Abstract
Summary
Subsequent fracture rates and associated mortality were compared before and after the introduction of fracture liaison service (FLS). In 100,198 women and men, FLS was associated with 13% and 10% lower risk of subsequent fragility fractures and 18% and 15% lower mortality. The study suggests that FLS may prevent fractures.
Purpose
Efficient fracture prevention strategies are warranted to control the global fracture burden. We investigated the effect of a standardized fracture liaison service (FLS) intervention on subsequent fracture risk and mortality.
Methods
The NoFRACT study was designed as a multicenter, pragmatic, register-supported, stepped-wedge cluster-randomized trial. The FLS intervention was introduced in three clusters with 4-month intervals starting May 2015 through December 2018 and included evaluation of osteoporosis and treatment in patients over 50 years with a low-energy fracture. Based on data from the Norwegian Patient Registry, patients with index fractures were assigned to the control period (2011–2015) or intervention period (2015–2018) depending on the time of fracture. Rates of subsequent fragility fractures (distal forearm, proximal humerus, or hip) and all-cause mortality were calculated.
Results
A total of 100,198 patients (mean age 69.6 years) suffered an index fracture of any type. During a maximum follow-up of 4.7 years, 11% (6948) of the women and 6% (2014) of the men experienced a subsequent fragility fracture, and 20% (14,324) of the women and 22% (8,326) of the men died. FLS was associated with 13% lower subsequent fragility fracture risk in women (hazard ratio (HR) 0.87, 95% confidence intervals (CI) 0.83–0.92) and 10% in men (HR 0.90, 95% CI 0.81–0.99) and 18% lower mortality in women (HR 0.82, 95% CI 0.79–0.86) and 15% in men (HR 0.85, 95% CI 0.81–0.89).
Conclusion
A standardized FLS intervention was associated with a lower risk of subsequent fragility fractures and mortality and may contribute to reduce the global fracture burden.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00198-024-07376-y.
Keywords: fracture liaison service, fragility fractures, osteoporosis, post-fracture mortality, secondary fracture prevention, stepped-wedge cluster-randomized trial
Introduction
In 2019, a total of 178 million new fractures were recorded worldwide, and a further rise is expected with the aging population [1]. The lifetime risk of sustaining a fragility fracture after the age of 50 years is 50% for women and 20% for men [2]. A fragility fracture doubles the risk of a subsequent fracture, and a hip fracture triples the risk of a new hip fracture, causing increased morbidity and mortality [3, 4]. The imminent fracture risk is highest in the first 2 years after the initial fracture, making early fracture prevention essential [5–7]. Treatment with anti-osteoporosis drugs (AOD) can reduce the relative risk of a subsequent fracture by 15–70% [2]. Nevertheless, assessment and treatment of osteoporosis are still suboptimal, leaving a large care gap between the high proportion in need of treatment and the small proportion of less than 20% of fragility fracture patients who receive AOD [8].
A fracture liaison service (FLS) facilitates the systematic identification of patients with a fracture, assessment of fracture risk, and treatment of osteoporosis. FLS is internationally suggested as the best solution to close the treatment gap [9]. FLS has been reported to increase the frequency of bone mineral density (BMD) measurements and initiation of AOD treatment and to improve adherence [10–12]. Some studies have shown that FLS reduces subsequent fracture rates [13–19] and mortality [14, 17, 18, 20], whereas others have not [12, 20, 21]. Studies on the effect of FLS have varied with respect to design, sample size, and observation time, and to the best of our knowledge, no previous randomized trial has been performed with subsequent fracture rates and mortality as outcomes.
Purpose
The aim of this study was therefore to investigate the effect of introducing a standardized FLS intervention using a stepped-wedged cluster-randomized design on the incidence of subsequent fragility fractures (distal forearm fractures, proximal humerus fractures, and hip fractures) and all-cause mortality.
Methods
Study design
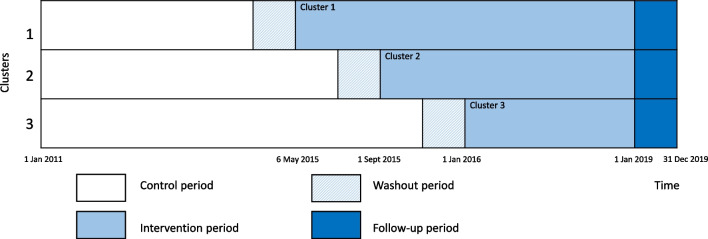
The Norwegian Capture the Fracture Initiative (NoFRACT) study was designed as a pragmatic, multicenter, register-supported, stepped-wedge cluster-randomized trial (SW-CRT). The stepped-wedge design was chosen to ensure that all study sites received the intervention during the study period. Further, the prospective SW-CRT design allowed for both a randomized trial design and to take time trends in fracture rates into consideration. The study was registered (Clinicaltrials.gov, NCT02536898) and the trial protocol has been published [22]. Seven Norwegian hospitals were randomized for the order of starting date, and divided into three clusters, with an interval of 4 months between the clusters (Fig. 1). Randomization was performed by the Norwegian Osteoporosis Association, who had no other involvement in the study. Standard care was delivered at each hospital during the control period (2011–2015) and the hospitals crossed over in only one direction and delivered the standardized FLS during the intervention period (2015–2018). The intervention was not blinded. The Consolidated Standards of Reporting Trials reporting guideline (CONSORT) with extension for SW-CRTs was followed for this trial. This is the first major report from the NoFRACT study.
Fig. 1.
Study design of the NoFRACT intervention as recommended by the Consolidated Standards of Reporting Trials (CONSORT) extension for stepped-wedged cluster randomized trials (SW-CRTs)
Study intervention
The control period started on 1 January 2011 and went on until the stepwise introduction of the FLS intervention at each cluster. The FLS intervention was carried out at departments of orthopedic surgery at the NoFRACT hospitals with recruitment from 6 May 2015 until 31 December 2018 and follow-up through December 2019 (Fig. 1). All women and men ≥ 50 years with low-energy fractures (except fractures of the fingers, toes, face, and skull) who were treated at one of the NoFRACT hospitals and residing within each hospital’s catchment area were eligible for the FLS intervention. Exclusion criteria were short life expectancy judged by the treating physician.
