ABSTRACT
In recent years, the development of next-generation secondary batteries employing resource-abundant metals such as Na has garnered significant attention. However, the high reactivity of Na raises safety concerns, necessitating the development of safer devices. To address this, ionic liquids (ILs) and organic ionic plastic crystals (OIPCs) have emerged as promising novel electrolytes. Despite their potential, studies investigating the influence of cation structures on various properties remain scarce, particularly in composites where Na salts are introduced into OIPCs. This study focuses on the effects of cation species and Na-salt concentration in OIPCs, specifically in N,N-diethylpyrrolidinium bis(fluorosulfonyl)amide ([C2epyr][FSA]) and N-ethyl-N-isopropylpyrrolidinium bis(fluorosulfonyl)amide ([Ci3epyr][FSA]), with the addition of sodium bis(fluorosulfonyl)amide (NaFSA). The phase transition behavior, dissociation state of Na salts, and electrochemical properties exhibited significant differences based on the cationic structure of the OIPCs. The combination of each OIPC with Na salt resulted in liquid mixtures, and the ionic conductivity increased significantly as the Na salt concentration increased. High ionic conductivities were achieved with [C2epyr][FSA]/NaFSA (20 mol%) and [Ci3epyr][FSA]/NaFSA (10 mol%), showing values of 2.7 × 10−3 and 2.2 × 10−3 S cm−1 at 25°C, respectively. Linear sweep voltammetry results indicated superior oxidative stability in the [Ci3epyr][FSA] system. Solvation numbers of Na+, influenced by differences in cationic side-chain structures, were determined to be 2.7 for the [C2epyr]+ system and 2.9 for the [Ci3epyr]+ system. The results suggest that controlling solvation numbers is a critical factor in the molecular design of high-performance ionic conductors.
KEYWORDS: Sodium-ion batteries, organic ionic plastic crystals, solvation number, pyrrolidinium, ionic conductivity
GRAPHICAL ABSTRACT
IMPACT STATEMENT
Controlling solvation numbers is a critical factor in the molecular design of high-performance ionic conductors utilizing pyrrolidinium-based electrolytes and sodium salt.
Introduction
Lithium-ion batteries (LIBs) have achieved significant success as a primary power source for portable electronic devices, hybrid electric vehicles (HEVs), and electric vehicles (EVs). However, the finite nature of lithium resources could lead to increased LIB costs, limiting their feasibility for large-scale energy storage. As the demand for advanced rechargeable batteries continues to grow, sodium-ion batteries (SIBs), which utilize abundant sodium resources, have drawn considerable attention due to their low cost [1–3]. Sodium not only exhibits a high natural abundance but also possesses a suitable electrochemical reduction potential (−2.71 V vs. the standard hydrogen electrode), making it an attractive candidate for next-generation energy storage systems [1]. Matsumoto et al. reported the development of a composite system comprising [C2mim][FSA] (1-ethyl-3-methylimidazolium bis(fluorosulfonyl)amide) and NaFSA. At 298 K, they demonstrated that a system containing 30 mol% NaFSA enables stable sodium metal deposition and dissolution near 0 V vs. Na/Na+.
Despite significant progress in SIB development, various challenges remain for practical applications, including energy and power density as well as long-term cycle stability [4]. Another challenge is safety concerns, and solid electrolytes have gained attention for their potential to mitigate safety concerns, as they eliminate leakage and thus are expected to enhance device safety [5,6]. Moreover, solid-state batteries can utilize sodium metal, which significantly improves energy density while suppressing dendrite formation, thereby preventing internal short circuits [7,8]. For example, Ling et al. reported a multilayer flexible solid electrolyte by UV curing a poly(ether-acrylate) interpenetrating network within a Na₃Zr₂Si₂PO₁₂/poly(vinylidene fluoridehexafluoropropylene) porous skeleton. This electrolyte demonstrated a high Na+ transference number of 0.63 and a relatively high ionic conductivity exceeding 10−4 S cm−1 at 60°C [9]. Makhlooghiazad et al. evaluated an all-solid-state electrolyte composed of an AB diblock copolymer (polystyrene-blockpoly(acrylethyl(butylimidazolium bis(trifluoromethanesulfonyl)imide))), sodium bis(fluorosulfonyl)amide (NaFSA), and N-propyl-N-methylpyrrolidinium bis(fluorosulfonyl)amide ([C3mpyr][FSA]) in a molar ratio of 1.0:2.5:1.0. The performance of the electrolyte was investigated using NaFePO4 as the cathode and Na metal as the anode, within a potential range of 2.0–4.0 V vs. Na/Na+ at temperatures of 50°C and 70°C. The results demonstrated that the initial discharge capacities at a rate of C/20 were 134 mAh g−1 and 156 mAh g−1 at 50°C and 70°C, respectively [10].
However, several limitations of solid electrolytes hinder the practical realization of all-solid-state sodium batteries. These include low ionic conductivity at room temperature, narrow electrochemical windows, poor chemical and electrochemical stability, and inadequate interfacial compatibility between electrodes and electrolytes [8]. As a promising class of soft solid electrolytes, organic ionic plastic crystals (OIPCs) have been proposed. OIPCs exhibit a plastic crystal (PC) phase, a mesophase between solid and liquid phases, where the constituent ions possess three-dimensional positional order but can freely rotate even in the crystal structure, leading to orientational disorder [11]. OIPCs are attractive solid electrolytes due to their excellent ionic conductivity, plasticity, and safety characteristics [12].
Various combinations of cations and anions have been reported for OIPCs [13,14], with significant research focusing on Li-ion conductors [15]. The development of Mg batteries and supercapacitors using OIPCs has also been reported [16,17], and the range of applications is steadily increasing. Recently, Kufel et al. developed Na-ion conductors by adding sodium bis(fluorosulfonyl)amide (NaFSA) to two types of FSA-based OIPCs: N-isopropyl-N-methylpyrrolidinium bis(fluorosulfonyl)amide and diethyl tetramethyl guanidinium bis(fluorosulfonyl)amide. Their study revealed that the cation structure of OIPCs influences the dynamics of all ionic species in the mixtures, particularly the coordination degree between cations and anions, which affects Na+ mobility [18].
