Abstract
Background
Frailty has been consistently implicated as a pivotal factor in the onset of delirium following anesthesia and surgery. Nonetheless, a comprehensive understanding of the relationship between frailty and delirium remains to be elucidated. This study addresses that knowledge gap.
Methods
A comprehensive search of literature databases identified 43 relevant studies involving 14,441 participants. The studies were subjected to a rigorous quality assessment using the Newcastle-Ottawa Scale. Statistical analysis was conducted using Review Manager (v5.4.1), including subgroup and sensitivity analyses.
Results
Meta-analysis revealed a significant association between preoperative physical frailty and postoperative delirium (pooled odds ratio: 2.47; 95% confidence interval: 2.04–2.99; I2 = 46.7%). The baseline frailty rate was 34.0% (4,910/14,441), while the overall incidence of postoperative delirium was 20% (2,783/14,441). Subgroup analyses based on characteristics such as race, frailty-assessment tools, and surgical types were conducted to explore potential sources of heterogeneity. This meta-analysis provided compelling evidence supporting a notable link between preoperative physical frailty and an increased risk of postoperative delirium in older surgical patients. Early identification through frailty screening can enable targeted interventions, potentially enhancing overall management and individualized treatment. Integrating frailty assessment into preoperative evaluation may improve predictive accuracy in surgical planning and anesthesia management.
Conclusions
Future research could focus on optimizing the integration of frailty assessment into preoperative protocols for timely intervention and improved patient outcomes.
Trial registration
The review protocol was registered with PROSPERO (CRD42023390486), date of registration: Aug 11, 2023.
Supplementary information
The online version contains supplementary material available at 10.1186/s12871-025-02994-3.
Keywords: Frailty, Delirium, Aged, Elderly, Meta-analysis
Background
Postoperative delirium (POD) is a critical neurological complication of anesthesia and surgery that can significantly impact patient outcomes [1]. The clinical importance of POD is highlighted by its correlation with major morbidity, encompassing prolonged hospital stays, functional and cognitive decline, nursing home admission, and mortality [2]. With the aging of the population, understanding the factors contributing to POD in vulnerable elderly patients is becoming increasingly vital for effective clinical management.
It is important to differentiate POD from early postoperative cognitive decline (POCD), which represents a closely related diagnosis. POD is most often seen within the first 3 postoperative days [3]. POCD occurs at the end of the first postoperative week, has no effect on consciousness, and may last up to 3 months after surgery [3, 4]. POD is considered a risk factor and strong predictor of POCD development [3].
Our previous research [5], along with other studies [6, 7], has pointed towards frailty as a potential factor influencing the occurrence of POD. Frailty, which is characterized by increased vulnerability and reduced physiological reserve, may play a pivotal role in the development of POD in elderly surgical patients [6, 8]. However, existing studies exhibit inconsistencies in terms of sample sizes, population characteristics, and study designs, which hinder a comprehensive understanding of the frailty-delirium relationship [9–13].
Our study provides a timely and comprehensive analysis of the effect of frailty on delirium in older surgical patients, filling a gap in the literature and offering valuable insights into this critical topic. The importance of this research is underscored by the increasing number of older surgical patients due to population aging and by the high risk of severe outcomes, including death and disease progression, due to the co-occurrence of frailty and delirium in this special population [10, 12, 14, 15]. Our meta-analysis addresses research gaps by focusing on the impact of frailty on POD in elderly surgical patients, and aims to offer evidence-based insights to inform clinical practice and improve care for this vulnerable group.
Methods
The current systematic review and meta-analysis was conducted and reported following the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) 2020 guidelines [16]. The review protocol was registered with PROSPERO (CRD42023390486).
Search strategy
A systematic literature search was performed in PubMed, EMBASE, Web of Science, and the Cochrane Library from the inception of the databases until June 29, 2024. To ensure the comprehensiveness of the literature retrieval, we manually searched references, citations, and other relevant articles from the authors of the studies that were initially retrieved. Free terms and subject terms were used as search terms, combined with Boolean conjunctions (OR/AND). No language restrictions were imposed. The details of the search strategy are as follows:
Frailty (MeSH) OR Frail OR Frailty syndrome OR Frail elderly OR Frailties OR Frailness OR Debility OR Debilities OR Sarcopenia OR Muscle wasting
AND
Delirium (MeSH) OR Perioperative neurocognitive disorder OR Postoperative delirium OR Postoperative cognitive dysfunction OR Delayed cognitive recovery OR Postoperative neurocognitive dysfunction OR Mild cognitive impairment OR Pre-existing cognitive impairment OR Preoperative cognitive impairment OR Neurocognitive impairment OR Cerebral dysfunction OR Cognitive decline OR Neurological complications OR Delirious OR POD OR Deliri* OR Acute confusional syndrome OR Acute confusional AND Aged (MeSH) OR Elderly OR Elder OR Older adults OR Functionally-impaired elderly OR Functionally-impaired.
Eligibility criteria
Full-text articles published in peer-reviewed journals were eligible. If multiple studies used the same cohort, the study with the longest follow-up period or the largest sample size was included in the meta-analysis. The inclusion criteria were based on the PICO process, as outlined below.
Population: Patients over 60 years who were undergoing surgery and did not have neurocognitive disorder at the baseline.
Intervention: Assessment of frailty before surgery using common, validated, and recognized criteria. Frailty is characterized by a state of vulnerability and poor homeostatic capacity to respond to stressors due to cumulative physiological decline, resulting in poorer health outcomes [17]. Various frailty-assessment tools, including the frailty phenotype [18], deficit-accumulation frailty index (FI) [19] Clinical Frailty Scale (CFS) [20], and Edmonton Frail Scale (EFS) [21], have been used in acute settings.
Comparison: Preoperative non-frailty.
Outcomes: The incidence of POD, diagnosed based on established criteria, such as the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) or the Confusion Assessment Method (CAM) [22, 23].
Study design: Prospective or retrospective cohort study.
Exclusion criteria
Randomized controlled trials, observational case-control studies, systematic reviews, review articles, and case series.
Non-English language articles.
Animal studies, editorials, commentary, letters, book chapters, and conference proceedings.
Studies with data on outcome indicators that could not be extracted.
Study selection
Efforts were made to comprehensively include all studies published to date on the association between frailty and POD. To identify eligible studies, we first searched several electronic databases since their inception for articles exploring frailty and delirium, by utilizing various combinations of Medical Subject Headings (MeSH) and non-MeSH terms. The search process was then complemented by: (i) reviewing the reference sections of all relevant studies, (ii) manually searching key journals and abstracts from major annual meetings in the field of delirium, and (iii) reaching out to experts.
The initial database search was independently conducted by 2 researchers (HW, SY), and any discrepancies were resolved through consultation with an investigator not involved in the initial search (HZ). The 2 researchers then independently screened the retrieved literature and extracted and cross-checked data according to predetermined criteria. In the event of disagreements, resolutions were achieved through discussion or consultation with a third researcher. Literature screening involved the removal of duplicates and a review of the titles and abstracts of the remaining studies. Following the exclusion of evidently irrelevant literature, a thorough examination of the full text determined whether a given study was included. The following data were extracted from the retrieved studies: author, year of publication, region, population source, number of men/women, mean age, and screening tools for cognitive frailty. If there was a deficiency in the data, attempts were made to communicate with the original study authors to acquire supplementary information.
Data extraction
For document management, EndNote 21 software was utilized, and Excel tables were employed for data extraction.
Statistical analysis
Odds ratios (ORs) with their corresponding 95% confidence intervals (CIs) were used as the general indicator to assess the associations between preoperative physical frailty and POD in older surgical patients. Preoperative physical frailty was considered as a categorical variable, and ORs were calculated by comparing groups with preoperative physical frailty to those without preoperative physical frailty. We performed random-effects models to pool the ORs for the incidence of POD in individual studies in order to compare patients with and without preoperative physical frailty. Heterogeneity among the included studies was assessed using Cochrane’s Q test and the I2 statistic. I2 > 50% and P ≤ 0.10 reflected the presence of significant heterogeneity, in which case, the random-effects model was utilized. Otherwise, a fixed-effects model was used.
