Abstract
Background
Mitochondrial dysfunction is a hallmark of cardiometabolic diseases. Circulating mitochondrial DNA (mtDNA) profiles could refine risk stratification, but current methods do not account for different fractions of circulating mtDNA. We investigated whether patients with type 2 diabetes and/or heart failure (HF) have a specific signature of the total circulating mtDNA profile, including intracellular and cell-free fractions.
Methods
We performed a complete clinical assessment, including blood tests, 12-lead ECG and ultrasound at rest and during cardiopulmonary exercise. Ultrasound congestion was defined at rest as inferior vena cava of ≥ 21 mm, lung B-lines ≥ 4, or discontinuous renal venous flow. In fasting whole blood and plasma samples collected at rest, we simultaneously measured the copy number of the cellular and cell-free components of mtDNA by real-time quantitative polymerase chain reaction (qPCR) using custom standards. We calculated the ratio of cell mtDNA to cell-free mtDNA as an index of mitochondrial efficiency.
Results
We enrolled 120 consecutive patients: 50 (42%) with HF and preserved ejection fraction (HFpEF), 40 (33%) with HF and reduced ejection fraction (HFrEF) and 30 (25%) at risk of developing HF; 42/120 (35%) had diabetes. Cell-free mtDNA was increased in patients with HF (with higher levels in HFrEF than HFpEF) and those with diabetes. Cell-free mtDNA was also higher in patients with systemic inflammation (expressed by high-sensitivity C-reactive protein [hs-CRP] ≥ 0.2 mg/dL with neutrophil-lymphocyte ratio [NLR] > 3) and more ultrasound signs of congestion. The cell/cell-free mtDNA ratio showed opposite trends (all p < 0.05), but there were no significant differences in cell mtDNA. Cell-free mtDNA and mtDNA ratio independently predicted the presence of ≥ 2 ultrasound signs of congestion and effort intolerance (peak oxygen consumption < 16 mL/kg/min) at ROC analysis and using multivariable regressions after adjustment for age, sex, hs-CRP, NLR, high-sensitivity Troponin T and NT-proBNP.
Conclusions
Patients with HF and diabetes have an altered circulating mtDNA signature characterised by higher cell-free mtDNA and lower mtDNA ratio, whereas cellular mtDNA remains unaffected. Cell-free mtDNA and mtDNA ratio are associated with impaired response to exercise, higher systemic inflammation and increased congestion. Circulating mitochondrial profile could be a new biomarker of mitochondrial status in cardiometabolic diseases.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-025-02656-1.
Keywords: Circulating mitochondrial DNA, Heart failure, Type 2 diabetes, Effort intolerance, Congestion
Introduction
Mitochondrial dysfunction is a pathogenetic hallmark of cardiometabolic disease, characterising cardiometabolic damage and its progression from early [1] to advanced stages [2]. Assessing mitochondrial functional status in cardiometabolic patients would be highly informative to identify better individuals at higher risk of disease progression [3]. However, a gold-standard method to non-invasively measure or estimate mitochondrial function is still missing.
Evidence indicates a direct relationship between mitochondrial function and the number of mitochondrial genomes [4]. Measurement of circulating mitochondrial DNA (mtDNA) could, therefore, characterise mitochondrial status. Accordingly, some studies have attempted to correlate circulating intracellular mtDNA copy number with cardiometabolic profile and cardiovascular risk, albeit with controversial results [1, 5, 6]. A possible reason for these inconsistencies may lie in the methodological approach of these different observations, which do not share a standardised method and, more critically, are based on the measurement of intracellular mtDNA copy number alone [7]. Indeed, mtDNA is also present in a cell-free fraction. While intracellular mtDNA copy number reflects the activity of mitochondria within cells, cell-free mtDNA is associated with mitochondrial overload due to metabolic stress and mitochondrial-driven inflammation [3, 8]. In the cardiometabolic context, where low-grade systemic inflammation plays a pivotal role in disease progression and has its major contributors in the mitochondria [9, 10], the recognition of the circulating cell-free mtDNA fraction when assessing the circulating mtDNA profile may be fundamental [7].
In the present study, we aim to investigate whether the circulating mtDNA signature is altered in cardiometabolic patients with heart failure (HF) and/or type 2 diabetes (T2D). To this end, we characterised the circulating mtDNA signature by simultaneously assessing intracellular and cell-free mtDNA copy number. In addition, we tested whether the circulating mtDNA profile reflects key features of cardiometabolic damage associated with mitochondrial dysfunction, such as response to exercise, systemic inflammation and congestion [2].
Methods
Study population
We prospectively enrolled 191 consecutive patients referred for dyspnoea to the University Hospital of Pisa between January 2022 and June 2024. According to the American College of Cardiology/American Heart Association HF staging system, patients fell within Stage A (asymptomatic subjects with cardiovascular risk factors), B (structural heart disease without signs or symptoms of HF), or C (clinically overt HF) [11]. All Stage C patients had a prior diagnosis of HF or were newly diagnosed with HF by an independent physician blinded to the study protocol. A new diagnosis of HF required at least two typical HF signs or symptoms: third heart sound, pulmonary rales, jugular venous distention, hepatomegaly, peripheral oedema, or lung congestion on lung ultrasound or chest X-ray. HFrEF was defined by a LVEF < 50%, while HFpEF required a LVEF ≥ 50%, N-terminal pro-B-type natriuretic peptide (NT-proBNP) > 125 pg/mL and the additional presence of relevant structural heart disease or diastolic dysfunction [12]. All patients were hemodynamically stable at recruitment, without acute myocardial ischemia at rest or during exercise, severe renal dysfunction (i.e., estimated glomerular filtration rate < 30 mL/min/1.73 m2), acute infections or significant autoimmune comorbidities. No one had a regular workout routine. A clinical diagnosis of T2D was based on the ESC/EASD criteria [13]. All patients underwent spirometry, and those with more than moderate airflow obstruction (forced expiratory volume in 1s [FEV1]/forced vital capacity [FVC] < 0.70 and FEV1 < 50% of predicted FEV1) and/or restrictive pattern (< 80% of predicted FVC) were excluded (n = 11). Other exclusion criteria were a more than moderate left-sided valve disease at rest or during exercise (n = 52) and a significant lower extremity artery disease and/or inability to perform exercise due to mobility limitations (n = 8). The final study population comprised 120 patients (Stages AB, n = 30; Stage C-HFrEF, n = 40; Stage C-HFpEF, n = 50), of whom 42/120 (35%) had T2DM.
The study fulfilled the requirements in the Declaration of Helsinki. The local ethics committee approved the protocol (number 19204), and written informed consent was obtained from all patients. All authors had full access to the data, took responsibility for its integrity, contributed to the manuscript, and agreed to this report as written.
Study protocol
The study protocol is part of a standardised workup in a dedicated dyspnoea clinic in Pisa [14]. All measurements were performed in a quiet room with a stable room temperature. No meal, caffeine, or smoking was allowed three hours before the evaluation. Therapy remained unchanged during the whole protocol. Resting evaluation included a complete clinical assessment, blood tests, a 12-lead ECG, and an ultrasound evaluation.
Laboratory evaluation
Patients were instructed to fast overnight and not to take any medications before blood and urine sampling on the morning of the tests. Blood samples were drawn after a 30-min supine rest. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula [15]. Plasma norepinephrine was evaluated using high-performance liquid chromatography with the electrochemical detector CLC 100 (Chromsystems, Munchen, Germany). Direct renin and aldosterone were assayed using a chemiluminescence immunoassay (LIASON, DiaSorin, Saluggia, Italy). NT-proBNP was measured with the ECLIA monoclonal assay using the Cobas e411 platform (Roche Diagnostics Italia, Monza, Italy). We measured urinary albumin (Cobas-8000 analyser, Roche Diagnostics, Basel, Switzerland) to estimate the urinary albumin-to-creatinine ratio (UACR); we defined micro-albuminuria and macro-albuminuria as UACR > 30 mg/g and > 300 mg/g, respectively. We defined the presence of a persistent proinflammatory response as a high-sensitivity C-reactive protein (hs-CRP) level ≥ 0.2 mg/dL with neutrophil-lymphocyte ratio (NLR) > 3 [16, 17].
