Abstract
The gut microbiota of insects plays a pivotal role in digesting food, supplying nutrients, and synthesizing enzymes, particularly those capable of degrading lignocellulosic biomass—a key factor in waste management. In Karnataka, India, the larvae of Holotrichia serrata and Leucopholis coneophora are major crop pests. However, the potential of their gut bacterial communities to degrade lignocellulose has yet to be fully explored. This study aimed to isolate and evaluate lignocellulose-degrading bacteria from these larvae. Seventeen cellulolytic bacterial strains were successfully isolated from the fermentation chamber of white grubs, most of which belonged to the Firmicutes and γ-Proteobacteria classes. Notable genera included Bacillus, Enterobacter, and Klebsiella. Among these, Bacillus toyonensis strain LC3B1 exhibited remarkable cellulolytic activity, with a cellulolytic index of 1.93 ± 0.037. This strain demonstrated the highest degradation on groundnut husk powder (33.25 ± 0.823%), followed by paddy straw powder (31.45 ± 0.608%) and corncob powder (28.15 ± 1.56%), highlighting its potential for effective agricultural residue degradation. FTIR analysis of carboxymethyl cellulose (CMC) hydrolyzed by LC3B1 revealed various decomposition products, including ketones, aldehydes, alcohols, and carboxylic acids. Scanning electron microscopy (SEM) of the treated biomass revealed significant morphological changes, such as pore formation and tunneling within the substrate. The broad cellulolytic capabilities observed across bacteria from white grub gut microbiota, including members of the Bacillaceae, Enterobacteriaceae, and Pseudomonadaceae families, underscore their potential as valuable resources for lignocellulosic biomass degradation, biofuel production, and sustainable waste management strategies. This study highlights the promise of insect gut microbiota as a reservoir for environmentally beneficial microbial applications.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-025-03805-y.
Keywords: White grub, Gut microbes, 16S rRNA gene sequencing, Cellulolytic activity, Lignocellulosic biomass, Cellulase enzyme
Background
Insects, among the most omnipresent and dominant organisms on Earth, represent the largest and most diverse class within the phylum Arthropoda. Their extensive diversity and remarkable adaptability make the class Insecta a widespread and ecologically successful group in the animal kingdom. Symbiotic partnerships, which range from mutualistic to parasitic interactions, are essential to insect biology. The insect gut microbiota, in particular, is crucial for digestion and nutrient acquisition, forming intricate colonies of bacteria, fungi, and protozoa within the digestive tract [1, 2].
These symbiotic microorganisms play a crucial role in breaking down lignocellulose biomass, a key component of plant cell walls that many insects rely on as a food source. Lignocellulose consists of complex compounds cellulose, hemicellulose, and lignin which are notoriously challenging to degrade. Yet, insects have evolved specialized digestive systems that can efficiently decompose these tough carbohydrates along with their gut microbiota. Notably, termites and specific beetles host gut bacteria from the Firmicutes and Proteobacteria phyla, aiding in cellulose degradation [3, 4]. Beyond digestion, the gut microbiota contributes by synthesizing unique compounds and enzymes with therapeutic and industrial applications [5–7]. Additionally, these microbial communities are crucial for bioremediation, plastic degradation, disease prevention, and pest control [8–11].
While significant research has explored the lignocellulose-degrading abilities of termites and beetles, the cellulolytic capabilities of white grubs remain relatively underexplored. White grubs, the larvae of scarab beetles, are polyphagous subterranean herbivores posing major agricultural threats. This study focuses on Holotrichia serrata and Leucopholis coneophora, prominent pests in Karnataka, India, known for damaging the roots of economically important crops like sugarcane and arecanut, which are rich in cellulose and nitrogenous compounds [12–15]. This study will assess the lignocellulose-degrading capacity of various white grub species by extracting and identifying their gut microbiome. Insights into these insect-microbiome interactions can not only deepen our understanding of pest biology and host–microbiome coevolution but also pave the way for bioengineering digestive enzymes for industrial applications, such as in biorefineries and biofuel production [16–21].
Materials and methods
Substrate preparation
Agricultural wastes, including paddy straw, groundnut husk, and corncob powder, were sourced from local fields at the University of Agricultural Sciences, Gandhi Krishi Vignana Kendra, Bengaluru, Karnataka, India. These materials were individually pretreated using a mild alkaline solution (0.1 N NaOH, w/v) to enhance their degradability. After pretreatment, the substrates were thoroughly rinsed with deionized water to reach a neutral pH, air-dried, finely ground, and passed through a 2.0 mm sieve to achieve a uniform particle size. The processed substrates were then either used immediately in hydrolysis experiments or stored in airtight containers for future applications [22].
Insect sample collection
Third-instar larvae of the white grub species L. coneophora and H. serrata were collected from infested fields in Moodabidri, Dakshina Kannada district, and Mahadeshwarapura, Mandya district, Karnataka, India, respectively (Fig 1). The identification of these species was confirmed by entomologist Dr. K.V. Prakash using binomial keys. The larvae were then transported to the laboratory in sterile, aerated plastic containers and starved for 24 h prior to dissection to ensure gut clearance.
Fig. 1.
Holotrichia serrata (A) and Leucopholis coneophora (B)
Isolation of gut bacteria
The larvae were first rinsed in double-distilled water for 30 s, then immersed in 70% ethanol for 60 s, and finally rinsed in double-distilled water for another 30 s to remove surface contaminants. The sterilized larvae were then dissected under laminar airflow using sterile microscissors to extract the gut, specifically isolating and removing the fermentation chamber [23].
The fermentation chambers were pooled into a 1.5 mL microtube containing 1.0 mL of phosphate-buffered saline (PBS, pH 7.4) and homogenized with a sterile micropestle. The homogenized gut samples were then enriched in Berg minimal salt medium (BMS) supplemented with 2 g/L NaNO₃, 0.02 g/L MgSO₄·7H₂O, 0.02 g/L MnSO₄·H₂O, 0.5 g/L K₂HPO₄, 0.02 g/L FeSO₄·7H₂O, and 0.5 g/L CaCl₂·2H₂O, with 1% (w/v) carboxymethyl cellulose (CMC) as the substrate [22]. The inoculated medium was incubated at 37 °C with shaking at 150 rpm for 48 h. Following incubation, the culture broth was serially diluted in PBS (pH 7.4) up to a final dilution of 10⁻⁷. One hundred microliters of each dilution were spread onto BMS-CMC agar plates and incubated at 37 °C for 48 h. Colonies were purified through repeated streaking on LB agar plates, and the isolated strains were stored in glycerol at -80 °C for further use.