Subgroup analyses were used to identify potential sources of heterogeneity as well as characteristics that might strengthen the association between preoperative physical frailty and POD. We conducted subgroup analyses according to frailty-assessment methods (FI vs. Fried vs. Fatigue, Resistance, Ambulation, Illness, and Loss of weight [FRAIL] vs. EFS vs. CFS vs. others), racial groups (Asian vs. non-Asian), and types of surgery (cardiovascular surgery vs. orthopedic surgery vs. abdominal surgery vs. elective surgery). Furthermore, Cochrane’s Q test and the I2 statistic were utilized to test for subgroup differences. The Newcastle-Ottawa Scale (NOS) was used to assess the methodological quality of the included studies. This scale is based on selection, comparability, and outcome or exposure criteria, and has a maximum score of 9. Studies with NOS scores of 7 or more were considered to be of high quality and have a low risk of bias. Two researchers (HW, SY) independently conducted NOS scoring, and any discrepancies were resolved through consultation with an investigator not involved in the initial assessment (HZ). Sensitivity analysis involved systematically excluding one study at a time to assess result stability. Funnel plots were generated to visualize potential publication bias, and the Egger test was used to assess the asymmetry of the funnel plot.
Meta-analysis was conducted using Review Manager (RevMan; version 5.4.1) software and R (version 4.3.3) software. P < 0.05 was considered statistically significant for a two-tailed test throughout the analyses.
Results
Study selection
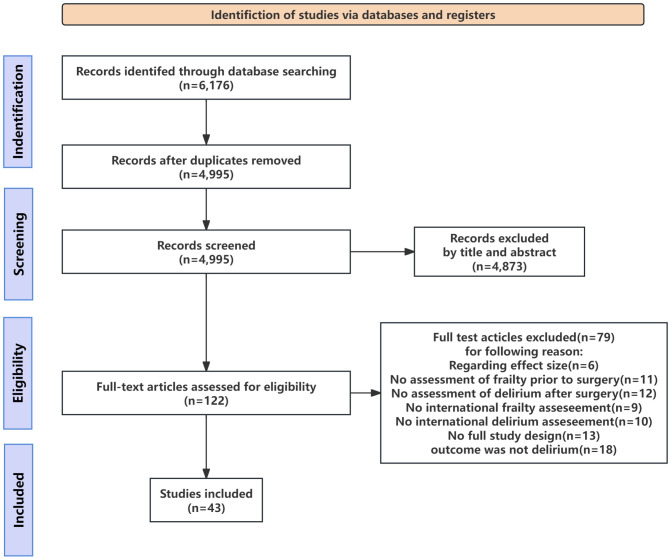
Through a comprehensive search of the 4 electronic databases, we identified 6176 studies. After removing duplicates automatically and manually, we excluded 1181 studies. The titles and abstracts of 4995 studies were screened, after which 122 studies that met the eligibility criteria were retained for a full-text review. Of these 122 studies, 79 studies were excluded due to various reasons: insufficient effect-size information (6 studies), no preoperative frailty assessment (11 studies), no POD assessment (12 studies), no international frailty-assessment tool used (9 studies), no reported international delirium assessment (10 studies), non-full study designs (e.g., letters, comments, reviews, or conference abstracts; 13 studies), and outcomes other than delirium (18 studies). Ultimately, 43 cohort studies (involving 14,441 patients) with adequate methodological quality were identified and included in our review (Fig. 1). The baseline frailty rate was 34.0% (4910 patients), and the overall incidence of POD was 20% (2783 patients). None of the studies had a low NOS score. The NOS results for cohort studies are presented in Supplementary Table 1. According to the NOS scores, the included studies had a high overall quality, with a median NOS score of 7.8 (range: 6–9). The characteristics of the studies included in the meta-analysis are detailed in Table 1 [22–62].
Fig. 1.
PRISMA flowchart of included studies. PRISMA, preferred reporting items for systematic reviews and meta-analyses
Table 1.
Characteristics of the studies included in the meta-analysis (n = 43)
| Participants | Exposures/interventions | Comparison | Outcome (frailty) | Outcome (non-frailty) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Author, year Country No. of patients/No. of male patients Age/Age criterion Mean ± SD or median (IQR) age Type of surgery |
Frailty measure category |
Delirium measure category |
Comparator | Delirium | Total | Delirium | Total | ||
|
Tsai 2023[40] China, Taiwan 345/206 ≥ 65 years 73 years (65–99 years) Elective abdominal surgery |
CGA | CAM |
Fit group: n = 159 (46.1%) Frail group: n = 186 (53.9%) |
15 | 186 | 4 | 159 | ||
|
Tian 2023[26] China 1372 /781 > 65 years Not mentioned Elective lung cancer surgery |
mFI | DSM-V |
Frail group: n = 388 (mFI-5, 2–5), Pre-frail group: n = 503 (mFI-5, 1) Robust group: n = 481 (mFI-5, 0) |
121 | 388 | 15 | 984 | ||
|
Tomoyuki Sugi, 2023[27] Japan 158 /111 ≥ 75 years 79 years (75–91 years) Gastroenterological surgery |
Fried | CAM |
Postoperative delirium (+): n = 53 Postoperative delirium (−): n = 105 |
27 | 48 | 26 | 110 | ||
|
J. Steenblock 2023[28] Germany 701/367 ≥ 70 years 77.1 ± 4.7 years Elective surgery |
FI |
A combination of I-CAM and chart review |
Non-frail group: n = 173 Frail group: n = 528 |
134 | 528 | 31 | 173 | ||
|
Hunter 2023[29] Australia 300/89 > 50 years 81.1 ± 9.8 years Hip fracture surgery |
CFS | 3D-CAM |
