Abstract
The active forms of STAT5A (signal transducer and activator of transcription 5A) and STAT5B are able to relieve the cytokine dependence of haematopoietic cells and to induce leukaemia in mice. We have demonstrated previously that activation of the PI3K (phosphoinositide 3-kinase) signalling cascade plays a major role in cell growth and survival induced by these proteins. Interaction between STAT5 and p85, the regulatory subunit of the PI3K, has been suggested to be required for this activation. We show in the present study that the scaffolding protein Gab2 [Grb2 (growth-factor-receptor-bound protein 2)-associated binder-2] is an essential component of this interaction. Gab2 is persistently tyrosine-phosphorylated in Ba/F3 cells expressing caSTAT5 (constitutively activated STAT5), independent of JAK2 (Janus kinase 2) activation where it interacts with STAT5, p85 and Grb2, but not with Shp2 [SH2 (Src homology 2)-domain-containing tyrosine phosphatase] proteins. Interaction of STAT5 with Gab2 was also observed in Ba/F3 cells stimulated with interleukin-3 or expressing the oncogenic fusion protein Tel–JAK2. The MAPKs (mitogen-activated protein kinases) ERK1 (extracellular-signal-regulated kinase 1) and ERK2 were constitutively activated in the caSTAT5-expressing cells and were found to be required for caSTAT5-induced cell proliferation. Overexpression of Gab2-3YF, a mutant of Gab2 incapable of binding PI3K, inhibited the proliferation and survival of caSTAT5-expressing cells as well as ERK1/2 and Akt/protein kinase B phosphorylation. Taken together, our results indicate that Gab2 is required for caSTAT5-induced cell proliferation by regulating both the PI3K/Akt and the Ras/MAPK pathways.
Keywords: Gab2, growth-factor-receptor-bound protein 2 (Grb2), mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K), proliferation, signal transducer and activator of transcription 5 (STAT5)
Abbreviations: ERK, extracellular-signal-regulated kinase; Grb2, growth-factor-receptor-bound protein 2; Gab2, Grb2-associated binder-2; GFP, green fluorescent protein; GST, glutathione S-transferase; IL-3, interleukin-3; IRS, insulin receptor substrate; JAK, Janus kinase; MAPK, mitogen-activated protein kinase; NP40, Nonidet P40; PH domain, pleckstrin homology domain; PI3K, phosphoinositide 3-kinase; SH2, Src homology 2; Shp2, SH2-domain-containing tyrosine phosphatase; STAT5, signal transducer and activator of transcription 5; caSTAT5, constitutively active STAT5
INTRODUCTION
STAT (signal transducer and activator of transcription) transcription factors play a central role in cytokine-dependent survival and proliferation of haematopoietic cells [1]. After cytokine addition, STAT proteins become tyrosine-phosphorylated and subsequently dimerize, forming homo- or hetero-dimers, translocate into the nucleus where they bind to specific elements in the promoter of target genes and activate transcription. The STAT protein family comprises seven members including the two closely related STAT5A and STAT5B molecules sharing 96% homology at the amino acid level. Mice in which STAT5a and STAT5b genes were deleted revealed redundant and specific functions of both proteins. STAT5a−/− mice have a profound defect in mammary gland development and in prolactin response, whereas STAT5b−/− mice display a defect in growth hormone response [2]. Simultaneous inactivation of STAT5a/b genes demonstrated the requirement of both proteins in myeloid and lymphoid cell proliferation. Indeed, erythroblasts, myeloid cells, mast cells, peripheral T-cells, NK cells and B cells display impaired proliferation and/or survival in mice lacking expression of STAT5 proteins [3–7]. STAT5 is also required to sustain the lympho-myeloid repopulating activity of haematopoietic stem cells [8].
Besides the physiological role of STAT5 in haematopoietic cell development, there is increasing evidence suggesting that inappropriate activation of STAT5 may contribute to the development of leukaemia and solid cancer [9–12]. STAT5 is frequently hyperactivated in cancer and leukaemia, most probably by alterations in tyrosine kinase or phosphatase activities. Constitutively active STAT5A or STAT5B mutants (caSTAT5) that closely mimic the wild-type STAT5 function have been identified by random mutagenesis. These mutants display oncogenic properties in vitro and in vivo and fully restore the defective phenotype of STAT5a/b−/− mice [13,14]. Since STAT5 is only a part of the distinct signalling cascades elicited by oncogenic tyrosine kinases or by cytokines, the use of caSTAT5 represents an attractive tool to determine the contribution of this transcription factor in cell growth and transformation. caSTAT5 promotes cell survival and/or proliferation by regulating the expression of genes involved in the control of cell cycle and survival like bcl-xL, cyclins D1 and D2, p21waf1 and pim-1 [4,15,16]. STAT5 proteins may also constitute important components of certain signal-transduction pathways. Indeed, STAT5 interacts with the SH2 (Src homology 2)-SH3 domain adapter protein CrkL and with PI3K (phosphoinositide 3-kinase) [17,18]. In addition, STAT5 has been shown to regulate Fas-mediated cell death [19]. Thus biological responses elicited by STAT5 may involve not only its nuclear function but also its capacity to regulate different signalling cascades.
We previously reported that caSTAT5 proteins (STAT5A1*6 and STAT5B1*6) interact with p85, the regulatory subunit of the PI3K and activate the PI3K/Akt pathway in Ba/F3 cells. This interaction was also demonstrated in cells transformed by the oncogenic fusion proteins Tel–JAK2 (where JAK2 stands for Janus kinase 2) as well as in cells stimulated with erythropoietin or IL-3 (interleukin-3) [20]. We demonstrate herein that STAT5 associates with the scaffolding protein Gab2 [Grb2 (growth-factor-receptor-bound protein 2)-associated binder-2], which plays an essential role in caSTAT5-induced cell proliferation and survival by activating the PI3K/Akt and Ras/MAPK pathways (where MAPK stands for mitogen-activated protein kinase).
EXPERIMENTAL
Cell culture and reagents
Parental Ba/F3 cells and Ba/F3 cell lines expressing STAT5A1*6 (caSTAT5A), STAT5B1*6 (caSTAT5B) (see Figure 1A) or the Tel–JAK2 proteins were maintained in RPMI 1640 medium as described previously [20,21]. Recombinant murine IL-3 was purchased from Valbiotech (Paris, France) and the MEK [MAPK/ERK (extracellular-signal-regulated kinase) kinase] inhibitor UO126 was from Sigma.
