Abstract
Background
Hypertension, the first global modifiable risk factor for cardiovascular disease (CVD) morbidity and mortality, is a consequential and remediable threat to the health of individuals and society. Therefore, we conducted this study to explore the role of calcium (Ca++), magnesium (Mg++), and vitamin D (Vit-D) supplementation as complementary therapies for hypertension, focusing on their effects on systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse rate.
Methods
This systematic review and meta-analysis examined relevant 6509 articles in PubMed, Scopus, Web of Science, and Cochrane CENTRAL up to October 2024. The primary outcome was the difference in blood pressure measurements (systolic and diastolic) and the pulse rate. The extracted data were analyzed using Open Meta Analyst software.
Results
This systematic review and meta-analysis included 40 studies; of them, 24 studies were analyzed. Ca++ was associated with a significant drop in the DBP (MD: -2.04, 95% CI [-3.39, -0.69], P = 0.01), but not in the SBP (P = 0.34) or pulse rate (P = 0.84). Mg++ significantly reduced DBP (MD: -1.64, 95% CI [-3.19, -0.09], P = 0.04), but had no significant effect on the SBP (P = 0.16) or pulse rate (P = 0.81). The estimated effect of Vit-D showed a significant reduction in SBP (MD: -2.83, 95% CI [-5.47, -0.199], P = 0.04) and DBP (MD: -1.64, 95% CI [-2.97, -0.3], P = 0.01).
Conclusion
Ca++ and Mg++ significantly reduced DBP but had no significant effect on SBP or the pulse rate. Whereas, vitamin D significantly reduced SBP and DBP.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-025-04809-x.
Keywords: Hypertension, Calcium, Magnesium, Vitamin D, Complementary therapies, Supplementation, Pulse rate
Introduction
According to the 2023 World Health Organization (WHO) report, 1 in 3 persons had hypertension (silent disease) [1], defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg [2]. Globally, for example, the prevalence of hypertension among adults was higher in low- and middle-income countries (LMIC) (31.5%, 1.04 billion people) than in high-income countries (28.5%, 349 million people). However, from 1990 to 2015, the estimated number of BP-related all-cause and cardiovascular disease (CVD) deaths significantly increased; this is a leading preventable risk factor for all-cause CVD mortality [3, 4].
Sociodemographic, environmental, and behavioral factors are likely to explain racial and ethnic disparities in mean blood pressure and hypertension prevalence. Other modifiable risk factors for hypertension include alcohol, obesity, an unhealthy diet high in sodium and low in potassium, a lack of physical activity, air pollution, psychological stress, sleep disorders, and noise exposure [5–11].
The global mean BP has stayed steady or slightly dropped during the past four decades due to the widespread usage of antihypertensive drugs, which doubled to 1.3 billion from 650 million between 1990 and 2019 [1, 4]. Despite investing $216 million in primary health care to enhance hypertension care, 4 out of 5 hypertensive cases remain untreated. Treatment could save 76 million BP-related deaths between 2023 and 2050 [1, 12].
Primary care should offer improved hypertension treatment programs because the economic benefits outweigh the costs by an 18 to 1 ratio. Combining antihypertensive medication with additional nutrients and dietary supplements(DSs), such as the Dietary Approaches to Stop Hypertension (DASH) I and II diets, can have additive or synergistic benefits [2, 13, 14]. DSs, such as calcium (Ca++), magnesium (Mg++), and vitamin D, are a subgroup of complementary and alternative medicine (CAM), which the public commonly uses and touts as natural ways to affect blood pressure [14–16].
Ca++, a structural mineral required to contract muscles and signal cells, is abundant in salmon, soybeans, kale, cheese, and yogurt. BP regulation by Ca++ is unknown. Most studies found an inverse relationship between BP and Recommended Dietary Allowance (RDA) Ca++ intake (1000–1300 mg/day). Others found minimal or no hypotensive impact from Ca++ ingestion in normal and hypertensive subjects. Therefore, the recommendation was to combine Ca++ intake (1300 mg/day) with DASH to reduce the risk of hypertension [17, 18].
Oily fish, egg yolks, and red meat contain fat-soluble vitamin D (Vit-D). Dietary vitamin D becomes active vitamin D3 when exposed to sunlight. It maintains musculoskeletal, cardiovascular, and neurological health by controlling calcium homeostasis. Numerous studies have established an inverse link between Vit-D (or its metabolites, or its active form, Vit D3) and blood pressure and its subsequent consequences, such as CVD. Few trials showed no or slight hypotensive effects. Excess Vit-D can cause renal failure, stiffness (hypercalcemia), and vascular resistance. A few studies found that Vit-D and calcium had better hypotensive activity than vitamin D alone or calcium alone [19–22].
Magnesium (Mg++), “nature’s physiological calcium channel blocker,” is a cofactor for over 300 enzyme systems, including delta-6-desaturase. This enzyme converts linoleic acid to gamma linoleic acid. This is the longest stage in generating prostaglandin E (PGE1), which relaxes blood arteries and prevents platelet formation. Many foods contain it, especially nuts, unpolished grains, and leafy greens. Hypertension, cerebral and coronary vasospasms, and muscle cramps can result from magnesium shortage. For every 100 mg of dietary Mg++, ischemic stroke risk decreased by 8%. Hypertensive patients have altered Mg++ transporter TRPM7 channels [23–27].
The consumption of DSs as supplemental therapy differs by region, which may explain hypertension trends and incidence worldwide. Thus, lifestyle interventions targeting these risk factors may reduce worldwide inequities, hypertension prevalence, and BP [28]. Researchers have studied complementary therapies for blood pressure management, while pharmaceuticals remain beneficial. Numerous studies have demonstrated the lowering of blood pressure in hypertensives by Ca++, Mg++, and Vit-D supplements. Some investigations suggest the opposite hypothesis [29].
The aim of this study was to explore the role of Ca++, Mg++, and Vit-D supplementation as complementary therapies for hypertension, focusing on their effects on systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse rate.
Methods
This systematic review and metanalysis followed the updated PRISMA 2020 guidelines and all steps reported in the Cochrane Handbook of Systematic Reviews [30, 31].
Eligibility criteria
This study considered the retrieved articles eligible if they conformed to our PICOST (Population, Intervention, Control, Outcomes, Study Design, and Timeframe) framework. P: hypertensive patients; I: complementary therapy (Ca++, Mg++, and Vit-D); C: placebo; O: outcome, including SBP, DBP, and pulse rate; S: clinical trials in English, excluding review articles, unpublished manuscripts, conference abstracts, and book chapters; and T: from inception up to October 2024.
Information sources and search strategy
A well-developed systematic search was performed through four online databases: PubMed, Scopus, Web of Science, and Cochrane CENTRAL. We looked for eligible articles up to October 2024 using the following search query: [(“complementary therap*” OR “alternative therap*” OR “complementary medicine” OR “alternative medicine” OR “supplementary therap*” OR “Vitamin D” OR “Calcitriol 24-Hydroxylase” OR “1 alpha,25-Dihydroxycholecalciferol-24-Hydroxylase” OR Cholecalciferol OR calcium OR magnesium) AND (hypertension OR hypertensive OR “high blood pressure” OR “elevated blood pressure”) AND (“clinical trial” OR “randomized controlled trial” OR RCT)]. A manual search was performed to examine the reference list of the included articles and related review articles.
Selection process
We retrieved the search results from the databases using EndNote software. After duplicates removal, we extracted the articles into Rayyan software to start the screening process [32]. Four authors in two groups independently screened the title and abstracts of the resulting studies based on the eligibility criteria. The senior author, when necessary, resolved any disagreement through discussion. Then, the same authors independently reviewed the full texts of the included articles. Any disagreement was resolved by consulting the senior author.
