Abstract
Background
Controversial conclusions have been drawn from recent researches that examined the impact of keratinized mucosa width on clinical parameters of peri-implant health status. The purpose of this meta-analysis is to assess the effect of keratinized mucosa width on these clinical parameters by combining data from different studies.
Methods
A systematic literature search was performed using PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), and VIP Data from the beginning of the database until May 2024. Mean difference (MD) along with the 95% confidence interval (CI) were calculated for plaque index (PI), gingival index (GI), bleeding index (BI), probing depth (PD), clinical attachment level (CAL) and bone loss (BL). The Q test and I2 statistic were used to determine heterogeneity. Publication bias was evaluated by Begg’s test and Egger’s test.
Results
A total of 30 articles were included for meta-analysis. Implants with adequate width of keratinized mucosa (≥ 2 mm) presented significantly reduced PI (MD: -0.30, 95% CI: -0.42 to -0.17), GI (MD: -0.26, 95% CI: -0.38 to -0.13), BI (MD: -0.20, 95% CI: -0.33 to -0.07), and BL (MD: -0.27, 95% CI: -0.42 to -0.12). Although significant heterogeneity was detected among the included studies, sensitivity analysis further demonstrated the robustness of the results.
Conclusions
The meta-analysis demonstrated that adequate width of keratinised mucosa (≥ 2 mm) around dental implants is associated with reduced plaque accumulation, tissue inflammation, and bone loss.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-025-05680-5.
Keywords: Keratinized mucosa, Dental implants, Inflammation, Meta-analysis
Introduction
Dental implants are now accepted as a routine procedure for replacing missing teeth in the last few decades [1]. However, peri-implant inflammation, a pathological condition that begins in the soft tissues around dental implants, may lead to biological complications of the implant surrounding tissue, such as peri-implantitis and peri-implant mucositis [2]. Peri-implant mucositis affects the soft tissue only, if left untreated, it might eventually progress to peri-implantitis, which impacts both the soft and hard tissues [3]. These inflammatory complications are highly prevalent and adversely influence the long-term success of dental implant treatment [4]. Therefore, how to avoid the development of peri-implant inflammation has become a hot topic among academics. Several conditions, such as systemic disease, history of periodontitis, smoking, poor prosthesis contour and plaque control, have been recognized as potential risk factors for peri-implant inflammation [5]. One factor that is routinely investigated is the significance of keratinized mucosa on peri-implant health. To date, the adequate width of keratinized mucosa around dental implants for the maintenance of peri-implant health has been the subject of much debate.
The keratinized gingiva establishes a physical barrier between the oral environment and the periodontium’s connective tissues by being tightly connected to the teeth and underlying bone. A sufficient width of keratinized gingiva was thought to be critical for natural dentitions in order to preserve gingival health around natural teeth and slow the progression of periodontal disease [6]. The soft tissue around dental implants differs significantly from the soft tissue surrounding natural teeth in both anatomical and histologic ways. Collagen fibers in implants run parallel to the implant surface without direct anchorage [7]. This weak attachment, combined with reduced cellularity and vascularity in the peri-implant connective tissue, may make the peri-implant tissues more susceptible to breakdown than natural teeth during inflammatory disease processes [8]. However, still many investigations concluded that peri-implant tissue health could be maintained regardless of the presence or lack of keratinized mucosa [9, 10].
Good oral hygiene surrounding dental implants could be difficult to be achieved without the presence of keratinized mucosa [11]. Therefore, it seems reasonable that adequate width of keratinized mucosa is a crucial for keeping oral health and implant stability. In this regard, the most recent meta-analysis demonstrated that implants with sufficient width of keratinized mucosa had a reduced plaque index (PI), but probing depth (PD), soft-tissue recession, and marginal bone loss (BL) did not differ significantly between groups [12]. The strength of evidence from this meta-analysis was relatively low due to the insufficient statistical power, as only nine studies were included in their analysis, four of which were used for quantitative synthesis. Recently, several studies [9, 13–15] subsequent to that meta-analysis have emerged, not all confirmed the previous findings, and the controversy on this topic continues. Thus, whether insufficient keratinized mucosa is a risk factor for developing peri-implant inflammation remains unclear. With the most up-to-date evidence, we aimed to synthesize all available evidence to further comprehensively assess the influence of width of keratinized mucosa on peri-implant inflammation.
Methods
The current meta-analysis was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [16].
PECO question
The focused clinical question of this meta-analysis was proposed by following PECO (Population, Exposure, Comparison, Outcome) principles [17]: Is sufficient keratinized mucosa width around dental implants protective against peri-implant disease?
Population: Patients with dental implants;
Exposure: Dental implants with inadequate width of keratinized mucosa (< 2 mm);
Comparison: Dental implants with adequate width of keratinized mucosa (≥ 2 mm);
Outcome: PI, gingival index (GI), bleeding index (BI), PD, clinical attachment level (CAL) and BL.
Search strategy
A systematic electronic search of PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), and Chinese Science and Technology Periodical Citation Database (VIP) was performed from the beginning of the database until May 2024. Additionally, the reference lists of eligible studies and previously published review articles were further cross-checked in order to find more relevant studies. The keywords were as follows: (“attached gingiva” OR “keratinized gingiva” OR “keratinized mucosa” OR “attached mucosa” OR “soft tissue condition” OR “soft tissue volume” OR “gingiva volume” OR “soft tissue height” OR “gingiva height” OR “soft tissue width” OR “gingiva width”) AND (“peri-implantitis” OR “peri-implant pathology” OR “dental implant infections” OR “peri-implant bone loss” OR “peri-implant disease”). The literature search was undertaken by two authors (Z.Y.Z. and Z.Z) independently. When there was any disagreement, the senior author (Z.M.W.) was consulted to reach a consensus.
Selection criteria
We chose original research articles based on the following inclusion criteria: (1) human studies (could be defined as prospective or retrospective follow-up studies, or cross-sectional study); (2) studies on the impact of keratinized mucosa width on the development of peri-implantitis; (3) compared adequate width of keratinized mucosa (≥ 2 mm) vs. inadequate width of keratinized mucosa (< 2 mm) [18]; (4) studies on at least one periodontal index, including PI, GI, BI, PD, AL or BL; (5) the minimum follow-up time of the studies was six months; (6) articles published in the English or Chinese language.
The following exclusion criteria were applied: (1) animal study; (2) could not be published as a full report; (3) studies without available data; (4) studies without effective groups.
