Abstract
Background
The atherogenic index of plasma (AIP) and systematic inflammation, as measured by high-sensitivity C-reactive protein (hsCRP), are predictors of diabetes, but their combined impacts on incident diabetes are poorly understood. Using a nationally representative cohort in China, we aimed to investigate the association of AIP and hsCRP with incident diabetes among middle-aged and elderly adults.
Methods
This cohort comprised 9,112 participants aged at least 45 years from 125 cities in the China Health and Retirement Longitudinal Study who were free of diabetes at baseline in 2011. Of these, 5,048 participants were followed up until 2015. The AIP was calculated as Log10[TG (mg/dL)/HDL-C(mg/dL)]. Multivariate logistic regression and linear mixed-effect (LME) models were performed to evaluate the associations of AIP, hsCRP, and incident diabetes as well as glycemic biomarkers. Receiver operating characteristic (ROC) curves were used to evaluate their diagnostic values. We conducted a mediation analysis to assess the direct and indirect associations between AIP and hsCRP with diabetes.
Results
489 (9.7%) cases developed diabetes during four years. Higher levels of AIP and hsCRP were independently associated with diabetes. Compared to the lowest quartile of AIP or hsCRP, the highest quartile of AIP (adjusted odds ratio, aOR 2.53, 95% CI: 1.90–3.38) and hsCRP (aOR 2.38, 1.79–3.16) was significantly associated with incident diabetes. The joint effects showed that participants with higher levels of AIP and hsCRP had significantly higher aOR of 2.76 (2.13–3.57). The LME models showed AIP and hsCRP were related to an increased level of fasting blood glucose and glycated hemoglobin. The combination of AIP and hsCRP has better predictive efficacy (area under the curve, AUC: 0.628, 0.601–0.654) for incident diabetes than alone. Mediation analyses showed that high AIP significantly mediated 25.4% of the association between hsCRP and diabetes, and hsCRP simultaneously mediated 5.7% of the association between AIP and diabetes.
Conclusions
This cohort suggests combined effects and mutual mediation between the AIP and hsCRP on incident diabetes in China. Our findings provide clinical implications for monitoring and managing AIP and hsCRP levels to mitigate the development of diabetes.
Graphic abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-025-02653-4.
Keywords: Atherogenic index of plasma, C-reactive protein, Diabetes, Inflammation, Middle-aged and older adults
Background
Diabetes mellitus (DM), an established risk factor for cardiovascular disease (CVD), is one of the most serious chronic diseases in the world [1]. According to the International Diabetes Federation (IDF), the number of diabetic patients has reached 537 million in 2021 [2, 3], resulting in US$966 billion in health expenditures globally [4]. With rapid aging, the prevalence of diabetes has kept rising, especially in low- and middle-income countries including China [5]. Therefore, identifying the risk factors is crucial for preventing and implementing tailored interventions to reduce the burden of diabetes.
Existing studies have suggested that individuals with insulin resistance (IR) such as diabetes and metabolic syndrome, usually have dyslipidemia [6]. In diabetic patients, lipid metabolism abnormalities primarily manifest as atherosclerotic dyslipidemia, characterized by increased triglyceride (TG) and low-density lipoprotein (LDL), and decreased high-density lipoprotein (HDL) levels [7–10]. Some clinical studies showed that small dense LDL (sdLDL) had the highly atherogenic potential compared to other lipoprotein cholesterols [11, 12]. These factors may contribute to IR and impaired insulin secretion [13]. In recent years, the atherogenic index of plasma (AIP)-a logarithmic transition of the ratio of TG to HDL-C, is a new, cost-effective lipid indicator that reflects atherogenic dyslipidemia with a higher sensitivity and specificity than traditional lipid biomarkers (i.e., TG and LDL) [10, 11]. The AIP was negatively related to the fractional esterification rate of HDL-C (FERHDL) and inversely proportional to the particle size of LDL. Evidence has suggested that AIP could be an effective substitute for sdLDL particle size [14, 15], which is related to the risk of CVD [16], prediabetes [17], and all-cause mortality [18]. A retrospective cohort among prediabetic patients in China showed that the AIP positively related to the progression of diabetes [19]. However, there is an absence of nationally population-representative studies exploring the association between AIP with incident diabetes among Chinese adults.
Moreover, systematic inflammation is often observed in diabetes incidence [20]. Growing evidence has indicated the use of high-sensitivity C-reactive protein (hsCRP) as a clinical measure of inflammation [21]. Existing studies have reported the association between hsCRP with the risk of diabetes [22–25], while the findings are not always consistent [25–27]. A meta-analysis of prospective studies showed that hsCRP was associated with a higher risk of diabetes [28]. Notably, atherogenic lipid changes and inflammation have been recognized as biologically entangled progress [29]. Atherosclerosis, driven by lipid-induced immune-inflammatory progress, increases the risk of diabetes [30]. Inflammation significantly induces atherosclerosis formation and progression, elevating the risk of dyslipidemia [31]. Existing studies have highlighted the need for combined assessment and management of chronic inflammation and atherogenic dyslipidemia in the primary prevention of CVD [32, 33]. However, research on their joint effects and exploration of incident diabetes among middle-aged and elderly populations remains scarce. This might hinder a more comprehensive understanding of how changes in AIP and hsCRP impact diabetes progression. In addition, no study has examined the mutual mediation relationship between AIP and hsCRP with incident diabetes. Understanding the mediation pathway could provide clinical insights into the mechanisms underlying diabetes development and conduct targeting intervention.
To fill this knowledge gap, we used a national cohort study to examine the independent and joint effects of the AIP and hsCRP with the onset of diabetes among middle-aged and elderly adults in China. Meanwhile, we performed a mediation analysis to underline the mediating effect linking the AIP and hsCRP levels to the development of diabetes.
Methods
Study design and population
This study leveraged the data from the China Health and Retirement Longitudinal Study (CHARLS), a nationally representative cohort of middle-aged and elderly adults in China. Detailed design and sampling methods have been reported elsewhere [34]. Briefly, the baseline survey of CHARLS was conducted among 17,708 respondents recruited from 28 provinces, and 450 counties/villages in 2011, with follow-up waves every 2 to 3 years. During each study wave, the socio-demographics, health and disease conditions and lifestyle behaviors were collected by trained staff.
