Summary
Background
Artificial Intelligence (AI) has been used to automate detection of retinal diseases from retinal images with great success, in particular for screening for diabetic retinopathy, a major complication of diabetes. Since persons with diabetes routinely receive retinal imaging to evaluate their diabetic retinopathy status, AI-based retinal imaging may have potential to be used as an opportunistic comprehensive screening for multiple systemic micro- and macro-vascular complications of diabetes.
Methods
We conducted a qualitative systematic review on published literature using AI on retina images to detect systemic diabetes complications. We searched three main databases: PubMed, Google Scholar, and Web of Science (January 1, 2000, to October 1, 2024). Research that used AI to evaluate the associations between retinal images and diabetes-associated complications, or research involving diabetes patients with retinal imaging and AI systems were included. Our primary focus was on articles related to AI, retinal images, and diabetes-associated complications. We evaluated each study for the robustness of the studies by development of the AI algorithm, size and quality of the training dataset, internal validation and external testing, and the performance. Quality assessments were employed to ensure the inclusion of high-quality studies, and data extraction was conducted systematically to gather pertinent information for analysis. This study has been registered on PROSPERO under the registration ID CRD42023493512.
Findings
From a total of 337 abstracts, 38 studies were included. These studies covered a range of topics related to prediction of diabetes from pre-diabetes or non-diabeticindividuals (n = 4), diabetes related systemic risk factors (n = 10), detection of microvascular complications (n = 8) and detection of macrovascular complications (n = 17). Most studies (n = 32) utilized color fundus photographs (CFP) as retinal image modality, while others employed optical coherence tomography (OCT) (n = 6). The performance of the AI systems varied, with an AUC ranging from 0.676 to 0.971 in prediction or identification of different complications. Study designs included cross-sectional and cohort studies with sample sizes ranging from 100 to over 100,000 participants. Risk of bias was evaluated by using the Newcastle–Ottawa Scale and AXIS, with most studies scoring as low to moderate risk.
Interpretation
Our review highlights the potential for the use of AI algorithms applied to retina images, particularly CFP, to screen, predict, or diagnose the various microvascular and macrovascular complications of diabetes. However, we identified few studies with longitudinal data and a paucity of randomized control trials, reflecting a gap between the development of AI algorithms and real-world implementation and translational studies.
Funding
Dr. Gavin Siew Wei TAN is supported by: 1. DYNAMO: Diabetes studY on Nephropathy And other Microvascular cOmplications II supported by National Medical Research Council (MOH-001327-03): data collection, analysis, trial design 2. Prognositc significance of novel multimodal imaging markers for diabetic retinopathy: towards improving the staging for diabetic retinopathy supported by NMRC Clinician Scientist Award (CSA)–Investigator (INV) (MOH-001047-00).
Keywords: Artificial intelligence, Retina image, Diabetes-assciated complications, Systemic review, Sereening
Research in context.
Evidence before this study
Before undertaking this study, we conducted a comprehensive search of PubMed, Web of Science, and Google Scholar from January 2000 to October 2024 to identify studies evaluating AI applications in retinal imaging for predicting diabetes-associated systemic complications. Our inclusion criteria focused on cross-sectional, retrospective, and prospective studies that reported performance metrics of machine learning and deep learning models, such as AUC, sensitivity, and specificity, with validation experiments. Studies were assessed for quality and risk of bias using the Newcastle–Ottawa Scale (NOS) and AXIS tool, revealing a heterogeneous body of evidence with variable performance and reporting quality.
Added value of this study
Our study synthesizes and evaluates the performance of AI models in predicting diabetes-associated complications by using retinal imaging. Unlike previous reviews, we provide a structured narrative synthesis of AI model performance, highlighting gaps in validation and reporting standards in the context of diabetes-associated complications. By aligning the included studies with TRIPOD guidelines, this study identifies key strengths and limitations in the field, offering insights for improving model transparency and clinical applicability.
Implications of all the available evidence
The available evidence underscores the potential of AI-based retinal imaging to serve as a non-invasive tool for predicting systemic complications in diabetic patients, with significant implications for early intervention and management strategies. While the lack of high-quality prospective studies and standardized reporting limits the generalizability of these findings. Future research should prioritize robust validation in diverse populations and emphasize adherence to reporting standards to enhance clinical translation and policy development.
Introduction
Diabetes mellitus (DM) will affect approximately 600 million people by the year 2040.1 The significant morbidity and burden of care associated with the systemic complications of DM, will pose a significant challenge to healthcare systems worldwide.2 Early detection and intervention to delay the progression of diabetes and its complications are an important public health need. Patients with diabetes are susceptible to systemic complications such as diabetic kidney disease or nephropathy (DKD), diabetic neuropathy (DN), diabetic retinopathy (DR), cardiovascular disease (CVD), peripheral artery disease (PAD) and lower-extremity amputations (LEA) etc. Timely screening and management for these complications are crucial, yet it is noteworthy that in the US one in every five diabetes patients remains unaware of their diagnosis.3 Even for severe chronic kidney disease (CKD), 2–5 patients are not aware of the disease.4 Inadequate screening, inaccurate diagnoses, and low patient awareness pose a significant burden on our health system. Therefore, it is imperative to explore cost-effective and practical methods to detect diabetes-associated complications, especially in less developed areas. The retina, often referred to as a “window” to the vascular system, has the potential to serve as rapid and non-invasive means of assessing the status of the systemic vasculature.5
Artificial intelligence (AI) technology has achieved significant advancement in its application in clinical settings. Numerous AI models, including machine learning techniques, have been applied in disease screening, prediction, and identification, with a particular focus on their performance in analyzing multi-modal images.6 AI has a potential role to be applied in the clinical studies aiming to improve the clinical productivity and diagnostic accuracy.7 In ophthalmology, multiple studies have develop and validated the use of AI algorithms on retina imaging for various applications including the diagnosis and management of conditions such as diabetic retinopathy,8 glaucoma,9 age-related macular degeneration,9 and other retinal diseases.10
For individuals with diabetes, international guidelines recommend regular screening for diabetic retinopathy, which is usually performed by telemedicine based digital fundus photographic screening.11,12 This presents an opportunity for evaluation of the retinal images for other diseases.13 Furthermore, patients with referable DR would often be subject to specialist review and further examination with OCT, OCTA, and other imaging modalities. This opens the possibility of utilizing retinal imaging, which these patients already receive as part of their DR screening to opportunistically detection of other systemic complications related to DM. This concept has long been evaluate in multiple research papers and translation has been limited by practicality and accuracy of the traditional models based on manually identified imaging biomarkers such as retinal vessel caliber and geometry.14, 15, 16, 17, 18, 19
The development of AI systems for analysis of retinal images has brought an opportunity to develop non-invasive, scalable and cost effective detection of systemic complications of diabetes. Deep learning (DL), a branch of machine learning (ML) has contributed to the revolution of AI assisted medical image interpretation. The DL systems have been applied to diagnosis and prediction of various systemic diseases, including respiratory, cardiovascular, and neurology systems by utilizing multiple type of imaging data.20, 21, 22 Research also demonstrated robust achievement in segmentation, prediction and identification of the systemic diseases by using ocular images.23, 24, 25 DL has been successfully used to perform automated identification of DR from retinal fundus images used for DR screening,8,26,27 and can improve the efficiency and cost effectiveness of DR screening programs.28 Therefore, the ubiquity of retinal imaging in patients with diabetes from DR screening combined with the ability of DL and AI to improve the detection of imaging biomarkers for systemic diseases and build models for diagnosis or prediction of systemic diseases in patients with diabetes in a rapid, scalable and cost effective manner, presents a potential paradigm shift in the screening for diabetic complications.
AI algorithms for DR detection from retina images are already well published, and a number of algorithms have regulatory approval and are deployed in clinical use in many countries. However, AI analysis of retina imaging data has the added potential to enhance the accuracy of predicting diabetic complications, including cardiovascular disease, DKD progression, and neurological diseases. Retina images can be obtained non-invasively during routine health examinations, making it easy to scale and combine with conventional risk models that rely solely on clinical or laboratory data.29 In our review, we have provided a comprehensive analysis and summary of the current studies on the use of retinal imaging based AI for detecting systemic diseases related to diabetes, excluding diabetic retinopathy, and discuss the unmet needs and future directions required to facilitate the translation of these AI based model into wider clinical use.
Methods
We conducted this systemic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 guidelines. The study was registered on PROSPERO (registration ID: CRD42023493512). To explore the existing AI technologies and their prospective applications in both clinical practice and research, we searched PubMed®, Web of Science and Google Scholar from January, 2000 to October, 2024 for published English papers. The keywords were based on three main factors of our review: (1) retina images, (2) diabetes-related, (3) systemic disease such as cardiovascular disease, nervous system diseases, endocrine system diseases, metabolic diseases, liver diseases, hematologic diseases, digestive system diseases and immune system diseases, (4) artificial intelligence. The searching strategy of this study are listed in Supplementary Table S1. The research involved the extraction of various components, including the input, factors, AI model, dataset, validation procedures, and clinical outcomes (such as age, mortality, Alzheimer's disease, etc.), along with an assessment of their respective performances.
The outcomes of interest included both microvascular complications and macrovascular complications, as well as other related systemic outcomes such as cardiovascular events, kidney disease and mortality risk. We compared AI model performance across studies, focusing on key performance metrics including AUC, sensitivity, specificity, and C-statistics. Due to the heterogeneity, we conducted a narrative synthesis to summarize and compare the results. Studies were grouped by the type of diabetes-related complications predicted. We also examined AI model performance, highlighting the range of AUCs and other performance metrics across different studies.
Ethics
This review did not involve direct interactions with human participants or the use of patient-identifiable data, therefore, formal ethical approval was not required. All included studies were publicly available and had undergone ethical review and approval as part of their original publication processes.
Selection criteria
Articles were eligible for the criteria below: (1): full text available; (2) cross-sectional, retrospective, prospective studies which involved DM (Type 1 and Type 2) patients were included; (3) performance of algorithms including ML or DL were reported with metrics such as accuracy, sensitivity, area under the receiver operating characteristic curve (AUC) and specificity for binary outcomes or mean absolute error (MAE) and R square for regression models; (4) studies should include the validation experiment. Irrelevant study design, primarily focus on diabetic retinopathy without systemic complications, lack of sufficient performance metrics, non-English language, conference abstracts with insufficient data.