CFS ≥ 5: n = 160 CFS < 5: n = 121 |
66 | 164 | 16 | 135 | ||
|
Gandossi 2023[30] Italy 984/241 > 65 years 84 years (79–89 years) Urgent surgery |
FI | 4AT |
FI < 0.25: n = 504 FI ≥ 0.25: n = 480 |
228 | 480 | 83 | 504 | ||
|
Abdelfatah 2023[31] USA 411/203 > 65 years 75.1 ± 6.60 years Colorectal resection |
RAI-A |
ICD-9 ICD-10 |
Not frail: 42/288 (14.6%) Frail: 37/123 (30.1%) |
6 | 123 | 2 | 288 | ||
|
Zhao 2022[66] China 381/98 ≥ 65 years High CFI group: 83 ± 5 years Low CFI group: 78 ± 7 years Hip fracture repair surgery |
CFI | CAM |
High CFI group: n = 102 Low CFI group: n = 279 |
17 | 102 | 8 | 279 | ||
|
Xiang 2022[33] China 226/Not mentioned 65–85 years No POD: 70.4 ± 2.7 years POD: 71.7 ± 3.0 years Laparoscopic surgery for gynecologic cancers |
mFI | DSM-V |
POD No: n = 187 Yes: n = 39 |
10 | 31 | 29 | 195 | ||
|
Sieber 2022[67] USA 324/196 ≥ 65 years 4AT < 4: 73.1 ± 6.2 years 4AT ≥ 4: 77.5 ± 6.3 years Elective surgery |
EFS | 4AT |
4AT < 4: n = 309 4AT ≥ 4: n = 15 |
10 | 83 | 5 | 241 | ||
|
Musacchio 2022[35] Italy 244/39 ≥ 65 years 85 ± 6.9 years Hip fracture surgery |
MPI | 4AT |
Delirium: n = 104 No delirium: n = 140 |
76 | 143 | 28 | 101 | ||
|
Esmaeeli 2021[36] USA 557/169 ≥ 65 years POD: 85 ± 7 years No POD: 80 ± 8 years Orthopedic trauma patients |
FRAIL | CAM |
POD: n = 80 No POD: n = 477 |
4 | 18 | 76 | 539 | ||
|
Thillainadesan 2021[70] USA 150/102 ≥ 65 years 79.5 years (7.7 years) Vascular surgery |
CFS FI |
CAM |
Frail: n = 34 Fit: n = 116 CFS ≥ 5: n = 45 CFS < 5: n = 105 |
10 | 45 | 5 | 105 | ||
|
Pedemonte 2021[38] USA 558/165 ≥ 65 years 80.16 years (8.57 years) Orthopedic trauma patients |
FRAIL | CAM |
Robust: n = 166 Pre-frail: n = 217 Frail: n = 126 |
25 | 126 | 24 | 217 | ||
|
Mauri 2021[39] Germany 661/322 Not mentioned 82.3 ± 6.6 years TAVR |
EFT | CAM-ICU |
Delirium: n = 66 No delirium: n = 595 |
46 | 199 | 20 | 462 | ||
|
Gandossi 2021[30] Italy 988/250 ≥ 65 years 84.9 years (80.6–89.2 years) Hip fracture surgery |
FI |
4AT DSM-5 |
FI < 0.25: n = 628 FI ≥ 0.25: n = 360 |
183 | 360 | 228 | 628 | ||
|
Cheng 2021[41] China, Taiwan 152/104 ≥ 20 years 63.07 years (11.17 years) Cardiac surgery |
Fried | CAM |
Non-delirium: n = 5142 Delirium: n = 510 |
2 | 21 | 8 | 131 | ||
|
Chen 2021[24] China 383/132 65–85 years 73.2 ± 3.3 years Elective total joint arthroplasty |
mFI | DSM-V |
High mFI (> 0.18): n = 207 Low mFI (< 0.18): n = 176 |
44 | 207 | 22 | 176 | ||
|
Banning 2021[42] The Netherlands 639/497 Not mentioned 69.4 ± 10.0 Elective vascular surgery |
GFI | Not mentioned |
Frail: n = 183 (28.6%) Non-frail: n = 456 (71.4%) |
17 | 183 | 27 | 456 | ||
|
Ishihara 2020[43] Japan 295/216 ≥ 65 years 74 years (65–89 years) Hepatic resection |
KCL | ICDSC |
Delirium group: n = 22 Non-delirium group: n = 273 |
10 | 15 | 12 | 295 | ||
|
Susano 2020[44] Portugal 219/124 ≥ 70 years 75 years (73–79 years) Elective spine surgery |
FRAIL | CAM |
No POD: n = 164 (75%) POD: n = 55 (25%) |
24 | 53 | 31 | 166 | ||
|
Saljuqi 2020[25] USA 163/85 ≥ 65 years 71 ± 7 years Emergency general surgery |
ESFI | CAM |
Delirium: n = 38 No delirium: n = 107 |
15 | 30 | 23 | 80 | ||
|
Sanchez 2020[45] Spain 446/198 ≥ 65 years 78 years (65–103 years) Urgent abdominal surgery |
FRAIL | CAM |
No delirium: n = 385 Delirium: n = 61 |
18 | 59 | 43 | 387 | ||
|
Nakano 2020[46] Japan 133/97 ≥ 55 years Non-frail: 71.3 ± 7.1 years Frail: 73.5 ± 8.1 years Cardiac surgery |
Fried | CAM |
Non-frail: n = 89 Frail: n = 44 |
19 | 44 | 37 | 89 | ||
|
Mahanna-Gabrielli 2020[47] USA 178/75 ≥ 65 years Robust: 70 years (67–74.5 years) Prefrail: 70.5 years (67–75 years) Frail: 71 years (66–74 years) Non-cardiac surgery |
FRAIL | CAM-ICU |
Robust: n = 64 Pre-frail: n = 72 Frail: n = 31 |
11 | 31 | 27 | 106 | ||
|
Goudzwaard 2020[48] The Netherlands 543/297 Not mentioned 79.1 ± 8.0 years TAVI |
EFS | DSM-IV |
Delirium: n = 75 No delirium: n = 468 |
24 | 97 | 51 | 446 | ||
|
Atsunori Itagaki 2020[49] Japan 114/73 ≥ 65 years 74.9 ± 5.5 years Cardiac surgery |
J-CHS | ICDSC |
Non-frailty, non-MCI: n = 23 Non-frailty, MCI: n = 32 Frailty, non-MCI: n = 11 Frailty, MCI: n = 23 |
21 | 72 | 4 | 17 | ||
|
Chan 2019[64] Canada 423/267 ≥ 65 years 82.5 ± 8.4 years Total arthroplasty, hemiarthroplasty, dynamic hip or cannulated screws, intramedullary nail |
CFS |
A validated chart-abstraction instrument |
Not frail (CFS 1–3): n = 71 Vulnerable (CFS 4): n = 72 Mildly frail (CFS 5): n = 92 Frail (CFS 6–9): n = 187 |
211 | 279 | 50 | 143 | ||
|
Saravana-Bawan 2019[50] Canada 322/176 ≥ 65 years 76.1 ± 7.66 years Intestinal, appendix, gallbladder, or hernia repair surgery |