In the current study, participants who underwent the first wave were included at baseline in 2011 and followed up until 2015. Those aged less than 45 years, with diabetes at baseline, individuals who used lipid-lowering medications, and those who have missing data on AIP, hsCRP, or related covariates were excluded. A total of 9,112 participants without diabetes were screened at baseline, of whom 5,048 completed the follow-up until 2015 were included in the analyses (Figure S1). The CHARLS was performed in accordance with the principles of the Declaration of Helsinki and has received ethical approval from the Ethics Review Committee of Peking University (Nu: IRB00001052–11015). All participants provided written informed consent before inclusion in the study.
Measurement of AIP and HsCRP
In the current study, venous blood samples of participants were collected at baseline and the end of follow-up in 2015 separately by medical staff from the Centre for Disease Control and Prevention (CDC) using the standard protocol and subsequently tested at the central laboratory [34]. The AIP was calculated as log [TG (mg/dL)/HDL(mg/dL)]. The fasting blood glucose (FBG), and lipid profiles including total cholesterol (TC), TG, HDL, and LDL were tested by standard methods, with a coefficient of variation ranging from 0.7 to 1.5% within assay and from 1.2 to 1.8% between assay separately (Table S1). The glycated hemoglobin (HbA1c) was measured using the Boronate affinity high-pressure liquid chromatography method. The concentration of hsCRP was tested by an immunoturbidimetric assay on a Hitachi 7180 chemistry analyzer (Hitachi, Tokyo, Japan).
Assessment of diabetes
The outcome of this study was incident diabetes. The diagnosis of diabetes was meeting any of the following items: self-reported doctor-diagnosed diabetes, and/or FBG ≥ 7.0 mmol/L, and/or random plasma glucose ≥ 11.1 mmol/L, and/or HbA1c ≥ 6.5% according to the recommendations of the American Diabetes Association [35, 36].
Covariates
A set of possible covariates including demographics, lifestyle behaviors, and chronic conditions were included in the current study. The demographic information included sex, age, height, weight, educational level, marital status (married or unmarried), and place of residence (urban or rural areas). Body mass index (BMI) is computed as weight (kg) divided height (m) in squares. Following the Working Group on Obesity in China (WGOC) guideline, overweight or obesity were defined by BMI ≥ 24 kg/m2 and ≥ 28 kg/m2 separately [37]. Moreover, lifestyle behaviors including cigarette smoke status (never, current, or former) and alcohol drinking (yes or no, specifying if they had ever consumed alcohol) [38] were recorded. In addition, we collected information about chronic diseases including hypertension, dyslipidemia, heart disease, and stroke. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg or self-reported diagnosis history of hypertension [39]. In line with previous studies [40, 41], dyslipidemia was defined as a TC/HDL ratio > 5.0 or self-reported doctor-diagnosed dyslipidemia in the CHARLS. Heart disease and stroke were assessed by using the standardized questions of “Have you been diagnosed with heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems by a doctor?” or “Have you been diagnosed with stroke by a doctor?”, respectively [42].
Statistical analyses
Continuous variables were described by mean ± standard deviation (SD) or median (interquartile range, IQR), and categorical variables were expressed as N (%), respectively. The baseline characteristics of the participants were illustrated by joint assessment of AIP (median value [0.35] as a cutoff point) and hsCRP levels (median value [1.4 mg/l] as a cutoff point) and compared among four groups: (group 1: AIP < 0.35 & hsCRP < 1.4 mg/L[reference], group 2: AIP < 0.35 & hsCRP ≥ 1.4 mg/L, group 3: AIP ≥ 0.35 & hsCRP < 1.4 mg/L, and group 4: AIP ≥ 0.35 & hsCRP ≥ 1.4 mg/L) using the Chi-square tests for categorical variables and One-way ANOVA or Kruskal-Wallis H test for continuous variables, respectively.
To determine the independent and joint effects of AIP and hsCRP on incident diabetes, multivariate logistic regression was conducted to calculate the odds ratio (OR) and 95% confidence interval (CI). In the current analysis, hsCRP was naturally logarithmic-transformed, and then each standard deviation (SD) increase in AIP (0.27) and ln- hsCRP (1.01 mg/l) were modeled as a continuous variable. Furthermore, we conducted quartile analyses to examine the trend of AIP and hsCRP with incident diabetes. Three models were developed as below: model 1: crude model; model 2 adjusted for age, sex, BMI, educational level, and marital status; and model 3 further adjusted for residence, smoking, alcohol drinking, hypertension, and LDL based on model 2. The additive and multiplicative effects of AIP and hsCRP on diabetes were assessed. Relative excess risk due to interaction (RERI), attributable proportion (AP), and synergy index (SI) with 95%CI were computed to evaluate the additive interaction using the approximate variance estimators shown in previous research [43]. Moreover, both the AIP and hsCRP as well as their product were included in the model to evaluate the multiplicative effects on diabetes. Restricted cubic spline (RCS) regression with three knots was used to assess the exposure-response relationship between AIP and hsCRP exposure with incident diabetes. The predictive efficacy of AIP, hsCRP, and their combination for diabetes entailed the computation of the area under the curve (AUC) via the receiver operating characteristic (ROC) curve. Similarly, the predictive values of AIP with individual lipid biomarkers of TG and HDL for diabetes were also assessed. In addition, we conducted a sensitivity analysis using the suggested clinical cutoffs of hsCRP (< 1, 1 to 3, ≥ 3 mg/L) for CVD risk [44] to examine the joint association of AIP and hsCRP with incident diabetes.
To identify the potential modifications of the association, subgroup analyses were performed according to the following variables: sex (male or female), age (≥ 60 years or < 60 years), overweight/obesity (Yes or no, defined by BMI ≥ 24.0 kg/m2), smoking status (current, never, or former), and alcohol drinking (yes or no), respectively. The modifications were tested by using the likelihood ratio test.