Data extraction
We extracted data from each study, including: Input data (e.g., retinal imaging modalities), Input variables (e.g., age, sex, disease status), AI models (e.g., deep learning, machine learning), datasets and sample sizes, validation procedures (e.g., internal and external validation), and clinical outcomes (e.g., mortality, cardiovascular disease, kidney disease). The quality assessment was conducted using the Newcastle–Ottawa Scale (NOS) for cohort studies, and the AXIS tool for cross-sectional studies to evaluate the quality and risk of bias of the included articles. Data were collected independently by two reviewers using a standardized extraction form. Discrepancies were resolved by a third reviewer. No automation tools were used.
TRIPOD evaluation
We assessed the adherence of the 38 included papers to the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) guidelines. The TRIPOD statement provides a set of recommendations designed to ensure transparent reporting of predictive modeling studies, covering essential components such as the study participants, outcome measures, model development, performance metrics, and validation strategies.
Statistics
The findings from studies were synthesized using a thematic approach, categorizing studies based on shared conceptual frameworks, such as DM status, microvascular complications, macrovascular complications and systemic risk factors. This approach allowed for an integrated discussion of outcomes across different studies, providing a broad view of AI-based retinal imaging applications in diabetes-related complications.
Various performance metrics were extracted from the included studies to assess the accuracy of AI-based retinal imaging. These metrics included: 1 AUC/AUROC (Area Under the Receiver Operating Characteristic Curve): This metric evaluates the ability of the model to distinguish between classes, with values closer to 1 indicating stronger predictive performance. 2 F1-score: represents the balance between precision and recall, indicating the test's accuracy when both false positives and false negatives are considered. 3 Kappa score (k-score): measures the level of agreement between observed and predicted classifications, accounting for the possibility of agreement occurring by chance.4. C-statistic: reflects the model's ability to discriminate between positive and negative outcomes, with higher values indicating better discrimination.
Role of funding
The funding sources did not have any direct involvement in the design, data collection, or analysis of the study. The specific contributions of the funding sources are as follows:
-
1.
DYNAMO: Diabetes study on Nephropathy And other Microvascular complications II (supported by the National Medical Research Council, MOH-001327-03): The funding supported the data collection process, analysis, and trial design for the study. The funding did not influence the interpretation of the results or the writing of the manuscript.
-
2.
Prognostic Significance of Novel Multimodal Imaging Markers for Diabetic Retinopathy: Towards Improving the Staging for Diabetic Retinopathy: This funding facilitated the investigation and development of multimodal imaging markers for diabetic retinopathy. The funding had no role in the analysis, interpretation of data, or the writing of this manuscriptRole of the funding source.
Results
Research selection
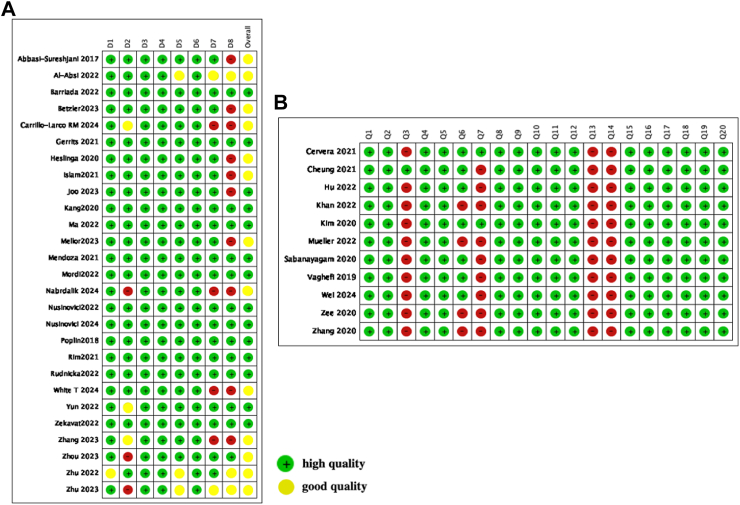
The selection process of our study is showed in Fig. 1. As we focused on systemic complications, we excluded research targeting primarily on diabetic retinopathy. From researching the studies available from three main database, we finally got total 1536 studies, 369 articles PubMed, 833 from google scholar, 334 from Web of Science. In the final review, we included 38 full-text studies in our systematic review. All the included papers are shown in Table 1. The Quality Assessment of Newcastle–Ottawa scale (NOS) and Appraisal tool for Cross-Sectional Studies (AXIS) are shown in Fig. 2.
Fig. 1.
Study flow diagram.
Table 1.
Summary of artificial intelligence screening systemic diseases from retina images.
| Author | years | Input | Analyzed variables | Study type | AI model | Dataset, sample size and country of origin | Validation | Clinical outcomes | Performance |
|---|---|---|---|---|---|---|---|---|---|
| Nusinovici et al.30 | 2024 | CFP | All-cause mortality, cardiovascular disease mortality, cancer mortality, cardiovascular disease events | Cohort development and validation study | Deep-learning (RetiPhenoAge marker derived from CNN) | UK Biobank (34,061 participants), SEED (9429 participants, Singapore), AREDS (3986 participants, USA) | Internal and external validation across UK Biobank, SEED, AREDS cohorts | Prediction of morbidity and mortality outcomes, including cardiovascular events and cancer mortality | HR 1.92 for all-cause mortality, HR 1.97 for cardiovascular disease mortality, HR 2.07 for cancer mortality; replicated in independent cohorts |
| Wei J.31 | 2024 | CFP | eGFR, Blood Pressure, Blood Uric Acid | Cross sectional study | Deep learning (ViT), metadata-image hybrid | 6091 diabetic patients (ShDMC), 9327 from UK Biobank, China and UK | 5-fold cross-validation (ShDMC), external test (UK Biobank) | Hyperuricemia classification | AUC 0.92 (hybrid model, ShDMC), AUC 0.89 (UK Biobank), R2 0.727 (hybrid model) |
| Nabrdalik K32 | 2024 | CFP | Gender, age, BMI, diabetes, DM duration, DR, CKD, HbA1c, UACR, eGFR, total cholesterol, LDL, Triglycerides | Observational Cohortstudy | Deep learning (ResNet 18, ResWide 50) | 229 DM patients, Silesia Diabetes-Heart Project, Poland | Training and validation, testing on unseen image set | CAN classification (early vs severe stage) | AUC 0.87 for CAN, AUC 0.94 for severe CAN (ResNet 18) |
| White T.33 | 2024 | CFP | HbA1c, SBP, DBP, eGFR | Prospective, non-interventional study | Machine learning | 301 participants, Kenya (validation), UK Biobank (training) | External validation in Kenyan population | Cardiovascular risk factors estimation | AUC 0.765 (hypertension), AUC 0.762 (diabetes), comparable to UK Biobank training performance |
| Carrillo-Larco34 | 2024 | CFP, text metadata | Sex, age, comorbidities and taking insulin | Exploratory study | Extra Tree Classifier and MedCLIP embeddings | 988 images from 563 people from Brazilian Multilabel Ophthalmological Dataset, Brazil | Tested for multiclass classification | Predicting years living with diabetes using retinal images and metadata | F1 score 57%, highest precision (64%) for 15+ years of diabetes, overall accuracy 55–64% |
| Zhou35 | 2023 | CFP and OCT | Systemic diseases (heart failure, myocardial infarction) and ocular diseases (diabetic retinopathy, glaucoma, AMD) | Retrospective Study | RETFound (Self-Supervised Learning, SSL) | UK Biobank (United Kingdom) and EyePACS (United States), exact sample size not explicitly mentioned, large-scale multi-country origin | Cross-dataset validation (trained on EyePACS, validated on UK Biobank and other datasets) | Detected multiple systemic and ocular diseases from retinal images, including Ischemic stroke, Myocardial infarction, Heart failure and Parkinson's disease | AUC for heart failure: 0.87, myocardial infarction: 0.86, glaucoma: 0.90, AMD: 0.88; high generalizability with good performance across all diseases |
| Joo36 | 2023 | CFP | Age, Gender, Diabetes, Hypertension, eGFR (estimated glomerular filtration rate) | Cohort study | ConvNeXT, Reti-CKD | 79,108 adults from Severance Hospital, Korea | internal validation in the UK Biobank and external validation in the Korean Diabetic Cohort | Chronic kidney disease | Prediction: C-statistics, 0.638 in the UK Biobank, 0.703 in the Korean Diabetic Cohort, AUC: 0.85 for CKD prediction. |
| Zhu37 | 2023 | CFP, OCT (Topcon 3D, 1000 Mk2) | Retina age gap, age, gender, ethnicity, Townsend index, smoking status, drinking status, obesity, physical activity, history of stroke, hypertension | population-based cohort | Xception | 131,238 images from 66,500 participants from UK Biobank study, UK | 5-fold cross-validation | Mortality, age | MAE: 3.55 years, HR for mortality: 1.02 per year increase in retinal age gap |
| Betzler38 | 2023 | CFP | eGFR, age, sex, ethnicity, duration of diabetes, HbA1c, and systolic blood pressure | Cohort study | ResNet 18 | 79,511 patients from SiDRP, Singapore | 5-fold cross-validation | Diabetic kidney disease | Detection AUC: 0.826–0.866 (internal), 0.726–0.828 (external), hybrid: 0.765 |