CFS | Inouye chart review method |
No delirium: n = 249 Delirium: n = 73 |
33 | 78 | 40 | 244 | ||
|
Goudzwaard 2019[48] The Netherlands 213/46.5% Not mentioned 82.03 years (78.2–85.6 years) TAVI |
EFS | DSM-IV |
Non-frail: n = 153 Frail (EFS ≥ 3): n = 60 |
27 | 61 | 15 | 152 | ||
|
Nomura 2019[51] USA 133/97 ≥ 65 years 69.33 ± 7.90 years CABG, valve procedures, or other surgery |
Fried | CAM |
Non-frail: n = 15 Pre-frail: n = 74 Frail: n = 44 |
19 | 40 | 37 | 88 | ||
|
Haugen 2018[52] USA 893/545 Not mentioned 50.3 ± 13.7 years KT |
Fried | A validated instrument for chart review |
No delirium: n = 851 Delirium: n = 42 |
13 | 146 | 29 | 747 | ||
|
Tanaka 2018[53] Japan 217/149 ≥ 65 years Non-frail: 75 years (65–88 years); Frail: 72 years (65–88 years) Hepatic resection |
KCL | ICDSC |
Frail group: n = 63 Non-frail group: n = 154 |
8 | 63 | 3 | 154 | ||
|
Bagienski 2017[54] Poland 141/52 ≥ 75 years 82.0 years (77.5–85.0 years) TAVI |
FI |
CHART DEL |
No delirium: n = 112 Delirium: n = 29 |
15 | 47 | 14 | 94 | ||
|
Brown 2016[55] USA 55/41 ≥ 55 years Non-frail: 64.7 ± 5.6 years; Frail: 67.7 ± 8.4 years CABG |
Fried | CAM |
Non-frail: n = 38 Frail: n = 17 |
8 | 17 | 1 | 38 | ||
|
Assmann 2016[56] The Netherlands 89/47 ≥ 75 years 80.4 years (6.3 years) MT, SAVR, TAVI |
FI | DSM-IV |
No delirium: n = 64 Delirium: n = 25 |
15 | 47 | 10 | 42 | ||
|
Khan 2016[74] Singapore 25/17 ≥ 65 years 79 years (74–83 years) Femur fracture fixation surgery, abdominal laparotomy; total knee replacement |
Fried | CAM-ICU |
Nonfrail: n = 11 Frail: n = 14 |
1 | 14 | 1 | 11 | ||
|
Eide 2015[58] Norway 143/62 ≥ 80 years 83.5 ± 2.7 years TAVI, SAVR |
FI |
CAM DSM-IV |
No delirium: n = 60 Delirium: n = 76 |
32 | 56 | 44 | 87 | ||
|
Jung 2015[59] Canada 133/98 ≥ 18 years Non-frail: 68.7 ± 7.4 years; Frail: 73.0 ± 8.2 years CABG, valve procedures |
Fried |
CAM-ICU, CAM |
Non-frail: n = 61 Frail: n = 72 |
20 | 72 | 5 | 61 | ||
|
Kistler 2015[60] USA 35/6 ≥ 65 years 86 ± 4 years Hip fracture surgery |
Fried | CAM |
Frail: n = 18 Non-frail: n = 17 |
10 | 18 | 4 | 17 | ||
|
Partridge 2015[61] UK 125/86 ≥ 60 years 76.3 ± 7.27 years Arterial vascular surgery |
EFS | CAM |
EFS < 6.5: n = 60 (48.0%) EFS > 6.5: n = 65 (52.0%) |
15 | 65 | 9 | 60 | ||
|
Leung 2011[62] USA 63/29 ≥ 65 years Delirium: 74.2 ± 6.0 years; No delirium: 71.9 ± 6.3 years General, arthroplasty, spine, or thoracic surgery |
EFS | CAM |
Delirium: n = 16 No delirium: n = 47 |
9 | 21 | 7 | 32 | ||
|
Pol 2011[63] The Netherlands 142/100 21–87 years 68 ± 11 years Vascular surgery |
GFI | DSM-IV-TR |
GFI > 4: n = 50 GFI ≤ 4: n = 92 |
6 | 50 | 4 | 92 | ||
ASA, American Society of Anesthesiologists; AVR, aortic valve replacement; CABG, coronary artery bypass graft; CAM, Confusion Assessment Method; CHART-DEL, chart-based delirium identification instrument; DOS, Delirium Observation Score; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; ICDSC, Intensive Care Delirium Screening Checklist; KT, kidney transplant; NR, not reported; POD, postoperative delirium; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation
Meta-analysis of the association between preoperative frailty and POS
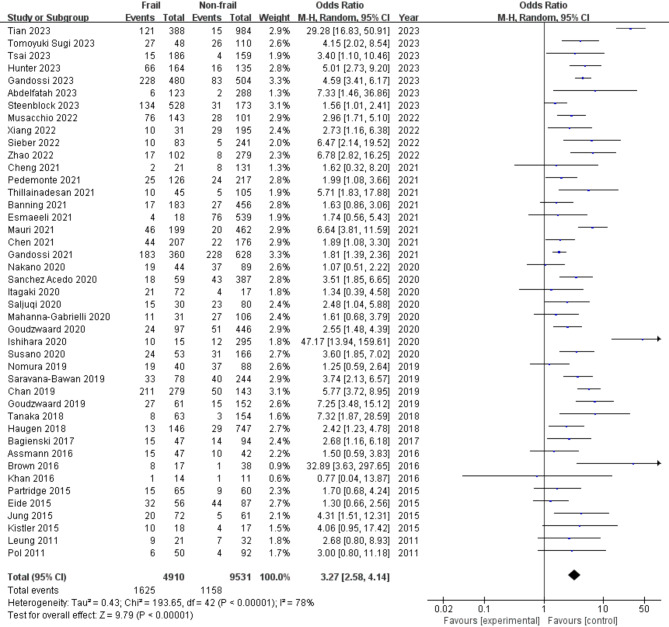
Of the 14,441 elderly participants included in the selected studies in this systematic review, a total of 4,910 participants (34.0%) were determined to have preoperative frailty. Meta-analyses of the 43 included studies showed evidence of a significant association between preoperative frailty and POS (OR: 2.47; 95% CI: 2.04–2.99; I² = 46.7%; Fig. 2).
Fig. 2.
Forest plot for crude association between preoperative frailty and postoperative delirium
Subgroup analyses
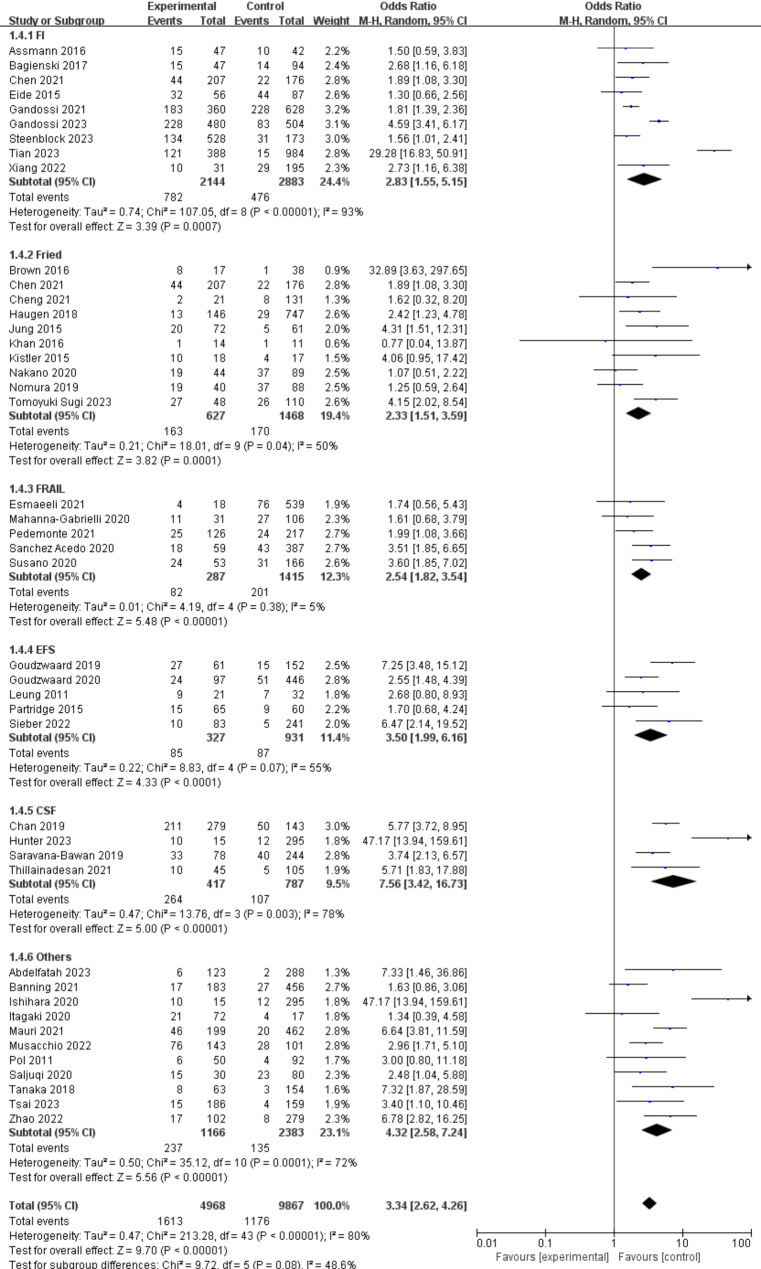
(1) Frailty-assessment methods
The associations between preoperative frailty and POD in different groups based on frailty-assessment methods are presented in Fig. 3. The most prevalent method was the FI and its related modifications (9 studies [24.4%]), followed by the Fried frailty phenotype and its related modifications (10 studies [19.4%]), FRAIL (5 studies [12.3%]), the EFS (5 studies [11.4%]), CFS (4 studies [7.56%]), and other instruments (11 studies [4.32%]). Among all the frailty-assessment method groups, the association between preoperative frailty and POD was strongest in the CFS group (OR: 7.56, 95% CI: 3.42–16.73) and weakest in the Fried group (OR: 2.33, 95% CI: 1.51–3.59). However, no statistically significant differences were detected within the 6 frailty-assessment method groups (P = 0.08).
Fig. 3.
