Abstract
Background
Lacrimal irrigation is a fundamental skill for diagnosing and managing lacrimal diseases. This study evaluates two lacrimal irrigation techniques and introduces a haptic-visual integrated self-serve training model to enhance skill acquisition among novice ophthalmology residents.
Methods
Ninety-two ophthalmology residents were randomized into Group A (n = 47) and Group B (n = 45). Both groups completed an 8-hour training program comprising theoretical instruction, demonstrations, and hands-on practice. Group A provided feedback to refine the training model, which was subsequently implemented in Group B1 (n = 23), while Group B2 (n = 22) served as the control. Outcomes were assessed through skill evaluations and post-training questionnaires measuring confidence scores and perceived efficacy.
Results
In Group A, 70.2% of participants preferred Technique 1 for its perceived ease of use, while 29.8% favored Technique 2 for pressurized irrigation scenarios (p < 0.05). Key barriers to proficiency included the absence of suitable training models (63.8%) and psychological anxiety (25.5%). In Group B, participants using the training model (Group B1) demonstrated significantly higher confidence scores compared to Group B2 (8.4 ± 1.2 vs. 6.1 ± 1.5, p < 0.05). Although skill assessment scores showed a positive trend in Group B1 (80.7 ± 8.3 vs. 76.8 ± 9.1), the difference was not statistically significant (p > 0.05).
Conclusion
Both lacrimal irrigation techniques are equally accessible to novices, with Technique 2 offering advantages in pressurized irrigation. The self-serve training model significantly enhances procedural confidence and addresses critical training barriers, including resource limitations and psychological safety. Future studies should validate these findings in larger cohorts and refine the model to incorporate enhanced simulation techniques and dynamic physiological feedback.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12909-025-06889-2.
Keywords: Lacrimal irrigation, Training model, Haptic feedback, Self-serve
Introduction
Lacrimal irrigation, a widely utilized and minimally invasive procedure, serves as a cornerstone in the clinical assessment of obstructive and inflammatory conditions affecting the lacrimal system [1, 2]. Its therapeutic potential was recognized as early as 1956, with reports indicating that forced irrigation successfully resolved nasolacrimal duct obstruction in 50% of patients [3]. In recent years, however, the primary application of lacrimal irrigation has shifted toward diagnostic purposes [4]. This procedure plays a pivotal role in identifying lacrimal pathologies by providing critical insights into the location and nature of obstructions within the lacrimal duct. Furthermore, lacrimal irrigation is an essential preoperative evaluation for intraocular surgeries, given that lacrimal obstruction is a well-documented risk factor for postoperative endophthalmitis [5, 6].
While advanced diagnostic modalities such as dacryocystography and dacryoscintigraphy offer superior accuracy in evaluating the anatomy and function of the lacrimal drainage system, their widespread adoption is hindered by limitations in time, resources, and accessibility, particularly in community healthcare settings [2, 7, 8]. These methods are unsuitable for routine screening of lacrimal drainage system diseases and are typically reserved for cases where abnormal findings are detected during lacrimal irrigation. Consequently, lacrimal irrigation remains an indispensable diagnostic tool in ophthalmology.
Given the clinical significance and accessibility of lacrimal irrigation, proficiency in this fundamental skill is essential for ophthalmology residents. However, many trainees frequently lack self-confidence during their initial attempts to master the procedure. This challenge is exacerbated by insufficient training opportunities and the absence of appropriate simulation models, resulting in a prolonged and inefficient learning process.
More critically, the direct training of residents on patients raises substantial ethical and legal concerns. The inexperience of trainees increases the risk of iatrogenic injuries, as exemplified by a reported case in which a detached hypodermic needle during ocular irrigation led to corneal injury, iris perforation, and severe endophthalmitis [9]. Additionally, issues related to informed consent arise, as patients may not be fully aware that they are being treated by a trainee, potentially leading to ethical dilemmas and legal complications. These challenges underscore the urgent need for alternative training methods that minimize patient involvement while ensuring effective skill acquisition. An appropriate simulation model could provide a safe and controlled environment for residents to practice lacrimal irrigation techniques, thereby reducing unnecessary risks to patients.
Moreover, the technical proficiency of the practitioner significantly influences the reliability of lacrimal irrigation outcomes. Variations in technique can lead to inconsistent results, particularly in the assessment of reflux patterns, which serve as crucial diagnostic indicators. Current research presents divergent benchmarks for normal reflux, with some studies suggesting that patent lacrimal systems exhibit less than 20% reflux [10, 11], while others set the threshold at 0% [12–14]. Although the interpretation of reflux remains somewhat subjective, it is essential to minimize technique-related variations to ensure diagnostic accuracy and reliability in clinical assessments.
