Abstract
GPCRs (G-protein-coupled receptors) are preferentially N-glycosylated on ECL2 (extracellular loop 2). We previously showed that N-glycosylation of ECL2 was crucial for cell-surface expression of the hAT1 receptor (human angiotensin II receptor subtype 1). Here, we ask whether positioning of the N-glycosylation sites within the various ECLs of the receptor is a vital determinant in the functional expression of hAT1 receptor at the cell surface. Artificial N-glycosylation sequons (Asn-Xaa-Ser/Thr) were engineered into ECL1, ECL2 and ECL3. N-glycosylation of ECL1 caused a very significant decrease in affinity and cell surface expression of the resulting receptor. Shifting the position of the ECL2 glycosylation site by two residues led to the synthesis of a misfolded receptor which, nevertheless, was trafficked to the cell surface. The misfolded nature of this receptor is supported by an increased interaction with the chaperone HSP70 (heat-shock protein 70). Introduction of N-glycosylation motifs into ECL3 yielded mutant receptors with normal affinity, but low levels of cell surface expression caused by proteasomal degradation. This behaviour differed from that observed for the aglycosylated receptor, which accumulated in the endoplasmic reticulum. These results show how positioning of the N-glycosylation sites altered many properties of the AT1 receptor, such as targeting, folding, affinity, cell surface expression and quality control.
Keywords: angiotensin II receptor subtype 1 (AT1 receptor), degradation, G-protein-coupled receptor (GPCR), N-glycosylation, protein folding, quality control
Abbreviations: AFU, arbitrary fluorescence units; AngII, angiotensin II; (h)AT1 receptor, (human) angiotensin II receptor subtype 1; AT1-AG, aglycosylated AT1 receptor; AT1-WT, wild-type AT1 receptor; [Ca2+]i, intracellular [Ca2+]; DMEM, Dulbecco's modified Eagle's medium; ECL, extracellular loop; ER, endoplasmic reticulum; ERAD, ER-associated degradation; fura 2/AM, fura 2 acetoxymethyl ester; GPCR, G-protein-coupled receptor; Grp78/BiP, 78 kDa glucose-regulated protein/heavy-chain binding protein; HBSS, Hepes-buffered saline solution; HSP70, heat-shock protein 70; ICL1, intracellular loop 1; IP/IB, immunoprecipitation and immunoblotting; UPR, unfolded protein response; WT, wild-type
INTRODUCTION
A large number of hormones, neurotransmitters, chemokines and sensory stimuli exert their effects on cells and organisms by binding to GPCRs (G-protein-coupled receptors). Heterotrimeric G-proteins transduce the binding of ligands to these receptors into intracellular signals, which underlie many physiological responses of tissues and organisms. In order for a GPCR to elicit intracellular signalling, it must first go through a series of biosynthetic events aimed at sending the right quantity of properly folded functional receptors to the plasma membrane. Accumulation and aggregation of misfolded proteins in the ER (endoplasmic reticulum) are linked to many diseases, such as nephrogenic diabetes insipidus through the V2 vasopressin receptor [1], early onset familial Alzheimer's disease through the β-amyloid protein, Parkinson's disease through α-synuclein [2], prion disease [3], α1-antitrypsin deficiency [4], retinitis pigmentosa through opsin [5] and Type II diabetes mellitus through amylin [6]. Accumulating evidence indicates that glycosylation plays a critical role in protein folding in the ER [7–9]. The vast majority of secreted and cell surface proteins that transit through the ER are N-glycosylated (95% of GPCRs). The functions associated with this co-translational modification are still the subject of numerous studies. N-linked oligosaccharides have been implicated in various cellular activities, such as lectin-based sorting along the secretory pathway [10], targeting to lyzosome as a consequence of mannose-6-phosphate modification [11], ERAD (ER-associated degradation) as a consequence of mannose trimming [12], quality control through the calnexin cycle [13], folding through the alteration of hydrophobicity [9], protection from protease-mediated degradation [14,15], immune evasion [16] and cell–cell interactions [17].
The AT1 receptor (angiotensin II receptor subtype I) is a GPCR involved in many physiological processes, such as regulation of blood pressure, salt and water homoeostasis and myoproliferation [18]. In a previous study, we showed that the three consensus sequences for N-linked glycosylation at asparagine residues 4, 176 and 188 were occupied by oligosaccharides and were critically involved in cell surface targeting of the AT1 receptor [19]. Disruption of the glycosylation consensus sequence at position 176 alone caused a dramatic decrease in cell surface expression. We also showed that a large proportion of aglycosylated AT1 receptor (AT1-AG) remained trapped in the ER. These results are supported by another study, which concluded that the level of expression of AT1 receptor at the plasma membrane positively correlated with the number of N-linked oligosaccharide attachment sites [20]. Furthermore, another study concluded that AT1 receptor glycosylation was involved in membrane targeting, since removal of the glycosylation sequons caused the accumulation of receptors in a perinuclear compartment [21].
GPCRs are preferentially N-glycosylated on ECL2 (extracellular loop 2). In-depth screening of human non-orphan GPCRs from the GPCR database (http://www.gpcr.org/) revealed that of the receptors glycosylated in at least one ECL, 66% contained the tripeptide sequon (Asn-Xaa-Ser/Thr) in the ECL2, and only 14% and 20% in ECL1 and ECL3 respectively. Evidence further suggests that the optimal properties of GPCRs are intimately related to the location of N-glycosylation sites. To determine whether the positioning of the glycosylation sites within the various extracellular loops of the receptor is a vital determinant in hAT1 (human AT1) biosynthesis, we evaluated the site specificity of N-linked oligosaccharides by constructing a series of mutant receptors incorporating glycosylation sequons into ECL1, ECL2, ECL3 and ICL1 (intracellular loop 1).
EXPERIMENTAL
Materials
The cDNA clone encoding the hAT1 receptor with a FLAG epitope at the N-terminus was constructed and subcloned in the mammalian expression vector pcDNA3 (Invitrogen Life Technologies, Burlington, ON, Canada). DMEM (Dulbecco's modified Eagle's medium), Lipofectamine™, fetal bovine serum, penicillin, streptomycin, glutamine and oligonucleotide primers were purchased from Life Technologies (Gaithersburg, MD, U.S.A.). AngII (angiotensin II), BSA, bacitracin, anti-FLAG M1 monoclonal antibody and lactacystin were obtained from Sigma–Aldrich (Oakville, ON, Canada). Rabbit anti-HSP70 (heat-shock protein 70) and anti-Grp78/BiP (78 kDa glucose-regulated protein/heavy-chain binding protein) antibodies were from Stressgen Biotechnology (Victoria, BC, Canada). EXPRE35S35S protein labelling mix (1175 Ci/mmol) was purchased from PerkinElmer Life Sciences (Boston, MA, U.S.A.). Restriction endonucleases, polymerases and 125I (2000 Ci/mmol) were from Amersham Biosciences (Piscataway, NJ, U.S.A.). [Sar1,Ile8]AngII was obtained from Bachem (King of Prussia, PA, U.S.A.). 125I-[Sar1,Ile8]AngII was prepared with Iodogen (Pierce Chemical, Rockford, IL, U.S.A.) and purified by HPLC on a C-18 column. The specific activity was determined by self-displacement in our binding system. Briefly, a saturation curve, with increasing concentrations of the radioactive ligand, and a dose-displacement curve, with a fixed concentration of the radioactive ligand inhibited by increasing concentrations of unlabelled homologous peptide, were performed simultaneously using a bovine adrenal cortex membrane preparation. We estimated the amount of radioactive ligand necessary to obtain an occupation ratio in the saturation curve corresponding to the occupation ratio obtained with a known amount of unlabelled peptide in the dose-displacement curve.
