Abstract
This study aims to investigate the adverse effects of immune checkpoint inhibitors (ICIs) on the female and male reproductive systems. In the FDA Adverse Event Reporting System (FAERS) database, adverse reactions under the “Reproductive system and breast disorders” category in the System Organ Classes were included, covering a period from January 1, 2015, to June 30, 2023. We identified 133,512 patients treated with ICIs. Immune checkpoint inhibitor-related reproductive adverse effects (irRAEs) were reported in 568 (0.43%) patients. Spermatogenesis abnormality (ROR025 = 7.91) had the highest signal strength associated with ICI use in males. Genital tract fistula was the only significant irRAE (ROR025 = 2.72) in females. PD-1 inhibitors pose greater risk than CTLA-4 inhibitors (OR = 1.65 [1.05–2.79], p = 0.045). Gynecologic cancers in females (OR = 3.77 [2.82–4.99], p < 0.0001) and urogenital cancers in males (OR = 1.56 [1.17–2.06], p = 0.0018) carried the highest risk compared to other cancers. Additional targeted drugs (OR = 2.32 [1.76–3.02], p < 0.0001), particularly lenvatinib (OR = 3.50 [2.48–4.94], p < 0.0001) and cabozantinib (OR = 3.71 [1.96–7.03], p < 0.0001) significantly increased the risk for females. Additional use of chemotherapy drugs was associated with a significant reduction in the risk for males (OR = 0.65 [0.42–0.96], p = 0.042) except for doxorubicin (OR = 2.58 [1.22–5.47], p = 0.013) and cyclophosphamide (OR = 2.36 [1.05–5.29], p = 0.038). This study demonstrates that ICIs could potentially lead to a wide range of adverse effects in the reproductive system in both males and females.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-91476-0.
Keywords: Immune checkpoint inhibitor, Neoplasm, Female genital system, Male genital system
Subject terms: Oncology, Cancer, Reproductive disorders, Urogenital reproductive disorders
Introduction
Immune checkpoint inhibitors (ICIs) are humanized monoclonal antibodies designed to enhance T-cell response against tumors by targeting immune inhibitory receptors PD-1 (Programmed cell death protein 1), PD-L1 (Programmed death-ligand 1), CTLA-4 (Cytotoxic T-lymphocyte-associated protein 4), and LAG-3 (Lymphocyte-activation gene-3)1. In the last decade, these drugs revolutionized strategic cancer treatment in a wide range of tumors not only at adjuvant and neoadjuvant settings but also in metastatic neoplasms2.
Besides the promising therapeutic potentials of the ICIs, they unfortunately cause a number of immune-related adverse events (irAEs) involving multiple organ systems. IrAEs are a spectrum of inflammatory toxicities resulting from the non-specific activation of the immune system, causing tissue/organ damage3. Overall incidence of irAEs ranges between 70 and 90% depending upon the type of the ICI. Compared to anti-PD-1 and PD-L1 therapy, anti-CTLA-4 therapy is associated with a higher rate of irAEs, and combination therapy leads to more frequent irAEs than monotherapy4. Moreover, irAEs seen in patients receiving anti-CTLA-4 therapy differ from those in patients treated with anti-PD-1, often presenting with greater severity5. However, the mechanisms underlying the differences in organ-specific toxicity between these therapeutic targets are not yet fully understood. 21% of these irAEs can even be life-threatening such as pneumonitis and liver dysfunction4. The most prevalent irAEs include gastrointestinal disturbances, cutaneous reactions, and endocrine dysfunctions including thyroiditis/hypothyroidism and hypophysitis-related pituitary dysfunction3.
Any ICI-related adverse reaction affecting the reproductive system in both genders can be defined as ICI-related reproductive adverse effect (irRAE). The available data on irRAEs is very limited. Testicular dysfunction and spermatogenic failure have been demonstrated in male cancer patients treated with ICIs6. However, no data is available on other adverse effects of ICIs on the reproductive system in male and female cancer patients. Therefore, we aimed to provide a detailed analysis of the irRAEs using the FDA Adverse Event Reporting System (FAERS) database.
Materials and methods
Data source
Our study investigates clinical features associated with irRAEs by using the FAERS database. Our focus was on ICIs approved by the FDA as of June 30, 2023, including anti-PD-1 agents (nivolumab, pembrolizumab, cemiplimab, dostarlimab, retifanlimab), anti-PD-L1 agents (atezolizumab, avelumab, durvalumab), anti-CTLA-4 agents (ipilimumab, tremelimumab), and an anti-LAG-3 agent (relatlimab). These ICIs served as keywords to extract relevant case reports from the database, covering the period from January 1, 2015, to June 30, 2023.
Classification and extraction of adverse reactions
Adverse reactions reported in the FAERS database are entered using preferred term (PT) codes from the Medical Dictionary for Regulatory Activities (MedDRA). The PTs provide distinct descriptors for an individual’s medical concepts, such as symptoms, side effects, and disease diagnosis. Different PTs are grouped into various System Organ Classes (SOCs). For this study, we defined irRAE as any ICI-related adverse reaction affecting reproductive organs in male and female patients. We included adverse reactions under the “Reproductive system and breast disorders” category in the SOC, marked as the primary SOC in the FAERS database. We obtained PTs for all reproductive adverse reactions from MedDRA (version 26.1) for our analysis, ensuring that the PTs analyzed were indeed related to reproductive adverse reactions (Supplementary Table S1)7.
Data processing procedure
A total of 13,702,373 cases were initially identified in the FAERS database, which was later reduced to 11,877,342 unique cases after excluding the duplications based on FDA guidelines. A total of 34,366,364 adverse events were reported in these cases.
We included only the cases where ICIs were the “primary suspect” in ROLE_COD, distinguishing between monotherapy (single ICI as “primary suspect”) and polytherapy (multiple ICIs with at least one as “primary suspect”). ROLE_COD classifies drugs as “primary suspect”, “secondary suspect”, “concomitant”, or “interacting” based on their possible contribution to the development of the adverse event. The time to onset was calculated as the period between the EVENT_DT and the START_DT. Reports were excluded if they contained discrepancies such as the EVENT_DT predating the START_DT or had inaccuracies in date entries. A total of 133,512 patients were identified who had received ICIs.
For analysis, we utilized the International Nonproprietary Names of chemotherapies, targeted therapies, endocrine therapies, and immunotherapies, categorized under “L01 ANTINEOPLASTIC AGENTS” by the WHO Collaborating Centre for Drug Statistics Methodology. This facilitated the categorization of therapies used alongside the ICIs (Supplementary Tables S2 and S3).
Signal mining/data extraction
For signal detection, we used the reporting odds ratio (ROR) method8. ROR calculations were conducted for PTs with at least three reports, as detailed in Supplementary Tables S4 and S5. A signal was considered significant if the lower limit of the 95% confidence interval (CI) for the ROR exceeded 1, identifying the key drug-adverse reaction associations relevant to irRAE9.
Statistical analysis
For categorical variables, we used Chi-square test or Fisher’s exact test, depending on the sample size and the expected frequencies in contingency tables. As both age and time-to-onset of irRAE, the continuous variables in our study, were not normally distributed, we utilized the Kruskal-Wallis test for their analysis. Univariate logistic regression analyses were performed to calculate the odds ratios (OR) for the occurrence of irRAE. All statistical procedures were conducted using R software (version 4.3.1), adhering to a significance threshold of p < 0.05.