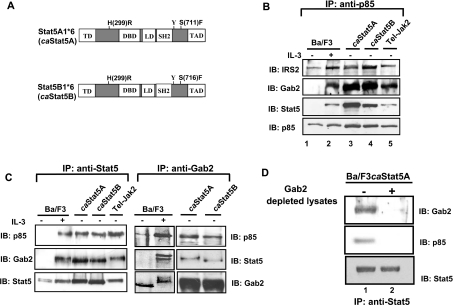
Figure 1. Interaction between STAT5, Gab2 and p85.
(A) Schematic representation of the caSTAT5 mutants (STAT5A1*6 and STAT5B1*6). The regulatory domains and mutations are shown. (B) Cell lysates were prepared from Ba/F3 cells stimulated (lane 2) or not (lane 1) with IL-3 (10 ng/ml) for 30 min or from Ba/F3 cells expressing caSTAT5A (lane 3), caSTAT5B (lane 4) or Tel–JAK2 (lane 5). The p85 subunit of PI3K was immunoprecipitated with specific antibodies and the presence of co-precipitated STAT5, IRS-2 and Gab2 was specifically assessed by Western blotting. The membrane was reprobed with anti-p85 antibodies. (C) The same cell lysates were used for immunoprecipitation with anti-STAT5 (left panel) and anti-Gab2 (right panel), then analysed by Western blotting for the presence of p85 and Gab2 or anti-p85 and STAT5 respectively. The membranes were reprobed with the antibodies used in immunoprecipitation. (D) STAT5 was immunoprecipitated from caSTAT5-containing cell lysates that have been or not immunodepleted of Gab2. The association of p85 with STAT5 was next determined by immunobloting with anti-p85 antibodies.
Plasmids and transfections
Gab2-3YF and Gab2-WT cDNAs were amplified by PCR from the p-EBB-Gab2-3YF and pEBB-Gab2-WT plasmids respectively and the Gab2/ΔSHP2 cDNA from the pMSCV/IRES plasmid, then subcloned at the NotI and XhoI sites of the pIRES-hrGFP vector (Stratagene, La Jolla, CA, U.S.A.; GFP stands for green fluorescent protein). In transient transfection assays, constructs (25 μg) were electroporated in parental Ba/F3, caSTAT5A-, caSTAT5B- or Tel–JAK2-expressing cells (250 V, 960 μF). Electroporated cells were expanded for 24 h in RPMI 1640 medium and the GFP+ cells were sorted by flow cytometry (Elite, Becton Dickinson, Le Pont de Claix, France). Proliferation and viability were then examined by counting viable cells using the Trypan Blue dye exclusion method.
Apoptosis studies
Cells were washed with PBS, 24 h after transfection with the Gab2-3YF construct and resuspended in 10 mM Hepes (pH 7.4), 140 mM NaCl and 2.5 mM CaCl2. Annexin V–PE was added to the cells for 15 min and the percentage of annexin V+, GFP+ cells was next determined by flow cytometry.
Western blotting and immunoprecipitation studies
NP40 (Nonidet P40) cell lysates were separated by SDS/PAGE and blotted on to a cellulose membrane (Hybond-C super membrane; Amersham Life Science). Blots were incubated with antibodies specific for: STAT5A and STAT5B (Zymed, San Francisco, CA, U.S.A.), STAT5 (BD Biosciences, San Diego, CA, U.S.A.), Gab2 (M19), Shp2 (SH2-domain-containing tyrosine phosphatase; C-18), IRS-2 (insulin receptor substrate-2; H-205), Akt (H-136), JAK2 (C-20), actin (C-11; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) and Grb2 (Cell Signaling Technology, Beverly, MA, U.S.A.). We used also antibodies raised against the following phosphorylated proteins: P-STAT5 (Zymed), P-Akt (Ser473), P-ERK1/ERK2 (Thr202/Tyr204) (Cell Signaling Technology), P-JAK2 (Tyr1007) (Santa Cruz Biotechnology). The anti-FLAG and the anti-phosphotyrosine (4G10) monoclonal antibodies were purchased from Stratagene and Upstate Biotechnology (Lake Placid, NY, U.S.A.) respectively. The rabbit polyclonal anti-p85 antibody was described elsewhere [18]. Immunoprecipitation experiments were performed as described previously [18]. In some experiments, the levels of phosphorylated ERK1/2 and Akt were quantified by using the NIH Image software.
In vitro association with p85
The GST (glutathione S-transferase) fusion protein containing the two SH2 domains of p85 was produced in bacteria and purified on glutathione–agarose beads. In vitro mixing experiments were performed as follows: cell extracts (1.5 mg of protein) were first precleared with a GST protein bound to glutathione–agarose for 1 h. Cell extracts were next mixed with GST-SH2-p85 fusion proteins at room temperature (18 °C) for 1 h and then the glutathione–agarose beads coupled with GST-SH2-p85 were extensively washed six times with NP40 lysis buffer containing 1% NP40, 50 mM Tris (pH 7.5), 150 mM NaCl, 10% (v/v) glycerol, 0.5 mM PMSF, 1 mM Na2VO4 and a cocktail of protease inhibitors (Roche, Indianapolis, IN, U.S.A.). Proteins were eluted using Laemmli's buffer [39], subjected to SDS/PAGE and immunoblotting was performed with specific antibodies.