Forest plots displaying pooled effect estimates for frailty-assessment methods
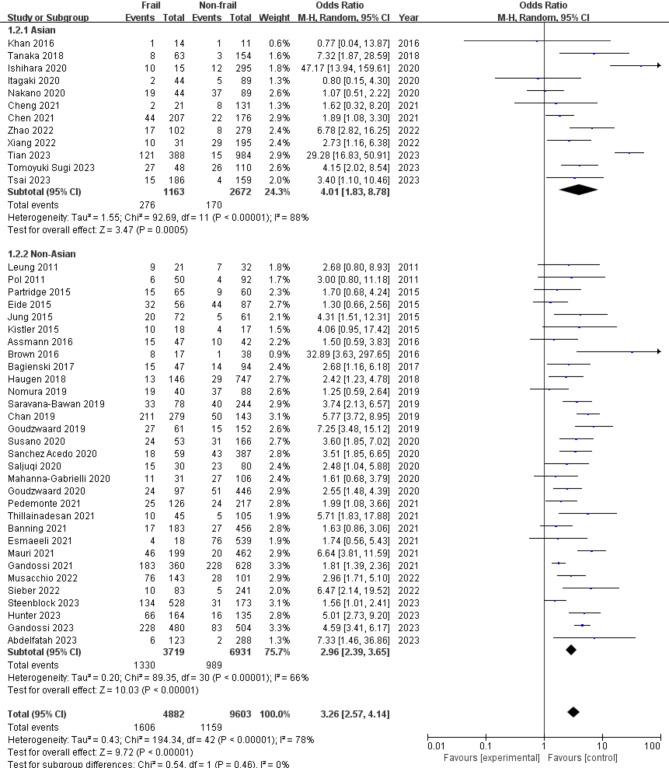
(2) Racial group
The associations between preoperative frailty and POD in different racial groups are illustrated in Fig. 4. In all, 12 (27.9%) studies included Asian patients, and 31 (72.1%) studies included non-Asian patients. The association was stronger in Asian patients (OR: 4.01, 95% CI: 1.83, 8.78) than in non-Asian patients (OR: 2.96, 95% CI: 2.39, 3.65). However, no statistically significant difference was found within the 2 racial groups (P = 0.46).
Fig. 4.
Forest plots displaying pooled effect estimates for racial groups
(3) Type of surgery
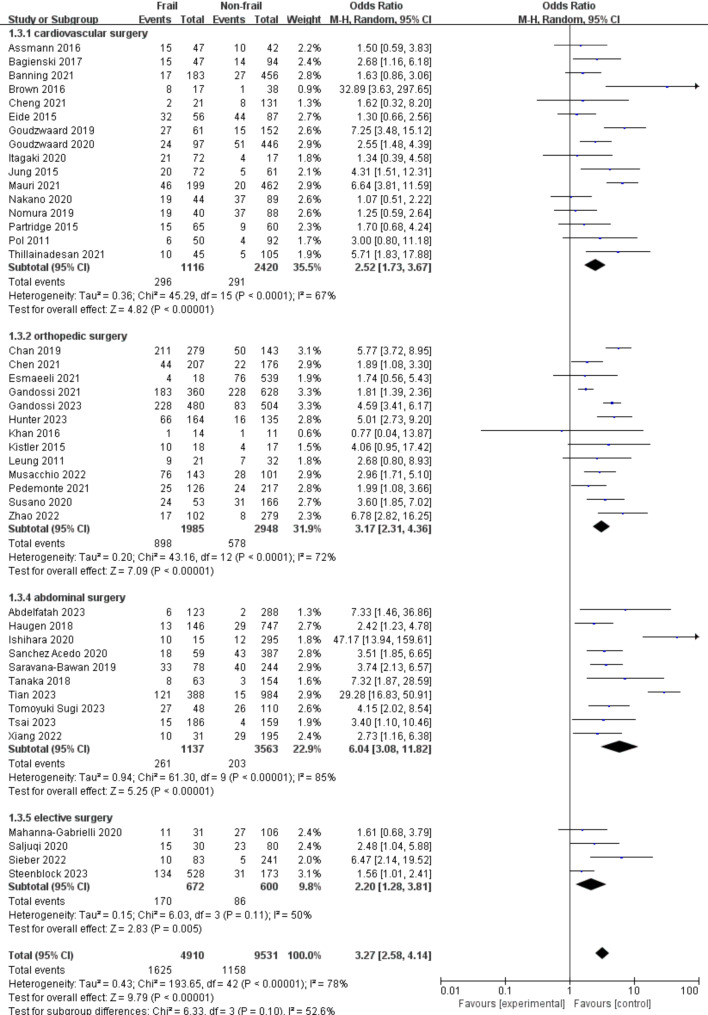
The associations between preoperative frailty and POD in different groups based on the type of surgery are shown in Fig. 5. Cardiovascular surgery was the most commonly performed surgery in the studied population (16 studies [36.7%]), followed by orthopedic surgery (13 studies [31.9%]), abdominal surgery (10 studies [22.9%]), and other elective surgery (4 studies [9.8%]). The association between preoperative frailty and POD was strongest in the abdominal surgery group (OR: 6.04, 95% CI: 3.08–11.82) and weakest in the elective surgery group (OR: 2.20, 95% CI: 1.28–3.81). However, no statistically significant differences were detected within the 4 surgical groups (P = 0.10).
Fig. 5.
Forest plots displaying pooled effect estimates for types of surgery
Publication Bias and sensitivity analysis
Publication bias in the included studies was assessed using a funnel plot. The plot exhibited a symmetrical pattern, suggesting no publication bias (Supplementary Fig. 1). Sensitivity analysis, performed by excluding one study at a time, showed that the combined prevalence rate remained stable, indicating the robustness of the meta-analysis results (Supplementary Table 2).
Discussion
This meta-analysis synthesized data from 43 studies involving a total of 14,441 patients to investigate the link between preoperative frailty and POD. The baseline frailty rate was 34.0%, and the incidence of POD was 20%. These results highlight the global prevalence of frailty in older adults and its significant impact on surgical outcomes, underscoring the importance of medical vigilance. Frailty is identified as a key prognostic factor associated with surgical complications and patient prognosis, reinforcing the value of preoperative frailty assessment.
Frailty and delirium share an intrinsic connection, with the inflammatory cytokine cascade postulated to initiate neuroinflammation and disruption of extensive neuronal networks in the brain, leading to acute declines in cognitive and functional capacities [24, 25, 38]. Studies have revealed that frailty is correlated with compromised DNA-repair mechanisms, mitochondrial dysfunction, elevated free-radical production, reduced telomere integrity, inflammation, impairments in innate immune function [26], and dysregulation of the hypothalamic-pituitary-adrenal axis [27, 63]. Additionally, frailty has been linked to hormonal imbalances [29, 30] and insulin resistance, along with deregulation of glucose metabolism [31, 32].
Our findings align with those of previous meta-analyses, which also found a significant link between frailty and delirium in elderly surgical patients. One recent meta-analysis of 11 studies, with a total of 794 patients, reported an adjusted OR of 2.45 (95% CI: 1.58–3.81) for POD in frail patients undergoing elective surgery [33], while another meta-analysis of 9 studies yielded an adjusted OR of 2.14 (95% CI: 1.43–3.19) [6]. Both highlighted the challenge of study heterogeneity and the need for further research to better understand the mechanisms by which frailty contributes to delirium and to evaluate the impact of different frailty assessments in various settings. Therefore, we aimed to provide a clearer understanding of the frailty-delirium relationship. Our literature review updated these previous meta-analyses as it identified more studies and included more recent publications [6, 33]. This ensured an accurate representation of older frail adults undergoing surgery that did not have neurocognitive disorders at baseline, and provided a robust sample size on which to base our conclusions. Our included studies were of high methodological quality, in contrast to the previous meta-analyses, which included studies with moderate to critical risk of bias [6].
Our study also aligns with the most recent European Society of Anaesthesiology and Intensive Care Medicine (ESAIC) guidelines, published in 2025 [64]. These guidelines recommend preoperative frailty screening with the CFS to predict postoperative outcomes, especially for assessing the risk of delirium. If a frailty phenotype is identified a multidisciplinary approach to patient care should be adopted, including an evaluation by a geriatrician. There is currently no consensus on the timing of frailty assessment in relation to surgery, identifying an evidence gap relevant to the implementation of frailty assessment in elderly surgical patients [65].