We performed mediation analyses to assess the direct and indirect associations between the AIP and incident diabetes through hsCRP, using the “Mediation” package in R software. The overall effects were decomposed into two sections: the average direct effect (ADE), and the average causal mediation effect (ACME). The mediation proportion was computed as ACME/(ACME + ADE), and its significance was evaluated using a bootstrap method involving 10,000 iterations [45]. Meanwhile, the mediating effect of hsCPR on incident diabetes through the AIP was similarly evaluated.
Linear mixed-effect (LME) models were conducted to explore the independent and joint effects of AIP and hsCRP on changes in FBG and HbA1c (2011, 2015) over a follow-up period separately, considering individual variability as a random effect. Moreover, we performed a sensitivity analysis by analyzing the association of AIP and hsCRP with diabetes by excluding those with heart disease and stroke at baseline (n = 569). Considering that the AIP was constructed from the traditional individual biomarkers, including causal-related TG, we also explored the mutual mediating roles of TG and hsCRP with diabetes.
All statistical analyses were performed using R software (version 4.2.3). P values < 0.05 (two-sided) were considered statistically significant.
Results
A total of 5,048 participants were finally included in this study. The average age was 58.9 (SD: 8.8) years, including 2,732 (51.6%) females. Table 1 shows the characteristics of the participants according to the joint groups of AIP and hsCRP. Overall, individuals with high AIP and hsCRP levels were more likely to be older, females, unmarried or widowed, live in urban areas, and be former smokers compared with those with lower AIP and hsCRP levels. Moreover, we observed a statistical significance for all health biomarkers among different groups (Table 1).
Table 1.
Characteristics of the participants according to the joint groups by AIP and hsCRP levels
| Characteristics | Total (n = 5048) |
Group 1 (n = 1549) |
Group 2 (n = 973) |
Group 3 (n = 899) |
Group 4 (n = 1627) |
P-value |
|---|---|---|---|---|---|---|
| Age (years) | 58.9 ± 8.8 | 58.9 ± 8.7 | 60.3 ± 9.3 | 57.7 ± 8.5 | 59.7 ± 8.8 | < 0.001 |
| Sex | ||||||
| Men | 2316 (45.9) | 758 (48.9) | 483 (49.6) | 368 (40.9) | 707 (43.5) | < 0.001 |
| Women | 2732 (54.1) | 791 (51.1) | 490 (50.4) | 531 (59.1) | 920 (56.5) | |
| BMI (kg/m2) | 23.56 ± 3.92 | 23.38 ± 4.12 | 23.67 ± 3.89 | 23.70 ± 3.83 | 23.60 ± 3.80 | 0.163 |
| Marital status | ||||||
| Married | 4481 (88.8) | 1389 (89.7) | 843 (86.6) | 805 (89.5) | 1444 (88.8) | < 0.001 |
| Unmarried or widow | 567 (11.2) | 160 (10.3) | 130 (13.4) | 94 (10.5) | 183 (11.2) | |
| Educational level | ||||||
| ≤ primary school | 3586 (71.0) | 1092 (70.5) | 737 (75.7) | 625 (69.5) | 1132 (69.6) | 0.007 |
| Middle school | 1005 (19.9) | 307 (19.8) | 176 (18.1) | 182 (20.2) | 340 (20.9) | |
| ≥ High school | 457 (9.1) | 150 (9.7) | 60 (6.2) | 92 (10.2) | 155 (9.5) | |
| Place of residence | ||||||
| Urban | 1647 (32.6) | 435 (28.1) | 278 (28.6) | 294 (32.7) | 640 (39.3) | < 0.001 |
| Rural | 3401 (67.4) | 1114 (71.9) | 695 (71.4) | 605 (67.3) | 987 (60.7) | |
| Smoke status | ||||||
| Current | 1547 (30.6) | 485 (31.3) | 326 (33.5) | 250 (27.8) | 486 (29.9) | < 0.001 |
| Previous | 412 (8.2) | 123 (7.9) | 81 (8.3) | 67 (7.5) | 141 (8.7) | |
| Never | 3089 (61.2) | 941 (60.7) | 566 (58.2) | 582 (64.7) | 1000 (61.5) | |
| Alcohol use | ||||||
| Have | 1962 (38.9) | 646 (41.7) | 417 (42.9) | 306 (34.0) | 593 (36.4) | < 0.001 |
| Have not | 3086 (61.1) | 903 (58.3) | 556 (57.1) | 593 (66.0) | 1034 (63.6) | |
| Hypertension (%) | 1948 (38.6) | 463 (29.9) | 379 (39.0) | 326 (36.3) | 780 (47.9) | < 0.001 |
| Dyslipidemia (%) | 418 (8.3) | 75 (4.8) | 66 (6.8) | 81 (9.0) | 196 (12.0) | < 0.001 |
| Heart disease (%) | 569 (11.3) | 147 (9.5) | 104 (10.7) | 92 (10.2) | 226 (13.9) | < 0.001 |
| Stroke (%) | 95 (1.9) | 24 (1.5) | 18 (1.8) | 16 (1.8) | 37 (2.3) | 0.505 |
| SBP (mmHg) | 129.4 ± 21.3 | 126.3 ± 20.6 | 129.5 ± 21.2 | 128.1 ± 20.6 | 132.8 ± 21.8 | < 0.001 |
| DBP (mmHg) | 75.5 ± 12.3 | 73.6 ± 11.9 | 74.8 ± 12.2 | 75.2 ± 12.0 | 78.0 ± 12.4 | < 0.001 |
| FBG (mmol/l) | 5.44 ± 1.22 | 5.22 ± 1.06 | 5.31 ± 1.12 | 5.39 ± 1.12 | 5.78 ± 1.39 | < 0.001 |
| HbA1c (%) | 5.82 ± 0.56 | 5.73 ± 0.43 | 5.79 ± 0.46 | 5.76 ± 0.53 | 5.94 ± 0.70 | < 0.001 |
| TC (mg/dl) | 184.4 ± 35.5 | 180.1 ± 31.8 | 181.9 ± 36.8 | 183.0 ± 33.4 | 191.3 ± 38.0 | < 0.001 |
| TG (mg/dl) | 138.5 ± 85.2 | 82.6 ± 22.1 | 86.0 ± 21.2 | 157.2 ± 47.5 | 213.0 ± 100.9 | < 0.001 |
| HDL (mg/dl) | 51.9 ± 11.9 | 58.6 ± 11.8 | 56.5 ± 12.1 | 47.1 ± 7.7 | 45.4 ± 8.4 | < 0.001 |
| LDL (mg/dl) | 102.9 ± 28.5 | 101.1 ± 26.0 | 104.2 ± 30.2 | 102.7 ± 27.2 | 104.0 ± 30.6 | 0.014 |
| AIP | 0.37 ± 0.27 | 0.15 ± 0.11 | 0.18 ± 0.13 | 0.51 ± 0.13 | 0.64 ± 0.20 | < 0.001 |
| hsCRP (mg/l) | 1.40 (0.80, 2.60) | 0.70 (0.40, 0.90) | 2.40 (1.70, 4.00) | 0.90 (0.60, 1.10) | 2.60 (1.90, 4.40) | < 0.001 |
Group 1: AIP < median (0.35) & hsCRP< median (1.4 mg/l); Group 2: AIP < median & hsCRP≥ median; Group 3: AIP ≥ median & hsCRP< median; Group 4: AIP ≥ median& hsCRP≥ median
The ROC curve analysis of the AIP, hsCRP, and their combination for diabetes prediction is shown in Fig. 1. The combination of them had a better predictive efficacy (AUC: 0.628, 95%CI: 0.601–0.654) for incident diabetes than AIP or hsCRP alone. In addition, compared with TG or HDL, the AUC of the AIP was larger (0.604, 95% CI: 0.578–0.631) in predicting diabetes risk (Figure S2).
