Abstract
Elevation of the intracellular cAMP level induces morphological changes of astrocyte-like differentiation in C6 glioma cells. Such changes may be accompanied with expression of cytoskeletal protein genes. We therefore analyzed morphological changes after a treatment with dibutyryl cAMP (dbcAMP) and then assessed the expression of cytoskeletal protein genes by a quantitative real-time polymerase chain reaction. The cell number remained unaltered upon incubation with 1 mM dbcAMP in medium supplemented with 0.1% fetal bovine serum (FBS), whereas the number and lengths of processes increased, when compared with those of cells incubated in medium supplemented with 0.1% or 10% FBS only. The amounts of β-actin, γ-actin, and β-tubulin mRNAs in C6 cells, but not α-tubulin mRNA, increased during the early proliferation in DMEM containing 10% FBS. The expression of cytoskeletal protein genes decreased when incubated with 0.1% FBS or 1 mM dbcAMP in 0.1% FBS, compared with those of cells cultured in 10% FBS. These results indicated that, during the early proliferation in normal culture condition, the expression of cytoskeletal protein genes in C6 cells, except α-tubulin, increased, while in differentiating or differentiated C6 glioma cells, cAMP-induced morphological changes were not accompanied with elevation of gene expression for cytoskeletal proteins, such as actin and tubulin.
Keywords: Actin, cAMP, Cytoskeleton, C6 glioma cell, Differentiation, Gene expression, Morphology, Tubulin
Introduction
Astrocytes are the most abundant cell components in the mammalian CNS and have various supporting functions. It is therefore important to realize regulatory mechanisms of astrocyte differentiation, which occurs largely during the postnatal period (Qian et al. 2000). The timing of differentiation is regulated by various extracellular cues and cell-intrinsic programs (Edlund and Jessell 1999). One important cue is cAMP-elevating stimulus (Vallejo and Vallejo 2002).
Cell morphology is principally defined by cytoskeletons, such as microfilaments, microtubules, and intermediate filaments during differentiation. Astrocytes lost their microfilament bundles when treated with cAMP or cAMP-elevating agents (Goldman and Chiu 1984), whereas the microtubule-depolymerizing agent, colchicines, prevented cAMP-induced morphological changes (Goetschy et al. 1986). The formation of mature astrocytes with long processes also involves microfilaments and microtubules, although the control of actin gene expression was a complex process (Faivre-Sarrailh et al. 1990; Poddar et al. 1996). Microtubules are also involved in the neurite outgrowth in cultured neurons (Wang and Kimberly 1998). However, little is known about expression of genes encoding cytoskeletal proteins, particularly in relation to changes in cell morphology during differentiation. Such information is therefore valuable to understand molecular and cellular bases of cAMP-induced astrocytic differentiation.
In the present study, we attempted to clarify the above-mentioned problem using rat origin C6 glioma cells. They were morphologically differentiated by elevation of intracellular cAMP (Willingham 1976) and were often used as a model system for studies of differentiation of astrocytes (Benda et al. 1968; Yoshimura et al. 1997). We therefore used C6 glioma cells to analyze changes in morphology and estimate expression of cytoskeletal protein genes after a dibutyryl cAMP (dbcAMP) treatment. Gene expression was assessed by the amounts of mRNAs for actin and tubulin subunits determined by a quantitative real-time polymerase chain reaction (PCR).
Materials and Methods
Condition of Cell Culture
A clonal cell line of rat C6 glioma cells (Benda et al. 1968), kindly provided by Dr. Ichiro Matsuoka, Hokkaido University, was used in the present study. Cells were subcultured at a density of 5 × 104 cells/ml in DMEM supplemented with 10% fetal bovine serum (FBS; Trace Biosciences PTY Ltd., New South Wales) for two days. The third day was designated as day 0, and the effects of culture conditions were preliminarily tested to confirm they were appropriate for differentiation of cells in terms of concentration of dbcAMP, concentration of FBS and cell density on day 2. Then cells were observed under a light microscope, and images were recorded with a Nikon Coolpix 990 digital camera (Tokyo).
