Abstract
Purpose
Pituitary surgery is the mainstay treatment for most pituitary adenomas, but many questions remain about perioperative and long-term management and outcomes. This study aimed to identify the most pressing research priorities in pituitary surgery with input from patients, caregivers, and healthcare professionals.
Methods
An initial survey of patients, caregivers, and healthcare professionals assembled priorities related to preoperative care, surgical techniques, and postoperative management in pituitary surgery. Priorities were thematically grouped into summary priorities, and those answered by existing evidence were omitted following a literature review. An interim survey asked patients, caregivers, and healthcare professionals to select their top 10 priorities from the remaining list. The highest-ranked priorities advanced to a consensus meeting, where the top 10 questions were prioritized.
Results
In the initial survey, 147 participants—60.5% of whom were patients, caregivers, or patient support group representatives—submitted 785 priorities, which were then condensed into 52 summary priorities.
After a literature review, 33 unanswered priorities were included in the interim survey, completed by 155 respondents, of whom 54.2% were patients, caregivers, or patient support group representatives. The top-ranked priorities were discussed by 14 participants (7 patients and 7 healthcare professionals) during a consensus meeting. The top 10 priorities covered a variety of themes including enhancing diagnosis and management of pituitary adenomas, advancing surgical techniques and technologies, optimizing the prediction of outcomes and complications, and improving patient support and follow-up.
Conclusions
The top 10 research priorities in pituitary surgery aim to align researchers and direct funding in order to maximize impact and champion patient representation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11102-025-01502-7.
Keywords: Pituitary surgery, Research priorities, Priority setting
Introduction
Pituitary adenomas are common benign tumors, accounting for around 15% of all intracranial neoplasms [1]. Their detection within the general population is increasing, affecting 76 to 116 cases per 100,000 [2]. Aside from the scenario of an incidental pituitary adenoma, the typical presentation includes visual deterioration for macroadenomas and/ or clinical features of hormonal imbalances. Management is guided by several factors, mainly by hormonal hypersecretion and adenoma size, typically involving a multidisciplinary approach [1].
Surgical resection, via an endonasal transsphenoidal approach, is the mainstay of treatment for symptomatic non-functioning pituitary adenomas (NFPA), most functioning adenomas and asymptomatic patients whose tumour was found by chance, but with anatomical features mandating a preventive approach (e.g., incidental adenoma compressing the chiasm) [3–7]. Over recent years, there have been significant advances in pituitary adenoma surgical techniques and technology. Despite these advances, there remain challenges and unmet needs for patients undergoing pituitary adenoma surgery. For example, early complications such as post-operative cerebrospinal fluid (CSF) rhinorrhea and dysnatraemia remain common, in up to 5% and 30% of cases respectively in some centres [8, 9]. Similarly, failure to achieve remission for hypersecreting adenomas and recurrence remain common, and efforts to improve these rates have plateaued in recent years [10]. Furthermore, there is an increasing appreciation of the adverse impact of pituitary adenomas across several quality of life (QoL) domains relating to both physical and mental health measures [11]. This impact on QoL persists in patients despite ‘good’ outcomes by conventional metrics, with the exact reasons for this misalignment unclear.
To address these challenges, research should ideally be focused on agreed priority areas, aligned to patient perspectives [12]. Current clinical research is largely reflective of the interests of researchers in academia or industry [13]. This has therefore led to a mismatch in research priorities for patients, clinicians, and researchers and at times to inefficient and ineffective research reports [14].
Priority setting studies aim to consolidate research priorities through consensus for areas of healthcare where there are substantial gaps in research. Research priority studies bring patients, caregivers, and healthcare professionals together to identify and prioritize the research that matters most to them, aligning both patients’ interests and researchers’ objectives. To date, there have been several research priority studies covering a variety of neurosurgical disease areas including degenerative cervical myelopathy, brain and spine cavernous malformations, and spinal cord injury [15–17]. However, there has been no study focusing on the research priorities for pituitary surgery.
This study aims to identify the most important research priorities in pituitary surgery from the joint perspective of patients, caregivers, and clinicians. The findings will inform healthcare researchers and funding agencies, helping to shape the direction of future research and resource allocation [18].
Methods
Overview
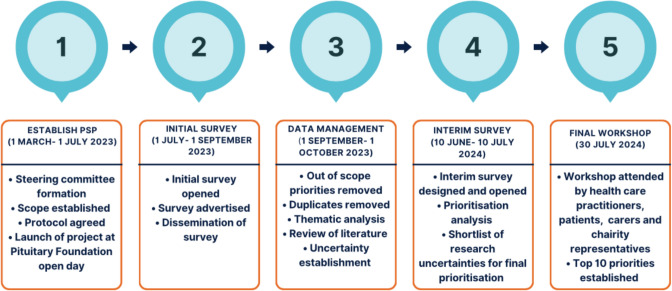
An international multistakeholder research priority consensus study designed by an expert steering committee, comprised two Delphi surveys and a consensus workshop (Fig. 1). The first survey aimed to gather a comprehensive list of priorities, while the interim survey and consensus workshop aimed to iteratively refine the long list into the most pressing research priorities. Study design and report generation were guided by the REPRISE guidelines [19]. Ethics approval was granted by the University of Cambridge Psychology Research Ethics Committee (PRE.2023.080).
Fig. 1.
Priority setting partnership (PSP) study timeline
Scope
The scope of the research priority study encompassed all areas of pituitary adenoma surgery research, including both functioning and NFPAs undergoing surgical resection.
Decisions on whether proposed submissions were in- or out-of-scope were made by the steering group. Out-of-scope priorities were considered those not related to pituitary adenoma surgery or those that were too broad.
Study management
This collaborative study included multiple stakeholders throughout—in the steering committee, management committee, and participants. Stakeholders included healthcare professionals involved in the diagnosis and surgical management of pituitary adenomas, such as endocrinologists, neurosurgeons, ophthalmologists, oncologists, otolaryngologists, radiologists, and clinical nurse specialists. Service users, including patients, caregivers, and family members, along with charity representatives from the Pituitary Foundation, were also involved.