Mitochondrial DNA measurement
The circulating mtDNA profile was assessed according to a previously published protocol [7]. Briefly, for each patient, we extracted total DNA using commercial kits according to the manufacturer’s instructions (Qiagen QIAamp® DNA Mini Kit, Qiagen, Milano, Italy; QIAamp MinElute Virus Spin Kit, Qiagen, Milano, Italy) and then measured mtDNA copy number in a whole blood sample for the intracellular mtDNA fraction (cell mtDNA) and in a plasma sample for the cell-free mtDNA fraction (cell-free mtDNA). Both samples were collected simultaneously during the resting evaluation. mtDNA quantification was assessed by quantitative real-time polymerase chain reaction (qPCR) using a calibration curve obtained by custom mtDNA copy number standards. Cell mtDNA was normalised to a housekeeping gene (HB2M), while cell-free mtDNA was normalised to a standard volume. We then calculated an index of mitochondrial efficiency using the cell mtDNA to cell-free mtDNA ratio, reflecting the activation of intracellular mitochondrial pathways standardised to mitochondrial overload (per nL of serum). The complete measurement protocol can be found in the supplementary appendix (comprehensive of Supplemental Tables 1–2, Supplemental Fig. 1).
Baseline echocardiography
All patients underwent a comprehensive transthoracic echocardiography examination using LISENDO 880 (Hitachi Medical Systems Tokyo, Japan) in Pisa according to international recommendations [18, 19]. The echocardiographic protocol is provided in the supplementary appendix.
Lung ultrasound (LUS)
B-lines were measured with a linear transducer in parallel orientation (transverse) to the ribs at an imaging depth of ∼15–18 cm using an eight-region scan [20]. In each region, B-lines were counted one by one if distinguishable; if confluent, we estimated their number by the percentage of space occupied on the screen, divided by 10 (up to a max of 10 B-lines/region). As previously validated, the sum of B-lines from the eight scanning regions yielded a score denoting the extent of the extravascular lung water [21, 22].
Renal venous flow (RVF)
Doppler assessment of RVF was performed using the same phased array transducer to obtain a longitudinal view of the right kidney. The flow scale of color Doppler imaging was adjusted to low-flow velocities (< 20 cm/s) to optimise the identification of the interlobar veins. The best-aligned vein was then sampled with pulsed-wave Doppler during an end-expiratory breath hold. The scale was adjusted to maximise the signal amplitude (usually around ≈ 20 cm/s), and the electrocardiographic signal was used to synchronise the RVF signal with the cardiac cycle. As previously validated, we used a semi-quantitative assessment, distinguishing continuous (normal conditions), discontinuous pulsatile or biphasic (worsening congestion) and monophasic (most severe cases) RVF [21, 22].
We defined congestion by ultrasound as the evidence of a discontinuous RVF, a dilated inferior vena cava (≥ 21 mm), or B-lines above or equal to the lower boundary of the highest tertile (≥ 4) [21]. The reproducibility of congestion assessment by ultrasound has been previously published [21].
Cardiopulmonary-exercise stress echocardiography (CPET-ESE) evaluation
The following working day after the resting evaluation, a combined CPET-ESE protocol was performed [14, 23]. Briefly, a symptom-limited graded ramp bicycle exercise test was conducted in the semi-supine position on a tilting cycle ergometer (Ergoline ergoselect 1200 GmbH, Germany). ESE was performed concurrently with breath-by-breath gas exchange measurements using the same ultrasound system used at baseline. All images were stored for offline analysis. The protocol is summarised in the Supplemental Appendix [24, 25]. We defined the presence of at least moderate effort intolerance as peak oxygen consumption (VO2) < 16 mL/kg/min [26].
Statistical analysis
Categorical data are presented as numbers and percentages and were compared using Pearson’s Chi-square test or the Fisher exact test. Continuous data are reported as median and interquartile range (IQR) and were compared using the Kruskal-Wallis test; post-hoc tests were performed with Bonferroni corrections (p-value for significance < 0.01 for p-values < 0.05 on the Kruskal–Wallis test). Based on previous literature [27], we calculated that a minimum sample size of n = 30 for each group would have been able to detect statistically significant differences between two groups with a two-sided α of 0.05 and a power of 88% using non-parametric tests. Correlations among markers were assessed using Spearman’s rank correlation coefficients, with log-transformed data for cell mtDNA, cell-free mtDNA, mtDNA ratio, NT-proBNP, hs-Troponin T (hs-TnT) and aldosterone. Receiver operating characteristic (ROC) analysis was used to calculate the area under the curve (AUC). We used multivariable logistic and linear regression models to predict ≥ 2 US signs of congestion and peak VO2, respectively. We included in the models independent variables (namely, age, sex, hs-CRP, NLR, hs-TnT and NT-proBNP) selected on prior knowledge and pathophysiology [12] while using forward stepwise selection (entry and removal value of p < 0.01 and p < 0.10, respectively) to prevent overfitting. Variance inflation factor > 5 was used to exclude multi-collinearity between selected variables. Bootstrap validation was performed across 5000 bootstrapped replicates to evaluate the stability of the prediction model. Then, calibration plots were generated to determine the agreement between the observed and model-predicted peak VO2 with and without bootstrap (bias-corrected and apparent curve, respectively). The bias-corrected curve showed good overall calibration and an almost linear relationship (mean absolute error = 0.99 mL/kg/min vs. ideal model). Missing data were not included in the models. All tests were two-sided, with a p-value of < 0.05 considered significant. Data were analysed with SPSS version 25.0 (IBM Corp., Armonk, NY).
Results
Study population
Demographic and clinical characteristics of the study population are shown in Table 1. Patients with HFpEF and HFrEF were older than those in HF Stages AB; also, HFpEF patients were more likely to be women with diabetes, hypertension and atrial fibrillation (AF). The eGFR, NT-proBNP and hs-Troponin T were similarly impaired in patients with HF, irrespective of LVEF. However, micro- and macro-albuminuria were more common, and hs-CRP levels were significantly higher in HFpEF than in HFrEF.
Table 1.