Fig. 1.
Receiver operating characteristic curves for AIP, hsCRP, and their combination for incident diabetes
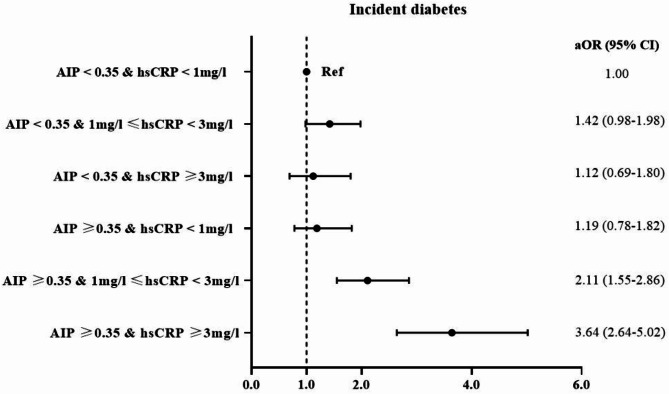
During a max follow-up of four years, 489 participants (9.7%) developed diabetes. Table 2 shows the independent effect of AIP and hsCRP on the incident risk of diabetes. After controlling for confounders, a significant association of each SD level increment in AIP and ln-hsCRP with diabetes was observed. Compared with the lowest quartile, the highest quartile of AIP and hsCRP were significantly related to incident diabetes, with aOR and 95% CI of 2.53 (95% CI: 1.90–3.38) for AIP and 2.38 (95% CI: 1.79–3.16) for hsCRP separately, and the trend test showed a statistical significance (P < 0.001). In addition, the RCS curve showed that AIP was linearly associated with incident diabetes (P nonlinear = 0.709). We observed a nonlinear relationship for hsCRP (P nonlinear < 0.001), with distinct inflection points evident (1.38 mg/l) (Figure S3). Table 3 shows the joint effects of AIP and hsCRP exposure on incident diabetes. Compared with group 1, the estimate of incident diabetes was highest in group 4 (aOR, 2.76, 95% CI: 2.13–3.57). The interaction analyses showed a significant additive effect of the AIP and hsCRP on diabetes (RERI: 0.93, 95% CI: 0.32–1.53, AP: 0.34, 95% CI: 0.13–0.55, SI:2.12, 95% CI: 1.02–4.38) while no multiplicative effects (Table S2-S3). Sensitivity analyses by reclassifying hsCRP levels into three subgroups, the estimates of diabetes were highest (aOR 3.64, 2.64–5.02) in the joint group of AIP ≥ 0.35 & hsCRP ≥ 3 mg/l, when compared to the individuals with AIP < 0.35 & hsCRP < 1 mg/l (Fig. 2).
Table 2.
The independent association of AIP, HsCRP and incident risk of diabetes among the participants
| Incident DM | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| AIP (continuous) # | 1.40 (1.28–1.54) | < 0.001 | 1.44 (1.31–1.57) | < 0.001 | 1.44 (1.30–1.58) | < 0.001 |
| Q1 | 1.00 (ref) | — | 1.00 (ref) | — | 1.00 (ref) | — |
| Q2 | 1.26 (0.92–1.72) | 0.147 | 1.28 (0.94–1.75) | 0.123 | 1.21 (0.89–1.67) | 0.228 |
| Q3 | 1.92 (1.43–2.56) | < 0.001 | 1.96 (1.46–2.63) | < 0.001 | 1.80 (1.34–2.42) | < 0.001 |
| Q4 | 2.57 (1.94–3.40) | < 0.001 | 2.69 (2.03–3.58) | < 0.001 | 2.53 (1.90–3.38) | < 0.001 |
| P for trend | — | < 0.001 | — | < 0.001 | — | < 0.001 |
| hsCRP (continuous) # | 1.44 (1.31–1.57) | < 0.001 | 1.41 (1.29–1.55) | < 0.001 | 1.38 (1.25–1.51) | < 0.001 |
| Q1 | 1.00 (ref) | — | 1.00 (ref) | — | 1.00 (ref) | — |
| Q2 | 1.17 (0.85–1.61) | 0.342 | 1.15 (0.83–1.59) | 0.401 | 1.09 (0.79–1.51) | 0.592 |
| Q3 | 1.87 (1.40–2.51) | < 0.001 | 1.84 (1.37–2.46) | < 0.001 | 1.69 (1.26–2.27) | < 0.001 |
| Q4 | 2.69 (2.03–3.56) | < 0.001 | 2.59 (1.95–3.43) | < 0.001 | 2.38 (1.79–3.16) | < 0.001 |
| P for trend | — | < 0.001 | — | < 0.001 | — | < 0.001 |
# the estimate was calculated for each SD increase in AIP and ln-hsCRP levels separately
Model 1: crude model
Model 2 adjusted for age, sex, BMI, educational level, and marital status
Model 3 further adjusted for place of residence, smoking status, alcohol use, hypertension, and LDL based on model 2
Table 3.