Using a Materials Informatics approach, Ootahara et al. identified N-ethyl-N-isopropylpyrrolidinium bis(fluorosulfonyl)amide ([Ci3epyr][FSA]) as an FSA-based OIPC that exhibits a high ionic conductivity among those reported [19]. However, the various properties of electrolytes based on [Ci3epyr][FSA] remain unexplored. Moreover, the design of electrolytes based on the combination of OIPCs with high concentrations of alkali metal salts has emerged as a promising approach [20–22]. The addition of alkali metal salts to OIPCs may result in the formation of electrolytes that are liquid at ambient temperature. For instance, Al-Masri et al. developed LiFSA-based ionic liquid (IL) electrolytes by the addition of a high LiFSA concentration to an OIPC (N-isopropyl-N-methylpyrrolidinium bis(fluorosulfonyl)amide, [Ci3mpyr][FSA]), demonstrating high coulombic efficiency during lithium plating/stripping and excellent lithium-ion transference numbers [20]. Similarly, the addition of a high concentration of NaFSA to the same OIPC ([Ci3mpyr][FSA]) also resulted in a liquid at ambient temperature. [Ci3mpyr][FSA] with 30 mol% NaFSA that is liquid state at room temperature showed an ionic conductivity of 1.95 × 10−3 S cm−1 at 30°C [18], while [C2mpyr][FSA] and [C3mpyr][FSA] with 30 mol% NaFSA showed ionic conductivities of 2.33 × 10−3 and 2.40 × 10−3 S cm−1 at 30°C [23,24]. The ionic conductivity of [Ci3mpyr][FSA]/NaFSA was slightly lower than those of the respective [C2mpyr][FSA] and [C3mpyr][FSA] mixtures.
These studies highlight the potential of not only OIPCs but also ILs as ionic electrolytes for practical applications. ILs have similar properties to OIPCs, such as non-volatility and non-flammability. Namely, we can develop safe electrolyte materials with OIPCs and ILs. Further improvement of ionic conductivity and a deeper understanding of the conduction mechanism are required for practical applications. This study aims to develop novel Na-ion conducting media by introducing 10–50 mol% NaFSA into two OIPCs, N,N-diethylpyrrolidinium bis(fluorosulfonyl)amide ([C2epyr][FSA]) [14,16,19,25] and [Ci3epyr][FSA] (see Figure 1), which have demonstrated high ionic conductivity. By varying the side-chain structure, diverse molecular designs can be achieved. However, changes in the carbon chain significantly influence the physical states. Therefore, this study focuses on OIPCs as the starting materials and compares the properties of pyrrolidinium-based OIPCs with different side-chain structures. Although the molecular weights of [C2epyr][FSA] and [Ci3mpyr][FSA] [18] are the same, their side-chain structures are different, and it is therefore very interesting to compare their properties. In addition, although the molecular weights of [Ci3epyr][FSA] and [Ci3mpyr][FSA] [18] are different, both compounds have isopropyl groups in the side chain, allowing the influence of the branching structure on their properties to be investigated. This work explores the effects of Na-salt concentration and the influence of the side-chain structures of the organic cations, providing insights into the conduction mechanisms and facilitating the design of high-performance Na-ion electrolytes.
Figure 1.

Chemical structure of the pyrrolidinium-based OIPCs used in this study.
Experimental
Materials
1-Ethylpyrrolidine (98%) and 2-iodopropane (>99.0%) were purchased from Tokyo Chemical Industry Co., Ltd. Iodoethane (>98.0%) and acetonitrile (AN, 99.8%) were purchased from FUJIFILM Wako Pure Chemical Corporation. All reagents were purified prior to use. Lithium bis(fluorosulfonyl)amide (LiFSA, 99%) and sodium bis(fluorosulfonyl)amide (NaFSA, 99.9%) were purchased from Kishida Chemical Co., Ltd. All solvents were purchased from Kanto Chemical Co., Inc. or FUJIFILM Wako Pure Chemical Corporation and were purified as necessary.
Synthesis of OIPC
[C2epyr][FSA] [14,26] and [Ci3epyr][FSA] [19] were synthesized according to previously reported methods. Briefly, an aqueous solution of Li[FSA] (rather than K[FSA]) was added dropwise to an aqueous solution of [C2epyr]I and [Ci3epyr]I. Their chemical structures were confirmed using 1H-NMR spectroscopy (Bruker AVANCE III HD NanoBay 400 MHz), fast atom bombardment mass spectrometry (JMS-T100LC), and elemental analysis (JM11, J-SCIENCE LAB Co. Ltd.).
Preparation of OIPC/NaFSA composies
OIPC and NaFSA were weighed and stirred at 60°C for 72 h to prepare the OIPC/NaFSA materials in an argon filled glovebox. The [C2epyr][FSA]/NaFSA and [Ci3epyr][FSA]/NaFSA mixtures are referred to as [C2epyr]-x and [Ci3epyr]-x, respectively (x represents the mol% of NaFSA).
Method
Phase transition behavior
The phase transition behavior was investigated by differential scanning calorimetry (DSC) using a DSC7020 (Hitachi High-Tech) at a scanning rate of 10°C min−1. Samples were sealed in aluminum pans under an argon atmosphere in a glove box. The reported data were obtained from the second heating scan. The temperature range for measurements was set between −150°C and 150°C.
Raman spectroscopy
Raman spectroscopy was conducted to investigate newly prepared [C2epyr]-x and [Ci3epyr]-x (x = 2.5, 5, 7.5, 10) samples. Measurements were performed under an argon atmosphere using sealed sample tubes prepared in a glovebox. Measurements were performed with a Jasco PR-1w Palmtop Raman spectrometer (JASCO Corporation) using an exposure time of 2 s and 32 accumulations. The laser excitation wavelength was set to 785 nm, and the laser power was adjusted to 50 mW. Spectral data were collected over the range of 3000 cm−1 to 200 cm−1 Raw Raman spectra were normalized based on appropriate Raman bands arising from the cations ([C2epyr]+ and [Ci3epyr]+). The data fitting was carried out using OriginPro 2023b. Peak separation and fitting were performed using Gaussian functions.