Ethical statement
Due to the public accessibility of the FAERS database and the anonymization of patient records, ethical approval and informed consent are not required for this study.
Results
Overall analysis
We identified 133,512 patients who had received ICIs, among whom 568 developed irRAEs (0.43%) (Table 1). Of these 568 patients, 285 (52.7%) were male and 256 (47.3%) were female. Overall, the incidence of irRAEs was significantly higher in female patients compared to males (0.57% vs. 0.39%, respectively; p < 0.0001). The median (IQR) age of the female patients experiencing irRAEs was significantly lower than those who had not developed irRAEs (57 [47–69.5] vs. 64 [55–72] years, respectively; p < 0.0001). However, such a difference was not observed in male patients who had and had not developed irRAEs (67 [58–73] vs. 67 [59–74] years, respectively; p = 0.24).
Table 1.
Demographics, clinical characteristics, and gender-based differences in patients with and without reproductive adverse effects (AEs) following ICI administration reported in the FAERS database.
| Clinical Characteristics | All patients (n=133,512) | Patients without reproductive AEs (n=132,944) | Patients with reproductive AEs (n=568) | Male patients with reproductive AEs (n=285) | Female patients with reproductive AEs (n=256) | p-value* |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 72,935 (62.1%) | 72,650 (62.1%) | 285 (52.7%) | Incidence rate: 0.39% | NA | <0.0001 |
| Female | 44,582 (37.9%) | 44,326 (37.9%) | 256 (47.3%) | NA | Incidence rate: 0.57% | |
| Missing | 15,995 | 15,968 | 27 | NA | NA | |
| Age, median (IQR) | 66 (57 – 73) |
66 (57.8 – 73) M: 67 (59–74)** F: 64 (55–72)*** |
63 (51 - 71.8) | 67 (58-73)** | 57 (47 - 69.5)*** | <0.0001 |
| ≥ 65 | 49,175 (55.8%) | 48,992 (55.9%) | 183 (46.0%) | 119 (56.4%) | 61 (34.1%) | <0.0001 |
| < 65 | 38,951 (44.1%) | 38,736 (44.1%) | 215 (54.0%) | 92 (43.6%) | 118 (65.9%) | |
| Missing | 45,386 | 45,216 | 170 | 74 | 77 | NA |
| Time to onset-day, median (IQR) | 42 (14 – 114) | 42 (14 – 114) | 30 (7.8 – 85) | 32 (8 – 93) | 29 (8-82) | 0.0013 |
| Indication for ICI Use by Organ Systems | ||||||
| Lung | 40,148 (30.1%) | 40,023 (30.1%) | 125 (24.8%) | 81 (31.0%) | 37 (16.3%) | <0.0001 |
| Skin | 22,724 (17.0%) | 22,620 (17.0%) | 104 (20.6%) | 57 (21.8%) | 43 (18.9%) | |
| Urogenital | 17,021 (12.8%) | 16,927 (12.7%) | 94 (18.6%) | 66 (25.3%) | 26 (11.5%) | |
| Gynecologic | 4354 (3.3%) | 4281 (3.2%) | 73 (14.5%) | 0 (0%) | 69 (30.4%) | |
| Gastrointestinal | 7613 (5.7%) | 7584 (5.7%) | 29 (5.7%) | 12 (4.6%) | 17 (7.5%) | |
| Breast | 3186 (2.4%) | 3171 (2.4%) | 15 (3.0%) | 0 (0%) | 14 (6.2%) | |
| Hepatopancreatobiliary | 6393 (4.8%) | 6379 (4.8%) | 14 (2.8%) | 11 (4.2%) | 3 (1.3%) | |
| Hematopoietic and Lymphoid | 2527 (1.9%) | 2514 (1.9%) | 13 (2.6%) | 9 (3.5%) | 3 (1.3%) | |
| Head and Neck | 4083 (3.1%) | 4071 (3.1%) | 12 (2.4%) | 9 (3.5%) | 3 (1.3%) | |
| Other Thoracic (Cardiac, mesothelioma, thymus) | 1366 (1.0%) | 1358 (1.0%) | 8 (1.6%) | 5 (1.9%) | 3 (1.3%) | |
| Musculoskeletal | 828 (0.6%) | 823 (0.6%) | 5 (1.0%) | 3 (1.2%) | 2 (0.9%) | |
| CNS | 995 (0.7%) | 992 (0.7%) | 3 (0.6%) | 0 (0%) | 2 (0.9%) | |
| Endocrine | 395 (0.3%) | 394 (0.3%) | 1 (0.2%) | 0 (0%) | 1 (0.4%) | |
| Other Indications | 4319 (3.2%) | 4310 (3.2%) | 9 (1.8%) | 8 (3.1%) | 4 (1.8%) | |
| Unspecified or Missing | 17,560 | 17,497 | 57 | 24 | 29 | NA |
| Number of different immunotherapy agents used | ||||||
| Monotherapy | 111,412 (83.5%) | 110,930 (83.4%) | 482 (84.9%) | 237 (83.2%) | 221 (86.3%) | 0.22 |
| Dual Therapy | 21,617 (16.2%) | 21,535 (16.2%) | 82 (14.4%) | 46 (16.1%) | 34 (13.3%) | |
| Triple Therapy or More | 483 (0.4%) | 479 (0.4%) | 4 (0.7%) | 2 (0.7%) | 1 (0.4%) | |
| Number of different chemotherapy agents used | ||||||
| None | 116,072 (86.9%) | 115,566 (86.9%) | 506 (89.1%) | 260 (91.2%) | 224 (87.5%) | 0.14 |
| One | 4280 (3.2%) | 13,118 (9.9%) | 42 (7.4%) | 10 (3.5%) | 8 (3.1%) | |
| Two or more | 13,160 (9.9%) | 4260 (3.2%) | 20 (3.5%) | 15 (5.3%) | 24 (9.4%) | |
| Number of different targeted therapy agents used | ||||||
| None | 117,061 (87.7%) | 116,611 (87.7%) | 450 (79.2%) | 246 (86.3%) | 180 (70.3%) | <0.0001 |
| One | 15,007 (11.2%) | 14,897 (11.2%) | 110 (19.4%) | 35 (12.3%) | 72 (28.1%) | |
| Two or more | 1444 (1.1%) | 1436 (1.1%) | 8 (1.4%) | 4 (1.4%) | 4 (1.6%) | |
| Outcomes | ||||||
| Non-serious outcome | 16,285 (12.2%) | 16,181 (12.2%) | 104 (18.3%) | 50 (17.5%) | 49 (19.1%) | <0.0001 |
| Serious outcome | 117,227 (87.8%) | 116,763 (87.8%) | 464 (81.7%) | 235 (82.5%) | 207 (80.9%) | |
| Death | 34,526 (25.9%) | 34,463 (25.9%) | 63 (11.1%) | 35 (12.3%) | 26 (10.2%) | <0.0001 |
| Hospitalization | 53,884 (40.4%) | 53,615 (40.3%) | 269 (47.4%) | 140 (49.1%) | 118 (46.1%) | 0.0007 |
| Life-Threatening | 8141 (6.1%) | 8108 (6.1%) | 33 (5.8%) | 17 (6.0%) | 14 (5.5%) | 0.84 |
| Disability | 2761 (2.1%) | 2742 (2.1%) | 19 (3.4%) | 8 (2.8%) | 10 (3.9%) | 0.046 |
| Required Intervention to Prevent Permanent Impairment/Damage | 155 (0.1%) | 154 (0.1%) | 1 (0.2%) | 1 (0.4%) | 0 (0%) | 0.48 |
| Other Serious | 89,320 (66.9%) | 88,971 (66.9%) | 349 (61.4%) | 183 (64.2%) | 150 (58.6%) | 0.0064 |
F, Female ; IQR, interquartile range ; M, male ; NA, not applicable.