Population characteristics
| Variable | HF Stages AB (n = 30) |
HFpEF (n = 50) |
HFrEF (n = 40) |
p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 69 (54–73) | 77.5 (72–83)* | 76 (67–81.5)* | < 0.0001 |
| Male | 22 (73) | 24 (48) | 30 (75) | 0.013 |
| BMI, Kg/m2 | 26 (23–27) | 26 (23–28) | 26 (23–30) | 0.728 |
| Waist circumference, cm | 86 (82.5–93) | 97 (89–118.5)* | 97 (91.5–111)* | 0.007 |
| Body surface area, m2 | 1.79 (1.71–2.01) | 1.83 (1.67–2.03) | 1.93 (1.76–2.08) | 0.272 |
| Current smoker | 3 (10) | 14 (28) | 10 (25) | 0.267 |
| Clinical evaluation | ||||
| Arterial hypertension | 13 (43) | 40 (80) | 15 (45) | < 0.0001 |
| Dyslipidaemia# | 4 (13) | 32 (64) | 26 (65) | < 0.0001 |
| Diabetes mellitus | 2 (7) | 28 (56) | 12 (30) | < 0.0001 |
| History of atrial fibrillation | 3 (10) | 29 (58) | 19 (48) | < 0.0001 |
| Stroke/TIA | 0 | 4 (10) | 1 (3) | 0.230 |
| CAD | 0 | 10 (20) | 18 (45) | < 0.0001 |
| Coronary revascularization | 0 | 9 (18) | 15 (38) | < 0.0001 |
| Previous MI | 0 | 5 (10) | 12 (30) | 0.001 |
| Pacemaker | 2 (7) | 6 (12) | 17 (43) | < 0.0001 |
| ICD | 0 | 0 | 16 (40) | < 0.0001 |
| CRT | 0 | 0 | 10 (25) | < 0.0001 |
| ECG | ||||
| Atrial fibrillation | 2 (7) | 24 (48) | 12 (30) | 0.005 |
| Atrial paced rhythm | 0 | 3 (6) | 3 (8) | 0.331 |
| Ventricular paced rhythm | 0 | 6 (12) | 14 (35) | 0.001 |
| Left bundle branch block | 0 | 4 (8) | 6 (15) | 0.080 |
| Therapy | ||||
| Beta-Blocker | 8 (27) | 41 (52) | 36 (90) | < 0.0001 |
| DHP CCB | 0 | 18 (36) | 4(10) | < 0.0001 |
| Non-DHP CCB | 1 (3) | 2 (4) | 0 | 0.456 |
| ACEi or ARB | 13 (43) | 29 (58) | 50 (50) | 0.431 |
| MRA | 6 (20) | 15 (30) | 26 (65) | < 0.0001 |
| ARNI | 0 | 1 (2) | 16 (40) | < 0.0001 |
| ASA | 1 (3) | 8 (16) | 10 (25) | < 0.0001 |
| Oral anticoagulant | 3 (10) | 25 (50) | 16 (40) | 0.001 |
| Statins | 2 (7) | 31 (62) | 24 (60) | < 0.0001 |
| Thiazides/thiazide-like diuretics | 2 (7) | 5 (10) | 0 | 0.129 |
| Furosemide | 0 | 34 (68) | 31 (78) | < 0.0001 |
| Insulin | 0 | 5 (10) | 2 (5) | 0.175 |
| Oral hypoglycaemic drugs | 3 (10) | 24 (48) | 24 (60) | < 0.0001 |
| Blood tests | ||||
| Haemoglobin, g/dL | 13.3 (12.3–14.1) | 12.2 (10.6–13.8) | 13.3 (12.6–14.2) | 0.012 |
| Na+, mEq/L | 140 (137–142) | 141 (137–142) | 141 (139–143) | 0.823 |
| K+, mEq/L | 4.3 (4.0–4.4) | 4.2 (4.1–4.8) | 4.3 (3.9–4.6) | 0.696 |
| Total cholesterol, mg/dL | 180 (151–192) | 148 (124–176) | 159 (140–168) | 0.032 |
| LDL, mg/dL | 97 (75–129) | 81 (60–108) | 88 (69–109) | 0.035 |
| HDL, mg/dL | 61 (46–71) | 47 (39–64) | 44 (40–54) | 0.021 |
| Triglycerides, mg/dL | 65 (62–85) | 86 (61–142) | 89 (76–128)* | 0.007 |
| Creatinine, mg/dL | 0.81 (0.70–0.91) | 1.06 (0.85–1.62)* | 1.02 (0.90–1.33)* | < 0.0001 |
| eGFR, mL/min/1.73 m2 | 87 (80–106) | 57 (41–81)* | 60 (53–78)* | < 0.0001 |
| Fasting blood sugar, mg/dL | 91 (84–97) | 98 (82–135) | 93 (83–122) | 0.474 |
| HbA1c, mmol/mol | 38 (37–40) | 42 (39–51)* | 40 (37–44) | 0.005 |
| UACR, mg/g | 7 (5–14) | 24 (10–105)* | 23 (5–65) | 0.007 |
| Micro-albuminuriaa | 3 (10) | 7(18) | 9 (25) | 0.285 |
| Macro-albuminuriaa | 0 | 10 (20) | 4(10) | 0.024 |
| Uric acid, mg/dL | 5.7 (4.7–6.1) | 5.7 (4.4–6.7) | 5.4 (4.4–6.2) | 0.912 |
| hs-CRP, mg/L | 0.11 (0.06–0.30) | 0.31 (0.11–0.75)* | 0.22 (0.10–0.61) | 0.005 |
| Neutrophil-Lymphocyte Ratio | 2.0 (1.5–2.5) | 2.9 (2.0–3.9)* | 2.8 (2.1–3.5)* | 0.001 |
| NT-proBNP, pg/mL | 153 (62–257) | 1229 (446–3167)* | 1758 (599–3541)* | < 0.0001 |
| hs-Troponin T, pg/mL | 9 (6–12) | 22 (13–42)* | 26 (16–48)* | < 0.0001 |
| Mitochondrial DNA | ||||
| Cell mtDNA, copies/cell | 31.2 (17.5–60.7) | 42.1 (22.2–61.7) | 47.4 (31.2–59.4) | 0.472 |
| Cell-free mtDNA, copies/nL | 6.2 (2.7–16.6) | 21.1 (4.4–83.1)* | 101.6 (9.6–206.9)*‡ | < 0.0001 |
| mtDNA ratio | 5.9 (3.5–9.7) | 1.5 (0.5–6.2)* | 0.6 (0.1–3.7)*‡ | 0.0001 |
Values are n (%), or median (25th quartile, 75th quartile)
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; ARNI: angiotensin receptor neprilysin inhibitor; ASA: acetylsalicylic acid; BMI: body mass index; BSA: body surface area; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; CRT: cardiac resynchronization therapy; DHP CCB: dihydropyridine calcium channel blocker; eGFR: estimated glomerular filtration rate; HbA1c: glycated haemoglobin (available only in patients with diabetes mellitus); HDL: high-density lipoprotein; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; hs-CRP: high sensitivity C-reactive protein; ICD: implantable cardioverter-defibrillator; LDL: low-density lipoprotein; MI: myocardial infarction; MRA: mineralocorticoid receptor antagonist; mtDNA: mitochondrial DNA; NT-proBNP: N-terminal prohormone of brain natriuretic peptide; PCI: percutaneous coronary intervention; TIA: transient ischemic attack; UACR, urine albumin-to-creatinine ratio
* p < 0.01 vs. HF Stages AB; ‡ p < 0.01 vs. HFpEF
# total cholesterol ≥ 200 mg/dL or LDL ≥ 130 mg/dL or on lipid-lowering therapy
aMicro-albuminuria and macro-albuminuria were defined as UACR > 30 mg/g and > 300 mg/g, respectively
Cardiopulmonary function at rest and during exercise
In HFrEF, we observed worse indexes of systolic function both at rest and during exercise, while cardiac output (CO), diastolic function and mean pulmonary artery pressure (mPAP)/CO slope were similarly impaired in HFpEF and HFrEF cohorts. HFpEF patients had more dilated left atria and showed the worse right ventricle-pulmonary artery coupling (expressed by lower values of tricuspid annular plane systolic excursion/systolic pulmonary artery pressure [TAPSE/sPAP]). Congestion assessment by US revealed a higher prevalence of discontinuous RVF and more B-lines in HF patients (Table 2). All patients who underwent CPET-ESE performed a maximal exercise (peak respiratory exchange ratio > 1.00); no data was missing. Despite a similar time of effort, peak VO2, VE/VCO2 and VO2/work slope were significantly more impaired in HF patients than in HF Stages AB (Table 3).
Table 2.