Construction of AT1 receptor mutants
PCR-mediated mutagenesis was carried out with the Expand High Fidelity PCR System (Roche Molecular Biochemicals, Laval, QC, Canada) using the overlapping primer method to insert glycosylation consensus sequences into the hAT1 receptor cDNA, which was cloned into the pcDNA3 plasmid at the HindIII/XbaI restriction sites. Two overlapping mutagenic primers were used individually with two vector-based primers at the extremities. Following the first two individual rounds of amplification, the PCR products were denatured and combined. Anneal-overlapping mutagenized regions were amplified in the third round of PCR and resulting fragments were cloned into pcDNA3 at the HindIII/XbaI restriction sites. Four mutations were introduced into the wild-type AT1 cDNA template and the AT1-AG cDNA template, generating eight mutant receptors (where underlining indicates the introduced glycosylation sites and bold the mutated nucleotides) (see Figure 1). ECL1 glycosylation (F96N, N98T): forward primer, 5′-CGC TGG CCC AAT GGC ACT TACC TAT G-3′; reverse primer, 5′-CA TAG GTA AGT GCC ATT GGG CCA GCG G-3′. ECL2 glycosylation (N176T): forward primer, 5′-GAG AAC ACC ACT ATT ACA GTT TG-3′; reverse primer, 5′-CA AAC TGT AAT AGT GGT GTT CTC-3′. ECL3 glycosylation (G269N, I271T): forward primer, 5′-G ATT CAA CTA AAC ATC ACA CGT GAC TG-3′; reverse primer, 5′-C AGT CAC GTG TGT GAT GTT TAG TTG AAT C-3′. ICL1 glycosylation (L59N): forward primer, 5′-C TTT TAT ATG AAG AAT AAG ACT GTG G-3′; reverse primer, 5′-C CAC AGT CTT ATT CTT CAT ATA AAA G-3′. Primers used for the non-mutated vector-localized fragment extremities were the same for all PCR reactions: forward primer, 5′-CTG CTT CGC GAT GTA CGG GC-3′; reverse primer, 5′-AAA CCC GGG TTT AAA CCC-3′. All constructs were submitted to fluorescent-primer-based nucleotide sequencing to confirm cDNA integrity.
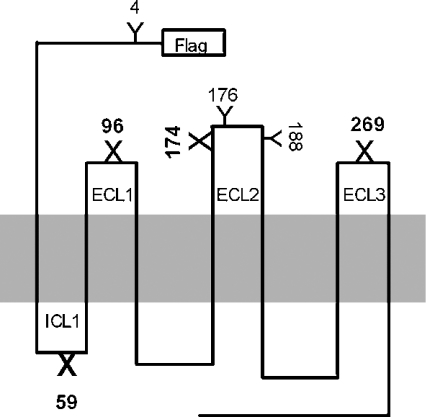
Figure 1. Representation of N-glycosylation mutant receptors.
Y shows natural N-glycosylation sites, whereas X depicts artificial N-glycosylation sites engineered into the hAT1 receptor cDNA. The numbering is based on the location of asparagine residues, which represent oligosaccharide insertion sites (Asn-Xaa-Ser/Thr).
Cell culture and transfections
COS-7 cells were grown in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin and 2 mM glutamine (complete DMEM) in a humidified atmosphere containing 5% CO2. One day before transfection, 100 mm dishes were seeded with 1.5×106 cells, 60 mm plates were seeded with 5×105 cells and 35 mm plates were seeded with 2.5×105 cells per well. The cells were transfected 18 h later in serum-free DMEM with 4 μg of DNA and 25 μl of lipofectamine (100 mm plates), 1.25 μg of DNA and 10 μl of lipofectamine (60 mm plates) or 0.5 μg of DNA and 4 μl of lipofectamine (35 mm plates). The plates were incubated for 5 h at 37 °C, after which the medium was replaced with serum-rich complete DMEM. Two days after transfection, the cells were washed with PBS and either used immediately for flow cytometry, intracellular calcium assays and immunoblot studies, or stored at −80 °C for binding, photolabelling and post-treatment metabolic labelling studies.
Binding experiments
Frozen COS-7 cells were thawed, gently scraped in cold washing buffer (25 mM Tris/HCl, pH 7.4, 100 mM NaCl and 5 mM MgCl2), centrifuged at 3000 g for 15 min at 4 °C and dispersed in binding buffer (washing buffer with 0.1% BSA and 0.01% bacitracin). Broken cells were incubated for 1 h at room temperature in binding buffer containing increasing concentrations of 125I-[Sar1,Ile8]AngII in a final volume of 0.5 ml. The bound radioactive ligand was separated from free ligand by filtration through GF/C filters, presoaked for at least 2 h in binding buffer. Non-specific binding was measured in the presence of 1 μM unlabelled AngII. Receptor-bound radioactivity was evaluated by gamma-counting.
Photoaffinity labelling
Broken COS-7 cells (1×106 cells) were prepared as for the binding experiments and incubated for 1 h at room temperature with 5 nM 125I-[L-Bpa8]AngII in 1 ml of binding buffer. They were then washed twice with PBS and irradiated for 45 min at 0 °C under filtered UV light (365 nm). Photolabelled receptors were solubilized in 150 μl of medium containing 50 mM Tris/HCl, pH 7.4, 1% Nonidet P40, 150 mM NaCl and 1 mM PMSF. After centrifugation at 15000 g for 10 min to remove insoluble material, the supernatant was mixed with an equal volume of 2×Laemmli solution and incubated for 60 min at 37 °C before electrophoresis on a 10% polyacrylamide gel at 20 mA. Gels were dried under vacuum before autoradiography with a Kodak Biomax MS film.
IP/IB (immunoprecipitation and immunoblotting)
COS-7 cells expressing glycosylation mutants were washed by centrifugation at 2000 g for 15 min at 4 °C and resuspended in RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Nonidet P40, 0.1% deoxycholate, 5 mM NaN3 and 1 mM CaCl2) with Complete protease inhibitor cocktail (Roche) under gentle agitation for 60 min at 4 °C. Cell lysates were then centrifuged at 15000 g for 15 min at 4 °C to remove insoluble material and immunoprecipitation was performed by adding anti-FLAG M1 antibody (1:1000) under gentle agitation overnight at 4 °C with 30 μl of Protein A/G–agarose (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.). After three washes with RIPA buffer, the immunoprecipitated proteins were resuspended in Laemmli's buffer and heated for 30 min at 55 °C. Purified proteins were separated by SDS/PAGE, transferred on to a PVDF membrane (Millipore) and blocked in Tris-buffered saline (TBS-T: 50 mM Tris, 150 mM NaCl, 1 mM CaCl2 and 0.1% Tween 20) supplemented with 5% non-fat dried milk for 60 min, and immunoblotted with either a monoclonal anti-FLAG M1 antibody (1:3000) or a polyclonal rabbit anti-HSP70 antibody (1:2000). The primary antibody was probed with either a mouse or donkey alkaline phosphatase-conjugated secondary antibody (1:5000 or 1:20000), and the proteins were visualized with the ECL® system (Amersham Pharmacia Biotech) on a Bio-Max ML film (Eastman Kodak).