Ionic conductivity
The ionic conductivity of liquid samples was measured using VSP-300 impedance analyzer (BioLogic) and SU-261 temperature-controlled chamber (ESPEC Corp.). For [C2epyr][FSA] and [Ci3epyr][FSA], measurements were performed in a cell equipped with Pt electrodes and donut-shaped spacers (outer radius 24 mm, inner radius 8 mm, and thickness 100 µm) fabricated with Kapton tape No. 650S-P (KENIS Co., Ltd.). The cell constant was determined using a cell filled with 0.1 M KCl aqueous solution in a 9 mL sample bottle with inserted platinum electrodes. The cell was placed in the thermostatic chamber and measured after a stabilization time of 30 min under conditions of a frequency range of 100 mHz to 1 MHz and an applied voltage of 10 mV at 25°C and 50°C. Nyquist plots were obtained, and the cell constant was determined from the semicircle in the plots.
This procedure was repeated for each sample over a similar frequency range and applied voltage, with measurements conducted across temperatures ranging from −30°C to 100°C.
Linear sweep voltammetry (LSV)
Linear sweep voltammetry (LSV) was conducted to investigate the electrochemical stability window of the electrolyte. The samples were loaded into an electrode-equipped cell (EC FRONTIER Co., Ltd.) under an argon atmosphere in a glovebox. LSV measurements were performed at 25°C using a VSP-300 potentiostat (BioLogic) within TB-1 temperature-controlled chamber (BAS Inc.). A Pt disk was used as the working electrode, and a Pt wire as the counter electrode. For the reference electrode, silver trifluoromethanesulfonate was dissolved in N,N-diethyl-N-methyl-N-(2methoxyethyl)ammonium bis(trifluoromethylsulfonyl)amide, and the concentration of Ag+ was adjusted to 0.1 M. A silver wire was dipped in the Ag+ solution, and the Ag+ solution was separated from the electrolyte under investigation with a porous glass frit. The applied voltage range was −0.50 to 4.0 V vs. Ag/Ag+, with a scan rate of 10 mV s−1.
Cyclic voltammetry (CV)
Cyclic voltammetry (CV) was performed to study the redox reactions of Na in the electrolyte. The samples were prepared in an electrode-equipped cell (EC FRONTIER Co., Ltd.) under an argon atmosphere in a glovebox. Measurements were conducted at 25°C using a VSP-300 potentiostat (BioLogic) inside a TB-1 temperature-controlled chamber (BAS Inc.). A Cu disk electrode (3 mm diameter) was used as the working electrode, while a Pt wire electrode served as the counter electrode. The 0.1 M Ag/Ag+ electrode described above was utilized as the reference electrode for the measurements. The voltage range was set between −3.8 and −2.7 V vs Ag/Ag+, with a scan rate of 100 mV s−1.
Results and Discussion
Thermal behavior
Figure 2 presents the DSC profiles of OIPCs and OIPC/Na-salt composites. For [C2epyr][FSA] ([C2epyr]-0), the solid–solid phase transition temperature (Ts-s) and the melting point (Tm) were observed at −34.4 and 129.3°C, respectively. [Ci3mpyr][FSA] exhibited solid–solid phase transitions at −32, −24, and 17°C and the melting point at 189°C [18]. The Tm of [C2epyr]-0 was 60°C lower than that of [Ci3mpyr][FSA]. This is thought to be due to the branched side-chain of pyrrolidinium cation. In the case of [Ci3epyr][FSA] ([Ci3epyr]-0), multiple Ts-s values and Tm were identified at −25.4, 115.8, and 120.7°C, which are consistent with the phase transition temperatures reported by Ootahara et al. [19]. Notably, the Tm of [Ci3epyr]-0 was approximately 70°C lower than that of [Ci3mpyr][FSA]. The Tm of [C2mpyr][FSA] is reported to be 205°C [13,26], which is approximately 75°C higher than that of [C2epyr]-0. These results clearly indicate that the elongation of the alkyl side chain has the effect of lowering the melting point of pyrrolidinium salts. In general, the temperature range from Tm to the first Ts-s is referred to as phase I, followed by subsequent phases such as phase II, phase III, and so forth. The two OIPCs investigated in this study exhibited multiple Ts-s values before melting, with [C2epyr]-0 being in phase I and [Ci3epyr]-0 in phase II at 25°C. Although the DSC curves of [Ci3epyr]-0 and [C2epyr]-0 are similar, the temperature range of phase I of [Ci3epyr]-0 is about 5°C, which is extremely narrow compared to [C2epyr]-0 and other pyrrolidinium-based OIPCs. This may also be attributed to the branched side-chain of pyrrolidinium cation. The investigation of the crystal structure is in progress. Timmermans reported that the entropy of fusion (ΔSf) for molecular plastic crystals typically falls below 20 J K−1 mol− 1 [27]. ΔSf values for [C2epyr]-0 and [Ci3epyr]-0 were determined to be 8.96 and 3.63 J K−1 mol−1, respectively, consistent with Timmermans’ criterion.
Figure 2.

DSC profiles of (a) [C2epyr]-x and (b) [Ci3epyr]-x.
