ABSTRACT
The skull is a very important structure, and it is the centre of many vital functions. There have been many studies on the skulls of mammals, but not many studies on the prenatal period. The aim of this study is to examine developmental sheep foetal skulls from the last two trimesters of pregnancy. A total of 40 sheep foetuses, 20 in the 2nd trimester (10 females and 10 males) and 20 in the 3rd trimester (10 females and 10 males), were examined. On the basis of CT scans of foetal skulls, morphometric measurements were performed by creating a three‐dimensional (3D) model. Total skull length was statistically significant between males and females in the third trimester (p < 0.01). In the second trimester, the tooth length parameter was statistically significant between males and females (p < 0.01). In the second trimester, M3 was found to be statistically significant in the sheep foetus mandible (p < 0.01). It was determined that there was developmental sexual dimorphism between males and females.
Keywords: craniometry, morphometry, sheep foetus, three‐dimensional (3D) modelling
The skull in foetal development has been little studied. In this study, it is planned to reveal the differences between groups, female and male, by analysing the sheep skull developmentally.
1. Introduction
The mammalian lineage evolved from reptilian ancestors approximately 178 million years ago (Kemp 2005). Divided into monotremes, marsupials and placentals, modern mammals encompass more than 6000 species that occupy a wide variety of ecological niches (Feldhamer 2007). Many evolutionary changes in living things, especially changes in the skull, have enabled living things to adapt to life (Higashiyama et al. 2021). The skull is a highly plastic region of the mammalian skeleton, housing the organs of special sense and facilitating many vital functions. The skull protects the brain, and special sensory organs (vision, smell, hearing, balance and taste) provide openings for air and food passage and house teeth in the jaws for chewing (Dyce et al. 2010). Morphological innovations and skull shape variability in mammals are reflections of changes in embryonic development (Green et al. 2015). Foetal development is an important research area of animal biology, and in this process, skull morphometry plays a critical role in understanding the evolutionary and developmental biology of organisms (Buss et al. 2012). Skull structure reflects many biological factors, such as brain growth, feeding habits and environmental adaptations (Succu et al. 2023). Developmental analyses of foetal skulls are performed using biometric measurements and advanced imaging techniques. These analyses reveal growth rates of different parts of the skull, changes in shape and differences between females and males (Szara et al. 2024). Mammalian skulls help us understand the relationship between foetal developmental patterns and brain development, and these data allow us to examine the effects of genetic and environmental factors on skull development (Koyabu 2023).
With the developing technology, three‐dimensional (3D) modelling techniques have begun to be used in the fields of industry and health (Demircioglu and Gezer Ince 2020). 3D modelling also contributes to studies used in clinical cases, complex pathological cases and anthropological and sexual dimorphism (Güzel et al. 2022; Demircioglu et al. 2021; Gündemir 2023; Gündemir et al. 2023).
This study aims to investigate the developmental characteristics of sheep foetal skulls in the last two periods of pregnancy and the differences between males and females by revealing sexual dimorphism comparatively.
2. Materials and Methods
2.1. Animals
In our study on skull and mandible measurements of 2nd‐ and 3rd‐trimester sheep, a total of 40 foetuses from the 2nd trimester (10 females and 10 males) and the 3rd trimester (10 females and 10 males) were used. Fetuses were collected from private slaughterhouses in the Southeastern Anatolia region. Foetuses are between 89 and 95 days in the second‐trimester group and 112 and 130 days in the third‐trimester group. In confirming the gestational days, a formulation appropriate to the literature was applied (İşbilir et al., 2024; Kandil et al., 2025). Computerized tomography of the collected foetuses was taken at Hayat Hospital in Siirt Province.
2.2. CT Imaging and 3D Model Generation
The skull and mandible of the 2nd‐ and 3rd‐trimester sheep were scanned with a multi‐slice Siemens computed tomography device with 64 detectors at 80 kV, 200 MA, 639 mGY and 0.625 mm section thickness. The resulting images were saved in DICOM format. Then, images in DICOM format were written and loaded with 3D‐Slicer 5.6.2 software. Skulls were segmented using the threshold segmentation module, with a threshold set at a minimum of 230.38 and a maximum of 5372.09. The resulting skull models were saved in STL format. Measurements were made on the resulting 3D models. Images of the head are shown in Figures 1 and 2, and images of the mandible are shown in Figure 3.
FIGURE 1.
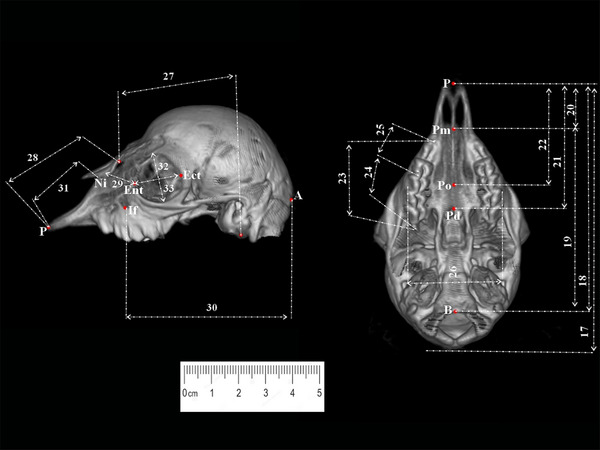
Lateral and ventral measurement points of the skull taken from sheep foetuses (third trimester). B, Basion; P, Prosthion; Pd, Postdentale; Ect, Ectorbitale; If, Infraorbitale; Ni, Nasointermaxillare; Pm, Premolare; Po, Palatinoorale.
FIGURE 2.