Molecular characterization and identification of isolated bacterial strains
Genomic DNA was extracted from the bacterial isolates following a modified protocol [24]. The bacterial cultures were grown in LB broth and incubated overnight at 37 °C with shaking. Each culture was placed in a 1.5 mL aliquot in a microcentrifuge tube, centrifuged for 7 min to pellet the cells, and the supernatant was carefully removed. The cell pellet was re-suspended in TE buffer containing 100 mg/mL lysozyme, 20 mg/mL proteinase K, and 10% SDS, followed by incubation at 37 °C for 1 h. Subsequently, 5 M NaCl and CTAB solution were added, and the mixture was incubated at 65 °C for 10 min. After removing impurities from the samples using a chloroform-isoamyl alcohol mixture (24:1), the aqueous phase was moved to a new tube. After adding an equivalent volume of phenol:chloroform alcohol (25:24:1), the mixture was centrifuged for five minutes at 4 °C at 8,000 rpm. This step was repeated until the supernatant was clear. DNA was precipitated by adding chilled isopropanol, gently mixed, and incubated overnight at -20 °C. The DNA was then pelleted by centrifugation at 10,000 rpm for 20 min at 4 °C, washed with 70% ethanol, air-dried, and dissolved in TE buffer.
For PCR amplification of the 16S rRNA gene, primers 27F (5'-AGAGTTTGATCMTGGCTCAG-3') and 1492R (5'-TACGGYTACCTTGTTACGACTT-3') were used. The thermal cycling conditions included a 5-min initial denaturation at 95 °C, 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 1 min, with a final extension at 72 °C for 7 min. The PCR products were purified and sequenced after their size and purity were confirmed on 1.5% (w/v) agarose gels. BLAST analysis was performed to identify close matches in the NCBI database (https://www.ncbi.nlm.nih.gov/) after sequences were aligned using BioEdit [25]. A neighbor-joining phylogenetic tree was built using the Kimura 2 evolutionary model [26] and MEGA 11 software [27], with a bootstrap value of 1000 replications for robustness. Additional alignment was carried out using ClustalW [28].
Screening of cellulose-degrading bacteria
The bacterial isolates were inoculated onto BMS agar plates supplemented with carboxymethylcellulose (CMC) as the substrate and incubated at 37 °C for 48 h. To assess CMC degradation by the bacterial isolates, the plates were flooded with 0.1% Congo red solution and allowed to stain for 30 min. Excess Congo red was then removed, and the plates were treated with a 1 M NaCl solution for 15 min. Degradation of the β-1,4 glycosidic bonds by cellulase activity prevents Congo red from binding to the cellulose polymer, resulting in clear zones in the medium [29]. Bacterial colonies exhibiting the most distinct and largest diameter zones of hydrolysis on the stained plates were selected for further investigation. The cellulolytic indices of the isolates were measured according to a previously published protocol with minor modifications [30]. The cellulolytic index was calculated as (diameter of zone of clearance − diameter of bacterial colony)/diameter of bacterial colony.
Substrate degradation ratio
In brief, 1 mL of freshly grown bacterial culture (OD₆₀₀nm: 0.5) was inoculated into BMS medium containing various substrates and incubated at 37 °C and 150 rpm on a rotary shaker for 7 days. After incubation, the cultures were centrifuged at 10,000 rpm for 15 min at 4 °C, and the supernatant was collected as a crude enzyme extract, which was then stored at 4 °C.
For substrate preparation, the substrates were treated with 3 mL of acetic-nitric reagent (a mixture of 150 mL of 80% acetic acid and 15 mL of concentrated nitric acid), then washed with distilled water for 5 min, followed by a 10 min wash with absolute ethanol. The residues were dried at 60 °C in a hot air oven [31], and their final weights were measured. The percentage of substrate degradation was calculated using the following formula from Updegraff, 1969 [32]:
Substrate Degradation (%) = {(Initial weight of substrate − Final weight of substrate)/Initial weight of substrate} × 100.
Cellulase (β-1,4-endoglucanase) enzyme assay
The supernatant from the previous experiment served as the crude enzyme for the assay. For each reaction, 0.5 mL of supernatant was mixed with 1 mL of 0.05 M citrate buffer (pH 4.5) containing 1% CMC as the substrate. The reaction mixture was incubated in a water bath for 60 min. After incubation, 3 mL of DNS (dinitro salicylic acid) reagent was added to stop the enzymatic reaction, and the tubes were heated at 100 °C for 5 min and subsequently cooled to 4 °C for another 5 min [33].
Absorbance was measured at 540 nm using a spectrophotometer, with a control sample as the baseline. The amount of reducing sugars produced was calculated using a glucose standard, with enzymatic activity expressed in units (U/mL). One unit was defined as the amount of enzyme that releases 1 μmol of reducing sugars (as glucose) per mL per minute under the assay conditions [34].
SEM analysis of the hydrolyzed substrate
The hydrolyzed substrates and control samples were characterized using Fourier transform infrared spectroscopy (FTIR) and field emission scanning electron microscopy (FESEM). A 1% bacterial inoculum was introduced into freshly prepared BMS liquid media supplemented with 1000 mg of filter paper (FP) as the substrate, followed by incubation at 150 rpm and 37 °C for 14 days. A control experiment was conducted under the same conditions, but without bacterial inoculation.
After 14 days of incubation, the culture broth was centrifuged at 5000 rpm for 5 min to separate the biomass. For FESEM analysis, the samples were prepared following a previously published protocol with slight modifications [22]. The filter paper was washed with distilled water and then dried overnight at 60 °C. To enhance conductivity and minimize charging effects during scanning electron microscopy, the samples were sputter-coated with a 100 Å gold layer in an argon gas atmosphere.
