest potential therapeutic targets.
Graphical abstract
Keywords: Chronic migraine, Vestibular migraine, Neural circuit, Microglia, CGRP, TRPV2
Introduction
Migraine is one of the most common clinical primary headaches, affecting more than 15% of the world’s population [1]. The World Health Organization lists it as one of the four most serious dysfunctional diseases, together with dementia, mental disorders, and quadriplegia [2]. Vestibular migraine (VM) is a common clinical disease characterized by recurrent dizziness/vertigo accompanied by nausea and vomiting with or without headache symptoms [3]. The Bárány Society and the International Headache Society jointly revised and published the diagnostic criteria for VM, which are defined as vestibular dysfunction caused by migraine [4–6]. Combined with the clinical manifestations and onset characteristics of VM, it is currently believed that the pathogenesis of VM is associated with migraine [7]. In our previous clinical studies, we identified significant impairments in parietal-insular vestibular cortex, as well as in the vestibular nuclei (VN) and cerebellar regions, in VM patients via techniques such as brainstem auditory evoked potentials, resting-state fMRI, and diffusion tensor imaging [8–11]. Furthermore, in basic research, Xie [12] investigated glutamatergic neurons in the VN via the VGLUT2-IRES-Cre mouse model. The study revealed that these neurons receive input from 57 brain nuclei and project to 59 brain regions, demonstrating extensive communication between the medial, lateral VN, and other brain areas. These connections highlight the VN’s role in integrating sensory information, which is critical for functions such as balance and spatial orientation. Notably, the vestibulocerebellum (VbC), which receives vestibular input from the VN, plays a key role in the perception of self-motion and spatial orientation [13]. Additionally, studies suggest that microglial activation and the resulting inflammatory response in the spinal trigeminal nucleus (Sp5c) contribute to central sensitization in CM and may trigger migraine attacks. Activated microglia release proinflammatory molecules with neuromodulatory effects, lowering pain thresholds and underscoring the importance of neuron-glia interactions in migraine pathophysiology [14, 15]. Taken together, these findings suggest that VM may represent a manifestation of “brain network dysfunction”. Such crosstalk between different brain regions appears to play a significant role, ultimately influencing the network dynamics involved in vestibular migraine pathophysiology.
A comprehensive exploration of neural networks is necessary to understand the integration of neurons, glial cells, neurotransmitter systems, and ion channels. This study aimed to investigate the existence of reciprocal communication between these complex brain nuclei and to examine the potential molecular mechanisms involved. The architecture of their networks and synaptic connections remains poorly characterized, particularly in relation to their role in vertigo and pain processing [16]. Modulating the activity of this circuit may influence vestibular dysfunction in VM, providing a foundation for future studies aimed at identifying new therapeutic targets.
Methods
Animals
All animal procedures and ethical considerations for this study received formal approval from the Institutional Animal Care and Use Committee at Harbin Medical University, under reference number YS166. C57BL/6J mice were obtained from Liaoning Changsheng Biotechnology Co., Ltd (Benxi, Liaoning, China). The weight of the mice ranged from 25 to 30 g, and their ages ranged from 2 to 3 months. Mice were assigned to three groups using an online random number generator (Randomly Assign Subjects to Treatment Groups). The VEH group received an intraperitoneal injection of saline and an intracochlear injection of saline. All the animals were housed under controlled conditions, with a temperature of 23 ± 1 °C and a 12-hour light–dark cycle. They had free access to food and water. All experiments were conducted with investigators blinded to group assignments, with unblinding only after data collection was completed.
CM model
To induce migraine-relevant symptoms in mice, nitroglycerin (NTG), a strong vasodilator that triggers migraine-like pain and associated behaviors in rodents [17] was used at a dose of 10 mg/kg via intraperitoneal injection [18]. The NTG solution was prepared from a 5 mg/ml NTG stock solution obtained from Beijing Yimin, China, and was freshly diluted with 0.9% saline to achieve a10 mg/kg dose. The mice were administered NTG every other day for a total of 9 days, resulting in 5 injections.
VM model
The VM model involves two main steps [19–21]. First, migraine was induced by intraperitoneal administration of 10 mg/kg NTG. Thirty minutes after each NTG administration, peripheral vestibular organ injury was induced via kainic acid, a drug typically used to induce excitotoxic neuronal damage [22]. After all surgeries were conducted under isoflurane anesthesia (3% induction, 1.5% maintenance in 70% N2O and 30% O2), the inner and outer ear canals were enlarged to identify the tympanic membrane. A fine needle was used to create a small opening in the tympanic membrane, and 0.10 ml of 12.5 mM kainic acid was injected slowly.
Periorbital allodynia test
Periorbital allodynia is characterized by a decreased mechanical threshold in the periorbital region [23]. We carried out a periorbital withdrawal threshold test to measure mechanical hypersensitivity [24] Threshold levels were measured immediately before and 2 h after VM and CM modeling. The von Frey test was conducted via a general-purpose plastic restraining tube (inner diameter 3.5 cm, length 10 cm), specifically designed for rodents weighing up to 40 g [25]. To assess changes in periorbital sensitivity, von Frey monofilaments calibrated by filament size for bending force were used. Mechanical thresholds were determined by applying the von Frey filaments to the periorbital regions on both sides of the face. For the stimulus to elicit a positive response, the filament needed to be made firm, in perpendicular contact with the skin, and bent to apply the precisely calibrated force set by the manufacturer. Positive responses were identified by behaviors such as intense facial scratching, head withdrawal from the stimulus, or head shaking. We used the “up-and-down” method [26], with the filament force thresholds ranging from 0.008 to 1.0 g across all tests.
Balance beam walk
Since VM patients experience significant balance disturbances, it is essential to measure the time taken to cross the balance beam [27]. The method of balance beam walking refers to the research of Zhang [20]. Balance beam walk tests were conducted after threshold testing, 2.5 h post-VM and CM modeling. A horizontal balance beam, 100 cm long and 2.5 cm in diameter, is placed 40 cm above the ground. Soft pads were placed under the beam to prevent the rats from falling and becoming injured. The time to traverse the entire length of the balance beam was recorded for each mouse. The maximum time limit for each trial was 90 s. The minimum interval between consecutive trials for each mouse was 5 min. The average duration of the three trials was calculated.
Vestibular dysfunction tests
The vestibular dysfunction test was conducted 3 h after the fifth VM and CM modeling. The vestibular dysfunction score was assessed according to previously described methods [28, 29]: This score was calculated by sequentially assessing six different tests, with each parameter rated from 0 to 2 points (0: normal, 1: moderate, 2: severe): (1) Head movement (intermittent abnormal backward extension of the neck); (2) Circling (stereotyped circling motion around the hindquarters, ranging from none to compulsive); (3) Retropulsion (characteristic backward walking, indicating vestibular dysfunction); (4) Tail-hang reflex (typically induces forelimb extension to contact the ground, whereas unilateral dysfunction causes axial body rotation); (5) Contact inhibition reflex (In animals with vestibular dysfunction, the animal remains gripping the grid in a supine position owing to impaired spatial orientation)and (6) Aerial righting reflex (In animals with vestibular dysfunction, this results in the animal landing on its back when it drops from a height of 40 cm onto a foam pad).