The data collection process
Four authors in two groups independently extracted the matched data using a well-developed Excel sheet. The senior author scrutinized the extracted data and resolved any disagreements through discussion. The extracted data were as follows: (1) Baseline characteristics, such as sample size, age, and sex; (2) A summary of the included article, detailing the design, sample sizes, health state, and dose and duration of treatment; and (3) Outcomes of interest, including blood pressure measurements and pulse rate.
Quality assessment
Two authors independently assessed the quality of the included articles using the Cochrane risk of bias assessment tool for randomized controlled trials. Finally, they classified RCTs as having a high, unclear, or low risk of bias [33] according to the following domains: randomization process, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Any disagreement was resolved by discussion with the third senior author.
Data synthesis
The extracted data were analyzed using Open Meta Analyst software [34]. We pooled continuous data using mean differences. We considered the data statistically significant if the P-value was < 0.05 and statistically heterogeneous if the P-value of the chi-square test was < 0.05 and the I-square test was > 60% [35]. A fixed-effect model was used except for the pooled heterogeneous studies, where a random-effect model was used. A sensitivity analysis was performed to identify the source of heterogeneity among the included studies. Because all the assessed outcomes included < 10 studies, we did not perform Egger’s test of publication bias [36].
Results
Study selection
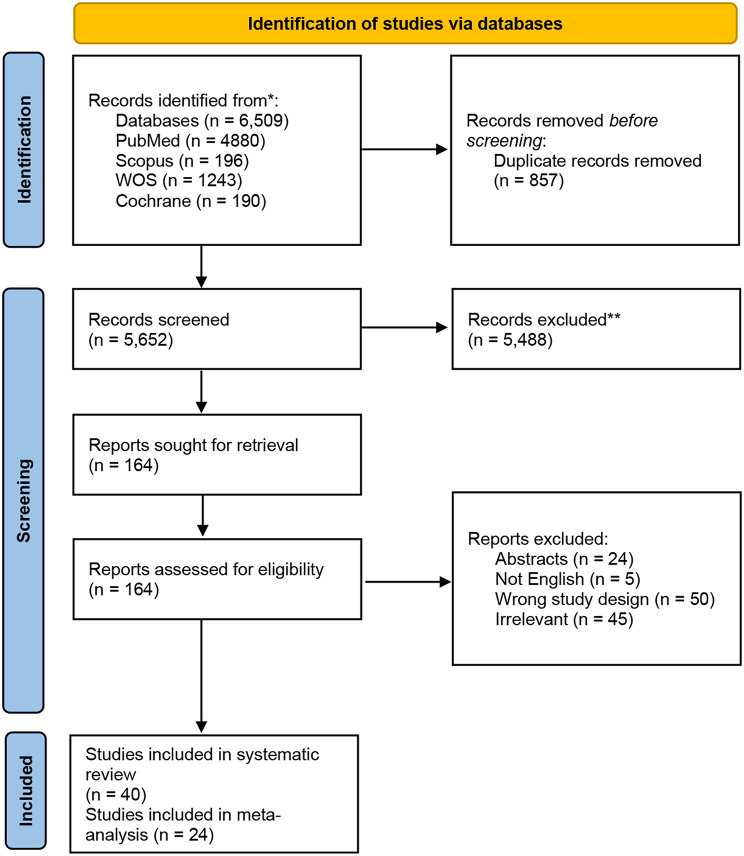
Our database search resulted in 6509 records, which were reduced to 5652 after removing the duplicates. Following title and abstract screening, 164 studies were assessed for eligibility in full-text screening. Finally, the total number of included studies reached 40 studies, including 24 studies for meta-analysis. Figure 1 represents the PRISMA flow chart of the selection process.
Fig. 1.
The PRISMA flow chart of selection process
Study characteristics
The selected articles included a total of 2979 participants. Most of them had mild to moderate hypertension at the time of recruiting. Their ages ranged between 18 and 82 years old. We classified the included articles into three groups based on the intervention (Ca++, Mg++, and Vit-D). The included articles were designed as controlled clinical trials, either randomized controlled or crossover trials. The follow-up duration varied between only one week, as in Gonçalves 2020 [37], and one year and a half, as in Sluyter 2017 [38]. Table 1 represents a summary of the included articles.
Table 1.
A summary of the included articles
| Study ID | Design | Duration (wks) | Population | Sample size | Intervention group | Dose | N | Control group | N |
|---|---|---|---|---|---|---|---|---|---|
| Dazai 1994 [72] | Interventional | 2 | Mild to moderate HTN / stage I, II essential HTN | 14 | Ca++ | 7.7 g | 14 | placebo | 5 |
| de Paula 2020 [73] | RCT | 8 | HTN with diabetes | 43 | Vit-D | 100,000 IU | 22 | pacebo | 21 |
| Ferrara 1992 [74] | Clinical trial | 24 | Mild to moderate primary HTN | 14 | Mg++ | 15mmol/day | 7 | placebo | 7 |
| Goncalves 2020 [37] | Pilot study | 1 | Hypertensive elderly women | 11 | Vit-D |
Single dose 200.000 IU |
11 | placebo | 5 |
| Grobbee 1986 [75] | RCT | 12 | Mild HTN | 90 | Ca++ | 4-7gm | 46 | placebo | 44 |
| Guerrero-romero 2008 [52] | RCT | 16 | Diabetic, HTN on captopril or single drug | 79 | Mg++ | 2.5 g | 40 | placebo | 39 |
| Hatzistavri 2009 [51] | RCT | 12 | Uncomplicated HTN | 48 | Mg++ | 600 mg | 24 | placebo | 24 |
| Sanjuliani 1996 [39] | RCTCrossover | 6 | Mild to mederate HTN | 15 | Mg++ | 600 mg | 15 | placebo | 15 |
| Sheikh 2020 [76] | RCT | Essential HTN | 208 | Vit-D | 1000U | 104 | placebo | 104 | |
| 50,000U | |||||||||
| Sluyter 2017 [38] | RCT | 1.5 year | Adult men and women aged 50 to 84 years and resident in Auckland, New Zealand. | 517 | Vit-D | 200 000 IU (initial dose) followed 1 month later by monthly 100 000-IU doses | 256 | placebo | 261 |
| Cappuccio 1987 [77] | RCT Cross over | 4 | Mild to moderate essential HTN | 18 | Ca++ | 40 mmol/day or 1600 mg/day | 18 | placebo | 18 |
| Chen 2014 [78] | RCT | 24 | HTN Grade: 1&2 | 126 | Vit-D | 2000 IU/d | 63 | placebo | 63 |
| Cunhaa 2016 [79] | RCT | 24 | HTN women | 35 | Mg++ | 600 mg/day | 17 | placebo | 18 |
| Widman 1993 [80] | RCT Cross over | 21 | HTN patients | 17 |
Mg hydroxide |
15 mmol/day | 17 | placebo | 17 |
| Weinberger 1993 [81] | RCT Cross over | 8 | Normotensive and hypertensive | 27 | Ca++ | 1.5 g/day for 8 weeks | 27 | placebo | 27 |
| Witham 2014 [82] | RCT | 24 | HTN patients | 68 | Vit-D | 100,000 U oral every 2 months | 34 | placebo | 34 |
| Witham 2013 [83] | RCT | 48 | Elderly with isolated systolic HTN and vit D levels < 30 ng/mL | 159 |