| Mellor39 | 2023 | CFP | gender, ethnicity, age, diabetes duration, BMI, SBP, DBP, cardiovascular, smoking status, HDL, eGFR, dyslipidemia, hypertension, atrial fibrillation | Prospective cohort study | ResNet-101 | SDRN-NDS 24,012 and 202,843 people with T1DM and T2DM, Scottish | 20% validation set | Incident Cardiovascular Disease | Prediction T1DM: AUC 0.822, T2DM: AUC 0.711, C-statistics improvement marginal (ΔLL = 6.7 for T1DM, 51.1 for T2DM) |
| Zekavat40 | 2022 | CFP, blood-derived DNA | Age, Gender, smoke status | Population based cohort study | U-Nets & PheWAS&GWAS | UK Biobank 97,895 images, UK | Both internal and external validation | Incident mortality, hypertension, congestive heart failure, renal failure, type 2 diabetes, sleep apnea, anemia, | AUC: 0.99 (vascular segmentation), HR: 1.83 for mortality with T2DM |
| Ma41 (short communication) | 2022 | CFP | sex, age, systolic blood pressure, total cholesterol, body mass index, current smoking status, diabetes | cohort study | Inception-ResNet-V2, ImageNet | 798,866 fundus from BRAVE, Beijing, China | 390,947 and 20,571 participants for development and internal validate | Ischemic cardiovascular diseases | Detection ICVD risk ≥5%: AUC 0.971 (95% CI: 0.967–0.975) internal, 0.859 (95% CI: 0.822–0.895) ICVD risk ≥7.5%: AUC 0.976 (95% CI: 0.973–0.985) internal, 0.876 (95% CI: 0.816–0.937) |
| Mordi42 | 2022 | CFP | blood sample for genotyping, genome-wide association, blood pressure, glycated hemoglobin, cholesterol | cohort study | VMAPIRE | 5152 individuals from GoDARTS, Scotland | Internal validate | MACE (major adverse cardiovascular event) | Prediction AUC 0.686 (retinal + PRS model), AUC: 0.663 (retinal only model), HR: 1.11 for retinal risk score |
| Nusinovici43 | 2022 | CFP | gender, Age, Albumin, Creatinine, Glucose, C-reactive protein, Lymphocyte, Red cell distribution width percent, white blood cell count, mortality status | RetrospectiveCohort study | VGG, RetiAGE | 46,551 from Korean Health Screening Study; 56,301 from UK Biobank Study, Korea and UK | Internal (Korea) and external (UK) validation | Morbidity related to CVD and cancer | Prediction AUC: 0.70 (CVD mortality), HR: 1.67 (all-cause mortality), HR: 2.42 (CVD mortality) |
| Hu44 | 2022 | CFP | age, gender, ethnicity, Townsend index, smoking status, drinking status, obesity, physical activity, history of stroke, hypertension. | cross-sectional study | Deep Learning Model | 46,969 participants from UK Biobank study, UK | Internal validation | Parkinson's disease | Predictive AUC = 0.717, HR: 1.10 per year increase in retinal age gap |
| Zhu45 | 2022 | CFP | LogMAR, keratometry and autorefraction, IOP, age, gender, ethnicity, education, smoking status, drinking status, heath status, cardiovascular disease, metabolic syndrome | Prospective cohort study | Deep Learning Model using Xception architecture | 19,200 fundus images of 11,052 participants from UK Biobank, UK | 5-fold cross-validation | Arterial stiffness index, Incident CVD events | AUC: 0.708 (CVD), HR: 1.03 per year increase in retinal age gap for CVD risk |
| Zhu46 | 2022 | CFP | age, gender, ethnicity, education, smoking status, drinking status, Obesity, cardiovascular disease, diabetes | Prospective cohort study | Xception | 80,169 fundus images from 46,969 participants in the UK Biobank cohort, UK | Internal validation | Incident stroke | Prediction AUC 0.676, 95% CI: 0.644–0.708, HR: 2.37 for highest retinal age gap quintile |
| Mueller47 | 2022 | CFP | age, gender, lowest ankle-brachial-pressure-index, history of acute coronary syndrome | exploratory study | AlexNet CNN | 92,363 from Department of Ophthalmology, University Hospital Bonn, Germany | Internal validation | Peripheral arterial disease | Detection AUC 0.890, Precision: 0.954, Recall: 0.822 |
| Al-Absi48 | 2022 | CFP | Age, Gender, Bone Mineral Density, Body Fat Composition, Lean Mass, Area measurements | Prospective cohort study | DMA model, Retinal image model, Hybrid model | 1839 retinal images from all participants, Qatar | 5-fold cross validation | Cardiovascular Disease | Identification: Accuracy: 78.3% (hybrid model), AUC: Not reported, DXA accuracy: 77.4%, retinal accuracy: 75.6% |
| Khan49 | 2022 | CFP | gender, ethnicity, age, LDL, HDL, smoking status, cardiac disease, HbA1c, hypertension, angiotensin receptor blocker (ARB) use, angiotensin-converting enzyme inhibitor use, and aspirin use | cross-sectional study | DenseNet-201 | 1277 retinal fundus from San Francisco Bay Area, USA | Only split into training and testing set, internal validation | Ethnicity, Age, Gender, ACEi, ARB, LDL, HDL, Smoking status, HbA1c, Cardiac disease, medication-aspirin, hypertension | Prediction AUC: Ethnicity 0.926, Age 0.902, Gender 0.852, ACEi 0.815, ARB 0.783, LDL0.766, HDL0.756, Smoking status 0.732, HbA1c 0.708, Cardiac disease 0.7, medication-aspirin 0.696 hypertension 0.687 |
| Rudnicka50 | 2022 | CFP | gender, ethnicity, age, LDL, HDL, smoking status, BMI, cholesterol, hypertension, BP | Prospective Cohort study | QUARTZ | 88,052 UK Biobank (UKB) participants and 7411 European Prospective Investigation into Cancer (EPIC), UK | Externally validated in EPIC-Norfolk, and internal (UKB) | Circulatory mortality, Incident stroke, incident myocardial infarction | Prediection C-statistic: 0.749–0.774 (circulatory mortality), 0.73–0.76 (stroke), 0.68–0.75 (MI) |
| Barriada51 | 2022 | CFP | Coronary Artery Calcium score | Cohort study | VGG16, VGG 19, ResNet | 152 retinal images from PRECISED study, USA | 5-fold cross-validation | Cardiovascular disease | Prediction Accuracy 0.72, Recall 0.52, Precision 0.77, F1 0.62 |
| Yun52 | 2022 | CFP | Gender, ethnicity, age, obese, cardiovascular, unfavorable lifestyle, HDL, HbA1c, glucose, hypertension | Prospective Cohort study | ResNet-18 | 62,262 participants from UK Biobank, UK | 12,185 patients for validation | Type 2 DM | Prediction AUC: 0.731 (retinal only), 0.844 (TRFs + deep learning model) |
| Rim53 | 2021 | CFP | CAC, Age, years, Sex, Systolic blood pressure, Diastolic blood pressure, Fasting glucose, Body-mass index, Hypertension, Diabetes, Dyslipidemia, Current smoke | Prospective cohort | RetiCAC | 13, 8024 retinal photographs from five datasets from South Korea, Singapore, and the UK | External validation | Coronary Artery Calcium | Prediction AUC: 0.742 for CAC prediction, HR: 1.33 (SEED cohort), HR: 1.28 (UK Biobank) |
| Cheung54 | 2021 | CFP | Retinal-vessel caliber, age, gender, ethnicity MAMP, BMI, smoking | Prospective cross-sectional study | SIVA-human, SIVA-DLS | 70,000 retinal photographs from 15 datasets: Singapore, Australia, New Zealand, Hong Kong, China, UK, South Korea | External validation 1060 from SEED study | Cardiovascular events, mortality | ICC: 0.82–0.95 for vessel calibre measurement, HR: 1.24 (narrow CRAE, CVD events) |
| Zhang23 | 2021 | CFP | eGFP, DM status, age, sex, height, weight, body-mass index, blood pressure | Consists of both cross-sectional and longitudinal datasets | ResNet 50 | 115,344 retinal fundus photographs from CC-FII, China | External validate in independent patient populations | chronic kidney disease and type 2 diabetes | Identification of CKD: AUC of 0.930 (95% CI: 0.921–0.940) DM: AUC 0.929 (95% CI: 0.920–0.937) Prediction for eGFR: coefficient of determination (R2): 0.507 |
| Zee55 | 2021 | Brain MRI, CFP | age, gender, education, hypertension, diabetes, WMH volume, Log-transformed WMH volume, Frontal lobe, Parietal-occipital lobe, Basal Ganglia | Community-Based Cohort | ResNet 50, ARIA | 240 subjects from The Chinese University of Hong Kong—Risk index for Subclinical Brain Lesions in Hong Kong | Cross-validation | White matter changes, cerebral small vessel disease | Early detection: AUC: 0.76 for WMH detection |
| Gerrits56 | 2021 | CFP | age, sex, blood pressure, smoking status, glycemic status, total lipid panel, sex steroid hormones and bioimpedance measurements | prospective and longitudinal cohort | MobileNet-V2 | 3000 participants from Qatar Biobank, Qatar | validated for 2400 pictures | Cardiometabolic risk factors: such as age, sex, blood pressure, smoking status, glycemic status, total lipid panel, sex steroid hormones and bioimpedance measurements | Prediction: SBP (R2 = 0.40, MAE = 8.96 mmHg), DBP (R2 = 0.24, MAE = 6.84 mmHg), Hemoglobin A1c (HbA1c) (R2 = 0.34, MAE = 0.61%) relative fat mass (R2 = 0.43, MAE = 5.68 units) testosterone (R2 = 0.54, MAE = 3.76 nmol/L) sex AUC 0.97, Systolic blood pressure AUC 0.4, Diastolic blood pressure AUC 0.24, Hemoglobin A1c AUC 0.34, Relative fat mass value AUC 0.43, Testosterone (nmol/L) AUC 0.43 |