Flow cytometric studies
COS-7 cells (48 h post-transfection) expressing mutant receptors were washed three times in PBS, detached with 0.5 mM EDTA, fixed with 2% paraformaldehyde on ice for 30 min, incubated in the presence or absence of mouse anti-FLAG M1 antibody (1:400) for 60 min at 4 °C, washed with PBS and incubated at 4 °C for 30 min with FITC-conjugated goat anti-mouse antibody (1:250). The individual fluorescence of 2×104 cells was measured using a FACScan flow cytometer and software (Becton-Dickinson).
Measurement of [Ca2+]i (intracellular [Ca2+])
Transfected COS-7 cells were grown on coverslips, washed twice with HBSS (Hepes-buffered saline solution; 120 mM NaCl, 5.3 mM KCl, 0.8 mM MgSO4, 1.8 mM CaCl2, 11.1 mM glucose and 20 mM Hepes, pH 7.4) and loaded with fura 2/AM (fura 2 acetoxymethyl ester, 5 μM in HBSS; Molecular Probes,) for 20 min at room temperature in the dark. The coverslips were washed, placed in fresh HBSS for 20 min at room temperature to de-esterify the fura 2/AM, inserted into a circular open-bottom chamber and placed on the stage of a Zeiss Axovert microscope fitted with an Attofluor Digital Imaging and Photometry System (Attofluor Inc., Rockville, MD, U.S.A.). Isolated fura 2-loaded cells (n=10–20) that responded to AngII and/or ATP were selected and their [Ca2+]i was measured by fluorescence video-microscopy using alternating excitation wavelengths of 334 nm and 380 nm and monitoring emitted fluorescence at 520 nm. All reagents were diluted to their final concentrations in HBSS and applied to the cells by surface perfusion.
Inositol phosphate accumulation
COS-7 cells were labelled for 18 h in Medium 199 containing 15 μCi/ml of myo-[3H]inositol. Cells were stimulated with different concentrations of AngII for 10 min at 37 °C in Medium 199 containing 25 mM Hepes, pH 7.4, 10 mM LiCl and 0.1% BSA. Incubations were stopped by addition of perchloric acid (5%, v/v). Water-soluble inositol phosphates were extracted with an equal volume of 1,1,2-trichlorotrifluoroethane and tri-n-octylamine. The samples were vigorously mixed and centrifuged at 15000 g for 15 min at 4 °C. The upper phase was applied to an AG 1-X8 resin column and inositol phosphates were sequentially eluted by addition of ammonium formate/formic acid solutions of increasing ionic strength. Radioactive Ins(4,5)P2 and Ins(1,4,5)P3 content was evaluated by liquid scintillation counting.
Metabolic labelling
Metabolic labelling was performed 24 h post-transfection by incubating COS-7 cells in DMEM (without cysteine or methionine) containing 50 μCi/ml [35S]methionine/cysteine for 2 h at 37 °C. When needed, the proteasome inhibitor lactacystin (10 μM) was added 1 h before pulse labelling and this concentration was maintained throughout the experiment. The pulse was terminated by washing the cells twice with PBS and chasing for different periods of time in complete DMEM. The cells were then washed with PBS and stored at −80 °C before immunoprecipitation and SDS/PAGE. For the detection of 35S radioactivity, the gels were treated with EN3HANCE® (PerkinElmer Life Sciences) according to the manufacturer's instructions, dried and exposed at −80 °C for 1–3 days using Biomax MR film and intensifying screens (Eastman Kodak). The relative intensities of the labelled bands were analysed by densitometric scanning using the Bio-Rad GS-250 Phosphoimager system and analysis software (Mississauga, ON, Canada).
RESULTS
Artificial N-glycosylation sequons are occupied by oligosaccharides
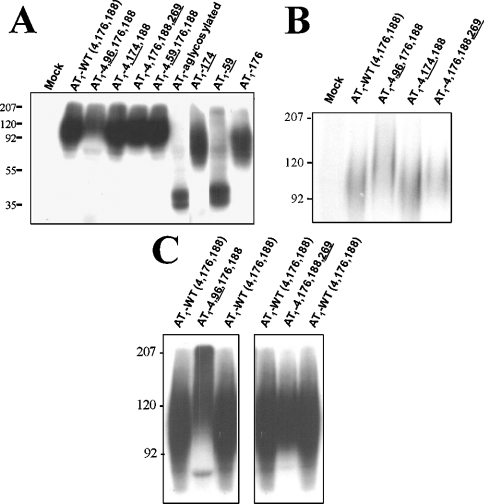
To verify that the artificial glycosylation sites engineered into the AT1 receptor cDNA were occupied by oligosaccharides, each AT1 receptor mutant was photolabeled with 125I-[L-Bpa8]AngII and analysed by SDS/PAGE and autoradiography. The wild-type AT1 receptor (AT1-WT) migrated as a broad band between 80 and 140 kDa (Figure 2A), with an average molecular mass of 105 kDa. The broadness of the band was consistent with the glycoprotein nature of the receptor [19]. To discriminate further between the molecular masses of the mutant receptors, the electrophoresis was prolonged for 2 h. Figures 2(B) and 2(C) show that mutants AT1-4,96,176,188 and AT1-4,176,188,269 (where underlining indicates the introduced glycosylation sites) had slower electrophoretic mobilities corresponding to average molecular masses of 130 kDa and 118 kDa respectively, suggesting that they contained four N-linked oligosaccharides. AT1-WT and AT1-4,174,188 had average molecular masses of 105 kDa, which was consistent with three N-linked oligosaccharides. The characteristic change in electrophoretic mobility of hyperglycosylated receptors observed by SDS/PAGE was seen as an upward shift in the bottom end of the diffuse glycoprotein band. AT1-AG migrated as a 35 and 38 kDa doublet (Figure 2A). The electrophoretic mobility of mutant AT1-59 was similar to that of AT1-AG, suggesting that the intracellular glycosylation consensus sequence did not permit the insertion of carbohydrate moieties. Mutants AT1-174 and AT1-176, which contained a single glycosylation sequence, had an average molecular mass of 80 kDa, whereas mutants AT1-96 and AT1-269 were undetectable using this approach. These results showed a good correlation between the electrophoretic mobility of the photolabelled mutant receptors and their predicted number of glycosylation sites.
Figure 2. Photoaffinity labelling of AT1 receptor glycosylation mutants.
(A) COS-7 cells expressing AT1-WT and various mutant receptors were incubated in the presence of 5 nM 125I-[L-Bpa8]AngII for 1 h at room temperature. The cells were irradiated under 365 nm filtered UV light for 45 min at 0 °C. After solubilization, the samples were resolved on 10% SDS/PAGE followed by autoradiography, as described in the Experimental section. Electrophoresis of selected mutant receptors was prolonged for 2 h with a small (B) and large (C) amount of specifically photolabelled receptor complex to highlight the shift in electrophoretic mobility. Protein standards of the indicated molecular-mass markers were run in parallel (values shown on the left-hand side of the gels in kDa). These results are representative of at least three independent experiments.