Transmission electron microscopy (TEM)
As this experiment involves a peripheral vestibular dysfunction model, electron microscopy was used to examine the cellular morphology of peripheral sensory receptors. Eight Mouse were perfused with 0.1 mol/L PBS. The trigeminal ganglia and cochleae were then dissected, trimmed into 1 mm³ blocks, and fixed in 2.5% glutaraldehyde at room temperature for 2 h. The samples were subsequently fixed with 2% osmium tetroxide followed for 1 h. The samples were dehydrated through graded ethanol, infiltrated with epoxy resin, and embedded. After polymerization at 60 °C for 48 h, 90-nm sections were cut, stained with uranyl acetate and lead citrate, and examined via TEM [30].
Immunofluorescence staining
The whole brain tissue was dissected immediately after perfusion with saline and 4% paraformaldehyde, fixed in 4% paraformaldehyde at 4 °C for 24 h, dehydrated through a graded series of ethanol, cleared in xylene, embedded in paraffin, and sectioned to a thickness of 6 μm. After the samples were blocked with blocking solution for 1 h at room temperature, they were incubated with primary antibody overnight at 4 °C. The next day, secondary antibodies were applied at a room temperature. The sections were subsequently stained with DAPI for 10 min at 37 °C. The slides were mounted with mounting medium and the staining effect was observed and photographed via a confocal microscope (Leica TCS SP8). Detailed information about the antibodies used for immunofluorescence staining can be found in the Table 1. To quantify the number of positive cells via immunofluorescence, five views were selected in the core area of each nuclear Sect. (150 × 150 μm per field of view). Images of these areas were observed under constant viewing conditions, scale bar: 20 μm. The cells positively stained by both antibodies were counted only when the nuclei were clearly visible, ensuring accuracy in counting.
Table 1.
Antibodies used in Immunofluorescence staining
| Antibody | Manufacturer | Catalog Number | Dilution |
|---|---|---|---|
| CGRP | CST | 14,959 | 0.388889 |
| c-Fos | CST | 2250 | 0.736111 |
| Neuron | Abcam | EPR2763 | 0.736111 |
| DAPI | CST | 4083 S | 2.125 |
| TRPV2 | Abcam | ab272862 | 0.388889 |
| IBA1 | Proteintech | 26177-1-AP | 0.388889 |
| NLRP3 | Proteintech | 19771-1-AP | 0.73611 |
| ASC | Bioss | bs-6741R | 0.38889 |
| cleaved caspase 1(P20) | CST | D57A2 | 0.73611 |
| cleaved IL-1β | Affinity | AF4006 | 0.38889 |
| GAPDH | Proteintech | 60004-1-Ig | 3.51389 |
Multiplex immunohistochemistry staining
Multiplex immunohistochemistry staining of the slides were performed to visualize five markers simultaneously, followed by antigen retrieval and blocking. The Sp5C, VN, and VbC sections were underwent deparaffinization to remove paraffin wax, followed by rehydration through graded alcohol washes to restore the tissue to a water-based state. The sections were then fixed in neutral buffered formalin to preserve the tissue structure and prevent degradation. Antigen retrieval was performed to increase epitope accessibility. The samples were subsequently incubated with four primary antibodies at 37 °C for 30–60 min, followed by incubation with HRP-conjugated secondary antibodies. Tyramide signal amplification (TSA) was applied in combination with Opal dyes for signal detection. TSA was selectively used for antibodies with low signal intensity, such as anti-5-HT, GABA, GLUT1, and tyrosine hydroxylase, to increase sensitivity. Finally, all the slides were counterstained with DAPI for 5 min and mounted with a sealing agent to preserve fluorescence [31].
RNA-sequencing
Total RNA from the Sp5C nucleus was extracted to identify the most differentially expressed ion channels by comparing the data with the ion channel database. Total RNA from the Sp5C nuclei was extracted via TRIzol reagent (Thermo Fisher Scientific, Cat. No. 15596026) according to the manufacturer’s protocol for Illumina sequencing. mRNA was then purified with poly-T oligo-attached magnetic beads before RNA-seq library construction. Gene expression analysis and assessment of alterations were conducted via StringTie. MA-plot analyses and heatmaps of gene expression levels were generated via OmicShare tools (https://www.omicshare.com/tools) and BMK Cloud (www.biocloud.net).
Stereotactic injection of cholera toxin subunit B (CTB-555)
After anesthesia, the mice were placed in a prone position on a stereotaxic frame to examine the projection between the VN and Sp5C. Following the coordinates for the Sp5c from previous studies (AP: -6.1, ML: ±1.6, DV: -5.3) [32], a retrograde tracer, CTB-555, was injected. For VN injection, the coordinates were set at (AP: -6.0, ML: ±1.2, DV: -4.5) [33]. Approximately 200 nL of CTB was injected at a rate of 50 nL/min, and the needle was held in place for at least 5 min post injection before withdrawal. After injection, the mice were allowed to recover for approximately 5 days, after which the VM model was induced, followed by perfusion and brain extraction for immunofluorescence experiments.
Statistical analysis
The experimental data were presented as the mean ± SEM and analyzed using GraphPad Prism 9 statistical analysis software. The normality of all data was assessed using the Shapiro-Wilk test prior to statistical analysis. Behavioral data for vestibular dysfunction scores, were used Kruskal Wallis test. The Balance Beam Walk and Periorbital Withdrawal Threshold tests were evaluated with two-way ANOVA incorporating the Geisser-Greenhouse correction, followed by Tukey’s post hoc tests. For immunofluorescence staining, data with equal SEM were analyzed using one-way ANOVA with Tukey’s post hoc tests for multiple comparisons. For data that did not assume equal SEM, the Brown-Forsythe ANOVA was applied, followed by Dunnett’s T3 post hoc tests. Statistical significance was defined as p < 0.05.
Results
Chronic nitroglycerin injection reproduced symptoms resembling CM, while the addition of Kainic acid to the cochlea induced pain and Dizziness behaviors similar to VM in mouse models
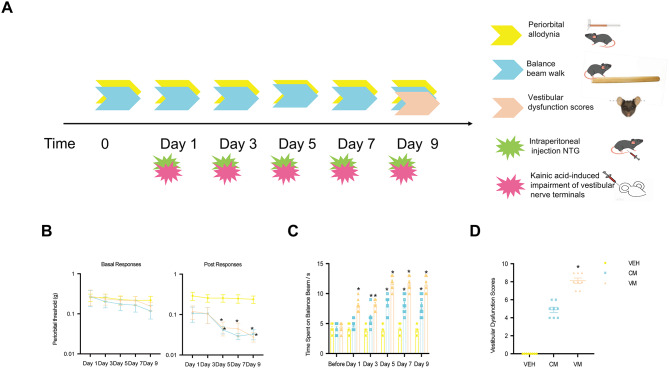
To assess the allodynia and susceptibility to dizziness in the CM and VM, we used von Frey filaments to determine the baseline mechanical pain threshold of the periorbital regions after each administration. We also evaluated the susceptibility to dizziness in the VM by measuring the time spent on a balance beam and assessing dizziness behavior scores. The experimental procedure is depicted in Fig. 1A. the baseline pain thresholds in the periorbital area of the mice were not significantly different between the groups (VEH vs. CM p = 0.9969; VEH vs. VM p = 0.9877, n = 8/group, Fig. 1B). However, both the CM and VM groups presented a significant decrease in the mechanical pain threshold compared with the control group starting from the fifth day, with a gradual decrease in pain intensity increased (VEH vs. CM p = 0.0103; VEH vs. VM p = 0.0138, n = 8/group, Fig. 1C).