Dorsal and caudal measurement points of the head taken from sheep foetuses (third trimester). A, Akrokranion; B, Basion; O, Opisthion; Ot, Otion; Br, Bregma; Ect, Ectorbitale; Ent, Entorbitale; If, Infraorbitale; Rh, Rhinion; Sp, Supraorbitale.
FIGURE 3.

Measurement points of mandible taken from sheep foetuses (third trimester).
2.3. Statistical Analysis
The mean values, standard deviations, coefficient of variations and craniofacial indices were calculated with SPSS (version 22). Independent samples t‐test was used for p values. Statistical significance level was defined as p < 0.05.
2.4. Obtaining Craniometric Parameters
In the article, measurements taken from the skull and mandible were taken from similar articles (Güzel and İşbilir 2024; Gündemir et al. 2020; Güzel et al. 2023: İşbilir and Güzel 2023). Linear measurement points taken from the skull and mandible are given as follows:
Craniometric parameters:
-
C1.
The total skull length
-
C2.
The greatest breadth of the skull
-
C3.
Akrokranion‐bregma
-
C4.
Frontal length greatest length of nasals bone
-
C5.
Upper neurocranium length
-
C6.
Facial length (Sopraorbitale‐Prosthion)
-
C7.
Akrokranion‐infraorbitale of one side
-
C8.
The Greatest length of nasals bone
-
C9.
Short lateral facial length
-
C10.
Least breadth of the parietal bone
-
C11.
Greatest neurocranium breadth
-
C12.
Greatest breadth across the orbits
-
C13.
Least breadth between the orbits
-
C14.
Facial breadth
-
C15.
Greatest breadth across the nasals
-
C16.
Greatest breadth across the premaxillae
-
C17.
The condylobasal length—from incisive bone to the occipital condyles
-
C18.
Basal length (Basion‐Prosthion)
-
C19.
Short skull length (Basion‐Premolare)
-
C20.
Premolare‐prosthion
-
C21.
Dental length (Postdentale‐Prosthion)
-
C22.
Oral palatal length
-
C23.
Length of the cheek tooth row
-
C24.
Length of the molar row
-
C25.
Length of the premolar row
-
C26.
Greatest palatal breadth
-
C27.
Neurocranium length
-
C28.
Viscerocranium length
-
C29.
The greatest length of the lacrimal bone
-
C30.
From the aboral (Between the foramen infraorbital and the upper point of the foramen magnum)
-
C31.
The lateral length of the premaxilla
-
C32.
The greatest inner length of the orbit
-
C33.
The greatest inner height of the orbit
-
C34.
The greatest mastoid breadth of the paraoccipital processes
-
C35.
The greatest breadth of the occipital condyles
-
C36.
The greatest breadth at the breadth of the paraoccipital processes
-
C37.
The greatest breadth of the foramen magnum
-
C38.
Height of the foramen magnum (Basion‐Opisthion)
In this study, craniofacial indices were calculated as follows (Aslan Kanmaz et al. 2024; Dayan et al. 2023):
Skull index: Greatest breadth of the skull (2)/Total length (1)×100
Facial index 1: Facial breadth (14)/Viscerocranium length (28)×100
Facial index 2: Greatest breadth of the skull (2) /viscerocranium length (28)×100
Basal index: Greatest breadth of the skull (2)/basal length (18)×100
Palatal index: Greatest palatal breadth (26)/dental length (21)×100
Orbital index: Greatest inner height of the orbit (33)/greatest inner length of the orbit (32)×100
Foramen magnum index: Height of the foramen magnum (38)/greatest breadth of the foramen magnum (37)×100
Mandible parameters:
M1 (GOC‐ID): Length between GOC‐ID
M2 (PC‐ID): Length between the aboral edge of proc. condylar‐ID
M3 (GOC‐MTR): Length between GOC‐ aboral alveolar edge of M3
M4 (GOC‐FMN): Length between GOC—aboral edge of for. Mental
M5 (MTR‐MH): Height of mandible in the plane of posterior alveolar edge of M3
M6 (GOV‐CR): Length between GOC‐CR
M7 (SI): Mandible width at last incisive tooth level
M8 (BM): Width of the mandible at the level of the first molar
3. Results
In our study, craniometric measurements of the skulls of second‐ and third‐trimester ewes belonging to the last two periods of pregnancy were made, and cranial indices were calculated. In addition, mandible measurements were made, and the measurements were completed. The measurements of the skull and mandible are given in Tables 1, 2, 3, 4, 5, 6. In second‐ and third‐trimester sheep, C1 values of sheep were found to be larger in males than females. When the skull measurements of two‐trimester sheep are analysed in Table 1, it is seen that C1, C4, C5, C7, C15, C16, C29, C32, C36 and FMI measurement parameters are statistically highly significant (p < 0.001). C18, C25 and PI measurement parameters were found to be statistically significant (p < 0.05). When third‐trimester sheep skulls were analysed, C21, C26, C27, C29 and OI measurement parameters were found to be statistically highly significant (p < 0.01). C12, C16 and PI measurement parameters were statistically significant (p < 0.05). In the second‐ and third‐trimester mandibles, the C1 parameter was found to be larger in males than females. When the second‐trimester sheep mandible was analysed, it was found that the M3 parameter was highly significant (p < 0.01). When the third trimester was analysed, the M8 measurement parameter was statistically significant (p < 0.05).
TABLE 1.