RESULTS
STAT5 interacts with Gab2 and p85 in Ba/F3 cells
Although the interaction between STAT5 and p85 has been observed in various cell types, it remained unclear whether STAT5A1*6 or STAT5B1*6 (referred in the text as caSTAT5A and caSTAT5B, Figure 1A) could trigger PI3K activation and cell growth via this interaction or indirectly via a secreted humoral factor. Secretion of such a STAT5-induced factor has been recently suggested to contribute to the activation of NF-κB (nuclear factor κB) in Ba/F3 cells [22]. We tested the capacity of conditioned media obtained from caSTAT5A- and caSTAT5B- expressing Ba/F3 cell cultures to induce the proliferation of IL-3-starved Ba/F3 cells, but failed to observe any growth-promoting effects (results not shown). In addition, expression of caSTAT5 rapidly relieved the IL-3 dependence of Ba/F3 cells within 24 h (results not shown), indicating that induction of growth by caSTAT5 was due rather to its signalling properties than the effects of putative humoral factor acting in an autocrine loop. We therefore extensively analysed the way in which p85 interacts with STAT5 in Ba/F3 cells expressing caSTAT5A or caSTAT5B and, as control, in Ba/F3 cells stimulated with IL-3 or expressing the oncogenic fusion protein Tel–JAK2. It has been previously shown that in cytokine- or growth-factor-stimulated cells, p85 interacts with the PH domain (pleckstrin homology domain)-containing adapter proteins Gab2 and/or IRS-2 that play a crucial role in PI3K activation [23,24]. To analyse this possible in situ association, p85 was immunoprecipitated from lysates of Ba/F3 cells stimulated or not with IL-3 and from Ba/F3 cells expressing caSTAT5A, caSTAT5B or Tel–JAK2. Immunoblotting was next performed with anti-STAT5, anti-IRS-2 or anti-Gab2 antibodies (Figure 1B). Co-precipitation of IRS-2 with p85 was observed in unstimulated Ba/F3 cells and was further increased upon IL-3 treatment (lane 2). IL-3 also induced association of p85 with Gab2 and STAT5. This interaction was constitutive in Ba/F3 cells expressing caSTAT5A, caSTAT5B or Tel–JAK2 (lanes 3–5). We next determined whether Gab2 or IRS-2 interacts with STAT5 in these different cell lines. STAT5A and STAT5B were precipitated with specific monoclonal antibodies using the different cell lysates as described above and the co-immunoprecipitation of Gab2 and p85 was next estimated by immunoblotting (Figure 1C, left panel). Again, IL-3 stimulation of Ba/F3 cells induced the interaction of Gab2 and p85 with STAT5, whereas constitutive association of these proteins was observed in caSTAT5- or Tel–JAK2-expressing cells. In contrast, we failed to detect the presence of IRS-2 in the STAT5 immunoprecipitates (results not shown). To confirm the specificity of the Gab2–STAT5 interaction, Gab2 was immunoprecipitated from the same cell lysates and the presence of co-precipitated STAT5 was assessed by immunoblotting (Figure 1C, right panel). STAT5 was associated with Gab2 in IL-3-stimulated Ba/F3 cells and in caSTAT5-expressing Ba/F3 cells. The presence of p85 in both Gab2 and STAT5 immunoprecipitates was also confirmed by reprobing the membrane with anti-p85 antibody. To determine whether Gab2 was required for the STAT5–PI3K interaction, we immunoprecipitated STAT5 from a caSTAT5Ba/F3 cell lysate depleted of Gab2 by prior immunoprecipitation with Gab2 antibodies. The presence of p85 in the immunoprecipitates was next analysed by immunoblotting (Figure 1D). In Gab2-depleted lysates, p85 did not interact with STAT5, indicating that the binding of STAT5 to p85 requires Gab2 (Figure 1D, lane 2). Collectively, these results suggest that STAT5 interacts with Gab2 but not with IRS-2 in Ba/F3 cells and together with p85 forms a signalling complex.
Gab2 is tyrosine-phosphorylated in caSTAT5-expressing Ba/F3 cells
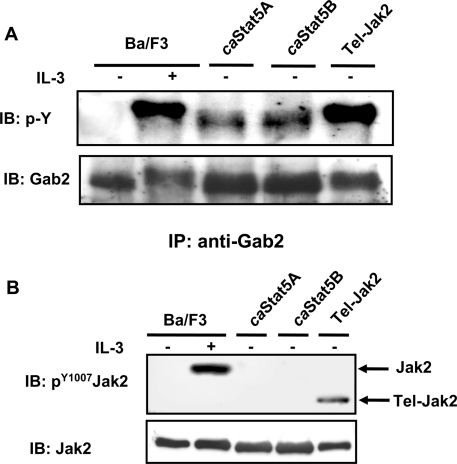
It is assumed that tyrosine phosphorylation of Gab2 is required before its interaction with p85. We thus evaluated whether Gab2 was tyrosine-phosphorylated in caSTAT5-expressing Ba/F3 cells. Gab2 was immunoprecipitated from caSTAT5A- or caSTAT5B-expressing Ba/F3 cells and revealed by Western blotting using the 4G10 anti-phosphotyrosine antibody. As a control, tyrosine phosphorylation of Gab2 was also examined in Ba/F3 cells stimulated with IL-3 or expressing Tel–JAK2 (Figure 2A). Gab2 was tyrosine-phosphorylated in IL-3-stimulated Ba/F3 cells as well as in cells expressing caSTAT5 or Tel–JAK2. However, tyrosine phosphorylation of Gab2 was less pronounced in caSTAT5-expressing Ba/F3 cells as estimated by the intensity of the Gab2 band and its faster mobility in the gel. We next analysed the contribution of JAK2 in the phosphorylation of Gab2 in caSTAT5-expressing cells. Western blots were performed using an antibody that specifically recognizes the residue phospho-Tyr1007 of JAK2 which plays a crucial role in its activation (Figure 2B). No activated JAK2 was detected in cells expressing the caSTAT5 proteins, whereas both JAK2 and Tel–JAK2 were phosphorylated in Ba/F3 cells stimulated with IL-3 or expressing the oncogenic fusion protein respectively. We also failed to detect the tyrosine phosphorylation of JAK2 immunoprecipitated from caSTAT5Ba/F3 cell extracts (results not shown). Thus the low but significant tyrosine phosphorylation of Gab2 is independent of JAK2 activation in Ba/F3 cells expressing caSTAT5.
Figure 2. Gab2 is tyrosine-phosphorylated in caSTAT5-expressing Ba/F3 cells.
(A) NP40 cell lysates were first subjected to immunoprecipitation with an anti-Gab2 antibody and then analysed by immunoblotting with an anti-phosphotyrosine antibody (4G10). The membrane was reprobed with anti-Gab2 antibody. (B) Total cell lysates were subjected to SDS/PAGE and then immunoblotted with anti-phospho-Tyr1007-JAK2 antibody. The membrane was reprobed with an anti-JAK2 antibody.
In vitro association of Gab2 and STAT5 with the SH2 domains of p85
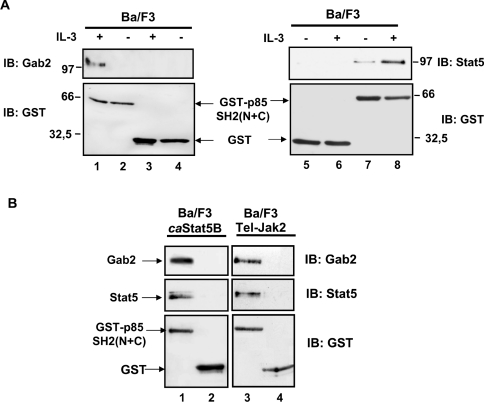
The structure of p85 is characterized by the presence of two SH2 domains. We thus aimed to determine whether the interaction of p85 with Gab2 and STAT5 was SH2-domain-dependent. A GST fusion protein containing both the N- and C-terminal SH2 domains of p85 [GST–SH2 (N+C)] was mixed with cell lysates from Ba/F3 cells stimulated or not with IL-3 and from Tel–JAK2- or caSTAT5B-expressing Ba/F3 cells. Proteins bound to the GST-p85-SH2 (N+C) were analysed by immunoblotting with anti-Gab2 and anti-STAT5 antibodies. In Ba/F3 cells, IL-3 induced the binding of the GST-p85-SH2 (N+C) protein to Gab2 and STAT5 (Figure 3A, lanes 1 and 8). No interactions were observed when extracts were mixed with the GST protein alone (lanes 3–6). In Tel–JAK2 or caSTAT5B cell lysates, Gab2 and STAT5 were constitutively bound to the GST–SH2 (N+C) protein (Figure 3B, lanes 1 and 3). We conclude from these experiments that the p85 SH2 domains are sufficient for the interaction of p85 with Gab2 and tyrosine-phosphorylated STAT5.