A steering group was formed with experts representing each stakeholder group and held overall responsibility for the study, including study design, consensus generation, and dissemination of results. The steering group consisted of representatives from both UK and international professional and charitable organizations, including the Pituitary Society, the Pituitary Foundation (an international charity for patients with disorders of the pituitary gland), the Society for Endocrinology, and the European Society of Ophthalmology. The steering group consisted of 12 neurosurgeons, 10 endocrinologists, 1 pathologist, 1 ophthalmologist, 1 oncologist, 1 otolaryngologist, 1 radiologist, 3 neurosurgical trainees, 1 clinical nurse specialist, and 3 service users with lived experience, all of whom were charity representatives from the Pituitary Foundation (Appendix 1).
A management group oversaw the day-to-day administration of the priority study. The management group consisted of six healthcare professionals: two pituitary neurosurgeons, one endocrinologist and three neurosurgical trainees.
Initial survey
The initial survey was created by the steering group to gather potential research priorities as well as the demographics of the respondents including age, biological sex, location, and stakeholder group. The survey asked the following four questions to structure stakeholder feedback:
What question(s) about the diagnosis of pituitary adenomas requiring surgery would you like to see answered by research?
What question(s) about the surgical treatment of pituitary adenoma would you like to see answered by research?
What question(s) about the long-term care and follow-up after pituitary adenoma surgery would you like to see answered by research?
What other question(s) about pituitary adenoma surgery that do not fit into the above categories would you like to see answered by research?
A dedicated webpage (www.pit-cop.com) was created for the study, providing information resources, promotional videos (Supplementary Video 1) and direct links to the survey, which was conducted via Qualtrics. The survey was launched at the 2023 annual Pituitary Foundation Open Day meeting. The survey was open from 1st July 2023 to 1st September 2023 to patients, their families and caregivers, and healthcare professionals involved in the diagnosis and treatment of pituitary adenomas. The survey targeted patients, healthcare professionals, and researchers, ensuring comprehensive outreach to key stakeholders. The survey was promoted and disseminated through the Pituitary Foundation, the Society for Endocrinology, and the Pituitary Society. Furthermore, it was promoted via the study’s official social media platform (X: @PitCop2023) and through the Steering Committee’s professional network, leveraging email communications and various social media channels to maximize outreach. Promotional materials supported by our local public engagement team and the Pituitary Foundation were used to support recruitment and study understanding. All responses were anonymized and there was no limit on the number of research priorities that could be submitted. Responses were collected until data saturation, defined as the point when steering group members agreed that no new priorities or themes were emerging.
An information specialist (N.N.) reviewed the initial survey responses, removed duplicates, excluded out-of-scope submissions with steering group consensus, and grouped the remaining research priorities into themes. In-scope priorities were cross-checked against the literature for sufficient evidence, and summary priorities were generated from unanswered priorities for the interim survey.
Interim survey
The interim survey aimed to rank unanswered research priorities from the initial survey by asking stakeholders to select their top 10. In-scope unanswered priorities identified in the initial survey were digitized and presented via Qualtrics, with the survey running from 10th June to 10th July 2024. Dissemination followed the same approach as the initial survey, with links shared through relevant organizations and sent directly to those who participated in the initial survey. Responses were analyzed by stakeholder group, and the most frequently ranked priorities were brought forward to the final research priority setting workshop.
Consensus meeting
The meeting aimed to establish the top 10 research priorities from the shortlist of priorities via an online consensus workshop. The final prioritization workshop included 14 representatives, with equal representation of healthcare professionals and service users (1:1 ratio). Representatives were recruited from the steering committee and from the steering committees’ network. The meeting was held online via Zoom® on 30th July 2024.
Results
Initial survey
A total of 147 responses were received (88 patients, 1 relative and caregiver, 57 healthcare professionals, and 1 who preferred not to say), across 14 countries (Table 1).
Table 1.
Demographics of survey participants
| Initial survey (n = 147) (%) | Interim priority setting survey (n = 155) (%) | |
|---|---|---|
| Stakeholder sub-group | ||
| Healthcare professional | 57 (38.7) | 71 (45.8) |
| Neuroendocrinologist | 21 (36.8) | 27 (38.0) |
| Neurosurgeon | 29 (50.9) | 37 (52.1) |
| Oncologist | 1 (1.8) | 1 (1.4) |
| Ophthalmologist | 2 (3.5) | 2 (2.8) |
| Otolaryngologist | 1 (1.8) | 1 (1.4) |
| Pathologist | 1 (1.8) | 1 (1.4) |
| Radiologist | 1 (1.8) | 1 (1.4) |
| Specialist Nurse | 1 (1.8) | 1 (1.4) |
| Patients/caregivers/patient representatives | 89 (60.5) | 84 (54.2) |
| Patient | 88 (98.9) | 80 (51.6) |
| Caregiver | 1 (1.1) | 2 (1.3) |
| Charity representative | 0 | 2 (1.3) |
| Prefer not to say | 1 (0.7) | 0 |
| Geographical region | ||
| Europe | 122 (82.1) | 120 (77.4) |
| North America | 15 (10.2) | 20 (13.0) |
| Oceania | 5 (3.4) | 3 (1.94) |
| Asia | 4 (2.7) | 4 (2.56) |
| Africa | 3 (2.0) | 4 (2.6) |
| South America | 1 (0.7) | 1 (0.7 |
| Ethnicity | ||
| White | 113 (90.5) | 134 (86.5) |
| Asian or Asian British | 8 (5.4) | 12 (7.7) |
| Black, Black British, Caribbean or African | 5 (3.5) | 5 (3.2) |
| Any other ethnic group | 1 (0.7) | 1 (0.6) |
| Prefer not to say | 0 | 3 (1.9) |
| Age of respondents | ||
| < 30 | 4 (2.7) | 10 (6.5) |
| 31–50 | 58 (39.5) | 61 (39.4) |
| 51–70 | 69 (46.9) | 67 (43.2) |
| 71 + | 16 (10.9) | 17 (11.0) |
| Sex | ||
| Female | 73 (49.7) | 79 (51.0) |
| Male | 74 (50.3) | 76 (49.0) |
Respondents submitted a total of 785 unique research priorities. After removal of out-of-scope submissions, 708 remained. A total of 52 research priorities were reviewed by the steering group and consolidated to create 33 refined research priorities. No priorities were found to be sufficiently answered by existing research during evidence checking, and therefore, all were progressed to the interim survey (Supplementary Table 1).