Measurement parameters of sheep skulls from the second trimester.
| Gender | N | Mean | Std. Deviation | P | |
|---|---|---|---|---|---|
| 1 | Male | 10 | 68.93 | 0.59 | ** |
| Female | 10 | 67.06 | 0.25 | ||
| 2 | Male | 10 | 27.90 | 1.16 | NS |
| Female | 10 | 25.44 | 0.35 | ||
| 3 | Male | 10 | 6.24 | 0.41 | NS |
| Female | 10 | 4.87 | 0.19 | ||
| 4 | Male | 10 | 38.67 | 0.80 | ** |
| Female | 10 | 36.44 | 0.29 | ||
| 5 | Male | 10 | 31.46 | 0.64 | ** |
| Female | 10 | 30.10 | 0.08 | ||
| 6 | Male | 10 | 64.58 | 1.10 | NS |
| Female | 10 | 61.80 | 0.70 | ||
| 7 | Male | 10 | 37.88 | 0.72 | ** |
| Female | 10 | 36.12 | 0.10 | ||
| 8 | Male | 10 | 27.52 | 0.72 | NS |
| Female | 10 | 25.99 | 0.86 | ||
| 9 | Male | 10 | 31.64 | 0.78 | NS |
| Female | 10 | 30.40 | 0.47 | ||
| 10 | Male | 10 | 14.21 | 0.80 | NS |
| Female | 10 | 12.89 | 0.55 | ||
| 11 | Male | 10 | 23.93 | 0.69 | NS |
| Female | 10 | 22.43 | 0.26 | ||
| 12 | Male | 10 | 26.08 | 0.57 | NS |
| Female | 10 | 24.91 | 0.41 | ||
| 13 | Male | 10 | 25.30 | 0.30 | NS |
| Female | 10 | 24.59 | 0.25 | ||
| 14 | Male | 10 | 18.53 | 0.46 | NS |
| Female | 10 | 17.31 | 0.20 | ||
| 15 | Male | 10 | 12.35 | 0.14 | ** |
| Female | 10 | 11.48 | 0.37 | ||
| 16 | Male | 10 | 11.27 | 0.21 | ** |
| Female | 10 | 10.25 | 0.07 | ||
| 17 | Male | 10 | 67.30 | 0.39 | NS |
| Female | 10 | 66.52 | 0.21 | ||
| 18 | Male | 10 | 65.47 | 0.64 | * |
| Female | 10 | 64.15 | 0.12 | ||
| 19 | Male | 10 | 35.49 | 0.49 | NS |
| Female | 10 | 33.79 | 0.45 | ||
| 20 | Male | 10 | 12.62 | 0.26 | NS |
| Female | 10 | 11.13 | 0.44 | ||
| 21 | Male | 10 | 23.54 | 0.23 | NS |
| Female | 10 | 21.97 | 0.48 | ||
| 22 | Male | 10 | 19.58 | 0.19 | NS |
| Female | 10 | 17.72 | 0.52 | ||
| 23 | Male | 10 | 13.52 | 0.24 | NS |
| Female | 10 | 11.81 | 0.16 | ||
| 24 | Male | 10 | 9.27 | 0.35 | NS |
| Female | 10 | 8.63 | 0.52 | ||
| 25 | Male | 10 | 2.76 | 0.19 | * |
| Female | 10 | 2.15 | 0.06 | ||
| 26 | Male | 10 | 19.50 | 0.18 | NS |
| Female | 10 | 18.22 | 0.31 | ||
| 27 | Male | 10 | 36.55 | 0.28 | NS |
| Female | 10 | 35.18 | 0.25 | ||
| 28 | Male | 10 | 32.41 | 0.28 | NS |
| Female | 10 | 30.81 | 0.36 | ||
| 29 | Male | 10 | 13.48 | 0.21 | ** |
| Female | 10 | 13.76 | 0.94 | ||
| 30 | Male | 10 | 16.73 | 0.38 | NS |
| Female | 10 | 15.34 | 0.24 | ||
| 31 | Male | 10 | 20.53 | 0.69 | NS |
| Female | 10 | 19.21 | 1.99 | ||
| 32 | Male | 10 | 12.11 | 0.60 | ** |
| Female | 10 | 10.36 | 0.16 | ||
| 33 | Male | 10 | 12.52 | 0.21 | NS |
| Female | 10 | 11.57 | 0.19 | ||
| 34 | Male | 10 | 29.03 | 0.71 | NS |
| Female | 10 | 27.37 | 0.48 | ||
| 35 | Male | 10 | 22.09 | 0.83 | NS |
| Female | 10 | 19.67 | 1.10 | ||
| 36 | Male | 10 | 19.52 | 1.29 | ** |
| Female | 10 | 17.73 | 0.46 | ||
| 37 | Male | 10 | 8.68 | 1.06 | NS |
| Female | 10 | 7.40 | 0.89 | ||
| 38 | Male | 10 | 13.43 | 0.81 | NS |
| Female | 10 | 12.00 | 0.47 |
Abbreviation: NS, non‐significant.
p < 0.05.
p < 0.01.
TABLE 2.
Index of sheep skulls from the second trimester.
| Gender | N | Mean | Std. Deviation | p | |
|---|---|---|---|---|---|
| SI | Male | 10 | 40.47 | 1.53 | NS |
| Female | 10 | 37.94 | 0.59 | ||
| FI1 | Male | 10 | 57.16 | 1.40 | NS |
| Female | 10 | 56.19 | 0.66 | ||
| FI2 | Male | 10 | 86.07 | 3.50 | NS |
| Female | 10 | 82.58 | 1.25 | ||
| BI | Male | 10 | 42.61 | 1.42 | NS |
| Female | 10 | 39.66 | 0.48 | ||
| PI | Male | 10 | 82.83 | 0.63 | * |
| Female | 10 | 82.95 | 1.69 | ||
| OI | Male | 10 | 103.84 | 5.15 | NS |
| Female | 10 | 110.72 | 4.40 | ||
| FMI | Male | 10 | 65.10 | 11.21 | ** |
| Female | 10 | 62.34 | 8.76 |
Abbreviation: NS, non‐significant.
p < 0.05.
p < 0.01.