* P-values belong to chi-squared, Fisher’s exact and T-test analyses between patients with and without reproductive AEs.
** p=0.24.
*** p<0.0001.
The time to develop any immune-related adverse effect was significantly shorter in patients who had experienced irRAEs, with a median (IQR) onset time of 30 days (7.8–85 days) compared to 42 days (14–114 days) for those without irRAEs (p = 0.0013).
There were variations in the incidences of irRAEs across different types of cancers. Overall, the most common indication for the use of ICIs was lung cancer (30.1%). Lung cancer was also the most common malignancy among the patients experiencing irRAEs (24.8%). The second most common indication for the use of ICIs was skin cancer (17.0%), and 20.6% of all irRAEs occurred in this group. Urogenital cancers ranked as the third most common indication (12.8%), with 18.6% of irRAEs observed in this group. Among all cases with irRAEs, there were significant gender disparities in the percentages of lung and urogenital cancers. Lung cancers were the indication for ICI therapy in 31.0% of males and 16.3% of females experiencing irRAEs (p = 0.0006), and urogenital cancers were the indication for ICI therapy in 25.3% of males and 11.5% of females experiencing irRAEs (p < 0.0001). No gender difference was observed in patients with skin cancers (21.8% in males vs. 18.9% in females; p = 0.32). Although gynecological malignancies constituted only 9.8% of the indications for the use of ICIs in female patients, 30.4% of all irRAEs in females were observed in this group, making them the most common indication among female patients experiencing irRAEs.
Reproductive adverse events according to the gender and type of immune checkpoint inhibitor drug
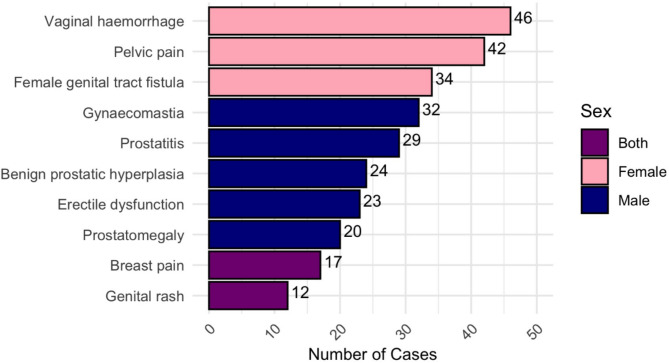
The most common irRAEs reported in female patients were vaginal hemorrhage (46 cases; 18%), pelvic pain (42 cases; 16.4%), and female genital tract fistula (34 cases; 13.3%). In male patients, gynecomastia (32 cases; 11.2%), prostatitis (29 cases; 10.2%), benign prostatic hyperplasia (24 cases; 8.4%), erectile dysfunction (23 cases; 8.1%), and prostatomegaly (20 cases; 7.0%) were the most common. Breast pain in 17 cases (3.0%) and genital rash in 12 cases (2.1%) were also reported as other irRAEs in both sexes (Fig. 1).
Fig. 1.
The most commonly reported immune checkpoint inhibitor-related reproductive adverse effects (irRAEs) in female and male patients. In females, vaginal hemorrhage, pelvic pain, and genital tract fistulas were the most frequent irRAEs, while in males, the most commonly reported irRAEs were gynecomastia, prostatitis, benign prostatic hyperplasia, erectile dysfunction, and prostatomegaly.
Males
In overall analysis of male patients, spermatogenesis abnormality (ROR025 = 7.91) was the most significant irRAE associated with the use of ICIs. This was followed by scrotal irritation (ROR025 = 3.83), scrotal oedema (ROR025 = 1.90), prostatitis (ROR025 = 1.25), acquired phimosis (ROR025 = 1.20), and scrotal erythema (ROR025 = 1.08) (Fig. 2).
Fig. 2.

The lower limits of the 95% confidence intervals (CI) for the reporting odds ratio (ROR) calculations. ROR calculations were conducted for preferred terms with at least three reports. The lower limit of the 95% CI for the ROR exceeding 1 was considered significant, highlighting key drug-adverse reaction associations.
All cases reporting spermatogenesis abnormality as irRAE were on dual ICI therapy regimens consisting of nivolumab plus ipilimumab (n = 4); and pembrolizumab plus ipilimumab (n = 2). It is unknown if isolated use of ICIs cause spermatogenesis abnormalities due to insufficient data and small number of cases for each drug.
When used as monotherapy, durvalumab (ROR025 = 2.37) and nivolumab (ROR025 = 1.01) were found to be associated with a significant risk of prostatitis. However, such an adverse effect was not observed for other ICIs (ROR025 = 0.47 for pembrolizumab, ROR025 = 0.52 for atezolizumab), suggesting that not all ICIs cause prostatitis.
It also appeared that dual therapy regimens but not monotherapy were associated with a significant risk of balanoposthitis development (ROR025 values for dual therapy and monotherapy were 1.65 and 0.36, respectively) (Supplementary Table S6).
Females
While the most frequent irRAE among female patients was vaginal hemorrhage (n = 46 [18%], ROR025 = 0.14), the only irRAE that shows significant association with the use of ICI was genital tract fistula (n = 34 [13.3%], ROR025 = 2.72). Interestingly, this side effect was observed only in the patients treated with atezolizumab (ROR025 = 4.51) and pembrolizumab (ROR025 = 3.69) but not nivolumab (ROR025 = 0.50). There were some other adverse effects which were only observed in certain specific monotherapy treatment regimens as follows: pelvic fluid collection with pembrolizumab (ROR025 = 2.12), vulvovaginal rash with pembrolizumab (ROR025 = 2.00), breast disorder with atezolizumab (ROR025 = 1.21), and genital rash with nivolumab (ROR025 = 1.06).
Perineal rash remained to be the only significant adverse effect of the ICIs occurring in both males and females (ROR025 = 1.38).
Details of the most commonly reported IrRAEs
Prostatitis (n = 29), scrotal oedema (n = 12), and spermatogenesis abnormalities (n = 6) were the most commonly reported irRAEs in male patients (Table 2).
Table 2.