To address these challenges, efficient training methodologies and accessible, effective training models are imperative. However, research on lacrimal irrigation training for ophthalmology residents remains limited, particularly in comparing techniques for skill acquisition and retention, and in developing standardized models that simulate clinical scenarios while minimizing patient risk.
This study introduces two distinct techniques and proposes a haptic-visual integrated self-serve training model. The acceptability and efficacy of these approaches are systematically evaluated to enhance lacrimal irrigation proficiency among residents.
Methods
Participants and post-training questionnaire survey
A total of 92 ophthalmology residents, currently in the pre-clinical stage of their residency training, participated in this study. All participants were novice learners in ophthalmic basic skills with limited clinical experience and were divided into two groups: Group A (47 participants) and Group B (45 participants).
Group A underwent an 8-hour training session where they were instructed in two different techniques for lacrimal irrigation by a senior instructor. During the training session, the participants practice lacrimal irrigation with each other under the guidance of the senior instructor. Skill assessments were conducted for all participants after the training session. In addition, post-training questionnaires were also used to assess their abilities and gather feedback (Supplemental Table 1). Data were collected on their baseline skills, practice times, and proficiency levels (Elementary, Intermediate, and Advanced). The operational difficulty, stability, and injection performance of each trainee’s chosen technique was also assessed. Furthermore, the operator assessed the subjects’ level of comfort by taking into account patient complaints and facial expressions. A numeric rating scale ranging from 0 to 10 was utilized, where 0 indicated no pain and 10 indicated severe pain.
Based on the feedback received from Group A, we developed a simplified training model. Subsequently, Group B also planned to receive the same training and was randomly divided into two subgroups using block randomization to ensure balanced group sizes and minimize selection bias: Group B1 (23 participants), which implemented the training model, and Group B2 (22 participants), which did not. Participants in Group B1 were guided to practice lacrimal irrigation with the assistance of the training model before proceeding to practice with their partners. Skill assessments were conducted for all participants in Group B after the training session. Additionally, post-training questionnaires (Supplemental Table 1) were used to evaluate the effectiveness of the training model in improving the mastery of lacrimal irrigation skills.
In the analysis of Group B, participants with pre-existing entry-level skill ability were excluded. Entry-level skill ability was defined as the ability to independently and successfully perform lacrimal irrigation with a history of ≤ 10 prior attempts. This threshold was established based on the study team’s clinical experience and the assumption that participants with more than 10 successful attempts likely possess a level of proficiency beyond that of a novice learner. Although no specific guidelines or literature directly support this threshold, it was deemed appropriate to ensure a homogeneous novice cohort for evaluating the training model’s effectiveness (Fig. 1).
Fig. 1.
Flow chart of the study
Lacrimal irrigation test
Preparation of medicines and supplies
A 5mL syringe, blunt-tipped 23-G cannula, 0.9% saline solution, proparacaine hydrochloride eye drops, cotton swab, and punctum dilator were assembled.
Lacrimal irrigation process
Prior to the examination, participants with contraindications were excluded. Topical ocular surface anesthesia was administered using proparacaine hydrochloride eye drops. In cases where the punctum was stenosis, a punctum dilator was utilized.
A 23-G lacrimal cannula was attached to a 5 ml syringe containing a saline solution with a small amount of ointment. The operator positioned themselves behind the patient to begin lacrimal irrigation.
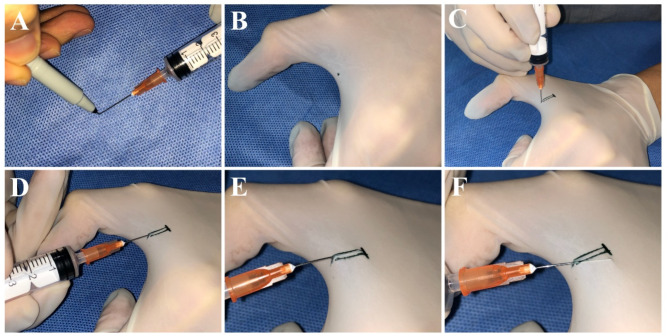
For the lower lacrimal puncta, the procedure started by instructing the patient to look upward. The operator gently pulled the lower eyelid outward and downward, exposing the lower punctum upward. Holding the syringe with the thumb, index finger, and middle finger, the operator inserted the cannula vertically into the lower punctum by about 1.5–2 mm. A distinct sense of penetration was felt (Fig. 2A). Then, the syringe was adjusted for a horizontal injection (Fig. 2B). As the cannula approached the lacrimal sac, the operator tilted the syringe downward by approximately 15° while steadily advancing upward. When the cannula tip encountered a hard stop as it passed beyond the common canaliculus and reached the anterior lacrimal crest, it was retracted by 2–3 mm. Subsequently, the operator adjusted their hand position to prepare for the injection of saline solution.