The fracture patients were identified by a coordinating nurse and offered information, clinical assessment, lifestyle advice, and AOD if indicated, while they were in the hospital for their index fracture or as out-patients [22]. Lifestyle advice about physical activity, fall prevention, healthy diet, smoking cessation, and moderate alcohol intake was given, and referral to fall prevention at the hospital or primary care if indicated. The fracture risk assessment included BMD measurements using dual-energy X-ray absorptiometry (DXA) of both hips and spine, and/or calculation of the 10-year probability of a major osteoporotic fracture (MOF) using the Fracture Risk Assessment Tool (FRAX), and serum analyses of 25-hydroxyvitamin D, calcium, parathyroid hormone, thyroid-stimulating hormone, albumin, and creatinine to assess secondary causes of osteoporosis and the kidney function. AOD was offered directly to patients with a hip fracture, a vertebral fracture, or two or more low-energy fractures while they were still in the hospital, without the need of BMD or FRAX score assessment. Patients with a low-energy fracture of any other type were offered assessment within 6 weeks after their index fracture and treatment with AOD if BMD T-score ≤ − 1.5 or FRAX score for MOF > 20%. The primary drug of choice for patients with a hip fracture was intravenous zoledronic acid administered during the hospitalization for surgery. For patients with their first low-energy fracture, other than hip fracture, oral bisphosphonate, preferentially alendronate once weekly, was the drug of choice. Denosumab was an option in case of reduced kidney function (estimated glomerular filtration rate (eGFR) 20–35 mL/min). Patients with BMD T-score ≤ − 3.5, or subsequent low-energy fracture while on anti-resorptive treatment, were referred to an osteoporosis specialist to consider osteoanabolic treatment [22].
Audit data from each hospital
Audit data from the fracture patients during the NoFRACT intervention period was collected at each hospital. Aggregated summaries of the registration were used to monitor the number and proportion of patients who were offered FLS, had their fracture risk assessed, and were prescribed AOD. The ethical clearance for the collection of such data was given for local surveillance only and could not be linked to the register data.
Administrative register data from the Norwegian Patient Register (NPR)
The main analyses were based on data retrieved from NPR after the end of the intervention period, on all women and men ≥ 50 years at the time of index fracture (2011–2018), who were treated at a NoFRACT hospital. An 11-digit person identification number enabled the merging of data to other data sources and the identification of index and subsequent fractures in the data. NPR also provided data on age, sex, and Charlson Comorbidity Index (CCI) based on registered diagnoses within the same year as the fracture [23]. However, the data from the NPR did not include information on whether the patients were exposed to the FLS intervention and had a BMD scan or AOD prescribed. We had no registered data on participation in the FLS intervention, and patients were placed in the control or intervention period solely based on the time of index fracture. Therefore, the analysis was an intention-to-treat analysis. As described above, we have included some aggregated audit data from the hospitals to help in interpreting the effect of FLS.
Statistics Norway provided data for all patients: dates of migration and death, marital status (never married, previously married, and married), country of birth (Scandinavian (Norway, Sweden, and Denmark), and non-Scandinavian), municipality of residence at time of fracture. Statistics Norway also provided an urban centrality index for each municipality: a score from 1 (most central) to 6 (least central) based on the number of inhabitants and the driving time from residential housing to jobs and services [24].
Outcome measures
As registered in www.clinicaltrials.gov, the primary outcome was the change in the rate of subsequent fragility fracture defined as a fracture of the distal forearm, proximal humerus, and/or hip in patients with any type of index fracture. The secondary outcome was all-cause mortality in patients with any type of index fracture.
Additional outcomes were the change in the rate of any type of subsequent fracture, subsequent hip fracture after any type of index fracture, and second hip fracture and mortality after an index hip fracture.
Patients who were registered with a code for fracture control (International Classification of Diseases version 10 (ICD-10) Z-codes), fracture follow-up treatment (ICD-10 T-codes), and/or a Nordic Medico-Statistical Committee (NOMESCO) surgical procedure codes (NCSP) for reoperation were excluded from the analysis. Hip fracture diagnoses in the NPR have previously been validated [25]. The combined Cohen’s kappa for comparison of register data with fractures in local fracture registries was 0.95. We have also performed a study investigating forearm fracture validity in administrative hospital data using X-rays and/or medical records for verification [26]. The sensitivity (completeness) of the forearm fracture registrations was 90%. The positive predictive value increased from 74% in crude data to 91% when using a washout period of 6 months, and consequently, we applied a 6-month wash-out within each fracture group (ICD-10 S-code). Additionally, as fracture location can be miscoded, a 30 day-washout between registrations of fracture of the arm (S42, S52, and S62); ribs, spine, and pelvis (S22 and S32); and leg and foot (S82 and S92) were applied.
Statistical analyses
All results are presented for women and men separately. We used stratified Cox proportional hazard regression analysis to calculate hazard ratios (HRs) with 95% confidence intervals (CIs). The stratified Cox models allowed hospitals to act as their own control with individual baseline hazard functions. An index fracture was defined as the first fracture after a fracture-free wash-out period of 3 years. The 3-year wash-out period led to the possibility that some patients could count in both the control and intervention periods; therefore, repeated measures by individuals were additionally accounted for in the stratified Cox model. Follow-up measures for fracture outcomes were years contributed by each patient from the date of the index fracture to the first subsequent fracture, death, migration, or end of follow-up on December 31, 2019, with a maximum follow-up of 4.7 years. The follow-up measure for mortality outcome was years contributed by each patient from the date of the index fracture to death, migration, or end of follow-up on December 31, 2019, with a maximum follow-up of 4.7 years. In the analyses of mortality, patients could contribute with person-time in multiple episodes if they sustained subsequent fractures before they died. The stratified Cox model ensured equal maximum follow-up time in the control and intervention period, which was a requirement for using this method. The proportionality of the HRs was verified using log–log plot of survival by intervention status, adjusting for age, sex, hospital, and time.
The following regression analyses were performed for the primary and secondary outcomes as registered in www.clinicaltrials.gov: (a) from any type of index fracture to a subsequent fragility fracture, (b) from any type of index fracture to death. Additional analyses were (c) from any type of index fracture to any type of subsequent fracture, (d) from any type of index fracture to a subsequent hip fracture, (e) from an index hip fracture to a second hip fracture, and (f) from an index hip fracture to death. (Definitions of type of fractures are shown in Table 1).
Table 1.