Data extraction
Data were collected and arranged in the following fields: first author, publication year, location, type of study, patient number, gender, age, implant number, follow-up time, clinical outcomes. Two independent authors (Z. Y.Z. and Z.Z) extracted relevant data from the included articles. Any discrepancy was resolved by discussion and consensus. If there was a lack of data information, we would try to contact the corresponding author in order to obtain required data.
Quality assessment
Two of the authors (Z.Y.Z. and Z.Z) independently assessed the quality of included studies by using the Newcastle-Ottawa Scale [19]. The Newcastle-Ottawa Scale uses a ‘star’ rating system to judge quality based on three aspects of the study: selection, comparability, and exposure. The quality assessment values ranged from 0 to 9 stars. Generally, studies with a score of 7 stars or more were considered to be of high quality.
Statistical analysis
The influence of keratinized mucosa on peri-implant clinical index was assessed using the difference values between adequate and inadequate keratinized mucosa width on peri-implant clinical index. And the total effect size was reported as mean difference (MD) with 95% confidence interval (CI). A p-value < 0.05 was considered to indicate statistically significant between groups. Statistical heterogeneity among studies was evaluated using the Q test and I2 statistic. When p-value > 0.05 (Q test) and I2 < 50%, it was considered to be non-existent heterogeneity. Then, the fixed-effects model was used to calculate the overall effect size. Otherwise, the random effects model was used. We conducted sensitivity analyses to assess the sources of heterogeneity and stability of the results. Publication bias was evaluated by Begg’s test and Egger’s test. A p-value < 0.05 was considered to indicate statistically significant publication bias. All analyses were conducted with Stata (version 12.0, Stata Corp, College Station, TX, USA).
Results
Search and screening
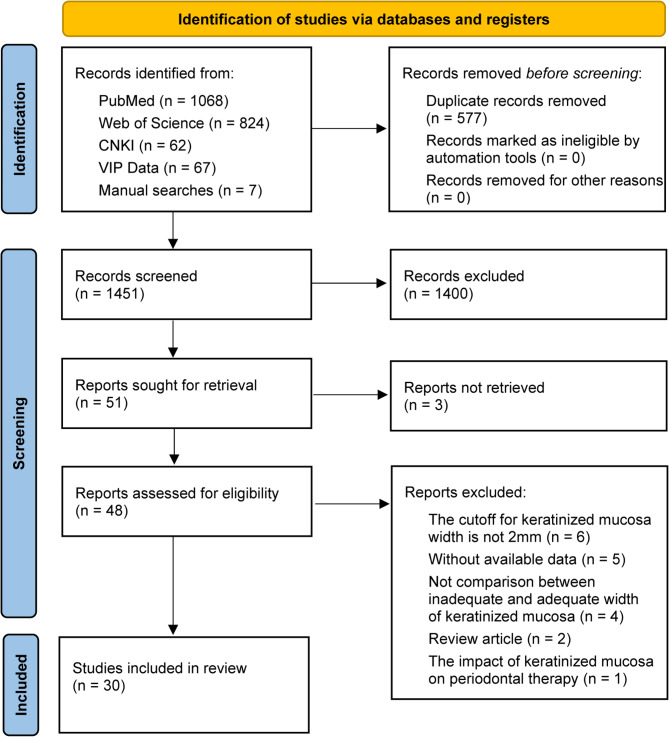
The initial search yielded a total of 2028 articles, including 1068 from PubMed, 824 from Web of Science, 62 from CNKI, 67 from VIP Data and seven from manual searching. Then, 1451 articles were chosen after duplicates were eliminated, and 51 potentially pertinent publications were selected following the article title and abstract were scrutinized, of which three articles were not found full text (inter-reviewer agreement, κ = 0.85). After reading the full text of the 48 remaining articles, we also disregarded 18 studies because they failed to meet one or more inclusion criteria (inter-reviewer agreement, κ = 0.727). Finally, the meta-analysis included 30 publications (Fig. 1). The excluded studies and reasons for exclusion were listed in Supplementary Table 1.
Fig. 1.
Flow chart from identification of eligible studies to final inclusion
Characteristics of included studies
A summary of the 30 included studies is given in Table 1. These studies were published between 1994 and 2024. All except one [20] of the studies were single-center clinical trials. Fifteen of them are prospective cohort studies [9, 13, 20–32], 13 cross sectional studies [15, 33–44], and two retrospective studies [14, 45]. The included studies involved a total of 1773 subjects, ranging in number from 15 to 130. According to eight studies [20, 22, 33, 34, 36, 37, 43, 44], 2.9–28% of the participants were smokers at the time of the study. There were 5241 implants in all. Three studies reported having only mandibular implants [20, 21, 25], 18 studies reported having both maxillary and mandibular implants [9, 13–15, 22–24, 27, 29–31, 34, 39, 40], and the remaining studies did not report the location of implant placement. Keratinized mucosa width was assessed at the mid-buccal aspect in 15 studies [13–15, 22, 24–28, 30, 36–38, 40, 43], and two studies provided mean values for the assessed mesial, mid-buccal, and disto-buccal aspects [35, 42]. The measurements of keratinized mucosa width were collected at the mid-buccal and mid-lingual aspects in three studies [20, 21, 41], and were either addressed as average values or reported separately for the two aspects. The follow up time varied from 6 months to 25 years.
Table 1.