Cell Culture for dbcAMP-induced Differentiation
On the basis of above preliminary experiments, cells were first subcultured at the concentration of 1 × 104 cells/ml in DMEM supplemented with 10% FBS for two days. On the third day, designated as day 0, culture media were changed with DMEM containing 10, 0.1% FBS, or 1 mM cAMP in 0.1% FBS. The effects of dbcAMP were observed for 3 days in morphological experiments and for four days in estimation of gene expression. Cells were fixed for staining or harvested for real-time PCRs every day during experimental periods. For real-time PCRs, cells were centrifuged at 6,000 rpm for 10 min and stored at −80°C until assayed. The experiments were repeated in triplicate or quadruplicate.
Actin Staining and Immunocytochemistry
Cells were fixed with fresh 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 for 15 min, and treated with 2% goat serum for 30 min. They were incubated with mouse anti-β tubulin (Sternberger Monoclonals, Baltimore, MD, USA; 1:500) at 4°C overnight. Then, they were simultaneously incubated with Alexa Flour 488 labeled anti-mouse IgG (Molecular Probes, Eugene, OR, USA; 1:200) and rhodamine–phalloidin, which binds to the F-actin (Molecular Probes, Eugene, OR, USA; 1:50) for 30 min at room temperature. The preparations were mounted and examined under a Nikon Microphot-FXA microscope. Images were recorded with the Coolpix Nikon camera and analyzed with Image J software.
Specificity of antiserum was confirmed by several control experiments. Because of difficulty in obtaining tubulins commercially, cells were treated with a microtubule-disrupting drug, nocodazole, at 10 μM for 2 h. The green immunofluorescence signal for the presence of microtubules was almost eliminated. In addition, no staining was observed when only the secondary antibody was applied to the cells without prior labeling with the primary antibody.
Analysis of Cell Morphology
The number of cells was counted from 6–9 micrographs and expressed as the number per square millimeter. The lengths of processes were defined as the extension from the point where the process emerged from the cell body to the tip of the major process. The number and lengths of processes were measured from 25–30 cells, except the minor processes which were shorter than the radius.
Confirmation of Nucleotide Sequences
The nucleotide sequences of target cytoskeletal protein subunits were confirmed by cloning and sequence analyses of cDNAs from C6 glioma. Total RNA was extracted from harvested cells by the guanidium thiocyanate hot phenol-chloroform method (Chirgwin et al. 1979), reverse transcribed, and subjected to sequence analyses by the dideoxy chain-termination method (Sanger et al. 1977) using an SQ-5500 DNA sequencer (Hitachi, Tokyo, Japan).
Total RNA Preparation and Quantitative Real-time PCR Assay
Total RNA was extracted individually from C6 glioma cells in each experimental group by the guanidium thiocyanate hot phenol–chloroform method and was treated with 1 U deoxyribonuclease I (TaKaRa Biochemicals, Shiga, Japan). Then it was recovered by a phenol chloroform–isoamyl alcohol extraction, chloroform extraction, and ethanol precipitation. The concentrations of total RNA were determined by measurement of optical density at 260/280 nm, and its quantity and also integrity were verified by gel electrophoresis. Total RNA of C6 glioma cells was reverse transcribed to obtain cDNA.
Real-time PCR was carried out with an ABI Prism 7700 Sequence Detection System (PE Applied Biosystems). Nucleotide sequences of primers and probes are shown in Table 1. The PCR mixture contained 1× TaqMan Buffer A (PE Applied Biosystems), 25 mM MgCl2, 2.5 mM dNTP, 100 nM each forward and reverse primers (Table 1), 100 nM of TaqMan probe (Table 1), and 0.25 U of AmpliTaq Gold DNA polymerase. Amplification profile was 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 58°C for 45 s for γ-actin, β-tubulin, and α-tubulin, except 59°C for 45 s for β-actin. To determine absolute amounts of mRNA, standard-sense RNA was in vitro synthesized by a MAXIscript (Ambion, Texas) using the plasmid DNAs containing cDNA insert. The synthesized RNA was quantified and used as the standard-sense RNA. Serially diluted standard RNAs (2 × 103–2 × 109 copies) were reverse transcribed with the same reverse primer used for cloning in 10 μl of reaction mixture to prepare the first-strand cDNA, diluted to 1:100, and used as the standards.