ESAIC guidelines recommend the CFS as a screening tool based on feasibility of use in the preoperative setting and its strong association with mortality and unfavorable discharge [64]. Alternative measures include the EFS, which correlates well with the development of postoperative complications, and the Fried Frailty Phenotype, which is best associated with the development of POD. The Frailty Phenotype is less feasible for use in the preoperative setting as it needs specific equipment and is a time burden (5 to 20 min vs. 44 s for the CFS) [64]. Our study highlighted the variety of tools currently in clinical use, including the Fried Frailty Phenotype, CFS, FI, and EFS, with our meta-analysis indicating a particular preference for the FI, Fried, and FRAIL tools.
High frailty prevalence was noted in a Singapore study of 234 older adults with surgical indications, with 68% of patients (95% CI: 62–74%) experiencing subsyndromal delirium [33]. A UK multicenter study of 1,507 patients also reported a high frailty rate of 66% (95% CI: 64–68%) [35]. In contrast, a study from Australia found a slightly lower frailty rate: 53% (95% CI: 48–59%) among 302 patients with atrial fibrillation [36]. In our previous study in China,1 48% of 148 elderly hip-fracture patients were found to be frail preoperatively. The incidence of POD was 24.3% by day 7, with frail patients being at a higher risk for this complication (42.3% vs. 7.8%, P < 0.001). Moreover, preoperative frailty was found to be an independent risk factor for POD (P = 0.002) [1]. Notably, our current study resolves the above inconsistencies in the prevalence of frailty among different populations, demonstrating comparable frailty prevalence between Asian and non-Asian populations (Asian: 61.9% [276/446] vs. non-Asian: 57.4% [1330/2319]) and reinforcing the global significance of frailty. Unfortunately, frailty research in China’s large population remains limited, suggesting that assessments and screenings have not received adequate focus. This is lamentable, as it could lead to missed chances for early interventions and better health outcomes for the elderly.
Methods of anesthesia and anesthetics have been identified as risk factors for POD in the elderly [66]. Major surgery requires a constant state of unconsciousness, maintained using inhaled and intravenous anesthetics, benzodiazepines and opioids. Chest and abdominal surgeries may be performed using regional anesthetic methods, such as spinal and epidural anesthesia. The impact of general anesthesia compared to regional anesthesia on POD remains to be elucidated. The use of fewer drugs, the shorter duration of surgery and shallower depth of sedation with regional anesthesia may result in a lower incidence of POD compared to general anesthesia [66, 67]. However, several studies, including a recent systematic review and meta-analysis, revealed no benefits of regional anesthesia over general anesthesia for POD in the elderly, identifying the need for further studies that assess the associations between the type of anesthesia methods used in clinical practice and the incidence of POD [66, 68].
Delving into specific surgical types, our study reaffirms the heightened risk of delirium after cardiovascular procedures as compared to non-cardiovascular surgeries. However, frailty emerged as a crucial determinant of POD across various surgical domains, emphasizing the need for preoperative frailty assessment regardless of surgical type.
Evidence supporting pharmacological and non-pharmacological prophylaxis for POD is inconsistent, especially among homogeneous subpopulations of surgical patients such as frail older adults, identifying a critical clinical need for well-designed studies that rigorously evaluate the risks and benefits of potential interventions across a variety of patients [37]. Pharmacological options for prevention of POD include dexmedetomidine, olanzapine and risperidone [28]. Dexmedetomidine is a sedative, analgesic, neuroprotectant and anxiolytic. Randomized controlled trials and meta-analyses indicate that dexmedetomidine may reduce the incidence and duration of POD in cardiac and non-cardiac adult surgical populations. Mechanisms include altering the inflammatory and stress response to surgery. Dosing may be perioperative or postoperative in the ICU. Adverse events associated with dexmedetomidine administration include hemodynamic instability [39], such as bradycardia and hypotension. Olanzapine and risperidone are atypical antipsychotics that may also have a role in POD prevention. Randomized controlled trials show these atypical antipsychotics may reduce the incidence of POD in cardiac and non-cardiac adult surgical populations, but POD may be prolonged and more severe in patients who develop POD after receiving these drugs [40]. Non-pharmacological prophylaxis of POD includes avoiding the use of precipitating drugs such as benzodiazepines and atropine, maintaining patient mobility and the sleep-wake cycle, minimizing fasting, appropriately managing anesthesia, diagnosing and managing intraoperative complications in a timely manner, and providing guidance in the postoperative period [41, 42].
The limitations of this review include that studies were restricted to those published in the English language, populations and sample sizes varied across studies, and diverse methods were used for assessing frailty and delirium. First, the retrospective nature of our analysis limits the inference of causality, and despite adjustments for multiple factors, residual confounding may still influence the outcomes. Second, the use of various frailty- and delirium-assessment tools introduces clinical heterogeneity [43], potentially biasing the results, although our pseudo-risk-minimization method ensured the robustness of our models. Third, the inconsistency in delirium-screening tools and follow-up times among studies adds to the complexity [22]. Lastly, delirium was assessed inconsistently, potentially leading to underestimation, as it was not continuously monitored. The optimal timeframe for the diagnosis of POD remains undefined, with peaks typically occurring 1–3 days after surgery [44].
Conclusions
Our study underscores the global link between preoperative frailty and POD, emphasizing the need for clinical frailty assessment to guide interventions and improve outcomes. The meta-analysis shows that preoperative frailty is significantly tied to higher POD risk, with early screening aiding in targeted care. Further research should aim to streamline frailty evaluation in preoperative assessments, boosting timely identification and support for frail patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- CIs
Confidence intervals
- CAM
Confusion Assessment Method
- FRAIL
Fatigue, Resistance, Ambulation, Illness, and Loss of weight
- CFS
Clinical Frailty Scale
- EFS
Edmonton Frail Scale
- MeSH
Medical Subject Headings
- ORs
Odds ratios
- POD
Postoperative delirium
- PRISMA
Preferred Reporting Items for Systematic Review and Meta-Analyses
- NOS
Newcastle-Ottawa Scale
Author contributions
Haotian Wu: This author helped with data analysis, data interpretation, preparation of the first draft, and subsequent revisions. Siyi Yan: This author helped with data interpretation, preparation of the first draft, and review of the final draft. Chunyu Feng: This author helped with study design, data analysis, data interpretation, and manuscript revisions. Han Cao: This author helped with study design, manuscript review, data interpretation, and final revision. Huan Zhang: This author helped with study design, study execution, data analysis, data interpretation, and manuscript revisions.
Funding
This work was supported by the Beijing Municipal Administration of Hospitals Incubating Program [PX2022037].
Data availability
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haotian Wu and Siyi Yan contributed equally to this work.
References
- 1.Humeidan M, Deiner SG. Postoperative delirium. Principles and practice of geriatric surgery: third edition: with 261 figures and 155 tables. Springer International Pub. 2020;395–409.