Fig. 1.
Chronic nitroglycerin injection reproduced CM-like symptoms, while the addition of kainic acid to the ears reproduced VM-like pain and dizziness behavior in mice. A Representative schematic diagrams and procedures for the behavioral tests. B Baseline pain thresholds in the periorbital area of mice. (VEH vs. CM P = 0.9969, VEH vs. VM P = 0.9877, Two-way ANOVA test, Time Factor: F (2.324, 16.27) = 0.9298, P = 0.4276, Group Factor: F (1.511,10.58) = 1.162, P = 0.3334, n = 8/group). C post-treatment pain thresholds in the periorbital area of mice. (VEH vs. CM P = 0.0103, VEH vs. VM P = 0.0138, Two-way ANOVA test, Time Factor: F (2.907, 20.35) = 2.159, P = 0.1256; Group Factor: F (1.362, 9.534) = 42.3, P = 0.0001, n = 8/group). D Time to pass the beam. VEH vs. CM P = 0.9569; VEH vs. VM P = 0.8694. Two-way ANOVA test, Time Factor: F (2.637, 18.46) = 43.97, P = 0.0001; Group Factor: F (1.362, 9.532) = 228.7, P = 0.0001, n = 8/group). E Vestibular function behavior scores. (VEH vs. CM P = 0.0619; VEH vs. VM P = 0.0001, Kruskal-Wallis test)
In the balance beam experiment, before VM or CM modeling, the time spent on the beam was approximately 4.5 s, with no significant differences between the groups (VEH vs. CM p = 0.9569; VEH vs. VM p = 0.8694, n = 8/group, Fig. 1D). However, third modeling after VM and CM, showed a significant increase in the time spent on the beam compared to the control group (VEH vs. CM p = 0.0300; VEH vs. VM p = 0.0001, n = 8/group, Fig. 1D). Vestibular dysfunction behaviors included head movement, circling, retropulsion, the tail-hang reflex, the contact inhibition reflex and the aerial righting reflex. Compared with the control group, only VM group showed significantly increased vestibular dysfunction behavior scores (VEH vs. CM p = 0.0619; VEH vs. VM p = 0.0001, n = 8/group, Fig. 1E).
Damage to peripheral receptors in the trigeminal ganglia and cochlear cells was observed in both VM and CM models
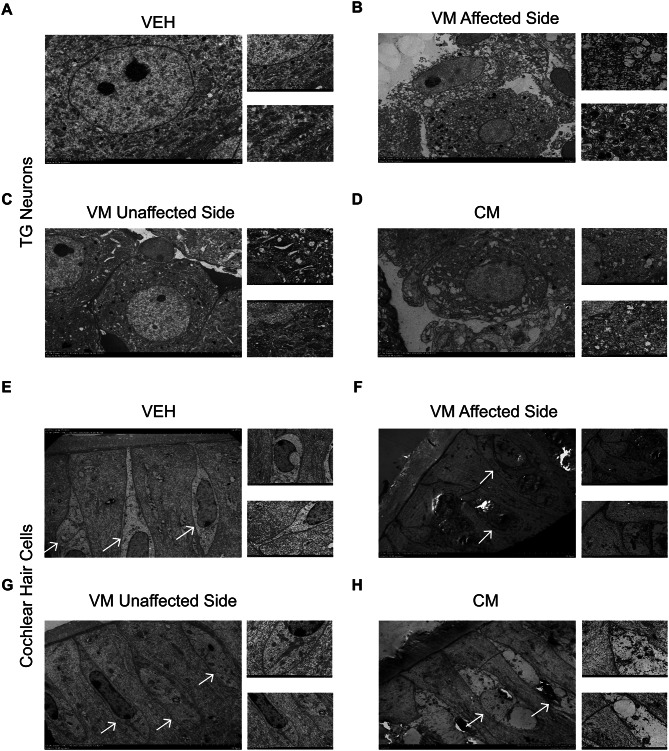
To investigate whether vestibular dysfunction and reduced pain thresholds induced by VM and CM result from direct damage to peripheral organs, we used transmission electron microscopy to examine the morphological changes in cells within the trigeminal ganglion and cochlear tissues of mice, two hours after CM and VM induction. In the trigeminal ganglion of the VM group, neuronal cell membranes were disrupted, and lysosomes accumulated within the neurons. (Figure 2A and B). Conversely, in contrast, the unaffected side of VM exhibited organized cell arrangements, albeit with an increased presence of lysosomes compared with those in VEH group (Fig. 2C). However, in the CM group, the neuronal cell membranes remained intact, yet noticeable swelling of the endoplasmic reticulum and mitochondria was observed compared with those in VEH group (Fig. 2D).
Fig. 2.
Peripheral receptor trigeminal ganglions and cochlear cells were damaged in VM and CM models. A. The structure of trigeminal ganglion neuronal cells in the VEH group. B-C. The structure of trigeminal ganglion neuronal cells on the affected and unaffected sides of the VM group. D. Structure of trigeminal ganglion neuronal cells of the CM group. E. The structure of cochlear cells in the VEH group. F-G. The structure of cochlear cells on the affected and unaffected sides of the VM group. H. Structure of cochlear cells of the CM group. As shown in the figure, the white arrows denote the hair cells
In the VEH group, the cochlea exhibited characteristic sensory epithelial morphology, with well-organized supporting cells and pear-shaped type I hair cells enveloped by calyx endings (Fig. 2E). On the affected side of the VM group, the supporting cells displayed disorganized arrangements, casting uncertainty on the identity of the hair cell types. This was accompanied by significant swelling and the presence of substantial vacuoles within the sensory epithelial cells (Fig. 2F). The unaffected side of the VM group showed cell damage falling between the levels observed in the CM and affected VM sides, characterized by slightly disorganized supporting cells and mildly swollen hair cells (Fig. 2G). In the CM group, the supporting cells remained organized, but the hair cells exhibited partial separation due to the presence of small vacuoles (Fig. 2H).