In the case of [C2epyr]-x, the x = 10 sample only exhibited a Ts-s. Although the sample remained in a solid state at 25°C, the Tm was not distinctly detected. However, based on the ionic conductivity results, the Tm is estimated to be near 50°C (vide infra). When the Na-salt concentration exceeded 20 mol%, the samples became liquid at room temperature, though Ts-s could still be observed up to 30 mol%. For [Ci3epyr]-x, the sample transitioned to a liquid state at room temperature when the Na-salt concentration exceeded 10 mol%, showing a broader liquid range compared to [C2epyr]-x. This is likely due to the smaller entropy of fusion (ΔSf) of [Ci3epyr][FSA], which facilitates the disruption of its crystalline structure upon NaFSA addition. Phase transitions such as Ts-s and glass transition temperature (Tg) were observed. Similar to [C2epyr]-x, Tg increased with the addition of Na salt, displaying a linear increase with higher salt concentrations. A similar trend has been reported for the [C2mim][FSA] (C2mim: 1-ethyl-3methylimidazolium) and NaFSA system, attributed to enhanced dynamic cross-linking, ion pair formation, and a reduction in overall molecular dynamics [28–30]. For both systems, crystallization of the samples was observed near 10°C for x = 20 and 30. This behavior has also been confirmed by Sijian et al. [31] for the [C2mpyr][FSA] (C2mpyr:N-ethyl-N-methylpyrrolidinium), poly(ethylene oxide), and LiFSA system, and by Danah Al-Masri et al. [32] for the [C2epyr][FSA] and LiFSA system. Such crystallization behavior is not typically observed in composites where alkali-metal salts are added to ionic liquids (ILs). This indicates that OIPCs possess higher crystallinity compared to ILs, with their intrinsic crystallinity persisting even in the liquid state.
Dissociation state of Na salt
Figure 3 shows the Raman spectra of OIPCs and OIPC/Na-salt composites. FSA− coordinates with Na+ in the electrolyte to form complex ions. FSA− exhibits characteristic peak around 730 and 1220 cm−1, corresponding to the symmetric stretching vibration of the N-S bond (νs(N-S)) and the symmetric stretching vibration of the S=O bond (νs(S=O)), respectively [33]. In the analysis, the characteristic νs(N-S) around 730 cm−1 overlapped with the pyrrolidinium cation band; therefore, the band at 1230 cm−1 was used. Coordination with Na+ causes a high-frequency shift of νs(S=O). The Raman spectral peaks ideally follow a Lorentzian function; however, for amorphous materials, they may exhibit Gaussian-like behavior. In this analysis, the peaks were fitted using a Gaussian function to determine the peak components.
Figure 3.

Raman spectra of (a) [C2epyr]-x and (b) [Ci3epyr]-x.
Each Raman spectrum can be deconvoluted into two components: FSA− in its uncoordinated state (free FSA) and FSA− that has shifted to higher frequencies due to coordination with Na+ (bound FSA). As the Na salt concentration increases, the amount of bound FSA increases, indicating a decrease in the degree of dissociation (Supporting Information, Table S1). The spectral shift in the Raman spectra may be associated with the formation of aggregates based on Na+ and FSA−. To determine the solvation number of Na+, the method reported by Fujii et al. [33] was applied to the Raman spectra in the region around 1230 cm−1. The plot of If/mNa versus mT/mNa gave a straight line, as seen in Figure 4, wherein If is the integral intensity of the free FSA, mNa is the concentration of Na ions, and mT is the total concentration of FSA−. The Na+ solvation numbers for [C2epyr]-x and [Ci3epyr]-x were determined to be 2.7 and 2.9, respectively, with [Ci3epyr]-x exhibiting a higher solvation number. The difference in this value is small but is a reasonable value for judging the difference in the solvation number between [C2epyr]-x and [Ci3epyr]-x. Fujii et al. previously reported a Li+ solvation number of 3 for [C2mim][FSA]/LiFSA composites [33]. Similarly, Timo et al. reported a solvation number of 2.7 for the [C4mpyr][FSA]/NaFSA composites (C4mpyr: N-butyl-N-methylpyrrolidinium) using the same calculation method [34]. These results suggest that comparable solvation numbers can be observed for the composites utilizing OIPCs. The comparison of the solvation numbers indicates stronger interactions between Na+ and FSA− in [Ci3epyr]-x compared to [C2epyr]-x, suggesting the formation of complex ions of NaFSA. In the case of the mixture of 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide ([C2mim][TFSA]) and NaTFSA, the solvation number of Na+ was found to be 3 [35]. However, DFT calculations showed that the formation of both [Na(TFSA)2]− and [Na(TFSA)3]2- is energetically favorable. As the Na+ solvation numbers for [C2epyr]-x and [Ci3epyr]-x were 2.7 and 2.9, respectively, both [Na(FSA)2]− and [Na(FSA)3]2- will exist in their mixtures. This suggests that there are more [Na(FSA)3]2- in [Ci3epyr]-x than [Na(FSA)2]−. For composites comparing TFSA− and FSA− with similar cation species, FSA-based composites have been reported to exhibit higher solvation numbers [33]. Additionally, the side-chain structure of the cation was found to influence the degree of dissociation of component ions.
Figure 4.

Plots of If/mNa against mT/mNa for the 1220 cm−1 band at [C2epyr]-x and [Ci3epyr]-x (n represents solvation number).
Ionic conductivity
Figure 5 presents the Arrhenius plots of ionic conductivities for OIPCs and OIPC/Na-salt composites. OIPCs typically exhibit discontinuous changes in ionic conductivity due to changes in crystalline structure and molecular mobility/diffusivity associated with solid–solid phase transitions [14]. The OIPCs used in this study, [C2epyr]-0 and [Ci3epyr]-0, displayed discontinuities in ionic conductivity before and after the solid–solid phase transitions, correlating with their phase transition behavior. The ionic conductivities at 25°C for [C2epyr]-0 and [Ci3epyr]-0 were 8.7 × 10−6 and 1.1 × 10−5 S cm−1, respectively. [Ci3epyr]-0 showed a higher ionic conductivity than [C2epyr]-0, despite being phase II at 25°C, suggesting that [Ci3epyr]-0 has the same high molecular mobility even in phase II as in phase I. The ionic conductivity at 30°C for [Ci3mpyr][FSA] was 1.7 × 10−6 S cm−1 [20]. Although the molecular weights of [C2epyr][FSA] and [Ci3mpyr][FSA] are the same, [C2epyr]-0 showed a higher ionic conductivity than [Ci3mpyr][FSA] with isopropyl group as the side chain.
Figure 5.

Arrhenius plots of ionic conductivity (a) [C2epyr]-x and (b) [Ci3epyr]-x. Open plots: Liquid, Closed plots: Solid.
