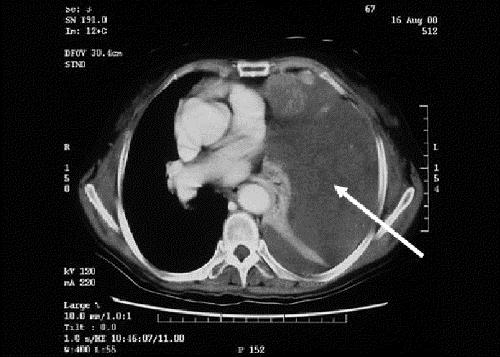
THE CASE: A 67-year-old woman was brought to hospital by her husband, who had found her sweaty and unresponsive in the morning. She was hypoglycemic, with a plasma glucose level of 1.2 mmol/L (normal range 3.8–6.1 mmol/L). After receiving dextrose intravenously, she regained consciousness and her plasma glucose level increased to 3.8 mmol/L. Apart from a recent dry cough, she was otherwise well and taking no medications. She was a known carrier of hepatitis B virus. A physical examination was notable for reduced air entry and percussion dullness over the left side of her chest. The results of initial investigations, including electrolyte levels, liver function tests and complete blood count, were all normal. She was admitted to hospital, where she experienced 2 further episodes of spontaneous hypoglycemia; serum insulin levels were undetectable on both occasions. A short Synacthen test (whereby a plasma cortisol level is checked 30 minutes after tetracosactrin is given) showed an adequate rise in cortisol concentration, which ruled out a hypoadrenal state. Chest radiography showed a large opacity over the left hemithorax (Fig. 1). A CT scan confirmed a large solid mass in the left pleural space causing compressive collapse of the left lung, but the patient's pancreas appeared normal (Fig. 2, white arrow). A needle biopsy of the pleural mass suggested a fibrous tumour, and the specimen stained positively for CD34, raising the suspicion of non-islet cell tumour hypoglycemia secondary to a pleural fibroma. Her insulin-like growth factor (IGF)-I and total IGF-II levels were normal at 10.6 (normal range 5.0–22.5) nmol/L and 111.9 (normal range 55–150) nmol/L respectively. An elevated level of “big” IGF-II (75 [normal < 14] nmol/L) and elevated IGF-II/IGF-I ratio (11.3 [normal < 10]) confirmed the diagnosis.
Figure 1.
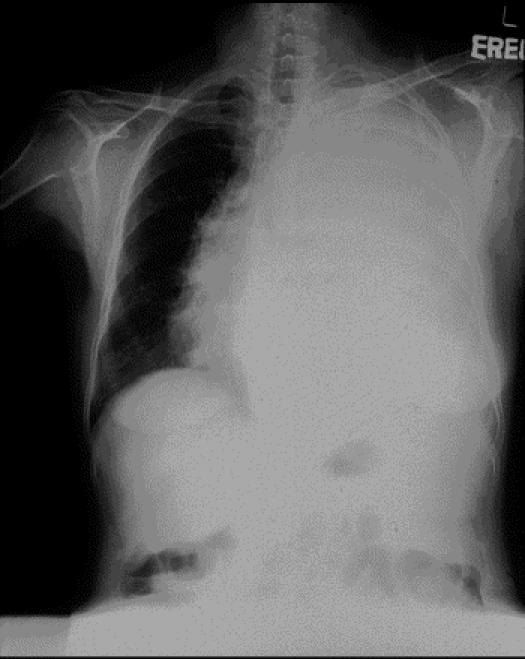
Figure 2.
The patient declined surgical resection, and long-term steroid treatment was felt to be contraindicated in view of her hepatitis B carrier status. A 2-week trial of the somatostatin analogue octreotide resulted in a slight reduction in the frequency of hypoglycemic episodes but no improvement in her biochemical profile, with “big” IGF-II levels elevated at 100 nmol/L, IGF-II/IGF-I ratio at 16.3 and plasma glucose at 6.9 mmol/L, and insulin levels suppressed to less than 10 pmol/L and C peptide levels to less than 94 pmol/L. She agreed to undergo surgery for removal of the mass. The 2285-g encapsulated tumour was confirmed histologically to be a pleural fibroma. After surgery her biochemical IGF levels returned to normal, and, after 8 days of unremarkable recovery, the patient was discharged. She has had no further hypoglycemic episodes 5 years later.
Recurrent hypoglycemia has several causes (Box 1). In the case of non-islet cell tumours, recurrent hypoglycemia may occasionally be the initial clinical presentation, but the tumour is usually clinically apparent by the time hypoglycemia develops.
Box 1.

To appreciate the pathophysiology of recurrent hypoglycemia in patients with non-islet cell tumours, an understanding of insulin-like growth factor (IGF) biochemistry is useful. Both IGF- I and IGF-II are structurally similar to insulin, sharing about 50% of their amino acid residues. Insulin is made only by pancreatic β-cells, whereas IGF-I is made primarily in the liver and IGF-II is produced by many different tissues. Insulin, IGF-I and IGF-II each have their own receptors, and they bind to each others' receptors with varying affinity. IGF-I, a 70-amino acid protein, has roles in skeletal and cartilage development. It exists at about half the circulating plasma levels of IGF-II, a 67-amino acid compound whose exact physiologic role is not well defined. So-called “big” IGF-II, a higher-molecular-weight form of IGF-II, represents only 10%–15% of the total circulating IGF in normal human serum. In the normal state, 70%–80% of circulating IGF is bound to insulin-like growth factor binding protein-3 (IGFBP-3) and a protein called acid labile subunit to form an inactive 150-kD complex. The sequestration of IGF forms in these complexes prevents them from causing hypoglycemia.