FTIR analysis of the hydrolyzed substrate
Fourier transform infrared (FTIR) spectroscopy is a rapid and nondestructive technique for detecting functional groups in the mid-IR region. In this study, FTIR spectroscopy was used to analyze filter paper, which serves as the sole carbon source for bacterial growth. After 14 days of bacterial treatment, the filter paper samples and the control samples were mixed with 1000 mg of spectroscopy-grade potassium bromide (KBr) in an agate mortar and pressed into discs.
The infrared (IR) spectra were recorded using an FTIR spectrometer in transmission mode, covering the range from 400 to 4000 cm⁻1 [22, 35]. SEM and FTIR analyses were conducted using the instruments available at the Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR) in Bengaluru.
Statistical analysis
The results for cellulolytic activity, substrate degradation, and enzyme activity were analyzed using descriptive statistics, analysis of variance (ANOVA), and Duncan's multiple range test (DMRT) to assess differences between factors at a significance level of p = 0.05. Statistical analyses were performed using OP STAT [36], and the results were interpreted with Microsoft Excel (version 2021). FTIR data were plotted and analyzed using the OriginPRO (version 2023b; OriginLab, Northampton, MA, USA) software.
Results
Isolation of cellulolytic gut bacteria
The morphological characteristics of bacterial isolates 1 to 7 from L. coneophora and 8 to 17 from H. serrata are provided in the Supplementary Data (Table S1). In total, seventeen bacterial isolates were obtained from the fermentation chambers (Fig. 2) of the two white grub species collected from distinct regions.
Fig. 2.
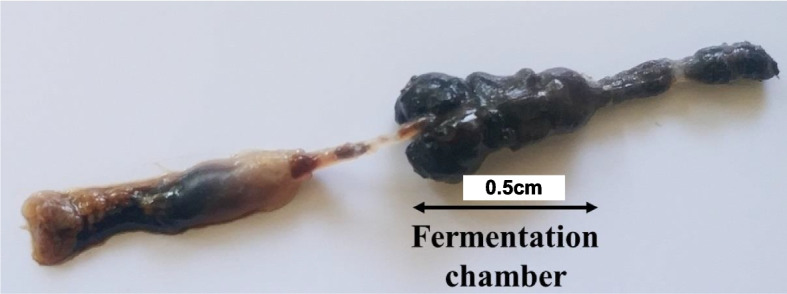
Representative fermentation chambers isolated from the two white grubs used in the present study
Molecular characterization of cellulolytic gut bacteria
DNA was extracted from all samples, and the amplification of the 16S rRNA gene using universal primers yielded high-quality amplicons,approximately 1450 bp in size (Figures S1 and S2). These PCR products were sequenced, and the resulting sequences were aligned using the BioEdit tool. The sequences were then compared to those in the GenBank database, accessed through the National Center for Biotechnology Information (NCBI) website.
Comparative analysis showed that five of the seven bacterial isolates from L. coneophora exhibited more than 95% similarity to known sequences, while one isolate displayed 90% similarity (Table S2). For the bacterial isolates from H. serrata, eight exhibited over 95% similarity, and the remaining two showed more than 90% similarity (Table S3).
Phylogenetic tree analysis
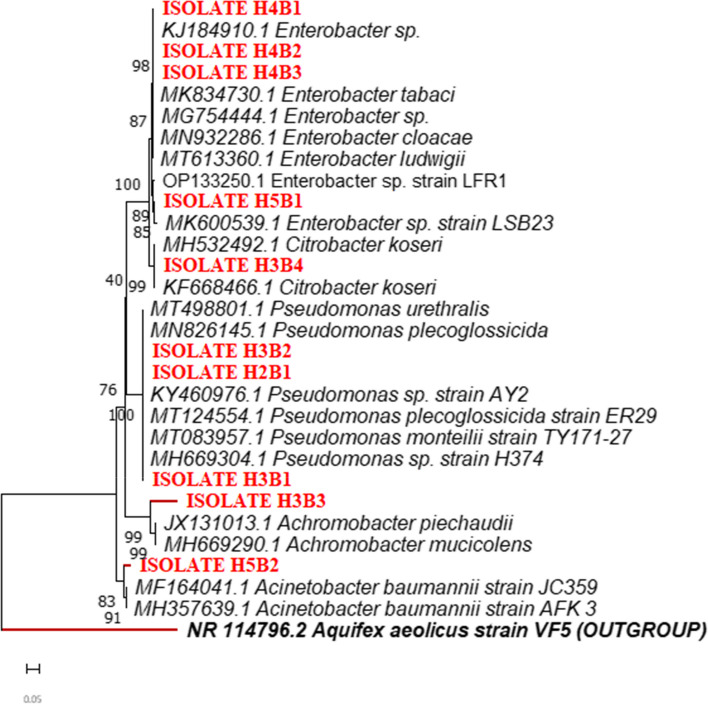
Phylogenetic analysis of the 16S rRNA sequences revealed that the bacteria colonizing the gut of L. coneophora formed two distinct major clades, each with various subgroups. The dominant phyla in the phylogenetic tree were Firmicutes and γ-Proteobacteria, which constituted the major clades. The Firmicutes clade was represented by the genus Bacillus, comprising three species and several subbranches, likely indicating different strains. The second major clade consisted of γ-Proteobacteria, represented by Klebsiella sp. and Citrobacter farmeri. The major clades of the phylogenetic tree were analyzed using 1000 bootstrap replications, with Acidobacterium capsulatum serving as the outgroup. The sequence for the outgroup was retrieved from the NCBI database. A phylogenetic tree depicting the seven identified gut bacteria in L. coneophora is shown in Fig. 3
Fig. 3.