For [C2epyr]-x and [Ci3epyr]-x, the addition of Na salt resulted in an increase in ionic conductivity by more than two orders of magnitude. This enhancement is attributed to a transition from a crystalline state to a liquid state, improving ionic diffusivity. Although not specifically observed in the DSC measurement (vide supra), [C2epyr]-10 is thought to undergo a melting process near 50°C and displayed a liquid-like behavior above 60°C. Below 50°C, the Arrhenius plot was linear, and the solid-state ionic conductivity at 25°C was as high as 1.8 × 10−4 S cm−1. For [C2epyr]-20–50 and [Ci3epyr]-10–50, the temperature dependence of the ionic conductivity followed a convex curve, consistent with Vogel-Fulcher-Tammann (VFT) behavior. The ionic conductivity of OIPCs is largely governed by viscosity, which increases as Na+ coordinates with FSA−, reducing molecular mobility. The highest ionic conductivities for each composite were observed in [C2epyr]-20 and [Ci3epyr]-10, reaching 2.7 × 10−3 and 2.2 × 10−3 S cm−1, respectively. Matsumoto et al. previously reported that the ionic conductivity of [C2mim][FSA] systems with added NaFSA is viscosity-dependent [28].
Figure 6 compares the ionic conductivities of [C2epyr]-x and [Ci3epyr]-x systems at 25°C. In the high-concentration region (x ≥ 20), [C2epyr]-x exhibited higher ionic conductivity. The smaller molecular weight of the [C2epyr] cation allows for greater ion mobility. Additionally, the lower solvation number of [C2epyr]-x suggests a reduced impact on mobility, contributing to its higher ionic conductivity.
Figure 6.
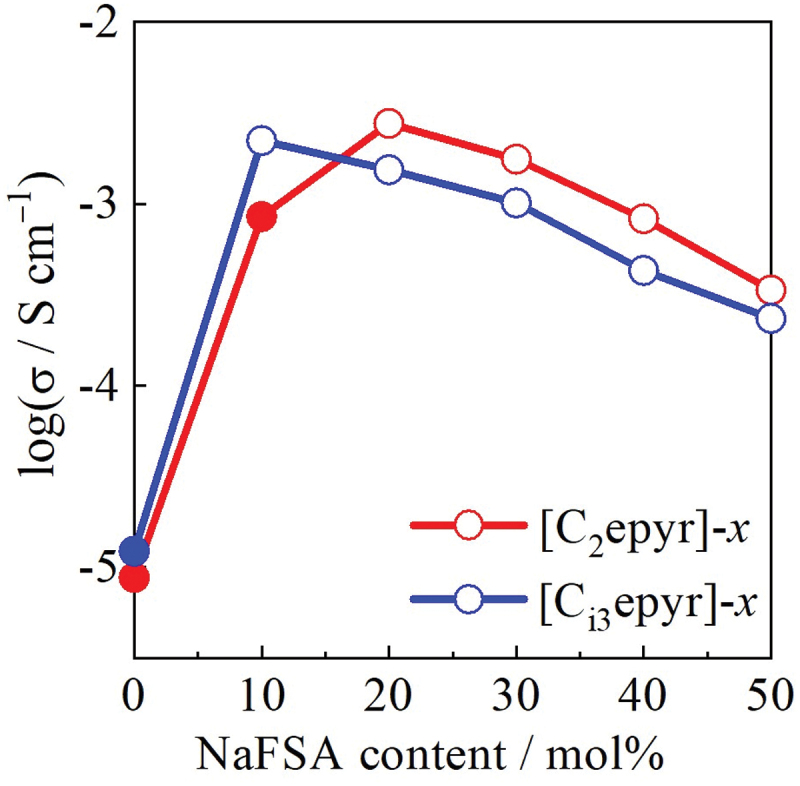
Relationship between ionic conductivity at 25°C and NaFSA mol% for [C2epyr]-x and [Ci3epyr]-x. Open plots: Liquid, Closed plots: Solid.
Electrochemical stability
Figure 7 shows the Linear Sweep Voltammetry (LSV) curves for [C2epyr]-x and [Ci3epyr]-x at 25°C. The oxidation decomposition potential was defined as the point where the current density reached +5 mA cm− 2. The decomposition current will be attributed to the FSA anion [36]. For both composites, the oxidation stability improved with increasing NaFSA concentration. The oxidation decomposition potentials for [C2epyr]-x were 2.05, 2.15, 2.16, 2.25, and 2.31 V (vs. Ag/Ag+) for x = 10, 20, 30, 40, and 50, respectively. Similarly, the oxidation decomposition potentials for [Ci3epyr]-x were 2.11, 2.17, 2.24, 2.30, and 2.41 V (vs. Ag/Ag+) for x = 10, 20, 30, 40, and 50 (Supporting Information, Figure S1), respectively. For [C2epyr]-10, the lower current density on the high-voltage side can be attributed to the difference in diffusivity between the solid and liquid states, as it remains in a solid state at 25°C. The oxidation decomposition potential values for [Ci3epyr]-x were higher, indicating a wide electrochemical window compared to [C2epyr]-x (vide infra). This can be attributed to the larger solvation number of [Ci3epyr]-x, which reduces the number of free FSA anions on the electrode surface. These results suggest that the OIPC/Na salt composites exhibit sufficient oxidative stability to serve as electrolytes for sodium-ion secondary batteries. Furthermore, they demonstrate stability even when applied to sodium-ion secondary batteries with operating voltages in the 4–5 V vs. Na/Na+ range.
Figure 7.