Characteristics of eligible studies included in this meta-analysis
| First author | Year | Location | Type of study | No. of patient | Gender (%Female) |
Age | Systemic condition | No. of implant | Implant position | Follow-up time |
Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mericske-Stern | 1994 | Switzerland | Prospective cohort study | 33 | 51.5% | 69.0 ± 7.0 | NA | 66 | Mandible | 5 years | PI, BI, PD, AL |
| Chung | 2006 | America | Prospective cohort study | 69 | 59.4% | 61.3 ± 13.6 |
Diabetes: 6 Non-diabetes: 63 |
339 |
Maxilla: 198 Mandible: 141 |
> 3 years | PI, GI, BI, PD, BL |
| Bouri | 2008 | America | Cross sectional study | 76 | NA | > 18 | NA | 200 | NA | 1 year | PI, GI, PD, BL |
| Adibrad | 2009 | Iran | Cross sectional study | 27 | 56.0% | 63.1 ± 6.9 | Without conditions known to affect bone metabolism | 66 |
Maxilla: 24 Mandible: 42 |
2 years | PI, GI, BI, PD, AL, BL |
| Kim | 2009 | Korea | Prospective cohort study | 100 | 48.0% | 52.2 ± 10.8 | NA | 276 |
Maxilla: 132 Mandible: 144 |
13 months |
PI, GI, PD, BL |
| Schrott | 2009 | America | Prospective cohort study | 73 | 52.1% | 58.0 ± 9.6 | Without conditions known to affect bone metabolism | 386 | Mandible | 5 years | PI, BI |
| Crespi | 2010 | Italy | Prospective cohort study | 29 | 37.9% | 25–67 | Without systemic diseases | 164 |
Maxilla: 132 Mandible: 32 |
4 years | PI, GI, BI, PD |
| Esper | 2012 | Brazil | Cross sectional study | 109 | NA | 16–50 | Without systemic diseases | 202 | NA | 1 year | PI, GI, PD |
| Boynueğri | 2013 | Turkey | Prospective cohort study | 15 | 53.3% | 54.0 ± 10.0 | Without systemic diseases | 36 | Mandible | 1 year | PI, GI |
| Amponpan | 2014 | Thailand | Cross sectional study | 51 | 70.6% | 33–71 | Without conditions known to affect bone metabolism | 82 | NA | 42 months | PI, GI, BI, PD, AL |
| Zhang | 2014 | China | Prospective cohort study | 25 | 44.0% | 45.5 ± 9.9 | Without conditions known to affect bone metabolism | 40 | NA | 1 year | PI, GI, PD, BL |
| Askin | 2015 | Turkey | Prospective cohort study | 18 | 55.6% | 47.5 ± 11.3 | Without systemic diseases | 60 |
Maxilla: 32 Mandible: 28 |
6 months | PI, GI, PD, BL |
| Romanos | 2015 | Germany | Retrospective cohort study | 118 | 46.6% | 62.6 ± 13.7 | NA | 320 | NA | > 2.6 years | PI, BI |
| Esfahanizadeh | 2016 | Iran | Cross sectional study | 36 | 47.2% | 30–76 | Without conditions known to affect bone metabolism | 110 | NA | 3 years | PI, GI, BI, PD |
| Souza | 2016 | Brazil | Cross sectional study | 80 | 68.8% | 52.0 ± 11.7 | Without uncontrolled diabetes | 269 |
Maxilla: 129 Mandible: 140 |
1 year | PI, PD, AL |
| Ueno | 2016 | Japan | Cross sectional study | 60 | 61.7% | 60.7 ± 12.9 |
Diabetes: 1 Non-diabetes: 59 |
90 |
Maxilla: 30 Mandible: 60 |
> 1 year | PI, GI, PD |
| Yang | 2017 | China | Prospective cohort study | 120 | 37.5% | 51.6 ± 4.4 | Without conditions known to affect bone metabolism | 156 | NA | 6 months | PI, BI, PD, BL |
| Crespi | 2018 | Italy | Prospective cohort study | 42 | 59.5% | 28–71 | Without systemic diseases | 123 |
Maxilla Mandible |
8 years | PI, GI, BI, PD |
| Perussolo | 2018 | Brazil | Prospective cohort study | 71 | 66.7% | 55.7 ± 10.7 | Without conditions known to affect bone metabolism | 202 |
Maxilla: 106 Mandible: 96 |
4 years | PI, PD, AL |
| Qian | 2018 | China | Prospective cohort study | 58 | 36.2% | 51.4 ± 8.3 | Without conditions known to affect bone metabolism | 158 |
Maxilla: 78 Mandible: 80 |
4 years | PI, BI, PD |
| Fatih | 2019 | Iraq | Cross sectional study | 39 | 61.5% | 20–65 | Without systemic diseases | 157 |
Maxilla: 101 Mandible: 56 |
1–9 years | PI, BL |
| Grischke | 2019 | Germany | Cross sectional study | 52 | 55.8% | 67.6 ± 10.3 | Without conditions known to affect bone metabolism | 231 | NA | 3–25 years | GI |
| Monje | 2019 | Spain | Cross sectional study | 37 | 37.6% | 49.9 ± 12.9 | Without conditions known to affect bone metabolism | 66 |
Maxilla Mandible |
≥ 3 years | PI, BI, PD, BL |
| Sukuroglu | 2019 | Turkey | Prospective cohort study | 29 | 10.3% | 36–65 | Without systemic diseases | 71 |
Maxilla Mandible |
6 months | PI, GI, PD |
| Ravidà | 2020 | America | Cross sectional study | 40 | 55.0% | 64.5 ± 9.0 | 10% presented with hyperglicaemia | 68 |
Maxilla: 39 Mandible: 39 |
> 1 year | PD, BL |
| Kabir | 2021 | Germany | Cross sectional study | 130 | 54.6% | 70 ± 10.32 | Without compromised immune system and hyperglycemia | 130 |
Anterior: 38 Posterior: 92 |
1–31 years | PI, GI, BI, PD |
| Babayiğit | 2023 | Turkey | Prospective cohort study | 31 | 58.1% | 21–69 | Without uncontrolled systemic diseases | 87 |
Maxilla: 40 Mandible: 47 |
2 years | PI, GI, PD, BL |
| Gurbuz | 2023 | Turkey | Prospective cohort study | 34 | 53.2% | 53.55 ± 1.72 | Without conditions known to affect bone metabolism | 34 |
Maxilla Mandible |
2 years | PI, BI, PD, BL |
| Carvalhaes | 2024 | Brazil | Retrospective cohort study | 84 | 67.9% | 61.9 ± 10.1 | Without conditions known to affect bone metabolism | 242 |
Maxilla: 80 Mandible: 162 |
5–15 years | PI, BI, PD, BL |
| Eroglu | 2024 | Turkey | Cross sectional study | 87 | 65.5% | 48.1 ± 7.7 | Without conditions known to affect bone metabolism | 87 |
Maxilla: 33 Mandible: 54 |
> 1 year | PI, GI, PD, BL |
AL, attachment loss; BI, bleeding index; BL, bone loss; GI, gingival index; PD, probing depth; PI, plaque index
Risk of bias assessment
Based on the Newcastle-Ottawa Scale, an average score of 6.43 was achieved after the assessment of study quality. Sixteen studies had an overall high risk of bias (4 to 6 stars) and 14 studies (7 to 9 stars) were judged to have a low risk of bias (inter-reviewer agreement, κ = 0.732). Summarized results of the assessment of risk of bias are presented in Supplementary Table 2.