| Mendoza57 | 2021 | OCT circle scan and scan of optic nerve head | Age, sex, race, diabetes diagnosis, hypertension, cardiovascular disease (CVD), and axial length | Prospective cohort study | Deep Learning Model | 1772 patients, 52,552 circle B-scans: 730 patients, 111,456 radial B-scans from Glaucoma Study and African Descent and Glaucoma Evaluation Study (ADAGES), US | Internal Validation of 5% | Age, sex, race, diabetes diagnosis, hypertension, cardiovascular disease, and axial length | MAE: Age 5.1 (4.5, 5.8), Axial length 0.7 (0.6, 0.9), AUC sex 0.72 (0.65, 0.79), race 0.96 (0.92, 0.98), diabetes diagnosis 0.76 (0.64,0.85), hypertension 0.71 (0.59, 0.81), CVD diagnosis 0.56 (0.47, 0.65) |
| Islam58 | 2021 | CFP | severity of diabetic retinopathy | Prospective cohort study | DiaNet | QATAR cohort 246, control group 246; EyePACS over 80,000 images, Qatar | 5-fold cross-validation | Diabetes | Detection Accuracy 84.47%, Precision 83.59%, sensitivity 85.86%. AUC 84.46%, Specificity 83.06%, F1 Score 84.71% |
| Cervera59 | 2021 | CFP | Age, diabetes duration, Hba1c, BMI, serum cholesterol, TGL cholesterol, HDL | Cross-sectional Study | Inception v3, Squeezenet v1.0 and Densenet | 23,784 retinal images from 1561 participants of SNDREAMS study, India | 5-fold cross-validation | Diabetic peripheral neuropathy | Prediction 0.8013 (validation), 0.7097 (test), AUC: 0.8673 (DR subgroup) |
| Sabanayagam60 | 2020 | CFP | CKD stage, age, gender, ethnicity, diabetes, hypertension | Population-based, cross-sectional | retinal image DLA, RF DLA, hybrid DLA | SEED: develop (5188 patients) and validate (1297 patients). (External testing SP2, 3735 patients BES, 1538 patients), Singapore | External validation in two independent datasets in SP2 Singapore and BES China | CKD | Detection AUC: image DLA 0.911 (95% CI 0.886–0.936), RF 0.916 (0.891–0.941), hybrid DLA 0.938 (0.917–0.959) |
| Kim61 | 2020 | CFP | Hypertension, Diabetes, and Smoking, Age and Sex | Cross-sectional study | ResNet-152 | 412,026 retinal fundus images from Seoul National University Bundang Hospital, Korea | 2397 internal validation-set | Prediction of Age and Sex | Correlation between predicted and chronologic age: R2 = 0.92 (0.92–0.93), sex prediction: above AUC 0.96 underlying vascular conditions |
| Zhang62 | 2020 | CFP | gender, Age, current smoker, exercise, salty taste, PSQI, BMI, basal metabolism, waist-hip, body fat ratio, visceral fat index, chronic disease, BP. Bilirubin | cross-sectional study | Inception-v3, TensorFlow | 625 participants from Xinxiang, Henan China | Internal validation | Hyperglycemia, hypertension, dyslipidemia, age, gender, drinking, salty taste, smoking, BMI, WHR, HCT, MCHC, T-BIL, D-BIL | Prediction AUC Hyperglycemia 0.880, hypertension 0.766, dyslipidemia 0.703, age 0.850, gender 0.704, drinking 0.948, salty taste 0.809, smoking 0.794, BMI 0.731, WHR 0.704, HCT 0.759, MCHC 0.686, T-BIL 0.764, D-BIL 0.703 |
| Heslinga63 | 2020 | CFP | age, gender, T2DM status | Observational prospective population-based cohort study | VGG-19 | 2336 pictures from The Maastricht Study, Netherlands | 20% for validation set | T2DM | Detection 0.758 (combining left and right eye), 0.746 (MTL approach with random initialization) |
| Kang64 | 2020 | CFP | Gender, age, dGFR, HbA1c | Retrospective cohort study | VGG-19 | 25,706 CFP from CGMH, Taoyuan, Taiwan. | 10% validation set | Early Renal Function Impairment | AUC: 0.81 (overall), 0.87 (HbA1c >10%), Sensitivity: 0.89, Specificity: 0.61 |
| Vaghefi65 | 2019 | CFP | age, gender, HbA1c, Dyslipidemia, Hypertension, Retinopathy level | Cross-sectional study | Inceptionv3 neural network | 81,711 participants from Auckland Diabetic screening, New Zealand |
20% validation set | Smoke status related to CVD | Detection: Accuracy 88.88%, specificity 93.87%, sensitivity 62.62%. AUC 0.86 |
| Poplin66 | 2018 | CFP | age, gender, smoking status, blood pressure, body mass index (BMI), glucose, and cholesterol levels | Observational study | soft attention (Neural network) | 48,101 patients from UK Biobank and 236,234 patients from EyePACS, UK and US | External validation 12,026 patients from UK Biobank and 999 patients from EyePACS | age, gender, smoking status, HbA1c, systolic blood pressure, major adverse cardiac events | Prediction AUC: 0.97 (gender), 0.71 (smoking status), 0.70 (MACE prediction), MAE: 3.26 years (age), 11.23 mmHg (systolic blood pressure) |
| Abbasi-Sureshjani67 | 2018 | CFP | Age, gender, diabetic status, blood sugar level | Retrospective cohort study | ResNet | healthy (5791 images), type 2 diabetic subjects (3133 images) from Maastricht Study, Netherlands | 20% for internal validation set | T2DM | Prediction: k score of 0.458, F1- score of 0.758 better than human experts (F1 = 0.222) |
Fig. 2.
Quality Assessment of Included Studies A: Using the Newcastle–Ottawa Scale for quality assessment, B: Using the Appraisal tool for Cross-Sectional Studies quality assessment.
We evaluated the adherence of the included studies to the TRIPOD guidelines, with all studies providing clear and informative titles and abstracts, detailed descriptions of participants, and clearly defined outcomes and predictors (Supplemantary Table S2). All studies reported sample sizes, described their model development processes, and provided model performance metrics, such as AUROC, accuracy, and sensitivity. However, only 4 studies (11%) reported missing data handling, with the remaining 34 studies (89%) lacking this information. Internal validation was reported in 26 studies (68%), while only 12 studies (32%) conducted external validation. Overall, the included studies followed most TRIPOD guidelines, while reporting missing data is neede for handling and performing external validation.
Research characteristics
Based on our inclusion criteria, 38 articles were included and fundamental information had been showed in Table 1. 6 of these articles utilized OCT as one of the inputs, while majority of the articles used the CFP as input. 26 had internal validation, and 122 studies included external validation. UK Biobank was used by 10 studies, while other studies included datasets originating from multiple regions including Singapore, India, US and China. The performance of the AI model has been summarized in the table, including AUC, accuracy, sensitivity and specificity, k score and F1- score has also been reported. The studies demonstrated high methodological quality Of the 38 studies, 21 were classified as high quality (scoring 8 out of 9 stars), and 17 were categorized as good quality (scoring 7 out of 9 stars). Most cohort studies showed a low risk of bias, with strong cohort definitions, reliable exposure, outcome assessments and appropriate statistical adjustments. In contrast, several cross-sectional studies showed a moderate risk of bias, primarily due to issues with missing data, non-responder handling and sample size justification (Fig. 3).
Fig. 3.
AI models for DM related complications.
Prediction of diabetes from pre-diabetes or non-diabetic individuals
DM patients are more likely to developing systemic disease, and early detection and treatment of these conditions and risk systemic factors can potentially slow down their progression.68 In the context of predicting and identifying DM patients from the healthy control group, there were 4 studies with an F1-score reported from 0.758 to 0.847 and AUC ranging from 0.746 to 0.845.52,58,63,67 Multiple CNN models such like VGG-19, DiaNet and ResNet were utilized to perform the analysis.
A previously study utilized the CNNs (convolutional neural networks) to automatically extract features from CFP, enabling capture the retinal vasculature change directly from images. This study achieved a predictive performance for diabetes status, with a k score of 0.458 and F1-score of 0.758.67 One publication proposed a multi-stage fine-tuning approach which combines image from different datasets to improve the model performance compared with only one dataset. The AUC for detecting diabetes from non-diabetic patients can reach 84.46% from multiple data sets, while the AUC was 79.01% when utilizing only one dataset.58 Specifically, a multi-target learning approach performed well with identifying T2DM patients with an AUC = 0.746, which improved to 0.758 by combining images from both eyes.63 Moreover, the model's discriminative performance improved from 0.731 to 0.844, by combining the deep learning algorithm with the traditional risk factors model.45 Patient with prediabetes would ideally be distinguished from non-diabetic patients by utilizing the fundus pictures, and these at risk group can subsequently be sent for a formal oral glucose tolerance enabling early identification of prediabetes patients for primary and secondary prevention.