Definitions of types of fractures
| Variable | Definitions using ICD-10 Codes |
|---|---|
| Any type of fracture | S22, S32, S42, S52, S62 (excluding fingers S62.5–S62.7), S72, S82, S92 (excluding toes S92.4–S92.5) |
| Fragility fracture | S52.5–S52.6 (distal forearm), S42.2 (proximal humerus), and/or S72.0–S72.2 (hip fracture) |
| Ribs, sternum, thoracic spine | S22 |
| Lumbar spine, pelvis | S32 |
| Upper arm | S42 |
| Forearm | S52 |
| Hand, wrist | S62 (excluding fingers S62.5–S62.7) |
| Hip | S72.0–S72.2 |
| Femur | S72.3–S72.9 |
| Patella, lower leg, ankle | S82 |
| Foot | S92 (excluding toes S92.4–S92.5) |
We also performed a multiple failures analysis where data were organized as ordered episodes and all fracture episodes from 2011 to 2019 were included. Since it was not possible to distinguish index fractures from other fractures, we included data from 2019 in this analysis, and consequently, the total number of patients differed from the main analyses.
Confounders were mapped using a causal-directed acyclic graph [27]. We estimated crude HRs for the association with the FLS intervention and HRs after adjustment for age, sex, urban centrality index [24], marital status, education level, Scandinavian-born, Charlson Comorbidity Index [23], fracture number, and type of index fracture. To account for time trends (declining subsequent hip fracture rates during the last 20 years) [28] and time-dependent randomization in the SW-CRT design, the analyses were adjusted for the day of hospital admission of the index fracture (cubic spline with 7 knots). Stata 16 (StataCorp, College Station, TX, USA) was used for the analyses.
Deviations from the protocol
The University Hospital of North Norway introduced the intervention on 1 October 2015, which was 5 months later than scheduled. Haukeland University Hospital initiated treatment with zoledronic acid directly only in patients with hip fracture > 70 years of age. We intended to start FLS treatment within 6 weeks after an index fracture; however, due to delay in the assessment of fracture risk, osteoporosis treatment may have been initiated after more than 6 weeks in some patients. As stated in the protocol article, we planned to exclude patients outside the hospitals’ referral region [22]; however, it was not possible to obtain hospital-specific data from The University Hospital of North Norway and Oslo University Hospital for the entire period, and data from the entire health trusts therefore had to be used. A multilevel regression model, where individuals count only once, was described in the protocol article [22]. However, SW-CRT was a relatively new study design when we planned NoFRACT. Statisticians who have experience with data analysis from a SW-CRT clearly recommend that the best way to accommodate cluster effects by hospital and individuals in our setting was to use a stratified Cox model including a term for repeated measures by individual (confer statistical analyses for more information). Using this method, patients in both the control period and intervention period had an equal chance to be included and have their fracture counted, when having a subsequent fracture within a similar 3-year period of washout. This is of importance to avoid variation in inclusion criteria as well as selection bias.
Results
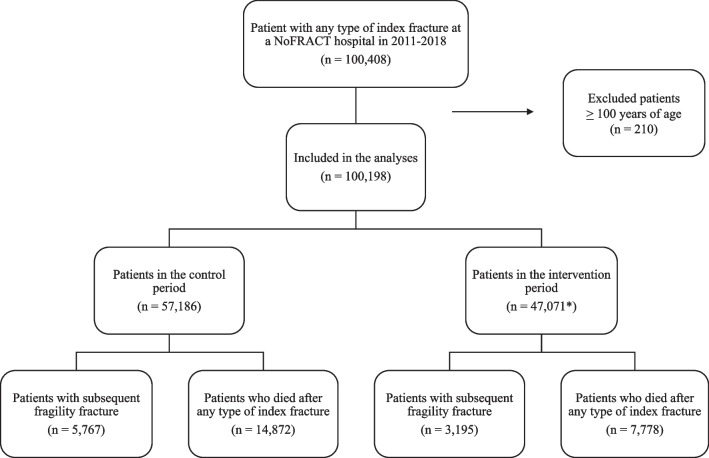
A total of 100,198 patients suffering an index fracture between January 1, 2011, and December 31, 2018, were included in this study (Fig. 2). The mean age was 69.6 years (range 50–99) and 66% were women. Patients who were more than 100 years old (n = 210) were excluded, as they were not likely to have received the intervention. Of the index fractures, forearm fracture was the most common type for women in both periods (31% and 30%), whereas ribs, sternum, and thoracic spine were the most common types in men (19% and 22%) followed by forearm fracture (15% in both periods). Other common fracture types in both sexes were the upper arm, hip, and patella/lower leg (14–15%). The characteristics of the patients in the control and intervention periods are shown in Table 2. Based on audit data (collected during the intervention period from 2015 to 2018), 78% of patients with index fractures were offered FLS, and 70% of all fracture patients had their fracture risk assessed (88% of those who were offered FLS accepted to be tested for osteoporosis and 65% of those assessed had a DXA scan performed). Less than 9% of the patients used AOD before their index fracture, ranging from 1 to 9% between the different hospitals. Of those who were investigated for osteoporosis, 56% had AOD prescribed according to the treatment algorithm [22].
Fig. 2.
Flowchart of patients with an index fracture treated at a NoFRACT hospital in 2011–2018. *4059 patients were included in both the intervention and the control period
Table 2.