Meta-analysis of clinical indices
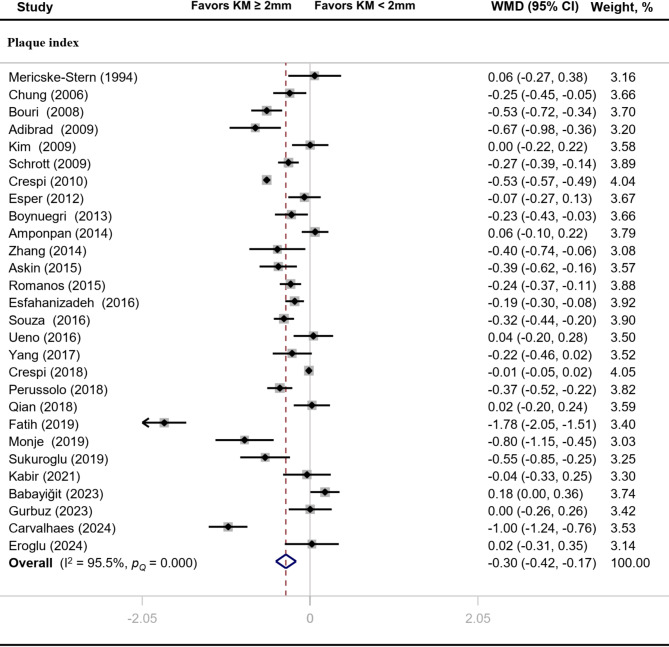
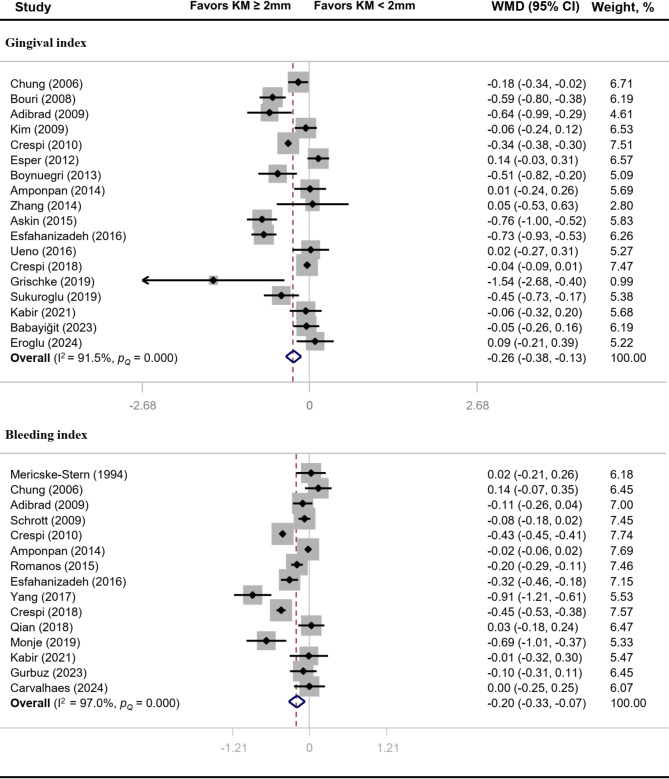
Compared with implants with inadequate width of keratinized mucosa, PI (MD: -0.30, 95% CI: -0.42 to -0.17), GI (MD: -0.26, 95% CI: -0.38 to -0.13) and BI (MD: -0.20, 95% CI: -0.33 to -0.07) values of implants with adequate width of keratinized mucosa were significantly reduced (P < 0.05) (Figs. 2 and 3). There was significant heterogeneity across studies for PI (I2 = 95.5%, P < 0.01), GI (I2 = 91.5%, P < 0.01) and BI (I2 = 97.0%, P < 0.01).
Fig. 2.
Forest plot of clinical index related to plaque accumulation
Fig. 3.
Forest plot of clinical indices related to gingival inflammation
Sensitivity analysis of clinical indices
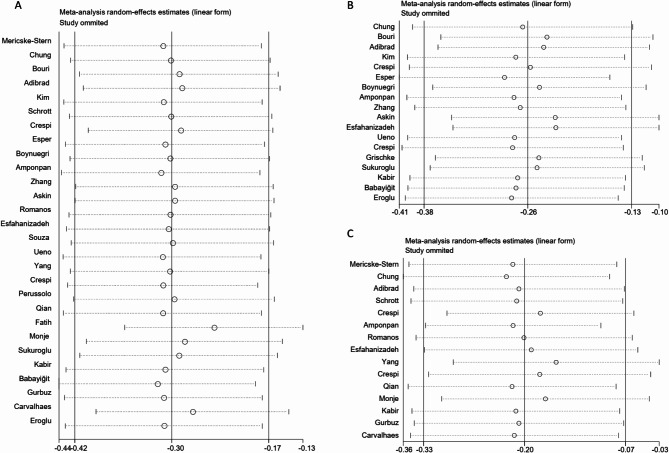
Heterogeneity appeared significant for PI, GI and BI, so we conducted further sensitivity analysis investigating the influence of a single study on the combined effect size by omitting one study in each turn. The findings of the sensitivity analysis showed that PI, GI and BI results were stable, deleting any of the studies did not have a significant impact on the experimental results (Fig. 4A, B and C).
Fig. 4.
Sensitivity analysis of clinical indices. A, plaque index. B, gingival index. C, bleeding index
Meta-analysis of clinical parameters
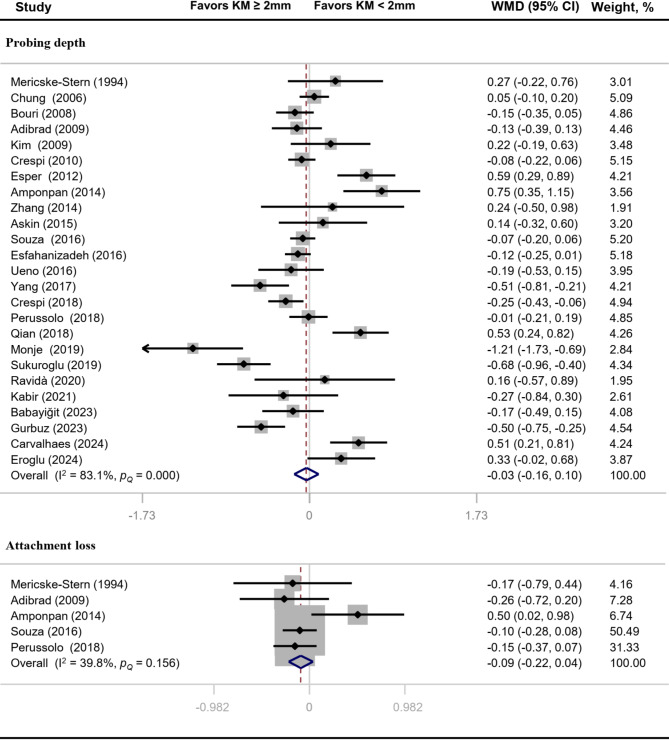
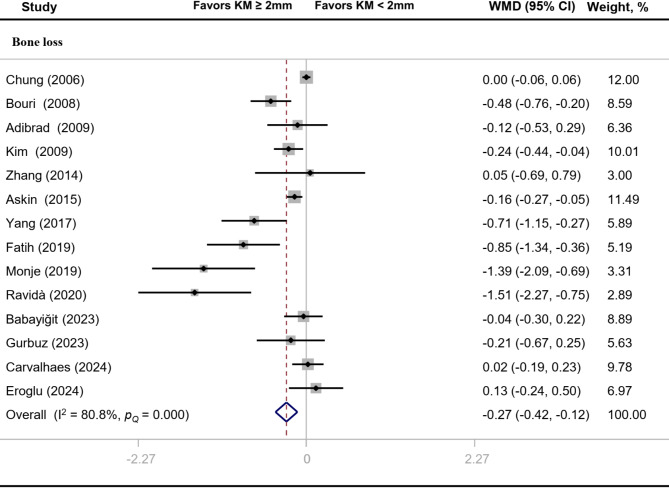
The overall effect sizes of comparison between implants with “adequate” and “inadequate” width of keratinized mucosa on PD and CAL were − 0.03 (95% CI: -0.16 to 0.10) and − 0.09 (95% CI: -0.22 to 0.04) respectively (Fig. 5). BL value of implants with adequate width of keratinized mucosa was significantly reduced (MD: -0.27, 95% CI: -0.42 to -0.12) (Fig. 6). Heterogeneity analysis showed that PD (I2 = 83.1%, P < 0.01) and BL (I2 = 80.8%, P < 0.01) had significant heterogeneity.