TABLE 3.
Measurement parameters of sheep skulls from the third trimester.
| Gender | N | Mean | Std. Deviation | p | |
|---|---|---|---|---|---|
| 1 | Male | 10 | 100.36 | 1.22 | NS |
| Female | 10 | 95.62 | 1.05 | ||
| 2 | Male | 10 | 46.61 | 0.95 | NS |
| Female | 10 | 43.59 | 0.83 | ||
| 3 | Male | 10 | 12.06 | 0.64 | NS |
| Female | 10 | 10.59 | 0.39 | ||
| 4 | Male | 10 | 67.37 | 1.08 | NS |
| Female | 10 | 62.65 | 1.15 | ||
| 5 | Male | 10 | 53.30 | 1.11 | NS |
| Female | 10 | 55.59 | 1.15 | ||
| 6 | Male | 10 | 95.23 | 1.05 | NS |
| Female | 10 | 92.23 | 0.86 | ||
| 7 | Male | 10 | 72.24 | 0.62 | NS |
| Female | 10 | 69.09 | 1.73 | ||
| 8 | Male | 10 | 51.74 | 0.94 | NS |
| Female | 10 | 47.72 | 0.48 | ||
| 9 | Male | 10 | 64.34 | 0.53 | NS |
| Female | 10 | 60.50 | 0.37 | ||
| 10 | Male | 10 | 26.42 | 0.59 | NS |
| Female | 10 | 23.38 | 0.740 | ||
| 11 | Male | 10 | 41.37 | 0.71 | NS |
| Female | 10 | 38.14 | 0.72 | ||
| 12 | Male | 10 | 52.87 | 0.68 | * |
| Female | 10 | 49.94 | 0.32 | ||
| 13 | Male | 10 | 44.33 | 0.87 | NS |
| Female | 10 | 41.59 | 0.58 | ||
| 14 | Male | 10 | 37.77 | 0.66 | NS |
| Female | 10 | 34.94 | 0.74 | ||
| 15 | Male | 10 | 25.30 | 1.38 | NS |
| Female | 10 | 23.64 | 1.18 | ||
| 16 | Male | 10 | 22.43 | 0.29 | * |
| Female | 10 | 20.28 | 0.87 | ||
| 17 | Male | 10 | 96.53 | 0.95 | NS |
| Female | 10 | 92.56 | 0.73 | ||
| 18 | Male | 10 | 85.60 | 0.71 | NS |
| Female | 10 | 81.05 | 0.63 | ||
| 19 | Male | 10 | 69.44 | 0.33 | NS |
| Female | 10 | 66.16 | 0.59 | ||
| 20 | Male | 10 | 17.55 | 0.78 | NS |
| Female | 10 | 14.83 | 0.56 | ||
| 21 | Male | 10 | 39.97 | 0.560 | ** |
| Female | 10 | 35.93 | 1.20 | ||
| 22 | Male | 10 | 35.78 | 0.43 | NS |
| Female | 10 | 32.26 | 0.54 | ||
| 23 | Male | 10 | 21.82 | 0.51 | NS |
| Female | 10 | 19.94 | 0.33 | ||
| 24 | Male | 10 | 18.68 | 0.74 | NS |
| Female | 10 | 15.75 | 0.69 | ||
| 25 | Male | 10 | 4.01 | 0.59 | NS |
| Female | 10 | 2.78 | 0.28 | ||
| 26 | Male | 10 | 35.54 | 0.36 | ** |
| Female | 10 | 32.63 | 0.81 | ||
| 27 | Male | 10 | 68.53 | 0.54 | ** |
| Female | 10 | 64.09 | 1.22 | ||
| 28 | Male | 10 | 61.36 | 0.63 | NS |
| Female | 10 | 57.83 | 0.93 | ||
| 29 | Male | 10 | 22.30 | 0.67 | ** |
| Female | 10 | 20.06 | 0.23 | ||
| 30 | Male | 10 | 31.96 | 0.79 | NS |
| Female | 10 | 28.67 | 0.66 | ||
| 31 | Male | 10 | 32.71 | 0.51 | NS |
| Female | 10 | 30.28 | 0.36 | ||
| 32 | Male | 10 | 24.78 | 0.70 | NS |
| Female | 10 | 21.27 | 0.61 | ||
| 33 | Male | 10 | 22.12 | 0.54 | NS |
| Female | 10 | 20.41 | 0.49 | ||
| 34 | Male | 10 | 49.95 | 0.84 | NS |
| Female | 10 | 45.73 | 0.48 | ||
| 35 | Male | 10 | 34.86 | 0.72 | NS |
| Female | 10 | 32.55 | 0.40 | ||
| 36 | Male | 10 | 32.03 | 0.53 | NS |
| Female | 10 | 29.58 | 0.54 | ||
| 37 | Male | 10 | 16.27 | 0.55 | NS |
| Female | 10 | 14.24 | 0.55 | ||
| 38 | Male | 10 | 18.70 | 0.49 | NS |
| Female | 10 | 16.45 | 0.39 |
Abbreviation: NS, non‐significant.
p < 0.05.
p < 0.01.
TABLE 4.