AT1 receptor glycosylation mutants are functional
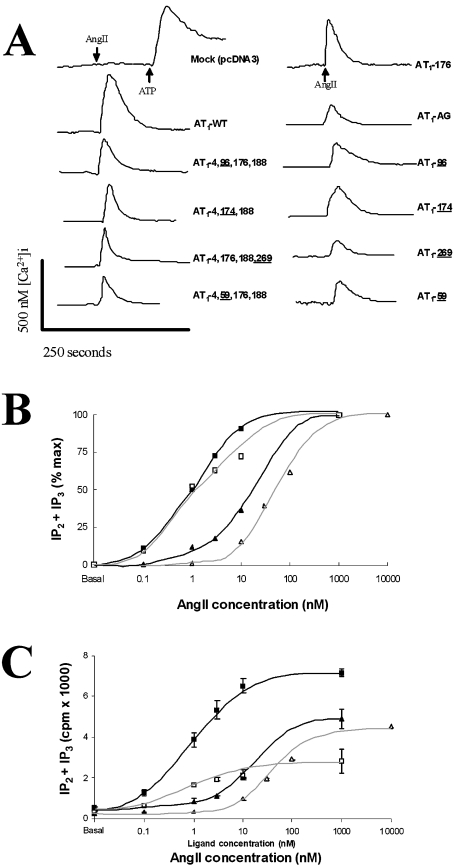
Each mutant receptor was tested for AngII-induced calcium mobilization. Figure 3(A) shows that 100 nM AngII could not elicit a calcium response in COS-7 cells transfected with pcDNA3 vector alone (mock transfection), whereas 100 μM ATP caused a rapid transient increase in [Ca2+]i via an endogenous Gq-coupled purinergic receptor. AngII (100 nM) elicited a robust calcium transient in cells transfected with AT1-WT receptor. All other mutant receptors responded to 100 nM AngII to various degrees that corresponded to their expression levels at the plasma membrane (see Figure 4). The functional properties of mutant receptors were also evaluated by AngII-induced inositol phosphates [Ins(4,5)P2 and Ins(1,4,5)P3] accumulation within transiently transfected COS-7 cells. Concentration–response curves were expressed as the percentage of the maximal response (Figure 3B) or as absolute values (Figure 3C) for AT1-WT, AT1-4,96,176,188 (ECL1), AT1-4,174,188 (ECL2) and AT1-4,176,188,269 (ECL3). All mutant receptors caused a progressive concentration-dependent increase in inositol phosphates, with EC50 values (concentration causing 50% of maximal inositol phosphates accumulation) rank order mirroring their Kd values (Figure 3B). Taking into account their levels of expression, all mutant receptors were as effective as the AT1-WT at activating phospholipase C.
Figure 3. Functionality of AT1 receptor glycosylation mutants.
(A) COS-7 cells were transiently transfected with pcDNA3 vector (mock) or the various mutant receptors, loaded with fura 2/AM and mounted on a video-microscopy system. fura 2 fluorescence was monitored under basal conditions and after stimulation with 100 nM AngII. Mock-transfected cells were also stimulated with 100 μM ATP. These typical traces show variations of the fluorescence ratio (F334/F380), as described in the Experimental section. Similar results were obtained with three different cell preparations. (B) Production of inositol phosphates is expressed as a percentage of maximal stimulation of COS-7 cells expressing AT1-4,176,188 (WT, ■), AT1-4,96,176,188 (ECL1, △), AT1-4,174,188 (ECL2, ▲), AT1-4,176,188,269 (ECL3, □); and (C) the results are expressed as absolute values [sum of IP2+IP3, where IP2 is Ins(4,5)P2 and IP3 is Ins(1,4,5)P3]. The results are expressed as the means±S.D. of duplicate values and are representative of two independent experiments.
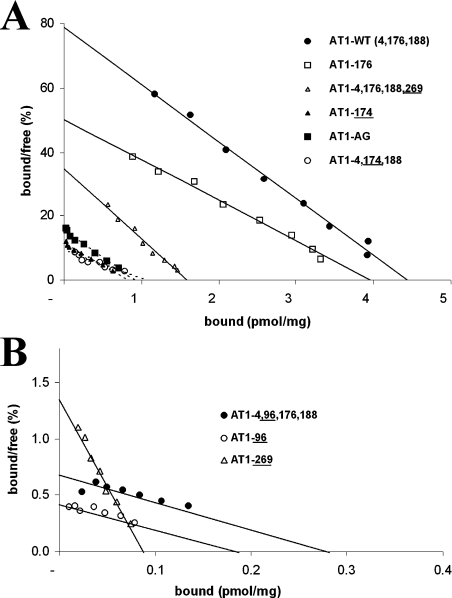
Figure 4. Pharmacological properties of AT1 receptor glycosylation mutants.
Broken COS-7 cells expressing the AT1-WT receptor and various glycosylation mutants were incubated for 1 h with increasing concentrations of 125I-[Sar1,Ile8]AngII. Non-specific binding was assessed in the presence of 1 μM unlabelled [Sar1,Ile8]AngII. Affinity (Kd) and Bmax values were evaluated from the saturation curve data by Scatchard analysis, which is shown to emphasize the decrease in Bmax (A) and the decrease in both Bmax and Kd of the ECL1 mutants (B). Each data point represents the mean of triplicate values. These results are representative of at least three independent experiments.
Pharmacological properties of AT1 receptor glycosylation mutants
Mutant receptors were expressed in COS-7 cells and their binding properties were evaluated with the radioactive analogue 125I-[Sar1,Ile8]AngII. Scatchard plots of the ligand concentration binding isotherms are shown in Figure 4. Table 1 summarizes the pharmacological properties of the mutant receptors. AT1-WT had a Kd of 0.64±0.24 nM and a Bmax of 4.37±0.61 pmol/mg of protein. All the mutant receptors were expressed at lower levels than the AT1-WT receptor. Most showed similar high binding affinities (ranging from 0.6 nM to 1.8 nM), except for the ECL1 mutant receptors AT1-4-96,176,188 and AT1-96, whose binding affinities were 10 times lower. ECL1 mutant receptors AT1-4,96,176,188 and AT1-96 had much lower levels of expression at the plasma membrane, with Bmax values 7% and 4% that of the AT1-WT receptor respectively (Table 1). The Bmax values of ECL2 mutant receptors AT1-4,174,188 and AT1-174, for which the critical glycosylation site at position 176 was relocated to position 174, were 24% and 15% that of the AT1-WT receptor respectively. These mutant receptors had high binding affinities of 1.75±0.20 nM and 0.93±0.33 nM respectively. Binding studies were also performed in HEK-293 cells expressing either AT1-4,176,188 (WT), AT1-4,96,176,188 (ECL1), AT1-4,174,188 (ECL2), AT1-4,176,188,269 (ECL3) or AT1-AG (aglycosylated) receptor mutants. Cell surface expression (Bmax) and affinities (Kd) were similar to those obtained in COS-7 cells (results not shown).
Table 1. Binding properties of AT1 receptor glycosylation mutants.
Broken COS-7 cells expressing the various glycosylation mutant receptors were incubated for 1 h with increasing concentrations of 125I-[Sar1,Ile8]AngII. Saturation data were transformed by Scatchard analysis to calculate Bmax, affinity (Kd) and expression levels as a percentage of AT1-WT receptor expression levels. The results are expressed as the means±S.D. for at least three independent experiments.