C-Fos and CGRP activation mapping was conducted in cortical structures, the thalamus, brainstem, and cerebellar regions to analyze neural activity associated with CM and VM
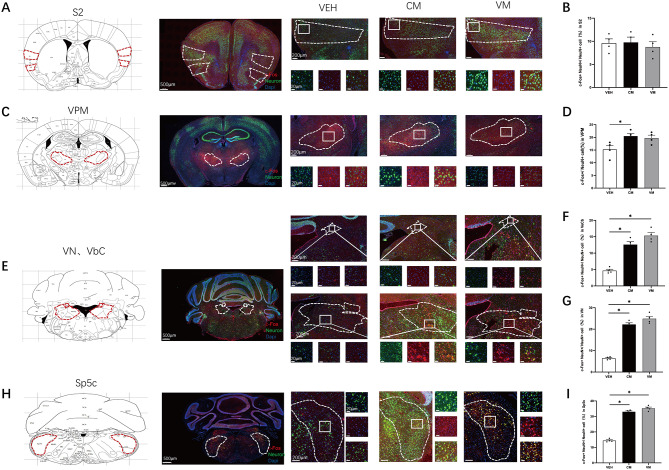
It remains unclear whether similar patterns of brain region activation occur in CM and VM induced by NTG and kainic acid. To investigate this, we employed c-Fos and NeuN costaining to evaluate neural activation across various brain regions 1 h after establishing the CM and VM models. We found no significant difference in the percentage of c-Fos + cells within NeuN + cells in the secondary somatosensory cortex (S2) region between the VM and CM groups (VEH vs. CM p = 0.0926; VEH vs. VM p = 0.1154, n = 4/group, Fig. 3A and B) groups. There was a significant increase in the percentage of c-Fos + cells among NeuN + cells in the VPM region of the CM group compared with the VEH group, but not in the VM group (VEH vs. CM p = 0.0383; VEH vs. VM p = 0.0740, n = 4/group, Fig. 3C and D). However, the percentage of c-Fos + cells among NeuN + cells in the VN and VbC nuclei compared was significantly greater than that in the control group (VbC: F (2,9) = 79.93, VEH vs. CM p = 0.0001; VEH vs. VM p = 0.0001, n = 4/group. VN: VEH vs. CM p = 0.0001; VEH vs. VM p = 0.0009, n = 4/group, Fig. 3E, F and G). Similarly, c-Fos + activation was evident in the Sp5c nuclei in both the CM and VM groups (VEH vs. CM, p = 0.0001; VEH vs. VM p = 0.0001, n = 4/group, Fig. 3H and I). These findings suggest differential activation patterns in certain brain regions following NTG and kainic acid induction, particularly notable effects observed in the VN, VbC, and Sp5c nuclei.
Fig. 3.
C-Fos Activation Mapping in Cortical Structures, Thalamus, Brainstem, and Cerebellar Structures in CM and VM. A Representative pictures of c-Fos (red) and NeuN (green) immunofluorescence labeling in the S2. B In S2, no significant differences in the percentage of c-Fos + cells in NeuN + cells were found. VEH vs. CM P = 0.0926; VEH vs. VM P = 0.1154, Brown-Forsythe and Welch ANOVA tests, n = 4/group). C Representative pictures of c-Fos (red) and NeuN (green) immunofluorescence labeling in the VPM. D In VPM, a significant difference in the percentage of c-Fos + cells in NeuN + cells were found in CM, but not in VM compared to control group. (VEH vs. CM P = 0.0383; VEH vs. VM p = 0.0740, One-way ANOVA test, F (2,9) = 5.117, P = 0.0325, n = 4/group). E Representative pictures of c-Fos (red) and NeuN (green) immunofluorescence labeling in the VbC and VN. F VbC showed a significant increase of the percentage of c-Fos + cells in NeuN + cells. (VEH vs. CM P = 0.0001; VEH vs. VM P = 0.0001, One-way ANOVA test, F (2,9) = 79.93, P = 0.0001, n = 4/group). G VN, showed a significant increase of the percentage of c-Fos + cells in NeuN + cells. (VEH vs. CM P = 0.0001; VEH vs. VM P = 0.0009, Brown-Forsythe and Welch ANOVA tests, n = 4/group). H Representative pictures of c-Fos (red) and NeuN (green) immunofluorescence labeling in the Sp5c. I In Sp5c, a significant difference in the percentage of c-Fos + cells in NeuN + cells were found. (VEH vs. CM p = 0.0001; VEH vs. VM p = 0.0001, One-way ANOVA test, F (2,9) = 102.6, P = 0.0001, n = 4/group)
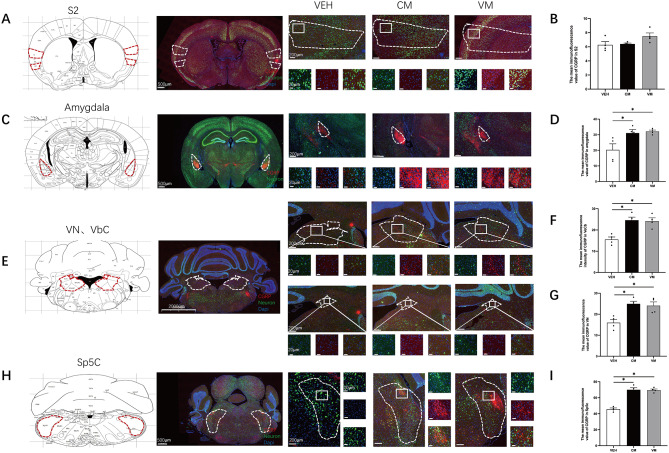
Additionally, we also employed CGRP and NeuN costaining to evaluate activation in various brain regions, given that CGRP is a key factor in migraine pathology. In both the VM and CM groups, we detected no significant difference in the intensity of CGRP observed in the S2 (VEH vs. CM p = 0.0656; VEH vs. VM p = 0.9993, n = 4/group, Fig. 4A and B). However, the intensity of CGRP was significantly elevated in the amygdala (VEH vs. CM p = 0.0005; VEH vs. VM p = 0.0001, n = 4/group, Fig. 4C and D) and in the VN and VbC nuclei than in the control group (VbC: VEH vs. CM p = 0.0347, VEH vs. VM p = 0.0067. VN: VEH vs. CM p = 0.0067, VEH vs. VM p = 0.0167, n = 4/group, Fig. 4E, F and G). Similarly, CGRP intensity was evident in the Sp5c nuclei in both the CM and VM groups (VEH vs. CM p = 0.0001, VEH vs. VM p = 0.0004, n = 4/group, Fig. 4H and I).
Fig. 4.