Vit-D Group |
of 100 000 U oral every 3 months for 1 year | 80 | placebo | 79 |
| Zemel 1990 [84] | RCT | 12 | Mild HTN | 13 | Mg++ | 40 mmol | 7 | placebo | 6 |
| Zhou 1994 [44] | RCT | 14 | HTN patients | 57 | Ca++ | 1000 mg/day | 44 | placebo | 38 |
| Zoccal 1988 [85] | RCT Cross over | 8 | Mild to moderate essential HTN | 23 | Ca++ | 1 g/d for 8 weeks orally | 23 | placebo | 23 |
| McCarron 1985 [86] | RCT Cross over | 16 w and 4 w washout | 48 hypertensive and 32 normotensive | 80 | Ca++ | 1 g | 80 | placebo | 80 |
| Meese 1978 [87] | RCT Cross over | 8 w and 2 w washout | Uncomplicated 1ry HTN | 41 began the study / 28 completed it | Ca++ | 800 mg/day | 35 | placebo | 17 |
| Kawano 1998 [88] | RCT Cross over | 16 | Untreated or treated hypertensive patients | 60 | Mg++ | 20 mmol/d | 60 | placebo | 60 |
| Mozaffari-Khosravi 2014 [40] | RCT | 8 | Patients with elevated BP and vit D deficiency | 42 | Vit-D | 50 000 IU/week | 19 | oral liquid paraffin | 20 |
| Nowson 1989 [89] | RCT | 8 | Untreated, mild hypertensive subjects | 25 | Mg++ | 10 mmol/day | 12 | placebo | 13 |
| Nowson 1988 [90] | RCT Cross over | 12 | Ca++ | 20 mmol/day | 12 | placebo | 12 | ||
| Nowson 1989 [91] | RCT | Forty-seven patients with mildhypertension HTN) and 48 normotensive patients | 95 | Ca++ | 10 mmol/day | 31 | placebo | 33 | |
| 20 mmol/day | 31 | ||||||||
| Larsen 2012 [92] | RCT | 20 | Hypertensive patients residing in Denmark | 130 | Vit-D | 75 µg/day (3,000 IU) | 55 | placebo | 57 |
| Lasaridis 1989 [93] | RCT Cross over | 10 days | Patients with uncomplicated essential HTN | 18 | Ca++ | 1 g | 9 | placebo | 9 |
| Pikilidou 2009 [42] | RCT | 8 | Patients with type 2 DM and HTN | 31 | Ca++ | 1,500 mg/d | 15 | placebo | 16 |
| Pilz 2015 [41] | RCT |
Participants with arterial hypertension and 25-hydroxyvitamin D levels below 30 ng/mL. |
200 | Vit-D | 2800 IU/d | 100 | placebo | 100 | |
| Lind 1989 [94] | RCT | HTN | 42 | Alphacalcidol (Vit-D analogue) | 1 µ% | 39 | placebo | ||
| Barrios 2016 [95] | RCT | 6 | Essential HTN | 45 | Vit-D | 1,000 IU daily | 18 | placebo | 18 |
| Cappuccio 1985 [96] | RCT | 8 | Mild to moderate essential HTN | 17 | Mg++ | 15 mmol Mg/day | 17 | placebo | 17 |
| Wimalawansa 1993 [97] | RCT | 18 | Mild to moderate essential HTN | 8 | Ca++ | 35 mmol/day | 8 | placebo | |
| Witteman 1994 [98] | RCT | 24 | Mild to moderate essential HTN | 91 | Mg++ | 20mmol/d | 47 | placebo | 44 |
| Theiler-Schwetz 2020 [99] | RCT | . | Adults with arterial HTN and a 25(OH)D serum concentration < 30 ng/Ml | 200 | Vit-D | 2800 IU/day | 100 | placebo | 100 |
| Morris 1991 [100] | Clinical trial | 48 w CaCO3, followed by 12 w placebo |
Volunteers 50–80 years with SBP (when not taking antihypertensive medication) was consistently > 140 mmHg or if DBP was > 90 mmHg during a 4-week baseline period |
128 | Ca++ | 1 g/d | 103 | placebo | 12 |
| Lind 1991 [101] | RCT | 24 | Adults with DBP > 95 mmHg or DBP 85 to 94 mmHg together with SBP > 165 mmHg and without antihypertensive medication | 71 | Mg++ | 15 mmol | 49 | placebo | 22 |
| Santos 2024 [102] | RCT | 24 | Age- and gender-matched adults with obesity-related hypertension and vitamin D deficiency | 36 | Cholecalciferol | 18 | Placebo | 18 |
Quality assessment
According to the risk of bias assessment tool, we assessed the included studies as high risk, low risk, or some concerns. Most of the included studies fell into the category of low risk (n = 11) or raised some concerns. Four of the included studies were scored as high risk of bias. The supplementary table presents the quality assessment of the included articles.
The effect of Ca++ on blood pressure and pulse
In seven studies, Ca++ was found to have a non-significant effect on lowering SBP (MD: -1.28, 95% CI [-3.88, 1.32], P = 0.34). Pooled studies were homogenous (P = 0.97, I2 = 0%) (Fig. 2).
Fig. 2.
A forest plot of the effect of calcium on systolic blood pressure
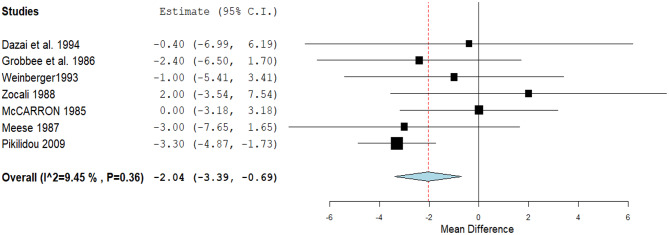
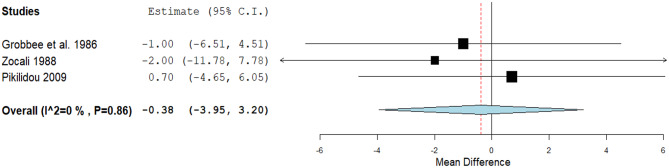
Seven studies achieved a statistically significant reduction in DBP. (MD: -2.04, 95% CI [-3.39, -0.69], P = 0.01). Pooled studies were homogenous (P = 0.36, I² = 9.4%). (Fig. 3). A non-significant decrease in the pulse rate was detected through three studies (MD: -0.38, 95% CI [-3.95, 3.2], P = 0.84). Pooled studies were homogenous (P = 0.86, I² = 0%) (Fig. 4).
Fig. 3.
A forest plot of the effect of calcium on diastolic blood pressure
Fig. 4.
A forest plot of the effect of calcium on pulse rate
The effect of Mg++ on blood pressure and pulse rate
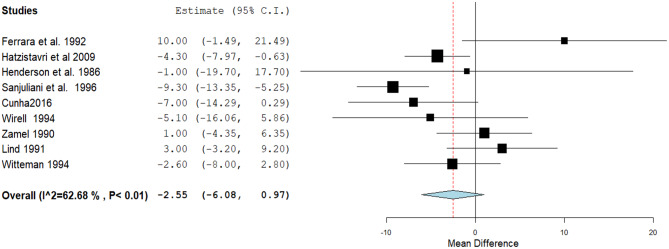
Nine studies detected a non-statistically significant reduction in the SBP. The mean difference was − 2.55, with a 95% confidence interval of -6.08 to 0.97, and a P value of 0.16. Pooled studies were heterogeneous (P < 0.01, I² = 62.6%) (Fig. 5). Heterogeneity was best resolved by excluding Sanjuliani et al.‘s 1996 [39] without (P = 0.11, I2 = 39.4%) altering the results.
Fig. 5.