Baseline patient characteristics in the control period and the fracture liaison service (FLS) intervention period
| No FLS intervention (n = 57,186) | FLS intervention (n = 47,071) | ||||||
|---|---|---|---|---|---|---|---|
| Women | Men | All | Women | Men | All | ||
| Age (years), mean ± SD, median (interquartile range) |
71.1 ± 12.8 70 (60–82) |
67.3 ± 12.3 65 (57–77) |
69.8 ± 12.8 68 (59–81) |
70.6 ± 12.6 70 (60–81) |
67.3 ± 12.2 66 (57–76) |
69.4 ± 12.5 68 (59–79) |
|
| Marital status, n (%)a | Unmarried | 3696 (9.9) | 2975 (15.3) | 6671 (11.8) | 3537 (11.6) | 2764 (17.0) | 6301 (13.5) |
| Previously married | 17,895 (48.0) | 5370 (27.6) | 23,265 (41.0) | 13,928 (45.8) | 4329 (26.6) | 18,257 (39.1) | |
| Married | 15,715 (42.1) | 11,147 (57.2) | 26,862 (47.3) | 12,955 (42.6) | 9172 (56.4) | 22,127 (47.4) | |
| Birth country, n (%) | Scandinavian | 35,406 (94.5) | 18,270 (92.7) | 53,676 (93.9) | 28,459 (93.2) | 14,896 (90.2) | 43,355 (92.1) |
| Other | 2076 (5.5) | 1434 (7.3) | 3510 (6.1) | 2094 (6.9) | 1622 (9.8) | 3716 (7.9) | |
| Educational level, n (%)b | < 12 years | 11,717 (31.5) | 4770 (24.6) | 16,487 (29.1) | 8169 (27.0) | 3661 (22.7) | 11,830 (25.5) |
| 12 years | 16,164 (43.5) | 8806 (45.3) | 24,970 (44.1) | 12,962 (42.8) | 7247 (44.9) | 20,209 (43.6) | |
| > 12 years | 9274 (25.0) | 5855 (30.1) | 15,129 (26.7) | 9133 (30.2) | 5237 (32.4) | 14,370 (31.0) | |
| Urban centrality index, n (%)c | Urban | 25,831 (70.5) | 13,046 (68.0) | 38,877 (69.7) | 21,287 (70.1) | 11,182 (69.0) | 32,469 (70.1) |
| Suburban | 7308 (20.0) | 4103 (21.4) | 11,411 (20.4) | 6141 (20.4) | 3402 (21.0) | 9543 (20.6) | |
| Rural | 3496 (9.5) | 2035 (10.6) | 5531 (9.9) | 2698 (9.0) | 1608 (9.9) | 4306 (9.3) | |
| Charlson Comorbidity Index groups, n (%) | 0 | 26,090 (69.6) | 12,669 (64.3) | 38,759 (67.8) | 21,347 (69.9) | 10,596 (64.2) | 31,943 (67.9) |
| 1 | 5377 (14.4) | 2798 (14.2) | 8175 (14.3) | 4322 (14.2) | 2258 (13.7) | 6580 (14.0) | |
| 2 | 6015 (16.1) | 4237 (21.5) | 10,252 (17.9) | 4884 (16.9) | 3664 (22.2) | 8548 (18.2) | |
| Index fracture typesd, n (%) | Ribs, sternum, thoracic spine | 3046 (8.1) | 3709 (18.8) | 6755 (11.8) | 2818 (9.2) | 3618 (21.9) | 6436 (13.7) |
| Lumbar spine, pelvis | 2857 (7.6) | 1645 (8.4) | 4502 (7.9) | 2226 (7.3) | 1352 (8.2) | 3578 (7.6) | |
| Upper arm | 5612 (15.0) | 2720 (13.8) | 8332 (14.6) | 4552 (14.9) | 2337 (14.2) | 6889 (14.6) | |
| Forearm | 11,236 (30.9) | 3032 (15.4) | 14,268 (25.0) | 9220 (30.2) | 2427 (14.7) | 11,647 (24.7) | |
| Hand, wrist | 2572 (6.9) | 2497 (12.7) | 5069 (8.9) | 2250 (7.4) | 2112 (12.8) | 4362 (9.3) | |
| Hip | 5443 (14.5) | 2778 (14.1) | 8221 (14.4) | 3879 (12.7) | 2056 (12.5) | 5935 (12.6) | |
| Femur | 740 (2.0) | 316 (1.6) | 1056 (1.9) | 517 (1.7) | 242 (1.5) | 759 (1.6) | |
| Patella, lower leg, ankle | 5245 (14.0) | 2846 (14.4) | 8091 (14.2) | 4463 (14.6) | 2347 (14.2) | 6810 (14.5) | |
| Foot | 2283 (6.1) | 1302 (6.6) | 3585 (6.3) | 1821 (6.0) | 1025 (6.2) | 2846 (6.1) | |
aMissing marital status (n = 774)
bMissing educational level (n = 1262)
cMissing urban centrality index (n = 2120)
d100,198 patients had 104,257 fracture episodes, 3015 fracture episodes had multiple fracture types, and 4059 patients were reported in both groups (no FLS and FLS)
Altogether, 8962 (9%) patients experienced a subsequent fragility fracture (distal forearm, proximal humerus, and/or hip) during a median follow-up of 3.1 years (maximum 4.7 years) (Fig. 1). During follow-up, the FLS intervention was associated with 13% lower risk of a subsequent fragility fracture in women (HR 0.87, 95% CI 0.83–0.92) and 10% in men (HR 0.90, 95% CI 0.81–0.99) after adjustment for relevant confounders (Table 3). In the age group > 65 years, FLS was associated with 14% lower risk in women (HR 0.86, 95% CI 0.80–0.91) and 12% lower risk in men (HR 0.88, 95% CI 0.78–0.98).
Table 3.
Associations of a fracture liaison service (FLS) with fracture risk and mortality in women and mena
| Person-years | n | HR (95% CI)b | p-value | HR (95% CI)c | p-value | HR (95% CI)d | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Women | |||||||||
| Fragility fractures | No FLS | 135,286 | 4489 | ref | ref | ref | |||
| FLS | 70,391 | 2459 | 0.87 (0.83, 0.92) | < 0.001 | 0.88 (0.83, 0.92) | < 0.001 | 0.87 (0.83, 0.92) | < 0.001 | |
| Mortalitye | No FLS | 195,913 | 9524 | ref | ref | ref | |||
| FLS | 124,615 | 4800 | 0.78 (0.75, 0.81) | < 0.001 | 0.81 (0.78, 0.84) | < 0.001 | 0.82 (0.79, 0.86) | < 0.001 | |
| Hip fractures | No FLS | 138,781 | 2259 | ref | ref | ref | |||
| FLS | 71,653 | 1013 | 0.73 (0.67, 0.79) | < 0.001 | 0.75 (0.69, 0.81) | < 0.001 | 0.75 (0.70, 0.82) | < 0.001 | |
| Men | |||||||||
| Fragility fractures | No FLS | 73,103 | 1278 | ref | ref | ref | |||
| FLS | 38,155 | 736 | 0.88 (0.80, 0.97) | 0.012 | 0.89 (0.81, 0.98) | 0.023 | 0.90 (0.81, 0.99) | 0.025 | |
| Mortalitye | No FLS | 102,344 | 5348 | ref | ref | ref | |||
| FLS | 56,880 | 2978 | 0.82 (0.78, 0.86) | < 0.001 | 0.84 (0.80, 0.89) | < 0.001 | 0.85 (0.81, 0.89) | < 0.001 | |
| Hip fractures | No FLS | 73,448 | 776 | ref | ref | ref | |||
| FLS | 38,228 | 410 | 0.78 (0.68, 0.89) | < 0.001 | 0.79 (0.69, 0.90) | < 0.001 | 0.79 (0.69, 0.90) | 0.001 | |
aResults are presented as hazard ratio (HR) with a 95% confidence interval (CI) for subsequent fractures and mortality by FLS intervention in 100,198 patients with any type of index fracture from 2011 to 2018
bAdjusted for age, clustering by person, fracture number, and stratified on hospital
cAdjusted for age, marital status, education level, Scandinavian-born, fracture type, comorbidity, clustering by person, fracture number, and stratified on hospital
dAdjusted for age, marital status, education level, Scandinavian-born, fracture type, comorbidity, clustering by person, fracture number, hospitalization day (in splines with 8 knots), and stratified on hospital
eSubsequently, fractures were not considered as competing events of mortality; therefore, all fracture episodes were included (138,186 in 112,900 individuals from 2011 to 2019)
Fragility fractures are defined as fractures of the distal forearm, proximal humerus, and/or hip, and the definition of all fracture groups is shown in Table 1
A total of 22,650 (21%) patients died during follow-up: 20% of the women (14,324) and 22% of the men (8326) and FLS was associated with lower mortality after an index fracture of any type by 18% in women (HR 0.82, 95% CI 0.79–0.86) and 15% in men (HR 0.85, 95% CI 0.81–0.89, Table 3).