Fig. 5.
Forest plot of clinical parameters related to gingival tissue destruction
Fig. 6.
Forest plot of clinical parameter related to alveolar bone destruction
Sensitivity analysis of clinical parameters
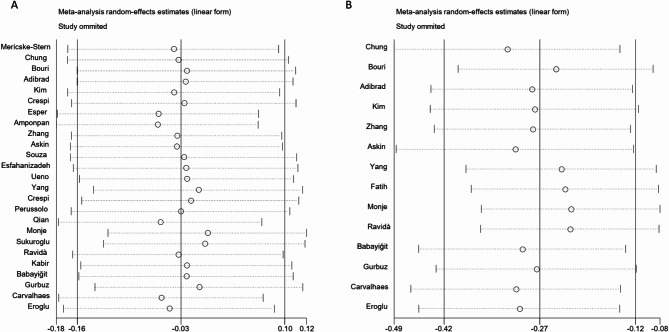
To explore the robustness of the results of PD and BL, we performed sensitivity analyses by omitting one study at a time and calculating the combined effect size for the remaining studies. We found that the PD and BL results were stable and credible, none of the individual studies appeared to have appreciable impacts on the overall combined effect sizes (Fig. 7).
Fig. 7.
Sensitivity analysis of clinical parameters. A. probing depth. B. bone loss
Publication bias analysis
Funnel plots revealed that PI, GI and CAL scatter were basically symmetric, while BI, PD and BL scatter were slightly asymmetric (Fig. 8). Egger’s test suggested that BL had obvious publication bias. Trim-and-fill analysis indicated there was no difference between the adjusted value of BL and the original value. For PD and BI, four potentially missing studies were detected. Additionally, there was no significant difference between BI adjusted values and the original values. Nevertheless, the PD adjusted value differs significantly from the original value (Table 2).
Fig. 8.
Funnel plots for the meta-analysis
Table 2.
Quantitative analysis of publication bias
| Outcome | Studies trimmed/total studies | Trim-and-fill analysis | Begg’s test (P-value) | Egger’s test (P-value) | |
|---|---|---|---|---|---|
| Mean difference | 95% Confidence interval | ||||
| Plaque index | 0/28 | -0.30 | -0.42, -0.17 | 0.42 | 0.65 |
| Gingival index | 0/18 | -0.26 | -0.38, -0.13 | 0.60 | 0.81 |
| Bleeding index | 4/19 | -0.31 | -0.43, -0.19 | 0.49 | 0.12 |
| Probing depth | 4/29 | -0.14 | -0.28, -0.004 | 0.56 | 0.53 |
| Attachment loss | 0/5 | -0.09 | -0.22, 0.04 | 1.00 | 0.62 |
| Bone loss | 0/14 | -0.27 | -0.42, -0.12 | 0.06 | 0.01 |
Subgroup analysis
The results of subgroup analyses according to study design, general condition, implant position, length of follow-up, and Newcastle-Ottawa Scale are presented in Supplementary Table 3. Both prospective cohort and cross-sectional studies showed that implants with adequate keratinized mucosa displayed considerably lower PI, GI, BI, and BL than implants with inadequate keratinized mucosa. Keratinized mucosa width had a significant relationship with PI, GI and BI in most subgroups but not in the subgroup of retrospective cohort studies for PI and GI, or subgroup with follow-up time > 3 years for GI and BI. For PD and BL, a substantial association was observed with keratinized mucosa width in subgroup with Newcastle-Ottawa Scale ≥ 7.
Discussion
The literature on the significance of keratinized mucosa for peri-implant health is inconsistent. The influence of keratinized mucosa width on peri-implant inflammation was assessed in the present meta-analysis. We included a total of 30 original studies, and six clinical indicators, including PI, GI, BI, PD, CAL and BL were used to determine the status of peri-implant inflammation. The present meta-analysis showed that adequate keratinized mucosa implants, compared with inadequate keratinized mucosa implants, significantly reduced PI by -0.30 (95% CI: -0.42 to -0.17), GI by -0.26 (95% CI: -0.38 to -0.13), BI by -0.20 (95% CI: -0.33 to -0.07) and BL by -0.27 (95% CI: -0.42 to -0.12). Nevertheless, comparisons of other clinical indicators (PD and CAL) did not reach statistically significant differences.
The accumulation of dental plaque is widely recognized as a primary pathogenic factor contributing to peri-implant inflammation [46]. Previous meta-analyses have discovered a statistically significant association between a minimum of 2 mm of keratinized mucosa and reduced plaque accumulation [12, 47, 48]. This conclusion is consistent with the results obtained from our meta-analysis. We found that implants with adequate keratinized mucosa (≥ 2 mm) had significantly lower plaque accumulation than those with inadequate keratinized mucosa. The reliability of this finding has been reinforced by analyzing the outcomes of prospective and cross-sectional studies separately. A lack of keratinized mucosa may simplify plaque accumulation, owing to the increased mobility of the lining mucosa, which facilitates plaque accumulation in the pockets [44]. Consistent results on plaque accumulation across multiple meta-analyses imply that having a sufficient width of keratinized mucosa is advantageous for improving oral hygiene around dental implants. When placing dental implants, the presence of sufficient amounts of keratinized tissue should be considered.