Phylogenetic tree showing the evolutionary relationships of the bacterial isolates from Holotrichia serrata
A similar phylogenetic tree was constructed for the gut bacteria of H. serrata, revealing two distinct clades representing different groups: γ-Proteobacteria and β-Proteobacteria. The major γ-Proteobacteria clade included the genera Pseudomonas, Citrobacter, Enterobacter, and Acinetobacter, while the minor β-Proteobacteria clade contained only the genus Achromobacter. This tree was also constructed with an outgroup, with the sequence retrieved from the NCBI database. Both clades of the phylogenetic tree, along with the outgroup (Aquifex aeolicus), are depicted in Fig. 3, with bootstrap values based on 1000 replicates.
Overall, the taxonomic classification of gut bacteria from L. coneophora and H. serrata provides valuable insights into the diversity of bacterial species present in these insects (Tables S4 & S5).
Cellulolytic indices of the bacterial isolates
The cellulolytic activity of the bacterial isolates was evaluated using the Congo red overlay method, where the presence of a halo zone on CMC agar plates (Fig. 4B) indicated the cellulolytic index. Of the seventeen bacterial isolates tested, thirteen exhibited significant cellulolytic activity. The cellulolytic index ranged from a maximum of 1.93 ± 0.037 for the LC3B1 isolate (Bacillus toyonensis) to a minimum of 0.24 ± 0.015 for the LC2B3 isolate (Klebsiella oxytoca) from L. coneophora. Among the H. serrata strains, the highest cellulolytic index was 1.49 ± 0.04 for the H4B3 isolate (Enterobacter sp.), while the lowest index was 0.26 ± 0.012 for the H4B2 isolate (Enterobacter ludwigii). The cellulolytic indices of the gut bacteria isolates are presented in Fig. 4A (Table S6). The cellulolytic indices varied significantly among the different bacterial strains, as indicated by the ANOVA results: F(2,12) = 661.1, p < 2e-16Duncan’s test further confirmed these differences, revealing significant variation in the cellulolytic indices between the strains. Strain LC3B1 exhibited the highest cellulolytic index, followed by strain LC1B1. Based on these findings, four bacterial isolates—LC1B1, LC3B1, H4B3, and H5B2—were selected for further study due to their promising cellulolytic activities (Fig. 5).
Fig. 4.

A Cellulolytic indices of gut bacteria isolated from two white grub species. B Cellulolytic indices of gut bacteria isolated from two white grub species (a-LC1B1, b-LC3B1, c-H4B3, and d-H5B2)
Fig. 5.
Substrate degradation ratio (%) of cellulolytic isolates with different agricultural residues
Substrate degradation ratio of agricultural residues
The four selected bacterial isolates were cultured in media containing powdered corncob, paddy straw, and groundnut husk substrates, followed by incubation for eight days. Among the isolates, H5B2 exhibited the highest degradation efficiency, reaching 48.15 ± 1.56% for groundnut husk, whereas LC3B1 exhibited 46 ± 0.608% for paddy straw powder and 43 ± 0.27% for corncob. The substrate degradation ratio was significantly different between the F(2, 3) = 12.236 strain (p < 0.05) and the F(2,2) = 8.050 strain (p < 0.05) but not between the substrates. The other isolates also demonstrated significant degradation capabilities, as shown in Fig. 5 (Table S7).
Cellulase (β-1,4-endoglucanase) enzyme assay
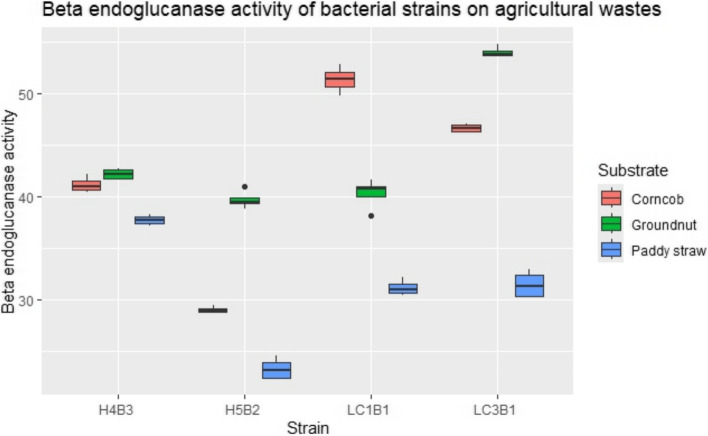
Cellulase (β-1,4-endoglucanase) activity assays were conducted using the DNS method at 50 °C and pH 4. β-1,4-endoglucanase was statistically significant among the various substrates F(2, 2) = 7.3, p < 0.024 but not between bacterial strains F(2,3) = 1.16, p = 0.39). Among the tested genes, LC3B1 had the highest β-1,4-endoglucanase activity on groundnut husk, followed by LC1B1, which had the second highest endoglucanase activity (3.614 ± 0.02 U/mL), particularly on the corn cob powder substrate. LC1B1 also exhibited significant activity on groundnut husk (2.168 ± 0.07 U/mL), highlighting its potential for enzyme production. Isolates H4B3 (2.4 ± 0.03 U/mL) and H5B2 (1.626 ± 0.01 U/mL) also exhibited notable endoglucanase activities on groundnut husk. Additionally, LC1B1 exhibited activity (1.628 ± 0.01 U/mL) on paddy straw, whereas the remaining three isolates displayed activities of less than 0.7 U/mL on all three substrates. Moreover, LC3B1 exhibited considerable endoglucanase activity (2.082 ± 0.02 U/mL) on corncob.
These findings highlight the substrate-specific cellulase activities of the bacterial isolates, with LC3B1 showing significant potential for enzyme production, particularly on groundnut husk and corncob substrates. The results are depicted in Fig. 6 (Table S8).
Fig. 6.