Fig. 2.
Lacrimal Irrigation Process: A. Vertically insert syringe into the lower punctum by approximately 1.5–2 mm. B. Adjust syringe for horizontal injection. C-D. Maintain a firm grip on the syringe barrel with the thumb and middle finger, while the index finger charges the syringe plunger. E-F. Create a crossed fixation of the barrel between the index and middle fingers, and reposition the thumb to the syringe plunger
There are two specific techniques outlined below:
Technique 1
Maintain a firm grip on the syringe barrel using the thumb and middle finger while releasing the index finger to transfer it to the syringe plunger. Push the plunger to perform lacrimal irrigation (Fig. 2C-D).
Technique 2
Maintain a secure hold on the syringe barrel with the thumb and index finger. Transfer the middle finger to the opposite side of the syringe, creating a crossed fixation of the barrel between the index and middle fingers. Once the syringe is stabilized, reposition the thumb to the syringe plunger and push the plunger to perform lacrimal irrigation (Fig. 2E-F).
During the lacrimal irrigation process, fluid is gently injected while carefully monitoring for fluid reflux. The patient is asked if they perceive any fluid entering the throat. Following the completion of the procedure, Tobramycin eye drops are administered, and the results of lacrimal irrigation are documented.
During the process, the following findings were noted:
Some participants initially struggled with the correct insertion angle, causing patient discomfort. Based on operator feedback, adjustments were made to refine the technique and improve procedural accuracy.
The presence of resistance or a “hard stop” when the cannula reached the anterior lacrimal crest was critical for understanding proper insertion depth.
Participants’ skill levels varied significantly, with more proficient operators exhibiting better control over the syringe and less discomfort reported by patients.
Design rationale and key features of the haptic-visual integrated self-serve training model
To enhance the design of the training model, we conducted preliminary experiments involving various finger webs on the left-hand glove and even tested placing gloves over disposable paper cups. Ultimately, we determined that the finger web positioned between the index finger and thumb provided the optimal design for the training model. This choice was based on several key considerations:
Tactile sensitivity
The skin between the index finger and thumb is highly sensitive, providing realistic haptic feedback, enabling operators to acutely perceive the penetration sensation during cannula insertion, as well as the trajectory and depth variations of the cannula within the designated path. This heightened sensitivity facilitates the immediate detection and correction of improper techniques during training.
Operational space
This area provides sufficient space for cannula manipulation while maintaining a relatively stable support structure, owing to the stronger and more coordinated force generated by the combination of the thumb and index finger compared to other digits. This stability allows trainees to practice precise movements and sustain training for longer durations.
Supplies for the preparation include a marker pen, a 5-mL syringe, a blunt-tipped 23-G lacrimal cannula, saline solution, disposable light-colored rubber gloves, and a ruler.
For the training model design, follow these steps:
Step 1
Simulate tense skin with the glove.
Put on a disposable light-colored rubber glove and spread open your index finger and thumb to create tension in the glove over the finger web.
Step 2
Create a simulated punctum.
Using a marker pen, apply ink to the tip of the blunt-tipped lacrimal cannula (Fig. 3A). Position the marked cannula at the central edge between the index finger and thumb, piercing the glove to create a marked punctum that mimics the shape of the lacrimal punctum. This punctum will be used for subsequent lacrimal duct irrigation training (Fig. 3B).
Fig. 3.
Model training key points: A. Use a marker to apply ink to the blunt-tipped lacrimal cannula. B. Create a marked punctum resembling the lacrimal punctum’s shape. C. Draw two parallel lines, 10–12 mm long, spaced 2–3 mm apart to mimic the lacrimal canaliculus. D. Gently advance the cannula 8–10 mm along the simulated lacrimal duct, ensuring cannula visibility through the latex glove. E. Noticing a distinct cannula shape indicates excessive lifting. F. Avoid tilting the cannula beyond the marked lines
Step 3
Create a simulated lacrimal canaliculus and the bony wall.
Using a marker pen, draw two parallel lines perpendicular to the finger web, approximately 10–12 mm in length and spaced 2–3 mm apart, to simulate the lacrimal canaliculus. At the far end of the lines from the puncture site, draw a vertical line to indicate the position where the distal end of the lacrimal sac contacts the anterior lacrimal crest (Fig. 3C). This marks the maximum insertion depth that the cannula should reach during lacrimal irrigation training. Do not exceed this marked line.