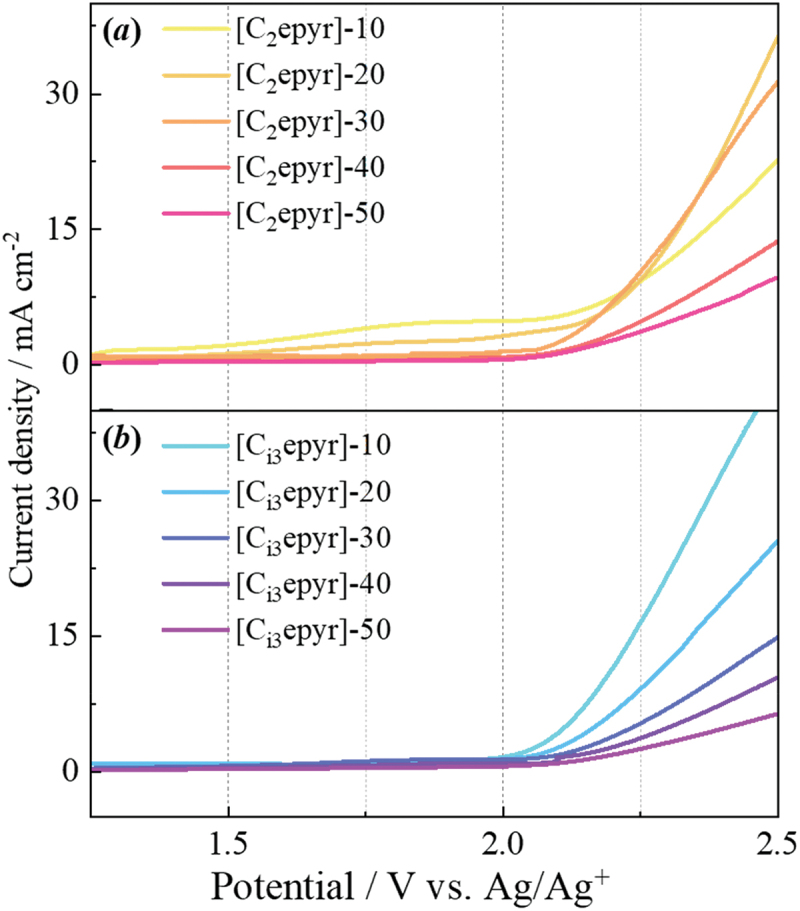
LSV curve of the oxidized side of (a) [C2epyr]-x and (b) [Ci3epyr]-x.
Redox behavior of Na
Figure 8 shows the cyclic voltammetry (CV) results for [C2epyr]-20 and [Ci3epyr]-20. Both samples exhibited reduction currents below −3.6 V vs Ag/Ag+, corresponding to the reduction of Na+ ions, and oxidation currents around −3.1 V vs Ag/Ag+, indicative of Na oxidation. The current density of 20 mA cm− 2 at the 10th cycle was defined as the reduction potential in this study. The potential windows of [C2epyr]-20 and [Ci3epyr]-20 were calculated to be 5.71 and 5.76 V (vs. Ag/Ag+), respectively, from the LSV measurements. It was found that the difference between ethyl and isopropyl groups on the side chain of pyrrolidinium cation has almost no effect on the potential window. Ding et al. reported a potential window of 5.3 V (vs. Na/Na+) at 25°C for a composite containing NaFSA added to the ionic liquid [C3mpyr][FSA] [37]. [C2epyr]-20 and [Ci3epyr]-20 showed a similar electrochemical window.
Figure 8.

Cyclic voltammograms of (a) [C2epyr]-20 and (b) [Ci3epyr]-20 at 25°C.
The redox reaction of Na suggests the dissolution and deposition of Na on the Cu electrode surface. Hosokawa et al. compared the dissolution and deposition behavior of metallic Na in [C2mim][FSA]/Na[FSA] and [C2mim][TFSA]/Na[TFSA] composites.
Their results showed oxidation currents only in FSA−-based composites [38]. Similarly, the present measurements confirm the high stability of the FSA anion toward metallic sodium. In general, FSA− forms a solid electrolyte interphase (SEI) layer via reductive decomposition on the electrode surface [39]. This SEI layer suppresses excessive decomposition of the electrolyte and stabilizes the reversible redox reactions. As a result, the SEI layer is formed during the first cycle, and subsequent cycles exhibit stabilized Na redox reactions. For [C2epyr]-20, the current values monotonically decreased with an increasing number of cycles. In contrast, [Ci3epyr]-20 exhibited the highest current density during the first cycle, followed by a decrease, and stabilized at a consistent current density after 30 cycles. These results suggest that the side-chain structure of the pyrrolidinium cation influences the redox behavior of sodium.
The role of side structure on Li cycling electrochemistry was investigated by the CV measurements of [Ci3mpyr][FSA] with 50 mol% LiFSA and [C3mpyr][FSA] with the same Li-salt concentration [20]. The Coulombic efficiency of both electrolytes maintained similar values (>90% after several cycles) in their CV results. The effect of alkyl chain structure on Li electrochemistry is not yet clear. The effect of the side-chain structure of organic cations on Na electrochemistry also needs to be elucidated in Na salt composites. In the future, the effects of solvation number and Na transference number on SEI formation should be investigated to understand the effect of side-chain structure.
Conclusion
This aim of this study was to develop Na-ion conductors for Na secondary batteries using OIPCs and to investigate the effects of the side-chain structure of pyrrolidinium cation and NaFSA addition. The thermal and electrochemical stability of OIPC/Na-salt composites demonstrated their applicability as electrolytes for secondary batteries. OIPCs with added Na salts transitioned to a liquid state at room temperature for [C2epyr]-based composites with NaFSA concentrations above 20 mol% and for [Ci3epyr]-based composites above 10 mol%. As the Na salt concentration increased, the liquid phase content also increased. Raman spectroscopy enabled the calculation of FSA-anion-solvation numbers of Na+, which were determined to be 2.7 for the [C2epyr] system and 2.9 for the [Ci3epyr] system, indicating a higher solvation number for the latter. These findings correlate with the observed ionic conductivity and electrochemical oxidation stability. The ionic conductivity increased by more than two orders of magnitude for both systems upon the addition of Na salt. At room temperature, [C2epyr]-20 exhibited an ionic conductivity of 2.7 × 10−3 S cm−1, while [Ci3epyr]-10 showed a conductivity of 2.2 × 10−3 S cm−1. Both composites exhibited sodium redox behavior at 25°C, demonstrating the feasibility of using OIPC and NaFSA for electrochemical devices. The tunability of molecular design in these systems highlights their potential for advancing the development of next-generation high-conductivity Na secondary batteries.