The GI, which has been used to clinically define the degree of gingival inflammation, can also be utilized to evaluate the peri-implant health [49]. Unfortunately, this important outcome of inflammation (GI) at peri-implant sites was not taken into consideration by the most recent meta-analysis [47]. According to previous studies, soft tissue grafting procedures for keratinized tissue gain could result in a significantly greater improvement of GI values [49]. This suggested that GI is an important indicator for evaluating the clinical effects of keratinized tissue. In our meta-analysis, eighteen studies reported the GI values and eight of these studies reported lower values of GI in implants with adequate keratinized mucosa (≥ 2 mm) [22, 24, 25, 27, 32–34, 37]. The findings of the combined investigation support the results of the eight previously mentioned studies. Based on the present meta-analysis, we speculate that inadequate keratinized mucosa is likely to be a contributing factor to peri-implant inflammation. However, as the GI was initially introduced to asses inflammation around teeth and not implants, these conclusions should be considered with some caution.
BI and bleeding on probing (BOP) have frequently been utilized as indicators of the existence of an inflammatory lesion in the peri-implant mucosa [2, 50]. In contrast to implants with inadequate keratinized mucosa, implants with adequate keratinized mucosa exhibited a substantial decrease in BOP, but not in BI value, according to the most recent meta-analysis [47]. Considering only seven original studies were included for the BI quantitative synthesis, the inconsistent results in previous meta-analysis might be attributed to the unreliable results caused by the limited number of original studies investigating BI. In the present meta-analysis, 15 of the 30 studies reported the BI, with seven of these studies showing significantly higher BI at implant sites with inadequate compared to adequate width of keratinized mucosa [20, 24, 28, 29, 37, 42, 45]. Pooled analyses revealed that implants with inadequate keratinized mucosa had a considerably higher BI. In light of the aforementioned findings, we speculate that implants with inadequate keratinized mucosa are more prone to gingival inflammation and bleeding. This might result in subsequent bone loss, then leading to failure of the implant.
The long-term outcome and stability of the dental implant mainly depend on the peri-implant bone level [40]. In clinical investigations, long-term peri-implant inflammation has been found to stimulate the loss of supporting bone around implants [51]. A recent meta-analysis stated that the lack of keratinized mucosa was significantly associated with higher prevalence of peri-implantitis defined by marginal BL ≥ 2 mm [52]. Another meta-analysis summarized individual studies but failed to detect a significant association of keratinized mucosa width with bone levels around dental implants [48]. In our meta-analysis, bone levels differed significantly between implants with “adequate” and “inadequate” width of keratinized mucosa, favoring implants with adequate keratinized mucosa (≥ 2 mm). This result supports the findings of previous meta-analyses, which revealed a considerable increase in BL at implants with inadequate keratinized mucosa (< 2 mm) in comparison to the control sites [47]. Our findings provide additional evidence that adequate keratinized mucosa might be beneficial to prevent BL around dental implants.
We must emphasize that while the width of the keratinized gingival plays a significant role, other risk factors such as oral hygiene, implant design, periodontal diseases, smoking habits, and the patient’s overall systemic health can also profoundly impact the development and progression of peri-implant diseases. Therefore, when assessing peri-implant diseases, it is crucial to consider all these factors in conjunction with the width of the keratinized mucosa. To ascertain the potential impact of systemic diseases on our analysis, we conducted subgroup analyses on patients with and without systemic diseases. The results indicate that systemic diseases did not significantly affect the outcomes of this study. However, given that the majority of studies do not report smoking and non-smoking status, it poses a challenge for us to conduct a separate analysis for smokers and non-smokers.
Some limitations should be considered in our meta-analysis. First, we limited our investigation to articles published in English or Chinese. We did not consider studies in other languages. Second, the analysis included studies with a variety of confounding factors (e.g., implant position, implant system, population selection, number of patients, calculated outcome measures, and follow-up time), which contributed to the significant degree of heterogeneity among the studies. Third, variations in the location and methodology of keratinized mucosa measurement employed in various investigations may impact the precision of the analysis findings. Finally, there is publication bias in some studies. The author perhaps more likely to publish if favorable results are acquired.
Conclusion
Within these limitations, it was concluded that inadequate keratinized mucosa around dental implants was associated with increased plaque accumulation, soft-tissue inflammation and marginal bone loss. Hence, clinicians might think about soft-tissue grafting to augment keratinized mucosa in cases where it is inadequate in order to support peri-implant health. However, given the limitations of the meta- analyses, further prospective clinical studies are required, with homogenous case definitions and similar analysis, in order to confirm what is stated in the current meta-analysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are very grateful to all the participants in this study.
Author contributions
W-ZM, Z-ZY, and ZZ designed the study. ZZ, and Z-ZY acquired the study data. W-PC and Z-YZ analyzed and interpreted the data. Z-ZY and W-PC wrote the first draft of the manuscript. All authors revised the manuscript and approved it for publication.
Funding
This work was funded by the Natural Science Foundation of Tianjin (No.23JCYBJC01130), the Key Discipline Construction Project of Tianjin Stomatological Hospital (No.2022P01), Tianjin High-level Talents Development Program for the Health Fields (No. TJSQNYXXR-D2-114) and Tianjin Health Research Project (No. TJWJ2023MS034).
Data availability
The data for the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zheng Zhang, Zhenyu Zhang and Pengcheng Wang contributed equally to this work.
Contributor Information
Zhitao Wang, Email: wangzhitao6@hotmail.com.
Zuomin Wang, Email: wzuomin@sina.cn.