Statistically significant reproductive adverse events reported with immune checkpoint inhibitors with demographic and regimen details.
| Adverse effects | No. cases | Age (Mean ± SD, Range) | ICI regimen | Therapeutic indication | Reporter country | Days until onset after ICI use (Median, Range) | No. comorbid endocrine IrAEs |
Concomitant drugs1 | No. mortality | |
|---|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy | Targeted therapy | |||||||||
| Perineal rash2 | 3 | 81±NA, 81-81 |
PEM : 2 DUR: 1 |
Lung: 1 Skin: 1 NS: 1 |
US: 2 AU: 1 |
70, 70-70 | NA | NA | Dabrafenib + Trametinib: 1 | 0 |
| Male | ||||||||||
| Acquired phimosis | 3 | 69±11.31, 61-77 |
ATE: 1 IP/NIV: 1 PEM : 1 |
Lung: 1 Urogenital 1 NS: 1 |
DE: 2 NS: 1 |
44, 0-88 | NA | NA | Bevacizumab: 1 | 0 |
| Prostatitis | 29 | 70.88±9.52, 55-86.33 |
NIV: 10 IP/NIV: 5 PEM : 5 DUR: 4 ATE: 3 CEM: 1 IP: 1 |
Urogenital 10 Lung: 8 Skin: 4 Gastrointestinal: 2 Hematopoietic and Lymphoid: 2 Head and Neck: 1 Other Thoracic4: 1 NS: 1 |
JP: 8 US: 6 FR: 5 ES: 2 BR: 1 CZ: 1 DE: 1 IE: 1 UK: 1 NS: 3 |
57, 0-486 |
Hypothyroidism: 1 Hyperthyroidism and hypothyroidism: 13 |
AZA: 2 CB + PTX: 2 DTX: 1 OXA + CE: 1 OXA + Tegafur: 1 |
Bevacizumab: 2 Cabozantinib: 1 Cetuximab: 1 |
3 |
| Scrotal erythema | 3 | 67±4.24, 64-70 |
PEM : 2 ATE: 1 |
Lung: 1 Hepatopancreatobiliary: 1 NS: 1 |
IT: 1 US: 1 NS: 1 |
48, 31-65 | Hypothyroidism: 1 | NA | Bevacizumab: 1 | 0 |
| Scrotal irritation | 3 | 58.33±13.65, 46-73 |
IP/NIV: 1 NIV: 1 PEM : 1 |
Lung: 1 Urogenital: 1 Other Thoracic4: 1 |
US: 2 CA: 1 |
22, 22-22 | NA | NA | Cabozantinib: 1 | 0 |
| Scrotal oedema | 12 | 60.42±13.84, 41-84 |
PEM : 4 IP/NIV: 3 NIV: 3 ATE: 1 IP: 1 |
Skin: 3 Gastrointestinal: 2 Hepatopancreatobiliary: 2 Urogenital: 2 Head and Neck: 1 Lung: 1 NS: 1 |
US: 7 BG: 1 CN: 1 DK: 1 FR: 1 JP: 1 |
161, 14-635 | Adrenal insufficiency: 1 | NA | Cabozantinib: 1 | 3 |
| Abnormal spermatogenesis | 6 | NA |
IP/NIV: 4 IP/PEM: 2 |
Skin: 6 | US: 6 | NA | NA | NA | NA | 0 |
| Female | ||||||||||
| Female genital tract fistula | 34 | 57.84±15.76, 27-86 |
PEM : 15 ATE: 9 NIV: 4 IP/NIV: 3 AVE: 1 CEM: 1 DUR: 1 |
Gynecologic: 21 Gastrointestinal: 4 Urogenital: 3 Head and Neck: 2 CNS: 1 Lung: 1 Skin: 1 NS: 1 |
US: 9 JP: 8 FR: 3 DE: 2 IL: 2 IT: 2 ES: 2 MX: 1 CH: 1 UK: 1 NS: 3 |
45, 1-292 | Hypothalamus and pituitary disorder: 1 |
CB + PTX: 2 PTX + CP: 2 CB: 1 CP: 1 OXA: 1 5-FU + OXA + IRI: 1 |
Lenvatinib: 6 Bevacizumab: 5 Cabozantinib: 1 Celecoxib: 1 |
6 |
5-FU, Fluorouracil; AZA, Azacitidine; ATE, Atezolizumab; AVE, Avelumab; CB, Carboplatin; CEM, Cemiplimab; CE, Capecitabine; CP, Cisplatin; DTX, Docetaxel; DUR, Durvalumab; ICI, Immune checkpoint inhibitor; IP, Ipilimumab; irAEs, Immune-related adverse effects; IRI, Irinotecan; NA, Not applicable; NIV, Nivolumab; NS, Not specified; OXA, Oxaliplatin; PEM, Pembrolizumab; PTX, Paclitaxel; SD, Standard deviation.
1No patient has been administered endocrine therapy for oncologic purposes.
2The sex distribution of perineal rash is: Male: 1, Female: 2.
3One patient experienced hypothyroidism, while another experienced both hypothyroidism and hyperthyroidism as endocrine irAEs.
4Other thoracic indications are mesothelioma, cardiac and thymus malignancies.
The mean (±SD) age of the patiens who had developed prostatitis were 70.9 years (±9.5). Median onset time of prostatitis was 57 days. The most common indications for the use of ICIs in these cases were urogenital cancer (n = 10; upper tract urothelial cancer [n = 5], renal cancer [n = 4], prostate cancer [n = 1]), lung cancer (n = 8), and skin cancer (n = 4). Additional chemotherapy was used in 7 cases, while 4 cases involved the use of additional targeted therapy.
The mean (±SD) age of the patiens who had developed scrotal oedema were 60.4 years (±13.8). Median onset time of scrotal oedema was 161 days. The indications for ICI therapy for the cases with scrotal oedema were skin cancer (n = 3), gastrointestinal cancer (n = 2), hepatopancreatobiliary cancer (n = 2), urogenital cancer (n = 2), head and neck cancer (n = 1), and lung cancer (n = 1). Among these cases, only one patient received additional targeted therapy.
There were no available data on the ages and time to onset of irRAE in patients who experienced spermatogenesis abnormality. All of the six cases reporting spermatogenesis abnormality as irRAE had skin cancer and were on dual ICI therapy regimens (nivolumab plus ipilimumab = 4, pembrolizumab plus ipilimumab = 2). None of these patients received additional chemotherapy or targeted therapy, and had any concomitant adverse events.
In female patients, genital tract fistula exhibited the highest signal strength associated with the use of ICIs (n = 34; ROR025 = 2.72). The mean (±SD) age of these cases was 57.8 years (±15.8). Median onset time of genital tract fistula formation was 45 days. The indications for ICI therapy in these cases were gynecologic cancer (n = 21; cervical cancer [n = 10], endometrial cancer [n = 8], ovarian cancer [n = 2], and vulvar cancer [n = 1]), gastrointestinal cancer (n = 4), urogenital cancer (n = 3), head and neck cancer (n = 2), lung cancer (n = 1), CNS cancer (n = 1), and skin cancer (n = 1). The most commonly used ICIs in these cases were pembrolizumab (n = 15), atezolizumab (n = 9), and nivolumab (n = 4). Nivolumab and ipilumumab dual therapy was used only in three cases. While additional targeted therapy was used in 13 cases, additional chemotherapy was used in eight cases.
Factors affecting the risk of IrRAEs
The logistic regression analysis revealed that male patients had a significantly lower risk of irRAEs compared to females (OR = 0.68 [0.57–0.81], p < 0.0001) (Table 3). The presence of additional targeted therapy significantly increased the risk of experiencing irRAEs (OR = 1.87 [1.52–2.29], p < 0.0001) and this risk appeared to be more pronounced in females (OR = 2.32 [1.76–3.02], p < 0.0001; males, OR = 1.23 [0.86–1.70], p = 0.23). However, additional chemotherapy did not appear to have any effect on the overall risk of irRAEs (OR = 0.82 [0.62–1.05], p = 0.13). Notably, when stratified by gender, additional chemotherapy was associated with a significantly reduced risk of irRAEs in males (OR = 0.65 [0.42–0.96], p = 0.042), while this effect was not observed in females (OR = 0.77 [0.52–1.10], p = 0.16). There was no marked difference for the development of irRAEs between the patients on polytherapy vs. monotherapy (OR = 0.90 [0.71–1.13], p = 0.36).