Index of sheep skulls from the third trimester.
| Gender | N | Mean | Std. Deviation | p | |
|---|---|---|---|---|---|
| SI | Male | 10 | 46.46 | 1.34 | NS |
| Female | 10 | 45.59 | 0.95 | ||
| FI1 | Male | 10 | 61.55 | 0.88 | NS |
| Female | 10 | 60.42 | 1.15 | ||
| FI2 | Male | 10 | 75.97 | 1.13 | NS |
| Female | 10 | 75.39 | 1.76 | ||
| BI | Male | 10 | 54.46 | 1.24 | NS |
| Female | 10 | 53.78 | 1.05 | ||
| PI | Male | 10 | 88.93 | 1.38 | * |
| Female | 10 | 90.90 | 3.10 | ||
| OI | Male | 10 | 89.27 | 2.07 | ** |
| Female | 10 | 96.05 | 4.42 | ||
| FMI | Male | 10 | 114.99 | 4.14 | NS |
| Female | 10 | 115.68 | 5.17 |
Abbreviation: NS, non‐significant.
p < 0.05.
p < 0.01.
TABLE 5.
Measurement point of sheep mandibles of the 2nd trimester.
| Gender | N | Mean | Std. Deviation | p | |
|---|---|---|---|---|---|
| M1 | Male | 10 | 5.5740 | 0.33374 | NS |
| Female | 10 | 4.6070 | 0.27653 | ||
| M2 | Male | 10 | 5.4890 | 0.24830 | NS |
| Female | 10 | 4.6310 | 0.21921 | ||
| M3 | Male | 10 | 4.4470 | 0.21899 | ** |
| Female | 10 | 3.8740 | 0.08003 | ||
| M4 | Male | 10 | 5.2930 | 0.11748 | NS |
| Female | 10 | 4.4310 | 0.25133 | ||
| M5 | Male | 10 | 1.4850 | 0.07706 | NS |
| Female | 10 | 1.0351 | 0.04045 | ||
| M6 | Male | 10 | 2.5680 | 0.13990 | NS |
| Female | 10 | 1.8400 | 0.11547 | ||
| M7 | Male | 10 | 1.1680 | 0.08574 | NS |
| Female | 10 | 1.0580 | 0.04211 | ||
| M8 | Male | 10 | 1.0830 | 0.03268 | NS |
| Female | 10 | 1.0012 | 0.03784 |
Abbreviation: NS, non‐significant.
p < 0.01.
TABLE 6.
Measurement point of sheep mandibles of the third trimester.
| Gender | N | Mean | Std. Deviation | p | |
|---|---|---|---|---|---|
| M1 | Male | 10 | 8.33 | 0.20 | NS |
| Female | 10 | 7.40 | 0.22 | ||
| M2 | Male | 10 | 7.56 | 0.18 | NS |
| Female | 10 | 6.96 | 0.12 | ||
| M3 | Male | 10 | 7.31 | 0.08 | NS |
| Female | 10 | 6.80 | 0.07 | ||
| M4 | Male | 10 | 6.31 | 0.14 | NS |
| Female | 10 | 5.69 | 0.22 | ||
| M5 | Male | 10 | 1.83 | 0.09 | NS |
| Female | 10 | 1.50 | 0.11 | ||
| M6 | Male | 10 | 3.77 | 0.08 | NS |
| Female | 10 | 3.25 | 0.07 | ||
| M7 | Male | 10 | 1.45 | 0.08 | NS |
| Female | 10 | 1.25 | 0.07 | ||
| M8 | Male | 10 | 1.32 | 0.064 | * |
| Female | 10 | 1.21 | 0.035 |
Abbreviation: NS, non‐significant.
p < 0.05.
4. Discussion
Bone remains recovered from zooarchaeological excavations from the past to the present can give information about past civilizations. To obtain this information, it is very important to know the osteological and osteometric characteristics of common sheep breeds in the world. Craniometric features are frequently used to determine the differences among species, breeds and even sexes. It is possible to come across craniometric studies in many sheep breeds. However, it is advantageous to have information about foetal development to understand the craniometric differences among sheep breeds. In the present study, it was aimed to determine the developmental differences between male and female foetuses in Hamdani crossbred sheep breed by examining the skull and mandible bones in the last two periods of pregnancy using the 3D modelling method. Due to the lack of studies in the foetal period, comparisons were made with different animal species and breeds.
The mean length of the skull was measured as 183.7 ± 6.5 mm and 200.8 ± 2.9 mm in Akkaraman and Kangal Akkaraman sheep, respectively (Baş et al. 2023). The skull length parameter was reported as 246.5 ± 21.6 mm in Barbados Black Belly sheep (Mohamed et al. 2016). In our study, the skull length parameter showed a statistically significant difference in 2nd‐trimester foetuses in males compared to females (p < 0.01). In third‐trimester foetuses, there was no such difference.
Neurocranium length was determined as 36.25 ± 0.28 mm and 35.18 ± 0.25 mm in male and female foetuses in the second trimester, respectively. In the third trimester, it was found to be 68.53 ± 0.54 mm and 64.09 ± 1.22 mm in the same order, and the neurocranium length was statistically greater in male foetuses than in female foetuses. Neurocranium length was reported as 110.82 ± 3.42 mm in adult Morkaraman sheep and 107.20 ± 3.69 mm in Tuj sheep (Özcan et al. 2010). In the Bardhoka sheep breed, the statistical difference in terms of neurocranium length in male and female animals was found to be compatible with the third‐trimester group of our study (Gündemir et al. 2020).