| Glycosylation mutant | Bmax (pmol/mg of protein) | Bmax (% of WT) | Affinity (Kd, nM) |
|---|---|---|---|
| AT1-WT (4,176,188) | 4.37±0.60 | 100.0 | 0.64±0.24 |
| AT1-4,96,176,188 | 0.27±0.11 | 6.3 | 7.13±0.56 |
| AT1-4,174,188 | 1.04±0.25 | 23.7 | 1.75±0.20 |
| AT1-4,176,188,269 | 1.39±0.40 | 31.9 | 0.57±0.14 |
| AT1-AG | 0.76±0.10 | 17.4 | 0.61±0.16 |
| AT1-96 | 0.16±0.06 | 3.7 | 6.47±0.42 |
| AT1-174 | 0.67±0.06 | 15.3 | 0.93±0.33 |
| AT1-269 | 0.07±0.02 | 1.5 | 1.12±0.66 |
| AT1-176 | 4.21±0.46 | 96.3 | 0.71±0.18 |
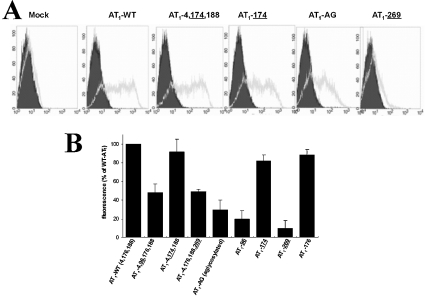
Evaluation of the cell surface expression of AT1 receptor mutants by flow cytometry
Mutant receptors were expressed in COS-7 cells and their level of expression at the plasma membrane was evaluated by flow cytometry. Figure 5(A) shows that mock-transfected cells exhibited no significant shift in mean AFU (arbitrary fluorescence units), whether they were incubated without (4.54 AFU; filled area) or with anti-FLAG antibody (6.15 AFU; unfilled area). However, the AFU shifted dramatically when cells were transfected with AT1-WT and incubated with the anti-FLAG antibody (81.90 AFU versus 4.06 AFU). Cell surface expression of AT1-AG and ECL3-glycosylated AT1-269 was very low (29±11% and 10±8% respectively) compared with that of AT1-WT. Figure 5(B) shows that ECL2-glycosylated AT1-4,174,188 was expressed at a level comparable with that of WT receptor (92±14%), a notably different result from that obtained in the binding studies (24% of AT1-WT). AT1-4,96,176,188 and AT1-4,176,188,269 were expressed at low levels corresponding to approx. 50% that of AT1-WT, whereas ECL1-glycosylated AT1-96 was expressed at a very low level. Figure 5(B) shows the cell surface expression of all the glycosylation mutants evaluated by flow cytometry. The cell surface expression of all the mutant receptors mirrored the expression levels observed in the binding studies, with the exception of both ECL2 mutants.
Figure 5. Cell surface expression levels of FLAG-tagged AT1 receptor glycosylation mutants by flow cytometry.
(A) Transiently transfected COS-7 cells were subjected to flow cytometry 48 h post-transfection. Cells (20×104) were labelled with anti-FLAG antibody (grey curves) or were left unlabeled (black curves). A representative experiment is shown for several glycosylation mutants. (B) Cell surface expression of all glycosylation mutants was evaluated by flow cytometry and compared with that of the AT1-WT receptor. Results are expressed as the means±S.D. for three independent experiments.
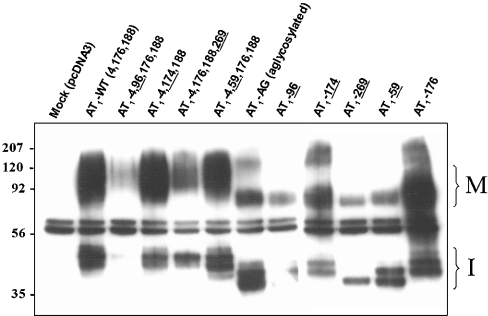
Immunoblot studies of AT1 receptor glycosylation mutants
Mutant receptors were transiently transfected into COS-7 cells and total receptor expression was evaluated by IP/IB assays. All immunoreactive bands were specifically recognized by the anti-FLAG antibody except for the 58/62 kDa doublet, which also appeared in the mock-transfected cells (Figure 6). The major immunoreactive band for AT1-WT appeared between 80 and 140 kDa, which is characteristic of the migration pattern on SDS/PAGE. Immunoreactive bands of greater electrophoretic mobility (42, 45 and 48 kDa) were also observed for AT1-WT. Not surprisingly, for the various glycosylation mutants, the molecular masses of these bands were proportional to the number of glycosylation sites present on the receptor. Furthermore, it appears that mono-glycosylated and aglycosylated receptors had a greater tendency to aggregate, as immunoreactive bands corresponding to the molecular masses of dimers and trimers were observed for these mutant receptors. Interestingly, AT1-269 had only a single 37 kDa band, whereas all the other mutant receptors had at least two immunoreactive low molecular mass bands. Furthermore, the mature forms of receptors containing four N-glycosylation consensus sequences (AT1-4,96,176,188 and AT1-4,176,188,269) had higher average molecular masses than receptors containing three consensus sequences (AT1-4,176,188 and AT1-4,174,188).
Figure 6. Immunoblot analysis of FLAG-tagged AT1 receptor glycosylation mutants.
COS-7 cells expressing the various glycosylation mutants were grown for 48 h post-transfection, immunoprecipitated from cell lysates with anti-FLAG antibody and the proteins were revealed with anti-FLAG antibody, as described in the Experimental section. Bands corresponding to the immature (37–48 kDa; I) and mature (80–150 kDa; M) forms of the AT1 receptor are indicated. The anti-FLAG antibody doublet (58 and 62 kDa) can also be seen. Protein standards of the indicated molecular masses were run in parallel. These results are representative of at least three independent experiments.
AT1-269, but not AT1-AG, is degraded by the proteasome
To follow the maturation of selected glycosylation mutants, metabolic labeling studies were performed. The degradation kinetics of the aglycosylated receptor was compared with that of AT1-269. COS-7 cells transiently transfected with these two receptors were radioactively labelled for 2 h. Figure 7 shows representative gels and corresponding histograms where AT1-AG and AT1-269 were chased for 2 h in the presence or absence of lactacystin, a specific non-reversible proteasome inhibitor. The amount of pulse-labelled AT1-AG, which accumulates in a perinuclear organelle co-localizing with ER-specific markers [19], remained stable in the presence (10.4±2.2% degradation) or absence (3.6±11.7% degradation) of lactacystin after a 2 h chase. However, the extensive degradation by the proteasome of ECL3 mutant AT1-269 during the 2 h chase (60.8±1.1% degradation) was significantly reduced in the presence of lactacystin (22.6±3.2% degradation).
Figure 7. Degradation kinetics of selected AT1 receptor glycosylation mutants.
COS-7 cells expressing AT1-AG (A) and AT1-269 (B) mutants were pulse labelled for 2 h with 50 μCi/ml EXPRE35S35S, and subsequently chased for up to 2 h in the presence or absence of lactacystin (10 μM). When needed, lactacystin was added 1 h before the pulse and maintained throughout the experiment. Labelled receptors were immunoprecipitated from cell lysates with anti-FLAG antibody, separated by SDS/PAGE (10% gel) and subjected to densitometric analysis using Phosphoimager technology. The results are expressed as the means±S.D. for three independent experiments. Representative autoradiograms are shown above each graph; carbonic anhydrase (35 kDa) was run in parallel.
ECL2 mutants are targeted to the plasma membrane in a misfolded conformation
Binding and flow cytometry studies corroborated membrane expression levels for all receptors that had affinities similar to that of the WT receptor, except for the ECL2 mutants (Figure 8A). The correlation coefficient (R2) was 0.96 when the ECL2 mutant receptors were excluded from the correlation, whereas R2 decreased to 0.49 when they were included. These results suggest that one population of ECL2 mutants was present at the plasma membrane in a severely misfolded conformation that was unable to bind ligand. We hypothesized that these ECL2 mutants, if misfolded, would mediate stronger interactions with quality control chaperones, such as HSP70. The IP/IB studies presented in Figure 8(B) show that HSP70 co-precipitated with AT1-WT (lanes 2 and 3), whereas mock-transfected cells were unable to co-immunoprecipitate HSP70 (lane 1). Interestingly, AT1-4,174,188 was able to immunoprecipitate more HSP70 than AT1-WT (Figure 8B, lanes 4 and 5). In order to control for AT1 receptor expression levels, the membranes were stripped and reblotted with the anti-FLAG antibody recognizing the epitope-tagged receptor, showing a similar amount of mature and immature WT and ECL2 mutant receptors.