Table 3.
Univariate logistic regression analysis of risk factors for reproductive adverse effects after ICI administration, with sex-based subgroup analyses.
| Variable | Reference | Odds Ratio [95% CI] | p-value |
|---|---|---|---|
| All patients | |||
| Male | Female | 0.68 [0.57-0.81] | <0.0001 |
| Targeted therapy | No targeted therapy | 1.87 [1.52-2.29] | <0.0001 |
| Chemotherapy | No chemotherapy | 0.82 [0.62-1.05] | 0.13 |
| Polytherapy | Monotherapy | 0.90 [0.71-1.13] | 0.36 |
| PD-L1 | CTLA-4 | 1.39 [0.86-2.42] | 0.21 |
| PD-1 | PD-L1 | 1.18 [0.95-1.49] | 0.14 |
| PD-1 | CTLA-4 | 1.65 [1.05-2.79] | 0.045 |
| Skin Cancer | Other Indications | 1.06 [0.85-1.32] | 0.57 |
| Lung Cancer | Other Indications | 0.62 [0.50-0.76] | <0.0001 |
| Urogenital Cancer | Other Indications | 1.33 [1.06-1.66] | 0.013 |
| Male patients | |||
| Targeted therapy | No targeted therapy | 1.23 [0.86-1.70] | 0.23 |
| Chemotherapy | No chemotherapy | 0.65 [0.42-0.96] | 0.042 |
| Polytherapy | Monotherapy | 0.95 [0.69-1.28] | 0.74 |
| PD-L1 | CTLA-4 | 1.45 [0.70-3.52] | 0.36 |
| PD-1 | PD-L1 | 1.25 [0.92-1.74] | 0.17 |
| PD-1 | CTLA-4 | 1.81 [0.92-4.27] | 0.12 |
| Skin Cancer | Other Indications | 1.25 [0.92-1.67] | 0.13 |
| Lung Cancer | Other Indications | 0.79 [0.60-1.02] | 0.07 |
| Urogenital Cancer | Other Indications | 1.56 [1.17-2.06] | 0.0018 |
| Female patients | |||
| Targeted therapy | No targeted therapy | 2.32 [1.76-3.02] | <0.0001 |
| Chemotherapy | No chemotherapy | 0.77 [0.52-1.10] | 0.16 |
| Polytherapy | Monotherapy | 0.86 [0.59-1.21] | 0.41 |
| PD-L1 | CTLA-4 | 0.99 [0.51-2.17] | 0.98 |
| PD-1 | PD-L1 | 1.19 [0.86-1.69] | 0.30 |
| PD-1 | CTLA-4 | 1.18 [0.64-2.49] | 0.63 |
| Skin Cancer | Other Indications | 0.92 [0.65-1.27] | 0.62 |
| Lung Cancer | Other Indications | 0.43 [0.30-0.61] | <0.0001 |
| Gynecologic Cancer | Other Indications | 3.77 [2.82-4.99] | <0.0001 |
| Urogenital Cancer | Other Indications | 1.20 [0.78-1.77] | 0.39 |
NA, not applicable; CI, confidence interval.
Regarding the specific ICIs, PD-1 inhibitors pose the greatest risk for irRAEs when compared with CTLA-4 inhibitors (OR = 1.65 [1.05–2.79], p = 0.045). However, such a difference was not found between PD-L1 inhibitors and CTLA-4 inhibitors (OR = 1.39 [0.86–2.42], p = 0.21), and also PD-1 and PD-L1 inhibitors (OR = 1.18 [0.95–1.49], p = 0.14).
When specific cancer types were analyzed, gynecologic cancers in females (OR = 3.77 [2.82–4.99], p < 0.0001) and urogenital cancers in males (OR = 1.56 [1.17–2.06], p = 0.0018) were the strongest risk factors for the development of irRAEs. Even though lung cancers constituted the most common indications for the use of ICIs, they were associated with a significantly lower risk of irRAEs in females (OR = 0.43 [0.30–0.61], p < 0.0001) but not in males (OR = 0.79 [0.60–1.02], p = 0.07).
We also found that certain chemotherapy agents and targeted therapies that were used in combination with ICIs showed significant associations with the risk of irRAEs. Doxorubicin (OR = 2.58 [1.22–5.47], p = 0.013) and cyclophosphamide (OR = 2.36 [1.05–5.29], p = 0.038) were associated with an increased risk, whereas pemetrexed (OR = 0.38 [0.17–0.84], p = 0.017) and carboplatin (OR = 0.63 [0.42–0.93], p = 0.02) were associated with a decreased risk (Table 4).
Table 4.
Univariate logistic regression analysis of added agents for ICI-related reproductive adverse effects.
| Antineoplastic agents | All patients | Male patients | Female patients | |||
|---|---|---|---|---|---|---|
| Odds Ratio [95% CI] | p-value | Odds Ratio [95% CI] | p-value | Odds Ratio [95% CI] | p-value | |
| Alkylating agents | 1.51 [0.72-3.19] | 0.28 | NA | NA | 1.32 [0.54-3.21] | 0.54 |
| Nitrogen mustard analogues | 2.11 [0.94-4.74] | 0.07 | NA | NA | 1.75 [0.72-4.27] | 0.22 |
| Cyclophosphamide | 2.36 [1.05-5.29] | 0.038 | NA | NA | 1.89 [0.78-4.60] | 0.16 |
| Antimetabolites | 0.66 [0.43-1.02] | 0.061 | 0.62 [0.34-1.13] | 0.12 | 0.61 [0.31-1.19] | 0.15 |
| Folic acid analogues | 0.36 [0.16-0.81] | 0.013 | NA | NA | NA | NA |
| Pemetrexed | 0.38 [0.17-0.84] | 0.017 | NA | NA | NA | NA |
| Pyrimidine analogues | 1.00 [0.60-1.67] | 1.0 | 0.85 [0.40-1.81] | 0.68 | 1.14 [0.56-2.32] | 0.71 |
| Gemcitabine | 1.05 [0.47-2.35] | 0.90 | NA | NA | NA | NA |
| Plant alkaloids and other natural products | 0.89 [0.64-1.25] | 0.51 | 0.58 [0.32-1.06] | 0.075 | 0.90 [0.58-1.41] | 0.66 |
| Podophyllotoxin derivatives | 0.60 [0.27-1.34] | 0.21 | NA | NA | NA | NA |
| Etoposide | 0.60 [0.27-1.34] | 0.21 | NA | NA | NA | NA |
| Taxanes | 0.92 [0.62-1.36] | 0.66 | 0.41 [0.17-1.00] | 0.05 | 0.92 [0.56-1.51] | 0.75 |
| Paclitaxel | 0.89 [0.59-1.35] | 0.58 | NA | NA | 0.98 [0.60-1.61] | 0.94 |
| Cytotoxic antibiotics and related substances | 1.76 [0.83-3.72] | 0.14 | NA | NA | 1.26 [0.52-3.06] | 0.61 |
| Anthracyclines and related substances | 1.82 [0.86-3.84] | 0.12 | NA | NA | 1.27 [0.52-3.10] | 0.59 |
| Doxorubicin | 2.58 [1.22-5.47] | 0.013 | NA | NA | 1.88 [0.77-4.59] | 0.16 |
| Platinum compounds | 0.75 [0.54-1.02] | 0.066 | 0.42 [0.24-0.73] | 0.002 | 0.97 [0.65-1.46] | 0.88 |