Interim survey
Respondents were asked to pick their top 10 research priorities from a list of 33. A total of 155 respondents from 17 countries completed the survey online, comprising 80 patients (51.6%), 2 caregivers (1.3%), 2 charity representatives (1.3%), and 71 healthcare professionals (45.8%) (Table 1).
The top-ranked priorities in the interim survey were different between patients, caregivers and charity representatives, and healthcare professionals. Patients, caregivers, and charity representatives prioritised questions focused on improving long-term outcomes, QoL, and communication. Healthcare professionals prioritised questions aimed at refining diagnosis and improving outcomes through innovative techniques. Full details of responses by the respondent groups can be found in Supplementary Table 1. There were no notable differences in the prioritization of questions across geographic regions. However, the small number of participants from certain regions limits the ability to draw definitive conclusions about potential regional variations.
Among the top 10 priorities selected by both groups—patients, caregivers and charity representatives, and healthcare professionals—three priorities overlapped (Supplementary Table 1). To maintain balance for the final workshop, the top 21 priorities were shortlisted, incorporating the top 10 priorities from each group. The combined list is shown in Supplementary Table 2.
Final consensus meeting
The final meeting included 14 participants: 7 patients (2 of whom were charity representatives), 3 consultant neurosurgeons, 3 consultant endocrinologists, and a consultant ophthalmologist. The day was co-ordinated and run by three steering group members (HJM, NN, DZK). Throughout the workshop, each person was asked in turn to share their views, allowing for inclusive participation. Before the workshop, delegates were asked to rank the short list of 21 priority questions from 1 to 21 (Supplementary Table 2). This served as a personal reference for each participant to guide their views during the workshop.
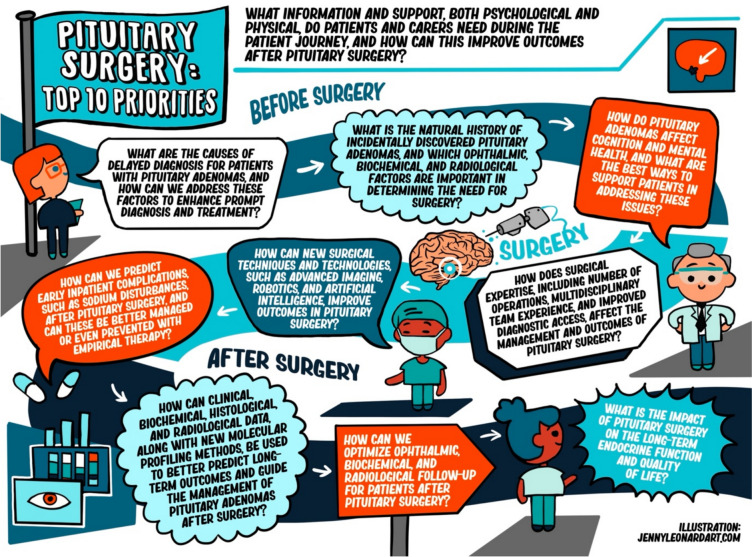
Prioritisation was undertaken in three sessions. Session 1 involved all delegates providing a rank order of 1–3 from their top 3 and bottom 3 priorities. These priorities were then separated into 3 lists: (1) highest-ranked priorities (2) priorities not mentioned (3) lowest-ranked priorities. Session 2 featured a detailed review of each of the aforementioned lists, exploring divergent views and justifying different perspectives. This discussion recommended combining 5 priorities due to their overlap (Supplementary Table 2). This resulted in the production of 15 priorities. Session 3 focused on establishing the top 10. Following recommendations, priorities were iterated, and the revised top 10 was circulated to workshop attendees for final ranking via an online survey. The final top 10 research priorities are detailed in Table 2, illustrated in Fig. 2, and supported by Supplementary Video 2.
Table 2.
Top 10 ranked research priorities in pituitary surgery
| Rank | Top 10 priorities |
|---|---|
| 1 | What is the impact of pituitary surgery on the long-term endocrine function and quality of life? |
| 2 | How can clinical, biochemical, histological, and radiological data, along with new molecular profiling methods, be used to better predict long-term outcomes and guide the management of pituitary adenomas after surgery? |
| 3 | What are the causes of delayed diagnosis for patients with pituitary adenomas, and how can we address these factors to enhance prompt diagnosis and treatment? |
| 4 | How can new surgical techniques and technologies, such as advanced imaging, robotics, and artificial intelligence, improve outcomes in pituitary surgery? |
| 5 | What information and support, both psychological and physical, do patients and carers need during the patient journey, and can this improve outcomes after pituitary surgery? |
| 6 | How does surgical expertise, including number of operations, multidisciplinary team experience, and improved diagnostic access, affect the management and outcomes of pituitary surgery? |
| 7 | How do pituitary adenomas affect cognition and mental health, and what are the best ways to support patients in addressing these issues? |
| 8 | What is the natural history of incidentally discovered pituitary adenomas, and which ophthalmic, biochemical, and radiological factors are important in determining the need for surgery? |
| 9 | How can we predict early inpatient complications, such as sodium disturbances, after pituitary surgery, and can these be better managed or even prevented with empirical therapy? |
| 10 | How can we optimize ophthalmic, biochemical, and radiological follow-up for patients after pituitary surgery? |
Fig. 2.
Infographic of the top 10 research priorities in pituitary surgery
Discussion
Pituitary surgery is critical for the management of pituitary nonfunctioning macroadenomas or secreting adenomas for most patients. Through international multistakeholder consensus, this PSP has identified the ‘top 10 priorities’ for future pituitary surgery research. The PSP collaborated with a variety of patient networks and professional bodies throughout the process to incorporate diverse perspectives and expertise in addressing the key priorities in pituitary surgery. Together, these established priorities provide a resource to inform research funding bodies and guide future pituitary surgery research. To our knowledge, this is the first attempt to formally identify research priorities in pituitary surgery.