Figure 3. In vitro association of Gab2, STAT5 and the SH2 domains of p85.
(A) Cell lysates were prepared from Ba/F3 cells stimulated or not with IL-3 for 30 min and incubated with glutathione–agarose beads coupled with either GST protein alone (lanes 3–6) or GST-p85-SH2 (N+C) (lanes 1, 2, 7 and 8). Proteins eluted from the beads were analysed by Western blotting with anti-Gab2 (left panel) or anti-STAT5 (right panel) antibodies. Membranes were reprobed with anti-GST antibody. (B) A similar experiment was conducted with cell extracts from caSTAT5B- and Tel–JAK2-expressing cells. Interaction of STAT5 and Gab2 with GST-p85-SH2 (N+C) (lanes 1 and 3) or GST alone (lanes 2 and 4) was determined by immunoblotting. The membrane was reprobed with the anti-GST antibody.
Gab2 interacts with Grb2 but not Shp2 in caSTAT5-expressing Ba/F3 cells
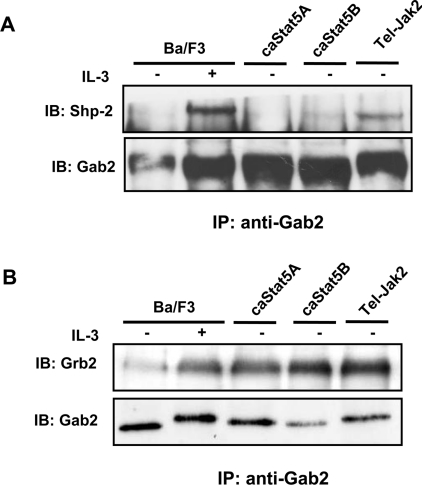
The Shp2 tyrosine phosphatase and the Grb2 adapter are the major Gab2-binding proteins in IL-3-stimulated Ba/F3 cells and such interactions have been shown to trigger the activation of the Ras/MAPK signalling cascade [25–27]. We therefore analysed the association of Gab2 with Shp2 and Grb2 in Ba/F3 cells ex-pressing caSTAT5 or Tel–JAK2 as well as in parental Ba/F3 cells stimulated with IL-3. Immunoprecipitations of Gab2 were performed using the different Ba/F3 cell lysates, and the presence of Shp2 and Grb2 in the immunoprecipitates was examined by immunoblotting. Association of Shp2 with Gab2 was detected in Ba/F3 cells stimulated with IL-3 or expressing the Tel–JAK2 oncogenic fusion, but not significantly in caSTAT5-expressing cells (Figure 4A). In contrast, Grb2 was found associated with Gab2 in all cell lines (Figure 4B). This interaction was present at weak levels in starved Ba/F3 cells and was sharply enhanced upon IL-3 stimulation. Association of Grb2 with Gab2 was constitutive in cells expressing caSTAT5 or Tel–JAK2. Similarly, Grb2 but not Shp2 was present in the STAT5 immunoprecipitates from caSTAT5-expressing Ba/F3 cell extracts (results not shown). Taken together, these findings suggest that Gab2 and STAT5 interact with Grb2 but not Shp2 in caSTAT5-expressing Ba/F3 cells.
Figure 4. Gab2 associates with Grb2 but not with Shp2 in caSTAT5-expressing Ba/F3 cells.
Anti-Gab2 immunoprecipitates obtained from NP40 cell lysates were analysed by immunoblotting for the presence of Shp2 (A) or Grb2 (B). Membranes were reprobed with the anti-Gab2 antibody.
MAPK signalling is persistently activated in caSTAT5-expressing Ba/F3 cells leading to cell proliferation
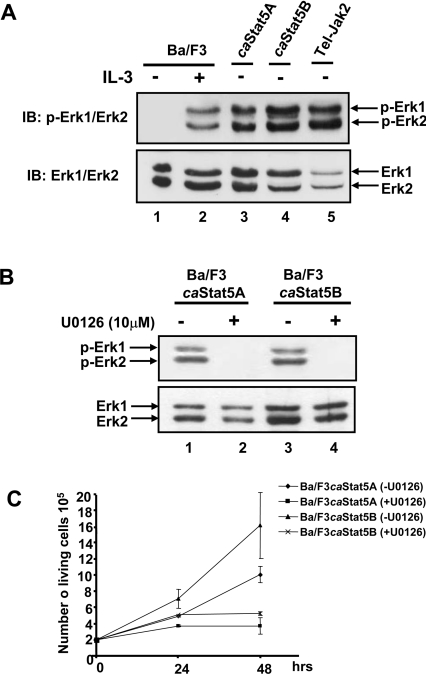
The absence of Shp2 in the Gab2/caSTAT5 immunocomplex prompted us to analyse whether or not the phosphorylation of ERK1 and ERK2 was affected in caSTAT5-expressing cells. Cell extracts were prepared as described above and analysed by Western blotting with specific anti-phospho-ERK antibodies (Figure 5A). IL-3 induced the phosphorylation of ERK1 and ERK2 (lane 2), while this activation was found to be constitutive in caSTAT5- and Tel–JAK2-expressing cells (lanes 3–5). To evaluate further the contribution of these protein kinases in the growth of caSTAT5-expressing cells, cells were incubated with the MEK inhibitor U0126. Addition of 10 μM U0126 abrogated ERK1 and ERK2 phosphorylation and cell proliferation (Figures 5B, lanes 2 and 4, and Figure 5C). These results indicate that activation of ERK1/2 plays an important role in caSTAT5-induced cell proliferation and does not require a Gab2–Shp2 interaction.
Figure 5. Constitutive activation of ERK1 and ERK2 in caSTAT5-expressing Ba/F3 cells: role in proliferation.