A forest plot of the effect of Magnesium on systolic blood pressure
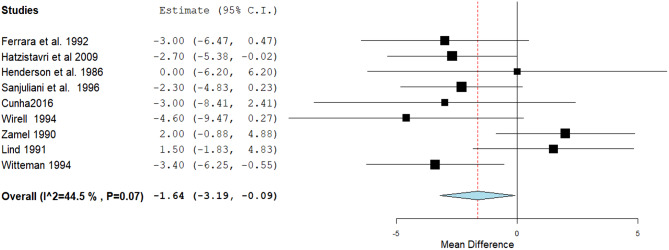
The estimated effect showed a statistically significant reduction in the DBP through nine studies (MD: -1.64, 95% CI [-3.19, -0.09], P= 0.04). Pooled studies were homogenous (P = 0.07, I² = 44.5%) (Fig. 6).
Fig. 6.
A forest plot of the effect of Magnesium on diastolic blood pressure
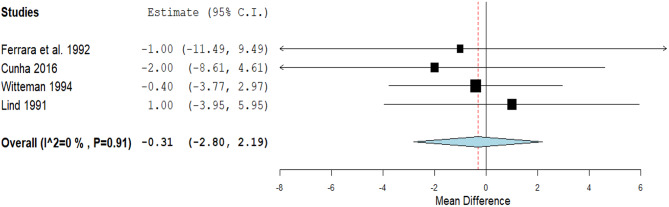
A non-significant decrease in the pulse rate was detected through four studies (MD: -0.31, 95% CI [-2.8, 2.19], p = 0.81). Pooled studies were homogenous (P = 0.91, I² = 0%) (Fig. 7).
Fig. 7.
A forest plot of the effect of Magnesium on pulse rate
The effect of vitamin D on blood pressure
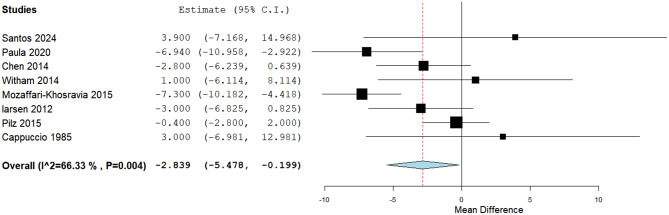
The estimated effect showed a statistically significant reduction in the SBP through eight studies (MD: -2.84, 95% CI [-5.48, -0.199], p = 0.04). Pooled studies were heterogeneous (P = 0.004, I² = 66.3%) (Fig. 8). Heterogeneity was resolved by Mozaffari-Khosravia 2015 [40] exclusion (P = 0.08, I² = 47.9%); a significant difference was obtained (MD: -2.3, 95% CI [-4.7, -0.07], p = 0.057).
Fig. 8.
A forest plot of the effect of Vit-D on systolic blood pressure
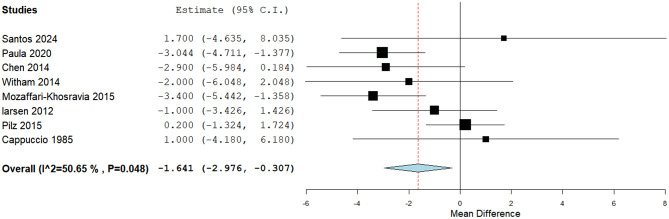
A statistically significant reduction in the DBP was detected through eight studies (MD: -1.64, 95% CI [-2.98, -0.3], P= 0.01). Pooled studies were heterogeneous (P = 0.04, I² = 50.6%) (Fig. 9). Heterogeneity was resolved by Pilz 2015 [41] exclusion (P = 0.48, I²=0%); no significant difference was obtained.
Fig. 9.
A forest plot of the effect of Vit-D on diastolic blood pressure
Discussion
Hypertension is characterized by blood vessel damage due to vascular inflammation, structural remodeling, stiffer arteries, less flexibility, and loss of elasticity [2]. Therefore, etiologies exist for all non-mechanical causes, including metabolic, endocrine, nutritional, toxic, infectious, and others. Therefore, in this meta-analysis, we studied the effect of the following DS as complementary therapies in the management of hypertension and found that Ca++ significantly reduces DBP but has no significant effect on SBP or the pulse rate. Mg++ significantly reduces SBP and DBP but has no significant effect on the pulse rate. Vitamin D significantly reduces SBP and DBP.
The effect of calcium supplementation on blood pressure and pulse
After comparing the SBP and DBP across 7 studies, this meta-analysis concluded that there was no statistically significant reduction in SBP (MD 1.28, 95% CI [3.88, 1.32], p = 0.34). After 8 weeks, Pikilidou et al. conducted a randomized control trial and found that the addition of Ca++ had no appreciable impact on the average ambulatory systolic and diastolic loads, pulse pressure, DBP, and SBP for the entire 24-hour day and night [42]. The belief that giving dietary Ca++ supplements to people with mild hypertension will lower blood pressure more effectively than a placebo is debunked [43]. However, Zhou et al.‘s RCT results demonstrate that oral Ca++ supplementation can significantly lower blood pressure in a significant number of essential hypertensive subjects [44]. Studies of the general population indicate a relationship between hypertension and calcium [45].
This is because Ca++ is a key part of smooth muscle contraction and cell signaling transduction. Also, the amount of Ca++ inside cells can control vascular tone, which makes vessels less constricted and more open. As a result, Ca++ directly affects blood pressure. Through controlling the SNS, Ca++ may improve the diuretic (Na+ excretion), control blood volume, and regulate cardiac output [45]. Moreover, reports suggest that Ca++ modifies the RAAS system, which in turn regulates the synthesis of AT-I and subsequently modifies blood pressure. Additionally, Ca++ indirectly controls BP, particularly by influencing parathyroid hormone secretion, which directly affects blood pressure [18, 46].
Compared to the DBP, we observed a significant decrease in it (MD 2.04, 95% CI [3.39, -0.69], p = 0.01).The study by Tanji et al. contradicts the finding by Zhou et al. that oral Ca++ supplementation significantly lowers BP [43]. Additionally, there was no statistically significant decrease in the pulse rate across the three studies (MD 0.38, 95% CI [3.95, 3.2], P= 0.84). while The study conducted by Pikilidou et al. indicated a lower pulse rate [42]. The different responses and inconsistent effects of calcium supplementation on blood pressure were because of the “ionic hypothesis” of high blood pressure, heart disease, and the metabolic, functional, and structural disorders that go along with them. Currently, we do not recommend calcium supplementation as an effective means to reduce BP [14, 45].
The effect of magnesium supplementation on blood pressure and pulse
Regarding its effect on SBP, there is no significant effect. In line with a study by Lind et al., they did not find any evidence that Mg++ supplements worked for people with high-normal blood pressure or mild hypertension and do not generally recommend their use [14]. However, a 2009 study by Hatzistavri et al. indicates that taking oral Mg++ supplements may cause a slight but consistent drop in ambulatory BP in mild hypertension patients [47]. A meta-analysis of 22 trials involving 1173 patients revealed SBP reductions of 3–4 mmHg.
This can be attributed to many factors, including: (1) the effects are not as consistent as those observed with sodium (Na+) and potassium (K+); (2) the duration and the dose of Mg++ supplements. (3) In cases of renal insufficiency or co-medications that cause Mg++ retention, Mg++ supplements should be avoided or used cautiously. (4) The secondary causes of hypertension in a study by Banjanin that involved patients with essential hypertension, it was discovered that taking oral Mg++ supplements significantly reduced SBP and DBP [48–50].
Regarding its effect on the DBP, this meta-analysis from nine studies found that there was a statistically significant decrease in DBP (MD 1.64, 95% CI [3.19, -0.09], p = 0.04). This is in line with a 2009 study, which discovered that giving adults with diabetes and high blood pressure Mg++ supplements with MgCl₂ significantly reduced their SBP and DBP [51]. Furthermore, Yamamoto et al. found that supplements containing calcium and Mg++ are unlikely to lower BP in adults with high-normal DBP [52]. Numerous epidemiologic studies show a negative link between high blood pressure and eating a lot of Mg++ (at least 500–1000 mg/d), with a maximum drop of 5.6/2.8 mmHg. For example, BP dropped significantly after eight weeks of taking Mg++ supplements in 60 patients with essential hypertension. Increased Mg++ intake, along with high K+ and low Na+ intakes, or with the addition of taurine at a dose of 1000–2000 mg/d, enhances the Mg++ anti-hypertensive effects [48, 53].