Principal findings
The key themes emerging from the PSP include enhancing diagnosis and management, advancing surgical techniques and technologies, improving patient support and follow-up, and optimizing the prediction of outcomes and complications.
Improving the diagnosis and management of pituitary adenomas requires addressing delays in diagnosis, identified as the 3rd most important research priority. The often incidental, insidious, and nonspecific nature of these tumors makes them particularly challenging to diagnose, leading to significant under-recognition by healthcare professionals. On average, patients with NFPAs face a 2–3-year diagnostic delay, those with Cushing’s disease experience a 3–4-year delay, and those with acromegaly encounter a 5.5-year delay from symptom onset [20, 21]. Left undiagnosed or untreated, NFPAs and functioning adenomas can result in progressive tumor growth and invasion, further complicating treatment and resulting in irreversible morbidity and mortality [5, 22]. Thus, timely diagnosis is essential to maximize the chances of cure and reduce the systemic morbidity and mortality. To overcome these diagnostic challenges, it is essential to raise awareness at all levels of healthcare, enhance early detection efforts, improve understanding of the natural course of incidental adenomas, and ensure timely treatment. Emerging technologies, including computer-aided tools like natural language processing (NLP), are being explored to aid in early detection by identifying patterns in medical data, offering new avenues for earlier intervention [9].
Despite many advances in modern pituitary surgery [3–7], challenges persist in identifying the tumor-gland interface and safely resecting large or laterally extending tumors, while protecting surrounding neurovascular structures. Even when tumors are visualized, achieving the necessary dexterity and reach with current endoscopic instruments can be difficult, potentially affecting the extent of resection and overall surgical outcomes. As reflected by priority 4, there is a critical need to advance surgical techniques and integrate new technologies to overcome these challenges. Recent innovations in surgical technology, including advanced imaging, robotic systems, and artificial intelligence (AI) offer promising solutions. Studies have shown that AI-driven real-time instrument tracking in endoscopic pituitary surgery can predict a surgeon’s skill level, offering valuable insights into their performance. Additionally, an AI-assisted video coaching program has led to improvements in surgical performance and outcomes, highlighting the emerging impact of AI technologies in advancing surgical training and improving patient outcomes [23, 24]. Newer imaging modalities, including 7-Tesla MRI and molecular PET imaging integrated with machine learning, may improve lesion detection and tumor delineation, optimizing pre-operative planning [25–28]. Intraoperative imaging techniques, including MRI, ultrasound, and fluorescence imaging can further aid in real-time tumor identification and accurate delineation of the tumor-normal gland interface, enhancing surgical precision thereby reducing post-operative complications [29–32]. Additionally, the use of intra-operative robotic systems offers added articulation and improved precision during resection, increasing dexterity in addressing challenging surgical areas. The integration of robotic platforms—such as teleoperated, shared-control, and handheld systems with articulated end effectors—has shown promising results in pre-clinical studies and holds the potential to significantly transform pituitary surgery [33–35]. For instance, a handheld surgical robot with articulated end effectors has demonstrated advantages over conventional non-articulated endoscopic tools, offering a greater workspace, enhanced ergonomics, and improved performance in standard surgical tasks [34–36].
A primary focus within the area of patient support and follow-up is understanding the impact of pituitary surgery on QoL, demonstrated in the 1st, 5th, and 7th research priorities. Traditional assessments of surgical success typically rely on objective metrics such as the extent of resection, symptom improvement, and recurrence rates; however, these measures often overlook the patient’s subjective experience and overall QoL. To bridge this gap, patient-reported outcome measures (PROMs) can be used to more accurately capture the true impact of surgery on QoL. In response to this need, recent studies have developed PROMs specifically tailored for patients undergoing pituitary surgery [37]. While these measures hold promise, they require external validation and broader adoption to be effectively integrated into future research, ensuring that they address QoL concerns and align care with patient-centered priorities [38, 39]. Beyond QoL, addressing the psychological and social needs of patients undergoing pituitary surgery is equally critical, as reflected in the 5th and 7th research priorities. Meeting these needs is consistent with priorities set by other PSPs [40–42]. There are significant gaps in the provision of psychological, emotional, and social support for patients with pituitary adenomas, often exacerbated by the psychosocial burden placed on partners and caregivers [43, 44]. This highlights the urgent need for targeted research and the development of supportive interventions—including psychological, educational, and social resources—to effectively address the complex psychosocial challenges faced by patients, families, and caregivers throughout the treatment journey.
Accurately predicting postoperative complications and long-term outcomes remains a critical unmet need in pituitary surgery, as reflected in the 2nd, 9th, and 10th research priorities. Postoperative complications, such as CSF leak, which can occur in up to 5% of cases, reductions in QoL secondary to ongoing sinonasal issues, and panhypopituitarism, highlight the importance of addressing these challenges [8]. Effective prediction allows for improved risk stratification and the development of personalized treatment strategies, such as tailored monitoring, preventive measures, and optimized discharge protocols. Additionally, predicting remission or recurrence is essential for long-term management, particularly for NFPAs, where recurrence rates remain high—ranging from 30 to 50% within 5–10 years, even after radiologically confirmed complete resection [45–48]. Similar challenges exist with functioning adenomas, especially ACTH-secreting adenomas, where the risk of recurrence remains significant despite significant advancements in imaging and surgical techniques over the past four decades [10, 49]. To address these issues, emerging solutions, including multimodal machine learning tools and novel digital biomarkers, have been developed to enhance outcome prediction and support clinical decision-making [9]. These innovations aim to improve precision in post-operative risk assessments and guide follow-up strategies, potentially reducing recurrence rates and improving patient outcomes. While early evidence shows promise for these tools, further research is needed to validate their effectiveness and ensure their safe integration into clinical practice. Continued advancements in predictive technologies are crucial for bridging the gap in post-operative care and translating these developments into improved outcomes for patients undergoing pituitary surgery.