The joint association of AIP with HsCRP and incident risk of diabetes among the participants
| Incident DM |
Case/N | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Group 1 | 88 /1549 | 1.0 (ref) | — | 1.0 (ref) | — | 1.0 (ref) | — |
| Group 2 | 83/973 | 1.55 (1.14–2.11) | 0.006 | 1.48 (1.09–2.03) | 0.013 | 1.43 (1.04–1.95) | 0.027 |
| Group 3 | 71/899 | 1.42 (1.03–1.97) | 0.033 | 1.45 (1.05–2.01) | 0.025 | 1.42 (1.01–1.95) | 0.042 |
| Group 4 | 247/1627 | 2.97 (2.31–3.83) | < 0.001 | 2.96 (2.30–3.82) | < 0.001 | 2.76 (2.13–3.57) | < 0.001 |
Model 1: crude model
Model 2 adjusted for age, sex, BMI, educational level, and marital status
Model 3 further adjusted for place of residence, smoking status, alcohol use, hypertension, and LDL based on model 2
Fig. 2.
Sensitivity analyses of the joint association of AIP, hsCRP exposure and incident diabetes. Note: Model adjusted for age, sex, BMI, educational level, marital status, place of residence, smoking status, alcohol use, hypertension, and LDL
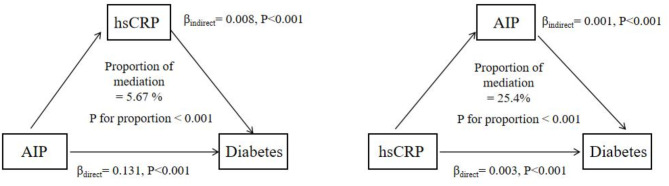
The mediated results showed that AIP mediated 25.4% of the association between hsCRP and incident diabetes, while hsCRP mediated 5.67% of the association between AIP and incident diabetes. Figure 3 shows the mutual mediating roles of AIP and hsCRP played in such associations with diabetes.
Fig. 3.
Mediate analysis of the association between AIP and hsCRP with incident diabetes. Note: model adjusted for age, sex, BMI, educational level, marital status, place of residence, smoking status, alcohol use, hypertension, and LDL
Table S4 presents the subgroup results of the joint associations of AIP and hsCRP with incident diabetes by sex, age, BMI, smoking status, and alcohol consumption, respectively. However, no significant modifications (P interaction > 0.05) were found for these variables.
In the sensitivity analysis by using FBG and HbA1c as outcomes, the LME model showed that higher quartile levels of the AIP and hsCRP were positively related to FBG and HbA1c (ref: lowest quartile) (Table S5). The adjusted β and 95% CIS in the highest quartile of AIP group were 0.312 (0.257 to 0.367) for FBG and 0.073 (0.046 to 0.099) for HbA1c, respectively. Moreover, the highest quartile levels of hsCRP were positively associated with FBG (β: 0.219, 0.165 to 0.273) and HbA1c (β: 0.071, 0.044 to 0.097) (ref: lowest quartile). The joint effects showed that the estimates for FBG and HbA1c were 0.253 (0.205 to 0.302) and 0.078 (0.054 to 0.102) in group 4, respectively (Table S5). After excluding participants with heart disease and stroke at baseline, we found very similar results of the association (Table S6). In the sensitivity analyses, we found significant mutual mediation effects of TG, hsCRP, and diabetes (Figure S4).
Discussion
In this nationwide cohort study, individual high AIP, hsCRP levels, and combined of them were associated with an increased risk of diabetes among the middle-aged and older Chinese population. Our findings indicated a significant additive interaction between AIP and hsCRP related to diabetes onset. We observed a better predictive efficacy of their combination for incident diabetes than AIP or hsCRP alone. Importantly, the current research was the first to contribute to revealing a mutual mediation relationship between the AIP and hsCRP in terms of incident diabetes. Sensitivity analysis showed robust associations between AIP and hsCRP exposure with elevated levels of FBG and HbA1c.
Dual changes in dyslipidemia and inflammation have been demonstrated to predate the development of diabetes, and both are intertwined biological processes [29, 46]. According to previous studies [47–49], AIP has been constructed as a novel marker of plasma atherosclerosis and exhibits a profound relationship with the atherosclerotic burden and cardiovascular risk. In recent years, a growing number of studies have assessed the relationship between individual AIP or hsCRP levels with diabetes [6, 27, 50–52]. However, most of them used cross-sectional design [6, 50], with a small sample size [51, 52], or conducted in western settings [22, 25, 53], and the findings are still controversial [25–27]. In the current cohort, we are the first to demonstrate a mutual mediation relationship between AIP and hsCRP with diabetes among middle-aged and elderly adults in China. Moreover, we evaluated the traditional individual biomarkers of TG in the sensitivity analyses, and a significant mutual mediation effect was observed. Consistent with previous studies [54, 55], we found a higher predictive value of AIP in the progress of diabetes than TG and HDL alone. The AIP is proposed as a predictor for IR, relating to hyperglycemia, diabetes, and CVD risk [47, 56]. In the current study, the combined effects of AIP and hsCRP showed a higher estimate of diabetes risk than alone, in line with previous research [32]. In addition, we added to find a non-linear relationship between hsCRP with diabetes. This suggested that moderate inflammation levels might play a pivotal role in the early stages of diabetes development. Despite this, future investigations are needed to confirm our findings.