Table 1.
Sequences of primers and TaqMan probes used in the present study
| Name | Sequence |
|---|---|
| β-actin forward primer | 5′-CGGTCAGGTCATCACTATCGG-3′ |
| β-actin reverse primer | 5′-CACAGGATTCCATACCCAGGA-3′ |
| β-actin TaqMan probe | 5′FAM-AATGAGCGGTTCCGATGCCC-TAMRA3′ |
| γ-actin forward primer | 5′-CTGCTGCATGAGCTGATTCTG-3′ |
| γ-actin reverse primer | 5′-CAAAGGCTTATTCCAGTTTCATGAG-3′ |
| γ-actin TaqMan probe | 5′FAM-CCCTGGCAAATGTACACACCTCATGCTAG-TAMRA3′ |
| α-tubulin forward primer | 5′-CTCGCATCCACTTCCCTCTG-3′ |
| α-tubulin reverse primer | 5′-GCTGTTCATGGTAGGCTTTCTC-3′ |
| α-tubulin TaqMan probe | 5′FAM-CCACTTATGCCCCTGTCATCTCTGC-TAMRA3′ |
| β-tubulin forward primer | 5′-TTCCATTGACAATGAGGCTCTGT-3′ |
| β-tubulin reverse primer | 5′-TGGTTGAGATCGCCATAGGTG-3′ |
| β-tubulin TaqMan probe | 5′FAM-TGACATCTGCTTCCGCACCCTGAA-TAMRA3′ |
In the assay, serially diluted copies of standard cDNA (β-actin, α-tubulin, and β-tubulin: 4 × 101–4 × 107 copies; γ-actin: 4 × 100–4 × 106) were applied in triplicate, and sample cDNAs prepared from total RNA were applied in duplicate.
Assessment of Assay Method
The standard curves were linear within the ranges of 4 × 101–4 × 107 copies of β-actin, α-tubulin, and β-tubulin (r 2 = 0.990, 0.992 and 0.995, respectively) and 4 × 100–4 × 106 of γ-actin (r 2 = 0.999) (Fig. 1). The primer sets for β-actin, γ-actin, α-tubulin, and β-tubulin amplified specific fragments of 87, 89, 69, and 81 bp (Fig. 1, inlets), while no fragments were amplified from negative controls without Multiscribe Reverse Transcriptase. C6 cDNAs synthesized from serially diluted C6 total RNA were checked for parallelism to the linear range of the standard curve. The curves obtained from the sample cDNA were parallel to the standard curves (Fig. 1).
Fig. 1.
Standard curves for quantitative real-time PCR for actin and tubulin mRNA. Reverse transcribed standard RNA (filled circle) or C6 total RNA (unfilled circle) was amplified. Specificities of primers were confirmed by use of standard cDNAs encoding actin or tubulin as templates (inset pictures)
In each assay, a standard sample (C6 glioma cell cDNA) was applied in triplicate to estimate coefficients of variation (CV) within and between runs. The intra-assay CV was 0.13–10.39% for β-actin assay, 0.07–0.90% for γ-actin assay, 0.03–6.21% for α-tubulin assay, and 0.10–1.20% for β-tubulin assay. The inter-assay CV was 4.27% for β-actin assay, 3.59% for γ-actin assay, 12.1% for α-tubulin assay, and 4.34% for β-tubulin assay. The amounts of mRNA were calculated as copies per ng total RNA.
Statistical Analysis
The results on parameters of cell morphology and the amounts of mRNA were expressed as the means ± SEM. Differences between the groups were tested by one-way analysis of variance (ANOVA), followed by LSD or Dunnett’s T3 (where equal variances are not assumed) post hoc test. P < 0.05 was considered statistically significant.