Supplementary Material
Funding Statement
This study was supported by JSPS KAKENHI (23K26765) and a Sophia University Special Grant for Academic Research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14686996.2025.2466417
References
- [1].Zhao LA, Zhang T, Li W, et al. Engineering of sodium-ion batteries: opportunities and challenges. Engineering-Prc. 2023;24:172–10. doi: 10.1016/j.eng.2021.08.032 [DOI] [Google Scholar]
- [2].Lao M, Zhang Y, Luo W, et al. Alloy-based anode materials toward advanced sodium-ion batteries. Adv Mater. 2017;29(48):1700622. doi: 10.1002/adma.201700622 [DOI] [PubMed] [Google Scholar]
- [3].Kim SW, Seo DH, Ma XH, et al. Electrode materials for rechargeable sodium-ion batteries: potential alternatives to current lithium-ion batteries. Adv Energy Mater. 2012;2(7):710–721. doi: 10.1002/aenm.201200026 [DOI] [Google Scholar]
- [4].Zhang H, Gao Y, Liu XH, et al. Long-cycle-life cathode materials for sodium-ion batteries toward large-scale energy storage systems. Adv Energy Mater. 2023;13(23):1700622. doi: 10.1002/aenm.202300149 [DOI] [Google Scholar]
- [5].Gebert F, Knott J, Gorkin R, et al. Polymer electrolytes for sodium-ion batteries. Energy Storage Mater. 2021;36:10–30. doi: 10.1016/j.ensm.2020.11.030 [DOI] [Google Scholar]
- [6].Li Z, Fu J, Zhou X, et al. Ionic conduction in polymer-based solid electrolytes. Adv Sci (Weinh). 2023;10(10):e2201718. doi: 10.1002/advs.202201718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li Z, Liu P, Zhu K, et al. Solid-state electrolytes for sodium metal batteries. Energy Fuels. 2021;35(11):9063–9079. doi: 10.1021/acs.energyfuels.1c00347 [DOI] [Google Scholar]
- [8].Hou WR, Guo XW, Shen XY, et al. Solid electrolytes and interfaces in all-solid-state sodium batteries: Progress and perspective. Nano Energy. 2018;52:279–291. doi: 10.1016/j.nanoen.2018.07.036 [DOI] [Google Scholar]
- [9].Ling W, Fu N, Yue JP, et al. A flexible solid electrolyte with multilayer structure for sodium metal batteries. Adv Energy Mater. 2020;10(9):1903966. doi: 10.1002/aenm.201903966 [DOI] [Google Scholar]
- [10].Makhlooghiazad F, Miguel Guerrero Mejia L, Rollo-Walker G, et al. Understanding polymerized ionic liquids as solid polymer electrolytes for sodium batteries. J Am Chem Soc. 2024;146(3):1992–2004. doi: 10.1021/jacs.3c10510 [DOI] [PubMed] [Google Scholar]
- [11].MacFarlane DR, Forsyth M.. Plastic crystal electrolyte materials: New perspectives on solid state ionics. Adv Mater. 2001;13(12–13):957–966. [Google Scholar]
- [12].Zhu H, MacFarlane DR, Pringle JM, et al. Organic ionic plastic crystals as solid-state electrolytes. Trends Chem. 2019;1(1):126–140. doi: 10.1016/j.trechm.2019.01.002 [DOI] [Google Scholar]
- [13].Yoshizawa-Fujita M, Kishi E, Suematsu M, et al. A plastic electrolyte material in a highly desirable temperature range: n-ethyl-n-methylpyrrolidinium bis(fluorosulfonyl)amide. Chem Lett. 2014;43(12):1909–1911. doi: 10.1246/cl.140833 [DOI] [Google Scholar]
- [14].Yoshizawa‐Fujita M, Yamada H, Yamaguchi S, et al. Synthesis and characteristics of pyrrolidinium‐based organic ionic plastic crystals with various sulfonylamide anions. Batter Supercaps. 2020;3(9):884–891. doi: 10.1002/batt.202000040 [DOI] [Google Scholar]
- [15].Thomas ML, Hatakeyama-Sato K, Nanbu S, et al. Organic ionic plastic crystals: flexible solid electrolytes for lithium secondary batteries. Energy Adv. 2023;2(6):748–764. doi: 10.1039/D3YA00078H [DOI] [Google Scholar]
- [16].Yoshifumi H, Ryotaro S, Yuko T, et al. Phase transition and ionic conductivity of pyrrolidinium-based ionic plastic crystals with magnesium salts. Bull Chem Soc Jpn. 2024;97(10):uoae101. doi: 10.1093/bulcsj/uoae101 [DOI] [Google Scholar]
- [17].Yoshizawa-Fujita M, Kubota S, Ishimoto S. All-solid-state high-voltage supercapacitors using an ionic plastic crystal-based electrolyte. Front Energy Res. 2022;10:854090. doi: 10.3389/fenrg.2022.854090 [DOI] [Google Scholar]
- [18].Kufel L, Gunathilaka IE, Forsyth M, et al. Thermal and transport properties of ionic electrolytes with tailored guanidinium and pyrrolidinium-based cation structures for Na-ion batteries. J Phys Chem C. 2024;128(34):14216–14228. doi: 10.1021/acs.jpcc.4c03715 [DOI] [Google Scholar]
- [19].Ootahara T, Hatakeyama-Sato K, Thomas ML, et al. Efficient exploration of highly conductive pyrrolidinium-based ionic plastic crystals using materials informatics. ACS Appl Electron Mater. 2024;6(8):5866–5878. doi: 10.1021/acsaelm.4c00861 [DOI] [Google Scholar]
- [20].Al-Masri D, Yunis R, Hollenkamp AF, et al. The influence of alkyl chain branching on the properties of pyrrolidinium-based ionic electrolytes. Phys Chem Chem Phys. 2020;22(32):18102–18113. doi: 10.1039/D0CP03046E [DOI] [PubMed] [Google Scholar]