Estimation DM related systemic risk factors
We reviewed the performance of model predicting systemic risk factors such as hypertension, hyperglycemia, dyslipidemia and other using retinal fundus photos alone, and found these models performed with an AUC from 0.24 to 0.97.31,33,39,52,62 Other studies demonstrated adding traditional risk factors to image only base deep learning algorithm, could enhance the predictive performance of DL model with AUROC reported up to 0.93.49,52 One study used AI models to analyze the vascular changes in retina, found that AI based quantification of lower microvascular density and branching complexity are associated with higher severity of disease among the incident cardiometabolic phenotypes patients.40
Chronological age is a risk factor for frailty and mortality in older population including among diabetics.69 With their continuous studies of prediction of age by using deep learning model, Zhu et al.37 showed their model conducted robust correlation between retinal age and chronological age (0.81), suggesting that retina age can service as a biomarker of aging. They also established that the predictive model for stroke using retinal age determined from the same DL model, achieved higher AUC compared with an established risk factor-based model (AUC = 0.676).46 The model is used in another study in DM patients, which proved that patients with DM had higher retinal age gap compared to persons without diabetes. Other algorithms focusing on prediction of chronological age, performed less effectively in individuals with DM or hypertension, demonstrating systemic vascular disease like DM, leads multiple changes in the retina vessels and contributes to an altered relationships between age and retinal vascular features.61
Detection of microvascular complications
The retina is the only physiological “window” in humans that provides noninvasive visualization of the microvasculature. Evaluating retina vessels using fundus, OCT and OCTA has the potential to provide a non-invasive and economical means for evaluating the microvascular status of the kidney and brain.70, 71, 72
Chronic kidney disease (CKD) is a significant contributor to disease-related mortality, particularly among individuals with diabetes.73 Published AI Models performed well in the evaluation of CKD with a good performance in detection of disease with an AUC from 0.87 to 0.91123,38,60,64; while for predicting the development of CKD and detection of early kidney impairment reported AUC ranged from 0.864 to 0.87.23,36
Reti-CKD score, a noninvasive risk assessment tool has been designed to identify CKD risk in individuals with preserved kidney function. In the study, the Reti-CKD demonstrated superior prediction performance for CKD incidence with a higher C-statistic of 0.703 compared to the eGFR-based method, indicating its effectiveness as a predictive tool over traditional blood tests.36 Deep learning algorithms with high accuracy of CKD detection (AUC = 0.911) from CFP provides a potentially non-invasive tool for rapid screening of CKD in community-based populations without the availability of laboratory infrastructure.60 The algorithm was able to detect CKD in DM patients, with an AUC of 0.886 in a hybrid model, which combined regression model and image-only model.38 To increase portability, smartphones have been employed to capture fundus images and combining these images with various clinical metadata, including age, gender, BMI, and blood pressure with a deep-learning approaches for the identification of CKD and T2DM was able to achieve an AUC of 0.898.23 For detection of early renal function impairment, an AI model reported an AUC of 0.87, particularly for the group with HbA1c levels above 10%, compared with AUC of 0.81 in overall population in their study.64
Small vessel disease (SVD) in the brain has been linked with dementia, stroke, depression, diabetic peripheral neuropathy and Parkinson's disease (PD).44,55,59,72 When utilizing retina images for early detection of SVD, AI models exhibited high performance with a sensitivity of 89.7% and accuracy of 93.3%. The automatic retinal image analysis model not only detects the presence of early white matter hyperintensities (WMH), but also analyzing the localization of WMH for all 6 brain regions by measuringthe retinal images, enabling diagnose and prediction of neurodegenerative dysfunctions.55 Furthermore, in a longitudinal study, a DL model was performed to predict the retinal age and risk of 5-year PD. The predictive value of retinal-age-based model is comparable with the risk-factor-based model for PD, indicating a novel biomarker for identifying high risk of having the PD (predictive AUC = 0.708 and 0.717).44
Peripheral neuropathy is another important microvascular complication of DM and can result in disability due to foot ulceration and the potential necessity for amputation.74 An AI model designed to detect diabetic peripheral neuropathy from retina images presence of DR reached an AUC of 0.867. The model demonstrated superior detection performance in individuals with DR, compared to those without, indicating that DR patients may have greater risk of developing peripheral neuropathy compared to individuals with mild or no DR.59
Detection of macrovascular complications
Cardiovascular diseases (CVD) is a major cause of mortality worldwide, with 60% of deaths globally over the past 30 years accounted for by CVD.75 Most of the studies identified aimed to detect or the predict the future risk of CVD or its risk factors.30,32,33,35,39, 40, 41, 42,45,48,50,51,53,54,56,57,65 For incident CVD, the AUC for prediction was reported to be as high as 0.991, with an AUC of 0.686 for major adverse cardiovascular events (MACE) and C-statistics between 0.75 and 0.77.39,40,42,50 A previously published neural network not only predicted several cardiovascular risk related variables from CFP, but also the onset of MACE within 5 years. Despite limited numbers of MACE, the system achieved AUC of 0.7, which is comparable to AUC of 0.72 for the European SCORE risk calculator. The results of the algorithm are consistent in 2 separate validation sets.66 A hybrid model which combined retina images with dual-energy X-ray absorptiometry (DXA), reached up to higher classification accuracy of 78.3%, compared with retina-image or DXA alone.48 By adding retinal vasculometry (RV) to Framingham risk scores (FRS), the C-statistics was higher compared with a simpler model based on age, RV, smoking status and medical history algrithms.50 The include models performed well in evaluating risk factors of CVD: age (MAE up to 2.78 ys), gender (AUC up to 0.97) and smoking status (accuracy achieved 88.88%) etc.56,57,65 For detecting calculated ischemic CVD risk ≥5%, the algorithm achieved an AUC of 0.971 in internal validation and 0.859 in external validation.41 Furthermore, in a DL model, each 1-year retinal age gap was associated with increased of a 3% increase in risk of incident CVD, alongside an arterial stiffness index with a β coefficient of 0.002. Their research suggested retinal age gap a potential biomarker to detect future CVD.45 Meanwhile, by measuring retinal vesselcalibre, a DL model showed better prediction performance for CVD risk factors than human modle (p < 0.01). Retinal vessel calibre washigh correlated with CVD risk factors, such as age, gender, BMI, MABP, smoking and DM status. Narrower CRAE ((hazard ratio) per s.d. (95% CI) 1.12 (1.02–1.24)) decrease were independently correlated with CVD incidence in SEED study. Their study further motivated DL systems for prediction of CVD on basis of retinal vessels features.54
Diabetes stands as a leading risk factor for developing peripheral artery disease (PAD), DM patients with PAD experience significant higher mortality rates.76 We identified only one study examining PAD with retinal imaging and the AI model utilizing multiple instance learning with a high spatial resolution, achieved an AUC of 0.89 for detection of early stage of PAD. The model distinguished PAD patients from controls by identifying alterations around the temporal arcade and optic disc.47
Discussion
Our review includes a total of 38 articles using AI models and retinal imaging for the identification or prediction of systemic diseases in persons with DM (Fig. 3). These studies utilized a range of retinal images, with most of them analyzing CFP, while 5 of them employed OCT for retinal examination. Most of these studies27 included a validation set in their research with 6 of them performing external validations. Majority of the models in this review demonstrate high performance of predicting disease over AUC 0.8, C-statistics and accuracy 0.75. The high performance of algorithms identified in our study suggest that AI-driven retinal imaging has the potential to make clinically significant impact on detection of DM-related complications. Early opportunistic detection of other systemic DM complications with retinal imaging during DR screening can enable early intervention and secondary prevention which may significantly reduce long term healthcare costs.
Previously one systemic factor-related research utilizing AI system, demonstrating prediction of risk factors and cardiovascular disease with reasonable results.66 Most of the research utilized only single imaging modality (CFP or OCT) for analyze, while multi-model photos of the retina such as combined with OCTA may enhance the performance of the prediction capabilities.77 Age is associated with progression of diseases, combined with retinal age and chronological age, the system may get a higher AUC in prediction of systemic disease. The retina can provide valuable information about age through images analysis, especially for DM patients. Zhu et al.37,46 introduced a novel biomarker, the retinal age gap, as a method for prediction.
Our review includes research on micro- and macrovascular complications. With all the convenient non-invasive picture screening, AI models can analyze both whole images as well as specific segment areas of retina.78 The retinal is a neurovascular organ and can reflect the damage diabetes has on both neuronal and vascular tissue. This is demonstrated by the accuracy of detection of peripheral neuropathy with model identified in the literature.47 Analyzing subtle changes such as vessel caliber change from the retina, the AI can detect pre-clinical disease from retinal imaging. Research showed model's performance distinguished the disease with higher accuracy under DM status.79 Thus, showed specific change in retina with DM, and remind research would not neglect these DM influence on the results during analyzing.
The articles we've included in this review are primarily focused on a single disease. However, it's possible in the future these AI systems can be further developed to detect and distinguish between various diseases through additional training and validation processes, or can be used in combination with each algorithm providing a separate output for further action. The potential for expanding disease detection through medical AI is an exciting prospect. However, recent systematic reviews have revealed a high false-positive rate with single fundus analysis in current screening programs. To address this, multi-model image-based analysis should be considered. A recent study suggests that future programs should incorporate combined analyses, such as OCT and OCTA, along with external photos, and provide more specialized training to enhance the accuracy of the models.80
The application of AI algorithms in diabetic retinopathy (DR) screening holds immense potential to revolutionize care delivery, particularly in low to middle-income countries and rural populations with limited access to healthcare. By leveraging AI-driven retinal imaging for early detection of DR, healthcare providers can implement cost-effective and scalable screening programs that overcome geographical and resource barriers. Portable retinal imaging devices equipped with AI algorithms can be deployed in community health centers, enabling timely identification of DR in underserved populations. Moreover, AI-driven screening programs can facilitate the triage of patients, directing limited healthcare resources to those at highest risk, thus optimizing care delivery in resource-constrained settings. Through this approach, AI algorithms not only enhance the efficiency and accessibility of DR screening but also empower healthcare systems to proactively address the burden of diabetic eye disease, ultimately improving patient outcomes and reducing the risk of vision loss in vulnerable populations.
Traditional examinations for systemic complications of DM, such as coronary artery calcium scans (CAC), blood tests, and glomerular filtration rate assessments, are often invasive and expensive, making them relatively inaccessible to the global diabetic population. AI-driven early and non-invasive detection of these systemic complication using retinal images has emerged as a promising approach to reduce the morbidity and mortality of DM, and thereby reducing the societal burden of the disease.63,81
Research results demonstrate that artificial intelligence, even in the absence of clinical manifestations, can accurately detect and predict the development of future diseases. Therefore, the translation of these AI driven retinal imaging algorithms into large-scale screening programs for less developed areas, has the potential to transform care in DM globally. In our review, majority of research related to DM has demonstrated relatively high predictive accuracy, with most studies achieving a prediction ability exceeding AUC 0.8. With high predictive capability of AI models, these can be tuned with a preference for sensitivity and used as a primary screening modality, identifying only those with high risk for future evaluation. This can reduce the risk and costs of confirmatory test such as CT scans used for assessing CAC scores which have radiation exposure, high capital outlay and limited screening capacity, or laboratory blood test which may not be easily available in rural or remote areas of lower to middle income countries. Predictive performance achieved high with AUC 0.742 (95% CI 0.732–0.753) in our study, highlights the potential for deep learning as an alternative means to predict disease instead of utilizing the CAC.53
In recent years, researchers have conducted significant advancements in AI studies. These models not only reduce the need for human resources in image identification but also offer a significant opportunity to alleviate the economic burden on our healthcare system. Even in less developed areas, with AI systems, diseases at a very early stage could be detected with potable device. AI systems have the potential to minimize the need for secondary screenings, and enhance opportunities for earlydetection of systemic diseases (Fig. 4).
Fig. 4.
Comparison of AI screening and traditional screening for diabetes-associated complications.82 $300,000 per 1000 people: estimated cost for comprehensive systemic complication screening for all DM patients. $60,000 per 1000 people estimated cost of using AI-based retinal imaging to screen for systemic complications in high-risk patients. DR screening: diabetic retinopathy screening, DM screening: general DM screening for complications related to systemic factors (the dollar sign refers to US dollar).
While the algorithms reported in this review performed well on research dataset, there is often a gap with real-world performance, where the patient population, image quality and prevalence of disease will be more variable, therefore external validation is crucial.83,84 We noted the lack of external validation among the models included in our study, only 6 of them performed the external validation. Models trained only in internal dataset demonstrated higher performance compared to those trained externally, suggesting a potential risk of overfittings and training bias. These discrepancies may hinder our understanding of the models’ applicability. There were no randomized clinical trials identified in our review evaluating the performance of these algorithms. Future research should prioritize training models using external datasets from multiple institutions or diverse ethnic backgrounds, and validate these algorithms with real world external dataset. Conducting RCTs will also be necessary to elucidate the true clinical effectiveness of Al algorithms in clinical care.
Our results demonstrate that while retinal image offers valuable insights into systemic problems, the predictive power of these models is significantly improved when combined with other phenotypes, including age, sex and traditional risk factors like blood pressure and HbA1c. This multi-modal approach allows for more accurate risk stratification and highlights the importance of integrating both retinal and clinical data in prediction models.