In many studies, craniofacial index parameters were determined in sheep breeds. These parameters were considered important in terms of understanding craniofacial deformities and examining brain development (Kanchan et al. 2014). The skull index value in adult Hamdani sheep was reported as 49.64 ± 0.62 in females and 49.04 ± 1.49 in males (Dayan et al. 2023). The Hamdani breed was found to have higher values than Hasak, Hashmer (Can et al. 2022) and Sharri (Jashari et al. 2022) sheep and lower values than Hemshin (Dalga et al. 2018), Mehraban (Karimi et al. 2011) and Romanov (Güzel and İşbilir 2024) sheep in terms of this parameter (Dayan et al. 2023). In the sheep breeds mentioned above, except for the Romanov sheep breed, skull index value did not show a statistical difference between male and female sheep, whereas the difference between sexes was reported in Romanov sheep (Güzel and İşbilir 2024). In our study, in accordance with the literature, skull index values did not show statistical differences between the sexes during pregnancy.
In our study, PI value showed a statistical difference between genders in both second‐ and third‐trimester foetuses. Female foetuses had a higher value than males. This study contrasts with the studies conducted on adult Hamdani sheep (Dayan et al. 2023) and Romanov sheep (Güzel and İşbilir 2024). No statistical difference was reported for PI parameters in adult Hamdani (Dayan et al. 2023) and Romanov (Güzel and İşbilir 2024) sheep.
C16, C29 and PI values were determined as the skull regions in which sexual dimorphism was prominent in both periods of foetal life. It was detected that many parameters differed in terms of sexual dimorphism in the early period. In addition, it was observed that the parameters showing sexual dimorphism decreased with foetal development in third‐trimester sheep skulls. Our findings suggest that sheep skulls show less sexual dimorphism during late foetal development compared to the early period of foetal life.
Considering the osteometric data of skull and mandible in humans and animals, it has been stated that males have statistically larger values than females for most parameters (Gezer Ince and Pazvant 2010; Onar et al. 1997; Pitakarnnop et al. 2017; Rooppakhun et al. 2010; Yılmaz and Demircioğlu, 2021). In the osteometric measurements performed in our study, a statistical difference was determined between sexes in the parameters M3 in second‐trimester foetuses and M8 in third‐trimester foetuses. Both values were higher in male animals following the literature. The M3 parameter was found to be 54.63 ± 0.91 mm in Hamdani rams and 50.79 ± 0.47 mm in sheep (Guzel, Demircioğlu and Gezer İnce 2023), and no statistical difference was reported between genders. In our study, the M3 parameter in second‐trimester foetuses was statistically higher in male foetuses than female foetuses following the Romanov sheep breed (İşbilir and Güzel 2023). In the Awassi sheep breed (Yılmaz 2020), this parameter was statistically higher in ewes than in rams.
In our study, the M8 parameter was determined as 1.08 ± 0.03 mm in male and 1.00 ± 0.03 mm in female foetuses in the second trimester and 1.32 ± 0.06 mm and 1.21 ± 0.35 mm in the third‐trimester foetuses, respectively. Second‐trimester foetuses did not have statistical differences between sexes as in Hamdani breed sheep (Güzel et al. 2023) and Awassi breed sheep (Yılmaz 2020). In the M8 parameter, no statistical difference was observed between genders in third‐trimester foetuses.
In conclusion, in this study, the osteometric properties of the skull and mandibular bones of the foetuses collected at different gestation periods in the Hamdani crossbred sheep breed were determined, and a developmental study was carried out in the foetal period. As reported in many studies, some osteometric parameters were found to be higher in males than females in the foetal period. According to the results of the study, it is thought that the use of craniometric parameters in sex discrimination in the foetal period will be more reliable than the morphometric parameters of the mandible due to the higher differences between sexes. The obtained data contain basic anatomical information that will be useful in taxonomic studies, diagnostic imaging, radiologically determined pathological disorders and treatment applications and evaluation of cranial facial and dental deformities. In addition, the data obtained can be used to determine whether sheep craniums found in zooarchaeological excavations belong to the foetal period and to differentiate the sexes. The parameters showing sexual dimorphism obtained from our study can be used in sex determination by measuring with ultrasonographic imaging in sheep foetuses. It will also contribute to future craniometric and osteometric studies in the foetal stages of mammals.
Author Contributions
Barış Can Güzel: project administration, conceptualization, investigation, original draft, writing–review and editing, methodology, validation, software, data curation. Fatma Işbilir: investigation, original draft, writing–review and editing.
Ethics Statement
With the ethics committee report numbered 2024/05/28, the Siirt University Experimental Animals Application and Research Center approved the procedures used in our investigation.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.70265.
Acknowledgements
We want to thank the Turkish Scientific and Technical Research Institute (TUBITAK) for providing funding support for the journal in the publication of our study.