Key usage points
Vertical insertion of lacrimal Punctum
During training, hold a syringe filled with 3 ml of air and align it with the marked simulated lacrimal punctum. Insert the cannula vertically to a depth of 2 mm, feeling a similar empty penetration sensation as with a real punctum (Fig. 3C). Adjust the depth based on tactile feedback on the finger web. Avoid inserting the cannula too deeply, as it may cause sharp pain.
Slow and horizontal insertion within marked line
After vertical insertion, rotate the cannula horizontally towards the finger web and gradually advance it along the marked simulated lacrimal duct by 8–10 mm. Ensure the cannula remains within the marked line and avoid tilting it (Fig. 3F), which indicates an incorrect insertion angle. The shape of the cannula should be visible through the latex glove, indicating the appropriate depth of insertion (Fig. 3D). Observing a noticeable cannula shape suggests excessive lifting, while causing discomfort on the finger indicates excessive pressing down (Fig. 3E).
Switch hand gestures and simulate injection
When the cannula’s tip reaches the endpoint marked line, retract it by 2–3 mm, and then switch hand gestures to inject the air within the syringe, simulating the injection of a saline solution. After completing the injection, slowly withdraw the cannula.
Enhance training based on haptic feedback
Adjust the training based on the tactile feedback of the skin. Consistent and effective training sessions will improve proficiency and stability in lacrimal irrigation, allowing operators to master the skill and prepare for real clinical practice.
Statistical analysis
Statistical analysis utilized SPSS Statistical Software, V.20 (IBM, SPSS). Continuous variables are presented as mean ± SD or median (range), determined by the Shapiro-Wilk normality test. Student’s t-test compared differences between two data groups. Nonparametric significance tests, including the Mann-Whitney U test, chi-square test, or Fisher’s exact test, were employed as appropriate. Statistical significance was set at p < 0.05. All participants provided complete datasets. No missing data required imputation or exclusion.
Results
Participants characteristics
All 92 participants completed the study, with 47 in Group A and 45 in Group B. The cohort consisted of 61 females (66.3%) and 31 males (33.7%), with a mean age of 24.30 ± 2.45 years (range: 21–33). Among the participants, 21 had prior experience with lacrimal irrigation, indicating a certain level of familiarity, while 71 had no prior experience in this area. Baseline characteristics, including age, gender distribution, and prior experience with lacrimal irrigation, showed no significant differences between Group A and Group B (p > 0.05), confirming that the groups were well-balanced at baseline (Table 1).
Table 1.
Baseline characteristics of the participants in group A and group B
| Group A (n = 47) | Group B(n = 14) | p value | |
|---|---|---|---|
| Gender | |||
| Male | 15 | 16 | 0.826 |
| Female | 32 | 29 | |
| Age | 24.64 ± 2.633 | 23.96 ± 2.225 | 0.183 |
| Prior experience | |||
| Yes | 14 | 7 | 0.104 |
| No | 33 | 38 |
Assessing trainees’ skill proficiency after training
After observing demonstrations of two different lacrimal irrigation techniques, trainees from group A independently selected one technique to practice. In group A, 70.2% of trainees (33/47) chose technique one (group A1), while 29.8% of trainees (14/47) chose technique two (group A2). There were no significant differences in terms of gender, age and baseline skill ability between groups A1 and A2 (p > 0.05). Trainees completed an average of 2.98 ± 1.54 (range: 1–10) practice sessions for lacrimal irrigation, and there was no statistically significant difference between groups A1 and A2 (p > 0.05). Based on the self-assessed mastery level in the post-training questionnaire, 35 trainees (74.4%) reported partial mastery of the skill, 9 trainees (19.1%) reported basic mastery, and 3 trainees (6.4%) reported proficiency. There was no statistically significant difference between groups A1 and A2 in terms of self-assessed mastery level (p > 0.05) (Table 2).
Table 2.