AI-based oculomics, particularly by using retinal imaging, has emerged as a promising tool for diagnosing systemic diseases, with AUCs in non-diabetic populations typically ranging from 0.70 to 0.95.85 However, the advantage in diabetic populations is clear, as microvascular pathology, which is more easily visualized in retinal images, offers better prediction of systemic complications compared to other populations. Further exploration of AI integration with clinical and genetic data will help refine predictive models and improve risk stratification in both diabetic and non-diabetic cohorts86,87
Our review encompasses research on systemic diseases related to DM utilizing AI systems with retina images. This review highlights the promising potential of AI systems in analyzing diseases based on retina characteristics. It offers valuable insights for current studies and contributes to the growing body of knowledge in this field. There are still limitations of our review, these limitations, in turn, prompt considerations for future research.: 1) We primarily focused on complications related to DM, which led to the exclusion of numerous valuable articles in the broader field of AI research, and those specifically focused on DR. The realm of DR has seen extensive research dedicated to AI models for screening, risk assessment, management, and prognostication, yielding promising outcomes. Indeed, there's a spectrum of systems showing promising performances in ongoing research within this domain. Systems like EyeArt, sponsored by Google, models developed by the Singapore National Eye Center, and algorithms like Bosch DR have all displayed encouraging results in their respective studies. These diverse initiatives signify the breadth of innovation and collaboration aimed at advancing medical AI for disease detection. The success of AI in DR detection is further underscored by the growing body of evidence from clinical studies and real-world implementation. Randomized clinical trials have validated the performance of AI algorithms, confirming their non-inferiority or even superiority to human doctors in diagnosing DR. Real-world studies have also demonstrated the feasibility and effectiveness of AI-driven DR screening programs, showcasing their potential to streamline workflows, improve resource allocation, and enhance patient care.88, 89, 90, 91, 92, 93 Furthermore, the utilization of multimodal retina images such as OCTA has also showed an appealing diagnostic performance in assessing DR.94 2) In our effort to analyze the latest trends, we include data from short communications and meeting reports, which may not provide the same level of detail as full research articles. 3) The majority of the articles we have included are cross-sectional or retrospective studies. It is essential for future research to conduct long-term and prospective studies to gain a more comprehensive understanding of the application of AI in predicting and identifying systemic diseases through retina images. Longitudinal studies and RCTs can provide insights into how these AI models perform over time and their effectiveness in real-world clinical settings. 5) While most of the studies analyzed data from retina photos, there is an increasing interest in studies utilizing OCT and OCTA, or multimodal imaging models. These technologies can provide more detailed information from different layers of the retina, offering a deeper understanding of the structural and vascular changes associated with systemic diseases. While OCT imaging offers high-resolution, cross-sectional retinal images, the equipment required can be costly and less portable compared to other modalities like fundus photography. However, the emergence of portable and home-monitored OCT devices holds promise for improving diagnostic efficacy and reducing both human and economic costs.95,96
The application of AI on retinal imaging has demonstrated advantages in predicting and identifying the systemic complications of diabetes. While some of the initial algorithms demonstrated limited efficacy, more recent publications have continued to show increasing promise in their clinical potential. However, given the qualitative nature of the current evidence, further quantitative research should be conducted, with a particular focus on more in-depth analysis and broader real-world implications. Further development may have the potential to enable AI and retinal imaging to contribution to a paradigm shift in screening for the systemic complications of diabetes.
Contributors
Qianhui YANG: writing—original draft, Yong Mong BEE, LIM Ciwei, SABANAYAGAM Charumathi, Carol CHEUNG: data curation.
Tien Yin WONG: supervision, writing review & editing.
Daniel SW TING, Li Ling LIM, Hua Ting LI, Mingguang HE, Aaron LEE, Jonathan SHAW, YEO.
Khung Keong: writing review & editing.
Gavin Siew Wei TAN: conceptualisation, writing review & editing.
All authors have read and approved the manuscript and at Dr Yang and Dr Tan had access to and verified the undelying data.
Data sharing statement
The data supporting the findings of this study are derived from previously published studies, all of which are publicly available and cited within this manuscript. No new primary data were generated or analyzed for this systematic review. Any additional information or specific data extraction details used during the review process can be made available upon reasonable request to the corresponding author.
Declaration of interests
Prof Tien Yin Wong has received consulting fee from Aldropika Therapeutics.
Bayer, Boehringer Ingelheim, Carl Zeiss, Genentech, Inc, Iveric Bio, Novartis, Opthea Limited, Quaerite Bipharm, Research Ltd, Plano, Roche, Sanofi, Shanghai Henlius. And he is a inventor, and hold patents and am a co-founder VISRE of start-up companies EyRiS and Visre, which have interests in, and develop digital solutions for eye diseases, including diabetic retinopathy.
Dr Yeo Khung Keong has received research funding from Amgen, Astra Zeneca, Abbott Vascular, Bayer, Boston Scientific, Shockwave Medical, Novartis (via institution); Consulting fees from Abbott Vascular, Medtronic, Novartis, Peijia Medical; Speaker fees from Shockwave Medical, Abbott Vascular, Boston Scientific, Medtronic, Alvimedica, Biotronik, Orbus Neich, Shockwave Medical, Amgen, Novartis, Astra Zeneca, Microport, Terumo, Omnicare.
Dr. Lee-Ling Lim has received Grants or contracts from: Boehringer Ingelheim, Abbott Nutrition, Zuellig Pharma Therapeutics, Novartis; and Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: AstraZeneca, Boehringer Ingelheim, Abbott, Amgen, Novartis, Novo Nordisk, Roche, Sanofi, Servier, Zuellig Pharma.
Dr. Aaron Y. Lee has received Grants or contracts from: Amazon, Lantham Vision Science Award, Meta, NIH/NEI K23EY029246, NIH OT2OD032644, Regeneron, Santen, Topcon, Zeiss, Research to Prevent Blindness. Consulting fees: Sanofi, Boehringer Ingelheim, Genentech, Inc., Gyroscope, Johnson & Johnson, US FDA.
Dr. Daniel Ting has Patents planned, issued or pending: SELENA for DR screening altorithm
Dr. Jonathan E Shaw received Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Astra Zeneca, Boehringer Ingelheim, Novo Nordisk, Roche, Zuellig Pharmaceutical, Eli Lilly, Abbott.
Dr. Yong Mong BEE received consulting fees from Abbott Payment and GSK, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Sebia, AstraZeneca, Boehringer Ingelheim, Participation on a Data Safety Monitoring Board or Advisory Board: Boehringer Ingelheim Honoraria paid to institution.
Dr Cynthia Lim received Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Sebia, AstraZeneca, Boehringer Ingelheim. Participation on a Data Safety Monitoring Board or Advisory Board: Boehringer Ingelheim Honoraria paid to institution.
Dr Gavin Tan has received grants from Santen and Singapore National Research Coucil. He has received consulting and lecture fees from Novartis, Bayer, Roche, Zeiss and Leica and has received honoraria from Abbvie-Allergan, Abbott and Nikon-optos. He is also a shareholder in VISRE and Eyris.
Acknowledgements
This study was supported by the following grants MOH-CSAINV21jun-005; OFLCG/001/2017 and MOHOFLCG22may-0003 from the Singapore National Medical Research Council (NMRC).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2025.103089.
Appendix A. Supplementary data
References
- 1.Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–234. doi: 10.1016/S0140-6736(23)01301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.11. Microvascular complications and foot care: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S135–S151. doi: 10.2337/dc20-S011. [DOI] [PubMed] [Google Scholar]
- 3.Standards of medical care in diabetes-2022 abridged for primary care providers. Clin Diabetes. 2022;40(1):10–38. doi: 10.2337/cd22-as01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu C.D., McCulloch C.E., Banerjee T., et al. CKD awareness among US adults by future risk of kidney failure. Am J Kidney Dis. 2020;76(2):174–183. doi: 10.1053/j.ajkd.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt-Erfurth U., Sadeghipour A., Gerendas B.S., Waldstein S.M., Bogunović H. Artificial intelligence in retina. Prog Retin Eye Res. 2018;67:1–29. doi: 10.1016/j.preteyeres.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Ting D.S.W., Pasquale L.R., Peng L., et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167–175. doi: 10.1136/bjophthalmol-2018-313173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramoff M.D., Whitestone N., Patnaik J.L., et al. Autonomous artificial intelligence increases real-world specialist clinic productivity in a cluster-randomized trial. NPJ Digit Med. 2023;6(1):184. doi: 10.1038/s41746-023-00931-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grzybowski A., Brona P., Lim G., et al. Artificial intelligence for diabetic retinopathy screening: a review. Eye (Lond) 2020;34(3):451–460. doi: 10.1038/s41433-019-0566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hood D.C., La Bruna S., Tsamis E., et al. Detecting glaucoma with only OCT: implications for the clinic, research, screening, and AI development. Prog Retin Eye Res. 2022;90 doi: 10.1016/j.preteyeres.2022.101052. [DOI] [PubMed] [Google Scholar]
- 10.Dong L., He W., Zhang R., et al. Artificial intelligence for screening of multiple retinal and optic nerve diseases. JAMA Netw Open. 2022;5(5) doi: 10.1001/jamanetworkopen.2022.9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ting D.S.W., Tan G.S.W. Telemedicine for diabetic retinopathy screening. JAMA Ophthalmol. 2017;135(7):722–723. doi: 10.1001/jamaophthalmol.2017.1257. [DOI] [PubMed] [Google Scholar]