Figure 8. Interaction of an ECL2 glycosylation mutant with HSP70.
(A) Binding and FACS data from Figures 4 and 5 were plotted to highlight the major discrepancy for the expression of ECL2 mutants. Binding data (Bmax) as a percentage of WT were compared with FACS data as a percentage of WT for all mutants exhibiting affinities similar to that of the AT1-WT receptor. The correlation coefficients (R2) were calculated with (○) and without (●) the ECL2 mutants (AT1-4,174,188 and AT1-174). (B) COS-7 cells transiently transfected with mock (pcDNA3), FLAG–AT1-WT or FLAG–AT1-4,174,188 were immunoprecipitated form cell lysates with anti-FLAG antibody. Proteins were then transferred on to PVDF membranes and immunoblotted with anti-HSP70 antibody. The membranes were then stripped and reblotted with anti-FLAG antibody. (C, D) COS-7 cells transiently transfected with selected glycosylation mutant receptors were lysed in RIPA buffer, transferred on to PVDF membranes and immunoblotted with anti-Grp78/BiP antibody. Immunoreactive bands were revealed by ECL Plus® and exposed for either 30 s (C) or 10 s (D) on a Biomax ML film to highlight the variation in band intensities. Protein standards of the indicated molecular masses were run in parallel.
Immunoblot studies were also performed to evaluate the expression level of Grp78/BiP, a chaperone up-regulated during the UPR (unfolded protein response), in COS-7 cells expressing the WT, AG and ECL2 mutant AT1 receptors. There were no differences in the immunoreactivity of Grp78/BiP following the expression of AT1-WT or AT1-4,174,188 (Figure 8C, lanes 11–13), whereas the expression of AT1-AG, which is known to accumulate in the ER, induced a significant UPR (Figure 8D, lanes 14 and 15).
DISCUSSION
In the present study, we used a mutagenesis approach to study the site specificity of N-glycosylation sites by inserting non-natural consensus sequences into AT1 receptor cDNA. To our knowledge, this is the first study to address the role of glycosylation positioning on the pharmacological properties, expression levels and cellular localization of a GPCR. We previously showed that the ECL2 oligosaccharide at position 176 is crucial for cell surface targeting of the receptor [19]. An evaluation of all human non-orphan GPCRs from the GPCR database (http://www.gpcr.org/) revealed a higher frequency of ECL2 glycosylation compared with ECL1 and ECL3. We therefore inserted novel glycosylation sequons into ECL1 and ECL3 and shifted the critical ECL2 position 176 sequon to position 174 to evaluate the intraloop and interloop specificity of AT1 receptor glycosylation.
Functionality
We first evaluated the ability of the mutant receptors to elicit a calcium response using a functional assay that evaluates a biological response further down the signaling pathway of this Gq-coupled receptor. All mutant receptors were able to mobilize calcium and the changes in intracellular calcium concentration correlated with the amount of receptor present at the cell surface. Even low-affinity ECL1-glycosylated mutants were able to elicit calcium transients as the concentration used for receptor stimulation was saturating. We [19] and others [20,21] have shown that removing endogenous glycosylation sites from the AT1 receptor does not affect its ability to produce a functional response. We thus concluded that both the addition and removal of AT1 receptor oligosaccharides did not affect its functional coupling to Gq-coupled receptor.
Glycosylation on ECL1
When an N-glycosylation sequon was added to ECL1, we observed significant decreases in the binding affinities of two mutant receptors (AT1-4,96,176,188 and AT1-96). A very significant decrease in cell surface expression was also observed for AT1-4,96,176,188 and AT1-96. Nonetheless, a small population of ECL1 mutants was targeted to the plasma membrane and showed a low-binding affinity. This decreased affinity could either be attributed to receptor misfolding or steric hindrance from the unnaturally located oligosaccharide, which could prevent proper interaction of the ligand in the binding pocket of AT1 receptor. This phenomenon has recently been shown for the IgG receptor FcγRIII, where the presence of the endogenous glycan at position 163 significantly decreases binding affinity towards immunoglobulin, whereas a mutation preventing glycosylation of this position increases binding affinity [22]. Conversely, all three N-glycosylation sites of the GM-CSF (granulocyte/macrophage colony-stimulating factor) receptor GMRβ (haematopoietin superfamily of receptors) must be occupied by oligosaccharides to form a high-affinity receptor [23]. Although the molecular mechanism involved is still unclear, our studies indicate that glycosylation has a major influence on the pharmacological properties of the AT1 receptor.
Glycosylation on ECL3
When a glycosylation sequon was inserted in ECL3 (AT1-4,176,188,269 and AT1-269), no significant change was noted in the binding affinity of the receptors. However, a very significant decrease in membrane expression was observed. In binding studies, AT1-269 was the least abundantly expressed receptor (1.5% of AT1-WT). Introduction of artificial N-glycosylation sequons can affect folding and/or targeting. Flahaut et al. [24] rescued cell surface targeting of ER-retained aglycosylated RAMP-1 by inserting a sequon normally present in RAMP-2 and RAMP-3, which are endogenously targeted to the cell surface. However, our results demonstrate that introduction of a glycosylation motif in the third extracellular loop was incompatible with efficient targeting to the cell surface. This could explain why only 20% of GPCRs are glycosylated in ECL3 compared with 66% for ECL2.
Glycosylation on ECL2
When we shifted the critical glycosylation site from position 176 to position 174, we observed a very significant decrease in cell surface expression, as evaluated by binding studies. This decrease was similar to that observed by removing position 176 glycosylation altogether. Indeed, the AT1-4,174,188, AT1-174 and AT1-AG receptors were expressed at levels corresponding to 24%, 15% and 17% that of the AT1-WT receptor, as evaluated by binding studies. Indeed, an octapeptide ligand, such as AngII, mediates many contact points with the AT1 receptor. For example, position 8 of AngII makes contact with the 7th transmembrane domain [25], whereas position 3 contacts ECL2 [26], thus ensuring that only properly folded receptors have high-binding affinity.
To support the results obtained in the binding studies, we also evaluated cell surface expression of the mutant receptors by flow cytometry. The results obtained with this approach are much less dependent on proper folding of the receptor. High heterogeneity was also observed in the level of cell surface expression of the mutant receptors by flow cytometry, but the results essentially mirrored those obtained in the binding studies for all the mutant receptors except the ECL2 mutants (Figure 8A). The flow cytometry measurements revealed that the level of expression of the ECL2 mutant receptors was similar to that of the AT1-WT receptor. The expression of AT1-176 and AT1-174 was 96% and 15% that of AT1-WT receptor respectively when evaluated by binding studies, but was 89% and 82% that of the AT1-WT receptor when evaluated by flow cytometry. These results suggest that a large proportion of the ECL2 mutant receptors were routed to the cell surface in a misfolded conformation that was unable to recognize the ligand. These results also suggest that ECL2 mutant receptors evaded quality control mechanisms normally present in the ER. Thus two distinct populations of ECL2 mutant receptors were present at the plasma membrane, both of which were recognized by the antibody, but only one of which bound the ligand. We hypothesized that a misfolded receptor mediated greater protein–protein interactions with various quality control chaperones. Indeed, HSP70, which is a cytoplasmic chaperone that binds to hydrophobic patches on newly synthesized proteins [27], co-precipitated to a greater degree with AT1-4,174,188 than AT1-WT. Interestingly, unlike ER-localized chaperones, such as calnexin [28] and Grp78/BiP [29], which interact with the ER intralumenal side of the receptor, HSP70 interacted with the cytoplasmic side of the receptor, thus allowing the interaction to occur throughout its biosynthesis, even at the plasma membrane. In other words, the interaction between HSP70 and AT1 receptor was not restricted to the ER, as is the case for calnexin and Grp78/BiP.