CGRP Activation Mapping in Cortical Structures, Thalamus, Brainstem, and Cerebellar Structures in CM and VM. A Representative pictures of CGRP (red) and NeuN (green) immunofluorescence labeling in the S2. B In S2, no significant differences in the mean immunofluorescence value of CGRP were found. (VEH vs. CM P = 0.0656; VEH vs. VM P = 0.9993, Brown-Forsythe and Welch ANOVA tests, n = 4/group). C Representative pictures of CGRP (red) and NeuN (green) immunofluorescence labeling in the Amygdala. D In Amygdala, compare to VEH group, a significant difference in the mean immunofluorescence value of CGRP were found in CM and VM groups. (VEH vs. CM P = 0.0005; VEH vs. VM P = 0.0001, One-way ANOVA test, F (2,9) = 36.43, P = 0.0001, n = 4/group). E Representative pictures of CGRP (red) and NeuN (green) immunofluorescence labeling in the VbC and VN. F (VEH vs. CM P = 0.0347, VEH vs. VM P = 0.0067, Brown-Forsythe and Welch ANOVA tests, n = 4/group). G (VEH vs. CM P = 0.0067, VEH vs. VM P = 0.0167, Brown-Forsythe and Welch ANOVA tests, n = 4/group). H Representative pictures of CGRP (red) and NeuN (green) immunofluorescence labeling in the Sp5c. I Sp5c showed a significant difference in the mean immunofluorescence value of CGRP. (VEH vs. CM p = 0.0001, VEH vs. VM p = 0.0004, One-way ANOVA test, F (2,9) = 58.18, P = 0.0001, n = 4/group)
VN integrates neuronal information from the VbC and projects it to the Sp5c, which also receives direct projections from the VbC
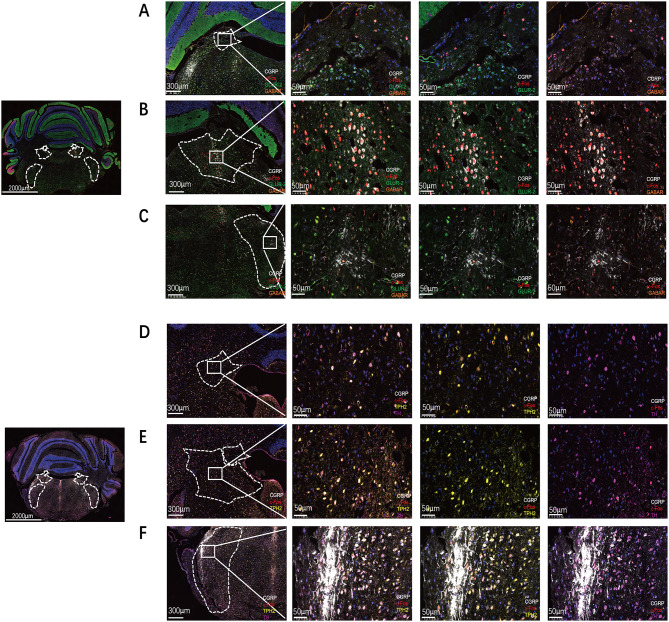
Immunofluorescence experiments collectively revealed the VN, SP5c, and the VbC as the regions most prominently activated in the CM and VM. However, these tissues consist of heterogeneous nuclear clusters, including glutamatergic neurons, GABAergic neurons, 5-HTergic neurons, and dopaminergic neurons. Paraffin sections were subjected to multiplex immunostaining for glutamate ionotropic receptor AMPA Type Subunit 2 (GluR2), serotonin, tyrosine hydroxylase, GABA, CGRP, and c-Fos to characterize the density of these neuronal types. Multispectral analysis revealed that, under the experimental modeling conditions in this study, the neurons activated in the VbC with the highest CGRP expression were primarily in the GABA neuron category (Fig. 5A and D), whereas in the VN and SP5c nuclei, the neurons activated with the highest CGRP expression were also predominantly glutamatergic neurons (Fig. 5B, C, E and F). These results suggest that in the CM and VM experimental models, the most activated neural clusters are VN, SP5c, and VbC. In the VN and SP5c, the predominant neuronal type is glutamatergic neurons, whereas VbC neurons exhibit GABAergic neuronal characteristics.
Fig. 5.
VN integrates neuron information from VbC and projects to Sp5c, Sp5c can also accept direct projections from VbC. A Multiplex immunostaining for GluR2, GABA, CGRP, and c-Fos in VbC. B Multiplex immunostaining for GluR2, GABA, CGRP, and c-Fos in VN. C Multiplex immunostaining for GluR2, GABA, CGRP, and c-Fos in Sp5c. D Multiplex immunostaining for 5-HTergic neurons, dopaminergic, CGRP, and c-Fos in VbC. E Multiplex immunostaining for 5-HTergic neurons, dopaminergic, CGRP, and c-Fos in VN. F Multiplex immunostaining for 5-HTergic neurons, dopaminergic, CGRP, and c-Fos in Sp5c
To further delineate potential neural circuit connections between Sp5c, VN, and VbC neurons, CTB-FITC was used as a retrograde tracer and stereotaxically injected into the unilateral VN of wild-type mice (Fig. 6A). Two weeks later, CTB-labeled neurons were observed in the bilateral VbC region. Additionally, CTB-FITC was injected into the unilateral Sp5c nucleus of wild-type mice (Fig. 6B). Two weeks later, CTB-labeled neurons were observed in the bilateral VN region and bilateral VbC. Our results showed that the VN integrates neuron information from VbC and projects to Sp5c, Sp5c can also accept direct projections from VbC.
Fig. 6.
VN integrates neuron information from VbC and projects to Sp5c, Sp5c can also accept direct projections from VbC. A CTB-FITC was injected into the unilateral VN of wild-type mice. The red arrows indicate the positive cells projecting from the VN to the VbC. B CTB-FITC was injected into the unilateral Sp5c of wild-type mice. The red arrows indicate the positive cells projecting from the Sp5c to the VbC and VN
Ion channels were modulated, and glial cells were activated within the Sp5c
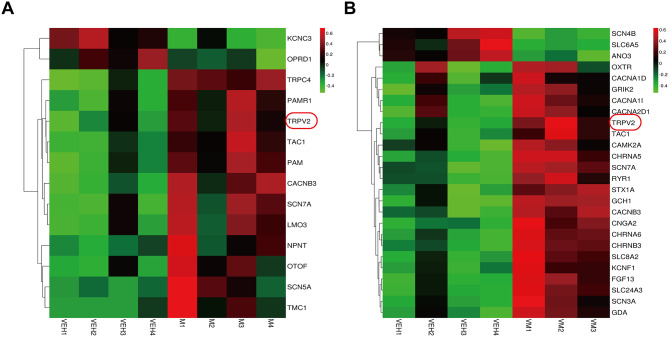
Our research findings demonstrate that the CM and VM models enhance the activity of the VbC-VN-Sp5c neural communication, raising the questions of what changes occur within the Sp5c nucleus and which underlying factors contribute to the progression of migraine in this model. Ion channels play pivotal roles in neural circuitry, participating in various processes, including pain, inflammation, and somatosensory modalities [34]. We used RNA sequencing results to shed light on this model, revealing that certain ion channels associated with pain sensitivity and vestibular dysfunction, such as TRPV2, TAC1, and Scn7a, are upregulated (Fig. 7A and B). Meents [35] highlighted the promising potential of targeting TRPV1 sensitization in combating migraines, emphasizing the need for further exploration in both research and the development of antimigraine medications. However, the role of TRPV2, a closely related member of the same family, remains largely unexplored in the context of migraines. This finding presents a significant gap in our understanding and underscores the importance of investigating the potential impact of TRPV2 on migraine pathology.
Fig. 7.