References
- 1.Stanford CM. Dental implants. A role in geriatric dentistry for the general practice? J Am Dent Assoc. 2007;138(Suppl):S34–40. [DOI] [PubMed] [Google Scholar]
- 2.Jepsen S, Berglundh T, Genco R, Aass AM, Demirel K, Derks J, Figuero E, Giovannoli JL, Goldstein M, Lambert F, et al. Primary prevention of peri-implantitis: managing peri-implant mucositis. J Clin Periodontol. 2015;42(Suppl 16):S152–157. [DOI] [PubMed] [Google Scholar]
- 3.Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, Mealey BL, Papapanou PN, Sanz M, Tonetti MS. A new classification scheme for periodontal and peri-implant diseases and conditions - introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45(Suppl 20):S1–8. [DOI] [PubMed] [Google Scholar]
- 4.Gharpure AS, Latimer JM, Aljofi FE, Kahng JH, Daubert DM. Role of thin gingival phenotype and inadequate keratinized mucosa width (< 2 mm) as risk indicators for peri-implantitis and peri-implant mucositis. J Periodontol. 2021;92(12):1687–96. [DOI] [PubMed] [Google Scholar]
- 5.Ng E, Tay JRH, Mattheos N, Bostanci N, Belibasakis GN, Seneviratne CJ. A mapping review of the pathogenesis of Peri-implantitis: the biofilm-mediated inflammation and bone dysregulation (BIND) hypothesis. Cells 2024, 13(4). [DOI] [PMC free article] [PubMed]
- 6.Lang NP, Loe H. The relationship between the width of keratinized gingiva and gingival health. J Periodontol. 1972;43(10):623–7. [DOI] [PubMed] [Google Scholar]
- 7.Berglundh T, Lindhe J, Ericsson I, Marinello CP, Liljenberg B, Thomsen P. The soft tissue barrier at implants and teeth. Clin Oral Implants Res. 1991;2(2):81–90. [DOI] [PubMed] [Google Scholar]
- 8.Ivanovski S, Lee R. Comparison of peri-implant and periodontal marginal soft tissues in health and disease. Periodontol 2000. 2018;76(1):116–30. [DOI] [PubMed] [Google Scholar]
- 9.Babayigit O, Ucan-Yarkac F. Influence of vertical mucosal thickness and keratinized mucosal width on peri-implant health and marginal bone loss: a prospective study with a 2-year follow-up. Medicina oral, patologia oral y cirugia bucal. 2023. [DOI] [PMC free article] [PubMed]
- 10.Felice P, Bonifazi L, Pistilli R, Ferri A, Gasparro R, Barausse C. Influence of Keratinized tissue on short Dental implants: a parallel cohort Retrospective Study on 217 implants with a Mean follow-up of 4.1 years. Int J Oral Maxillofac Implants. 2023;38(3):462–7. [DOI] [PubMed] [Google Scholar]
- 11.Pranskunas M, Poskevicius L, Juodzbalys G, Kubilius R, Jimbo R. Influence of Peri-implant Soft tissue Condition and Plaque Accumulation on Peri-implantitis: a systematic review. J oral Maxillofacial Res. 2016;7(3):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravida A, Arena C, Tattan M, Caponio VCA, Saleh MHA, Wang HL, Troiano G. The role of keratinized mucosa width as a risk factor for peri-implant disease: a systematic review, meta-analysis, and trial sequential analysis. Clin Implant Dent Relat Res. 2022;24(3):287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurbuz E, Ceylan E, Yurttas M. Evaluation of the significance of keratinized mucosa on peri-implant tissue health: a prospective clinical trial. Aust Dent J. 2023;68(2):105–12. [DOI] [PubMed] [Google Scholar]
- 14.Carvalhaes JM, Ponzoni D, Tonini KR, de Carvalho PSP. Clinical study on the association between keratinized mucosa and peri-implant health when external hexagon implants are installed in the posterior region of the maxilla and mandible: a cohort study. Int J Oral Maxillofac Surg. 2024;53(3):231–8. [DOI] [PubMed] [Google Scholar]
- 15.Tastan Eroglu Z, Ozkan Sen D, Oncu E. Association of Peri-implant Keratinized Mucosa Width and Mucosal thickness with early bone loss: a cross-sectional study. J Clin Med 2024, 13(7). [DOI] [PMC free article] [PubMed]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan RL, Whaley P, Thayer KA, Schunemann HJ. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121(Pt 1):1027–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta P, Lim LP. The width of the attached gingiva–much ado about nothing? J Dent. 2010;38(7):517–25. [DOI] [PubMed] [Google Scholar]
- 19.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle—Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available from: http://www.ohrica/programs/clinical_epidemiology/oxfordhtm 2009.
- 20.Schrott AR, Jimenez M, Hwang JW, Fiorellini J, Weber HP. Five-year evaluation of the influence of keratinized mucosa on peri-implant soft-tissue health and stability around implants supporting full-arch mandibular fixed prostheses. Clin Oral Implants Res. 2009;20(10):1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mericske-Stern R, Steinlin Schaffner T, Marti P, Geering AH. Peri-implant mucosal aspects of ITI implants supporting overdentures. A five-year longitudinal study. Clin Oral Implants Res. 1994;5(1):9–18. [DOI] [PubMed] [Google Scholar]
- 22.Chung DM, Oh TJ, Shotwell JL, Misch CE, Wang HL. Significance of keratinized mucosa in maintenance of dental implants with different surfaces. J Periodontol. 2006;77(8):1410–20. [DOI] [PubMed] [Google Scholar]
- 23.Kim BS, Kim YK, Yun PY, Yi YJ, Lee HJ, Kim SG, Son JS. Evaluation of peri-implant tissue response according to the presence of keratinized mucosa. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2009;107(3):e24-28. [DOI] [PubMed]
- 24.Crespi R, Cappare P, Gherlone E. A 4-year evaluation of the peri-implant parameters of immediately loaded implants placed in fresh extraction sockets. J Periodontol. 2010;81(11):1629–34. [DOI] [PubMed] [Google Scholar]
- 25.Boynueğri D, Nemli SK. Significance of keratinized mucosa around dental implants: a prospective comparative study. Clin Oral Implants Res. 2013;24(8):928–33. [DOI] [PubMed] [Google Scholar]
- 26.Yun-xin ZHANG, Rong SHU, Yu-feng X. Evaluation of the influence of keratinized mucosa width on peri-implant soft and hard tissue health. J SHANGHAI JIAO TONG Univ (MEDICAL SCIENCE). 2014;34(2):173–6. [Google Scholar]
- 27.Buyukozdemir Askin S, Berker E, Akincibay H, Uysal S, Erman B, Tezcan I, Karabulut E. Necessity of keratinized tissues for dental implants: a clinical, immunological, and radiographic study. Clin Implant Dent Relat Res. 2015;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 28.Yang Qian C, Fujun, Li C. li T: Observational study on the clinical effect of keratinized gingival width on implant repair. Contemporary Medical Symposium. 2017;15(10):12–14.