(A) Total cell lysates from Ba/F3 cells unstimulated (lane 1) or stimulated (lane 2) with IL-3 (10 ng/ml for 30 min) or from caSTAT5A- (lane 3), caSTAT5B- (lane 4) and Tel–JAK2- (lane 5) expressing cells were subjected to SDS/PAGE and immunoblotted with anti-phospho-ERK1/ERK2 antibody. The membrane was reprobed with an anti-ERK1/ERK2 antibody. (B) caSTAT5A- and caSTAT5B-expressing Ba/F3 cells were left untreated (ethanol) (lanes 1 and 3) or treated with U0126 (10 μM) for 24 h (lanes 2 and 4). Total cell lysates were prepared and analysed by Western blotting with anti-phospho-ERK1/ERK2 antibody. The membrane was reprobed with an anti-ERK1/ERK2 antibody. (C) caSTAT5A- and caSTAT5B-expressing Ba/F3 cells were treated or not with U0126 (10 μM) for the indicated time periods and the number of viable cells was determined daily, using the Trypan Blue dye exclusion method. Results shown are representative of three experiments.
Gab2 is required for the proliferation and survival of caSTAT5-expressing Ba/F3 cells
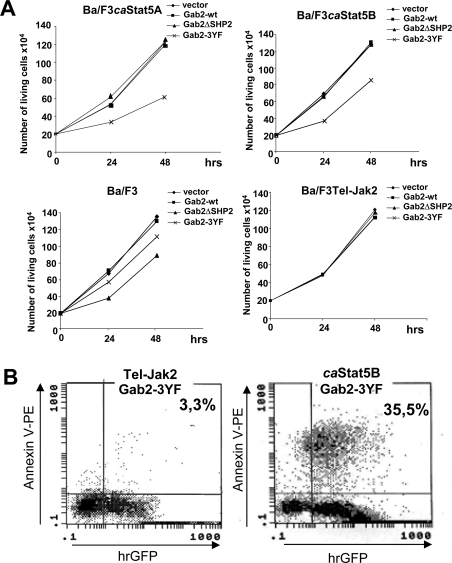
We next evaluated the role of Gab2 in caSTAT5-induced proliferation and survival in Ba/F3 cells. We used a Gab2 mutant (Gab2-3YF) in which the residues Tyr441, Tyr465 and Tyr574, three potential p85-binding sites, were changed to phenylalanine and a Gab2ΔSHP2 mutant containing phenylalanine substitutions at the two potential Shp2-binding sites (Tyr604/Tyr633). Expression of such mutants has been previously shown to inhibit the activation of the PI3K and Ras/MAPK pathways respectively [25,28]. The Gab2 mutants or the wild-type Gab2 were introduced in a bi-cistronic expression vector carrying the hrGFP gene (pIREShrGFP) and were fused in their C-terminal part to the FLAG-tagged sequence. Ba/F3 cells and caSTAT5- and Tel–JAK2-expressing Ba/F3 cells were transiently transfected with the different Gab2 constructs or the empty vector and, 24 h later, GFP-expressing cells were sorted by flow cytometry. The growth rate of GFP+ cells was next determined in a time-course experiment (Figure 6A). Expression of Gab2-3YF inhibited the proliferation of caSTAT5A- and caSTAT5B-expressing Ba/F3 cells, while expression of the Gab2-WT or the Gab2ΔSHP2 mutant were without effect. In contrast, the growth of parental Ba/F3 cells was affected by the Gab2ΔSHP2 and to a lesser extent by the Gab2-3YF. Tel–JAK2-expressing Ba/F3 cells remained insensitive to the expression of these mutants.
Figure 6. Overexpression of Gab2-3YF inhibits growth and induces apoptosis of the Ba/F3 cells expressing caSTAT5.
(A) Cells were electroporated with the different Gab2 constructs or the empty vector as indicated. On the following day, GFP+ cells were sorted by flow cytometry and analysed for growth by cell counting using the Trypan Blue dye exclusion method. Results shown are representative of three different transfection assays. (B) caSTAT5B- and Tel–JAK2-expressing Ba/F3 cells were labelled with annexin V–PE 24 h post-transfection, and the percentage of double positive (annexin V+, GFP+) apoptotic cells was determined by flow cytometry.
We next examined whether expression of Gab2-3YF interfered in the survival of caSTAT5-expressing cells. caSTAT5B- or Tel–JAK2-expressing Ba/F3 cells transfected with the Gab2-3YF construct were labelled with PE-conjugated annexin V and analysed by flow cytometry to determine the content of apoptotic cells (annexin V+/GFP+; Figure 6B). Expression of Gab2-3YF induced apoptosis of caSTAT5B-expressing Ba/F3 cells, but did not affect the survival of Tel–JAK2-expressing cells.
Gab2 is required for caSTAT5-induced Akt and ERK1/2 phosphorylation
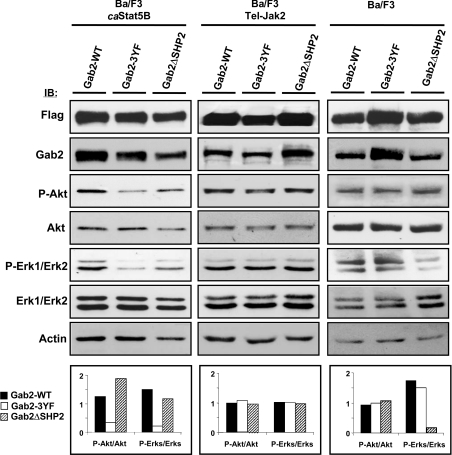
We next evaluated the activation of Akt and ERKs in the different Gab2-expressing cell lines (Figure 7). Overexpression of the transfected FLAG-tagged wild-type or the Gab2 mutants was detected by immunoblotting using anti-FLAG or anti-Gab2 antibodies (Figure 7). Phosphorylated Akt and ERK1/2 levels were next determined in transfected cells by immunoblotting using anti-phospho-Akt and anti-phospho-ERK1/2 antibodies. For a better estimation of the levels of phosphorylated Akt, and phosphorylated/ERK1/2 in each transfected cell, we took into account variations of protein loading and calculated the ratio of phosphorylated versus total Akt and ERK1/2 proteins by densitometric scanning analysis. Results showed that expression of Gab2-3YF inhibited the phosphorylation of both Akt and ERKs in the caSTAT5B-expressing cells but not in Tel–JAK2-expressing cells (Figure 7, left and middle panels). In contrast, expression of the Gab2ΔSHP2 mutant failed to inhibit ERK1/ERK2 in Tel–JAK2- and caSTAT5B-expressing cells but decreased the phosphorylation of these protein kinases in IL-3-stimulated Ba/F3 cells (right panel). These results indicated that Shp2 binding to Gab2 is required for the activation of ERK1/2 in IL-3-stimulated Ba/F3 cells but not for caSat5-induced ERK1/2 activation. Taken together, our results clearly showed that activation of Gab2 plays an important role in the proliferation and survival of caSTAT5-induced Ba/F3 cells by regulating both the PI3K/Akt and the Ras/MAPK pathways.