We showed that the ECL2 mutants were misfolded. The UPR, which results in the upregulation of Grp78/BiP [30], is caused by misfolded proteins in the ER. We therefore verified whether ECL2 mutants were able to induce UPR. Both AT1-WT and the ECL2 mutants were unable to induce UPR, whereas AT1-AG, which accumulates in the ER (Figure 7), significantly increased the expression of Grp78/BiP. The fact that ECL2 mutants were able to traffic to the cell surface in a misfolded conformation suggests that it was the accumulation of protein in the ER, and not the misfolding of proteins in the ER, that induced UPR. These results clearly demonstrate that the aglycosylated receptor, as opposed to the ECL2 mutant, caused the induction of UPR, as indicated by the increase in immunodetected Grp78/BiP.
Glycosylation on ICL1
We also constructed a mutant receptor incorporating a glycosylation consensus sequence in the first intracellular loop. If an N-linked glycan moiety was added to this consensus sequence, either the AT1 receptor topology or the glycosylation mechanics would have to be re-evaluated. Several studies have introduced artificial glycosylation sequons to assess multipass transmembrane protein topology [31–34]. Our results showed that the asparagine residue in this new glycosylation consensus sequence in ICL1 could not serve as an acceptor site for the oligosaccharyl-transferase enzyme. This confirmed that the cytoplasmic side of the AT1 receptor did not permit the addition of N-linked oligosaccharides.
Immature AT1 receptor isoforms
The molecular mass of the major immunoreactive band for AT1-WT was between 80 and 140 kDa, which is characteristic of this glycoprotein's migration pattern on SDS/PAGE [19]. An identical band was also present in the photolabelling experiments (Figure 2), suggesting that this was the form of receptor observed at the plasma membrane. However, Western blots also revealed a series of low molecular mass bands immunoprecipitated from cell lysates that were not seen in photolabelling experiments, suggesting that they were immature receptors not present at the plasma membrane. Moreover, although we observed high molecular mass bands corresponding to dimers and trimers of aglycosylated and monoglycosylated receptors in the IP/IB experiments, no such bands were observed in the photolabelling experiments, suggesting that they were not present at the cell surface. Similar to the aggregation of the vacuolar glycoprotein phaseolin in tunicamycin-treated cells [35], our under-glycosylated receptors would have an increased tendency to aggregate. The immature forms of aglycosylated AT1 receptors migrated as a doublet on SDS/PAGE gels. These doublets could thus not be due to glycosylation, but rather some other type of post-translational modification other than O-glycosylation or lipidation at Cys355, as these possibilities have been evaluated and rejected by Jayadev et al. [20]. We also observed from the Western blots that the low molecular mass AT1-269 mutant band was the only one that did not migrate as a doublet on SDS/PAGE gels. We thus propose that AT1-269 receptors may be degraded before being sufficiently advanced in the maturation process to acquire this modification. Metabolic labelling experiments showed that AT1-269 was degraded by the proteasome, since a greater proportion of pulse-labelled receptor remained after a 2 h chase in the presence of lactacystin (Figure 7B). Similar results were obtained when all pulse-labeled receptors were trapped in the ER and prevented from maturing to the cell surface by brefeldine A (results not shown). These results suggest that the degradation kinetics were accelerated for the AT1-269 mutant receptor. Furthermore, AT1-269 was detected as a 37 kDa species in Western blot analyses, suggesting the absence of oligosaccharides. AT1-269 may have been translocated to the cytosol and deglycosylated, as is the case for the δ opioid receptor [36]. Moreover, a post-translocational proteasomal bottleneck causes the accumulation of deglycosylated cytoplasmic forms of misfolded opsin [37]. This is not the case for the AT1-AG receptor, which is not degraded by the proteasome (Figure 7A), although both AT1-AG and AT1-269 receptors were probably misfolded due to the absence of critical oligosaccharides. The presence of an oligosaccharide on AT1-269 would allow it to be recognized by the glycan-based quality control machinery [(EDEM) ER-degradation-enhancing a-mannosidase-like protein)] and targeted for degradation by the proteasome. This is true for a variant of ribophorin I (RI332), where degradation by the proteasome is significantly delayed by treating the cells with an N-glycosylation inhibitor (tunicamycin) or by mutating the only RI332 glycosylation sequon [38].
The results of the present study suggest that addition of oligosaccharides to the AT1 receptor is both interloop and intraloop site-specific, since engineering non-natural sequons into ECL1, ECL2 and ECL3 altered several biosynthetic processes, such as targeting, folding, affinity, cell surface expression and quality control. Further studies are needed to clarify the precise role of oligosaccharides in the above-mentioned biosynthetic processes.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR). P.M.L. and P.C.L. are recipients of studentships from the Natural Sciences and Engineering Research Council of Canada (NSERC). E.E. is the recipient of a J.C. Edwards Chair in Cardiovascular Research. R.L. is a senior scholar of the Fonds de la Recherche en SantÉ du QuÉbec.