Endoglucanase activity (U/mL) of cellulolytic isolates with different agricultural substrates
FTIR and SEM results for the breakdown of cellulosic bonds in filter paper
Structural analysis of the four bacterial isolates was performed using the attenuated total reflectance (ATR) mode in the infrared range of 400–4000 cm−1, enabling the detection of bond types and functional groups (Fig. 7). The peak at 1160 cm−1 is attributed to C–O–C stretching in the cellulose/hemicellulose molecules. The peak between 2896 and 2900 cm−1indicates C-H (β-glycosidic bond) deformation in the polysaccharide molecules. The stretching of carbon–oxygen bonds between 1749 and 1599 cm−1 reveals decomposition products, such as ketones, aldehydes, alcohols, and carboxylic acids derived from cellulose and hemicellulose. Additionally, the shifts in the O–H and C–H stretching vibrations at 3333 and 2894 cm−1can be attributed to changes in the intra- and intermolecular bonds of the cellulosic polymer. The FTIR results revealed broad absorption bands at 897, 1651, and 2900 cm−1, corresponding to COC, CCO, OCH deformation, C-5, C-6 motion stretching, OH bending, and CH stretching, respectively. The intense peak at 3000–3700 cm−1 is attributed to the stretching of OH functional groups and amine groups.
Fig. 7.
FTIR profiles of the control and filtered filter paper treated with the LC1B1, LC3B1, H4B3, and H5B2 bacterial isolates
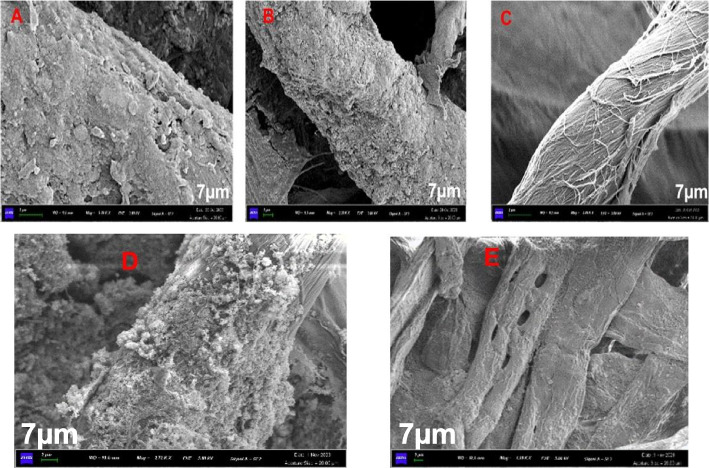
Field emission scanning electron microscopy (FESEM) rrevealed significant structural changes in the surface morphology of both the control and treated filter paper (FP) samples (Fig. 8). Compared to the control substrates, the treated FP surface exhibited a rough appearance, likely due to bacterial adherence and superficial hydrolysis of the biomass. The bacterial cells adhered to the substrate, potentially creating pores and tunnels that allowed access to the internal fibers of the cellulosic biomass. This process likely resulted in the release of microfibers from the long cellulose chains in the filter paper (FP). The electron micrographs further demonstrated substrate disruption, as evidenced by the accumulation of hydrolyzed biomass on the surface.
Fig. 8.
Electron micrograph showing the adhesion of cells and degradation of the filter paper due to bacteria (A-LC1B1-, B-LC3B1, C-CONTROL, D-H4B3 and E-H5B2
Discussion
This study explores the gut microbiota of the white grub species H. serrata and L. coneophora, focusing on their roles in cellulose degradation and potential industrial applications. Insects often host a wide range of microorganisms in their digestive systems that contribute to their survival by aiding digestion, detoxifying harmful compounds, and defending against pathogens through antimicrobial production (Jang and Kikuchi, 2020) [37]. Research on the microbiota of white grub species, such as the work by Lemke et al. (2003) [38] on Pachnoda ephippiata, has shown that both the hindgut and midgut harbor diverse microorganisms involved in cellulose breakdown, microbial fermentation, and proteolysis. However, despite extensive studies on the gut bacteria of various white grub species [31, 39–43], there are no existing reports on the bacterial diversity of H. serrata and L. coneophora, which are native to Karnataka, India.
In this investigation, 17 bacterial isolates were identified from the fermentation chambers of L. coneophora and H. serrata. Using 16S rRNA gene sequencing, these isolates were found to represent diverse bacterial strains, with Enterobacteriaceae being the most prevalent family, followed by Bacillaceae and Pseudomonadaceae, aligning with previous studies on other white grub species [44–46]. Phylogenetic analysis revealed a variety of taxa, primarily Firmicutes and Proteobacteria, in line with other studies on white grub microbiota [12, 30].
To evaluate cellulolytic activity, we measured the clearance zones around bacterial colonies on carboxymethyl cellulose (CMC) plates, which serve as an indicator of CMCase enzyme activity. Of the isolated strains, 13 exhibited notable cellulolytic activity. The highest cellulolytic index was observed in isolate LC3B1 (B. toyonensis) from L. coneophora (1.93 ± 0.037), followed by isolate H4B3 (Enterobacter sp.) from H. serrata (1.49 ± 0.04). Substrate degradation assays of the selected strains also indicated strong activity, supporting findings from other white grub bacterial isolates [47–49]. Additionally, pretreating the substrates enhanced degradation, with rumen-derived bacteria showing higher efficiency than gut bacteria [30, 50].
Further analysis of cellulase enzyme demonstrated diverse, substrate-specific activities among the bacterial isolates, highlighting their potential for lignocellulosic biomass conversion [48]. Notable cellulase producers from insect gut studies, such as Klebsiella pneumoniae and Bacillus species, have shown endoglucanase activity levels of 3.5 U/mL [51] and cellulolytic activities of 0.092 U/mL [52]. In comparison, cellulose-degrading bacteria from invertebrates like bookworms and termites exhibit extracellular cellulase activities ranging from 0.012 to 0.196 IU/mL (filter paper cellulase) and 0.162 to 0.400 IU/mL (endoglucanase) [45]. Preliminary enzyme assays underscore the importance of optimizing production conditions like pH, temperature, substrate, and metal ions to enhance cellulolytic efficiency.
To investigate the breakdown of cellulosic bonds, FTIR and SEM analyses confirmed enzymatic cellulose degradation by the isolated strains [53, 54]. The FTIR spectra highlighted structural changes in the biomass, with notable absorbance bands, including C-O, C = C, and C–C-O stretching at 1035 cm−1; C-H stretching at 2840 and 2937 cm−1 O–H in-plane bending at 1440 cm−1; and unconjugated C = O stretching at 1682 cm−1 which are characteristic of cellulose, hemicellulose, and lignin [55–57].