| Cisplatin | 1.15 [0.66-1.99] | 0.63 | NA | NA | 2.08 [1.06-4.05] | 0.033 |
| Carboplatin | 0.63 [0.42-0.93] | 0.02 | 0.42 [0.22-0.82] | 0.011 | 0.64 [0.38-1.10] | 0.11 |
| Oxaliplatin | 1.47 [0.70-3.11] | 0.31 | NA | NA | 2.68 [1.10-6.54] | 0.03 |
| Protein kinase inhibitors | 2.46 [1.94-3.13] | <0.0001 | 1.53 [0.99-2.37] | 0.055 | 2.81 [2.09-3.79] | <0.0001 |
| VEGFR tyrosine kinase inhibitors | 1.40 [0.70-2.83] | 0.34 | 2.10 [0.99-4.46] | 0.054 | NA | NA |
| Axitinib | 1.43 [0.71-2.87] | 0.32 | 2.12 [1.00-4.51] | 0.05 | NA | NA |
| Other tyrosine kinase inhibitors | 3.32 [2.56-4.30] | <0.0001 | 1.88 [1.11-3.16] | 0.018 | 3.61 [2.65-4.92] | <0.0001 |
| Cabozantinib | 2.69 [1.72-4.21] | <0.0001 | 2.07 [1.10-3.90] | 0.024 | 3.71 [1.96-7.03] | <0.0001 |
| Lenvatinib | 3.79 [2.75-5.22] | <0.0001 | NA | NA | 3.50 [2.48-4.94] | <0.0001 |
| Monoclonal antibodies and antibody drug conjugates | 1.05 [0.74-1.49] | 0.79 | 0.86 [0.50-1.48] | 0.59 | 1.17 [0.71-1.95] | 0.54 |
| VEGF/VEGFR inhibitors | 1.04 [0.71-1.52] | 0.84 | 0.91 [0.52-1.59] | 0.74 | 1.10 [0.61-1.96] | 0.75 |
| Bevacizumab | 1.05 [0.74-1.49] | 0.79 | 0.80 [0.44-1.45] | 0.46 | 1.13 [0.63-2.02] | 0.68 |
CI, confidence interval; NA, not applicable; VEGFR, Vascular endothelial growth factor receptor; VEGF, Vascular endothelial growth factor.
* The specific agents in each group were selected based on case numbers, with only those having at least three reported cases being included in this table. The full lists of all included agents are provided in Supplementary Tables S2 and S3.
For targeted therapies, protein kinase inhibitors lenvatinib (OR = 3.79 [2.75–5.22], p < 0.0001) and cabozantinib (OR = 2.69 [1.72–4.21], p < 0.0001) significantly increased the risk of irRAEs. Such an elevated risk was not observed in the patients who received monoclonal antibodies and antibody drug conjugates (OR = 1.05 [0.74–1.49], p = 0.79).
The effect of mono vs. dual ICIs and other additional cancer treatment modalities on the incidence of IrRAEs
The incidence of irRAEs in patients treated with ICI monotherapy was 0.43%. Among these cases, ICI monotherapy was used in 482 cases (84.9%) of whom nivolumab was administered in 178 (31.3%), pembrolizumab in 181 (31.9%), and atezolizumab in other 77 patients (13.6%) (Fig. 3).
Fig. 3.
The incidences of immune checkpoint inhibitor-related reproductive adverse effects (irRAEs) in patients treated with immune checkpoint inhibitor (ICI) monotherapy, including anti-PD-1, anti-PD-L1, and anti-CTLA-4 (ipilimumab) agents, as well as dual ICI therapy.
The incidence of irRAEs in patients treated with dual ICI therapy was 0.38%. Dual therapies were administered in 82 cases (14.4%) with the combination of nivolumab and ipilimumab being the most commonly used (73 cases [12.9%]). The incidence of irRAEs did not differ significantly between ICI monotherapy and dual ICI therapy (0.43% vs. 0.38%, respectively; p = 0.27).
Additional chemotherapy was used in 10.9% and 13.1% of the cases who did and did not develop irRAEs, respectively (p = 0.14). Additional chemotherapy was indeed associated with a significant reduction in the risk for males (OR = 0.65 [0.42–0.96], p = 0.042) except for doxorubicin (OR = 2.58 [1.22–5.47], p = 0.013) and cyclophosphamide (OR = 2.36 [1.05–5.29], p = 0.038), both of which posed an elevated risk for the development of irRAEs.
The use of additional targeted therapies appeared to have increased the incidence of irRAEs. For instance, 20.8% and 12.3% of the patients who did and did not experience irRAEs, respectively, had received targeted therapy (p < 0.0001). We also found a gender-based difference in the development of irRAEs after the use of additional targeted therapies, with a significantly higher percentage of female patients receiving these drugs compared to the males (29.7% vs. 13.7%, respectively; p < 0.0001).
Interestingly, cancer-related serious outcomes developed in a significantly lower proportion of the patients who had experienced irRAEs compared to those who had not (81.7% vs. 87.8%, respectively; p < 0.0001). Cancer related mortality (11.1% vs. 25.9%, respectively; p < 0.0001) and other serious outcomes (61.4% vs. 66.9%, respectively; p = 0.0064) were also significantly lower in the patients who had experienced irRAEs compared to those who had not. On the other hand, irRAEs were associated with higher hospitalization (47.4% vs. 40.3%, respectively; p = 0.0007) and disability (3.4% vs. 2.1%, respectively; p = 0.046) rates when compared to those without irRAEs.
Discussion
This is the first pharmacovigilance study which demonstrates a wide range of irRAEs in male and female cancer patients using FAERS data. According to the reporting odd ratios, it appeared that the most significant irRAEs associated with the use of ICIs were abnormal spermatogenesis, scrotal irritation, scrotal oedema, prostatitis, acquired phimosis, and scrotal erythema in males, and genital tract fistulas in females. Perineal rash remained as the only irRAE occurring in both sexes.