In all 100,198 patients, 18,196 (18%) had a subsequent fracture of any type, 13,144 (20%) women and 5052 (14%) men, and 4476 (4.5%) experienced a subsequent hip fracture, 3286 (5%) women and 1190 (3%) men. The associated risk reduction for a subsequent hip fracture was 25% for women (HR 0.75, 95% CI 0.70–0.82) and 21% for men (HR 0.79, 95% CI 0.69–0.90) (Table 3).
Of 16,326 patients with an index hip fracture (mean age 80.7 years), 1030 (9%) women and 443 (8%) men had a second hip fracture, and 5137 (47%) of the women and 2919 (54%) of the men died during follow-up. FLS was associated with a 20% lower risk of a second hip fracture after adjustment for relevant confounders in women and 26% in men (Table 3). The associated reduction in risk for a second hip fracture by FLS was 26% in patients ≥ 80 years (HR 0.74, 95% CI 0.64–0.85), 21% for women (HR 0.79, 95% CI 0.66–0.93), and 34% for men (HR 0.66, 95% CI 0.49–0.86). However, there was no significant reduction in risk of a second hip fracture in those < 80 years neither for women (HR 0.81, 95% CI 0.63–1.05) nor in men (HR 0.86, 95% CI 0.62–1.18), and no significant reduction in mortality after an index hip fracture (Table 4).
Table 4.
Associations of a fracture liaison service (FLS) intervention with risk of a second hip fracture and mortality after hip fracture in women and mena
| Person-years | n | HR (95% CI)b | p-value | HR (95% CI)c | p-value | HR (95% CI)d | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Women | |||||||||
| Second hip fracture | No FLS | 18,774 | 732 | ref | ref | ref | |||
| FLS | 8419 | 298 | 0.82 (0.72, 0.95) | 0.007 | 0.83 (0.72, 0.95) | 0.007 | 0.80 (0.69, 0.92) | 0.002 | |
| Mortality after hip fracture | No FLS | 20,177 | 3538 | ref | ref | ref | |||
| FLS | 8858 | 1599 | 0.97 (0.92, 1.04) | 0.40 | 0.98 (0.93, 1.04) | 0.56 | 0.98 (0.92, 1.05) | 0.58 | |
| Men | |||||||||
| Second hip fracture | No FLS | 7901 | 310 | ref | ref | ref | |||
| FLS | 3887 | 133 | 0.82 (0.72, 0.95) | 0.007 | 0.76 (0.61, 0.94) | 0.10 | 0.74 (0.60, 0.92) | 0.007 | |
| Mortality after hip fracture | No FLS | 8420 | 1863 | ref | ref | ref | |||
| FLS | 4078 | 1056 | 0.99 (0.92, 1.07) | 0.88 | 0.99 (0.92, 1.07) | 0.82 | 0.99 (0.92, 1.07) | 0.86 | |
aHazard ratio (HR) and 95% confidence interval (CI) for a second hip fracture and mortality by FLS in 16,326 patients with an index hip fractures from 2011 to 2018
bAdjusted for age, sex, and stratified on hospital
cAdjusted for age, sex, marital status, education level, Scandinavian-born, comorbidity, and stratified on hospital
dAdjusted for age, sex, marital status, education level, Scandinavian-born, comorbidity, hospitalization day (in splines with 7 knots), and stratified on hospital
A multiple failures analysis where we counted all 138,186 fracture episodes (time to next fracture) in 112,900 patients showed even stronger results for subsequent fragility fracture (HR 0.78, 95% CI 0.74–0.81) and subsequent hip fracture (HR 0.71, 95% CI 0.66–0.76) (Table 5).
Table 5.
Associations of a fracture liaison service (FLS) intervention with fracture risk and mortality in women and men in multiple failures analysisa
| Person-years | n | HRb (95% CI) | p-value | HRc (95% CI) | p-value | HRd (95% CI) | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Women | |||||||||
| Fragility fractures | No FLSe | 133,929 | 5477 | ref | ref | ref | |||
| FLSe | 77,407 | 3148 | 0.75 (0.72, 0.79) | < 0.001 | 0.76 (0.73, 0.80) | < 0.001 | 0.76 (0.73, 0.80) | < 0.001 | |
| Hip fractures | No FLSe | 133,929 | 1906 | ref | ref | ref | |||
| FLSe | 77,407 | 951 | 0.67 (0.62, 0.72) | < 0.001 | 0.69 (0.64, 0.75) | < 0.001 | 0.69 (0.64, 0.75) | < 0.001 | |
| Men | |||||||||
| Fragility fractures | No FLSe | 71,788 | 1468 | ref | ref | ref | |||
| FLSe | 41,108 | 933 | 0.78 (0.72, 0.86) | < 0.001 | 0.81 (0.74, 0.88) | < 0.001 | 0.81 (0.74, 0.89) | < 0.001 | |
| Hip fractures | No FLSe | 71,788 | 644 | ref | ref | ref | |||
| FLSe | 41,108 | 380 | 0.74 (0.65, 0.86) | < 0.001 | 0.77 (0.67, 0.89) | < 0.001 | 0.78 (0.67, 0.89) | < 0.001 | |
aHazard ratio (HR) of subsequent fragility fracture (including hip fracture) and hip fracture only by FLS in 138,186 fracture episodes (112,900 patients), presenting with fracture after 50 years of age at a NoFRACT hospital from 2011 to 2019 in multiple failures analysis (i.e., patients are considered at risk until study end/emigration or death)
bAdjusted for age, sex, clustering by person, and fracture number, and stratified on hospital
cAdjusted for age, sex, clustering by person, fracture number, marital status, educational level, Scandinavian-born, fracture type, comorbidity, and stratified on hospital
dAdjusted for age, sex, clustering by person, fracture number, marital status, educational level, Scandinavian-born, fracture type, comorbidity, hospitalization day (in splines with 7 knots), and stratified on hospital
eFracture liaison service intervention initiated in 2015–2016
Fragility fractures are defined as fractures of the distal forearm, proximal humerus, and/or hip, and the definition of all fracture groups is shown in Table 1
Discussion
In this large, cluster-randomized trial, a standardized FLS intervention was associated with a reduced risk of subsequent fragility fractures and reduced mortality in both women (13%) and men (10%) with any type of index fracture. A lower risk of subsequent hip fracture (25% in women and 21% in men) and a second hip fracture (20% in women and 26% in men). NoFRACT is the first stepped-wedge cluster-randomized trial that has shown an association of lower subsequent fragility fracture rates by an FLS intervention. A randomization on an individual level was deemed unacceptable, as treatment with AOD is well documented to reduce fracture risk [29]. However, this design is different from previous FLS studies, and one should be cautious when comparing results. Time is a potential confounder that must be accounted for to avoid misinterpreting reduction in fracture rates by time to be an effect of FLS [30]. Confounding by time trends has not been considered in prior FLS studies [16]. Our study design allowed for a randomized trial and took into account time trends of declining rates of subsequent fractures during the study [28]. However, we cannot be certain that the effect of time has not impacted the results, as the general health of the population might have changed over the time of the study. Nevertheless, by introducing the intervention over an entire year, adjusting for calendar time, and letting the hospitals be their own controls, we diminished the effect of both seasons’ variation and time trends.