Figure 7. Overexpression of Gab2-3YF inhibits Akt and ERK1/ERK2 phosphorylation in caSTAT5-expressing Ba/F3 cells.
Ba/F3 cells (right panel) and Tel–JAK2- (middle panel) and caSTAT5B- (left panel) expressing cells were electroporated with the FLAG-tagged Gab2 constructs as indicated. GFP+ cells were sorted 24 h later and the content of transfected Gab2 was determined by immunoblotting with anti-FLAG or anti-Gab2 antibodies. The levels of phosphorylated Akt and ERK1/2 in the different transfected cells were also determined by immunoblotting with the indicated antibodies and quantified by densitometric analysis. The ratio of phosphorylated versus total Akt and ERK1/ERK2 proteins is expressed in arbitrary units (bottom panels).
DISCUSSION
The PI3K/Akt pathway plays an important role in the cytokine-induced cell survival and/or proliferation. Binding of the p85 subunit to some but not all cytokine receptors is a prerequisite for PI3K activation. For other receptors, the PH domain-containing proteins of the Gab and IRS families play a major role in the activation of the PI3K/Akt cascade. We have reported previously that constitutively active STAT5 mutants (caSTAT5) interact with p85, activate PI3K and induce the growth of Ba/F3 cells [20]. We show herein that caSTAT5 induced the activation of the PI3K by interacting with both p85 and the Gab2 scaffolding proteins. Gab2 belongs to the Gab/Dos family of adapter proteins which includes Gab1, Gab3 and the Drosophila homologue Dos. It contains an N-terminal PH domain, several SH3 domain-binding motifs and multiple binding sites for SH2 domain-containing proteins. Gab2 undergoes tyrosine phosphorylation in response to a large variety of growth factors and cytokines, and subsequently associates with p85, Shp2 and Grb2 [29]. In the present study, we provide evidence that both activated endogenous STAT5 in IL-3-stimulated or Tel–JAK2-expressing Ba/F3 cells and caSTAT5 can bind to Gab2. This is reminiscent of the association between Gab2 and STAT5 observed in mycosis fungoides-derived T-cells stimulated with IL-15 or IL-2, indicating that the STAT5–Gab2 interaction is induced in various cell types stimulated with different cytokines [30]. The mechanism involved in this interaction remains, however, unclear. It is so far assumed that the association of Gab2 with STAT5 and p85 requires the tyrosine phosphorylation of Gab2. However, the tyrosine residues of Gab2 do not fall in the consensus sequence recognized by the SH2 domain of STAT5 and, accordingly, the SH2 domain of STAT5 does not bind to the phosphorylated tyrosine residues of Gab2 [31]. STAT5 may therefore interact indirectly through an additional adapter protein.
Interestingly, we found that Gab2 tyrosine phosphorylation does not require JAK2 activation in caSTAT5-expressing cells, suggesting that another tyrosine kinase is involved in this process. Gab2 has been shown to be phosphorylated by a broad range of other tyrosine kinases including Zap-70, Syk and the Src kinases [32–34]. Experiments are now in progress by our group to identify the tyrosine kinase involved in Gab2 phosphorylation in caSTAT5-expressing cells.
In the present study, we found that Grb2 but not Shp2 binds to Gab2 in caSTAT5-expressing Ba/F3 cells. Like Gab2, caSTAT5 was found to interact with Grb2 but not with Shp2, suggesting that both proteins form a complex capable of recruiting Grb2 and p85 and to initiate the activation of the PI3K and Ras/MAPK cascades.
The Shp2–Gab2 interaction is believed to be an important step in the activation of the Ras/MAPK pathway, although this has been controversial [27]. Our results clearly show that ERK1 and ERK2 are activated in caSTAT5-expressing Ba/F3 cells in the absence of an Shp2–Gab2 interaction, indicating that binding of Shp2 to Gab2 is not needed for the activation of ERK1/2. Accordingly, overexpression in caSTAT5-expressing Ba/F3 cells (but not in parental Ba/F3 cells) of the Gab2ΔSHP2 mutant, which no longer binds to Shp2, does not inhibit ERK1/ERK2 phosphorylation. It is likely that Gab2 transmits signal to ERK1/2 activation via PI3K in caSTAT5-expressing Ba/F3 cells. In keeping with this, we showed that overexpression of Gab2-3YF, a Gab2 mutant that no longer interacts with p85, inhibits ERK1/2 as well as Akt phosphorylation. These results are consistent with a previous study demonstrating that expression of a dominant-negative form of p85 inhibited the IL-3-induced ERK1/2 activation in Ba/F3 cells [26]. Altogether, our results suggest that Gab2 plays a critical role in caSTAT5-induced cell growth and survival by regulating both the PI3K/Akt and Ras/MAPK pathway activities. Interestingly, Gab2 has been shown to also play a major role in the c-kit ligand-induced ERK and Akt activation in normal mast cells whose development requires both Gab2 and STAT5 [6,35]. In addition, Gab2 seems to be essential for the transforming properties of the Bcr-Abl oncogene [36]. In contrast, we provide evidence that Gab2 is not crucial for the Tel–JAK2 activities, since both the Gab2-3YF and the Gab2ΔSHP2 mutants failed to inhibit its cell-induced proliferation. Moreover, expression of Gab2-3YF did not inhibit Akt and ERK1/2 phosphorylation in Tel–JAK2-expressing cells. Given the fact that both PI3K/Akt and Ras/MAPK pathways play a critical role in Tel–JAK2-induced proliferation, it is likely that Tel–JAK2-expressing cells use other signalling intermediates, which compensate the inhibitory effect of the mutant Gab2-3YF [37,38].
Recently, we analysed a primary cell system from caSTAT5-transplanted mice which suffered from a multilineage leukaemia [14]. Proliferation of these primary cells is strictly dependent on PI3K activity and we now investigate the involvement of Gab2 in this process.