Results
The Appropriate Condition for Differentiation of C6 Glioma Cells
The conditions of dbcAMP and FBS and the density of cells to induce differentiation of C6 glioma cells were similar to our previous results (Hu et al. in preparation) and also to those seen in primary cultures of rat cerebellar astrocytes (Sato et al. 1975). We therefore subcultured C6 glioma cells at 1 × 104 cells/ml in DMEM supplemented with 10% FBS and then replaced the media to those supplemented with 0.1% FBS or those containing 1 mM dbcAMP in addition to 0.1% FBS on day 0 to induce cell differentiation.
Morphological Change of C6 Glioma Cells During Differentiation
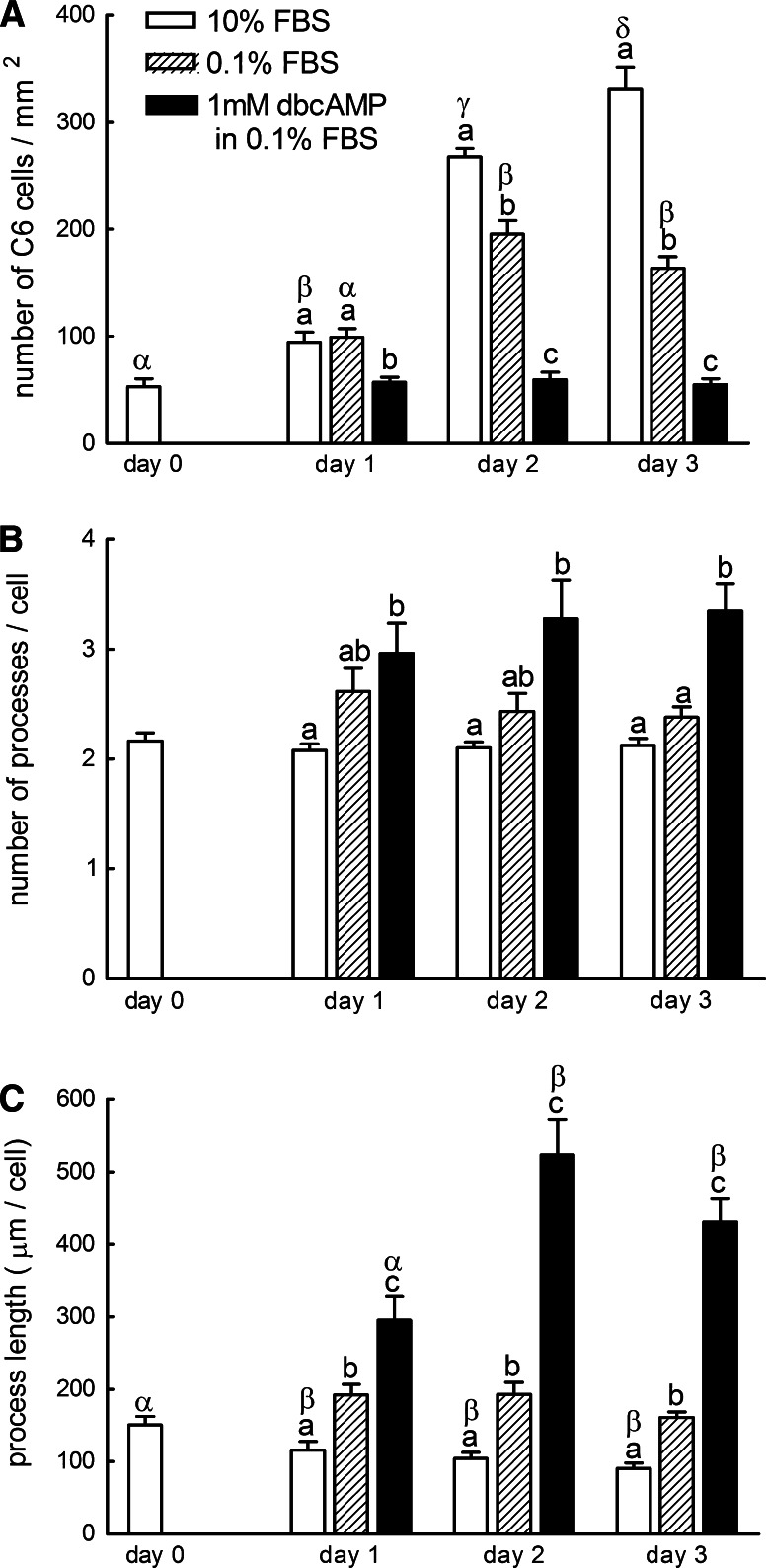
When C6 cells were subcultured in the DMEM supplemented with 10% FBS, they showed proliferation with a typical sigmoid growth curve which peaked out around day 3 (Figs. 2, 3a). The proliferation rate of C6 cells was highest between day 1 and 2. When treated with DMEM supplemented with 0.1% FBS, the number of cells also increased in a sigmoid manner, however, with lower proliferation rate. C6 glioma cells treated with dbcAMP did not show noticeable proliferation throughout the experimental period.
Fig. 2.
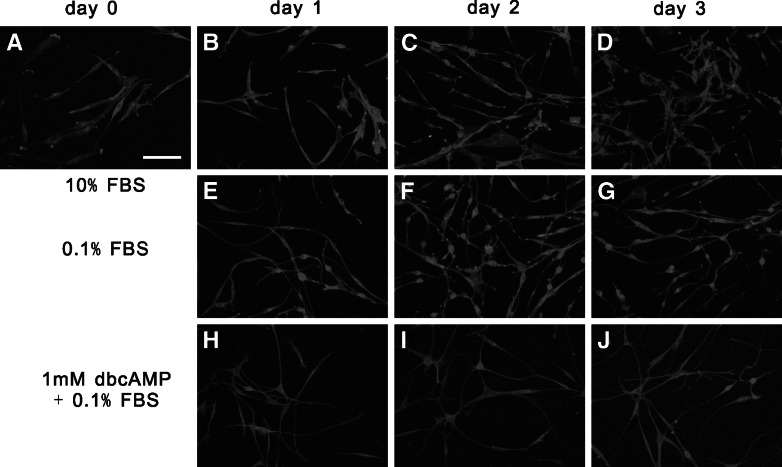
Morphological changes of C6 glioma cells during a treatment with dbcAMP. (a) C6 glioma cells on day 0; (b, e, h) C6 glioma cells on day 1; (c, f, i) C6 glioma cells on day 2; (d, g, j) C6 glioma cells on day 3. Cells were cultured in 10%FBS (a–d), 0.1% FBS (e–g), or 1mM cAMP + 0.1% FBS (h–j), respectively. C6 glioma cells were observed under rhodamine–phalloidin staining of F-actin. Bar = 100 μm