Some previous studies have reported a reduction in subsequent fracture rates ranging from 18 [16] to 35% [17], whereas others found no change [20, 21]. These studies varied in size and design and were smaller than the current study, with the largest study including 21,083 patients [16]. We included younger patients with any type of fracture in a real-world setting with a lower risk of subsequent fracture than previous studies which included non-vertebral-[17], major osteoporotic-[12, 16, 18], or only hip fractures [20, 21]. This may have attenuated our results. Despite the difference in fracture risk among women and men, the association of FLS with lower risk for subsequent fragility fractures was substantial in both sexes. This agrees with previous published studies on fracture risk; women have a higher risk for fractures than men [31]; however, the effect of AOD on fracture risk reduction seems to be similar between the sexes [32]. Fracture prevention in men is however less studied than in women.
We also found that FLS was associated with lower all-cause mortality after any type of index fracture by 18% in women and 15% in men. This is in accordance with the results from Huntjens et al. [14] and with recently published register data from China [33]. The reduction in mortality risk was higher than previously reported from trials on AOD. Speculating, we could suggest that there may be an effect of additional measures performed or initiated by the FLS directly or indirectly influencing mortality. However, no effect of the FLS on mortality after hip fracture was detected, and there was a high death rate after hip fractures in both periods. Notably, our study was not powered for studying hip fracture alone, but fragility fractures in general.
The stronger effect of the FLS observed in reducing the risk of a second hip fracture in patients with an index hip fracture may be attributed to the fact that the majority of these patients were treated with intravenous zoledronic acid during hospitalization. This treatment provides rapid, efficient long-term anti-fracture effects and no challenges pertaining to adherence. It should be recalled that most of the other fracture patients had their fracture risk evaluated, and 56% fulfilled the treatment indication. The majority of these were offered an oral bisphosphonate, with lower long-time adherence [34], as well as lower relative risk reduction for non-hip, non-vertebral fractures than for hip and vertebral fractures [35]. In addition, new vertebral fractures were not captured in the NPR data. This concords with data showing that up to 70% of vertebral fractures remain undiagnosed. Most patients with vertebral fractures are not referred to X-rays or to the hospital for treatment. Moreover, a high proportion of vertebral fractures are either missed or not reported by radiologists [36].
Limitations
This study has several limitations. Although 78% of the fracture patients were offered FLS, only around 70% of eligible patients at the NoFRACT hospitals had an assessment of the fracture risk; however, more than half of those assessed had AOD prescribed. Some patients were unwilling to participate, and sometimes there was a lack of capacity by the coordinators and staff to offer FLS to all the eligible patients with fractures. As the risk of subsequent fragility fractures is highest within the first 2 years [6], we aimed to start AOD treatment within 6 weeks, but this was not accomplished for all patients. The effect of oral bisphosphonates on non-hip, non-vertebral fracture risk has been shown to become evident after more than 1 year of treatment, whereas the fracture-reducing effect of zoledronic acid and denosumab is present after 6 months [29]. Hence, longer follow-up has the potential to show a larger effect of FLS on any type of fracture than the reported estimate. Furthermore, the validity of the register data is most likely better for hip fractures compared to other fracture types as most hip fractures are surgically treated at hospitals and thus more likely to be registered correctly compared to other fracture types. Longer intervals between the start of each cluster would have strengthened the study design. Because we depended on registered data for our analyses, we had limited information about confounding factors. However, the main analyses were adjusted for the key confounding variables potentially causing baseline imbalance. Still, we cannot rule out the possibility of residual confounding. Based on the audit data, we reported that not more than 9% of the patients with a fracture were on AOD at the time of their index fracture in the intervention period (2015–2018), suggesting that few high-risk patients were treated with AOD before their index fracture. Moreover, we have some knowledge from studies using the Norwegian Prescription Database showing low uptake of AOD in Norway in the period from 2005 to 2015: only 5–17% of women and men were using AOD after a hip fracture [37], and 3–11% were using AOD after a distal forearm fracture [38]. In the FLS period, 56% of all fracture patients were started on AODs which is more than double compared to previous data making it more likely that the associated fracture risk reduction seen in the intervention group could be due to the FLS implementation and not only caused by time-trends.
Another limitation is that some patients with very low BMD in need for bone-forming agents may have missed the opportunity to be identified. In the NoFRACT algorithm, patients with very high imminent fracture risk were recommended to start AOD while they were in hospital and recommended BMD measurements within 6 weeks (except for the oldest patients with hip fractures). Unfortunately, we do not have good figures on the proportion of patients thus identified as very high risk and treated with anabolic bone medication. However, at the time, we considered that it was better that these patients received AOD shortly after their fracture, rather than to risk no or delayed treatment.
The strengths of this study include a large sample size, and that the effect of our FLS program was investigated in a real-world setting, with a representative sample of the Norwegian population. The adjustment for time prevented misinterpretation of lower fracture rates by time to be an effect of FLS. The use of register data was both a strength and a limitation; it enabled a large sample size, prevented loss to follow-up, limited selection bias, and ensured that the same method was used for fracture identification at all hospitals during the study period. However, the use of register data from the entire health trusts for some sites and intention-to-treat-analysis might have diluted the effect of the intervention as patients who were not offered the intervention were included in the analysis.