- 2.Rudolph JL, Marcantonio ER. Postoperative delirium: acute change with long-term implications. Anesth Analg. 2011;112:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glumac S, Kardum G, Karanovic N. Postoperative cognitive decline after cardiac surgery: A narrative review of current knowledge in 2019. Med Sci Monit. 2019;25:3262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhushan S, Li Y, Huang X, Cheng H, Gao K, Xiao Z. Progress of research in postoperative cognitive dysfunction in cardiac surgery patients: A review Article. Int J Surg. 2021;95. [DOI] [PubMed]
- 5.Feng C, Wu H, Qi Z, Wei Y, Yang B, Yin H, et al. Association of preoperative frailty with the risk of postoperative delirium in older patients undergoing hip fracture surgery: a prospective cohort study. Aging Clin Exp Res. 2024. 10.1007/s40520-023-02692-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gracie TJ, Caufield-Noll C, Wang NY, Sieber FE. The association of preoperative frailty and postoperative delirium: A Meta-Analysis. Anesth Analg. 2021;133:314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persico I, Cesari M, Morandi A, Haas J, Mazzola P, Zambon A, et al. Frailty and delirium in older adults: A systematic review and Meta-Analysis of the literature. J Am Geriatr Soc. 2018;66:2022–30. [DOI] [PubMed] [Google Scholar]
- 8.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. The lancet. Elsevier B.V. 2013;752–62. [DOI] [PMC free article] [PubMed]
- 9.Wilson JM, Boissonneault AR, Schwartz AM, Staley CA, Schenker ML. Frailty and malnutrition are associated with inpatient postoperative complications and mortality in hip fracture patients. Journal of orthopaedic trauma. Lippincott Williams and Wilkins. 2019;143–8. [DOI] [PubMed]
- 10.Boissonneault A, Mener A, Schwartz A, Wilson J, Staley C, Schenker M. Impact of frailty on 30-day morbidity and mortality of patients with intertrochanteric femur fractures. Orthopedics. 2019;42:344–8. [DOI] [PubMed] [Google Scholar]
- 11.Morandi A, Davis D, Taylor JK, Bellelli G, Olofsson B, Kreisel S, et al. Consensus and variations in opinions on delirium care: a survey of European delirium specialists. Int Psychogeriatr. 2013;25:2067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellelli G, Mazzola P, Morandi A, Bruni A, Carnevali L, Corsi M, et al. Duration of postoperative delirium is an independent predictor of 6-month mortality in older adults after hip fracture. J Am Geriatr Soc. 2014;62:1335–40. [DOI] [PubMed] [Google Scholar]
- 13.Dunlop RAN, Van Zundert A. A systematic review of predictive accuracy via c-statistic of preoperative frailty tests for extended length of stay, post-operative complications, and mortality. Saudi J Anaesth. 2023;17:575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014. [DOI] [PMC free article] [PubMed]
- 15.Watt J, Tricco AC, Talbot-Hamon C, Pham B, Rios P, Grudniewicz A et al. Identifying older adults at risk of harm following elective surgery: A systematic review and meta-analysis. BMC Med. 2018;16. [DOI] [PMC free article] [PubMed]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. [DOI] [PMC free article] [PubMed]
- 17.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. In: The Lancet. 2013. [DOI] [PMC free article] [PubMed]
- 18.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. Volume 56. Journals of Gerontology - Series A Biological Sciences and Medical Sciences; 2001. [DOI] [PubMed]
- 19.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8. [DOI] [PMC free article] [PubMed]
- 20.Rockwood K, Theou O. Clinical frailty scale. Frailty. Springer International Pub. 2024;121–3.
- 21.Røyset IM, Falk Eriksen G, Benth JŠ, Saltvedt I, Grønberg BH, Rostoft S et al. Edmonton frail scale predicts mortality in older patients with cancer undergoing radiotherapy-A prospective observational study. PLoS ONE. 2023;18. [DOI] [PMC free article] [PubMed]
- 22.Chen Y, Qin J. Modified frailty index independently predicts postoperative delirium and delayed neurocognitive recovery after elective total joint arthroplasty. J Arthroplasty. 2021;36:449–53. [DOI] [PubMed] [Google Scholar]
- 23.Saljuqi AT, Hanna K, Asmar S, Tang A, Zeeshan M, Gries L, et al. Prospective evaluation of delirium in geriatric patients undergoing emergency general surgery. J Am Coll Surg. 2020;230:758–65. [DOI] [PubMed] [Google Scholar]
- 24.Tian JY, Hao XY, Cao FY, Liu JJ, Li YX, Guo YX, et al. Preoperative frailty assessment predicts postoperative mortality, delirium and pneumonia in elderly lung Cancer patients: A retrospective cohort study. Ann Surg Oncol. 2023;30:7442–51. [DOI] [PubMed] [Google Scholar]
- 25.Sugi T, Enomoto T, Ohara Y, Furuya K, Kitaguchi D, Moue S, et al. Risk factors for postoperative delirium in elderly patients undergoing gastroenterological surgery: A single-center retrospective study. Ann Gastroenterol Surg. 2023;7:832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steenblock J, Braisch U, Brefka S, Thomas C, Eschweiler GW, Rapp M et al. Frailty index and its association with the onset of postoperative delirium in older adults undergoing elective surgery. BMC Geriatr. 2023;23. [DOI] [PMC free article] [PubMed]
- 27.Hunter CL, Ni Chroinin D, McEvoy L, Chuan A. Poorer outcomes in patients with early postoperative delirium: 120-day follow-up of the delirium reduction by analgesia management in hip fracture (DRAM-HF) study. Australas J Ageing. 2023;42:736–41. [DOI] [PubMed] [Google Scholar]
- 28.Gandossi CM, Zambon A, Oliveri G, Codognola M, Szabo H, Cazzulani I, et al. Frailty, post-operative delirium and functional status at discharge in patients with hip fracture. Int J Geriatr Psychiatry. 2021;36:1524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdelfatah E, Ramos-Santillan V, Cherkassky L, Cianchetti K, Mann G. High risk, high reward: frailty in colorectal Cancer surgery is associated with worse postoperative outcomes but equivalent Long-Term oncologic outcomes. Ann Surg Oncol. 2023;30:2035–45. [DOI] [PubMed] [Google Scholar]
- 30.Zhao H, Wei P, Feng Y. Association between frailty and clinical outcomes and quality of life in older adults following hip fracture surgery: A retrospective cohort study. Anesth Analg. 2022;134:1035–42. [DOI] [PubMed] [Google Scholar]
- 31.Xiang D, Xing H, Zhu Y. A predictive nomogram model for postoperative delirium in elderly patients following laparoscopic surgery for gynecologic cancers. Support Care Cancer. 2023;31. [DOI] [PubMed]
- 32.Sieber F, Gearhart S, Bettick D, Wang NY. Edmonton frailty scale score predicts postoperative delirium: a retrospective cohort analysis. BMC Geriatr. 2022;22. [DOI] [PMC free article] [PubMed]
- 33.Musacchio C, Custodero C, Razzano M, Raiteri R, Delrio A, Torriglia D et al. Association between multidimensional prognostic index (MPI) and pre-operative delirium in older patients with hip fracture. Sci Rep. 2022;12. [DOI] [PMC free article] [PubMed]
- 34.Esmaeeli S, Franco-Garcia E, Akeju O, Heng M, Zhou C, Azocar RJ, et al. Association of preoperative frailty with postoperative delirium in elderly orthopedic trauma patients. Aging Clin Exp Res. 2022;34:625–31. [DOI] [PubMed] [Google Scholar]
- 35.Thillainadesan J, Mudge AM, Aitken SJ, Hilmer SN, Cullen JS, Yumol MF, et al. The prognostic performance of frailty for delirium and functional decline in vascular surgery patients. J Am Geriatr Soc. 2021;69:688–95. [DOI] [PubMed] [Google Scholar]
- 36.Pedemonte JC, Sun H, Franco-Garcia E, Zhou C, Heng M, Quraishi SA, et al. Postoperative delirium mediates 180-day mortality in orthopaedic trauma patients. Br J Anaesth. 2021;127:102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauri V, Reuter K, Körber MI, Wienemann H, Lee S, Eghbalzadeh K, et al. Incidence, risk factors and impact on Long-Term outcome of postoperative delirium after transcatheter aortic valve replacement. Front Cardiovasc Med. 2021;8:645724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai CY, Liu KH, Lai CC, Hsu J, Te, Hsueh SW, Hung CY et al. Association of preoperative frailty and postoperative delirium in older cancer patients undergoing elective abdominal surgery: A prospective observational study in Taiwan. Biomed J. 2023;46. [DOI] [PMC free article] [PubMed]