In summary, this study is the first to identify cellulolytic bacteria from the gut microbiota of H. serrata and L. coneophora, species with significant potential for breaking down cellulose-rich organic waste. The abundance of cellulase-producing strains within families like Enterobacteriaceae, Bacillaceae, and Pseudomonadaceae underscores their potential role in lignocellulosic biomass degradation. The variability in degradation efficiencies across strains and substrates highlights the substrate-specific nature of cellulose breakdown, which can produce reducing sugars like glucose—critical intermediates for bioethanol production. This approach could reduce dependence on traditional raw materials, such as sugar and corn, in bioethanol manufacturing. Efficient lignocellulose degradation through selective microbial strains represents a promising strategy for sustainable waste management and biofuel production, enhancing both the sustainability and value of biofuels.
Supplementary Information
Acknowledgements
I acknowledge CIPHET, Ludhiana for providing all the support.
Authors’ contributions
1.GVV conducted the experiment and analyzed data and drafted paper 2. PKV- Helped GVV to collect the insects 3. BNR- Edited the Manuscript 4.BP- Provided space to conduct the experiment 5. GKR- Assisted GVV while collecting the data.
Funding
This work was funded by the Science and Engineering Research Board under Teacher Associateship Research Excellence, TAR/2020/000014.
Data availability
STRAIN IDACCESSION NUMBERSPECIESCLOSEST STRAIN IN GENBANKIDENTITY (%) Leucophalis coneophora CDBLC1B1PP542502Klebsiella sp.Klebsiella sp. HaMC299.50 CDBLC1B2PP542506Bacillus siralisBacillus siralis J28TS899.12 CDBLC2B1PP542505Bacillus toyonensisBacillus toyonensis strain MB1490 CDBLC2B2PP542504Bacillus cereusBacillus cereus strain ABC1095 CDBLC2B3PP542497Klebsiella oxytocaKlebsiella oxytoca strain S1-2–298.21 CDBLC3B1PP542499Citrobacter farmeriCitrobacter farmeri isolate CCB19B97.7 CDBLC6B1PP542500Klebsiella pasteuriiKlebsiella pasteurii strain SPARK1448C298.28 Holotrichia serrata CDBH2B1PP542512Pseudomonas sp. Pseudomonas sp. strain H37499.13 CDBH3B1PP542515Pseudomonas monteiliiPseudomonas monteilii99.14 CDBH3B2PP542516Pseudomonas urethralis Pseudomonas urethralis strain BML-PP04299.07 CDBH3B3PP542518Achromobacter piechaudii Achromobacter piechaudii strain ERG193.38 CDBH3B4PP542520Citrobacter koseri Citrobacter koseri strain IHB B 684298.49 CDBH4B1PP542525Enterobacter tabaci Enterobacter tabaci strain TBMAX9296.49 CDBH4B2PP542528Enterobacter ludwigii Enterobacter ludwigii strain AA196.56 CDBH4B3PP542530Enterobacter sp. Enterobacter sp. JBIWA00397.68 CDBH5B1PP542527Enterobacter sp. Enterobacter sp. strain LFR199.1 CDBH5B2PP542526Acinetobacter baumannii Acinetobacter baumannii strain JC35990.
Declarations
Ethics approval and consent to participate
The insects were predominantly pestiferous and collected from farmer fields after consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Steinhaus EA. A study of the bacteria associated with thirty species of insects. J Bacteriol. 1941;42:757–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladouceur EE, Wood SC, Laudier D, Simko E. Arthropoda: insecta. Invertebrate Histol. 2021;301–17. 10.1002/9781119507697.ch12.
- 3.Jing TZ, Qi FH, Wang ZY. Most dominant roles of insect gut bacteria: digestion, detoxification, or essential nutrient provision? Microbiome. 2020;8(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie SX, Syrenne R, Sun S, Yuan JS. Exploration of natural biomass utilization systems (NBUS) for advanced biofuel- from systems biology to synthetic design. Curr Opin Biotechnol. 2014;27:195–203. [DOI] [PubMed] [Google Scholar]
- 5.Ulyshen MD. Wood decomposition as influenced by invertebrates. Biol Rev. 2016;91:70–85. [DOI] [PubMed] [Google Scholar]
- 6.Xie S, Lan Y, Sun C, Shao Y. Insect microbial symbionts as a novel source for biotechnology. World J Microbiol Biotechnol. 2019;35:1–7. [DOI] [PubMed] [Google Scholar]
- 7.Nandy G, Chakraborti M, Shee A, Aditya G, Acharya K. Gut microbiota from lower groups of animals: an upcoming source for cellulolytic enzymes with industrial potentials. Biointerface Res Appl Chem. 2021;11:13614–37. [Google Scholar]
- 8.Shao Y, Chen B, Sun C, Ishida K, Hertweck C, Boland W. Symbiont-derived antimicrobials contribute to the control of the lepidopteran gut microbiota. Cell Chem Biol. 2017;24(1):66–75. [DOI] [PubMed] [Google Scholar]
- 9.Berasategui A, Shukla S, Salem H, Kaltenpoth M. Potential applications of insect symbionts in biotechnology. Appl Microbiol Biotechnol. 2016;100:1567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang XG, Wen PP, Yang YF, Jia PP, Li WG, Pei DS. Plastic biodegradation by in vitro environmental microorganisms and in vivo gut microorganisms of insects. Front Microbiol. 2023;13:1001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel P, Moran NA. The gut microbiota of insects’ diversity in structure and function. FEMS Microbiol Rev. 2013;37:699–735. [DOI] [PubMed] [Google Scholar]
- 12.Soko KM, Bhattacharya RC, Ramakrishnan B, Sharma K, Subramanian S. Functional characterization of bacteria isolated from different gut compartments of white grub (Anamola dimidiate) larvae. J Environ Biol. 2020;41(6):1526–35. [Google Scholar]