Strengths and limitations
This study has several strengths. Importantly, the study comprised a diverse group of relevant stakeholders, including patients, charity representatives, and healthcare professionals, who provided valuable insights throughout the patient pathway and ensured that various treatment-related concerns were addressed. Furthermore, user-friendly outreach materials—such as posters, animated videos, articles, and presentations—were developed with support from public engagement specialists to promote awareness and facilitate participation (www.pit-cop.com). Strong engagement was evident throughout the process, with over 300 stakeholders contributing their perspectives, predominantly from patients. At each stage of the process, the PSP ensured balanced representation from all stakeholder groups. During the consensus meeting, there was equal representation of healthcare professionals and service users (1:1 ratio). Accordingly, these strengths assure that the final top 10 research priorities accurately reflect broad stakeholder involvement and a representative sample of the diverse perspectives and interests of all participants.
We acknowledge a number of limitations. The PSP was conducted via online surveys to identify and prioritize patient needs. This online approach may have inadvertently excluded individuals with challenged technical literacy and those with visual impairments in navigating the survey. Furthermore, despite considerable efforts to ensure survey dissemination, ethnic minority groups appeared to be underrepresented in the service user group, highlighting a limitation in capturing the perspectives and experiences of a more diverse population. Recognizing and addressing these barriers is crucial for fostering inclusivity, preventing health disparities, and ensuring that all individuals can voice their concerns and access the resources needed to improve their care.
Implications and future research
The top 10 research priorities, available at www.pit-cop.com, offer researchers and funding agencies clear guidance on where to concentrate their efforts, both in the short and long term, while also informing decisions on resource allocation. We invite researchers and clinicians to collaborate on advancing the top 10 research priorities in pituitary surgery, as identified through our consensus process. The next steps involve translating these priorities into researchable questions and engaging with funders, patients, and healthcare professionals to design and deliver studies to address these important issues. It is important to recognize that all priorities discussed were considered of value. As such, our main summary report will also include the priorities not represented in the top 10, i.e., the 33 summary priorities and priorities 11–15 ranked at the consensus (Supplementary Table 3). This will be sent to our partner organisations including the Pituitary Society and the Pituitary Foundation to inform and guide any researchers focusing on these specific areas.
Future studies in tumor pathophysiology are required to better understand the interactions between adenoma tissue and adjacent structures, as well as the relationship between hormone production and cellular proliferation. These investigations will be essential for improving prognostic accuracy, supporting the development of targeted adjuvant therapies, and potentially uncovering new therapeutic targets for more personalized treatment strategies.
Assessing the impact of the PSP will be vital to ensure that identified priorities are being used to guide meaningful research and drive improvements in clinical practice. The impact of the PSP will be evaluated via an online survey distributed to our partner organizations following dissemination of the results. Respondents will be asked to describe any research grant applications, or any other research activity inspired by the PSP results. Furthermore, research outputs relating to the PSP will also be tracked via systematic reviews to identify studies addressing the priorities and to assess how frequently the top 10 list is cited in research articles. Additionally, we will identify changes in health policies, clinical guidelines, or practice standards that have integrated any of the PSP priorities. Finally, as new research addresses existing priorities, it will then be necessary to reevaluate and re-identify research priorities for pituitary surgery. This will ensure that research remains relevant and continues to reflect the current needs and interests of all stakeholder groups.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the National Brain Appeal, Wellcome / EPSRC Centre for Interventional and Surgical Sciences (WEISS), University College London Hospitals, University College London, The University of Cambridge, The Pituitary Foundation, and the Pituitary Society for supporting the research.
PitCop Collaborative Group members: Alexandros Boukas, Alison Julia Bryant, Amanda Ann Brodie, Anastasia Dimakopoulou, Andrew Allenson, Andrew John Blamey, Andrew Thornton, Anita Evans, Anne-Marie Burke, Annmarie Maybey, Anthony James Woods, Ashley Grossman, Aswin Chari, Axel Petzold, Barbara Jean Briddock, Barry Culpin, Bilam Versha Datta, Briet Claire, Carmen Mulholland, Caroline Hayhurst, Catherine Bray, Chelsey Campbell, Chloe Cam, Christopher Allen Lindsay, Claude fabien Litré, Claudiu Matei, Colin Omorodion, Colin Victor Betteley, Dana L Rowland, Daniel Flanagan, David Collins, David Edward Perry, David Henry Eidmans, David Jonathan Collins, David Kass, David Roytowski, Deborah Hepburn, Deborah Samantha McKay Hewison, Debra Maxine Gordon, Dewara Handi Preethi Dissanayake, Dhaval Shukla, Dhulashiha Jegavanthan, Elizabeth Swingler, Emily Andrew, Faisal Hasan, Francesca Swords, Gemma Leanne Jones, Georgios Tsermoulas, Heather Harris, Heather Theoret Rockwell, Helen Baxter, Helen Louise Simpson, Hilary Waddington, Ian Nigel Dibb, Ismat Shafiq, Jacek K.Kunicki, Jade-Lea Halkier, James A. Balogun, James Alexander John King, James Ayokunle Balogun, James MacFarlane, James Towill, Jason Sheehan, Jayne Eileen Baker, Jeanette Curran, Jemma Farrell, Jenny Lindsay, Jessica Atkinson, Jill Sisco, Joachim Starup-Hansen, Joanne Hutchinson, Joao Paulo Almeida, John Earl Rose, John Newell-Price, John Patrick Boyd,, Jonathan Chainey, Jonathan Shapey, Julia Marie Harvey, Karen Ann Robins, Kristian Aquilina, Kyle Schweitzer, Laura J Scammell, Lisa Abrams, Litre, L-J Evans, Lucy Jane Crossley, Lynne Boudebza, Maddison Broadbent, Margaret Gray, Maria Hemmings, Marie-Ange Mignot, Mark Gruppetta, Martin Doughty, Mauricio Alvarez, Mia Littrell, Michael Buchfelder, Michael Crisp, Michelle Fattorini, Miles Levy, Mohsen Javadpour, Mollie Pullin, Muhwezi Trevor Emmanuel, Natalie Murphy, Nick Phillips, Olabisi Sanusi, Patricia Gildroy, Paul Meakin, Paul V Carroll, Pauline Swindells, Pejman Zoroufchian Moghadam, Peter Johnson Fenwick, Pouneh Fazeli, Rachael Burnham, Ramez Wadie Kirollos, Rebecca O’Hooley, Rhannon Lobo,Roland Chesney, Rosalind Way, Sally Barker-Dodds, Saurabh Sinha, Sian Sheppard, Soham Bandyopadhyay, Sunita M. C. De Sousa, Susie Frost, Suzanna Byrne, Tahir Zaman, Tara Kearney, Theofanis Giannis, Thierry Brue, Timothy Pearce, Tracey Christy, Tracey Morris, Trevor Maurice Williams, Tsegazeab Laeke, Varun R. Kshettry, MD, Wendy Tilley, Zeid Abussuud.