- 29.Crespi R, Cappare P, Crespi G, Gastaldi G, Romanos GE, Gherlone E. Midfacial tissue Assessment of the Effect of amount of Keratinized Mucosa on Immediate Temporarization of Fresh Socket implants: 8-Year follow-up. Int J Periodontics Restor Dent. 2019;39(2):227–32. [DOI] [PubMed] [Google Scholar]
- 30.Perussolo J, Souza AB, Matarazzo F, Oliveira RP, Araujo MG. Influence of the keratinized mucosa on the stability of peri-implant tissues and brushing discomfort: a 4-year follow-up study. Clin Oral Implants Res. 2018;29(12):1177–85. [DOI] [PubMed] [Google Scholar]
- 31.Jie-lei QIAN, Rong SHU, Yi-wei WANG, Yu-feng X. Influence of keratinized mucosa on peri-implant soft tissue health among patients with chronic periodontitis susceptibility. J SHANGHAI JIAO TONG Univ (MEDICAL SCIENCE). 2018;38(8):930–3. [Google Scholar]
- 32.Sukuroglu E, Baltacioglu E. Analyses of clinical and osteoimmunological parameters on keratinized mucosa around dental implants. Niger J Clin Pract. 2019;22(5):652–60. [DOI] [PubMed] [Google Scholar]
- 33.Bouri A Jr., Bissada N, Al-Zahrani MS, Faddoul F, Nouneh I. Width of keratinized gingiva and the health status of the supporting tissues around dental implants. Int J Oral Maxillofac Implants. 2008;23(2):323–6. [PubMed] [Google Scholar]
- 34.Adibrad M, Shahabuei M, Sahabi M. Significance of the width of keratinized mucosa on the health status of the supporting tissue around implants supporting overdentures. J Oral Implantol. 2009;35(5):232–7. [DOI] [PubMed] [Google Scholar]
- 35.Esper LA, Ferreira SB Jr., Rde K, de Almeida O. The role of keratinized mucosa in peri-implant health. Cleft palate-craniofacial Journal: Official Publication Am Cleft Palate-Craniofacial Association. 2012;49(2):167–70. [DOI] [PubMed] [Google Scholar]
- 36.Nattinee Amponpan R, Kuphasuk Y, Kuphasuk, Kerdvongbundit V. The association of buccal keratinized tissue width and periodontal biotype on soft tissue surrounding dental implant. Mahidol Dent J. 2014;34(3):311–20. [Google Scholar]
- 37.Esfahanizadeh N, Daneshparvar N, Motallebi S, Akhondi N, Askarpour F, Davaie S. Do we need keratinized mucosa for a healthy peri-implant soft tissue? Gen Dent. 2016;64(4):51–5. [PubMed] [Google Scholar]
- 38.Souza AB, Tormena M, Matarazzo F, Araujo MG. The influence of peri-implant keratinized mucosa on brushing discomfort and peri-implant tissue health. Clin Oral Implants Res. 2016;27(6):650–5. [DOI] [PubMed] [Google Scholar]
- 39.Ueno D, Nagano T, Watanabe T, Shirakawa S, Yashima A, Gomi K. Effect of the Keratinized Mucosa Width on the Health Status of Periimplant and Contralateral Periodontal tissues: a cross-sectional study. Implant Dent. 2016;25(6):796–801. [DOI] [PubMed] [Google Scholar]
- 40.Fatih MTZF, Gul SS. Significance of the Width of Keratinized Mucosa on PeriImplant Tissue Health: a cross-sectional analysis. Sulaimani Dent J. 2019;6(1):21–8. [Google Scholar]
- 41.Grischke J, Karch A, Wenzlaff A, Foitzik MM, Stiesch M, Eberhard J. Keratinized mucosa width is associated with severity of peri-implant mucositis. A cross-sectional study. Clin Oral Implants Res. 2019;30(5):457–65. [DOI] [PubMed] [Google Scholar]
- 42.Monje A, Blasi G. Significance of keratinized mucosa/gingiva on peri-implant and adjacent periodontal conditions in erratic maintenance compliers. J Periodontol. 2019;90(5):445–53. [DOI] [PubMed] [Google Scholar]
- 43.Ravida A, Saleh I, Siqueira R, Garaicoa-Pazmino C, Saleh MHA, Monje A, Wang HL. Influence of keratinized mucosa on the surgical therapeutical outcomes of peri-implantitis. J Clin Periodontol. 2020;47(4):529–39. [DOI] [PubMed] [Google Scholar]
- 44.Kabir L, Stiesch M, Grischke J. The effect of keratinized mucosa on the severity of peri-implant mucositis differs between periodontally healthy subjects and the general population: a cross-sectional study. Clin Oral Invest. 2021;25(3):1183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romanos G, Grizas E, Nentwig GH. Association of Keratinized Mucosa and Periimplant Soft tissue Stability around implants with platform switching. Implant Dent. 2015;24(4):422–6. [DOI] [PubMed] [Google Scholar]
- 46.Tonetti MS, Chapple IL, Jepsen S, Sanz M. Primary and secondary prevention of periodontal and peri-implant diseases: introduction to, and objectives of the 11th European workshop on periodontology consensus conference. J Clin Periodontol. 2015;42(Suppl 16):S1–4. [DOI] [PubMed]
- 47.Ramanauskaite A, Schwarz F, Sader R. Influence of width of keratinized tissue on the prevalence of peri-implant diseases: a systematic review and meta-analysis. Clin Oral Implants Res. 2022;33(Suppl 23):8–31. [DOI] [PubMed] [Google Scholar]
- 48.Stefanini M, Pispero A, Fabbro MD, Gobbato L, Ghensi P, Lodi G, Sculean A, Zucchelli…G: The Effect of Keratinized Mucosa on Peri-Implant Health and Patient-Reported Outcome Measures: A Systematic Review and Meta-Analysis. Appl Sci. 2023;13(15):8631.
- 49.Thoma DS, Naenni N, Figuero E, Hammerle CHF, Schwarz F, Jung RE, Sanz-Sanchez I. Effects of soft tissue augmentation procedures on peri-implant health or disease: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(Suppl):32–49. [DOI] [PubMed] [Google Scholar]
- 50.Figuero E, Graziani F, Sanz I, Herrera D, Sanz M. Management of peri-implant mucositis and peri-implantitis. Periodontol 2000. 2014;66(1):255–73. [DOI] [PubMed] [Google Scholar]
- 51.Serino G, Turri A. Extent and location of bone loss at dental implants in patients with peri-implantitis. J Biomech. 2011;44(2):267–71. [DOI] [PubMed] [Google Scholar]
- 52.Mahardawi B, Jiaranuchart S, Damrongsirirat N, Arunjaroensuk S, Mattheos N, Somboonsavatdee A, Pimkhaokham A. The lack of keratinized mucosa as a risk factor for peri-implantitis: a systematic review and meta-analysis. Sci Rep. 2023;13(1):3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for the current study are available from the corresponding author upon reasonable request.