- 12.Goh J.K., Cheung C.Y., Sim S.S., Tan P.C., Tan G.S., Wong T.Y. Retinal imaging techniques for diabetic retinopathy screening. J Diabetes Sci Technol. 2016;10(2):282–294. doi: 10.1177/1932296816629491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan R., Teo K.Y.C., Husain R., et al. Evaluating the outcome of screening for glaucoma using colour fundus photography-based referral criteria in a teleophthalmology screening programme for diabetic retinopathy. Br J Ophthalmol. 2023;108(7):933–939. doi: 10.1136/bjo-2023-323339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong T.Y., Sun J., Kawasaki R., et al. Guidelines on diabetic eye care: the international Council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125(10):1608–1622. doi: 10.1016/j.ophtha.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Ophthalmology ICo . 2017. ICO guidelines for diabetic eye care. [Google Scholar]
- 16.Broe R., Rasmussen M.L., Frydkjaer-Olsen U., et al. Retinal vessel calibers predict long-term microvascular complications in type 1 diabetes: the Danish Cohort of Pediatric Diabetes 1987 (DCPD1987) Diabetes. 2014;63(11):3906–3914. doi: 10.2337/db14-0227. [DOI] [PubMed] [Google Scholar]
- 17.Grauslund J., Hodgson L., Kawasaki R., Green A., Sjolie A.K., Wong T.Y. Retinal vessel calibre and micro- and macrovascular complications in type 1 diabetes. Diabetologia. 2009;52(10):2213–2217. doi: 10.1007/s00125-009-1459-8. [DOI] [PubMed] [Google Scholar]
- 18.McGeechan K., Liew G., Macaskill P., et al. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann Intern Med. 2009;151(6):404–413. doi: 10.7326/0003-4819-151-6-200909150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein R., Klein B.E., Moss S.E., Wong T.Y. Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2007;114(10):1884–1892. doi: 10.1016/j.ophtha.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Visuña L., Yang D., Garcia-Blas J., Carretero J. Computer-aided diagnostic for classifying chest X-ray images using deep ensemble learning. BMC Med Imaging. 2022;22(1):178. doi: 10.1186/s12880-022-00904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ait Nasser A., Akhloufi M.A. A review of recent advances in deep learning models for chest disease detection using radiography. Diagnostics (Basel) 2023;13(1):159. doi: 10.3390/diagnostics13010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachtiger P., Petri C.F., Scott F.E., et al. Point-of-care screening for heart failure with reduced ejection fraction using artificial intelligence during ECG-enabled stethoscope examination in London, UK: a prospective, observational, multicentre study. Lancet Digit Health. 2022;4(2):e117–e125. doi: 10.1016/S2589-7500(21)00256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang K., Liu X., Xu J., et al. Deep-learning models for the detection and incidence prediction of chronic kidney disease and type 2 diabetes from retinal fundus images. Nat Biomed Eng. 2021;5(6):533–545. doi: 10.1038/s41551-021-00745-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Li Z., Nan N., Wang X. TranSegNet: hybrid CNN-vision transformers encoder for retina segmentation of optical coherence tomography. Life (Basel) 2023;13(4):976. doi: 10.3390/life13040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang J., Ko A., Park S.M., et al. Association of cardiovascular mortality and deep learning-funduscopic atherosclerosis score derived from retinal fundus images. Am J Ophthalmol. 2020;217:121–130. doi: 10.1016/j.ajo.2020.03.027. [DOI] [PubMed] [Google Scholar]
- 26.Bellemo V., Lim G., Rim T.H., et al. Artificial intelligence screening for diabetic retinopathy: the real-world emerging application. Curr Diabetes Rep. 2019;19(9):72. doi: 10.1007/s11892-019-1189-3. [DOI] [PubMed] [Google Scholar]
- 27.Ting D.S.W., Cheung C.Y., Lim G., et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318(22):2211–2223. doi: 10.1001/jama.2017.18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Y., Nguyen Q.D., Hamzah H., et al. Artificial intelligence for teleophthalmology-based diabetic retinopathy screening in a national programme: an economic analysis modelling study. Lancet Digit Health. 2020;2(5):e240–e249. doi: 10.1016/S2589-7500(20)30060-1. [DOI] [PubMed] [Google Scholar]
- 29.Kumar Y., Koul A., Singla R., Ijaz M.F. Artificial intelligence in disease diagnosis: a systematic literature review, synthesizing framework and future research agenda. J Ambient Intell Humaniz Comput. 2023;14(7):8459–8486. doi: 10.1007/s12652-021-03612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nusinovici S., Rim T.H., Li H., et al. Application of a deep-learning marker for morbidity and mortality prediction derived from retinal photographs: a cohort development and validation study. Lancet Healthy Longev. 2024;5(10) doi: 10.1016/S2666-7568(24)00089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei J., Xu Y., Wang H., et al. Metadata information and fundus image fusion neural network for hyperuricemia classification in diabetes. Comput Methods Programs Biomed. 2024;256 doi: 10.1016/j.cmpb.2024.108382. [DOI] [PubMed] [Google Scholar]
- 32.Nabrdalik K., Irlik K., Meng Y., et al. Artificial intelligence-based classification of cardiac autonomic neuropathy from retinal fundus images in patients with diabetes: the Silesia Diabetes Heart Study. Cardiovasc Diabetol. 2024;23(1):296. doi: 10.1186/s12933-024-02367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White T., Selvarajah V., Wolfhagen-Sand F., et al. Prediction of cardiovascular risk factors from retinal fundus photographs: validation of a deep learning algorithm in a prospective non-interventional study in Kenya. Diabetes Obes Metab. 2024;26(7):2722–2731. doi: 10.1111/dom.15587. [DOI] [PubMed] [Google Scholar]
- 34.Carrillo-Larco R.M., Bravo-Rocca G., Castillo-Cara M., Xu X., Bernabe-Ortiz A. A multimodal approach using fundus images and text meta-data in a machine learning classifier with embeddings to predict years with self-reported diabetes - an exploratory analysis. Prim Care Diabetes. 2024;18(3):327–332. doi: 10.1016/j.pcd.2024.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y., Chia M.A., Wagner S.K., et al. A foundation model for generalizable disease detection from retinal images. Nature. 2023;622(7981):156–163. doi: 10.1038/s41586-023-06555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joo Y.S., Rim T.H., Koh H.B., et al. Non-invasive chronic kidney disease risk stratification tool derived from retina-based deep learning and clinical factors. NPJ Digit Med. 2023;6(1):114. doi: 10.1038/s41746-023-00860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Z., Shi D., Guankai P., et al. Retinal age gap as a predictive biomarker for mortality risk. Br J Ophthalmol. 2023;107(4):547–554. doi: 10.1136/bjophthalmol-2021-319807. [DOI] [PubMed] [Google Scholar]
- 38.Betzler B.K., Chee E.Y.L., He F., et al. Deep learning algorithms to detect diabetic kidney disease from retinal photographs in multiethnic populations with diabetes. J Am Med Inform Assoc. 2023;30(12):1904–1914. doi: 10.1093/jamia/ocad179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mellor J., Jiang W., Fleming A., et al. Can deep learning on retinal images augment known risk factors for cardiovascular disease prediction in diabetes? A prospective cohort study from the national screening programme in Scotland. Int J Med Inform. 2023;175 doi: 10.1016/j.ijmedinf.2023.105072. [DOI] [PubMed] [Google Scholar]
- 40.Zekavat S.M., Raghu V.K., Trinder M., et al. Deep learning of the retina enables phenome- and genome-wide analyses of the microvasculature. Circulation. 2022;145(2):134–150. doi: 10.1161/CIRCULATIONAHA.121.057709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Y., Xiong J., Zhu Y., et al. Deep learning algorithm using fundus photographs for 10-year risk assessment of ischemic cardiovascular diseases in China. Sci Bull. 2022;67(1):17–20. doi: 10.1016/j.scib.2021.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Mordi I.R., Trucco E., Syed M.G., et al. Prediction of major adverse cardiovascular events from retinal, clinical, and genomic data in individuals with type 2 diabetes: a population cohort study. Diabetes Care. 2022;45(3):710–716. doi: 10.2337/dc21-1124. [DOI] [PubMed] [Google Scholar]
- 43.Nusinovici S., Rim T.H., Yu M., et al. Retinal photograph-based deep learning predicts biological age, and stratifies morbidity and mortality risk. Age Ageing. 2022;51(4) doi: 10.1093/ageing/afac065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu W., Wang W., Wang Y., et al. Retinal age gap as a predictive biomarker of future risk of Parkinson's disease. Age Ageing. 2022;51(3) doi: 10.1093/ageing/afac062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Z., Chen Y., Wang W., et al. Association of retinal age gap with arterial stiffness and incident cardiovascular disease. Stroke. 2022;53(11):3320–3328. doi: 10.1161/STROKEAHA.122.038809. [DOI] [PubMed] [Google Scholar]
- 46.Zhu Z., Hu W., Chen R., et al. Retinal age gap as a predictive biomarker of stroke risk. BMC Med. 2022;20(1):466. doi: 10.1186/s12916-022-02620-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mueller S., Wintergerst M.W.M., Falahat P., et al. Multiple instance learning detects peripheral arterial disease from high-resolution color fundus photography. Sci Rep. 2022;12(1):1389. doi: 10.1038/s41598-022-05169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Absi H.R.H., Islam M.T., Refaee M.A., Chowdhury M.E.H., Alam T. Cardiovascular disease diagnosis from DXA scan and retinal images using deep learning. Sensors. 2022;22(12):4310. doi: 10.3390/s22124310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan N.C., Perera C., Dow E.R., et al. Predicting systemic health features from retinal fundus images using transfer-learning-based artificial intelligence models. Diagnostics. 2022;12(7):1714. doi: 10.3390/diagnostics12071714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudnicka A.R., Welikala R., Barman S., et al. Artificial intelligence-enabled retinal vasculometry for prediction of circulatory mortality, myocardial infarction and stroke. Br J Ophthalmol. 2022;106(12):1722–1729. doi: 10.1136/bjo-2022-321842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barriada R.G., Simó-Servat O., Planas A., Hernández C., Simó R., Masip D. Deep learning of retinal imaging: a useful tool for coronary artery calcium score prediction in diabetic patients. Appl Sci. 2022;12(3):1401. [Google Scholar]
- 52.Yun J.S., Kim J., Jung S.H., et al. A deep learning model for screening type 2 diabetes from retinal photographs. Nutr Metab Cardiovasc Dis. 2022;32(5):1218–1226. doi: 10.1016/j.numecd.2022.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rim T.H., Lee C.J., Tham Y.C., et al. Deep-learning-based cardiovascular risk stratification using coronary artery calcium scores predicted from retinal photographs. Lancet Digit Health. 2021;3(5):e306–e316. doi: 10.1016/S2589-7500(21)00043-1. [DOI] [PubMed] [Google Scholar]