Appendix 1: Demographics of the pituitary surgery steering group members
| Steering committee member | Stakeholder group/ speciality |
|---|---|
| Adam Mamelak | Neurosurgery |
| Alexandra Valetopoulou | Neurosurgery Trainee |
| Angelos Kolias | Neurosurgery |
| Ann McCormack | Endocrinology |
| Anouk Borg | Neurosurgery |
| Danyal Z Khan | Neurosurgery Trainee |
| Fion Bremner | Ophthalmology |
| Gabriel Zada | Neurosurgery |
| Gerald Raverot | Endocrinology |
| Hani Marcus | Neurosurgery |
| Inma Serrano | Clinical Nurse Specialist |
| Joy Ginn | Patient and Charity Representative |
| Juan Fernandez-Miranda | Neurosurgery |
| Katherine Miszkiel | Radiology |
| Maria Fleseriu | Endocrinology |
| Mark Gurnell | Endocrinology |
| Márta Korbonits | Endocrinology |
| Mathew Geltzeiler | Otolaryngology |
| Melmed Shlomo | Endocrinology |
| Michael Buchfelder | Neurosurgery |
| Michael Kosmin | Oncology |
| Neil Dorward | Neurosurgery |
| Nicola Newall | Neurosurgery Trainee |
| Olympia Koulouri | Endocrinology |
| Pat McBride | Patient and Charity Representative |
| Pierre Bouloux | Endocrinology |
| Richard Mannion | Neurosurgery |
| Simon Cudlip | Neurosurgery |
| Stephanie Baldeweg | Endocrinology |
| Steve Harris | Patient and Charity Representative |
| Theodore Schwartz | Neurosurgery |
| Tom Santarius | Neurosurgery |
| William Drake | Endocrinology |
| Zane Jaunmuktane | Pathology |
Author contributions
NN: Conceptualization, Data curation, Formal analysis, Writing—original draft, Writing—review & editing. AV, DZK: Conceptualization, Data curation, Formal analysis, Writing—review & editing. HJM, AGK, SEB: Conceptualization, Supervision, Writing—review & editing. AB, PMGB, FB, MB, SC, ND, WMD, JCF, MF, MG, JG, MG, SH, ZJ, MK, MK, OK, HLH, ANM, RM, PM, AIM, SM, KAM, GR, TS, THS, IS, GZ: Writing—review & editing. All the Authors reviewed the manuscript and approved its final version.
Funding
This work was supported by The National Brain Appeal and the Wellcome EPSRC Centre for Interventional and Surgical Sciences. The funding bodies had no role in the design of the study, collection, analysis, interpretation of data, or in writing the manuscript. H.J.M. is supported by grants from the Wellcome (203145Z/16/Z) EPSRC (NS/A000050/1) Centre for Interventional and Surgical Sciences and National Institute for Health and Care Research (NIHR) Biomedical Research Centre at University College London. H.J.M is also employed by Panda Surgical and holds shares in the company. D.Z.K. is supported by an NIHR Academic Clinical Fellowship. A.G.K. is supported by the NIHR Health Technology Assessment program, the NIHR Global Health program, and the Wellcome Trust Institutional Strategic Support Fund. MG is supported by the Cambridge NIHR Biomedical Research Centre.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
PitCop Collaborative members are listed in the Acknowledgments section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stephanie E. Baldeweg, Angelos G. Kolias, and Hani J. Marcus are joint senior authors.
References
- 1.Melmed S, Kaiser UB, Lopes MB, Bertherat J, Syro LV, Raverot G et al (2022) Clinical biology of the pituitary adenoma. Endocr Rev 43(6):1003–1037. 10.1210/endrev/bnac010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.aly AF, Beckers A. The epidemiology of pituitary adenomas. Endocrinol Metab Clin North Am. 2020 Sep 1;49(3):347–55. 10.1016/j.ecl.2020.04.002. [DOI] [PubMed]
- 3.Petersenn S, Fleseriu M, Casanueva FF, Giustina A, Biermasz N, Biller BM et al (2023) Diagnosis and management of prolactin-secreting pituitary adenomas: a Pituitary Society international Consensus Statement. Nat Rev Endocrinol 19(12):722–740. 10.1038/s41574-023-00886-5 [DOI] [PubMed] [Google Scholar]
- 4.Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR et al (2021) Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol 9(12):847–875. 10.1016/S2213-8587(21)00235-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleseriu M, Varlamov EV, Hinojosa-Amaya JM, Langlois F, Melmed S (2023) An individualized approach to the management of Cushing disease. Nat Rev Endocrinol 19(10):581–599. 10.1038/s41574-023-00868-7 [DOI] [PubMed] [Google Scholar]
- 6.Fleseriu M, Biller BMK, Freda PU, Gadelha MR, Giustina A, Katznelson L et al (2021) A pituitary society update to acromegaly management guidelines. Pituitary 24:1–3. 10.1007/s11102-020-01091-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giustina A, Barkhoudarian G, Beckers A, Ben-Shlomo A, Biermasz N, Biller B et al (2020) Multidisciplinary management of acromegaly: a consensus. Rev Endocr Metab Disord 21:667–678. 10.1007/s11154-020-09588-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CRANIAL Consortium (2023) CSF rhinorrhoea after endonasal intervention to the skull base (CRANIAL): a multicentre prospective observational study. Front Oncol 12:1049627. 10.3389/fonc.2022.1049627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan DZ, Hanrahan JG, Baldeweg SE, Dorward NL, Stoyanov D, Marcus HJ (2023) Current and future advances in surgical therapy for pituitary adenoma. Endocr Rev 44(5):947–959. 10.1210/endrev/bnad014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh CH, Khan DZ, Digpal R, Layard Horsfall H, Ali AMS, Baldeweg SE et al (2023) The clinical outcomes of imaging modalities for surgical management Cushing’s disease—a systematic review and meta-analysis. Front Endocrinol 13(13):1090144. 10.3389/fendo.2022.1090144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vega-Beyhart A, Enriquez-Estrada VM, Bello-Chavolla OY, Torres-Victoria TR, Martínez-Sánchez FD, López-Navarro JM et al (2019) Quality of life is significantly impaired in both secretory and non-functioning pituitary adenomas. Clin Endocrinol 90(3):457–467. 10.1111/cen.13915 [DOI] [PubMed] [Google Scholar]