Some tumours may over-produce “big” IGF-II. The initiating event appears to involve sudden overexpression of the prepro-IGF-II gene by tumour cells. Pro-IGF-II from tumour cells fails to undergo the usual glycosylation process and consequently is only partly processed, resulting in “big” IGF-II. In patients harbouring such non-islet cell tumours, “big” IGF-II represents about 77% of the total IGF. “Big” IGF-II does not bind well to IGFBP-3, so there are fewer IGF–IGFBP complexes available to associate with acid labile subunit to form the normal 150-kD complex. Instead, “big” IGF-II forms a smaller 50-kD complex, which leaves more IGF-II available. This in turn leads to hypoglycemia and the biochemical abnormalities observed among these patients (Fig. 3).1
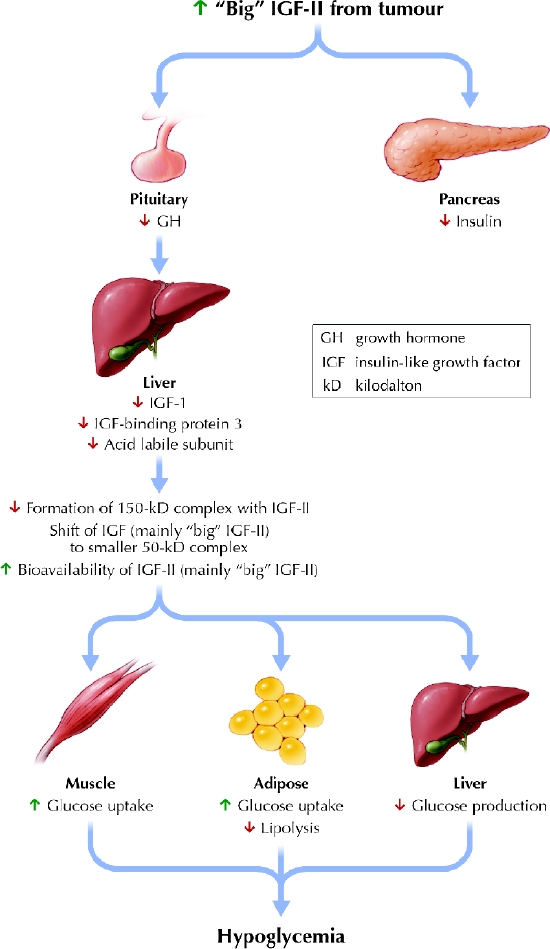
Fig. 3: Mechanism of non-islet cell tumour hypoglycemia. Photo: Lianne Friesen and Nicholas Woolridge
The diagnosis of non-islet cell tumour hypoglycemia can be confirmed by a combination of suppressed serum insulin levels and suppressed C peptide and growth hormone concentrations in the setting of hypoglycemia, along with elevated “big” IGF-II levels. A total IGF-II/IGF-I ratio of greater than 10 is felt to be diagnostic.2A description of how “big” IGF-II levels are measured is included in an online appendix (www.cmaj.ca/cgi/content/full/173/359/DC1).
Glucagon increases hepatic glucose production and can be used for the short-term management of non-islet cell tumour hypoglycemia. Long-term treatment requires a continuous intravenous infusion. Growth hormone increases the formation of IGF-II–IGFBP complexes3 but often requires supraphysiological doses, which may lead to volume overload. In view of the large tumour mass present, which was causing significant mediastinal shift, growth hormone was felt to be relatively contraindicated in our patient. Somatostatin receptors are present in a variety of neuroendocrine tumours, and the use of somatostatin analogues can result in reduced secretion by these tumours. Somatostatin receptors have previously been demonstrated in a pleural fibroma causing non-islet cell tumour hypoglycemia, and this provided the rationale for octreotide therapy for our patient. Octreotide may have failed to suppress “big” IGF-II production because somatostatin receptors on the tumour were nonfunctional.4 Glucocorticoids can suppress tumour secretion of IGF-II, thus reversing the hypoglycemia.4 The treatment of choice is tumour resection, which leads to normal glucose concentrations and biochemical marker levels.
Ronald C.W. Ma Peter C.Y. Tong Juliana C.N. Chan Clive S. Cockram Department of Medicine and Therapeutics Michael H.M. Chan Department of Chemical Pathology Chinese University of Hong Kong Prince of Wales Hospital Shatin, Hong Kong
Supplementary Material
Footnotes
This article has been peer reviewed.
References
- 1.Zapf J, Futo E, Peter M, Froesch ER. Can “big” insulin-like growth factor II in serum of tumor patients account for the development of extrapancreatic tumor hypoglycemia? J Clin Invest 1992;90:2574-84. [DOI] [PMC free article] [PubMed]
- 2.Teale JD, Marks V. Inappropriately elevated plasma insulin-like growth factor II in relation to suppressed insulin-like growth factor I in the diagnosis of non-islet cell tumour hypoglycaemia. Clin Endocrinol (Oxf) 1990;33:87-98. [DOI] [PubMed]
- 3.Baxter RC, Holman SR, Corbould A, Stranks S, Ho PJ, Braund W. Regulation of the insulin-like growth factors and their binding proteins by glucocorticoid and growth hormone in nonislet cell tumor hypoglycemia. J Clin Endocrinol Metab 1995;80:2700-8. [DOI] [PubMed]
- 4.Perros P, Simpson J, Innes JA, Teale JD, McKnight JA. Non-islet cell tumour-associated hypoglycaemia: 111 In-octreotide imaging and efficacy of octreotide, growth hormone and glucocorticosteroids. Clin Endocrinol (Oxf) 1996;44:727-31. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