Detailed scores of lacrimal irrigation performance of the participants in group A1 and A2
| Group A1(n = 33) | Group A2(n = 14) | p value | |
|---|---|---|---|
| Gender | |||
| Male | 8 | 7 | 0.083 |
| Female | 25 | 7 | |
| Age | 24.39 ± 2.549 | 25.21 ± 2.833 | 0.249 |
| Baseline of skills ability | |||
| Novices | 25 | 8 | 0.354 |
| Entry-level individual | 8 | 6 | |
| Practice times | 3.12 ± 0.30 | 2.64 ± 0.25 | 0.335 |
| Mastery level | |||
| Partial mastery | 27 | 8 | 0.159 |
| Basic proficiency | 5 | 4 | |
| Advanced proficiency | 1 | 2 |
The analysis of lacrimal irrigation techniques
Based on the survey results (Table 3), 66.7% (22/33) of participants in group A1 found technique one easy to master. Similarly, 85.7% (12/14) of participants in group A2 found technique two easy to master. There was no significant difference in the perceived difficulty of mastering these two techniques (p > 0.05). The handling and stability survey for both techniques were also evaluated, and no significant difference was found between group A1 and A2 (p > 0.05). However, technique two was found to be easier for performing the injection compared to technique one (p < 0.05). Additionally, participants rated the comfort of the lacrimal irrigation procedure using the Numeric Rating Scale based on patient complaints and facial expressions. The comfort rating for technique one was 2.06 ± 1.73, while for technique two, it was 1.57 ± 1.79. There was no significant difference in comfort ratings between the two groups (p > 0.05). Furthermore, the Skill assessment scores were 81.63 ± 7.72 in group A1 and 84.22 ± 8.85 in group A2. There was also no significant difference in Skill assessment scores (p > 0.05).
Table 3.
The analysis of lacrimal irrigation techniques
| Group A1(n = 33) | Group A2(n = 14) | p value | |
|---|---|---|---|
| Mastery difficulty | |||
| Easy | 22 | 12 | 0.328 |
| Difficult | 11 | 2 | |
| Stability | |||
| Stable | 15 | 8 | 0.464 |
| Unstable | 18 | 6 | |
| Perform injection | |||
| Easy | 19 | 13 | 0.018 |
| Difficult | 14 | 1 | |
| Comfort rating | 2.06 ± 1.73 | 1.57 ± 1.79 | 0.385 |
| Skill assessment scores | 81.63 ± 7.72 | 84.22 ± 8.85 | 0.320 |
Factors influencing lacrimal irrigation skill mastery: insights from Post-Training questionnaires
In order to further explore the factors affecting lacrimal irrigation skill mastery, we analyzed data from post-training questionnaires. The analysis revealed that the predominant factor was the absence of appropriate training models, as noted by 63.8% (30 out of 47) participants. A further 25.5% (12 out of 47) cited psychological barriers encountered during the procedure. Other noted challenges included the punctal stenosis, as reported by 14.9% (7 out of 47), and a lack of adequate practice time, mentioned by 6.4% (3 out of 47). Additionally, the limited access to practicing with live patients was recognized by 4.3% (2 out of 47) as an impediment to skill acquisition. (Fig. 4)
Fig. 4.
Key challenges hindering lacrimal irrigation skill acquisition
Effectiveness of the lacrimal training model
Based on feedback from Group A, we developed a haptic-visual integrated self-serve training model. Participants were allowed to select their preferred lacrimal irrigation technique during training, as preliminary analysis revealed no significant difference in skill acquisition efficiency between the two techniques (p > 0.05). Evaluation of the training model demonstrated that Group B1 (model users) achieved significantly higher confidence scores compared to Group B2 (non-users) (p < 0.05). While Group B1 showed a trend toward improved skill assessment scores, this difference did not reach statistical significance (p = 0.167). Notably, 85% of Group B1 participants rated the training model as highly effective for skill development (Table 4).
Table 4.
The analysis of efficacy of the training model
| Group B1(n = 20) | Group B2(n = 19) | p value | |
|---|---|---|---|
| Skill assessment scores | 80.741 ± 8.321 | 76.817 ± 9.075 | 0.167 |
| Confidence score | 6.850 ± 1.725 | 5.526 ± 1.954 | 0.031 |
| Skill-enhancing or not? | |||
| Yes | 17(85%) | / | |
| No | 3(15%) | / |
Discussion
This study demonstrates that both lacrimal irrigation techniques can be effectively mastered by novice ophthalmology residents, with no significant difference in skill acquisition efficiency (p > 0.05). Although Technique 1 was preferred by 72% of participants due to its perceived ease of use, Technique 2 demonstrated superior performance in pressurized irrigation scenarios, a critical requirement for diagnosing partial obstructions [2, 13]. This aligns with our skill assessment data, which revealed comparable performance between the two techniques, suggesting that user preference may be influenced by ergonomic factors rather than technical superiority.
Teaching invasive skills, such as lacrimal irrigation, to junior physicians is a resource-intensive process fraught with challenges. The delicate nature of the lacrimal drainage system results in low fault tolerance during procedures, while the risk of ocular injury induces psychological apprehension among trainees [9, 15]. Furthermore, the absence of standardized training models raises ethical concerns, particularly when practicing on live patients. These challenges are exacerbated when operators lack sufficient proficiency, underscoring the need for effective training solutions [16–18].