The RNA sequencing results reveal the involvement of specific ion channels in pain sensitivity and vestibular dysfunctions within this model. A The RNA sequencing results of VEH group and CM group of ion channels. n = 4/group. B The RNA sequencing results of VEH group and VM group of ion channels. VM, n = 3/group, VEH, n = 4/group
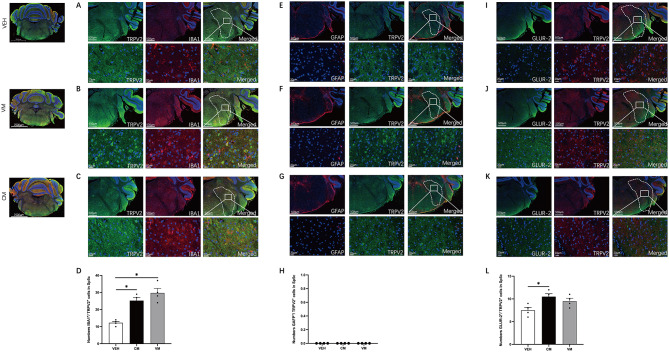
Immunofluorescence confirmed the RNA sequencing results, which revealed demonstrating high expression of TRPV2 in Sp5c in both the CM and VM models. Given that the RNA sequencing results were based on the entire Sp5c nucleus, we utilized costaining with Ionized calcium-binding adapter molecule 1 (IBA1), glial fibrillary acidic protein (GFAP), and GluR2 to explore which cell types in the Sp5c nucleus express TRPV2 most abundantly. The percentage of TRPV2 + cells within IBA1 + cells was significantly elevated in the Sp5c nucleus compared with the control group (VEH vs. CM p = 0.0114, VEH vs. VM p = 0.0005, n = 4/group, Fig. 8A, B, C and D). However, there was no significant difference in the percentage of TRPV2 + cells within GAFP + cells in the Sp5c nucleus compared with that in the control group (VEH vs. CM p > 0.05, VEH vs. VM p > 0.05, n = 4/group, Fig. 8E, F, G and H). There was a significant increase in the percentage of TRPV2 + cells within GluR2 + cells in the CM group compared with the VEH group, whereas no such increase was observed in the VM group (VEH vs. CM p = 0.0420, VEH vs. VM p = 0.3061, n = 4/group, Fig. 8I, J, K and L).
Fig. 8.
Modulation of ion channels and activation of glial cells in the Sp5c. A-C. Representative pictures of IBA1 (red) and TRPV2 (green) immunofluorescence labeling in the Sp5c. D. A significant difference in the numbers IBA1+/ TRPV2 + cells were found in Sp5c. (VEH vs. CM p = 0.0114, VEH vs. VM p = 0.0005, One-way ANOVA test, F (2,9) = 18.28, P = 0.0007, n = 4/group). E-G. Representative pictures of GFAP (red) and TRPV2 (green) immunofluorescence labeling in the Sp5c. H. No significant differences in the numbers GABA+/ TRPV2 + cells were found. (VEH vs. CM P > 0.05, VEH vs. VM P > 0.05, Brown-Forsythe and Welch ANOVA tests, n = 4/group). I-K. Representative pictures of TRPV2 (red) and GLUR-2 (green) immunofluorescence labeling in the Sp5c. L. A significant difference in the numbers GLUR-2+/ TRPV2 + cells were found in Sp5c (VEH vs. CM p = 0.0420, VEH vs. VM P = 0.3061, Brown-Forsythe and Welch ANOVA tests, n = 4/group)
In summary, our findings suggest that the upregulation of TRPV2 within the Sp5c nucleus may be one of the reasons for NTG and Kainic acid -induced migraine with vestibular dysfunction in this model.
Discussion
This study presents an innovative approach to create a mouse model of VM. The model combines two key interventions: NTG intraperitoneal administration and kainic acid-induced impairment of vestibular nerve terminals. This approach replicates the vestibular and pain components of VM in a controlled setting and allows investigation of their interplay in migraine pathophysiology. However, it may not fully reflect human VM features such as chronicity and long-term progression. Using this model, we explored the role of the VbC GABAergic-VN glutamatergic-Sp5c microglial communication in VM-related vertigo and pain. The core mechanism involves the activation of TRPV2 channels in Sp5c microglia, which enhances synaptic transmission and increases pain sensitivity and vestibular dysfunction. These findings could inform new therapeutic strategies, such as repetitive transcranial magnetic stimulation (rTMS), with potential for noninvasive modulation of neural circuits in VM [36].
Early findings from brainstem auditory-evoked potentials indicate that both VM and migraine patients exhibit brainstem dysfunction, with more severe impairment observed in VM patients [8, 9, 11]. Additionally, resting-state fMRI and diffusion tensor imaging revealed significant differences between VM patients and migraine patients. VM patients show pronounced activation in the vestibular pathways, particularly in the right hemisphere, the dominant vestibular hemisphere, and the parietal-insular vestibular cortex. In contrast, chronic migraine (CM) patients exhibit reduced activity in the Crus I region of the left cerebellum. Moreover, CM patients display structural abnormalities in white matter fibers, particularly in the knee of the corpus callosum and the anterior limb of the internal capsule, indicating that these regions are involved in pain processing [10]. These observations suggest that VM may be characterized as a disorder involving the dysfunction of multiple neural clusters. However, the exact composition of brain functional network connections and synaptic connections in VM remains largely unknown. Furthermore, current VM treatment strategies, which are based primarily on those developed for CM, are not always effective or well tolerated. This has led to an intensified research focus on identifying new targets and developing more precise treatments. Neuromodulation techniques are becoming increasingly important in the clinical management of neurological disorders. Thus, our research provides a theoretical foundation for studying brain functional networks in both CM and VM and offers valuable insights into potential pathways for more targeted therapeutic approaches.
Increasing imaging evidence suggests that VM is a brain network dysfunction disorder involving regions such as the thalamus, dorsal pons, midbrain, temporal cortex, and cerebellum, all of which contribute to the central mechanisms of VM [19, 37]. The VN transmits information from the cochlea and other vestibular organs to the cerebellar cortex [38]. Purkinje cells in the cerebellar cortex integrate this information and relay it to the deep cerebellar nuclei, which, in turn, project to the midbrain and other nuclei, such as the VN and Sp5c [39]. These cells may also connect directly with cerebellar neurons, including Purkinje cells in the nodular lobe, whose axons project directly to the vestibular medial nucleus [40]. Target neurons in the vestibular system are referred to as vestibular target neurons [41]. In this study, we confirmed significant activation of Sp5c, VN, and VbC through whole-brain staining of c-Fos and CGRP and used retrograde tracing with CTB to preliminarily demonstrate the communication relationships among the Sp5c, VN, and VbC nuclei.
Neuron-glia interactions play a key role in pain mechanisms, with microglia acting as important regulators of the brain’s microenvironment during migraine attacks [42]. These interactions may disrupt neurotransmitter balance and influence migraine pathophysiology. Microglia are the primary immune cells in the central nervous system and respond rapidly to changes in the microenvironment and coordinate neuroinflammation and oxidative stress responses. In Sp5c, microglia release proinflammatory cytokines such as IL-6 and TNF-α, increasing vascular permeability [15]. Elevated levels of immunoglobulin E, TNF-α, histamine, and interleukin 1β, along with reduced phagocytosis of polymorphonuclear leukocytes, have been linked to migraine, indicating a complex interaction between immune and nociceptive pathways [43]. Notably, TNF-α may sensitize trigeminal nociceptive afferents, promoting central sensitization and migraine attacks. Calcium signaling is critical for microglial function, with calcium-permeable transient receptor potential (TRP) channels facilitating various physiological responses [44]. Our RNA sequencing data revealed significant differences in TRPV2 ion channel expression in the Sp5c nucleus, and immunofluorescence confirmed that TRPV2-expressing microglia are predominant [45]. Although the TRPV1 channel may play a role in meningeal afferent signaling and headache, the TRPV1 antagonist SB-705,498 has failed to show efficacy in clinical studies on migraine. This casts doubt on the viability of selective TRPV1 antagonists as a treatment strategy [46]. Therefore, we hypothesize that microglia increase calcium influx through TRPV2 channels, enhancing synaptic transmission in VM and CM neural communication, and thus promoting central sensitization and migraine development.