- 13.Tippannavar PS, Patil RR. Species composition of white grubs in rainfed ecosystem of Karnataka. India J Exp Zool B. 2013;16(2):505–8. [Google Scholar]
- 14.Veeresh GK, Vijayendra M, Reddy NV, Rajanna C. Bioecology and management of areca white grubs (Leucopholis spp.) (Coleoptera: Scarabaeidae: Melolonthinae). J Soil Biol Ecol. 1982;2(2):78–86. [Google Scholar]
- 15.Swamy HM, Ramasamy A, Kalleshwaraswamy CM, Adarsh SK. Arecanut white grubs Leucopholis species (Melolonthinae: Scarabaeidae: Coleoptera) morphological, molecular identification and phylogenetic analysis. J Asia Pac Entomol. 2019;22(3):880–8. [Google Scholar]
- 16.Ps P, Arv K, Subaharan K, Venugopal V. Influence of abiotic factors on behavior and adult emergence pattern of coconut white grub (Leucopholis coneophora) Burmeister (Coleoptera: Scarabaeidae). Phytoparasitica. 2018;46:341–53. [Google Scholar]
- 17.Dahmen N, Lewandowski I, Zibek S, Weidtmann A. Integrated lignocellulosic value chains in a growing bioeconomy: status quo and perspectives. GCB Bioenergy. 2019;11(1):107–17. [Google Scholar]
- 18.Willis JD, Oppert C, Jurat-Fuentes JL. Methods for discovery and characterization of cellulolytic enzymes from insects. Insect Sci. 2010;17(3):184–95. [Google Scholar]
- 19.Chatterjee S, Sharma S, Prasad RK, Datta S, Dubey D, Meghvansi MK, Veer V. Cellulase enzyme-based biodegradation of cellulosic materials: an overview. Cellulose. 2015;5(6):271–82. [Google Scholar]
- 20.Olanbiwoninu F, Atinuke SA. Production of bacterial amylases and cellulases using sweet potato (Ipomoea batatas (L.) Lam.) peels. Afr J Biochem Res. 2015;9:104–9. [Google Scholar]
- 21.Lugani Y, Singla R, Sooc BS. Optimization of cellulase production from newly isolated Bacillus sp. Y3. Int J Bioprocess Biotech. 2015;5(11):1. [Google Scholar]
- 22.Maurya DP, Singla A, Negi S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech. 2015;5:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dar MA, Shaikh AA, Pawar KD, Pandit RS. Exploring the gut of Helicoverpa armigera for cellulose-degrading bacteria and evaluation of a potential strain for lignocellulosic biomass deconstruction. Process Biochem. 2018;73:142–53. [Google Scholar]
- 24.Zhang Z-y, Yuan Y, Ali MW, Peng T, Peng W, Raza MF, et al. Cultivable anaerobic and aerobic bacterial communities in the fermentation chambers of Holotrichia parallela (coleoptera: scarabaeidae) larvae. PLoS ONE. 2018;13(1):e0190663. 10.1371/journal.pone.0190663. [DOI] [PMC free article] [PubMed]
- 25.William S, Feil H, Copeland A. Bacterial genomic DNA isolation using CTAB. Sigma. 2012;50:6876. [Google Scholar]
- 26.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res Spec Publ. 1997;25(24):4876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–20. [DOI] [PubMed] [Google Scholar]
- 28.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–8. [Google Scholar]
- 29.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teather RM, Wood PJ. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982;43(4):777–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handique G, Phukan A, Bhattacharyya B, Baruah AALH, Rahman SW, Baruah R. Characterization of cellulose-degrading bacteria from the larval gut of the white grub beetle Lepidiota mansueta (Coleoptera: Scarabaeidae). Arch Insect Biochem Physiol. 2017;94(2):21370. [DOI] [PubMed] [Google Scholar]
- 32.Updegraff DM. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969;32(3):420–4. [DOI] [PubMed] [Google Scholar]
- 33.Dar MA, Syed R, Pawar KD, Dhole NP, Xie R, Pandit RS, Sun J. Evaluation and characterization of the cellulolytic bacterium Bacillus pumilus SL8 isolated from the gut of oriental leafworm Spodoptera litura: an assessment of its potential value for lignocellulose bioconversion. Environ Technol Innov. 2022;27:102459. [Google Scholar]
- 34.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–8. [Google Scholar]
- 35.Ghose TK. Measurement of cellulase activities. Pure Appl Chem. 1987;59(2):257–68. [Google Scholar]
- 36.Prasad RK, Chatterjee S, Mazumder PB, Sharma S, Datta S, Vairale MK, Dwivedi SK. Study on cellulase (Β-1,4-endoglucanase) activity of gut bacteria of Sitophilus oryzae in cellulosic waste biodegradation. Bioresour Technol. 2019;7:100274. [Google Scholar]
- 37.Jang S, Kikuchi Y. Impact of the insect gut microbiota on ecology, evolution, and industry. Curr Opin Insect Sci. 2020;41:33–9. [DOI] [PubMed] [Google Scholar]
- 38.Lemke T, Ulrich S, Egert M, Friedrich MW, Brune A. Physicochemical conditions and microbial activities in the highly alkaline gut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl Environ Microbiol. 2003;69:6650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cazemier AE, Verdoes JC, Reubsaet FA, Hackstein JH, van der Drift C, Op den Camp HJ. Promicromonospora pachnodae sp. nov., a member of the (hemi)cellulolytic hindgut flora of larvae of the scarab beetle Pachnoda marginata. Antonie Van Leeuwenhoek. 2003;83(2):135–48. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Jackson TA. Autochthonous bacterial flora indicated by PCR-DGGE of 16S rRNA gene fragments from the alimentary tract of Costelytra zealandica (Coleoptera: Scarabaeidae). J Appl Microbiol. 2008;105(5):1277–85. [DOI] [PubMed] [Google Scholar]