Baseline and exercise stress echocardiography
| Variable | HF Stages AB (n = 30) |
HFpEF (n = 50) |
HFrEF (n = 40) |
p-value |
|---|---|---|---|---|
| Left ventricle and VAC | ||||
| LVMi, g/m2.7 | 118 (99–138) | 123 (82–146) | 138 (121–166)*‡ | 0.002 |
| RWT | 0.37 (0.33–040) | 0.40 (0.36–0.44) | 0.31 (0.26–0.36)*‡ | < 0.0001 |
| Mitral E/A @rest | 1.0 (0.8–1.6) | 0.8 (0.7–1.5) | 0.8 (0.6–1.5) | 0.064 |
| Average E/e’ @rest | 10 (8–14) | 12 (9–18) | 11 (10–13) | 0.587 |
| Average E/e’ @peak | 9 (8–11) | 15 (11–18)* | 13 (10–18)* | 0.004 |
| SV, mL/beat @rest | 58 (45–76) | 59 (48–81) | 53 (39–69) | 0.210 |
| SV, mL/beat @peak | 78 (66–94) | 71 (60–88) | 67 (56–82) | 0.039 |
| CO, L/min @rest | 4.4 (3.4–6.3) | 4.3 (3.2–5.6) | 4.0 (3.2–5.6) | 0.277 |
| CO, L/min @peak | 11.1 (8.1–11.6) | 7.9 (6.3–9.8)* | 7.3 (6.0–9.4)* | 0.001 |
| LVEDVi, mL/m2 @rest | 73 (51–93) | 65 (55–79) | 99 (83–125)*‡ | < 0.0001 |
| LV ejection fraction, % @rest | 66 (62–69) | 63 (57–68) | 37 (30–42)*‡ | < 0.0001 |
| LV ejection fraction, % @peak | 75 (70–78) | 70 (66–74)* | 42 (35–46)*‡ | < 0.0001 |
| WMSI @rest | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 2.0 (2.0–2.2)*‡ | < 0.0001 |
| WMSI @peak | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 2.0 (1.5–2.00)*‡ | < 0.0001 |
| Average S’, cm/s @rest | 8.5 (7.0–9.5) | 6.5 (5.5–7.5)* | 5.5 (4.5–6.5)*‡ | < 0.0001 |
| Average S’, cm/s @peak | 12.5 (11.0–13.8) | 8.5 (7.0–9.5)* | 7.0 (6.0–9.0)*‡ | < 0.0001 |
| LVGLS, % @rest | 20 (17–24) | 14 (12–18)* | 8 (7–10)*‡ | < 0.0001 |
| LVGLS, % @low-load effort | 21 (20–26) | 17 (13–20)* | 9 (8–12)*‡ | < 0.0001 |
| Left atrium | ||||
| LAVi, mL/m2 | 37 (23–47) | 44 (37–52)* | 40 (36–60) | 0.001 |
| LA reservoir strain, % @rest | 34 (21–35) | 18 (13–25)* | 16 (10–25)* | < 0.0001 |
| LA booster strain @rest (SR only) | 13 (11–20) | 10 (8–14) | 11 (6–15) | 0.033 |
| Right ventricle and pulmonary circulation | ||||
| TAPSE, mm @rest | 23 (21–26) | 21 (18–23) | 17 (15–21)* | 0.001 |
| TAPSE, mm @peak | 29 (25–31) | 24 (22–37)* | 22 (19–26)* | < 0.0001 |
| sPAP, mmHg @rest | 30 (24–40) | 43 (33–53)* | 36 (25–48) | 0.007 |
| sPAP, mmHg @peak | 40 (45–50) | 58 (45–70)* | 50 (45–60)* | < 0.0001 |
| TAPSE/sPAP, mm/mmHg @rest | 0.70 (0.50–1.00) | 0.45 (0.39–0.65)* | 0.48 (0.34–0.70)* | 0.001 |
| TAPSE/sPAP, mm/mmHg @peak | 0.58 (0.48–0.70) | 0.39 (0.31–0.51)* | 0.43 (0.33–0.52)* | < 0.0001 |
| mPAP/CO slope | 1.4 (0.7–2.2) | 2.8 (1.9–4.4)* | 2.7 (2.1–4.0)* | < 0.0001 |
| Congestion assessment | ||||
| IVC, mm | 15 (15–20) | 18 (15–21) | 18 (15–21) | 0.190 |
| IVC collapse > 50% | 29 (97) | 45 (90) | 32 (80) | 0.088 |
| RVF continuous/discontinuous | 30 (100)/0 | 41 (82)/9 (18) | 32 (80)/8 (20) | 0.035 |
| B-lines @rest | 0 (0–3) | 3 (2–9) | 4 (1–7) | 0.012 |
| B-lines @peak | 3 (2–7) | 11 (6–24)* | 10 (5–18)* | 0.002 |
| ΔB-lines | 3 (0–4) | 8 (3–13)* | 6 (3–9)* | 0.001 |
Values are mean ± standard deviation, n (%), or median [25th quartile, 75th quartile]
CO: cardiac output; EDV: end-diastolic volume; HFpEF: heart failure with preserved ejection fraction; HFpEF: heart failure with reduced ejection fraction; IVC: inferior vena cava; LA: left atrium; LAVi: left atrial volume index; LV: left ventricle; LVEDVi: left ventricle end-diastolic volume index; LVGLS: left ventricle global longitudinal strain; LVMi: left ventricle mass index; MR: mitral regurgitation; RVF: renal venous flow; RWT: relative wall thickness; sPAP: systolic pulmonary artery pressure; SV: stroke volume; TAPSE: tricuspid annular plane systolic excursion; WMSI: wall motion score index
^parameters @peak were obtained only 147 patients with HFpEF and 138 patients with ASpEF
*p < 0.01 vs. Controls; ‡ p < 0.01 vs. HFpEF
Table 3.
Cardiopulmonary exercise test
| Variable | HF Stages AB (n = 30) |
HFpEF (n = 50) |
HFrEF (n = 40) |
p-value |
|---|---|---|---|---|
| HR, beats/min @rest | 75 (70–80) | 75 (65–85) | 82 (65–90) | 0.241 |
| HR, beats/min @peak | 140 (121–150) | 110 (105–135)* | 105 (95–135)* | 0.004 |
| Chronotropic incompetence# | 8 (27) | 27 (54) | 24 (60) | 0.015 |
| SBP, mmHg @rest | 130 (120–149) | 130 (118–141) | 118 (100–135) | 0.054 |
| DBP, mmHg @rest | 80 (75–90) | 70 (65–85) | 70 (67–80) | 0.052 |
| SBP, mmHg @peak | 195 (176–205) | 165 (146–185)* | 150 (140–167)* | < 0.0001 |
| DBP, mmHg @peak | 85 (80–103) | 75 (70–90) | 78 (65–90) | 0.066 |
| Workload, W | 125 (100–150) | 65 (46–89)* | 70 (60–110)* | < 0.0001 |
| Time of effort, min | 10 (9–11) | 9 (8–10) | 9 (8–10) | 0.071 |
| RER @peak | 1.1 (1.1–1.2) | 1.1 (1.1–1.2) | 1.1 (1.0–1.2) | 0.298 |
| VO2, mL/kg/min @peak | 21.2 (17.5–22.5) | 12.7 (9.5–14.5)* | 13.8 (10.2–15.5)* | < 0.0001 |