Conclusion
In this secondary fracture prevention multicenter cluster-randomized trial, implementation of a standardized FLS was associated with a lower risk of subsequent fragility fracture and mortality. The current FLS intervention was unique because patients with a very high risk of subsequent fractures were given treatment for osteoporosis directly without further investigation of the fracture risk. This study supports the notion that the implementation of FLS is a useful method for secondary fracture prevention.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all personnel at the participating sites for their work in FLS: Marit Osima, May-Greta Pedersen, and Anita Kanniainen (University Hospital of North Norway); Lars Gunnar Johnsen, Sølvi Liabakk, Nina Raaness Larsen, Kristine Aavik Haugen, Gry Mette Torstensen, and Hilde Kjøsnes Thoresen (St. Olavs University Hospital); Ruth Aga, Janne Blegen Høglund, Ingvild Hestnes, Elise Berg Vesterhus, Ellen Johansson, Mette Bentdal Larsen, and Karine Rosenlund (Oslo University Hospital); Mariann Hansen, Astrid Skei Bakketun, Torbjørn Hiis Bergh, Anne Helen Eidsheim, and Trude Instebø Hansen (Haukeland University Hospital); Charlotte Råmkes, Solveig Solberg, and Lasse Ørsal (Molde Hospital); Lars Michael Hübschle, May-Britt Stenbro, and Hanne Louise Hoelstad (Drammen Hospital); and Ellen T. Langslet and Merete Finjarn (Bærum Hospital). Finally, we thank Morten Valberg for the help with the statistical analyses.
Funding
Open access funding provided by University of Oslo (incl Oslo University Hospital) The study was supported with grants from the following: The Regional Health Authorities, grant number 243852; Northern Norway Regional Health Authority, grant number 14083; Vestre Viken Hospital Trust, grant number 19003007; St. Olavs University Hospital, grant number 46055600–51; and by South-Eastern Norway Regional Health Authority, grant number 2017032. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Data availability
Due to the protection of privacy under the General Data Protection Regulation and Norwegian law, the individual-level data can only be made available after approval by the Regional Committee for Medical and Health Research Ethics.
Declarations
Ethical approval
All procedures performed in the study were in accordance with the 1964 Helsinki Declaration and its later amendments. The study was approved by the Regional Committee of Medical and Health Research Ethics (REK 2015/334). The merging of data using the personal identification number and exemption from obtaining consent for the collection of data was approved by the Regional Committee of Medical and Health Research Ethics (REK 2015/334). A Data Protection Impact Assessment (DPIA) in agreement with the General Data Protection Regulation was performed by The University of Oslo. The study was approved by the Patient Data Protection Officer (PVO) at each of the hospitals and the PVO provided exemption from obtaining consent from the participants and authorized a secure research server for storage of the audit data.
Conflict of interest
CA, CD, TB, TW, GH, JMS, IL, AKH, FIN, LN, TKO, and ÅB no support from any organization for the submitted work; FF reports lecturing fees from UCB and Amgen; TTB reports speaker fees from UCB, Amgen, Roche Diagnostics, and Pharma Prim and participation in an advisory board for UCB. Further, FF is president of the Fragility Fracture Network which is sponsored by Amgen, UCB, and Danone; JEG reports lecture fees from Ortomedic Norway, Smith and Nephew, Heraeus Medical, and LINK Norway; WF reports lecture fees from Ortomedic Norway and Zimmer Biomet Norway; EMA reports participation in the advisory board for UCB and Amgen; RMJ reports consulting fees from Norwegian Directorate of Health, Norwegian Diabetes Association, and Northern Norway Regional Health Authority and lecture fees from Novo Nordisk, Eli-Lilly, Astra-Zeneca, and Sanofi; US reports speaker and consulting fees from Amgen, UCB, and Novartis; EFE reports consultant fees from Novartis, UCB, Amgen, Takeda, and Pharma Medico and research support from Takeda and Amgen. Further, EFE has worked as a medical consultant for the Norwegian Osteoporosis Society; LBS reports speaker fees from Eli Lilly, Amgen, and UCB. Further, LBS is the president of Fragility Fracture Network Norway.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu A-M, Bisignano C, James SL, Abady GG, Abedi A, Abu-Gharbieh E et al (2021) Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev 2(9):e580–e592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sambrook P, Cooper C (2006) Osteoporosis. Lancet 367(9527):2010–2018 [DOI] [PubMed] [Google Scholar]
- 3.Svedbom A, Borgstöm F, Hernlund E, Ström O, Alekna V, Bianchi ML et al (2018) Quality of life for up to 18 months after low-energy hip, vertebral, and distal forearm fractures-results from the ICUROS. Osteoporos Int 29(3):557–566 [DOI] [PubMed] [Google Scholar]
- 4.Alarkawi D, Bliuc D, Tran T, Ahmed LA, Emaus N, Bjornerem A et al (2020) Impact of osteoporotic fracture type and subsequent fracture on mortality: the Tromso Study. Osteoporos Int 31(1):119–130 [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA, Johansson H, Oden A, Harvey NC, Gudnason V, Sanders KM et al (2018) Characteristics of recurrent fractures. Osteoporos Int 29(8):1747–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson H, Siggeirsdottir K, Harvey NC, Oden A, Gudnason V, McCloskey E et al (2017) Imminent risk of fracture after fracture. Osteoporos Int 28(3):775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omsland TK, Emaus N, Tell GS, Ahmed LA, Center JR, Nguyen ND et al (2013) Ten-year risk of second hip fracture. A NOREPOS study. Bone 52(1):493–497 [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Curtis EM, Cooper C, Harvey NC (2019) State of the art in osteoporosis risk assessment and treatment. J Endocrinol Invest 42(10):1149–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD et al (2013) Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int 24(8):2135–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganda K, Puech M, Chen JS, Speerin R, Bleasel J, Center JR et al (2013) Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 24(2):393–406 [DOI] [PubMed] [Google Scholar]
- 11.Wu CH, Tu ST, Chang YF, Chan DC, Chien JT, Lin CH et al (2018) Fracture liaison services improve outcomes of patients with osteoporosis-related fractures: a systematic literature review and meta-analysis. Bone 111:92–100 [DOI] [PubMed] [Google Scholar]