Funding: This study was supported by Scientific and Technological Research Council of Turkey (TUBITAK).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Aslan Kanmaz, Y. , Güzel B. C., Baygeldi S. B., and Karan M.. 2024. “Three‐Dimensional Modeling and Morphometric Analysis of Skull of Badger (Meles meles) With Computed Tomography Images.” Veterinary Medicine and Science 10: e1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baş Ekici, H. , Beşoluk K., and Bozbıyık N.. 2023. “A Morphometric Comparison of the Skulls of Akkaraman and Kangal Akkaraman Sheep on a Three‐Dimensional Model Using Computed Tomography.” Veterinary Journal of Mehmet Akif Ersoy University 8, no. 1: 37–43. 10.24880/maeuvfd.1222154. [DOI] [Google Scholar]
- Buss, C. , Entringer S., and Wadhwa P. D.. 2012. “Fetal Programming of Brain Development: Intrauterine Stress and Susceptibility to Psychopathology.” Science Signaling 5, no. 245: pt7–pt7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can, M. , Özüdogru Z., and Ilgün R.. 2022. “A Morphometric Study on Skulls of Hasmer and Hasak Sheep Breeds.” International Journal of Morphology 40, no. 6: 1536–1545. [Google Scholar]
- Dalga, S. , Aslan K., and Akbulut Y.. 2018. “A Morphometric Study on the Skull of the Adult Hemshin Sheep.” Van Veterinary Journal 29: 125–129. [Google Scholar]
- Dayan, M. O. , Demircioğlu İ., Koçyiğit A., Güzel B. C., and Karaavci F. A.. 2023. “Morphometric Analysis of the Skull of Hamdani Sheep Via Three‐Dimensional Modelling.” Anatomia, Histologia, Embryologia 52, no. 2: 215–222. 10.1111/ahe.12873. [DOI] [PubMed] [Google Scholar]
- Demircioglu, I. , and Gezer Ince N.. 2020. “Three‐Dimensional Modelling of Computed Tomography Images of Limb Bones in Gazelles (Gazella subgutturosa).” Anatomia, Histologia, Embryologia 49, no. 6: 695–707. 10.1111/ahe.12564. [DOI] [PubMed] [Google Scholar]
- Demircioglu, I. , Yilmaz B., Gündemir O., and Dayan M. O.. 2021. “A Three‐Dimensional Pelvimetric Assessment on Pelvic Cavity of Gazelle (Gazella subgutturosa) by Computed Tomography.” Anatomia, Histologia, Embryologia 50, no. 1: 43–49. 10.1111/ahe.12597. [DOI] [PubMed] [Google Scholar]
- Dyce, K. M. , Sack W. O., and Wensing C. J. G.. 2010. Textbook of Veterinary Anatomy. 4th ed. Saunders/Elsevier. [Google Scholar]
- Feldhamer, G. A. 2007. Mammalogy: Adaptation, Diversity, Ecology. JHU Press. [Google Scholar]
- Flouri, D. , Darby J. R. T., Holman S. L., et al. 2022. “Placental MRI Predicts Fetal Oxygenation and Growth Rates in Sheep and Human Pregnancy.” Advanced Science (Weinheim, Baden‐Wurttemberg, Germany) 9, no. 30: e2203738. 10.1002/advs.202203738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gündemir, M. G. , Szara T., Spataru C., et al. 2023. “Shape Differences of the Carina Sterni in Birds of Various Locomotion Types.” Anatomia, Histologia, Embryologia 52, no. 2: 190–196. 10.1111/ahe.12870. [DOI] [PubMed] [Google Scholar]
- Gündemir, O. 2023. “Shape Analysis of Fossa Masseterica and Processus Coronoideus in Domestic Cats (Felis catus) and Domestic Dogs (Canis familiaris) .” Anatomia, Histologia, Embryologia 52, no. 6: 899–906. 10.1111/ahe.12947. [DOI] [PubMed] [Google Scholar]
- Gündemir, O. , Duro S., Jashari T., Kahvecioğlu O., Demircioğlu İ., and Mehmeti H.. 2020. “A Study on Morphology and Morphometric Parameters on Skull of the Bardhoka Autochthonous Sheep Breed in Kosovo.” Anatomia, Histologia, Embryologia 49, no. 3: 365–371. 10.1111/ahe.12538. [DOI] [PubMed] [Google Scholar]
- Güzel, B. C. , Demircioğlu İ., and Gezer İnce N.. 2023. “Three‐Dimensional Reconstruction and Morphometric Analysis of Mandible of Hamdani Sheep: A Computed Tomography (CT) Study.” Harran Üniversitesi Veteriner Fakültesi Dergisi 12, no. 1: 1–8. 10.31196/huvfd.1198191. [DOI] [Google Scholar]
- Güzel, B. C. , and İşbilir F.. 2024. “Morphometric Analysis of the Skulls of a Ram and Ewe Romanov Sheep (Ovis aries) With 3D Modelling.” Veterinary Medicine and Science 10, no. 2: e1396. 10.1002/vms3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güzel, B. C. , Koçyiğit A., Demircioğlu İ., and Demiraslan Y.. 2022. “Investigating Metacarpi of Hamdani Sheep Via Different Measurement and Modelling Methods: A Methodological Study.” Anatomia, Histologia, Embryologia 51, no. 4: 484–491. 10.1111/ahe.12816. [DOI] [PubMed] [Google Scholar]
- Gezer İnce, N. , and Pazvant G.. 2010. “Ratlarda (Wistar albino) Mandibula'nın Morfometrisi.” İstanbul Üniversitesi Veteriner Fakültesi Dergisi 36, no. 1: 51–56. 10.16988/iuvfd.01808. [DOI] [Google Scholar]