- [21].Al-Masri D, Yunis R, Hollenkamp AF, et al. Designing solid-state electrolytes through the structural modification of a high-performing ionic liquid. Chemelectrochem.2020;7(19):4118–4123. [Google Scholar]
- [22].Al-Masri D, Yunis R, Zhu HJ, et al. A new approach to very high lithium salt content quasi-solid state electrolytes for lithium metal batteries using plastic crystals. J Mater Chem A. 2019;7(44):25389–25398. doi: 10.1039/C9TA11175A [DOI] [Google Scholar]
- [23].Yang H, Hwang J, Wang YS, et al. N-Ethyl-N-propylpyrrolidinium Bis(fluorosulfonyl)amide ionic liquid electrolytes for sodium secondary batteries: effects of na ion concentration. J Phys Chem C. 2019;123(36):22018–22026. doi: 10.1021/acs.jpcc.9b05941 [DOI] [Google Scholar]
- [24].Matsumoto K, Okamoto Y, Nohira T, et al. Thermal and Transport Properties of Na[N(SO2F)2]–[N -Methyl-N-propylpyrrolidinium][n(so 2F)2] ionic liquids for na secondary batteries. J Phys Chem C. 2015;119(14):7648–7655. doi: 10.1021/acs.jpcc.5b01373 [DOI] [Google Scholar]
- [25].Nishikawa K, Fujii K, Matsumoto K, et al. Heterogeneous dynamics of diffusive motion in organic ionic plastic crystal studied using spin–spin relaxation time: N,N -diethylpyrrolidinium bis(fluorosulfonyl)amide. Bull Chem Soc Jpn. 2024;97(9):uose088. doi: 10.1093/bulcsj/uoae088 [DOI] [Google Scholar]
- [26].Yamada H, Miyachi Y, Takeoka Y, et al. Pyrrolidinium-based organic ionic plastic crystals: Relationship between side chain length and properties. Electrochim Acta. 2019;303:293–298. doi: 10.1016/j.electacta.2019.02.076 [DOI] [Google Scholar]
- [27].Timmermans PJ. Plastic crystals a historical review. J Phys Chem Solids. 1961;18(1):1–8. doi: 10.1016/0022-3697(61)90076-2 [DOI] [Google Scholar]
- [28].Matsumoto K, Hosokawa T, Nohira T, et al. The Na[FSA]–[C2C1im][FSA] (C2C1im+: 1-ethyl-3-methylimidazolium and FSA−: bis(fluorosulfonyl)amide) ionic liquid electrolytes for sodium secondary batteries. J Power Sources. 2014;265(265):36–39. doi: 10.1016/j.jpowsour.2014.04.112 [DOI] [Google Scholar]
- [29].Stallworth PE, Fontanella JJ, Wintersgill MC, et al. NMR, DSC and high pressure electrical conductivity studies of liquid and hybrid electrolytes. J Power Sources. 1999;81:739–747. doi: 10.1016/S0378-7753(99)00144-5 [DOI] [Google Scholar]
- [30].Monti D, Jonsson E, Boschin A, et al. Towards standard electrolytes for sodium-ion batteries: physical properties, ion solvation and ion-pairing in alkyl carbonate solvents. Phys Chem Chem Phys. 2020;22(39):22768–22777. doi: 10.1039/D0CP03639K [DOI] [PubMed] [Google Scholar]
- [31].Li SJ, Yang KH, Zhang ZX, et al. Organic ionic plastic crystal-poly(ethylene oxide) solid polymer electrolytes: application in all-solid-state lithium batteries. Ind Eng Chem Res. 2018;57(41):13608–13614. doi: 10.1021/acs.iecr.8b01964 [DOI] [Google Scholar]
- [32].Al-Masri D, Yunis R, Hollenkamp AF, et al. A symmetrical ionic liquid/Li salt system for rapid ion transport and stable lithium electrochemistry. Chem Commun. 2018;54(29):3660–3663. doi: 10.1039/C8CC00531A [DOI] [PubMed] [Google Scholar]
- [33].Fujii K, Hamano H, Doi H, et al. Unusual Li + Ion Solvation structure in bis(fluorosulfonyl)amide based ionic liquid. J Phys Chem C. 2013;117(38):19314–19324. doi: 10.1021/jp4053264 [DOI] [Google Scholar]
- [34].Carstens T, Lahiri A, Borisenko N, et al. [Py 1,4]FSI-NaFSI-based ionic liquid electrolyte for sodium batteries: Na + Solvation and Interfacial nanostructure on Au(111). J Phys Chem C. 2016;120(27):14736–14741. doi: 10.1021/acs.jpcc.6b04729 [DOI] [Google Scholar]
- [35].Monti D, Jónsson E, Palacín MR, et al. Ionic liquid based electrolytes for sodium-ion batteries: Na solvation and ionic conductivity. J Power Sources. 2014;245:630–636. doi: 10.1016/j.jpowsour.2013.06.153 [DOI] [Google Scholar]
- [36].Reber D, Figi R, Kühnel RS, et al. Stability of aqueous electrolytes based on LiFSI and NaFSI. Electrochim Acta. 2019;321:134644. doi: 10.1016/j.electacta.2019.134644 [DOI] [Google Scholar]
- [37].Ding C, Nohira T, Kuroda K, et al. NaFSA–C1C3pyrFSA ionic liquids for sodium secondary battery operating over a wide temperature range. J Power Sources. 2013;2013(238):296–300. doi: 10.1016/j.jpowsour.2013.03.089 [DOI] [Google Scholar]
- [38].Hosokawa T, Matsumoto K, Nohira T, et al. Stability of Ionic liquids against sodium metal: a comparative study of 1-ethyl-3-methylimidazolium ionic liquids with bis(fluorosulfonyl)amide and Bis(trifluoromethylsulfonyl)amide. J Phys Chem C. 2016;120(18):9628–9636. doi: 10.1021/acs.jpcc.6b02061 [DOI] [Google Scholar]
- [39].Shkrob IA, Marin TW, Zhu Y, et al. Why Bis(fluorosulfonyl)imide is a “Magic Anion” for Electrochemistry. J Phys Chem C. 2014;118(34):19661–19671. doi: 10.1021/jp506567p [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