- 41.Egert M, Stingl U, DyhrbergBruun L, Pommerenke B, Brune A, Friedrich MW. Structure and topology of microbial communities in the major gut compartments of Melolontha melolontha larvae (Coleoptera: Scarabaeidae). Appl Environ Microbiol. 2005;71(8):4556–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Surabhi K, Rangeshwaran R, Frenita ML, Shylesha AN, Jagadeesh P. Isolation and characterization of the culturable microbes associated with gut of adult dung beetle Onitis philemon (Fabricius). J Pharmacogn Phytochem. 2018;27:1609–14. [Google Scholar]
- 43.MsangoSoko K, Chandel R, Gandotra S, Yadav K, Gambhir S, Subramanian KSS. Diversity of microbial groups associated with the gut of the eri silkworm, Samia ricini (Lepidoptera: Saturniidae) and white grub, Anomala dimidiata (Coleoptera: Scarabaeidae) larvae as revealed by phospholipid fatty acids. J Entomol Zool Stud. 2020;8(2):1679–83. [Google Scholar]
- 44.Banerjee S, Maiti TK, Roy RN. Production, purification, and characterization of cellulase from Acinetobacter junii GAC 16.2, a novel cellulolytic gut isolate of Gryllotalpa africana, and its effects on cotton fiber and sawdust. Ann Microbiol. 2020;70(1):1–16. [Google Scholar]
- 45.Goswami K, DekaBoruah HP, Saikia R. Production of cellulase by Novosphingobium sp. Cm1 and its potential application in lignocellulosic waste hydrolysis. Prep Biochem Biotech. 2022;52(6):724–35. [DOI] [PubMed] [Google Scholar]
- 46.Gupta P, Samant K, Sahu A. Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int J Microbiol. 2012;1:578925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wakita Y, Shimomura Y, Kitada Y, Yamamoto H, Ohashi Y, Matsumoto M. Taxonomic classification for microbiome analysis, which correlates well with the metabolite milieu of the gut. BMC Microbiol. 2018;18:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hankin L, Anagnostakis SL. Solid media containing carboxymethylcellulose to detect Cx cellulase activity of microorganisms. Microbiol. 1977;98(1):109–15. [DOI] [PubMed] [Google Scholar]
- 49.Schwarz W. The cellulosome and cellulose degradation by anaerobic bacteria. Appl Microbiol Biotechnol. 2001;56:634–49. [DOI] [PubMed] [Google Scholar]
- 50.Pourramezan Z, Ghezelbash GR, Romani B, Ziaei S, Hedayatkhah A. Screening and identification of newly isolated cellulose-degrading bacteria from the gut of xylophagous termite Microcerotermes diversus (Silvestri). Microbiol. 2012;81:736–42. [PubMed] [Google Scholar]
- 51.Rajeswari G, Jacob S, Chandel AK, Kumar V. Unlocking the potential of insect and ruminant host symbionts for recycling of lignocellulosic carbon with a biorefinery approach: a review. Microb Cell Fact. 2021;20(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbosa KL, dos Santos Malta VR, Machado SS, Junior GAL, da Silva APV, Almeida RMRG, da Luz JMR. Bacterial cellulase from the intestinal tract of the sugarcane borer. Int J Biol Macromol. 2020;161:441–8. [DOI] [PubMed] [Google Scholar]
- 53.Lee SH, H’ng PS, Lee AN, Sajap AS, Tey BT, Salmiah U. Production of pyroligneous acid from lignocellulosic biomass and their effectiveness against biological attacks. J Appl Sci. 2010;10(20):2440–6. [Google Scholar]
- 54.Gopinath SM, Shareef I, Ashalatha Ranjit S. Isolation, screening, and purification of cellulase from cellulase-producing Klebsiella variicola RBER3 (KF036184.1). Int J Sci Res. 2012;3:2319–7064. [Google Scholar]
- 55.Lee CM, Kubicki JD, Fan B, Zhong L, Jarvis MC, Kim SH. Hydrogen-bonding network and OH stretch vibration of cellulose: comparison of computational modeling with polarized IR and SFG spectra. J Phys Chem B. 2015;119(49):15138–49. [DOI] [PubMed] [Google Scholar]
- 56.Sharma S, Sharma V, Kuila A. Cellulase production using natural medium and its application on enzymatic hydrolysis of thermochemically pretreated biomass. 3 Biotech. 2016;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hospodarova V, Singovszka E, Stevulova N. Characterization of cellulosic fibers by FTIR spectroscopy for their further implementation to building materials. Am J Anal Chem. 2018;9(6):303–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
STRAIN IDACCESSION NUMBERSPECIESCLOSEST STRAIN IN GENBANKIDENTITY (%) Leucophalis coneophora CDBLC1B1PP542502Klebsiella sp.Klebsiella sp. HaMC299.50 CDBLC1B2PP542506Bacillus siralisBacillus siralis J28TS899.12 CDBLC2B1PP542505Bacillus toyonensisBacillus toyonensis strain MB1490 CDBLC2B2PP542504Bacillus cereusBacillus cereus strain ABC1095 CDBLC2B3PP542497Klebsiella oxytocaKlebsiella oxytoca strain S1-2–298.21 CDBLC3B1PP542499Citrobacter farmeriCitrobacter farmeri isolate CCB19B97.7 CDBLC6B1PP542500Klebsiella pasteuriiKlebsiella pasteurii strain SPARK1448C298.28 Holotrichia serrata CDBH2B1PP542512Pseudomonas sp. Pseudomonas sp. strain H37499.13 CDBH3B1PP542515Pseudomonas monteiliiPseudomonas monteilii99.14 CDBH3B2PP542516Pseudomonas urethralis Pseudomonas urethralis strain BML-PP04299.07 CDBH3B3PP542518Achromobacter piechaudii Achromobacter piechaudii strain ERG193.38 CDBH3B4PP542520Citrobacter koseri Citrobacter koseri strain IHB B 684298.49 CDBH4B1PP542525Enterobacter tabaci Enterobacter tabaci strain TBMAX9296.49 CDBH4B2PP542528Enterobacter ludwigii Enterobacter ludwigii strain AA196.56 CDBH4B3PP542530Enterobacter sp. Enterobacter sp. JBIWA00397.68 CDBH5B1PP542527Enterobacter sp. Enterobacter sp. strain LFR199.1 CDBH5B2PP542526Acinetobacter baumannii Acinetobacter baumannii strain JC35990.