Despite these findings, this study has several limitations. First, the absence of AAV retrograde strategies for precise genetic manipulation within neural circuits limits our ability to test circuit modulation and explore targeted interventions. Second, the lack of electrophysiological validation of TRPV2 ion channel associations restricts our ability to confirm the underlying mechanisms, hindering a deeper understanding of migraine pathophysiology. Third, although a fully validated VM model has not yet been established, our model is considered an initial attempt at a VM model, and we acknowledge that further validation is necessary. Finally, the exclusion of female mice limits the exploration of sex differences in disease progression, which is particularly important for diseases like VM and CM that may exhibit sex differences. These limitations will be addressed in future research, allowing for more comprehensive investigations, and overcoming these challenges.
Conclusion
In conclusion, our behavioral data indicate that recurrent intraperitoneal injections of NTG and kainic acid effectively replicate vestibular dysfunction in VM and pain in CM. Furthermore, using immunofluorescence and CTB methods, we observed activation of the VbC GABAergic-VN glutamatergic-Sp5c astrocytic communication in VM. Through RNA-Seq technology, we elucidated the molecular mechanisms involved in this circuit. Moreover, preliminary findings suggest that the TRPV2 ion channel plays a significant role in microglia during neuron-glia crosstalk in VM and CM models.
Acknowledgements
We wish to thank Dr. Bin Liu (Department of Rehabilitation, Binzhou Medical University Hospital, Binzhou, China) for his help in RNA- Seq experiments.
Abbreviations
- VM
Vestibular Migraine
- CM
Chronic Migraine
- Sp5c
Spinal Trigeminal Nucleus Caudalis
- VN
Vestibular Nuclei
- c-Fos
Cellular Proto-oncogene
- NeuN
Neuronal Nuclei
- S2
Secondary somatosensory cortex
- TG
Trigeminal Ganglion
- NTG
Nitroglycerin
- CTB
Cholera Toxin Subunit B
- TRPV2
Transient Receptor Potential Vanilloid 2
- TSA
Tyramide Signal Amplification
- AP
Anteroposterior
- ML
Mediolateral
- DV
Dorsoventral
- IBA1
Ionized Calcium-Binding Adapter Molecule 1
- GluR2
Glutamate Ionotropic Receptor AMPA Type Subunit 2
- CGRP
Calcitonin Gene-Related Peptide
- TNC
Trigeminocervical Complex
- fMRI
Functional Magnetic Resonance Imaging
- FITC
Fluorescein Isothiocyanate
- KA
Kainic acid
Author contributions
This study was designed by YHP, QLZ and NZ. The experiments were completed by QLZ, NZ and HYL. QLZ and QHC performed statistical analysis and finished writing the manuscript. YHP and QJY provided supervision and final check. All the authors read the final version of this paper and approved it.
Funding
This work was supported by General Program of National Natural Science Foundation of China (82071549, 82371483); Key Program of Natural Science Foundation of Heilongjiang Province (ZD2019H006); and Key Program of Planning Subject for the 13th Five-Year Plan of Heilongjiang Province Education Sciences (GJB1319086).
Data availability
The RNA‐seq data have been deposited to Gene Expression Omnibus (GEO) database with the accession code GSE290448. All data generated in this study are included in this article. Source data are provided with this paper or upon request from the corresponding authors.
Declarations
Ethical approval and consent to participate
All animal experiments performed in this study were approved by the Ethics Committee for Animal Experimentation of First Affiliated Hospital of Harbin Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qingling Zhai and Qihui Chen contributed equally to this work.
References
- 1.Silberstein SD, Migraine (2004) Lancet 363(9406):381–391 [DOI] [PubMed]
- 2.Liu Q, Wu Y, Wang H, Jia F, Xu F (2022) Viral tools for neural circuit tracing. Neurosci Bull 38(12):1508–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva VPR, Castro LHM, Calderaro M (2022) Vestibular migraine. Arq Neuropsiquiatr 80(5 Suppl 1):232–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beh SC (2022) Vestibular migraine. Curr Neurol Neurosci Rep 22(10):601–609 [DOI] [PubMed] [Google Scholar]
- 5.Lempert T, Olesen J, Furman J, Waterston J, Seemungal B, Carey J et al (2013) [Vestibular migraine: diagnostic criteria: consensus document of the Bárány society and the international headache society]. Nervenarzt 84(4):511–516 [DOI] [PubMed] [Google Scholar]
- 6.The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia (2013);33(9):629–808 [DOI] [PubMed]
- 7.Villar-Martinez MD, Goadsby PJ (2024) Vestibular migraine: an update. Curr Opin Neurol 37(3):252–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Chen QH, Lin JH, Zhou C, Pan YH (2020) Research on the relationship between vestibular migraine with/without cognitive impairment and brainstem auditory evoked potential. Front Neurol 11:159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J, Shi S, Chen Q, Pan Y (2020) Differential expression and bioinformatic analysis of the circrna expression in migraine patients. Biomed Res Int 2020:4710780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang N, Pan Y, Chen Q, Zhai Q, Liu N, Huang Y et al (2023) Application of EEG in migraine. Front Hum Neurosci 17:1082317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou C, Zhang L, Jiang X, Shi S, Yu Q, Chen Q et al (2020) A novel diagnostic prediction model for vestibular migraine. Neuropsychiatr Dis Treat 16:1845–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi X, Wei H, Chen Z, Wang J, Qu W, Huang Z et al (2021) Whole-brain monosynaptic inputs and outputs of glutamatergic neurons of the vestibular nuclei complex in mice. Hear Res 401:108159 [DOI] [PubMed] [Google Scholar]
- 13.Cullen KE (2023) Internal models of self-motion: neural computations by the vestibular cerebellum. Trends Neurosci 46(11):986–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun S, Fan Z, Liu X, Wang L, Ge Z (2024) Microglia TREM1-mediated neuroinflammation contributes to central sensitization via the NF-κB pathway in a chronic migraine model. J Headache Pain 25(1):3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen Q, Wang Y, Pan Q, Tian R, Zhang D, Qin G et al (2021) MicroRNA-155-5p promotes neuroinflammation and central sensitization via inhibiting SIRT1 in a nitroglycerin-induced chronic migraine mouse model. J Neuroinflammation 18(1):287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang MC, Nguyen EK, Canto-Bustos M, Papale AE, Oswald AM, Ross SE (2020) Divergent neural pathways emanating from the lateral parabrachial nucleus mediate distinct components of the pain response. Neuron 106(6):927–39e5 [DOI] [PubMed] [Google Scholar]