References
- 1.Morello J. P., Salahpour A., Laperriere A., Bernier V., Arthus M. F., Lonergan M., Petaja-Repo U., Angers S., Morin D., Bichet D. G., Bouvier M. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J. Clin. Invest. 2000;105:887–895. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forman M. S., Lee V. M., Trojanowski J. Q. ‘Unfolding’ pathways in neurodegenerative disease. Trends Neurosci. 2003;26:407–410. doi: 10.1016/S0166-2236(03)00197-8. [DOI] [PubMed] [Google Scholar]
- 3.Soto C., Kascsak R. J., Saborio G. P., Aucouturier P., Wisniewski T., Prelli F., Kascsak R., Mendez E., Harris D. A., Ironside J., et al. Reversion of prion protein conformational changes by synthetic β-sheet breaker peptides. Lancet. 2000;355:192–197. doi: 10.1016/s0140-6736(99)11419-3. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y., Choudhury P., Cabral C. M., Sifers R. N. Intracellular disposal of incompletely folded human α1-antitrypsin involves release from calnexin and post-translational trimming of asparagine-linked oligosaccharides. J. Biol. Chem. 1997;272:7946–7951. doi: 10.1074/jbc.272.12.7946. [DOI] [PubMed] [Google Scholar]
- 5.Noorwez S. M., Kuksa V., Imanishi Y., Zhu L., Filipek S., Palczewski K., Kaushal S. Pharmacological chaperone-mediated in vivo folding and stabilization of the P23H-opsin mutant associated with autosomal dominant retinitis pigmentosa. J. Biol. Chem. 2003;278:14442–14450. doi: 10.1074/jbc.M300087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenzo A., Razzaboni B., Weir G. C., Yankner B. A. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature (London) 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 7.Trombetta E. S., Parodi A. J. Quality control and protein folding in the secretory pathway. Annu. Rev. Cell Dev. Biol. 2003;19:649–676. doi: 10.1146/annurev.cellbio.19.110701.153949. [DOI] [PubMed] [Google Scholar]
- 8.Helenius A., Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 9.Parodi A. J. Protein glucosylation and its role in protein folding. Annu. Rev. Biochem. 2000;69:69–93. doi: 10.1146/annurev.biochem.69.1.69. [DOI] [PubMed] [Google Scholar]
- 10.Hauri H., Appenzeller C., Kuhn F., Nufer O. Lectins and traffic in the secretory pathway. FEBS Lett. 2000;476:32–37. doi: 10.1016/s0014-5793(00)01665-3. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 1977;74:2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabral C. M., Liu Y., Sifers R. N. Dissecting glycoprotein quality control in the secretory pathway. Trends Biochem. Sci. 2001;26:619–624. doi: 10.1016/s0968-0004(01)01942-9. [DOI] [PubMed] [Google Scholar]
- 13.Trombetta E. S., Helenius A. Conformational requirements for glycoprotein reglucosylation in the endoplasmic reticulum. J. Cell Biol. 2000;148:1123–1129. doi: 10.1083/jcb.148.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kundra R., Kornfeld S. Asparagine-linked oligosaccharides protect Lamp-1 and Lamp-2 from intracellular proteolysis. J. Biol. Chem. 1999;274:31039–31046. doi: 10.1074/jbc.274.43.31039. [DOI] [PubMed] [Google Scholar]
- 15.Loh Y. P., Gainer H. Evidence that glycosylation of pro-opiocortin and ACTH influences their proteolysis by trypsin and blood proteases. Mol. Cell. Endocrinol. 1980;20:35–44. doi: 10.1016/0303-7207(80)90092-1. [DOI] [PubMed] [Google Scholar]
- 16.Reitter J. N., Means R. E., Desrosiers R. C. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 17.Picker L. J., Warnock R. A., Burns A. R., Doerschuk C. M., Berg E. L., Butcher E. C. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991;66:921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- 18.de Gasparo M., Catt K. J., Inagami T., Wright J. W., Unger T. International Union of Pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 19.Lanctot P. M., Leclerc P. C., Escher E., Leduc R., Guillemette G. Role of N-glycosylation in the expression and functional properties of human AT1 receptor. Biochemistry. 1999;38:8621–8627. doi: 10.1021/bi9830516. [DOI] [PubMed] [Google Scholar]
- 20.Jayadev S., Smith R. D., Jagadeesh G., Baukal A. J., Hunyady L., Catt K. J. N-linked glycosylation is required for optimal AT1a angiotensin receptor expression in COS-7 cells. Endocrinology. 1999;140:2010–2017. doi: 10.1210/endo.140.5.6689. [DOI] [PubMed] [Google Scholar]
- 21.Deslauriers B., Ponce C., Lombard C., Larguier R., Bonnafous J. C., Marie J. N-glycosylation requirements for the AT1a angiotensin II receptor delivery to the plasma membrane. Biochem. J. 1999;339:397–405. [PMC free article] [PubMed] [Google Scholar]
- 22.Drescher B., Witte T., Schmidt R. E. Glycosylation of FcγRIII in N163 as mechanism of regulating receptor affinity. Immunology. 2003;110:335–340. doi: 10.1046/j.1365-2567.2003.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu L., Heaney M. L., Vera J. C., Golde D. W. High-affinity binding to the GM-CSF receptor requires intact N-glycosylation sites in the extracellular domain of the β subunit. Blood. 2000;95:3357–3362. [PubMed] [Google Scholar]
- 24.Flahaut M., Rossier B. C., Firsov D. Respective roles of calcitonin receptor-like receptor (CRLR) and receptor activity-modifying proteins (RAMP) in cell surface expression of CRLR/RAMP heterodimeric receptors. J. Biol. Chem. 2002;277:14731–14737. doi: 10.1074/jbc.M112084200. [DOI] [PubMed] [Google Scholar]
- 25.Laporte S. A., Boucard A. A., Servant G., Guillemette G., Leduc R., Escher E. Determination of peptide contact points in the human angiotensin II type I receptor (AT1) with photosensitive analogs of angiotensin II. Mol. Endocrinol. 1999;13:578–586. doi: 10.1210/mend.13.4.0270. [DOI] [PubMed] [Google Scholar]
- 26.Boucard A. A., Wilkes B. C., Laporte S. A., Escher E., Guillemette G., Leduc R. Photolabeling identifies position 172 of the human AT(1) receptor as a ligand contact point: receptor-bound angiotensin II adopts an extended structure. Biochemistry. 2000;39:9662–9670. doi: 10.1021/bi000597v. [DOI] [PubMed] [Google Scholar]
- 27.Wegele H., Muller L., Buchner J. Hsp70 and Hsp90 – a relay team for protein folding. Rev. Physiol. Biochem. Pharmacol. 2004;151:1–44. doi: 10.1007/s10254-003-0021-1. [DOI] [PubMed] [Google Scholar]
- 28.Ou W. J., Cameron P. H., Thomas D. Y., Bergeron J. J. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature (London) 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- 29.Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kD glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986;46:291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- 30.Mori K., Sant A., Kohno K., Normington K., Gething M. J., Sambrook J. F. A 22 bp cis-acting element is necessary and sufficient for the induction of the yeast KAR2 (BiP) gene by unfolded proteins. EMBO J. 1992;11:2583–2593. doi: 10.1002/j.1460-2075.1992.tb05323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newbolt A., Stoop R., Virginio C., Surprenant A., North R. A., Buell G., Rassendren F. Membrane topology of an ATP-gated ion channel (P2X receptor) J. Biol. Chem. 1998;273:15177–15182. doi: 10.1074/jbc.273.24.15177. [DOI] [PubMed] [Google Scholar]
- 32.Lehmann S., Chiesa R., Harris D. A. Evidence for a six-transmembrane domain structure of presenilin 1. J. Biol. Chem. 1997;272:12047–12051. doi: 10.1074/jbc.272.18.12047. [DOI] [PubMed] [Google Scholar]
- 33.Schwalbe R. A., Wingo C. S., Xia S. L. Mutations in the putative pore-forming segment favor short-lived wild-type Kir2.1 pore conformations. Biochemistry. 2002;41:12457–12466. doi: 10.1021/bi026304a. [DOI] [PubMed] [Google Scholar]
- 34.Popov M., Li J., Reithmeier R. A. Transmembrane folding of the human erythrocyte anion exchanger (AE1, Band 3) determined by scanning and insertional N-glycosylation mutagenesis. Biochem. J. 1999;339:269–279. [PMC free article] [PubMed] [Google Scholar]
- 35.Sparvoli F., Faoro F., Daminati M. G., Ceriotti A., Bollini R. Misfolding and aggregation of vacuolar glycoproteins in plant cells. Plant J. 2000;24:825–836. doi: 10.1046/j.1365-313x.2000.00933.x. [DOI] [PubMed] [Google Scholar]
- 36.Petaja-Repo U. E., Hogue M., Laperriere A., Bhalla S., Walker P., Bouvier M. Newly synthesized human δ opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J. Biol. Chem. 2001;276:4416–4423. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- 37.Saliba R. S., Munro P. M., Luthert P. J., Cheetham M. E. The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation. J. Cell Sci. 2002;115:2907–2918. doi: 10.1242/jcs.115.14.2907. [DOI] [PubMed] [Google Scholar]
- 38.de Virgilio M., Kitzmuller C., Schwaiger E., Klein M., Kreibich G., Ivessa N. E. Degradation of a short-lived glycoprotein from the lumen of the endoplasmic reticulum: the role of N-linked glycans and the unfolded protein response. Mol. Biol. Cell. 1999;10:4059–4073. doi: 10.1091/mbc.10.12.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]