- 54.Cheung C.Y., Xu D., Cheng C.Y., et al. A deep-learning system for the assessment of cardiovascular disease risk via the measurement of retinal-vessel calibre. Nat Biomed Eng. 2021;5(6):498–508. doi: 10.1038/s41551-020-00626-4. [DOI] [PubMed] [Google Scholar]
- 55.Zee B., Wong Y., Lee J., et al. Machine-learning method for localization of cerebral white matter hyperintensities in healthy adults based on retinal images. Brain Commun. 2021;3(3) doi: 10.1093/braincomms/fcab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerrits N., Elen B., Craenendonck T.V., et al. Age and sex affect deep learning prediction of cardiometabolic risk factors from retinal images. Sci Rep. 2020;10(1):9432. doi: 10.1038/s41598-020-65794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mendoza L., Christopher M., Brye N., et al. Deep learning predicts demographic and clinical characteristics from optic nerve head OCT circle and radial scans. Invest Ophthalmol. 2021;62(8):2120. [Google Scholar]
- 58.Islam M.T., Al-Absi H.R., Ruagh E.A., Alam T. DiaNet: a deep learning based architecture to diagnose diabetes using retinal images only. IEEE Access. 2021;9:15686–15695. [Google Scholar]
- 59.Cervera D.R., Smith L., Diaz-Santana L., Kumar M., Raman R., Sivaprasad S. Identifying peripheral neuropathy in colour fundus photographs based on deep learning. Diagnostics (Basel) 2021;11(11):1943. doi: 10.3390/diagnostics11111943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sabanayagam C., Xu D., Ting D.S.W., et al. A deep learning algorithm to detect chronic kidney disease from retinal photographs in community-based populations. Lancet Digit Health. 2020;2(6):e295–e302. doi: 10.1016/S2589-7500(20)30063-7. [DOI] [PubMed] [Google Scholar]
- 61.Kim Y.D., Noh K.J., Byun S.J., et al. Effects of hypertension, diabetes, and smoking on age and sex prediction from retinal fundus images. Sci Rep. 2020;10(1):4623. doi: 10.1038/s41598-020-61519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L., Yuan M., An Z., et al. Prediction of hypertension, hyperglycemia and dyslipidemia from retinal fundus photographs via deep learning: a cross-sectional study of chronic diseases in central China. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0233166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heslinga F.G., Pluim J.P., Houben A., et al. Medical imaging 2020: computer-aided diagnosis. SPIE; 2020. Direct classification of type 2 diabetes from retinal fundus images in a population-based sample from the maastricht study. [Google Scholar]
- 64.Kang E.Y., Hsieh Y.T., Li C.H., et al. Deep learning-based detection of early renal function impairment using retinal fundus images: model development and validation. JMIR Med Inform. 2020;8(11) doi: 10.2196/23472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaghefi E., Yang S., Hill S., Humphrey G., Walker N., Squirrell D. Detection of smoking status from retinal images; a Convolutional Neural Network study. Sci Rep. 2019;9(1):7180. doi: 10.1038/s41598-019-43670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poplin R., Varadarajan A.V., Blumer K., et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat Biomed Eng. 2018;2(3):158–164. doi: 10.1038/s41551-018-0195-0. [DOI] [PubMed] [Google Scholar]
- 67.Abbasi-Sureshjani S., Dashtbozorg B., ter Haar Romeny B.M., Fleuret F. VipIMAGE 2017: PROCEedings of the VI ECCOMAS thematic conference on Computational Vision and Medical Image Processing Porto, Portugal, October 18-20, 2017. Springer; 2018. Exploratory study on direct prediction of diabetes using deep residual networks. [Google Scholar]
- 68.Mora T., Roche D., Rodríguez-Sánchez B. Predicting the onset of diabetes-related complications after a diabetes diagnosis with machine learning algorithms. Diabetes Res Clin Pract. 2023;204 doi: 10.1016/j.diabres.2023.110910. [DOI] [PubMed] [Google Scholar]
- 69.Jonsson B.A., Bjornsdottir G., Thorgeirsson T.E., et al. Brain age prediction using deep learning uncovers associated sequence variants. Nat Commun. 2019;10(1):5409. doi: 10.1038/s41467-019-13163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sim R., Cheung G., Ting D., et al. Retinal microvascular signs in COVID-19. Br J Ophthalmol. 2022;106(9):1308–1312. doi: 10.1136/bjophthalmol-2020-318236. [DOI] [PubMed] [Google Scholar]
- 71.Johansen K.L., Chertow G.M., Gilbertson D.T., et al. US renal data system 2021 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2022;79(4 Suppl 1):A8–A12. doi: 10.1053/j.ajkd.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sleight E., Stringer M.S., Clancy U., et al. Cerebrovascular reactivity in patients with small vessel disease: a cross-sectional study. Stroke. 2023;54(11):2776–2784. doi: 10.1161/STROKEAHA.123.042656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MacLaughlin H.L., Friedman A.N., Ikizler T.A. Nutrition in kidney disease: core curriculum 2022. Am J Kidney Dis. 2022;79(3):437–449. doi: 10.1053/j.ajkd.2021.05.024. [DOI] [PubMed] [Google Scholar]
- 74.Sloan G., Selvarajah D., Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol. 2021;17(7):400–420. doi: 10.1038/s41574-021-00496-z. [DOI] [PubMed] [Google Scholar]
- 75.Hunter L.D., Doubell A.F., Pecoraro A.J.K., et al. Morpho-mechanistic screening criteria for the echocardiographic detection of rheumatic heart disease. Heart. 2023;109(16):1241–1247. doi: 10.1136/heartjnl-2022-322192. [DOI] [PubMed] [Google Scholar]
- 76.Singh M.V., Dokun A.O. Diabetes mellitus in peripheral artery disease: beyond a risk factor. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.1148040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wisely C.E., Wang D., Henao R., et al. Convolutional neural network to identify symptomatic Alzheimer's disease using multimodal retinal imaging. Br J Ophthalmol. 2022;106(3):388–395. doi: 10.1136/bjophthalmol-2020-317659. [DOI] [PubMed] [Google Scholar]
- 78.Yip W., Sabanayagam C., Teo B.W., et al. Retinal microvascular abnormalities and risk of renal failure in Asian populations. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0118076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheung C.Y., Ran A.R., Wang S., et al. A deep learning model for detection of Alzheimer's disease based on retinal photographs: a retrospective, multicentre case-control study. Lancet Digit Health. 2022;4(11):e806–e815. doi: 10.1016/S2589-7500(22)00169-8. [DOI] [PubMed] [Google Scholar]
- 80.Lam C., Wong Y.L., Tang Z., et al. Performance of artificial intelligence in detecting diabetic macular edema from fundus photography and optical coherence tomography images: a systematic review and meta-analysis. Diabetes Care. 2024;47(2):304–319. doi: 10.2337/dc23-0993. [DOI] [PubMed] [Google Scholar]
- 81.Tomić M., Vrabec R., Hendelja Đ., Kolarić V., Bulum T., Rahelić D. Diagnostic accuracy of hand-held fundus camera and artificial intelligence in diabetic retinopathy screening. Biomedicines. 2023;12(1):34. doi: 10.3390/biomedicines12010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benhamza M., Dahlui M., Said M.A. Determining direct, indirect healthcare and social costs for diabetic retinopathy management: a systematic review. BMC Ophthalmol. 2024;24(1):424. doi: 10.1186/s12886-024-03665-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sathya Preiya V., Kumar V.D.A. Deep learning-based classification and feature extraction for predicting pathogenesis of foot ulcers in patients with diabetes. Diagnostics (Basel) 2023;13(12):1983. doi: 10.3390/diagnostics13121983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan T.E., Wong T.Y. Diabetic retinopathy: looking forward to 2030. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.1077669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghenciu L.A., Dima M., Stoicescu E.R., Iacob R., Boru C., Hațegan O.A. Retinal imaging-based oculomics: artificial intelligence as a tool in the diagnosis of cardiovascular and metabolic diseases. Biomedicines. 2024;12(9):2150. doi: 10.3390/biomedicines12092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patterson E.J., Bounds A.D., Wagner S.K., Kadri-Langford R., Taylor R., Daly D. Oculomics: a crusade against the four horsemen of chronic disease. Ophthalmol Ther. 2024;13(6):1427–1451. doi: 10.1007/s40123-024-00942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DeBuc D.C. AI for identification of systemic biomarkers from external eye photos: a promising field in the oculomics revolution. Lancet Digit Health. 2023;5(5):e249–e250. doi: 10.1016/S2589-7500(23)00047-X. [DOI] [PubMed] [Google Scholar]
- 88.Bellemo V., Lim Z.W., Lim G., et al. Artificial intelligence using deep learning to screen for referable and vision-threatening diabetic retinopathy in Africa: a clinical validation study. Lancet Digit Health. 2019;1(1):e35–e44. doi: 10.1016/S2589-7500(19)30004-4. [DOI] [PubMed] [Google Scholar]
- 89.Abràmoff M.D., Folk J.C., Han D.P., et al. Automated analysis of retinal images for detection of referable diabetic retinopathy. JAMA Ophthalmol. 2013;131(3):351–357. doi: 10.1001/jamaophthalmol.2013.1743. [DOI] [PubMed] [Google Scholar]
- 90.Santos A.R., Mendes L., Madeira M.H., et al. Microaneurysm turnover in mild non-proliferative diabetic retinopathy is associated with progression and development of vision-threatening complications: a 5-year longitudinal study. J Clin Med. 2021;10(10):2142. doi: 10.3390/jcm10102142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ipp E., Liljenquist D., Bode B., et al. Pivotal evaluation of an artificial intelligence system for autonomous detection of referrable and vision-threatening diabetic retinopathy. JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gulshan V., Peng L., Coram M., et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402–2410. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- 93.Bawankar P., Shanbhag N., K S.S., et al. Sensitivity and specificity of automated analysis of single-field non-mydriatic fundus photographs by Bosch DR Algorithm-Comparison with mydriatic fundus photography (ETDRS) for screening in undiagnosed diabetic retinopathy. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0189854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tan B., Chua J., Lin E., et al. Quantitative microvascular analysis with wide-field optical coherence tomography angiography in eyes with diabetic retinopathy. JAMA Netw Open. 2020;3(1) doi: 10.1001/jamanetworkopen.2019.19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Z., Huang W., Wang Z., et al. Evaluation of a self-imaging OCT for remote diagnosis and monitoring of retinal diseases. Br J Ophthalmol. 2023;108(8):1154–1160. doi: 10.1136/bjo-2023-324012. [DOI] [PubMed] [Google Scholar]
- 96.Maloca P., Hasler P.W., Barthelmes D., et al. Safety and feasibility of a novel sparse optical coherence tomography device for patient-delivered retina home monitoring. Transl Vis Sci Technol. 2018;7(4):8. doi: 10.1167/tvst.7.4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.