The self-serve training model developed in this study significantly enhanced operator confidence (Group B1: 6.9 ± 1.7 vs. B2: 5.5 ± 2.0, p = 0.031), supporting the evidence that procedural confidence is a crucial mediator in the development of technical competence for invasive skills. Higher levels of confidence are closely linked to improved surgical performance and increased motivation, which includes greater persistence and effort during skill acquisition [19, 20]. This finding is consistent with broader evidence in surgical education, where confidence, when coupled with deliberate practice, positively correlates with objective measures of procedural skill [21]. Moreover, the alignment between confidence and competence is essential for ensuring safe clinical practice, as both overconfidence and underconfidence can lead to clinical errors. In this context, the Confidence-Competence Ratio (CCR) serves as a valuable framework to guide learners toward achieving a balanced skillset [22].
While 85% of Group B1 participants perceived the model as effective, its limited impact on objective skill scores (B1: 80.7 ± 8.3 vs. B2: 76.8 ± 9.1, p = 0.167) highlights two key insights. First, the assessment scope mismatch between the model’s focus on procedural execution and the comprehensive evaluation rubric—which included pre-procedural, procedural, and post-procedural components—may have diluted its measurable impact [23]. Second, the temporal dynamics of skill acquisition suggest that confidence gains often precede measurable improvements, particularly in early-stage learners [24]. While our 8-hour intervention effectively boosted confidence, it may have been insufficient to surpass the “novice plateau” effect. The non-significant trend toward improved skill scores (p = 0.167) implies that extended practice with the model could translate confidence gains into statistically significant competency improvements—a hypothesis warranting further investigation.
Despite its diagnostic limitations—such as the inability to distinguish between nasolacrimal duct stenosis and functional delay [13], or identify pre-sac etiologies in cases of epiphora [12, 25], —lacrimal irrigation remains indispensable for preoperative assessment, particularly in reducing the risk of post-surgical endophthalmitis during intraocular surgery [5, 26–28]. In this context, non-invasive alternatives like the regurgitation on pressure over the lacrimal sac (ROPLAS) test have been proposed as complementary screening tools [1, 29]. ROPLAS, a simple outpatient procedure with minimal patient discomfort, is particularly valuable in resource-limited settings and has been shown to be effective for initial screening of nasolacrimal duct obstruction (NLDO) [30]. However, its limitations—including low sensitivity and predictive value—highlight the need for confirmatory testing with lacrimal irrigation in ambiguous cases [31]. Thus, while ROPLAS offers a quick, patient-friendly screening option, lacrimal irrigation remains indispensable for comprehensive preoperative assessment [2].
Given the critical role of lacrimal irrigation in diagnosing lacrimal diseases, mastering this skill is essential for ophthalmologists [28]. Our self-serve training model developed in this study addresses three key barriers to proficiency: (1) improved fault tolerance through simulated tactile feedback, allowing trainees to practice and correct errors in a low-risk environment, (2) psychological safety by eliminating risks associated with live patient procedures, and (3) standardization by providing reproducible practice conditions absent in clinical apprenticeships.
However, the model has notable limitations. First, it cannot simulate two-handed techniques requiring simultaneous eyelid manipulation and cannula advancement, a fundamental clinical skill that needs future integration. Second, its ecological validity is constrained by the absence of physiological responses, such as mucosal resistance variations, which influence real-world irrigation dynamics.
To address these limitations, we propose three future directions: (1) enhanced simulation through the development of specialized training gloves with force-sensitive membranes to mimic mucosal resistance gradients, (2) longitudinal validation studies with a larger sample size to assess skill retention and clinical error rates over a 6-month period, and (3) competency mapping using motion analytics (e.g., cannula path deviation metrics) to complement subjective assessments. Looking ahead, technologies like virtual reality (VR) and artificial intelligence (AI) could enhance lacrimal irrigation training by providing immersive simulations and real-time feedback, further improving skill acquisition and standardization.
Conclusion
This study demonstrates that both lacrimal irrigation techniques are equally accessible to novices, with Technique 2 offering distinct advantages in pressurized irrigation scenarios. The self-serve training model significantly enhances procedural confidence—a vital precursor to clinical competence—though technical proficiency requires extended deliberate practice beyond initial confidence gains. Despite limitations in short-term evaluation and partial anatomical fidelity, the model provides a scalable, risk-free platform for skill acquisition, addressing critical ethical and resource constraints. Future advancements, including enhanced simulation techniques, physiological feedback systems, and refined evaluation metrics, hold promise for bridging the gap between simulated training and clinical performance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
W-F.H. and Z-H.L. designed the research. W-F.H., Z-H.L., and Y.G. conducted the experiments. W-F.H., R.L., M-F.L., and X-H.W. analyzed the results. W-F.H., X-W.L., and R.L. wrote the manuscript. X-C.L. performed English editing. All authors reviewed and approved the manuscript.