Fig. 3.
Changes in the morphological aspects of C6 glioma cells. The numbers of cells (a) and processes (b) were counted in 10, 0.1% FBS, and 1mM cAMP + 0.1% FBS groups from day 0 to day 3. The length of processes per cell (c) was measured too. The number of cells was counted from 6–9 micrographs from three independent experiments. The number and lengths of processes were measured from 25 to 30 cells from three independent experiments. Column with letters a–c is statistically different from each group on the same day (P < 0.05). Columns with letters α-δ are statistically different from each other under the same treatment (P < 0.05)
Most cells cultured in 10% FBS showed bipolar appearance, whereas those cultured in the presence of dbcAMP exhibited a more differentiated astrocytic morphology (Fig. 2). They had more numerous neurite-like thin processes (Figs. 2, 3b) that were longer than those of cells subcultured in 10% or 0.1% FBS which did not contain dbcAMP (Fig. 3c). The increase in the number of processes might be initiated within 24 h after the changes of medium to that containing 1 mM dbcAMP, and then the lengths of processes rapidly increased to attain 5-fold those of C6 cells cultured in media, which did not contain dbcAMP. The lengths of processes of cells incubated in 0.1% FBS were significantly longer than those of cells in 10% FBS, although the increase in the number of processes was not significant.