- 12.Grill C (2021) Involving stakeholders in research priority setting: a scoping review. Res Involve Engage 7:1–8. 10.1186/s40900-021-00318-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcus HJ, Khan DZ, Borg A, Buchfelder M, Cetas JS, Collins JW et al (2021) Pituitary society expert Delphi consensus: operative workflow in endoscopic transsphenoidal pituitary adenoma resection. Pituitary 24(6):839–853. 10.1007/s11102-021-01162-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, Gülmezoglu AM et al (2014) How to increase value and reduce waste when research priorities are set. Lancet 383(9912):156–165. 10.1016/S0140-6736(13)62229-1 [DOI] [PubMed] [Google Scholar]
- 15.Davies BM, Khan DZ, Mowforth OD, McNair AGK, Gronlund T, Kolias AG et al (2019) RE-CODE DCM (REsearch Objectives and Common Data Elements for Degenerative Cervical Myelopathy): a consensus process to improve research efficiency in DCM, through establishment of a standardized dataset for clinical research and the definition of the research priorities. Global Spine J 9(1):65S-76S. 10.1177/2192568219832855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salman RA, Kitchen N, Thomson J, Ganesan V, Mallucci C, Radatz M (2016) Top ten research priorities for brain and spine cavernous malformations. Lancet Neurol 15(4):354–355. 10.1016/S1474-4422(16)00039-9 [DOI] [PubMed] [Google Scholar]
- 17.Van Middendorp JJ, Allison HC, Ahuja S, Bracher D, Dyson C, Fairbank J et al (2016) Top ten research priorities for spinal cord injury: the methodology and results of a British priority setting partnership. Spinal Cord 54(5):341–346. 10.1038/sc.2015.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The James Lind Alliance Guidebook (2021). www.jla.nihr.ac.uk. Accessed 2 Apr 2024
- 19.Tong A, Synnot A, Crowe S, Hill S, Matus A, Scholes-Robertson N et al (2019) Reporting guideline for priority setting of health research (REPRISE). BMC Med Res Methodol 19:1–1. 10.1186/s12874-019-0889-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreitschmann-Andermahr I, Psaras T, Tsiogka M, Starz D, Kleist B, Siegel S et al (2015) From first symptoms to final diagnosis of Cushing’s disease: experiences of 176 patients. Eur J Endocrinol 172(3):285–289. 10.1530/EJE-14-0766 [DOI] [PubMed] [Google Scholar]
- 21.Esposito D, Ragnarsson O, Johannsson G, Olsson DS (2020) Prolonged diagnostic delay in acromegaly is associated with increased morbidity and mortality. Eur J Endocrinol 182(6):523–531. 10.1530/EJE-20-0019 [DOI] [PubMed] [Google Scholar]
- 22.Fleseriu M, Langlois F, Lim DS, Varlamov EV, Melmed S (2022) Acromegaly: pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol 10(11):804–826. 10.1016/S2213-8587(22)00244-3 [DOI] [PubMed] [Google Scholar]
- 23.Das A, Sidiqi B, Mennillo L, Mao Z, Brudfors M, Xochicale M, Khan DZ et al (2024) Automated surgical skill assessment in endoscopic pituitary surgery using real-time instrument tracking on a high-fidelity bench-top phantom. Healthcare Technol Lett. 10.1049/htl2.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan DZ, Newall N, Koh CH, Das A, Aapan S, Layard Horsfall H et al (2024) Video-based performance analysis in pituitary surgery—part 2: artificial intelligence assisted surgical coaching. World Neurosurg. 10.1016/j.wneu.2024.07.219 [DOI] [PubMed] [Google Scholar]
- 25.Kim M, Kim HS, Kim HJ, Park JE, Park SY, Kim YH et al (2021) Thin-slice pituitary MRI with deep learning–based reconstruction: diagnostic performance in a postoperative setting. Radiology 298(1):114–122. 10.1148/radiol.2020200723 [DOI] [PubMed] [Google Scholar]
- 26.Rutland JW, Loewenstern J, Ranti D, Tsankova NM, Bellaire CP, Bederson JB et al (2020) Analysis of 7-tesla diffusion-weighted imaging in the prediction of pituitary macroadenoma consistency. J Neurosurg 134(3):771–779. 10.3171/2019.12.JNS192940 [DOI] [PubMed] [Google Scholar]
- 27.Koulouri O, Steuwe A, Gillett D, Hoole AC, Powlson AS, Donnelly NA et al (2015) A role for 11C-methionine PET imaging in ACTH-dependent Cushing’s syndrome. Eur J Endocrinol 173(4):M107–M120. 10.1530/EJE-15-0616 [DOI] [PubMed] [Google Scholar]
- 28.MacFarlane J, Bashari WA, Senanayake R, Gillett D, van der Meulen M, Powlson AS et al (2020) Advances in the imaging of pituitary tumors. Endocrinol Metab Clin 49(3):357–373. 10.1016/j.ecl.2020.06.002 [DOI] [PubMed] [Google Scholar]
- 29.Berkmann S, Fandino J, Zosso S, Killer HE, Remonda L, Landolt H (2011) Intraoperative magnetic resonance imaging and early prognosis for vision after transsphenoidal surgery for sellar lesions. J Neurosurg 115(3):518–527. 10.3171/2011.4.JNS101568 [DOI] [PubMed] [Google Scholar]
- 30.Zaidi HA, De Los RK, Barkhoudarian G, Litvack ZN, Bi WL, Rincon-Torroella J et al (2016) The utility of high-resolution intraoperative MRI in endoscopic transsphenoidal surgery for pituitary macroadenomas: early experience in the advanced multimodality image guided operating suite. Neurosurg Focus 40(3):E18. 10.3171/2016.1.FOCUS15515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabrilo I, Delaunay R, Heaysman CL, Ourselin S, Vitiello V, Vercauteren T et al (2022) A novel intraoperative ultrasound probe for transsphenoidal surgery: first-in-human study. Surgical Innovation 29(2):282–288. 10.1177/15533506211031091 [DOI] [PubMed] [Google Scholar]