| VE/VCO2 slope | 28 (26–32) | 38 (33–42)* | 40 (34–44)* | < 0.0001 |
| VD/VT (%) | 20 (16–23) | 23 (20–26)* | 24 (22–27)* | 0.001 |
| O2 pulse, mL/beat @peak | 11.2 (8.6–12.8) | 8.1 (6.3–10.6)* | 8.3 (7.2–10.1) | 0.002 |
| VO2/work slope, mL/min/W | 10 (9–10.5) | 7.4 (6–8.6)* | 7.5 (6.2–9.3)* | < 0.0001 |
| VO2/work flattening | 0 | 6 (12) | 5 (13) | 0.132 |
| EOV | 0 | 1 (2) | 1 (3) | 0.701 |
Values are n (%), or median (25th quartile, 75th quartile)
DBP: diastolic blood pressure; EOV: exercise oscillatory ventilation; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; HR: heart rate; MBP: mean blood pressure; RER: respiratory exchange ratio; SBP: systolic blood pressure; VCO2: carbon dioxide production; VD: Physiologic dead space; VE: minute ventilation; VO2: oxygen consumption; VT: Tidal volume
*p < 0.01 vs. Controls; ‡ p < 0.01 vs. HFpEF
#Failure to achieve ≥ 80% of the difference between age-predicted maximal HR and resting HR (HR reserve) during exercise
Circulating mitochondrial DNA signature in cardiometabolic patients
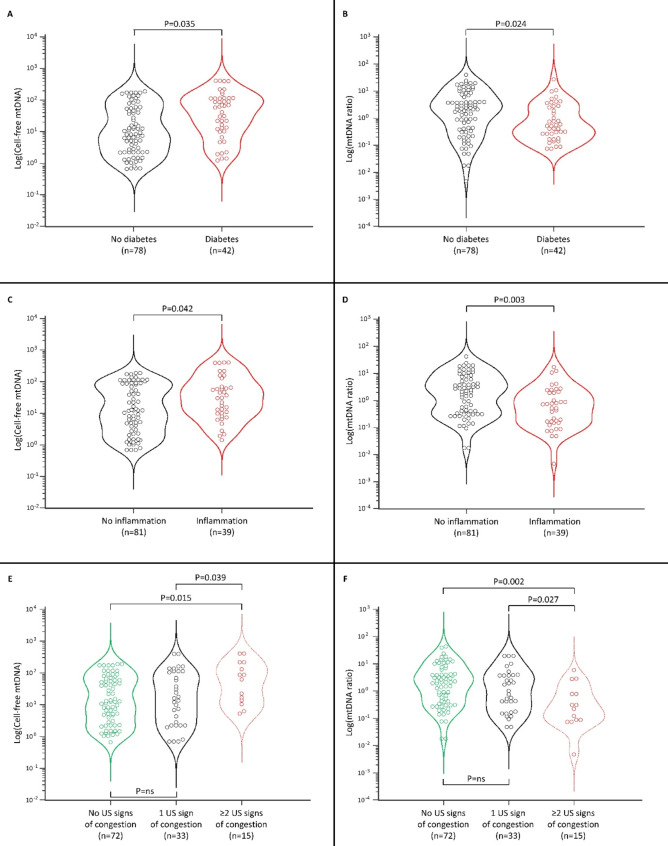
Cell mtDNA levels were similar across HF groups, while cell-free mtDNA levels were higher in HFpEF than Stages AB and the highest values were observed in HFrEF (all p < 0.01, Table 1). Conversely, the cell/cell-free mtDNA ratio was significantly lower in HFpEF and HFrEF than in Stages AB. Cell-free mtDNA levels were higher in patients with diabetes, while the mtDNA ratio was lower (Fig. 1A, B). Likewise, cell-free mtDNA levels were higher in patients with persistent inflammation, while the mtDNA ratio was lower (Fig. 1C, D). With increasing US signs of congestion, we observed higher cell-free mtDNA levels and a lower mtDNA ratio (Fig. 1E, F). The same analyses were non-significant when considering cell mtDNA levels.
Fig. 1.
Distribution of the cell-free mtDNA and mtDNA ratio presented as violin plots showing median and interquartile range in patients with and without diabetes (A, B), with and without inflammation (C, D) and according to the absence or presence of increasing ultrasound (US) signs of congestion (E, F). Inflammation was defined as the co-presence of C-Reactive Protein ≥ 0.2 mg/dL and Neutrophil-Lymphocyte Ratio > 3
Circulating mitochondrial DNA and cardiometabolic risk markers
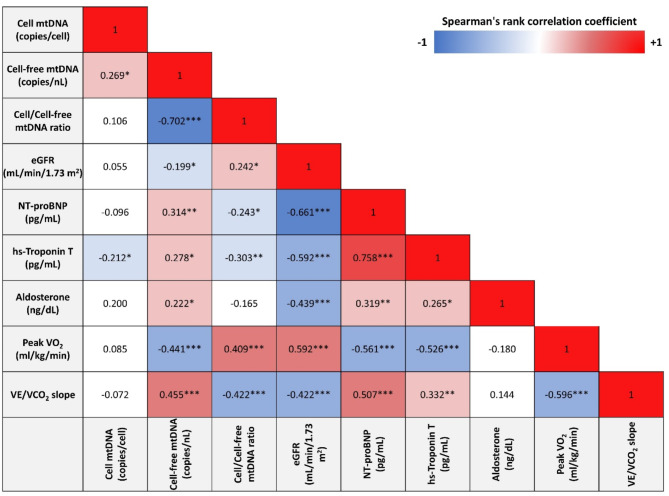
Cell-free mtDNA had a mild direct correlation with cell mtDNA levels, NT-proBNP, hs-TnT and aldosterone while showing a moderate correlation with peak VO2 (inverse) and VE/VCO2 (direct). The opposite relationships were observed when considering the mtDNA ratio (Fig. 2).
Fig. 2.
Univariate correlation matrix using Spearman’s rank correlation. *P < 0.01, **P < 0.001, ***P < 0.0001. Red shading indicates positive correlations, and blue shading indicates inverse correlations. White boxes are non-significant (P > 0.05). eGFR: estimated glomerular filtration rate; VE: ventilation; VOC2: carbon dioxide production; VO2: oxygen consumption
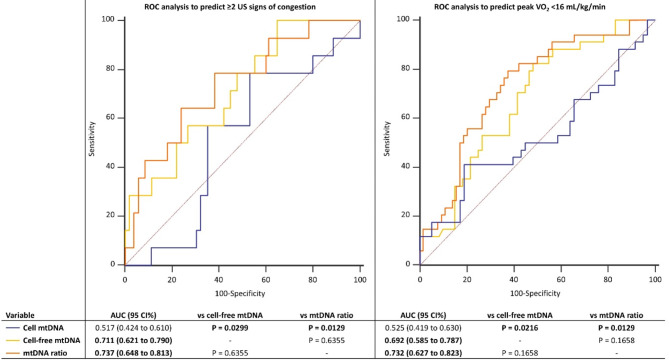
Circulating mitochondrial DNA and congestion
Cell-free mtDNA (optimal cut-off: >6.1 copies/nL, 100% sensitivity, 40%specificity) and mtDNA ratio (optimal cut-off: ≤0.5, 65% sensitivity, 77% specificity) independently predicted ≥ 2 US signs of congestion at ROC analysis (Fig. 3). The difference between AUCs of cell-free mtDNA vs. mtDNA ratio was 0.026, 95% CI -0.079–0.140 (p = 0.586). We also tested conventional cardiometabolic biomarkers to predict multi-organ congestion (Supplemental Table 3). NT-proBNP and HBA1c had the highest AUC for predicting ≥ 2 ultrasound (US) signs of congestion (0.753, 95% CI 0.630–0.869 and 0.751, 95% CI 0.636–0.866), followed by triglycerides (0.668, 95% CI 0.546–0.790). Conversely, hs-CRP, LDL and HDL were not independently associated with multi-organ congestion. Pairwise comparison of ROC curves of the previous significant conventional biomarkers vs. cell-free mtDNA and mtDNA ratio showed no significant differences between AUCs (Supplemental Table 4).
Fig. 3.
Receiver-operator characteristic (ROC) curves illustrating the accuracy of cell mtDNA, cell-free mtDNA and mtDNA ratio in predicting ≥ 2 ultrasound (US) signs of congestion and peak oxygen consumption (VO2) < 16 mL/kg/min. AUC: area under the curve
In a multivariable logistic regression adjusted for age, sex, hs-CRP, NLR, hs-TnT and NT-proBNP, both cell-free mtDNA (coefficient 1.203, odds ratio [OR] 3.329, 95% confidence interval [CI] 1.017–10.902) and mtDNA ratio (coefficient − 1.196, OR 0.302, 95% CI 0.101 to 0.903) independently predicted ≥ 2 US signs of congestion, while cell mtDNA did not (coefficient − 0.430, OR 0.650, 95% CI 0.167 to 2.539).