In terms of the mutual association, our findings indicated a significant mediating effect of the AIP on incident diabetes partially via hsCRP, and vice versa. The exact mechanisms remain unclear. It is suggested that atherogenesis and chronic inflammation may interact through oxidative stress and endothelial dysfunction to jointly promote the development of diabetes [29]. Evidence has indicated that inflammation largely mediates lipid metabolism, influencing the constitution of lipid profiles and exacerbating IR [57, 58]. In turn, atherogenic dyslipidemia complex, mostly from obesity, could elevate a low-grade inflammatory condition via the lipotoxic effects [46]. As an indicator of abdominal obesity, the increase of visceral adipose tissue (VAT) is related to IR and diabetes [59]. Moreover, dysfunction in VAT leads to increased release of free fatty acids (FFA) [60, 61], inducing the production of reactive oxygen species (ROS) and activating stress-sensitive factors such as nuclear factor-kappaB (NF-κappaB) [62, 63]. This triggers inflammatory cascades and exacerbates IR [64, 65]. The vicious cycle between FFA and ROS further impairs insulin signaling and damages pancreatic β-cell function through lipotoxicity [66, 67]. AIP is constructed from TG and HDL, it is noted that reduced HDL levels are related to low insulin sensitivity and secretion, which in turn impairs β-cell function and leads to IR [68]. Moreover, elevated AIP levels are closely related to endothelial dysfunction, characterized by reduced nitric oxide (NO) bioavailability and increased vascular inflammation, which could impair glucose metabolism [69]. In addition, hsCRP can exacerbate these effects by suppressing endothelial NO synthase (eNOS) expression [70], promoting lipid particle leakage, and the uptake of oxidized LDL (ox-LDL) [71]. This in turn aggravates endothelial damage and systemic inflammation [63]. Through these potential mechanisms, dyslipidemia and inflammation amplify each other, driving the onset and development of diabetes.
Our study has some notable strengths. First, this was one of the few studies to explore the independent and joint effects of the AIP and hsCRP with incident diabetes among middle-aged and elderly Chinese adults. The findings showed a significant additive interaction between AIP and hsCRP on diabetes, and a better predictive efficacy of the combination of them for incident diabetes, highlighting the incorporating of AIP and inflammatory burden into diabetes risk management and helping provide strategies for disease prevention. Moreover, a non-linear association with an inflection point of 1.38 mg/l was detected between hsCRP with diabetes, providing important implications for clinical practice to reduce diabetes risk. Second, our study was the first to reveal the mutual mediation roles of the AIP, hsCRP in the relationship with incident diabetes. These findings contributed to a better understanding of dyslipidemia and inflammation mechanisms underlying the development of diabetes. Third, a set of sensitivity analyses were conducted to confirm the robustness of these associations.
Several limitations should also be acknowledged. First, the observational design limits causal inference about the AIP, hsCRP, and incident diabetes. However, both AIP and hsCRP have been validated widely as predictors of diabetes [72, 73]. Second, this study was conducted only among the Chinese middle-aged and elderly population, which might limit its applicability to other populations. Third, we only assessed the AIP and hsCRP at baseline, the longitudinal changes in them during the follow-up period need to be explored in future studies. Considering that there is a lack of a clear threshold for AIP and hsCRP levels, potential misclassification could exist. The dose-response relationship analysis showed an inflection point (1.38 mg/l) for hsCRP, close to the median level of hsCRP in our study. Moreover, the sensitivity analysis by reclassifying hsCRP using suggested clinical cutoffs (< 1, 1 to 3, ≥ 3 mg/L) for CVD risk further confirmed the robustness of the results. Fourth, the definition of diabetes in our study did not include oral glucose tolerance testing due to an absence of data, which might underestimate diabetes incidence. Fifth, the unavailability of precise dates regarding diabetes onset in the CHARLS limited our ability to control for the duration preceding the incidence of diabetes in the regression. Finally, the residual and unobserved confounding factors such as genes and diets could not be considered in this study owing to unavailable data.
Conclusions
In summary, using a prospective, national cohort in China, we found a better predictive value of the combination of AIP and hsCRP for incident diabetes than alone. The AIP significantly mediated the association of chronic inflammation with incident diabetes, and vice versa. The findings highlight that decreasing AIP and keeping hsCRP below 1.38 mg/l may be promising for the prevention and treatment of diabetes. Our cohort provides important implications that incorporating AIP and inflammatory burden into clinical and therapeutic practice may refine diabetes risk assessment and evaluate potentiating dual-target benefits that warrant further attention.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all the participants in the CHARLS project team for their great efforts.
Abbreviations
- AIP
Atherogenic index of plasma
- hsCRP
High sensitivity C-reactive protein
- CHARLS
China Health and Retirement Longitudinal Study
- TG
Triglyceride
- HDL
High-density lipoprotein cholesterol
- TC
Total cholesterol
- HDL
Low-density lipoprotein cholesterol
- FBG
Fasting blood glucose
- IR
Insulin resistance
Author contributions
WS: Conceptualization; Investigation; Methodology; Writing-review & editing; Supervision. WT, ZM, and WS: Investigation; Methodology; Software; Writing-original draft. LY: Investigation; Writing-review & editing; ZT: Investigation; Writing-review & editing; All authors read and approved the final manuscript.
Funding
This study was supported by the National Nature Science Foundation of China (No. 72204048).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Review Committee of Peking University (IRB00001052-11015). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Data statements
This study used public-accessed data which can be applied from the website http://charls.pku.edu.cn/en/.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tongshuai Wang, Mengru Zhang and Wenxing Shi have contributed equally to this article.