One of the most important findings in our study is the significant association between ICI therapy and abnormal spermatogenesis as shown previously by other studies. Two cases of suspected autoimmune orchitis were reported in metastatic melanoma patients while receiving nivolumab and ipilimumab dual therapy and pembrolizumab monotherapy, respectively10,11. Recently, a case of azoospermia was reported in a patient with metastatic melanoma after two years of nivolumab and ipilimumab dual therapy, with an unclear onset time12. After this first case report, confirmatory findings were obtained in a small retrospective cohort of seven patients with metastatic melanoma who received ICI therapy. The study identified abnormal spermatogenesis in testicular autopsy tissue specimens in six out of seven patients6. In a recent cross-sectional study investigating fertility by spermiogram, analysis of sexual hormones, and questionnaires on sexual function and activity, one case of azoospermia and another case of exacerbated oligoasthenoteratospermia out of 25 patients treated with ICI for cutaneous malignancies or uveal melanoma were attributed to ICI therapy13. However, the molecular mechanisms underlying ICI-related abnormalities in spermatogenesis are not clearly identified. Since a wide variety of peculiar proteins are expressed at each steps of spermatogenesis, testes possess a remarkable “immune privilege” status which allows to tolerate these neo-antigens14. While the underlying mechanisms for the regulation of immune privilege status have not been fully explored yet, a growing body of evidence indicates that multiple mechanisms including blood-testis barrier, local immunosuppressive effects of immune cells, testis-specific cells, and cytokines play a crucial role in the maintenance of testicular immune privilege status15–17. Therefore, it is likely that ICIs might directly disrupt the regulation of testicular immune privilege and lead to abnormal spermatogenesis together with systemic elevation of inflammatory cytokine levels in addition to secondary hypogonadism caused by ICI-related pituitary gland dysfunction (hypophysitis)2. Our data further reinforces the link between ICI therapy and abnormal spermatogenesis, which is particularly pronounced in patients treated with dual therapy. Given the recent advancements in cancer survivorship and the growing number of ICI approvals, it becomes essential to conduct prospective studies investigating the potential link between ICI therapy and abnormal spermatogenesis. Despite the current lack of high-quality clinical data on this topic, it is of paramount importance to inform male patients about the risks of irRAEs and cryopreservation of sperm prior to initiating ICI therapy.
Unlike ICI-related spermatogenesis abnormalities in male cancer patients, no data could have been retrieved from the FAERS database regarding the ovarian toxicity of ICIs. Recurrent/advanced stages of cancers, prior chemotherapy and/or advanced female age might explain why ovarian/menstrual function was not reported or assessed in these cases. Very limited clinical data is available regarding the ovarian effects of the ICIs. One study analyzed ovarian reserve of women aged 20–35 treated with 3 mg/kg ipilimumab (57%) or 10 mg/kg ipilimumab (43%) for melanoma in the adjuvant setting on trial ECOG-ACRIN E1609. After a mean follow-up of 8 months, post therapy AMH, estradiol, and LH significantly decreased after treatment (p < 0.001, p = 0.016, and p = 0.012, respectively). The median AMH was 4.24 ng/mL in the pre-treatment group and 3.51 ng/mL in the post-treatment group. There was no significant difference in the levels of FSH and prolactin before and after treatment (p = 0.68 and p = 0.12, respectively)18.
There are also several preclinical studies on animal models investigating whether ICIs have a direct effect on female gonadal function and, if so, analyzing their mechanism of action. For ipilimumab, preclinical studies in monkeys demonstrated that the antibody specifically binds to the connective tissue in the ovary but did not cause any histopathological changes in oocyte morphology19. Repeat-dose toxicity studies of atezolizumab in female cynomolgus monkeys resulted in irregular menstrual cycle patterns and a lack of formation of new corpora lutea, indicating disruption of ovulation. However, this effect was observed when the drug was used at doses 6 times higher than the recommended dose20. In the study of Xu et al., the researchers injected pembrolizumab or anti-mouse PD-1 antibody into prepubertal female mice. In immunocompetent mice, the number of primordial follicles was significantly reduced after injection of pembrolizumab and anti-mouse PD-1 antibody. However, no change in the number of follicles was observed in immunodeficient nude mice. Furthermore, in this study, an increase in TNF-α after upregulation of cyclooxygenase-2 was observed in the ovaries after administration of anti-mouse PD-1 antibody, as well as the infiltration of CD3 + T cells within some follicles and between ovarian stromal cells in mice. They proposed that PD-1 immune checkpoint blockade affects ovarian reserve through a mechanism involving CD3 + T cells infiltration, and this is the first study to link ICI to inflammation-mediated follicular depletion in pre-clinical models of prepubertal pediatric patients21. In another preclinical study of Winship et al., researchers used tumor-bearing and tumor-free adult female mouse models to evaluate the effects of PD-L1 and CTLA-4 blockage on the ovaries. This study demonstrated that the depletion of ovarian follicles by ICIs is mediated by immune cells, and they found that ICIs increased immune cells infiltration and TNF-α expression in the ovaries, reduced ovarian follicle reserve, and impaired the ability of oocytes to mature and ovulate22. It is possible that ICIs may exert cytotoxic effects on ovarian follicles through similar mechanisms in human ovary. Preclinical/molecular studies are urgently needed in the ovaries of female cancer patients to analyze gonadotoxic potentials of ICIs. But at the moment, ICIs should be considered a potentially gonadotoxic agents and fertility preservation options such as oocyte freezing should be offered to the patients to protect their future fertility.
It should be emphasized that ICI-related disruption of hypothalamic-pituitary-gonadal axis may indirectly cause secondary hypogonadism and infertility by causing deficiency of follicle-stimulating hormone (FSH) and luteinizing hormone (LH)23. However, we focused on cases classified under the “Reproductive system and breast disorders” category in the FAERS database to directly identify cases where ICIs were reported as the primary cause. Secondary hypogonadism arising from ICI-related endocrine dysfunction was beyond the scope of our study.
ICIs act through different mechanisms of actions24. Therefore, their cytotoxicity profiles may also differ depending upon their type25. While our findings may indicate an association between PD-1 inhibitors and a higher incidence of irRAEs compared to CTLA-4 inhibitors, a larger sample size and a prospective study design are necessary to reach definitive conclusions. Our analysis also did not identify any marked difference in the overall incidence of irRAEs between patients on ≥ 2 ICIs and those on monotherapy, although it is well-established that dual ICI therapy is associated with a higher occurrence rate of immune-related adverse events compared to monotherapy25,26. Abnormal spermatogenesis and balanoposthitis were the isolated adverse events observed only after dual but not mono ICI therapy.
Increased toxicity has been documented in the majority of combination therapies involving immunotherapy and chemotherapy. However, our analysis did not reveal an increased risk of irRAEs with the combination of immunotherapy and chemotherapy. Moreover, the concurrent use of chemotherapy with immunotherapy seems to reduce the incidence of irRAEs in male patients. This finding might be, in part, explained by the detrimental effects of chemotherapy on immune system, a phenomenon that might be associated with dosage levels and dosing intervals27. The difference observed between the genders may be attributed to the utilization of varying treatment regimens with different indications and dosages. However, there was an increased likelihood of irRAEs associated with two particular chemotherapeutic drugs namely, doxorubicin and cyclophosphamide, both of which are well known for their gonadal toxicity in both males and females28,29. Therefore, patients who will be treated with ICIs combined with these chemotherapy agents should be informed about their potential gonadotoxic effects and offered the most appropriate fertility preservation option before starting the therapy.
Another important finding of our analysis is that the concurrent use of tyrosine kinase inhibitors (TKIs) with ICIs, particularly lenvatinib and cabozantinib, significantly increases the risk of irRAEs. Prior studies have reported that TKIs may induce adverse effects on spermatogenesis and ovarian stimulation, through the inhibition of c-KIT and PDGFα30. It has also been postulated that hypogonadism could manifest as a chronic adverse effect of TKIs, given the significant correlation observed between testosterone levels and the duration of TKI treatment31. Gynecomastia, testicular hydrocele, miscarriage, and fetal abnormalities have been associated with the use of TKIs in various case reports32. However, to the best of our knowledge, there are no consistent findings in the existing literature regarding the potential adverse effects of lenvatinib and cabozantinib on the reproductive system. Besides the individual toxicities associated with TKIs and immune-related adverse effects of ICIs, the combined therapy may result in a synergistic cytotoxic effect. While the precise underlying mechanism remains unclear, it has been hypothesized that TKIs enhance the sensitization of tumor cells to the immune system by facilitating tumor cell death and reducing the immunosuppressive environment33. Although data on the adverse effects of lenvatinib and cabozantinib on the reproductive system are very scarce, the concurrent use of these TKIs with ICIs has the potential to induce significant side effects on the reproductive system, particularly in cases of urogenital and gynecologic cancers.