Mg++ statistically significant decrease in DBP, this can be explained. Mg++ acts as a direct vasodilator, similar to a Calcium Channel Blockers (CCBs), as it competes with Na+ for binding sites and functions in vascular smooth muscle. Lowering oxLDL, HS-CRP, TBxA2, A-II, and norepinephrine and increasing PGE are some of the things that magnesium does. It also controls calcium, sodium, potassium, and pH levels inside cells and increases nitric oxide. It also enhances endothelial function. The systemic vascular resistance index and left cardiac work index both show improvements. Additionally, Mg++ improves glucose, insulin resistance, and MS. It also binds to potassium in a cooperative manner to reduce EDV and BP [48, 53–56].
Regarding its effect on the pulse rate, this meta-analysis of three studies found no statistically significant decrease. This is because Mg++ blocks NMDA receptors, regulates CCBs, maintains mitochondrial calcium levels, stops ischemia-induced glutamate release, and widens cerebral arteries. These actions lower cholesterol, stop the production of cytokines, stop nuclear factor Kb, lower oxidative stress, and stop platelets from sticking together to prevent thrombosis. Reductions in CVD and cardiac arrhythmias demonstrate these benefits; some suggested mechanisms include carotid IMT, cholesterol, and cytokine production [23–26, 57–61].
The effect of vitamin D supplementation on blood pressure and pulse rate
This meta-analysis of seven studies that used vitamin D found that both SBP and DBP went down statistically significantly (MD 3.16, 95% CI [5.84, -0.48], P= 0.02) and (MD 1.77, 95% CI [3.14, -0.41], P= 0.01). Many meta-analyses (MAs) from different cross-sectional studies in 2011 agreed that there is an inverse relationship between the amount of vitamin D in the blood and HT. Another MA from eight prospective studies in 2015 also found an inverse relationship between the amount of vitamin D (25-hydroxyvitamin D) in the blood and the risk of HT [40]. What a 2015 study showed was that giving 50,000 IU of vitamin D by mouth once a week for 8 weeks to people who were vitamin D deficient could help prevent the deficiency, work with blood pressure medicines, and keep SBP, DBP, and MAP in check [62]. However, a MA found in 2016 that a daily Vit D3 intake (dose > 800 IU/day) for more than 6 months could significantly lower SBP and DBP in both hypertension and normotensive patients [63]. In 2019, MA concluded that oral vitamin D3 consumption significantly reduced both SBP and DBP in subjects with hypertension and vitamin D deficiency [64].
This is understandable given that vitamin D controls parathyroid hormone secretion as well as calcium homeostasis, specifically calcium absorption and metabolism, by acting on voltage-dependent calcium channels and RAAS directly through renin production. As a result, co-supplementing calcium and vitamin D demonstrated improved blood pressure regulation. Vitamin D also enhances endothelial function by reducing vascular resistance, calcification, and the inflammatory response. This, in turn, increases the production of Nitric oxide and maintains the tone of the vessels. Therefore, vitamin D helps suppress complications related to CVD [65–67].
In contrast, many MAs; in 2009, vitamin D was found to significantly lower DBP (− 3.1 mm Hg) in hypertensive subjects but not to significantly change mean BP or SBP. This showed modest hypotensive activity in hypertensive patients but no changes in normotensive subjects [66]. In 2010, researchers found that oral vitamin D supplementation lowered SBP but not DBP [67]. But in 2015, a meta-analysis from 46 RCTs [68] found that there is no evidence of a BP-lowering effect, while another meta-analysis in 2020 discovered that vitamin D supplementation did not reduce BP in the general population [69].
The analysis must consider certain limitations. The study employed a relatively small sample size, potentially diminishing the statistical power to identify a significant effect and encountered a high noncompliance rate; it excluded observational studies and uncontrolled trials. The included population was of variable ages. Some studies couldn’t find small differences between the intervention and placebo groups. The study also didn’t find a significant difference in blood pressure levels between subgroups with different follow-up times. However, whether vitamin D supplements can lower blood pressure for longer periods of time (> 2 years) remains unclear. Finally, the cohort studies conducted in Europe, America, and Asia may limit the generalizability of the results to other populations [66–69].
Therefore, to maintain normal blood levels of vitamin D, the Food and Drug Administration (FDA) and WHO recommended consuming 10–20 µg/day of the vitamin in oral supplementation, which would raise vitamin D3 levels, which have an inverse relationship with blood pressure. Adjuvant therapy, which combines vitamin D3 with other minerals or macronutrients, can further reduce blood pressure in hypertensive patients who are vitamin D deficient. You can take supplements containing 500–1000 mg of magnesium daily. Amino acid-chelated magnesium formulations have the potential to enhance absorption and reduce diarrheal episodes [70, 71].
However, this meta-analysis has many strengths, as it included a total of 2979 hypertensive patients from 40 studies after the two screening steps of 6509 studies from four databases, up to October 2024, to explore the effect of three essential supplements on three essential parameters: SBP, DBP, and pulse rate.
Conclusion
This meta-analysis concluded that Ca++ significantly reduces DBP but has no significant effect on SBP or the pulse rate. However, Mg++ significantly reduces SBP and DBP but has no significant effect on the pulse rate. On the other hand, the meta-analysis for vitamin D supplementation revealed a statistically significant reduction in both SBP and DBP.
Recommendations
This meta-analysis suggests adding Mg++ and vitamin D supplements to hypertension therapy to lower SBP and DBP.
Future studies should use larger, better-controlled RCTs with longer follow-ups and standardize the dose of these supplements to find out how they should be taken to get the best blood pressure effects. These studies should also look at the long-term effects of these supplements on blood pressure, CVD, and other comorbidities, as well as how they interact with other hypertension treatments.
By following these guidelines, future studies can confirm the benefits of calcium, magnesium, and vitamin D supplements for hypertension. This will improve patient outcomes and guide therapeutic practice.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- BP
Blood pressure
- Ca++
Calcium
- CCBs
Calcium Channel Blockers
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- DSs
Dietary supplements
- LMIC
Low- and middle-income countries
- Mg++
Magnesium
- MA
Meta-analyses
- K+
Potassium
- RCTs
Randomized clinical trials
- RDA
Recommended Dietary Allowance
- Na+
Sodium
- SBP
Systolic blood pressure
- DASH
The Dietary Approaches to Stop Hypertension
- WHO
World Health Organization
- PGE
Prostaglandin E
- Vit-D
Vitamin D
- HTP
Hypertension
- IU
International Unit
Author contributions
Conceptualization: Samar A. Amer (SA), Moamen Asala (MA); Validation and methods: Dina E. Abo-elnour (DEA), Menna M. Sarhan (MM), Omar Hany Mohamed (OH), Mohamed Yousif Elnaghy (MY); Data extraction: Abdelrahman Salah Abdelrahman (AS), Hossam-Eldin Mohamed Hamdy (HE), Samar Kenawy (SK), Mohammed Baker (MB), Rawan Medhat El-Gayar (RE), Omnia Samy El-Sayed (OS); Formal analysis: DEA, MA, MM, OH, MY; Writing the original draft: S.A, Abdallah Abbas (AA), DEA; Reviewing, and editing: DEA, MA; Approve the final version of the manuscript: All authors; Funding: all authors; Supervision: DEA, SA, MA; Software: S.A; Consent publication of manuscript: All authors.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Not applicable.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author (Dr_samar11@yahoo.com) on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Samar A. Amer, Email: dr_samar11@yahoo.com
Abdallah Abbas, Email: abdallahabdelmoneam6@gmail.com.