F-actin visualized with rhodamine–phalloidin was distributed at the periphery of the cell body and the processes when cultured in DMEM containing 10% FBS (high resolution figure not shown). Immunofluorescent staining of β-tubulin showed that microtubules appeared to emanate from a concentrated site near the center of cell. The cell processes contained rather more compact bundles of microtubules. However, a treatment with 0.1% FBS or 1mM cAMP in 0.1% FBS did not induce noticeable changes in the distributions of F-actin and microtubules.
Change in the Amounts of mRNAs for Cytoskeletal Subunits During Differentiation
The expression of cytoskeletal protein genes during differentiation is shown in Fig.4. Although differences in the copy numbers were large among mRNAs, we obtained similar values among different experimental runs, and further intra- and inter-assay CVs of the present real-time PCR were similar to those of conventional immunoassays.
Fig. 4.
Changes in the amounts of β-actin, γ-actin, α-tubulin, and β-tubulin mRNAs in C6 glioma cells during a treatment with dbcAMP. The amounts of β-actin (a), γ-actin (b), α-tubulin (c), and β-tubulin (d) mRNA were determined by real-time PCR (n = 3–4). Columns with letters a–c are statistically different from each other on the same day (P < 0.05). Columns with letters α–γ are statistically different from each other under the same treatment (P < 0.05)
During a 4-day experimental period, the amounts of cytoskeletal mRNAs in C6 cells, except α-tubulin mRNA, increased in DMEM containing 10% FBS during the early proliferation, especially at the period of high proliferation from day 1 to 2. The amounts of β-actin or β-tubulin mRNAs increased 2 or 3 times separately, from day 0 to 2. When C6 cells were cultured in 0.1% FBS, the amounts of most mRNAs for cytoskeletal proteins decreased significantly compared with those of cells cultured in 10% FBS. β-actin and β-tubulin mRNAs decreased until day 2, while γ-actin and α-tubulin mRNAs decreased earlier from day 1 (Fig. 4). When incubated with 1mM dbcAMP in 0.1% FBS, the amounts of mRNAs encoding subunits of cytoskeleton proteins decreased from day 1, except β-tubulin mRNA which decreased from day 2. Only the amounts of β-actin mRNA in dbcAMP were consistently lower than those in 0.1% FBS.
Discussion
In the present study, we tried to clarify a relationship between cAMP-induced morphological changes and expression of genes encoding cytoskeletal proteins using C6 glioma cells. The cell number remained unaltered upon incubation with 1 mM dbcAMP in medium supplemented with 0.1% serum, whereas the number and lengths of processes increased, when compared with those of cells incubated in medium supplemented with 0.1% or 10% FBS only. The organization of microfilaments and microtubules did not show obvious differences among treatments. The amounts of cytoskeletal protein mRNAs in C6 cells, except α-tubulin mRNA, increased during the early proliferation in normal culture condition. Moreover, the amounts of mRNAs for β-actin, γ-actin, α-tubulin, and β-tubulin decreased when incubated with 0.1% FBS or 1 mM dbcAMP in 0.1% FBS, compared with those of cells in 10% FBS. These results indicated that, in differentiating or differentiated C6 glioma cells, cAMP-induced morphological changes were not accompanied with elevation of gene expression for cytoskeletal proteins, such as actin and tubulin.