Circulating mitochondrial DNA and cardiopulmonary performance
Cell-free mtDNA (optimal cut-off: ≤43.9 copies/nL, 83% sensitivity, 50% specificity) and mtDNA ratio (optimal cut-off: >1.3, sensitivity 80%, specificity 60%) independently predicted low peak VO2 (< 16 mL/kg/min) at ROC analysis (Fig. 3). The difference between AUCs of cell-free mtDNA vs. mtDNA ratio was 0.040, 95% CI -0.007–0.115 (p = 0.092). We also tested conventional cardiometabolic biomarkers to predict impaired response to exercise (Supplemental Table 5). NT-proBNP had the highest AUC for predicting peak VO2 < 16 mL/kg/min (0.795, 95% CI 0.684–0.905), followed by HBA1c (0.667, 95% CI 0.551–0.793), LDL (0.665, 95% CI 0.540–0.790) and triglycerides (0.664, 95% CI 0.537–0.791). Conversely, hs-CRP and HDL were not independently associated with impaired response to exercise. Pairwise comparison of ROC curves of the previous significant conventional biomarkers vs. cell-free mtDNA and mtDNA ratio showed no significant differences between AUCs (Supplemental Table 6).
In a multivariable linear regression adjusted for age, sex, hs-CRP, NLR, hs-TnT and NT-proBNP, both cell-free mtDNA (coefficient − 1.413, rpartial −0.249, p = 0.025) and mtDNA ratio (coefficient 1.099, rpartial 0.292, p = 0.021) independently predicted peak VO2 < 16 mL/kg/min, while cell mtDNA did not (coefficient 0.064, rpartial 0.009, p = 0.943).
Discussion
Accurate assessment of mitochondrial dysfunction could implement risk stratification strategies in cardiometabolic diseases such as HF and T2D, where mitochondrial homeostasis is directly involved in disease pathogenesis. However, convincing evidence in this setting remains elusive. In the present study, we report for the first time that patients with different HF phenotypes and/or T2D exhibit an altered circulating mtDNA signature, characterised by higher cell-free mtDNA and lower mtDNA ratio. Furthermore, we report that higher cell-free mtDNA levels and lower mtDNA ratios are present in patients with an unfavourable cardiometabolic phenotype, characterised by an impaired response to exercise, multi-organ congestion and higher levels of inflammation.
Effective and early risk stratification is challenging in cardiometabolic patients due to the cumulative burden of comorbidities [28]. Therefore, there is an urgent unmet need for novel circulating biomarkers [29]. Circulating mitochondrial (dys)function signature may provide a central pathogenetic hallmark of cardiometabolic damage [30]. However, assessing human mitochondrial function is a major challenge [3]. In our study, we simultaneously measured the intracellular and free fractions of circulating mtDNA to address both the major mitochondria-driven processes altered in cardiometabolic disease (i.e., metabolic dysfunction/insufficiency) and systemic mitochondrial inflammation.
We observed that circulating cellular mtDNA is not altered in HF and T2D, while conflicting results have been reported in the literature. A Mendelian randomisation (MR) study showed an inverse association between mtDNA and HF [7]. Likewise, a recent meta-analysis showed a negative association between leukocyte mtDNA copy number and incident HF risk [31]. Noteworthy, one of the studies extracted the number of mtDNA copies from buffy coat [32], another from whole blood [33] and a third performed Mendelian randomisation (MR) based on mtDNA abundance [5]. Interestingly, a later MR analysis on the same cohort using an updated assessment of mtDNA abundance reported conflicting results, showing no association between mtDNA copy number and cardiometabolic damage, except for dyslipidaemia [1]. Another MR analysis confirmed these data [6]. A compensatory mechanism may explain why circulating cellular mtDNA levels are not altered in HF and T2D since cellular mitochondria try to meet the exaggerated metabolic demand by over-activating themselves. This hypothesis is consistent with recent findings in an experimental aged HFpEF mouse model [34]. Similarly, a pilot analysis found increased levels of circulating mtDNA in patients with HF, although not normalised to nuclear DNA. However, in the same study, HF patients with lower cellular mtDNA had higher mortality. This aligns with another study, which showed that circulating cellular mtDNA is higher in patients with stable HF than controls but significantly lower in patients with acute decompensated HF [35]. Thus, normal/higher mtDNA in HF and T2D may underline an overcompensatory mechanism that, when exhausted, leads to decompensated cardiometabolic disease.
In the present research, cell-free mtDNA was increased in patients with HF and/or T2D, with levels significantly higher in the HFrEF cohort than HFpEF. Cell-free mtDNA is associated with a higher inflammatory state, as demonstrated in vitro [3] and reported in several human studies in neurological [8], autoimmune [36], age-related [37] and acute [38] and chronic infectious diseases [39]. Advanced cardiometabolic diseases, such as HF and T2D, are characterised by persistent systemic inflammation [40–43] that is strongly driven by mitochondrial dysfunction [42, 44, 45], which is in agreement with our results. The potential role of cell-free mtDNA has begun to be explored in patients with T2D, where its levels are higher and associated with microvascular complications and inflammatory signature [46, 47]. However, there is no data on HF, except for a recent report showing that higher cell-free mtDNA is associated with a higher rate of heart transplant failure [48].
We characterised the circulating mtDNA profile by exploring its relationship with cardiopulmonary function during exercise, congestion and inflammation, three landmarks of HF associated with mitochondrial dysfunction [2, 30, 49]. Higher cell-free mtDNA was associated with a worse profile for all three conditions. A recent translational investigation on SARS-CoV-2 myocardial injury showed that circulating cell-free mtDNA may be a hallmark of inflammation-induced cardiac dysfunction. Consistent with this, we report a correlation between cell-free mtDNA and NLR, a well-established marker of inflammatory cardiovascular risk [50], and hs-TnT. The capacity of the mtDNA profile to predict congestion and cardiopulmonary response to exercise, which are key pathogenetic elements of HF [51–53], has implications in the context of the need for circulating biomarkers for cardiovascular risk stratification [54].
To further explore the potential of simultaneous assessment of cell and cell-free mtDNA in the same patient, we decided to calculate the ratio of circulating intracellular mtDNA (standardised per cell) to circulating cell-free mtDNA (standardised to one nL of plasma) as an index of mitochondrial efficiency [2]. In particular, the mtDNA ratio consistently showed the opposite behaviour of cell-free mtDNA. Noteworthy, the discriminatory power of the mtDNA ratio consistently outperformed cell-free mtDNA, albeit slightly and non-significantly. Taken together, our exploratory findings show that the circulating mtDNA profile has a specific signature in cardiometabolic patients with HF and/or T2DM, recapitulating key cardiometabolic features associated with mitochondrial dysfunction. Larger and prospective studies are needed to analyse the patterns of circulating mtDNA components across the broad spectrum of cardiometabolic diseases, including, for example, atherosclerosis, coronary artery disease, obesity and nephropathy since these features often co-exist [10, 43]. In this context, implementing artificial intelligence techniques may help better understand the role of mtDNA profile as a risk stratification biomarker to identify individuals at higher risk of cardiometabolic progression [55]. Albeit its incremental value compared to conventional biomarkers needs still to be demonstrated, circulating mtDNA has the advantage of requiring a rapid and blood-based measurement, which can be quantified using qPCR machines that are widely available in diagnostic laboratories and not limited to highly specialised diagnostic centres. Ideally, its implementation could be relatively rapid in the current healthcare landscape and allow earlier intensive intervention by identifying the patients with a worse trajectory across the cardiometabolic spectrum.