References
- 1.First WHO report details devastating impact of hypertension and ways to stop it. Available from: https://www.who.int/news/item/19-09-2023-first-who-report-details-devastating-impact-of-hypertension-and-ways-to-stop-it
- 2.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of Population-Based studies from 90 countries. Circulation. 2016;134(6):441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London England). 2018;392(10159):1736–88. [DOI] [PMC free article] [PubMed]
- 4.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee M-Y, Na S-H, Kim Y-K, Lee M-M, Kim H-Y. Acute effects of cigarette smoking on arterial stiffness and blood pressure in male smokers with hypertension. Am J Hypertens. 2007;20(6):637–41. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Mei H, Jiang Y-R, Sun W-Q, Song Y-J, Liu S-J, et al. Relationship between duration of sleep and hypertension in adults: a meta-analysis. J Clin Sleep Med. 2015;11(9):1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Y, Zhang B, Ke W, Feng B, Lin H, Xiao J, et al. Associations of short-term and long-term exposure to ambient air pollutants with hypertension: a systematic review and meta-analysis. Hypertension. 2016;68(1):62–70. [DOI] [PubMed] [Google Scholar]
- 8.Yang B-Y, Qian Z, Howard SW, Vaughn MG, Fan S-J, Liu K-K, et al. Global association between ambient air pollution and blood pressure: a systematic review and meta-analysis. Environ Pollut. 2018;235:576–88. [DOI] [PubMed] [Google Scholar]
- 9.Liu M-Y, Li N, Li WA, Khan H. Association between psychosocial stress and hypertension: a systematic review and meta-analysis. Neurol Res. 2017;39(6):573–80. [DOI] [PubMed] [Google Scholar]
- 10.Manohar S, Thongprayoon C, Cheungpasitporn W, Mao MA, Herrmann SM. Associations of rotational shift work and night shift status with hypertension: a systematic review and meta-analysis. J Hypertens. 2017;35(10):1929–37. [DOI] [PubMed] [Google Scholar]
- 11.Van Kempen E, Casas M, Pershagen G, Foraster M. WHO environmental noise guidelines for the European region: a systematic review on environmental noise and cardiovascular and metabolic effects: a summary. Int J Environ Res Public Health. 2018;15(2):379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gheorghe A, Griffiths U, Murphy A, Legido-Quigley H, Lamptey P, Perel P. The economic burden of cardiovascular disease and hypertension in low- and middle-income countries: a systematic review. BMC Public Health [Internet]. 2018;18(1):975. Available from: 10.1186/s12889-018-5806-x [DOI] [PMC free article] [PubMed]
- 13.Elnaem MH, Mosaad M, Abdelaziz DH, Mansour NO, Usman A, Elrggal ME, et al. Disparities in prevalence and barriers to hypertension control: a systematic review. Int J Environ Res Public Health. 2022;19(21):14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houston M. The role of nutrition and nutraceutical supplements in the treatment of hypertension. World J Cardiol. 2014;6(2):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen CB, Glisson JK, Minor DS. Dietary supplements and hypertension: potential benefits and precautions. J Clin Hypertens (Greenwich Conn). 2012;14(7):467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iqbal S, Klammer N, Ekmekcioglu C. The effect of electrolytes on blood pressure: a brief summary of meta-analyses. Nutrients. 2019;11(6):1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanam F, Hossain B, Mistry SK, Mitra DK, Raza WA, Rifat M, et al. The association between daily 500 mg calcium supplementation and lower pregnancy-induced hypertension risk in Bangladesh. BMC Pregnancy Childbirth. 2018;18(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu H-F, Venkatakrishnan K, Golovinskaia O, Wang C-K. Impact of micronutrients on hypertension: evidence from clinical trials with a special focus on meta-analysis. Nutrients. 2021;13(2):588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouillon R, Norman AW, Lips P. Vitamin D deficiency. N Engl J Med. 2007;357(19):1980–1. [DOI] [PubMed] [Google Scholar]
- 20.Judd SE, Nanes MS, Ziegler TR, Wilson PWF, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2008;87(1):136–41. [DOI] [PubMed] [Google Scholar]
- 21.Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol. 2013;28:205–21. [DOI] [PubMed] [Google Scholar]
- 22.Zmijewski MA. Vitamin D and human health. Vol. 20, International journal of molecular sciences. MDPI; 2019. p. 145.
- 23.Cunha AR, Umbelino B, Correia ML, Neves MF. Magnesium and vascular changes in hypertension. International journal of hypertension. 2012;2012. [DOI] [PMC free article] [PubMed]
- 24.Kupetsky-Rincon EA, Uitto J. Magnesium: novel applications in cardiovascular disease–a review of the literature. Annals Nutr Metabolism. 2012;61(2):102–10. [DOI] [PubMed] [Google Scholar]
- 25.Song Y, Liu S. Magnesium for cardiovascular health: time for intervention. Vol. 95, the American journal of clinical nutrition. Oxford University Press; 2012. pp. 269–70. [DOI] [PubMed]
- 26.Houston M. The role of magnesium in hypertension and cardiovascular disease. J Clin Hypertens. 2011;13(11):843–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosanoff A, Weaver CM, Rude RK. Suboptimal magnesium status in the United States: are the health consequences underestimated? Nutr Rev. 2012;70(3):153–64. [DOI] [PubMed] [Google Scholar]
- 28.Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, et al. Primary prevention of hypertension: clinical and public health advisory from the National High blood pressure education program. JAMA. 2002;288(15):1882–8. [DOI] [PubMed] [Google Scholar]
- 29.Cascino TM, Hummel SL. Nutrient deficiencies in heart failure: a micro problem with macro effects? Vol. 7, Journal of the American Heart Association. Am Heart Assoc; 2018. p. e010447. [DOI] [PMC free article] [PubMed]
- 30.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Reviews. 2021;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Wiley; 2019. [DOI] [PMC free article] [PubMed]
- 32.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Reviews. 2016;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JP. Cochrane handbook for systematic reviews of interventions. Cochrane Collaboration and John Wiley & Sons Ltd.; 2008.