The condition of culture in the present study was considered to be the best or near the best to induce differentiation of the present line of astrocytic C6 cells. Our present results strongly support that subculture of cells at 1 × 104 cells/ml and then culture in DMEM containing 1 mM dbcAMP and 0.1% FBS are preferable to induce differentiation of C6 glioma cells, as described in the previous report. Application of 1 mM dbcAMP and serum-free environment both can induce morphological differentiation (Zimmer and Van Eldik 1989). During dbcAMP-induced differentiation, C6 glioma cells exhibited obvious elaborated morphology with marked increases in the number and lengths of processes. These phenomena rapidly proceeded during the first two days. Similar phenomena had been seen during differentiation of C6 glioma cells which developed long processes, and their morphology resembled those of mature astrocytes (Zimmer and Van Eldik 1989). It was also coincident that, due to differentiation, cell proliferation stopped, while a culture in the medium with normal serum level prominently increased the number of cells (Tabuchi et al. 1981). Despite these characteristics to differentiation, expression of genes encoding actin and tubulin subunits seemed to be suppressed during differentiation in this experiment.
Among three major isotypes of actin filaments (α, β and γ), only two (β and γ) which control the cell shape are expressed in the brain at a ratio of about 3:1 (Otey et al. 1987). Microtubules, formed from α and β subunits of tubulin, determine the transport of organelles and cell movement. Morphological changes require newly synthesized or dynamically rearranged cytoskeletal filaments (Alberts et al. 2002). It has been reported that the initial outgrowth of neurite was accompanied with the increase of actin and tubulin genes expression (Mizobuchi et al. 1990; Knoops and Octave 1997). β-actin mRNA has been reported to increase during earlier development to epithelioid morphology, while decrease when astrocytes undergo final transformation into the mature form (Paul et al. 1996). Our present results may include discrepancies, which may contain complex relationship between morphological changes and gene expression. In the developing rat brain, the rates of transcription of β- and γ-actin mRNA at different ages also lacked a correlation with their corresponding steady-state levels (Sarkar et al. 1997). One possible reason is a presence of complex post-transcriptional modulatory mechanisms during differentiation, although the protein synthesis of the cytoskeletal proteins during differentiation needs to be evaluated in further experiment. Otherwise cAMP-induced differentiation may be more related to the reorganization of cytoskeletal protein, although the obvious changes were not observed in the present experiments, which is probably because rearrangements occur more dynamically during the earlier phase of differentiation.
In addition, we are interested in finding that cytoskeletal protein mRNAs, except α-tubulin mRNAs, increased during the early proliferation when cultured in normal culture condition that contains 10% FBS, especially at the period of high proliferation from day 1 to 2. The synthesis of cytoskeleton protein is controlled mainly at the level of mRNA stability in response to changes of the ratio of polymerized/unpolymerized protein (Alberts et al. 2002; Cleveland 1989). These phenomena may be due to the decrease of unpolymerized protein pool because of abundant new cytoskeleton formation during cell proliferation, whereas the unpolymerized protein pool of α-tubulin may be larger than those of other subunits. These results also remind investigators to be cautious to apply cytoskeleton protein mRNA as internal control in the experiments about gene expression of cells, which are during high proliferation.
In conclusion, the expression of cytoskeletal protein genes in C6 cells, except α-tubulin, increased during the early proliferation in normal culture condition. In differentiating or differentiated C6 glioma cells, cAMP-induced morphological changes were not accompanied with elevation of gene expression for cytoskeletal proteins, such as actin and tubulin.
Acknowledgment
The present study was partially supported by the 21st Century Center of Excellence Program for Advanced Life Science on the Base of Bioscience and Nanotechnology.