- Green, S. A. , Simoes‐Costa M., and Bronner M. E.. 2015. “Evolution of Vertebrates as Viewed From the Crest.” Nature 520, no. 7548: 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama, H. , Koyabu D., Hirasawa T., Werneburg I., Kuratani S., and Kurihara H.. 2021. “Mammalian Face as an Evolutionary Novelty.” Proceedings of the National Academy of Sciences 118, no. 44: e2111876118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland, J. J. , Smith G. W., Scheetz D., et al. 2011. “Does Size Matter in Females? An Overview of the Impact of the High Variation in the Ovarian Reserve on Ovarian Function and Fertility, Utility of Anti‐Müllerian Hormone as a Diagnostic Marker for Fertility and Causes of Variation in the Ovarian Reserve in Cattle.” Reproduction, Fertility, and Development 23, no. 1: 1–14. 10.1071/RD10226. [DOI] [PubMed] [Google Scholar]
- İşbilir, F. , and Güzel B. C.. 2023. “Morphometric Analysis of the Mandible of Ram and Ewe Romanov Sheep (Ovis aries) With 3D Modelling: A CT Study.” Anatomia, Histologia, Embryologia 52, no. 5: 742–751. 10.1111/ahe.12932. [DOI] [PubMed] [Google Scholar]
- İşbilir, F. , Kandil B., İşbilir İ., Koca D., and Güzel B. C.. 2024. “Evaluation of Placentome Morphology in the Last Two Periods of Pregnancy in Hair Goats (Capra aegagrus hircus).” Reproduction in Domestic Animals = Zuchthygiene 59, no. 10: e14731. 10.1111/rda.14731. [DOI] [PubMed] [Google Scholar]
- Jashari, T. , Duro S., Gündemir O., et al. 2022. “Morphology, Morphometry and Some Aspects of Clinical Anatomy in the Skull and Mandible of Sharri Sheep.” Biologia 77: 423–433. 10.1007/s11756-021-00955-y. [DOI] [Google Scholar]
- Kanchan, T. , Krishan K., Gupta A., and Acharya J.. 2014. “A Study of Cranial Variations Based on Craniometric Indices in a South Indian Population.” Journal of Craniofacial Surgery 25, no. 5: 1645–1649. 10.1097/SCS.0000000000001210. [DOI] [PubMed] [Google Scholar]
- Kandil, B. , Turgut A. O., Koca D., Isbilir F., Atli M. Z., and Guzel B. C.. 2025. “Comprehensive Evaluation of Changes in Placentomes in the Second and Third Trimesters of Pregnancy in Cross‐Bred Hamdani Sheep.” Veterinary Medicine and Science 11, no. 1: e70208. 10.1002/vms3.70208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi, I. , Onar V., Pazvant G., Hadipour M., and Mazaheri Y.. 2011. “The Cranial Morphometric and Morphologic Characteristics of Mehraban Sheep in Western Iran.” Global Veterinaria 6, no. 2: 111–117. [Google Scholar]
- Kemp, T. S. 2005. The Origin and Evolution of Mammals. Oxford University Press. [Google Scholar]
- Koyabu, D. 2023. “Evolution, Conservatism and Overlooked Homologies of the Mammalian Skull.” Philosophical Transactions of the Royal Society B 378, no. 1880: 20220081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen, I. C. , and Robinson J. S.. 2005. “Developmental Origins of the Metabolic Syndrome: Prediction, Plasticity, and Programming.” Physiological Reviews 85, no. 2: 571–633. 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- Mohamed, R. , Driscoll M., and Mootoo N.. 2016. “Clinical Anatomy of the Skull of the Barbados Black Belly Sheep in Trinidad.” International Journal of Current Research Medical Sciences 2, no. 8: 8–19. http://s‐o‐i.org/1.15/ijcrms‐2016‐2‐8‐2. [Google Scholar]
- Onar, V. , Kahvecioğlu O., Mutuş R., and Alpak H.. 1997. “Morphometric Analysis of the Mandible in German Shepherd Dogs.” Turkish Journal of Veterinary & Animal Sciences 23: 329–334. [Google Scholar]
- Özcan, S. , Aksoy G., Kürtül İ., Aslan K., and Özüdoğru Z.. 2010. “A Comparative Morphometric Study on the Skull of the Tuj and Morkaraman Sheep.” Kafkas Universitesi Veteriner Fakultesi Dergisi 16: 111–114. [Google Scholar]
- Pitakarnnop, T. , Buddhachat K., Euppayo T., Kriangwanich W., and Nganvongpanit K.. 2017. “Feline (Felis catus) Skull and Pelvic Morphology and Morphometry: Gender‐Related Difference?” Anatomia, Histologia, Embryologia 46, no. 3: 294–303. 10.1111/ahe.12269. [DOI] [PubMed] [Google Scholar]
- Rooppakhun, S. , Surasith P., Vatanapatimakul N., Kaewprom Y., and Sitthiseripratip K.. 2010. “Craniometric Study of Thai Skull Based on Three‐Dimensional Computed Tomography (CT) Data.” Journal of the Medical Association of Thailand = Chotmaihet Thangphaet 93, no. 1: 90–98. [PubMed] [Google Scholar]
- Succu, S. , Contu E., Bebbere D., et al. 2023. “Fetal Growth and Osteogenesis Dynamics During Early Development in the Ovine Species.” Animals: An Open Access Journal From MDPI 13, no. 5: 773. 10.3390/ani13050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szara, T. , Gündemir O., Günay E., Gün G., Avanus K., and Pazvant G.. 2024. “Sex Determination in Domestic Rock Pigeons (Columba livia) Using Radiographic Morphometry.” Acta Zoologica 105, no. 1: 38–45. [Google Scholar]
- Yılmaz, B. 2020. “İvesi Koyunlarında (Ovis aries) Mandibula'nın Morfometrik İncelemesi.” Harran Üniversitesi Veteriner Fakültesi Dergisi 9, no. 2: 189–193. 10.31196/huvfd.813490. [DOI] [Google Scholar]
- Yılmaz, O. , and Demircioğlu İ.. 2021. “Examination of the Morphometric Features and Three‐Dimensional Modelling of the Skull in Van Cats by Using Computed Tomographic Images.” Ankara Üniversitesi Veteriner Fakültesi Dergisi 68, no. 3: 213–222. 10.33988/auvfd.775971. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