References
- 1.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. [DOI] [PubMed] [Google Scholar]
- 2.Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183: 109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Peng W, Zhao Z, et al. Prevalence and treatment of diabetes in China, 2013–2018. JAMA. 2021;326(24):2498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–34. [DOI] [PMC free article] [PubMed]
- 5.Zhou B, Lu Y, Hajifathalian K, et al. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin B, Wu Z, Xia Y, et al. Non-linear association of atherogenic index of plasma with insulin resistance and type 2 diabetes: A cross-sectional study. Cardiovasc Diabetol. 2023;22(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stahel P, Xiao C, Hegele RA, Lewis GF. The atherogenic dyslipidemia complex and novel approaches to cardiovascular disease prevention in diabetes. Can J Cardiol. 2018;34(5):595–604. [DOI] [PubMed] [Google Scholar]
- 8.Parhofer KG. Interaction between glucose and lipid metabolism: more than diabetic dyslipidemia. Diabetes Metab J. 2015;39(5):353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism. 2014;63(12):1469–79. [DOI] [PubMed] [Google Scholar]
- 10.NIH Consensus conference. Triglyceride, high-density lipoprotein, and coronary heart disease. NIH Consensus Development Panel on Triglyceride, High-Density Lipoprotein, and Coronary Heart Disease. JAMA. 1993;269(4):505–10. [PubMed]
- 11.Arai H, Kokubo Y, Watanabe M, et al. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb. 2013;20(2):195–203. [DOI] [PubMed] [Google Scholar]
- 12.Hirayama S, Miida T. Small dense LDL: an emerging risk factor for cardiovascular disease. Clin Chim Acta. 2012;414:215–24. [DOI] [PubMed] [Google Scholar]
- 13.Rachek LI. Free fatty acids and skeletal muscle insulin resistance. Prog Mol Biol Transl Sci. 2014;121:267–92. [DOI] [PubMed] [Google Scholar]
- 14.Chen M, Fang CY, Guo JC, et al. Predictive value of atherogenic index of plasma and atherogenic index of plasma combined with low-density lipoprotein cholesterol for the risk of acute myocardial infarction. Front Cardiovasc Med. 2023;10:1117362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Placzkowska S, Solkiewicz K, Bednarz-Misa I, Kratz EM. Atherogenic plasma index or non-high-density lipoproteins as markers best reflecting age-related high concentrations of small dense low-density lipoproteins. Int J Mol Sci. 2022;23(9):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Zhang L, Wang L, et al. The predictive value of cumulative atherogenic index of plasma (AIP) for cardiovascular outcomes: a prospective community-based cohort study. Cardiovasc Diabetol. 2024;23(1):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou Y, Lu S, Li D, et al. Exposure of cumulative atherogenic index of plasma and the development of prediabetes in middle-aged and elderly individuals: evidence from the CHARLS cohort study. Cardiovasc Diabetol. 2024;23(1):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You FF, Gao J, Gao YN, et al. Association between atherogenic index of plasma and all-cause mortality and specific-mortality: a nationwide population-based cohort study. Cardiovasc Diabetol. 2024;23(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, Kuang M, Yang R, et al. Evaluation of the role of atherogenic index of plasma in the reversion from Prediabetes to normoglycemia or progression to Diabetes: a multi-center retrospective cohort study. Cardiovasc Diabetol. 2024;23(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laaksonen DE, Niskanen L, Nyyssonen K, et al. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia. 2004;47(8):1403–10. [DOI] [PubMed] [Google Scholar]
- 21.Lawler PR, Bhatt DL, Godoy LC, et al. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J. 2021;42(1):113–31. [DOI] [PubMed] [Google Scholar]
- 22.Tong KI, Hopstock LA, Cook S. Association of C-reactive protein with future development of diabetes: a population-based 7-year cohort study among Norwegian adults aged 30 and older in the Tromso Study 2007–2016. BMJ Open. 2023;13(9): e070284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peper KM, Guo B, Leann LD, et al. C-reactive protein and racial differences in type 2 diabetes incidence: the REGARDS study. J Clin Endocrinol Metab. 2022;107(6):e2523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertoni AG, Burke GL, Owusu JA, et al. Inflammation and the incidence of type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2010;33(4):804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–34. [DOI] [PubMed] [Google Scholar]
- 26.Julia C, Czernichow S, Charnaux N, et al. Relationships between adipokines, biomarkers of endothelial function and inflammation and risk of type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):231–8. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Yatsuya H, Tamakoshi K, et al. Positive association between high-sensitivity C-reactive protein and incidence of type 2 diabetes mellitus in Japanese workers: 6-year follow-up. Diabetes Metab Res Rev. 2013;29(5):398–405. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Tao S, Peng J, et al. High-sensitivity C-reactive protein and risk of type 2 diabetes: a nationwide cohort study and updated meta-analysis. Diabetes Metab Res Rev. 2021;37(8): e3446. [DOI] [PubMed] [Google Scholar]
- 29.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15(5):635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barkas F, Elisaf M, Liberopoulos E, et al. Atherogenic dyslipidemia increases the risk of incident diabetes in statin-treated patients with impaired fasting glucose or obesity. J Cardiol. 2019;74(3):290–5. [DOI] [PubMed] [Google Scholar]
- 31.Zhan Y, Xu T, Tan X. Two parameters reflect lipid-driven inflammatory state in acute coronary syndrome: atherogenic index of plasma, neutrophil-lymphocyte ratio. BMC Cardiovasc Disord. 2016;16:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan Y, Chen G, Wu D, et al. Temporal relationship between atherogenic dyslipidemia and inflammation and their joint cumulative effect on type 2 diabetes onset: a longitudinal cohort study. BMC Med. 2023;21(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridker PM, Bhatt DL, Pradhan AD, et al. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. 2023;401(10384):1293–301. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nuha AE, Rozalina GM, Grazia A, et al. 2. Diagnosis and classification of diabetes: standards of care in diabetes-2025. Diabetes Care. 2025;48(Supplement_1):S27–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boris D, Vanita RA, George B, et al. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl1):S17–38. [DOI] [PubMed] [Google Scholar]
- 37.Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1–36. [PubMed] [Google Scholar]