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell, 4th edn. Garland Science, New York [Google Scholar]
- Benda P, Lightbody J, Sato G, Levine L, Sweet W (1968) Differentiated rat glial cell strain in tissue culture. Science 161:370–371 [DOI] [PubMed] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ (1979) Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18:5294–5299 [DOI] [PubMed] [Google Scholar]
- Cleveland DW (1989) Autoregulated control of tubulin synthesis in animal cells. Curr Opin Cell Biol 1:10–14 [DOI] [PubMed] [Google Scholar]
- Edlund T, Jessell TM (1999) Progression from extrinsic to intrinsic signaling in cell fate specification: a view from the nervous system. Cell 96:211–224 [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Ferraz C, Liautard JP, Rabie A (1990) Effect of thyroid deficiency on actin mRNA content in the developing rat cerebellum. Int J Dev Neurosci 8:99–106 [DOI] [PubMed] [Google Scholar]
- Goetschy JF, Ulrich G, Aunis D, Ciesielski-Treska J (1986) The organization and solubility properties of intermediate filaments and microtubules of cortical astrocytes in culture. J Neurocytol 15:375–387 [DOI] [PubMed] [Google Scholar]
- Goldman JE, Chiu FC (1984) Dibutyryl cyclic AMP causes intermediate filament accumulation and actin reorganization in astrocytes. Brain Res 306:85–95 [DOI] [PubMed] [Google Scholar]
- Knoops B, Octave JN (1997) Alpha 1-tubulin mRNA level is increased during neurite outgrowth of NG 108–15 cells but not during neurite outgrowth inhibition by CNS myelin. Neuroreport 10:795–798 [DOI] [PubMed] [Google Scholar]
- Mizobuchi T, Yagi Y, Mizuno A (1990) Changes in alpha-tubulin and actin gene expression during optic nerve regeneration in frog retina. J Neurochem 55:54–59 [DOI] [PubMed] [Google Scholar]
- Otey CA, Kalnoski MH, Bulinski JC (1987) Identification and quantification of actin isoforms in vertebrate cells and tissues. J Cell Biochem 13:113–124 [DOI] [PubMed] [Google Scholar]
- Paul S, Das S, Poddar R, Sarkar PK (1996) Role of thyroid hormone in the morphological differentiation and maturation of astrocytes: temporal correlation with synthesis and organization of actin. Eur J Neurosci 8:2361–2370 [DOI] [PubMed] [Google Scholar]
- Poddar R, Paul S, Chaudhury S, Sarkar PK (1996) Regulation of actin and tubulin gene expression by thyroid hormone during rat brain development. Brain Res Mol Brain Res 35:111–118 [DOI] [PubMed] [Google Scholar]
- Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, Temple S (2000) Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 28:69–80 [DOI] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Chaudhury S, Sarkar PK (1997) Regulation of beta- and gamma-actin mRNA by thyroid hormone in the developing rat brain. Neuroreport 8:1267–1271 [DOI] [PubMed] [Google Scholar]
- Sato S, Sugimura T, Yoda K, Fujimura S (1975) Morphological differentiation of cultured mouse glioblastoma cells induced by dibutyryl cyclic adenosine monophosphate. Cancer Res 35:2494–2499 [PubMed] [Google Scholar]
- Tabuchi K, Furuta T, Norikane H, Tsuboi M, Moriya Y, Nishimoto A (1981) Evaluation of the drug-induced morphological differentiation of rat glioma cells (C-6) from the aspects of S-100 protein level and con A binding pattern. J Neurol Sci 51:119–130 [DOI] [PubMed] [Google Scholar]
- Vallejo I, Vallejo M (2002) Pituitary adenylate cyclase-activating polypeptide induces astrocyte differentiation of precursor cells from developing cerebral cortex. Mol Cell Neurosci 21:671–683 [DOI] [PubMed] [Google Scholar]
- Wang W, Kimberly ED (1998) Quantitative analysis of mRNA expression of neuron-specific growth-associated genes in rat primary neurons by competitive RT-PCR. Brain Res Protoc 2:199–208 [DOI] [PubMed] [Google Scholar]
- Willingham MC (1976) Cyclic AMP and cell behavior in cultured cells. Int Rev Cytol 44:319–363 [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Sakai H, Nakashima S, Nozawa Y, Shinoda J, Sakai N, Yamada H (1997) Differential expression of Rho family GTP-binding proteins and protein kinase C isozymes during C6 glial cell differentiation. Brain Res Mol Brain Res 45:90–98 [DOI] [PubMed] [Google Scholar]
- Zimmer DB, Van Eldik LJ (1989) Analysis of the calcium-modulated proteins, S100 and calmodulin, and their target proteins during C6 glioma cell differentiation. J Cell Biol 108:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]