- 32.Schmidt I, Vergeer RA, Postma MR, van den Berg G, Sterkenburg AJ, Korsten-Meijer AGW et al (2024) Fluorescence detection of pituitary neuroendocrine tumour during endoscopic transsphenoidal surgery using bevacizumab-800CW: a non-randomised, non-blinded, single centre feasibility and dose finding trial [DEPARTURE trial]. Eur J Nucl Med Mol Imaging 11:1–9. 10.1007/s00259-024-06947-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farooq MU, Baek H, Seung S, Cha K, You H, Kwon DS et al (2020) A stiffness adjustable 6-DOF robotic system for pituitary tumor resection under MRI. IEEE Access 20(8):192557–192568 [Google Scholar]
- 34.Aylmore H, Dimitrakakis E, Carmichael J, Khan DZ, Stoyanov D, Dorward NL et al (2022) Specialised surgical instruments for endoscopic and endoscope-assisted neurosurgery: a systematic review of safety, efficacy and usability. Cancers 14(12):2931. 10.3390/cancers14122931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimitrakakis E, Dwyer G, Newall N, Khan DZ, Marcus HJ, Stoyanov D (2024) Handheld robotic device for endoscopic neurosurgery: system integration and pre-clinical evaluation. Front Robot AI. 10.3389/frobt.2024.1400017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dimitrakakis E, Aylmore H, Lindenroth L, Dwyer G, Carmichael J, Khan DZ et al (2022) Robotic handle prototypes for endoscopic endonasal skull base surgery: pre-clinical randomised controlled trial of performance and ergonomics. Ann Biomed Eng 50(5):549–563. 10.1007/s10439-022-02942-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karvandi E, Hanrahan JG, Khan DZ, Boloux PM, Bremner F, Cabrilo I et al (2022) A patient-reported outcome measure for patients with pituitary adenoma undergoing transsphenoidal surgery. Pituitary 25(4):673–683. 10.1007/s11102-022-01251-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang EW, Zanation AM, Gardner PA, Schwartz TH, Eloy JA, Adappa ND et al (2019) ICAR: endoscopic skull-base surgery. Int Forum Allergy Rhinol. 9(S3):S145-365. 10.1002/alr.22326 [DOI] [PubMed] [Google Scholar]
- 39.Sarris CE, Little AS, Kshettry VR, Rosen MR, Rehl RM, Haegen TW et al (2021) Assessment of the validity of the sinonasal outcomes test-22 in pituitary surgery: a multicenter prospective trial. Laryngoscope 131(11):E2757–E2763. 10.1002/lary.29711 [DOI] [PubMed] [Google Scholar]
- 40.Jones J, Bhatt J, Avery J, Laupacis A, Cowan K, Basappa N et al (2017) The kidney cancer research priority-setting partnership: Identifying the top 10 research priorities as defined by patients, caregivers, and expert clinicians. Can Urol Assoc J 11(12):379. 10.5489/cuaj.4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finer S, Robb P, Cowan K, Daly A, Robertson E, Farmer A (2017) Top ten research priorities for type 2 diabetes: results from the Diabetes UK-James Lind Alliance Priority Setting Partnership. Lancet Diabetes Endocrinol 5(12):935–936. 10.1016/S2213-8587(17)30324-8 [DOI] [PubMed] [Google Scholar]
- 42.Potter S, Fairhurst K, Cowan K, Vincent S, Lewis I, Cutress RI et al (2023) Identifying research priorities in breast cancer surgery: a UK priority setting partnership with the James Lind Alliance. Breast Cancer Res Treat 197(1):39–49. 10.1007/s10549-022-06756-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donegan D, Gowan T, Gruber R, Cottingham A, Flanagan M, Erickson D et al (2021) The need for patient-centered education among patients newly diagnosed with a pituitary tumor. J Endocrine Soc. 5(6):bvab061. 10.1210/jendso/bvab061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andela CD, Repping-Wuts H, Stikkelbroeck NMML, Pronk MC, Tiemensma J, Hermus AR et al (2017) Enhanced self-efficacy after a self-management programme in pituitary disease: a randomized controlled trial. Eur J Endocrinol 177(1):59–72. 10.1530/EJE-16-1015 [DOI] [PubMed] [Google Scholar]
- 45.Losa M, Mortini P, Barzaghi R, Ribotto P, Terreni MR, Marzoli SB et al (2008) Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J Neurosurg 108(3):525–532. 10.3171/JNS/2008/108/3/0525 [DOI] [PubMed] [Google Scholar]
- 46.Esposito D, Olsson DS, Ragnarsson O, Buchfelder M, Skoglund T, Johannsson G (2019) Non-functioning pituitary adenomas: indications for pituitary surgery and post-surgical management. Pituitary 15(22):422–434. 10.1007/s11102-019-00960-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Sullivan EP, Woods C, Glynn N, Behan LA, Crowley R, O’Kelly P et al (2009) The natural history of surgically treated but radiotherapy-naïve nonfunctioning pituitary adenomas. Clin Endocrinol 71(5):709–714. 10.1111/j.1365-2265.2009.03583.x [DOI] [PubMed] [Google Scholar]
- 48.Brochier S, Galland F, Kujas M, Parker F, Gaillard S, Raftopoulos C et al (2010) Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: a study of 142 patients. Eur J Endocrinol 163(2):193–200. 10.1530/EJE-10-0255 [DOI] [PubMed] [Google Scholar]
- 49.Stroud A, Dhaliwal P, Alvarado R, Winder MJ, Jonker BP, Grayson JW et al (2020) Outcomes of pituitary surgery for Cushing’s disease: a systematic review and meta-analysis. Pituitary 23:595–609. 10.1007/s11102-020-01066-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.