Acknowledgments
We thank V. Lacronique (INSERM EMI0210, Paris, France) for Tel–JAK2-expressing cells, and R. Moriggl (Institute of Molecular Pathology, Vienna, Austria) for a critical reading of the paper. This work was supported by ARC (Association de la Recherche contre le Cancer), Ligue contre le Cancer (Comité du Nord-Pas de Calais), Conseil Régional de Picardie, Fondation de France and Cent pour Sang la Vie. R.N. is supported by the ‘Ministere de la Recherche’ and C.P. is supported by ARERS-Verre Espoir.
References
- 1.Kisseleva T., Bhattacharya S., Braunstein J., Schindler C. W. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- 2.Buitenhuis M., Coffer P. J., Koenderman L. Signal transducer and activator of transcription 5 (Stat5) Int. J. Biochem. Cell Biol. 2004;36:2120–2124. doi: 10.1016/j.biocel.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Kieslinger M., Woldman I., Moriggl R., Hofmann J., Marine J. C., Ihle J. N., Beug H., Decker T. Antiapoptotic activity of Stat5 required during terminal stages of myeloid differentiation. Genes Dev. 2000;14:232–244. [PMC free article] [PubMed] [Google Scholar]
- 4.Moriggl R., Topham D. J., Teglund S., Sexl V., McKay C., Wang D., Hoffmeyer A., van Deursen J., Sangster M. Y., Bunting K. D., et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10:249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- 5.Sexl V., Piekorz R., Moriggl R., Rohrer J., Brown M. P., Bunting K. D., Rothammer K., Roussel M. F., Ihle J. N. Stat5a/b contribute to interleukin 7-induced B-cell precursor expansion, but abl- and bcr/abl-induced transformation are independent of stat5. Blood. 2000;96:2277–2283. [PubMed] [Google Scholar]
- 6.Shelburne C. P., McCoy M. E., Piekorz R., Sexl V., Roh K. H., Jacobs-Helber S. M., Gillespie S. R., Bailey D. P., Mirmonsef P., Mann M. N., et al. Stat5 expression is critical for mast cell development and survival. Blood. 2003;102:1290–1297. doi: 10.1182/blood-2002-11-3490. [DOI] [PubMed] [Google Scholar]
- 7.Socolovsky M., Nam H., Fleming M. D., Haase V. H., Brugnara C., Lodish H. F. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 8.Bunting K. D., Bradley H. L., Hawley T. S., Moriggl R., Sorrentino B. P., Ihle J. N. Reduced lymphomyeloid repopulating activity from adult bone marrow and fetal liver of mice lacking expression of STAT5. Blood. 2002;99:479–487. doi: 10.1182/blood.v99.2.479. [DOI] [PubMed] [Google Scholar]
- 9.Gouilleux-Gruart V., Debierre-Grockiego F., Gouilleux F., Capiod J. C., Claisse J. F., Delobel J., Prin L. Activated Stat related transcription factors in acute leukaemia. Leuk. Lymphoma. 1997;28:83–88. doi: 10.3109/10428199709058334. [DOI] [PubMed] [Google Scholar]
- 10.Kelly J. A., Spolski R., Kovanen P. E., Suzuki T., Bollenbacher J., Pise-Masison C. A., Radonovich M. F., Lee S., Jenkins N. A., Copeland N. G., et al. Stat5 synergizes with T cell receptor/antigen stimulation in the development of lymphoblastic lymphoma. J. Exp. Med. 2003;198:79–89. doi: 10.1084/jem.20021548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xi S., Zhang Q., Gooding W. E., Smithgall T. E., Grandis J. R. Constitutive activation of Stat5b contributes to carcinogenesis in vivo. Cancer Res. 2003;63:6763–6771. [PubMed] [Google Scholar]
- 12.Ahonen T. J., Xie J., LeBaron M. J., Zhu J., Nurmi M., Alanen K., Rui H., Nevalainen M. T. Inhibition of transcription factor Stat5 induces cell death of human prostate cancer cells. J. Biol. Chem. 2003;278:27287–27292. doi: 10.1074/jbc.M304307200. [DOI] [PubMed] [Google Scholar]
- 13.Schwaller J., Parganas E., Wang D., Cain D., Aster J. C., Williams I. R., Lee C. K., Gerthner R., Kitamura T., Frantsve J., et al. Stat5 is essential for the myelo- and lymphoproliferative disease induced by TEL/JAK2. Mol. Cell. 2000;6:693–704. doi: 10.1016/s1097-2765(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 14.Moriggl R., Sexl V., Kenner L., Duntsch C., Stangl K., Gingras S., Hoffmeyer A., Bauer A., Piekorz R., Wang D., et al. Stat5 tetramer association is associated with leukemogenesis. Cancer Cell. 2005;7:87–99. doi: 10.1016/j.ccr.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Dumon S., Santos S. C., Debierre-Grockiego F., Gouilleux-Gruart V., Cocault L., Boucheron C., Mollat P., Gisselbrecht S., Gouilleux F. IL-3 dependent regulation of Bcl-xL gene expression by STAT5 in a bone marrow derived cell line. Oncogene. 1999;18:4191–4199. doi: 10.1038/sj.onc.1202796. [DOI] [PubMed] [Google Scholar]
- 16.Nosaka T., Kawashima T., Misawa K., Ikuta K., Mui A. L., Kitamura T. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 1999;18:4754–4765. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oda A., Wakao H., Fujihara M., Ozaki K., Komatsu N., Tanaka S., Ikeda H., Miyajima A., Ikebuchi K. Thrombopoietin and interleukin-2 induce association of CRK with STAT5. Biochem. Biophys. Res. Commun. 2000;278:299–305. doi: 10.1006/bbrc.2000.3803. [DOI] [PubMed] [Google Scholar]
- 18.Rosa Santos S. C., Dumon S., Mayeux P., Gisselbrecht S., Gouilleux F. Cooperation between STAT5 and phosphatidylinositol 3-kinase in the IL-3-dependent survival of a bone marrow derived cell line. Oncogene. 2000;19:1164–1172. doi: 10.1038/sj.onc.1203418. [DOI] [PubMed] [Google Scholar]
- 19.Lanvin O., Gouilleux F., Mullie C., Maziere C., Fuentes V., Bissac E., Dantin F., Maziere J. C., Regnier A., Lassoued K., et al. Interleukin-7 induces apoptosis of 697 pre-B cells expressing dominant-negative forms of STAT5: evidence for caspase-dependent and -independent mechanisms. Oncogene. 2004;23:3040–3047. doi: 10.1038/sj.onc.1207450. [DOI] [PubMed] [Google Scholar]