Although FAERS’ extensive data set is invaluable for detecting a wide array of safety signals linked to ICIs, this study faces limitations common to pharmacovigilance databases. Challenges like underreporting and misreporting can lead to underestimation of adverse event incidences. The lack of detailed clinical information and denominator data in FAERS curtails our ability to perform in-depth analyses or accurately assess the true risk of adverse events. Reporting biases related to geographical and selective reporting add complexity to data interpretation. The retrospective study design limits our ability to establish a causality between the ICIs and adverse events directly. The varying nature of anticancer drugs in FAERS may introduce bias, complicating the isolation of ICI effects. Despite these challenges, our study demonstrates for the first time several irRAEs in both sexes. Prospective clinical and molecular studies are urgently needed to validate these findings and further understand their gonadotoxic potentials and safety profiles.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
BK, BHE, VT, BU, ÖÖ, and FS contributed to the study conception and design. Material preparation, data collection and analysis were performed by BK, BHE, ŞNB, and LÖ. The first draft of the manuscript was written by BK, BHE, ŞNB, LÖ, and ÖÖ. All authors contributed to previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
All data used in our study are publicly available at https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bahadır Köylü, Email: bkoylu@ku.edu.tr.
Özgür Öktem, Email: ooktem@ku.edu.tr.
References
- 1.Shiravand, Y. et al. Immune checkpoint inhibitors in Cancer therapy. Curr. Oncol. Tor. Ont.29, 3044–3060 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garutti, M., Lambertini, M. & Puglisi, F. Checkpoint inhibitors, fertility, pregnancy, and sexual life: a systematic review. ESMO Open.6, 100276 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar, V. et al. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front. Pharmacol.8, 49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin, Q. et al. Immune-related adverse events of immune checkpoint inhibitors: a review. Front. Immunol.14, 1167975 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-Related adverse events associated with immune checkpoint Blockade. N Engl. J. Med.378, 158–168 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Scovell, J. M. et al. Association of impaired spermatogenesis with the use of immune checkpoint inhibitors in patients with metastatic melanoma. JAMA Oncol.6, 1297–1299 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.English | MedDRA. https://www.meddra.org/how-to-use/support-documentation/english
- 8.Rothman, K. J., Lanes, S. & Sacks, S. T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf.13, 519–523 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Bate, A. & Evans, S. J. W. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf.18, 427–436 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Brunet-Possenti, F., Opsomer, M. A., Gomez, L., Ouzaid, I. & Descamps, V. Immune checkpoint inhibitors-related orchitis. Ann. Oncol. Off J. Eur. Soc. Med. Oncol.28, 906–907 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Quach, H. T. et al. Severe Epididymo-Orchitis and encephalitis complicating Anti-PD-1 therapy. Oncologist24, 872–876 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabinowitz, M. J. et al. Onset of azoospermia in man treated with Ipilimumab/nivolumab for BRAF negative metastatic melanoma. Urol. Case Rep.34, 101488 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salzmann, M. et al. Male fertility during and after immune checkpoint inhibitor therapy: A cross-sectional pilot study. Eur. J. Cancer Oxf. Engl. 1990. 152, 41–48 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Li, N., Wang, T. & Han, D. Structural, cellular and molecular aspects of immune privilege in the testis. Front. Immunol.3, 152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meinhardt, A. & Hedger, M. P. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol. Cell. Endocrinol.335, 60–68 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Mital, P., Hinton, B. T. & Dufour, J. M. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol. Reprod.84, 851–858 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fijak, M., Bhushan, S. & Meinhardt, A. Immunoprivileged sites: the testis. Methods Mol. Biol. Clifton NJ. 677, 459–470 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Buchbinder, E. I. et al. Impact of immune checkpoint Inhibition on ovarian reserve in patients with melanoma enrolled in the ECOG-ACRIN E1609 adjuvant trial. J. Clin. Oncol.41, 12013–12013 (2023). [Google Scholar]
- 19.Walter, J. R., Xu, S., Paller, A. S., Choi, J. N. & Woodruff, T. K. Oncofertility considerations in adolescents and young adults given a diagnosis of melanoma: fertility risk of food and drug Administration-approved systemic therapies. J. Am. Acad. Dermatol.75, 528–534 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen, Z., Huang, J., Kwak-Kim, J. & Wang, W. Immune checkpoint inhibitors and reproductive failures. J. Reprod. Immunol.156, 103799 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Xu, P. C. et al. Effects of PD-1 Blockade on ovarian follicles in a prepubertal female mouse. J. Endocrinol.252, 15–30 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winship, A. L. et al. Checkpoint inhibitor immunotherapy diminishes oocyte number and quality in mice. Nat. Cancer. 3, 1–13 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Tuerxun, H. et al. Immune checkpoint inhibitors as a threat to reproductive function: A systematic review. Crit. Rev. Oncol. Hematol.188, 104064 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Kciuk, M. et al. Recent advances in molecular mechanisms of Cancer immunotherapy. Cancers15, 2721 (2023). [DOI] [PMC free article] [PubMed]
- 25.Xu, C. et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ363, k4226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu, Z. et al. Adverse events of immune checkpoint inhibitors therapy for urologic Cancer patients in clinical trials: A collaborative systematic review and Meta-analysis. Eur. Urol.81, 414–425 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Salas-Benito, D. et al. Paradigms on immunotherapy combinations with chemotherapy. Cancer Discov. 11, 1353–1367 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Bhardwaj, J. K., Bikal, P. & Sachdeva, S. N. Chemotherapeutic drugs induced female reproductive toxicity and treatment strategies. J. Biochem. Mol. Toxicol.37, e23371 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Delessard, M. et al. Exposure to chemotherapy during childhood or adulthood and consequences on spermatogenesis and male fertility. Int. J. Mol. Sci.21, 1454 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Leo, S., Trevisan, M., Moneta, C. & Colombo, C. Endocrine-related adverse conditions induced by tyrosine kinase inhibitors. Ann. Endocrinol.84, 374–381 (2023). [DOI] [PubMed] [Google Scholar]
- 31.Afshar, M. et al. Chronic tyrosine kinase inhibitor (TKI) use in metastatic renal cell carcinoma (mRCC): can this lead to the adverse effect of hypogonadism? Expert Rev. Anticancer Ther.19, 529–532 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Boskabadi, S. J., Dashti, A., Karevan, S., Kargar-Soleimanabad, S. & Salehifar, E. Clinical uses and safety concerns of tyrosine kinase inhibitors with a focus on novel drugs: A narrative review. J. Oncol. Pharm. Pract. Off Publ Int. Soc. Oncol. Pharm. Pract.1078155223117479010.1177/10781552231174790 (2023). [DOI] [PubMed]
- 33.Vanneman, M. & Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer. 12, 237–251 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in our study are publicly available at https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.