- 34.Meta-Analyst O. Open meta-analyst - the tool| evidence synthesis in health. [Internet]. 2018. Available from: https://www.brown.edu/academics/public-health/research/evidence-synthesis-in-health/open-metaanalyst-tool
- 35.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonçalves MDCR, Neto MM, Cavalcante IGM, Sebadelhe VRRJ, De Souza MF, Neves JPR, et al. 200.000 IU of vitamin D does not reduce resting blood pressure and inhibit post-exercise hypotension in elderly women: a pilot study. Anais Da Acad Brasileira De Ciencias. 2020;92(1):1–13. [DOI] [PubMed] [Google Scholar]
- 38.Sluyter JD, Camargo CA, Stewart AW, Waayer D, Lawes CMM, Toop L et al. Effect of monthly, high-dose, long-term vitamin D supplementation on central blood pressure parameters: a randomized controlled trial substudy. J Am Heart Association. 2017;6(10). [DOI] [PMC free article] [PubMed]
- 39.Sanjuliani AF, De Abreu Fagundes VG, Francischetti EA. Effects of magnesium on blood pressure and intracellular ion levels of Brazilian hypertensive patients. Int J Cardiol. 1996;56(2):177–83. [DOI] [PubMed] [Google Scholar]
- 40.Mozaffari-Khosravi H, Loloei S, Mirjalili M-R, Barzegar K. The effect of vitamin D supplementation on blood pressure in patients with elevated blood pressure and vitamin D deficiency: a randomized, double-blind, placebo-controlled trial. Blood Press Monit. 2015;20(2):83–91. [DOI] [PubMed] [Google Scholar]
- 41.Pilz S, Gaksch M, Kienreich K, Grübler M, Verheyen N, Fahrleitner-Pammer A, et al. Effects of vitamin D on blood pressure and Cardiovascular Risk factors: a Randomized Controlled Trial. Hypertension. 2015;65(6):1195–201. [DOI] [PubMed] [Google Scholar]
- 42.Pikilidou MI, Befani CD, Sarafidis PA, Nilsson PM, Koliakos GG, Tziolas IM, et al. Oral calcium supplementation ambulatory blood pressure and relation to changes in intracellular ions and sodium–hydrogen exchange. Am J Hypertens. 2009;22(12):1263–9. [DOI] [PubMed] [Google Scholar]
- 43.Tanji JL, Lew EY, Wong GY, Treguboff C, Ward JA, Amsterdam EA. Dietary calcium supplementation as a treatment for mild hypertension. J Am Board Family Pract. 1991;4(3):145–50. [PubMed] [Google Scholar]
- 44.Zhou C, Fan S, Zhou L, Ni Y, Huang T, Shi Y. Clinical observation of treatment of hypertension with calcium. Am J Hypertens. 1994;7(4Pt1):363–7. [DOI] [PubMed] [Google Scholar]
- 45.McCarron DA. Role of adequate dietary calcium intake in the prevention and management of salt-sensitive hypertension. Am J Clin Nutr. 1997;65(2):S712–6. [DOI] [PubMed] [Google Scholar]
- 46.Garland CJ, Bagher P, Powell C, Ye X, Lemmey HAL, Borysova L, et al. Voltage-dependent Ca2 + entry into smooth muscle during contraction promotes endothelium-mediated feedback vasodilation in arterioles. Sci Signal. 2017;10(486):eaal3806. [DOI] [PubMed] [Google Scholar]
- 47.Lind L, Lithell H, Pollare T, Ljunghall S. Blood pressure response during long-term treatment with magnesium is dependent on magnesium status: a double-blind, placebo-controlled study in essential hypertension and in subjects with high-normal blood pressure. Am J Hypertens. 1991;4(8):674–9. [DOI] [PubMed] [Google Scholar]
- 48.Laurant P, Touyz RM. Physiological and pathophysiological role of magnesium in the cardiovascular system: implications in hypertension. J Hypertens. 2000;18(9):1177–91. [DOI] [PubMed] [Google Scholar]
- 49.Widman L, Wester PO, Stegmayr BK, Wirell M. The dose-dependent reduction in blood pressure through administration of magnesium a double blind placebo controlled cross-over study. Am J Hypertens. 1993;6(1):41–5. [DOI] [PubMed] [Google Scholar]
- 50.Banjanin N, Belojevic G. Changes of blood pressure and hemodynamic parameters after oral magnesium supplementation in patients with essential hypertension—an intervention study. Nutrients. 2018;10(5):581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatzistavri LS, Sarafidis PA, Georgianos PI, Tziolas IM, Aroditis CP, Zebekakis PE, et al. Oral magnesium supplementation reduces ambulatory blood pressure in patients with mild hypertension. Am J Hypertens. 2009;22(10):1070–5. [DOI] [PubMed] [Google Scholar]
- 52.Guerrero-Romero F, Rodríguez-Morán M. The effect of lowering blood pressure by magnesium supplementation in diabetic hypertensive adults with low serum magnesium levels: a randomized, double-blind, placebo-controlled clinical trial. J Hum Hypertens. 2009;23(4):245–51. [DOI] [PubMed] [Google Scholar]
- 53.Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med. 1978;64(2):193–8. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto ME, Applegate WB, Klag MJ, Borhani NO, Cohen JD, Kirchner KA, et al. Lack of blood pressure effect with calcium and magnesium supplementation in adults with high-normal blood pressure: results from phase I of the trials of Hypertension Prevention (TOHP). Ann Epidemiol. 1995;5(2):96–107. [DOI] [PubMed] [Google Scholar]
- 55.Gu D, He J, Wu X, Duan X, Whelton PK. Effect of potassium supplementation on blood pressure in Chinese: a randomized, placebo-controlled trial. J Hypertens. 2001;19(7):1325–31. [DOI] [PubMed] [Google Scholar]
- 56.He J, Gu D, Kelly TN, Hixson JE, Rao DC, Jaquish CE, et al. Genetic variants in the renin–angiotensin–aldosterone system and blood pressure responses to potassium intake. J Hypertens. 2011;29(9):1719–30. [DOI] [PubMed] [Google Scholar]
- 57.Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens. 2011;29(4):636–45. [DOI] [PubMed] [Google Scholar]
- 58.Zozaya JLG, Viloria MP. Alterations of calcium, magnesium, and zinc in essential hypertension: their relation to the renin-angiotensin-aldosterone system. Invest Clin. 1997;38:27–40. [PubMed] [Google Scholar]
- 59.Resnick LM. Calcium metabolism in hypertension and allied metabolic disorders. Diabetes Care. 1991;14(6):505–20. [DOI] [PubMed] [Google Scholar]
- 60.Kass L, Weekes J, Carpenter L. Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur J Clin Nutr. 2012;66(4):411–8. [DOI] [PubMed] [Google Scholar]
- 61.O’Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306(20):2229–38. [DOI] [PubMed] [Google Scholar]
- 62.Golzarand M, Shab-Bidar S, Koochakpoor G, Djafarian K. Effect of vitamin D3 supplementation on blood pressure in adults: an updated meta-analysis. Nutr Metabolism Cardiovasc Dis. 2016;26(8):663–73. [DOI] [PubMed] [Google Scholar]
- 63.He S, Hao X. The effect of vitamin D3 on blood pressure in people with vitamin D deficiency: a system review and meta-analysis. Medicine. 2019;98:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morvaridzadeh M, Sepidarkish M, Fazelian S, Rahimlou M, Omidi A, Ardehali SH, et al. Effect of calcium and vitamin D co-supplementation on blood pressure: a systematic review and meta-analysis. Clin Ther. 2020;42(3):e45–63. [DOI] [PubMed] [Google Scholar]
- 65.Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency: an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52(24):1949–56. [DOI] [PubMed] [Google Scholar]
- 66.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25(3):320–5. [DOI] [PubMed] [Google Scholar]
- 67.Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, et al. 1, 25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem. 2007;282(41):29821–30. [DOI] [PubMed] [Google Scholar]
- 68.Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009;27(10):1948–54. [DOI] [PubMed] [Google Scholar]
- 69.Wu SH, Ho SC, Zhong L. Effects of vitamin D supplementation on blood pressure. South Med J. 2010;103(8):729–37. [DOI] [PubMed] [Google Scholar]
- 70.Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175(5):745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang D. Effect of vitamin D on blood pressure and hypertension in the general population: an update meta-analysis of cohort studies and randomized controlled trials. Prev Chronic Dis. 2020;17. [DOI] [PMC free article] [PubMed]
- 72.Dazai Y, Iwata T, Hiwada K. Augmentation of Baroreceptor Reflex function by oral calcium supplementation in essential hypertension. Clin Exp Pharmacol Physiol. 1994;21(3):173–8. [DOI] [PubMed] [Google Scholar]
- 73.de Paula TP, Moreira JSR, Sperb LF, Muller MEP, Steemburgo T, Viana LV. Efficacy of single-dose cholecalciferol in the blood pressure of patients with type 2 diabetes, hypertension and hypovitaminoses D. Scientific Reports [Internet]. 2020;10(1):1–8. Available from: 10.1038/s41598-020-76646-6 [DOI] [PMC free article] [PubMed]
- 74.Ferrara LA, Iannuzzi R, Castaldo A, Iannuzzi A, Russo D, Mancini M. Long-term magnesium supplementation in essential hypertension. Cardiology. 1992;81(1):25–33. [DOI] [PubMed] [Google Scholar]
- 75.Hofman A. Saturday 27 September Effect of Calcium Supplementation. 1986;(September).