- 20.Santos S. C., Lacronique V., Bouchaert I., Monni R., Bernard O., Gisselbrecht S., Gouilleux F. Constitutively active STAT5 variants induce growth and survival of hematopoietic cells through a PI 3-kinase/Akt dependent pathway. Oncogene. 2001;20:2080–2090. doi: 10.1038/sj.onc.1204308. [DOI] [PubMed] [Google Scholar]
- 21.Lacronique V., Boureux A., Monni R., Dumon S., Mauchauffe M., Mayeux P., Gouilleux F., Berger R., Gisselbrecht S., Ghysdael J., et al. Transforming properties of chimeric TEL-JAK proteins in Ba/F3 cells. Blood. 2000;95:2076–2083. [PubMed] [Google Scholar]
- 22.Nakamura T., Ouchida R., Kodama T., Kawashima T., Makino Y., Yoshikawa N., Watanabe S., Morimoto C., Kitamura T., Tanaka H. Cytokine receptor common beta subunit-mediated STAT5 activation confers NF-kappa B activation in murine proB cell line Ba/F3 cells. J. Biol. Chem. 2002;277:6254–6265. doi: 10.1074/jbc.M109878200. [DOI] [PubMed] [Google Scholar]
- 23.Verdier F., Chretien S., Billat C., Gisselbrecht S., Lacombe C., Mayeux P. Erythropoietin induces the tyrosine phosphorylation of insulin receptor substrate-2. An alternate pathway for erythropoietin-induced phosphatidylinositol 3-kinase activation. J. Biol. Chem. 1997;272:26173–26178. doi: 10.1074/jbc.272.42.26173. [DOI] [PubMed] [Google Scholar]
- 24.Miyakawa Y., Rojnuckarin P., Habib T., Kaushansky K. Thrombopoietin induces phosphoinositol 3-kinase activation through SHP2, Gab, and insulin receptor substrate proteins in BAF3 cells and primary murine megakaryocytes. J. Biol. Chem. 2001;276:2494–2502. doi: 10.1074/jbc.M002633200. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Jenkins B., Shin J. L., Rohrschneider L. R. Scaffolding protein Gab2 mediates differentiation signaling downstream of Fms receptor tyrosine kinase. Mol. Cell. Biol. 2001;21:3047–3056. doi: 10.1128/MCB.21.9.3047-3056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craddock B. L., Hobbs J., Edmead C. E., Welham M. J. Phosphoinositide 3-kinase-dependent regulation of interleukin-3-induced proliferation: involvement of mitogen-activated protein kinases, SHP2 and Gab2. J. Biol. Chem. 2001;276:24274–24283. doi: 10.1074/jbc.M009098200. [DOI] [PubMed] [Google Scholar]
- 27.Gu H., Pratt J. C., Burakoff S. J., Neel B. G. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol. Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 28.Gu H., Maeda H., Moon J. J., Lord J. D., Yoakim M., Nelson B. H., Neel B. G. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol. Cell. Biol. 2000;20:7109–7120. doi: 10.1128/mcb.20.19.7109-7120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishida K., Hirano T. The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci. 2003;94:1029–1033. doi: 10.1111/j.1349-7006.2003.tb01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brockdorff J. L., Gu H., Mustelin T., Kaltoft K., Geisler C., Ropke C., Odum N. Gab2 is phosphorylated on tyrosine upon interleukin-2/interleukin-15 stimulation in mycosis-fungoides-derived tumor T cells and associates inducibly with SHP-2 and Stat5a. Exp. Clin. Immunogenet. 2001;18:86–95. doi: 10.1159/000049187. [DOI] [PubMed] [Google Scholar]
- 31.Crouin C., Arnaud M., Gesbert F., Camonis J., Bertoglio J. A yeast two-hybrid study of human p97/Gab2 interaction with its SH2 domain-containing binding partners. FEBS Lett. 2001;495:148–153. doi: 10.1016/s0014-5793(01)02373-0. [DOI] [PubMed] [Google Scholar]
- 32.Yu W. M., Hawley T. S., Hawley R. G., Qu C. K. Role of the docking protein Gab2 in beta(1)-integrin signaling pathway-mediated hematopoietic cell adhesion and migration. Blood. 2002;99:2351–2359. doi: 10.1182/blood.v99.7.2351. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki S., Nishida K., Hibi M., Sakuma M., Shiina R., Takeuchi A., Ohnishi H., Hirano T., Saito T. Docking protein Gab2 is phosphorylated by ZAP-70 and negatively regulates T cell receptor signaling by recruitment of inhibitory molecules. J. Biol. Chem. 2001;276:45175–45183. doi: 10.1074/jbc.M105384200. [DOI] [PubMed] [Google Scholar]
- 34.Podar K., Mostoslavsky G., Sattler M., Tai Y. T., Hayashi T., Catley L. P., Hideshima T., Mulligan R. C., Chauhan D., Anderson K. C. Critical role for Hck-mediated phosphorylation of Gab1 and Gab2 docking proteins in IL-6-induced proliferation and survival of MM cells. J. Biol. Chem. 2004;9:9. doi: 10.1074/jbc.M305783200. [DOI] [PubMed] [Google Scholar]
- 35.Nishida K., Wang L., Morii E., Park S. J., Narimatsu M., Itoh S., Yamasaki S., Fujishima M., Ishihara K., Hibi M., et al. Requirement of Gab2 for mast cell development and KitL/c-Kit signalling. Blood. 2002;99:1866–1869. doi: 10.1182/blood.v99.5.1866. [DOI] [PubMed] [Google Scholar]
- 36.Sattler M., Mohi M. G., Pride Y. B., Quinnan L. R., Malouf N. A., Podar K., Gesbert F., Iwasaki H., Li S., Van Etten R. A., et al. Role for Gab2 in transformation by BCR/ABL. Cancer Cell. 2002;1:479–492. doi: 10.1016/s1535-6108(02)00074-0. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen M. H., Ho J. M., Beattie B. K., Barber D. L. TEL-JAK2 mediates constitutive activation of the phosphatidylinositol 3′-kinase/protein kinase B signaling pathway. J. Biol. Chem. 2001;276:32704–32713. doi: 10.1074/jbc.M103100200. [DOI] [PubMed] [Google Scholar]
- 38.Ho J. M., Nguyen M. H., Dierov J. K., Badger K. M., Beattie B. K., Tartaro P., Haq R., Zanke B. W., Carroll M. P., Barber D. L. TEL-JAK2 constitutively activates the extracellular signal-regulated kinase (ERK), stress-activated protein/Jun kinase (SAPK/JNK), and p38 signaling pathways. Blood. 2002;100:1438–448. [PubMed] [Google Scholar]
- 39.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]