- 38.Shi W, Li Y, Huang Y, et al. Association of indoor solid fuel pollution with risk factors for cardiovascular disease among Chinese adults: a nationally multi-center study. Build Environ. 2024;256: 111513. [Google Scholar]
- 39.Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh report of the Joint National Committee on the prevention, detection, evaluation, and treatment of high blood pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41(6):1178–9. [DOI] [PubMed] [Google Scholar]
- 40.Zhao M, Cheng Y, Li M, et al. Temporal changes in lipid concentrations and the prevalence of dyslipidemia among individuals with diabetes, prediabetes, and normal blood glucose from 2011 to 2015. Lipids Health Dis. 2024;23(1):394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allison MA, Budoff MJ, Wong ND, et al. Prevalence of and risk factors for subclinical cardiovascular disease in selected US Hispanic ethnic groups: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167(8):962–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui C, Liu L, Li H, et al. Childhood exposure to interparental physical violence and adult cardiovascular disease. JAMA Netw Open. 2024;7(12): e2451806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–6. [DOI] [PubMed] [Google Scholar]
- 44.Ridker PM. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol. 2016;67(6):712–23. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z, Zheng C, Kim C, et al. Causal mediation analysis in the context of clinical research. Ann Transl Med. 2016;4(21):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qu L, Fang S, Lan Z, et al. Association between atherogenic index of plasma and new-onset stroke in individuals with different glucose metabolism status: insights from CHARLS. Cardiovasc Diabetol. 2024;23(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng X, Zhang X, Han Y, Hu H, Cao C. Nonlinear relationship between atherogenic index of plasma and the risk of prediabetes: a retrospective study based on Chinese adults. Cardiovasc Diabetol. 2023;22(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Won KB, Heo R, Park HB, et al. Atherogenic index of plasma and the risk of rapid progression of coronary atherosclerosis beyond traditional risk factors. Atherosclerosis. 2021;324:46–51. [DOI] [PubMed] [Google Scholar]
- 50.Sun Y, Li F, Zhou Y, et al. Nonlinear association between atherogenic index of plasma and type 2 diabetes mellitus in overweight and obesity patients: evidence from Chinese medical examination data. Cardiovasc Diabetol. 2024;23(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang YL, Koh WP, Yuan JM, Pan A. Plasma ferritin, C-reactive protein, and risk of incident type 2 diabetes in Singapore Chinese men and women. Diabetes Res Clin Pract. 2017;128:109–18. [DOI] [PubMed] [Google Scholar]
- 52.Belfki H, Ben AS, Bougatef S, et al. Association between C-reactive protein and type 2 diabetes in a Tunisian population. Inflammation. 2012;35(2):684–9. [DOI] [PubMed] [Google Scholar]
- 53.Effoe VS, Correa A, Chen H, Lacy ME, Bertoni AG. High-sensitivity C-reactive protein is associated with incident type 2 diabetes among African Americans: the Jackson Heart Study. Diabetes Care. 2015;38(9):1694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu XW, Deng FY, Lei SF. Meta-analysis of atherogenic index of plasma and other lipid parameters in relation to risk of type 2 diabetes mellitus. Prim Care Diabetes. 2015;9(1):60–7. [DOI] [PubMed] [Google Scholar]
- 55.Onat A, Can G, Kaya H, Hergenc G. “Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. 2010;4(2):89–98. [DOI] [PubMed] [Google Scholar]
- 56.Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, et al. Atherogenic Index of Plasma (AIP): a marker of cardiovascular disease. Med J Islam Repub Iran. 2015;29:240. [PMC free article] [PubMed] [Google Scholar]
- 57.Chen S, Shimada K, Crother TR, et al. Chlamydia and lipids engage a common signaling pathway that promotes atherogenesis. J Am Coll Cardiol. 2018;71(14):1553–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGillicuddy FC, de la Llera MM, Hinkle CC, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119(8):1135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez-Dominguez P, Gomez-Aviles P, Bautista-Garcia K, et al. Visceral adipose tissue mediates the relationship between left ventricular global longitudinal strain and insulin resistance among adults living with type 2 diabetes. Cardiovasc Diabetol. 2025;24(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ben-Yehuda O. High-sensitivity C-reactive protein in every chart? The use of biomarkers in individual patients. J Am Coll Cardiol. 2007;49(21):2139–41. [DOI] [PubMed] [Google Scholar]
- 61.Memon RA, Grunfeld C, Moser AH, Feingold KR. Tumor necrosis factor mediates the effects of endotoxin on cholesterol and triglyceride metabolism in mice. Endocrinology. 1993;132(5):2246–53. [DOI] [PubMed] [Google Scholar]
- 62.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–8. [DOI] [PubMed] [Google Scholar]
- 63.Kobayashi S, Inoue N, Ohashi Y, et al. Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of C-reactive protein. Arterioscler Thromb Vasc Biol. 2003;23(8):1398–404. [DOI] [PubMed] [Google Scholar]
- 64.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50(5):567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pillarisetti S, Saxena U. Role of oxidative stress and inflammation in the origin of Type 2 diabetes–a paradigm shift. Expert Opin Ther Targets. 2004;8(5):401–8. [DOI] [PubMed] [Google Scholar]
- 66.Chan PC, Wang YC, Chen YL, et al. Importance of NADPH oxidase-mediated redox signaling in the detrimental effect of CRP on pancreatic insulin secretion. Free Radic Biol Med. 2017;112:200–11. [DOI] [PubMed] [Google Scholar]
- 67.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li N, Fu J, Koonen DP, et al. Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atherosclerosis. 2014;233(1):130–8. [DOI] [PubMed] [Google Scholar]
- 69.Herrera MD, Mingorance C, Rodriguez-Rodriguez R, Alvarez DSM. Endothelial dysfunction and aging: an update. Ageing Res Rev. 2010;9(2):142–52. [DOI] [PubMed] [Google Scholar]
- 70.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165–8. [DOI] [PubMed] [Google Scholar]
- 71.Clapp BR, Hirschfield GM, Storry C, et al. Inflammation and endothelial function: direct vascular effects of human C-reactive protein on nitric oxide bioavailability. Circulation. 2005;111(12):1530–6. [DOI] [PubMed] [Google Scholar]
- 72.Li YW, Kao TW, Chang PK, Chen WL, Wu LW. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: a 9-year longitudinal study. Sci Rep. 2021;11(1):9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doi Y, Kiyohara Y, Kubo M, et al. Elevated C-reactive protein is a predictor of the development of diabetes in a general Japanese population: the Hisayama Study. Diabetes Care. 2005;28(10):2497–500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.