Study limitations
The cross-sectional design of the study does not allow for causal inference. However, the comprehensive characterisation of the cardiorespiratory profile of our patients allows reliable speculation on the role of the circulating profile of mtDNA in the context of HF and T2D. We measured cellular mtDNA in whole blood samples rather than peripheral blood mononuclear cells (PBMCs), but both approaches are reliable [27]. The rationale behind this choice was to avoid the sample processing required for PBMC extraction, which, in terms of time and facility dependency, could hinder the diffusion of a biomarker that is otherwise easy to measure in blood samples collected during clinical routine and does not require rapid access to a specialised laboratory [56]. The relatively small sample size does not allow detailed subgroup analysis or the application of machine learning analysis to build specific disease-prediction models [55]. Finally, there is a lack of a large group of healthy subjects without cardiovascular disease or exposure to cardiovascular risk factors, which would be needed to define the true normality of the different parameters of the circulating mtDNA profile. Given the above limitations, our results should be considered exploratory and further validation will be required to generalise our findings. However, this should be regarded as a pilot study, and the consistency of our results with a modest sample size strengthens the relevance of our main findings, supporting the need to explore the added value of assessing both components of circulating mtDNA ratio in larger patient cohorts to establish the clinical relevance and cost-effectiveness in comparison to conventional cardiometabolic biomarkers [3, 27].
Conclusions
Our exploratory results highlight the promising role of circulating mtDNA in assessing the overall profile of patients with HF and T2D. Cell-free mtDNA and mtDNA ratio are altered in patients with cardiometabolic disease and recapitulate important cardiometabolic features associated with mitochondrial dysfunction. If our results are confirmed in larger independent cohorts, the circulating mitochondrial profile might represent a potential new cardiometabolic biomarker that can be easily measured in routinely collected blood samples by qPCR, which is ubiquitous in diagnostic laboratories and requires no more than standard processing.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
A.M. and N.R.P. performed the statistical analysis, wrote the main manuscript text and prepared Figs. 1, 2 and 3. S.A., F.C., and E.D. performed laboratory measurements.N.D.B., L.D.P., V.N., and R.R. contributed to patient enrollment and clinical assessment. All authors reviewed the manuscript.
Funding
The research leading to these results has received funding from the European Union - NextGenerationEU through the Italian Ministry of University and Research under PNRR - M4C2-I1.3 Project PE_00000019 “HEAL ITALIA” to Stefano Taddei, CUP I53C22001440006. The views and opinions expressed are those of the authors only and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them.
Data availability
Data and materials supporting the results and analyses presented in the present paper available upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shared first author: Alessandro Mengozzi and Silvia Armenia
Shared last author: Nicola Riccardo Pugliese and Stefano Masi
References
- 1.Qin P, Qin T, Liang L, Li X, Jiang B, Wang X, et al. The role of mitochondrial DNA copy number in cardiometabolic disease: a bidirectional two-sample Mendelian randomization study. Cardiovasc Diabetol. 2024;23:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou B, Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018;128:3716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trumpff C, Michelson J, Lagranha CJ, Taleon V, Karan KR, Sturm G, et al. Stress and Circulating cell-free mitochondrial DNA: a systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion. 2021;59:225–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Erchia AM, Atlante A, Gadaleta G, Pavesi G, Chiara M, De Virgilio C, et al. Tissue-specific MtDNA abundance from exome data and its correlation with mitochondrial transcription, mass and respiratory activity. Mitochondrion. 2015;20:13–21. [DOI] [PubMed] [Google Scholar]
- 5.Luo J, Noordam R, Jukema JW, van Dijk KW, Hägg S, Grassmann F, et al. Low leukocyte mitochondrial DNA abundance drives atherosclerotic cardiovascular diseases: a cohort and Mendelian randomization study. Cardiovasc Res. 2023;119:998–1007. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Sun X, Zhang Y, Jiang W, Lai M, Wiggins KL, et al. Association between whole blood–derived mitochondrial DNA copy number, low-density lipoprotein cholesterol, and cardiovascular disease risk. J Am Heart Assoc. 2023;12:e029090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannah R, Malik AN. Accurate measurement of cellular and cell-free circulating mitochondrial DNA content from human blood samples using real-time quantitative PCR. In: Volkmar W, Edeas M, editors. Mitochondrial medicine: volume 3: manipulating mitochondria and disease-specific approaches. Springer, New York; 2021. p. 247–268. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X, Yu M, Zhao Y, Zheng Y, Meng L, Du K, et al. Circulating cell-free MtDNA release is associated with the activation of cGAS-STING pathway and inflammation in mitochondrial diseases. J Neurol. 2022;269:4985–96. [DOI] [PubMed] [Google Scholar]
- 9.Mengozzi A, Pugliese NR, Chiriacò M, Masi S, Virdis A, Taddei S. Microvascular ageing links metabolic disease to age-related disorders: the role of oxidative stress and inflammation in promoting microvascular dysfunction. J Cardiovasc Pharmacol. 2021;78:S78–S87. [DOI] [PubMed] [Google Scholar]
- 10.Mengozzi A, de Ciuceis C, Dell’oro R, Georgiopoulos G, Lazaridis A, Nosalski R et al. The importance of microvascular inflammation in ageing and age-related diseases: a position paper from the ESH working group on small arteries, section of microvascular inflammation. J Hypertens. 2023;41:1521–43. [DOI] [PubMed] [Google Scholar]
- 11.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/american heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79:e263–421. [DOI] [PubMed] [Google Scholar]
- 12.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- 13.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 14.Punta L, Del BN, De FV, Di, Maremmani D, Gargani L, Mazzola M, et al. Combining cardiopulmonary exercise testing with echocardiography: a multiparametric approach to the cardiovascular and cardiopulmonary systems. Eur Heart J - Imaging Methods Pract. 2023;1:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira JP, Girerd N, Pellicori P, Duarte K, Girerd S, Pfeffer MA et al. Renal function Estimation and Cockroft-Gault formulas for predicting cardiovascular mortality in population-based, cardiovascular risk, heart failure and post-myocardial infarction cohorts: the heart ‘omics’ in ageing (HOMAGE) and the high-risk myocardial. BMC Med. 2016;14:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- 17.Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci MDPI 2022;23:3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–71. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–60. [DOI] [PubMed] [Google Scholar]
- 20.Pellicori P, Platz E, Dauw J, ter Maaten JM, Martens P, Pivetta E, et al. Ultrasound imaging of congestion in heart failure: examinations beyond the heart. Eur J Heart Fail. 2021;23:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pugliese NR, Pellicori P, Filidei F, Del Punta L, De Biase N, Balletti A et al. The incremental value of multi-organ assessment of congestion using ultrasound in outpatients with heart failure. Eur Heart J Cardiovasc Imaging. 2023;24:961–71. [DOI] [PubMed] [Google Scholar]
- 22.Di Fiore V, Del Punta L, De Biase N, Pellicori P, Gargani L, Dini FL et al. Integrative assessment of congestion in heart failure using ultrasound imaging. Intern Emerg Med. 2024;20:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verwerft J, Bertrand PB, Claessen G, Herbots L, Verbrugge FH. Cardiopulmonary exercise testing with simultaneous echocardiography: blueprints of a dyspnea clinic for suspected HFpEF. JACC Heart Fail. 2023;11:243–9. [DOI] [PubMed] [Google Scholar]