- 12.Axelsson KF, Jacobsson R, Lund D, Lorentzon M (2016) Effectiveness of a minimal resource fracture liaison service. Osteoporos Int 27(11):3165–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van der Kallen J, Giles M, Cooper K, Gill K, Parker V, Tembo A et al (2014) A fracture prevention service reduces further fractures two years after incident minimal trauma fracture. Int J Rheum Dis 17(2):195–203 [DOI] [PubMed] [Google Scholar]
- 14.Huntjens KM, van Geel TA, van den Bergh JP, van Helden S, Willems P, Winkens B et al (2014) Fracture liaison service: impact on subsequent nonvertebral fracture incidence and mortality. J Bone Joint Surg Am 96(4):e29 [DOI] [PubMed] [Google Scholar]
- 15.Nakayama A, Major G, Holliday E, Attia J, Bogduk N (2016) Evidence of effectiveness of a fracture liaison service to reduce the re-fracture rate. Osteoporos Int 27(3):873–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Axelsson KF, Johansson H, Lundh D, Möller M, Lorentzon M (2020) Association between recurrent fracture risk and implementation of fracture liaison services in four Swedish hospitals: a cohort study. J Bone Miner Res 35(7):1216–1223 [DOI] [PubMed] [Google Scholar]
- 17.Huntjens KM, van Geel TC, Geusens PP, Winkens B, Willems P, van den Bergh J et al (2011) Impact of guideline implementation by a fracture nurse on subsequent fractures and mortality in patients presenting with non-vertebral fractures. Injury 42(Suppl 4):S39-43 [DOI] [PubMed] [Google Scholar]
- 18.Astrand J, Nilsson J, Thorngren KG (2012) Screening for osteoporosis reduced new fracture incidence by almost half: a 6-year follow-up of 592 fracture patients from an osteoporosis screening program. Acta Orthop 83(6):661–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lih A, Nandapalan H, Kim M, Yap C, Lee P, Ganda K et al (2011) Targeted intervention reduces refracture rates in patients with incident non-vertebral osteoporotic fractures: a 4-year prospective controlled study. Osteoporos Int 22(3):849–858 [DOI] [PubMed] [Google Scholar]
- 20.Hawley S, Javaid MK, Prieto-Alhambra D, Lippett J, Sheard S, Arden NK et al (2016) Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age Ageing 45(2):236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Quevedo D, Bautista-Enrique D, Perez-Del-Rio V, Bravo-Bardaji M, Garcia-de-Quevedo D, Tamimi I (2020) Fracture liaison service and mortality in elderly hip fracture patients: a prospective cohort study. Osteoporos Int 31(1):77–84 [DOI] [PubMed] [Google Scholar]
- 22.Andreasen C, Solberg LB, Basso T, Borgen TT, Dahl C, Wisløff T et al (2018) Effect of a fracture liaison service on the rate of subsequent fracture among patients with a fragility fracture in the Norwegian Capture the Fracture Initiative (NoFRACT): a trial protocol. JAMA Netw Open 1(8):e185701-e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC et al (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43(11):1130–1139 [DOI] [PubMed] [Google Scholar]
- 24.Høydahl E (2020) Sentralitetsindeksen. Statistisk sentralbyrå. Available from: https://www.ssb.no/befolkning/artikler-og-publikasjoner/sentralitetsindeksen.oppdatering-med-2020-kommuner. Accessed 27 Feb 2020
- 25.Hoiberg MP, Gram J, Hermann P, Brixen K, Haugeberg G (2014) The incidence of hip fractures in Norway -accuracy of the national Norwegian patient registry. BMC Musculoskelet Disord 15:372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omsland TK, Solberg LB, Bjornerem A, Borgen TT, Andreasen C, Wisloff T et al (2023) Validation of forearm fracture diagnoses in administrative patient registers. Arch Osteoporos 18(1):111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT (2016) Robust causal inference using directed acyclic graphs: the R package ‘dagitty.’ Int J Epidemiol 45(6):1887–1894 [DOI] [PubMed] [Google Scholar]
- 28.Meyer AC, Ek S, Drefahl S, Ahlbom A, Hedström M, Modig K (2021) Trends in hip fracture incidence, recurrence, and survival by education and comorbidity: a Swedish register-based study. Epidemiology 32(3):425–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laura I, Felicia B, Alexia C, Aude M, Florence B, Murielle S et al (2021) Which treatment to prevent an imminent fracture? Bone Rep 15:101105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemming K, Taljaard M, Forbes A (2017) Analysis of cluster randomised stepped wedge trials with repeated cross-sectional samples. Trials 18(1):101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnell O, Kanis J (2005) Epidemiology of osteoporotic fractures. Osteoporos Int 16(Suppl 2):S3-7 [DOI] [PubMed] [Google Scholar]
- 32.Fuggle NR, Beaudart C, Bruyere O, Abrahamsen B, Al-Daghri N, Burlet N et al (2024) Evidence-based guideline for the management of osteoporosis in men. Nat Rev Rheumatol 20(4):241–251 [DOI] [PubMed] [Google Scholar]
- 33.Lu K, Wu YM, Shi Q, Gong YQ, Zhang T, Li C (2024) A novel fracture liaison service using digital health: impact on mortality in hospitalized elderly osteoporotic fracture patients. Osteoporos Int 35(1):53–67 [DOI] [PubMed] [Google Scholar]
- 34.Cramer JA, Gold DT, Silverman SL, Lewiecki EM (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 18(8):1023–1031 [DOI] [PubMed] [Google Scholar]
- 35.Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC et al (2000) Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocriol Metabol 85(11):4118–24 [DOI] [PubMed] [Google Scholar]
- 36.O’Neill TW, Felsenberg D, Varlow J, Cooper C, Kanis JA, Silman AJ (1996) The prevalence of vertebral deformity in European men and women: the European vertebral osteoporosis study. J Bone Miner Res 11(7):1010–1018 [DOI] [PubMed] [Google Scholar]
- 37.Devold HM, Søgaaard AJ, Tverdal A, Falch JA, Furu K, Meyer HE (2013) Hip fracture and other predictors of anti-osteoporosis drug use in Norway. Osteoporos Int 24(4):1225–1233 [DOI] [PubMed] [Google Scholar]
- 38.Hoff M, Skurtveit S, Meyer HE, Langhammer A, Søgaard AJ, Syversen U et al (2015) Use of anti-osteoporotic drugs in central Norway after a forearm fracture. Arch Osteoporos 10:235 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the protection of privacy under the General Data Protection Regulation and Norwegian law, the individual-level data can only be made available after approval by the Regional Committee for Medical and Health Research Ethics.