Funding
This study was supported by grants from the Natural Science Foundation of China (82471068), Teaching Reform Projects in 2023 by the Steering Committee for the Construction of New Medical Departments in Guangdong Province, and the Natural Science Foundation of Guangdong Province of China (No. 2021A1515012043).
Data availability
The data presented in this study are available from the corresponding author on reasonable request.
Declaratioons
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Zhongshan Ophthalmic Center, Sun Yat-Sen University (No. 2021KYPJ100). All investigations followed the tenets of the Declaration of Helsinki. Prior to participation, all subjects were provided with detailed information about the study objectives, procedures, potential risks, and benefits. Written informed consent was obtained from each participant after ensuring they fully understood the study requirements and their right to withdraw at any time without penalty.
Consent for publication
The participants presented in Figs. 2 and 3 had given informed consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Weifeng Huang and Zuohong Li contributed equally to this work.
Contributor Information
Xuanwei Liang, Email: liangxuanwei@163.com.
Rong Lu, Email: lurong@gzzoc.com.
References
- 1.Kim U, Vardhan A, Datta D, et al. Regurgitation on pressure over the lacrimal sac versus lacrimal irrigation in determining lacrimal obstruction prior to intraocular surgeries. Indian J Ophthalmol. 2022;70:3833–6. 10.4103/ijo.IJO_1722_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura J, Kamao T, Mitani A, et al. Accuracy of the lacrimal syringing test in relation to dacryocystography and dacryoendoscopy. Clin Ophthalmol. 2023;17:1277–85. 10.2147/OPTH.S409662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs HB. Symptomatic epiphora. Br J Ophthalmol. 1959;43:415–34. 10.1136/bjo.43.7.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel CK, Ali B, Malhotra MJ. R (2024) Finesse in lacrimal syringing: the concept of soft observations during lacrimal irrigation. Semin Ophthalmol 1–3. 10.1080/08820538.2024.2380576 [DOI] [PubMed]
- 5.Kam JK, Cheng NM, Sarossy M, et al. Nasolacrimal duct screening to minimize post-cataract surgery endophthalmitis. Clin Exp Ophthalmol. 2014;42:447–51. 10.1111/ceo.12244. [DOI] [PubMed] [Google Scholar]
- 6.Lopez PF, Beldavs RA, al-Ghamdi S, et al. Pneumococcal endophthalmitis associated with nasolacrimal obstruction. Am J Ophthalmol. 1993;116:56–62. 10.1016/s0002-9394(14)71744-1. [DOI] [PubMed] [Google Scholar]
- 7.Nagi KS, Meyer DR. Utilization patterns for diagnostic imaging in the evaluation of epiphora due to lacrimal obstruction: a National survey. Ophthalmic Plast Reconstr Surg. 2010;26:168–71. 10.1097/IOP.0b013e3181b8c747. [DOI] [PubMed] [Google Scholar]
- 8.Aslanduz AA, Mahmoudian B, Sadigh AL, et al. Comparing the diagnostic accuracy of MR dacryocystography (MRD) and dacryoscintigraphy (DSG) in NLDO-related acquired epiphora. Int Ophthalmol. 2024;44:88. 10.1007/s10792-024-02932-1. [DOI] [PubMed] [Google Scholar]
- 9.Lu C-W, Hao J-L, Liu X-F, et al. Pseudomonas aeruginosa endophthalmitis caused by accidental iatrogenic ocular injury with a hypodermic needle. J Int Med Res. 2017;45:882–5. 10.1177/0300060517694570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vonica OA, Obi E, Sipkova Z, et al. The value of lacrimal scintillography in the assessment of patients with epiphora. Eye (Lond). 2017;31:1020–6. 10.1038/eye.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Usmani E, Shapira Y, Selva D. Functional epiphora: an under-reported entity. Int Ophthalmol. 2023;43:2687–93. 10.1007/s10792-023-02668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irfan S, Cassels-Brown A, Nelson M. Comparison between nasolacrimal syringing/probing, macrodacryocystography and surgical findings in the management of epiphora. Eye (Lond). 1998;12(Pt 2):197–202. 10.1038/eye.1998.47. [DOI] [PubMed] [Google Scholar]
- 13.Shapira Y, Juniat V, Macri C, Selva D. Syringing has limited reliability in differentiating nasolacrimal duct stenosis from functional delay. Graefes Arch Clin Exp Ophthalmol. 2022;260:3037–42. 10.1007/s00417-022-05654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Yang S, Park J, et al. Correlation between lacrimal syringing test and dacryoscintigraphy in patients with epiphora. J Craniofac Surg. 2020;31:e442–5. 10.1097/SCS.0000000000006389. [DOI] [PubMed] [Google Scholar]