- 24.Gargani L, Pugliese NR, Mazzola M, De Biase N, Agoston G, Arcopinto M, et al. Exercise stress echocardiography of the right ventricle and pulmonary circulation. J Am Coll Cardiol. 2023;82:1973–85. [DOI] [PubMed] [Google Scholar]
- 25.Hoedemakers S, Pugliese NR, Stassen J, Vanoppen A, Claessens J, Bekhuis Y et al. mPAP/CO slope and oxygen uptake add prognostic value in aortic stenosis. Circulation. 2024;149:1172–82. [DOI] [PubMed] [Google Scholar]
- 26.Weber KT, Janicki JS. Cardiopulmonary exercise testing for evaluation of chronic cardiac failure. Am J Cardiol. 1985;55:A22–A31. [DOI] [PubMed] [Google Scholar]
- 27.Rosa HS, Ajaz S, Gnudi L, Malik AN. A case for measuring both cellular and cell-free mitochondrial DNA as a disease biomarker in human blood. FASEB J. 2020;34:12278–88. [DOI] [PubMed] [Google Scholar]
- 28.Newgard CB. Metabolomics and metabolic diseases: where do we stand? Cell Metab. 2017;25:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meijers WC, Bayes-Genis A, Mebazaa A, Bauersachs J, Cleland JGF, Coats AJS, et al. Circulating heart failure biomarkers beyond natriuretic peptides: review from the biomarker study group of the heart failure association (HFA), European society of cardiology (ESC). Eur J Heart Fail. 2021;23:1610–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar AA, Kelly DP, Chirinos JA. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation. 2019;139:1435–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Liu X, Chen X, Wang Y, Wu S, Li F, et al. Leukocyte mitochondrial DNA copy number and cardiovascular disease: a systematic review and meta-analysis of cohort studies. iScience. 2024;27:110522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong YS, Longchamps RJ, Zhao D, Castellani CA, Loehr LR, Chang PP, et al. Mitochondrial DNA copy number and incident heart failure. Circulation. 2020;141:1823–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundquist K, Sundquist J, Wang X, Palmer K, Memon AA. Baseline mitochondrial DNA copy number and heart failure incidence and its role in overall and heart failure mortality in middle-aged women. Front Cardiovasc Med. 2022;9:1012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Lin H, Xiong W, Pan J, Huang S, Xu S, et al. p53-Dependent mitochondrial compensation in heart failure with preserved ejection fraction. J Am Heart Assoc. 2022;11:e024582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anguita E, Chaparro A, Candel FJ, Ramos-Acosta C, Martínez-Micaelo N, Amigó N, et al. Biomarkers of stable and decompensated phases of heart failure with preserved ejection fraction. Int J Cardiol. 2022;361:91–100. [DOI] [PubMed] [Google Scholar]
- 36.Giaglis S, Daoudlarian D, Voll RE, Kyburz D, Venhoff N, Walker UA. Circulating mitochondrial DNA copy numbers represent a sensitive marker for diagnosis and monitoring of disease activity in systemic lupus erythematosus. RMD Open. 2021;7:e002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan Z, Yang J, Guo Y, Liu Y, Zhong X. Altered levels of Circulating mitochondrial DNA in elderly people with sarcopenia: association with mitochondrial impairment. Exp Gerontol. 2022;163:111802. [DOI] [PubMed] [Google Scholar]
- 38.Hernández-Beeftink T, Guillen-Guio B, Rodríguez-Pérez H, Marcelino-Rodríguez I, Lorenzo-Salazar JM, Corrales A et al. Whole-blood mitochondrial DNA copies are associated with the prognosis of acute respiratory distress syndrome after sepsis. Front Immunol. 2021;12:737369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan S-W, Syed RR, Catanzaro DG, Ho M-L, Shu C-C, Tsai T-Y et al. Circulating mitochondrial cell-free DNA dynamics in patients with mycobacterial pulmonary infections: potential for a novel biomarker of disease. Front Immunol. 2022;13:1040947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pugliese NR, Pellicori P, Filidei F, De Biase N, Maffia P, Guzik TJ, et al. Inflammatory pathways in heart failure with preserved left ventricular ejection fraction: implications for future interventions. Cardiovasc Res. 2022;118:3536–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17:269–85. [DOI] [PubMed] [Google Scholar]
- 42.Karwi QG, Ho KL, Pherwani S, Ketema EB, Sun Q, Lopaschuk GD. Concurrent diabetes and heart failure: interplay and novel therapeutic approaches. Cardiovasc Res. 2022;118:686–715. [DOI] [PubMed] [Google Scholar]
- 43.Assar M, El, Angulo J, Rodríguez-Mañas L. Diabetes and ageing-induced vascular inflammation. J Physiol. 2016;594:2125–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang DD, Airhart SE, Zhou B, Shireman LM, Jiang S, Melendez Rodriguez C, et al. Safety and tolerability of nicotinamide riboside in heart failure with reduced ejection fraction. JACC Basic Transl Sci. 2022;7:1183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng Y, Xie M, Li Q, Xu X, Ou W, Zhang Y, et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ Res. 2021;128:232–45. [DOI] [PubMed] [Google Scholar]
- 46.Bae JH, Jo S, Kim SJ, Lee JM, Jeong JH, Kang JS et al. Circulating cell-free MtDNA contributes to AIM2 inflammasome-mediated chronic inflammation in patients with type 2 diabetes. Cells. 2019;8:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malik AN, Parsade CK, Ajaz S, Crosby-Nwaobi R, Gnudi L, Czajka A, et al. Altered Circulating mitochondrial DNA and increased inflammation in patients with diabetic retinopathy. Diabetes Res Clin Pract. 2015;110:257–65. [DOI] [PubMed] [Google Scholar]
- 48.Shah P, Agbor-Enoh S, Lee S, Andargie TE, Sinha SS, Kong H, et al. Racial differences in donor-derived cell-free DNA and mitochondrial DNA after heart transplantation, on behalf of the graft investigators. Circ Heart Fail. 2024;17:e011160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribeiro Junior RF, Dabkowski ER, Shekar KC, O´Connell KA, Hecker PA, Murphy MP. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic Biol Med. 2018;117:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adamstein NH, MacFadyen JG, Rose LM, Glynn RJ, Dey AK, Libby P, et al. The neutrophil–lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021;42:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pugliese NR, De Biase N, Conte L, Gargani L, Mazzola M, Fabiani I, et al. Cardiac reserve and exercise capacity: insights from combined cardiopulmonary and exercise echocardiography stress testing. J Am Soc Echocardiogr. 2021;34:38–50. [DOI] [PubMed] [Google Scholar]
- 52.De Biase N, Mazzola M, Del Punta L, Di Fiore V, De Carlo M, Giannini C, et al. Haemodynamic and metabolic phenotyping of patients with aortic stenosis and preserved ejection fraction: a specific phenotype of heart failure with preserved ejection fraction? Eur J Heart Fail. 2023;25:1947–58. [DOI] [PubMed] [Google Scholar]
- 53.Pugliese NR, Pellicori P, Filidei F, Del Punta L, De Biase N, Balletti A, et al. The incremental value of multi-organ assessment of congestion using ultrasound in outpatients with heart failure. Eur Heart J Cardiovasc Imaging. 2023;24:961–71. [DOI] [PubMed] [Google Scholar]
- 54.Núñez J, de la Espriella R, Rossignol P, Voors AA, Mullens W, Metra M, et al. Congestion in heart failure: a Circulating biomarker-based perspective. A review from the biomarkers working group of the heart failure association, European society of cardiology. Eur J Heart Fail. 2022;24:1751–66. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Gu Y, Huang L, Liu S, Chen Q, Yang Y, et al. Construction of machine learning diagnostic models for cardiovascular pan-disease based on blood routine and biochemical detection data. Cardiovasc Diabetol. 2024;23:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braudeau C, Salabert-Le Guen N, Chevreuil J, Rimbert M, Martin JC, Josien R. An easy and reliable whole blood freezing method for flow cytometry immuno-phenotyping and functional analyses. Cytometry B Clin Cytom. 2021;100:652–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials supporting the results and analyses presented in the present paper available upon reasonable request.