- 39.Cheng HW, Liu CY, Chen YS, Shih CC, Chen WY, Chiou AF. Assessment of preoperative frailty and identification of patients at risk for postoperative delirium in cardiac intensive care units: a prospective observational study. Eur J Cardiovasc Nurs. 2021;20:745–51. [DOI] [PubMed] [Google Scholar]
- 40.Banning LBD, El Moumni M, Visser L, van Leeuwen BL, Zeebregts CJ, Pol RA. Frailty leads to poor long-term survival in patients undergoing elective vascular surgery. J Vasc Surg. 2021;73:2132–e21392. [DOI] [PubMed] [Google Scholar]
- 41.Ishihara A, Tanaka S, Ueno M, Iida H, Kaibori M, Nomi T, et al. Preoperative risk assessment for delirium after hepatic resection in the elderly: a prospective multicenter study. J Gastrointest Surg. 2021;25:134–44. [DOI] [PubMed] [Google Scholar]
- 42.Susano MJ, Grasfield RH, Friese M, Rosner B, Crosby G, Bader AM, et al. Brief preoperative screening for frailty and cognitive impairment predicts delirium after spine surgery. Anesthesiology. 2020;133:1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sánchez Acedo P, Eguaras Córdoba I, Zazpe Ripa C, Herrera Cabezón J, Tarifa Castilla A. Prospective study of factors associated with postoperative delirium after urgent abdominal surgery. Cir Esp. 2020;98:450–5. [DOI] [PubMed] [Google Scholar]
- 44.Nakano M, Nomura Y, Suffredini G, Bush B, Tian J, Yamaguchi A, et al. Functional outcomes of frail patients after cardiac surgery: an observational study. Anesth Analg. 2020;130:1534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahanna-Gabrielli E, Zhang K, Sieber FE, Lin HM, Liu X, Sewell M, et al. Frailty is associated with postoperative delirium but not with postoperative cognitive decline in older noncardiac surgery patients. Anesth Analg. 2020;130:1516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goudzwaard JA, De Ronde-Tillmans MJAG, De Jager TAJ, Lenzen MJ, Nuis RJ, Van Mieghem NM, et al. Incidence, determinants and consequences of delirium in older patients after transcatheter aortic valve implantation. Age Ageing. 2020;49:389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Itagaki A, Sakurada K, Matsuhama M, Yajima J, Yamashita T, Kohzuki M. Impact of frailty and mild cognitive impairment on delirium after cardiac surgery in older patients. J Cardiol. 2020;76:147–53. [DOI] [PubMed] [Google Scholar]
- 48.Saravana-Bawan B, Warkentin LM, Rucker D, Carr F, Churchill TA, Khadaroo RG. Incidence and predictors of postoperative delirium in the older acute care surgery population: A prospective study. Can J Surg. 2019;62:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nomura Y, Nakano M, Bush B, Tian J, Yamaguchi A, Walston J, et al. Observational study examining the association of baseline frailty and postcardiac surgery delirium and cognitive change. Anesth Analg. 2019;129:507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haugen CE, Mountford A, Warsame F, Berkowitz R, Bae S, Thomas AG, et al. Incidence, risk factors, and sequelae of post-kidney transplant delirium. J Am Soc Nephrol. 2018;29:1752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka S, Ueno M, Iida H, Kaibori M, Nomi T, Hirokawa F, et al. Preoperative assessment of frailty predicts age-related events after hepatic resection: a prospective multicenter study. J Hepatobiliary Pancreat Sci. 2018;25:377–87. [DOI] [PubMed] [Google Scholar]
- 52.Bagienski M, Kleczynski P, Dziewierz A, Rzeszutko L, Sorysz D, Trebacz J, et al. Incidence of postoperative delirium and its impact on outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2017;120:1187–92. [DOI] [PubMed] [Google Scholar]
- 53.Brown CH, Max L, Laflam A, Kirk L, Gross A, Arora R, et al. The association between preoperative frailty and postoperative delirium after cardiac surgery. Anesth Analg. 2016;123:430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Assmann P, Kievit P, van der Wulp K, Verkroost M, Noyez L, Bor H, et al. Frailty is associated with delirium and mortality after transcatheter aortic valve implantation. Open Heart. 2016;3:e000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan BA, Perkins AJ, Prasad NK, Shekhar A, Campbell NL, Gao S, et al. Biomarkers of delirium duration and delirium severity in the ICU∗. Crit Care Med. 2020;48:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eide LSP, Ranhoff AH, Fridlund B, Haaverstad R, Hufthammer KO, Kuiper KKJ, et al. Comparison of frequency, risk factors, and time course of postoperative delirium in octogenarians after transcatheter aortic valve implantation versus surgical aortic valve replacement. Am J Cardiol. 2015;115:802–9. [DOI] [PubMed] [Google Scholar]
- 57.Jung P, Pereira MA, Hiebert B, Song X, Rockwood K, Tangri N, et al. The impact of frailty on postoperative delirium in cardiac surgery patients. J Thorac Cardiovasc Surg. 2015;149:869–e8752. [DOI] [PubMed] [Google Scholar]
- 58.Kistler EA, Nicholas JA, Kates SL, Friedman SM. Frailty and Short-Term outcomes in patients with hip fracture. Geriatr Orthop Surg Rehabil. 2015;6:209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Partridge JSL, Fuller M, Harari D, Taylor PR, Martin FC, Dhesi JK. Frailty and poor functional status are common in arterial vascular surgical patients and affect postoperative outcomes. Int J Surg. 2015;18:57–63. [DOI] [PubMed] [Google Scholar]
- 60.Leung JM, Tsai TL, Sands LP. Preoperative frailty in older surgical patients is associated with early postoperative delirium. Anesth Analg. 2011;112:1199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pol RA, Van Leeuwen BL, Visser L, Izaks GJ, Van Den Dungen JJAM, Tielliu IFJ, et al. Standardised frailty indicator as predictor for postoperative delirium after vascular surgery: A prospective cohort study. Eur J Vasc Endovasc Surg. 2011;42:824–30. [DOI] [PubMed] [Google Scholar]
- 62.Chan S, Wong EKC, Ward SE, Kuan D, Wong CL. The predictive value of the clinical frailty scale on discharge destination and complications in older hip fracture patients. J Orthop Trauma. 2019;33:497–502. [DOI] [PubMed] [Google Scholar]
- 63.Gandossi CM, Zambon A, Ferrara MC, Tassistro E, Castoldi G, Colombo F, et al. Frailty and post-operative delirium influence on functional status in patients with hip fracture: the GIOG 2.0 study. Aging Clin Exp Res. 2023;35:2499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lamperti M, Romero CS, Guarracino F, Cammarota G, Vetrugno L, Tufegdzic B, et al. Preoperative assessment of adults undergoing elective noncardiac surgery: updated guidelines from the European society of anaesthesiology and intensive care. Eur J Anaesthesiol. 2024;42:1–35. [DOI] [PubMed] [Google Scholar]
- 65.Wang HT, Nguyen QD, Menard CA, Morinville A, Hirdes JP, Hebert P. 97 How robust are frailty assessments? A systematic review of the application of frailty in the surgical population. Age Ageing. 2019;48:ii28–9. [Google Scholar]
- 66.Zhuang X, He Y, Liu Y, Li J, Ma W. The effects of anesthesia methods and anesthetics on postoperative delirium in the elderly patients: A systematic review and network meta-analysis. Front Aging Neurosci. 2022;14:935716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li T, Dong T, Cui Y, Meng X, Dai Z. Effect of regional anesthesia on the postoperative delirium: A systematic review and meta-analysis of randomized controlled trials. Front Surg. 2022;9:937293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ellard L, Katznelson R, Wasowicz M, Ashworth A, Carroll J, Lindsay T, et al. Type of anesthesia and postoperative delirium after vascular surgery. J Cardiothorac Vasc Anesth. 2014;28:458–61. [DOI] [PubMed] [Google Scholar]
- 69.Khan SA, Chua HW, Hirubalan P, Karthekeyan RB, Kothandan H. Association between frailty, cerebral oxygenation and adverse post-operative outcomes in elderly patients undergoing non-cardiac surgery: an observational pilot study. Indian J Anaesth. 2016;60:102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.