- 15.Alfarhan A, Al-Swailem S, Alobaid M, et al. Needle-Stick injuries in ophthalmic practice. Risk Manag Healthc Policy. 2023;16:1667–77. 10.2147/RMHP.S409326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh M, Kaur M, Abhaypal K, Gupta P. Commentary: rule out lacrimal duct obstruction before every intraocular procedure. Indian J Ophthalmol. 2022;70:3836–7. 10.4103/ijo.IJO_2228_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakshi SK, Lin SR, Ting DSW, et al. The era of artificial intelligence and virtual reality: transforming surgical education in ophthalmology. Br J Ophthalmol. 2021;105:1325–8. 10.1136/bjophthalmol-2020-316845. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Li S, Sun L, et al. Skills assessment after a grape-based microsurgical course for ophthalmology residents: randomised controlled trial. Br J Ophthalmol. 2022. 10.1136/bjophthalmol-2022-321135. bjophthalmol-2022-321135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Issenberg SB. The scope of simulation-based healthcare education. Simul Healthc. 2006;1:203–8. 10.1097/01.SIH.0000246607.36504.5a. [DOI] [PubMed] [Google Scholar]
- 20.Macluskey M, Anderson AS, Shepherd SD. The impact of a 1-Year COVID-19 extension on undergraduate dentistry in Dundee: final year students’ perspectives of their training in oral surgery. Dent J (Basel). 2022;10:230. 10.3390/dj10120230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clanton J, Gardner A, Cheung M, et al. The relationship between confidence and competence in the development of surgical skills. J Surg Educ. 2014;71:405–12. 10.1016/j.jsurg.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb M, Chan TM, Zaver F, Ellaway R. Confidence-competence alignment and the role of self-confidence in medical education: A conceptual review. Med Educ. 2022;56:37–47. 10.1111/medu.14592. [DOI] [PubMed] [Google Scholar]
- 23.Kneebone R. Perspective: simulation and transformational change: the paradox of expertise. Acad Med. 2009;84:954–7. 10.1097/ACM.0b013e3181a843d6. [DOI] [PubMed] [Google Scholar]
- 24.McGaghie WC, Issenberg SB, Petrusa ER, Scalese RJ. Effect of practice on standardised learning outcomes in simulation-based medical education. Med Educ. 2006;40:792–7. 10.1111/j.1365-2929.2006.02528.x. [DOI] [PubMed] [Google Scholar]
- 25.Usmani E, Shapira Y, Macri C, et al. Soft stop on syringing and probing May have a high false-positive rate in diagnosing pre-sac obstruction. Int Ophthalmol. 2023;43:1127–33. 10.1007/s10792-022-02510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zucoloto LH, Artioli MTR, Zornoff DM, et al. Nasolacrimal obstruction concomitant to cataract: diagnosis and management in the preoperative period of cataract surgery. Arq Bras Oftalmol. 2021;84:311–5. 10.5935/0004-2749.20210044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitz S, Dick HB, Krummenauer F, Pfeiffer N. Endophthalmitis in cataract surgery: results of a German survey. Ophthalmology. 1999;106:1869–77. 10.1016/S0161-6420(99)90395-0. [DOI] [PubMed] [Google Scholar]
- 28.Nair AG. Lacrimal irrigation before intraocular surgery: where are we today? Oman J Ophthalmol. 2024;17:311–2. 10.4103/ojo.ojo_334_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shenoy P, Mehta S, Shah C, et al. Comparison of post-cataract surgery endophthalmitis rates using syringing or regurgitation on pressure over the lacrimal sac as a preoperative screening tool for nasolacrimal duct obstruction: an impact assessment of protocol alteration due to the COVID-19 pandemic. Indian J Ophthalmol. 2021;69:2824–7. 10.4103/ijo.IJO_1218_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair AG, Kamal S, Agarwal A. Indian survey on practice patterns of lacrimal & eyelid disorders (iSUPPLE): report 3 - Cataract and nasolacrimal duct obstruction. Saudi J Ophthalmol. 2017;31:145–9. 10.1016/j.sjopt.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alam MS, Singh P, Amitava AK, Ali MJ. Self-regurgitation on pressure over lacrimal sac in cases of primary-acquired nasolacrimal duct obstruction: correlation with physician examination and patients’ perceptions. Saudi J Ophthalmol. 2021;35:217–9. 10.4103/SJOPT.SJOPT_87_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available from the corresponding author on reasonable request.