- 76.Sheikh V, Mozaianimonfared A, Gharakhani M, Poorolajal J. Effect of vitamin D supplementation versus placebo on essential hypertension in patients with vitamin D deficiency: a double-blind randomized clinical trial. J Clin Hypertens. 2020;22(10):1867–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.MacGregor GA, Cappuccio FP, Markandu ND. Sodium intake, high blood pressure, and calcium channel blockers. Am J Med. 1987;82(3B):16–22. 10.1016/0002-9343(87)90206-3. PMID: 3551600. [DOI] [PubMed]
- 78.Chen WR, Liu ZY, Shi Y, Yin DW, Wang H, Sha Y et al. Vitamin D and nifedipine in the treatment of Chinese patients with grades I-II essential hypertension: A randomized placebo-controlled trial. Atherosclerosis [Internet]. 2014;235(1):102–9. Available from: 10.1016/j.atherosclerosis.2014.04.011 [DOI] [PubMed]
- 79.Cunha AR, D’El-Rei J, Medeiros F, Umbelino B, Oigman W, Touyz RM, et al. Oral magnesium supplementation improves endothelial function and attenuates subclinical atherosclerosis in thiazide-treated hypertensive women. J Hypertens. 2017;35(1):89–97. [DOI] [PubMed] [Google Scholar]
- 80.Widman L, Wester PO, Stegmayr BK, Wirell M. The dose-dependent reduction in blood pressure through administration of magnesium. Am J Hypertens. 1993;6(1):41–5. [DOI] [PubMed] [Google Scholar]
- 81.Weinberger MH, Wagner UL, Fineberg NS. The blood pressure effects of calcium supplementation. Am J Hypertens. 1993;6(9):799–805. [DOI] [PubMed] [Google Scholar]
- 82.Witham MD, Ireland S, Graeme Houston J, Gandy SJ, Waugh S, Macdonald TM, et al. Vitamin D therapy to reduce blood pressure and left ventricular hypertrophy in resistant hypertension: Randomized, controlled trial. Hypertension. 2014;63(4):706–12. [DOI] [PubMed] [Google Scholar]
- 83.Witham MD, Price RJG, Struthers AD, Donnan PT, Messow CM, Ford I, et al. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension the VitDISH randomized controlled trial. JAMA Intern Med. 2013;173(18):1672–9. [DOI] [PubMed] [Google Scholar]
- 84.Zemel PC, Zemel MB, Urberg M, Douglas FL, Geiser R, Sowers JR. Metabolic and hemodynamic effects of magnesium supplementation in patients with essential hypertension. Am J Clin Nutr. 1990;51(4):665–9. [DOI] [PubMed] [Google Scholar]
- 85.Curatola G, Zoccali C, Crucitti S, Pustorino D, Siclari F, Cuzzucri A, Maggiore Q. Effect of posture on peritoneal clearance in CAPO patients. Perit Dial Int. 1988;8:58–9.
- 86.Hospitals US. Of Internal Medicine. Ann Intern Med. 1982;97(3):391–8. [Google Scholar]
- 87.Meese RB, Gonzales DG, Casparian JM, Ram CVS, Pak CM, Kaplan NM. The inconsistent effects of calcium supplements upon blood pressure in primary hypertension. American Journal of the Medical Sciences [Internet]. 1987;294(4):219–24. Available from: 10.1097/00000441-198710000-00001 [DOI] [PubMed]
- 88.Kawano Y, Matsuoka H, Takishita S, Omae T. Effects of magnesium supplementation in hypertensive patients: Assessment by office, home, and ambulatory blood pressures. Hypertension. 1998;32(2):260–5. [DOI] [PubMed] [Google Scholar]
- 89.Nowson CA, Morgan TO. Magnesium supplementation in mild hypertensive patients on a moderately low Sodium Diet. Clin Exp Pharmacol Physiol. 1989;16(4):299–302. [DOI] [PubMed] [Google Scholar]
- 90.Nowson CA, Morgan TO. Change in blood pressure in relation to change in nutrients effected by manipulation of dietary sodium and potassium. Clin Exp Pharmacol Physiol. 1988;15(3):225–42. 10.1111/j.1440-1681.1988.tb01065.x. PMID: 2856053. [DOI] [PubMed]
- 91.Nowson C, Morgan T. Effect of calcium carbonate on blood pressure in normotensive and hypertensive people. Hypertension. 1989;13(6 I):630–9. [DOI] [PubMed] [Google Scholar]
- 92.Larsen T, Mose FH, Bech JN, Hansen AB, Pedersen EB. Effect of cholecalciferol supplementation during winter months in patients with hypertension: a randomized, placebo-controlled trial. Am J Hypertens. 2012;25(11):1215–22. [DOI] [PubMed] [Google Scholar]
- 93.Lasaridis AN, Kaisis CN, Zananiri KI, Syrganis CD, Tourkantonis AA. Increased natriuretic ability and hypotensive effect during short-term high calcium intake in essential hypertension. Nephron. 1989;51(4):517–23. [DOI] [PubMed] [Google Scholar]
- 94.Lind L, Wengle B, Wide L, Ljunghall S. Reduction of blood pressure during long-term treatment with active vitamin D (Alphacalcidol) is dependent on plasma renin activity and calcium status. Am J Hypertens. 1989;2(1):20–5. [DOI] [PubMed] [Google Scholar]
- 95.Bricio-Barrios JA, Palacios-Fonseca AJ, del Toro-Equihua M, Sanchez-Ramirez CA. Effect of Calcitriol Supplementation on Blood Pressure in Older Adults. Journal of Nutrition in Gerontology and Geriatrics [Internet]. 2016;35(4):243–52. Available from: 10.1080/21551197.2016.1206499 [DOI] [PubMed]
- 96.Cappuccio FP. Lack of effect of oral magnesium double blind study. Br Med J. 1985;291(July):235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wimalawansa SJ. Antihypertensive effects of oral calcium supplementation may be mediated through the fotent vasodilator cgrp. Am J Hypertens. 1993;6(12):996–1002. [DOI] [PubMed] [Google Scholar]
- 98.Witteman JCM, Grobbee DE, Derkx FHM, Bouillon R, De Bruijn AM, Hofman A. Reduction of blood pressure with oral magnesium supplementation in women with mild to moderate hypertension. Am J Clin Nutr. 1994;60(1):129–35. [DOI] [PubMed] [Google Scholar]
- 99.Theiler-Schwetz V, Trummer C, Grübler MR, Keppel MH, Zittermann A, Tomaschitz A, et al. Effects of vitamin D supplementation on 24-Hour blood pressure in patients with low 25-Hydroxyvitamin D levels: a Randomized Controlled Trial. Nutrients. 2022;14(7):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morris CD, McCarron DA. Effect of calcium supplementation in an older population with mildly increased blood pressure. Am J Hypertens. 1992;5(4):1230–7. [DOI] [PubMed] [Google Scholar]
- 101.Article J, Med AI, Chambers AE, Count BC. Count C. a. 1991. Image (Rochester, NY). 1991;1–2.
- 102.Santos C, Carvalho R, Fonseca AM, Castelo Branco M, Alves M, Jarak I. Standard Doses of Cholecalciferol Reduce Glucose and increase glutamine in obesity-related hypertension: results of a Randomized Trial. Int J Mol Sci. 2024;25(6):3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author (Dr_samar11@yahoo.com) on reasonable request.