- 17.Nelson-Maney NP, Bálint L, Beeson AL, Serafin DS, Kistner BM, Douglas ES et al (2024) Meningeal lymphatic CGRP signaling governs pain via cerebrospinal fluid efflux and neuroinflammation in migraine models. J Clin Invest. 134(15) [DOI] [PMC free article] [PubMed]
- 18.Chen H, Tang X, Li J, Hu B, Yang W, Zhan M et al (2022) IL-17 crosses the blood-brain barrier to trigger neuroinflammation: a novel mechanism in nitroglycerin-induced chronic migraine. J Headache Pain 23(1):1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang Y, Wang Y, Zhang X, Qin G, Zhang D et al (2023) Inhibition of glutamatergic trigeminal nucleus caudalis- vestibular nucleus projection neurons attenuates vestibular dysfunction in the chronic-NTG model of migraine. J Headache Pain 24(1):77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Zhang Y, Tian K, Wang Y, Fan X, Pan Q et al (2020) Calcitonin gene-related peptide facilitates sensitization of the vestibular nucleus in a rat model of chronic migraine. J Headache Pain 21(1):72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neill AB, Pan JB, Sullivan JP, Brioni JD (1999) Pharmacological evaluation of an in vivo model of vestibular dysfunction in the rat. Methods Find Exp Clin Pharmacol 21(4):285–289 [DOI] [PubMed] [Google Scholar]
- 22.Gaboyard-Niay S, Travo C, Saleur A, Broussy A, Brugeaud A, Chabbert C (2016) Correlation between afferent rearrangements and behavioral deficits after local excitotoxic insult in the mammalian vestibule: a rat model of vertigo symptoms. Dis Model Mech 9(10):1181–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harriott AM, Chung DY, Uner A, Bozdayi RO, Morais A, Takizawa T et al (2021) Optogenetic spreading depression elicits trigeminal pain and anxiety behavior. Ann Neurol 89(1):99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai Q, Wang K, Zhang D, Chen J, Dong X, Pan Y (2023) Perampanel ameliorates nitroglycerin-induced migraine through Inhibition of the cAMP/PKA/CREB signaling pathway in the trigeminal ganglion in rats. Korean J Pain 36(3):335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A (2014) Characterization of a novel model of chronic migraine. Pain 155(2):269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63 [DOI] [PubMed] [Google Scholar]
- 27.Cohen JM, Bigal ME, Newman LC (2011) Migraine and vestibular symptoms–identifying clinical features that predict vestibular migraine. Headache 51(9):1393–1397 [DOI] [PubMed] [Google Scholar]
- 28.Saldaña-Ruíz S, Soler-Martín C, Llorens J (2012) Role of CYP2E1-mediated metabolism in the acute and vestibular toxicities of nineteen nitriles in the mouse. Toxicol Lett 208(2):125–132 [DOI] [PubMed] [Google Scholar]
- 29.Al Deeb S, Al Moutaery K, Khan HA, Tariq M (2000) Exacerbation of iminodipropionitrile-induced behavioral toxicity, oxidative stress, and vestibular hair cell degeneration by gentamicin in rats. Neurotoxicol Teratol 22(2):213–220 [DOI] [PubMed] [Google Scholar]
- 30.Xie W, Li R, Tang W, Ma Z, Miao S, Li C et al (2023) Proteomics profiling reveals mitochondrial damage in the thalamus in a mouse model of chronic migraine. J Headache Pain 24(1):122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerdes MJ, Sevinsky CJ, Sood A, Adak S, Bello MO, Bordwell A et al (2013) Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc Natl Acad Sci U S A 110(29):11982–11987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Li Y, Zhang W, Ma Z, Xiao S, Xie W et al (2023) Dopaminergic projections from the hypothalamic A11 nucleus to the spinal trigeminal nucleus are involved in bidirectional migraine modulation. Int J Mol Sci. 24(23) [DOI] [PMC free article] [PubMed]
- 33.Montardy Q, Wei M, Liu X, Yi T, Zhou Z, Lai J et al (2021) Selective optogenetic stimulation of glutamatergic, but not gabaergic, vestibular nuclei neurons induces immediate and reversible postural imbalance in mice. Prog Neurobiol 204:102085 [DOI] [PubMed] [Google Scholar]
- 34.Ross SE (2011) Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol 21(6):880–887 [DOI] [PubMed] [Google Scholar]
- 35.Meents JE, Neeb L, Reuter U (2010) TRPV1 in migraine pathophysiology. Trends Mol Med 16(4):153–159 [DOI] [PubMed] [Google Scholar]
- 36.Burch R (2021) Preventive migraine treatment. Continuum (Minneap Minn) 27(3):613–632 [DOI] [PubMed] [Google Scholar]
- 37.Borsook D, Burstein R (2012) The enigma of the dorsolateral Pons as a migraine generator. Cephalalgia 32(11):803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carleton SC, Carpenter MB (1983) Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the Cat and monkey. Brain Res 278(1–2):29–51 [DOI] [PubMed] [Google Scholar]
- 39.Noseda R (2022) Cerebro-Cerebellar networks in migraine symptoms and headache. Front Pain Res (Lausanne) 3:940923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hull C, Regehr WG (2022) The cerebellar cortex. Annu Rev Neurosci 45:151–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng H, Blázquez PM, Dickman JD, Angelaki DE (2014) Diversity of vestibular nuclei neurons targeted by cerebellar nodulus Inhibition. J Physiol 592(1):171–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun M, Rong J, Zhou M, Liu Y, Sun S, Liu L et al (2024) Astrocyte-Microglia crosstalk: A novel target for the treatment of migraine. Aging Dis 15(3):1277–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Latrémolière A, Mauborgne A, Masson J, Bourgoin S, Kayser V, Hamon M et al (2008) Differential implication of Proinflammatory cytokine interleukin-6 in the development of cephalic versus extracephalic neuropathic pain in rats. J Neurosci 28(34):8489–8501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maksoud MJE, Tellios V, An D, Xiang YY, Lu WY (2019) Nitric oxide upregulates microglia phagocytosis and increases transient receptor potential vanilloid type 2 channel expression on the plasma membrane. Glia 67(12):2294–2311 [DOI] [PubMed] [Google Scholar]
- 45.Lana D, Landucci E, Mazzantini C, Magni G, Pellegrini-Giampietro DE, Giovannini MG (2022) The protective effect of CBD in a model of in vitro ischemia May be mediated by agonism on TRPV2 channel and microglia activation. Int J Mol Sci. 23(20) [DOI] [PMC free article] [PubMed]
- 46.Dussor G, Yan J, Xie JY, Ossipov MH, Dodick DW, Porreca F (2014) Targeting TRP channels for novel migraine therapeutics. ACS Chem Neurosci 5(11):1085–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The RNA‐seq data have been deposited to Gene Expression Omnibus (GEO) database with the accession code GSE290448. All data generated in this study are included in this article. Source data are provided with this paper or upon request from the corresponding authors.