Abstract
Background
Lower dietary energy intake (DEI) may be associated with increased mortality risk. This study aims to analyze the influence of baseline DEI, time average DEI, and other factors on survival in peritoneal dialysis (PD) patients.
Method
It was a single-center retrospective cohort study. Patients who started PD from January 2006 to June 2021 were included in this study and followed up until June 2023. Their baseline (six months after the beginning of PD) demographic, dietary intake, laboratory data, and time varying dietary intake data were collected and analyzed. The relationships between these data and survival were examined using Cox model to estimate death hazard ratios.
Result
A total of 794 patients were included in this study, 424 males and 370 females, with a mean age of 58.87 ± 16.02 years. Their mean normalize baseline dietary energy intake (nDEI) was 25.46 ± 6.72 kcal/kg/day, time average nDEI was 24.87 ± 4.74 kcal/kg/day. The median follow-up duration was 46.58 (27.38, 78.52) months in the overall cohort. Based on multivariate Cox proportional hazard analysis, age (HR = 1.056, 95% Cl = 1.047–1.065, p < 0.001), diabetes (HR = 1.364, 95% Cl = 1.114–1.671, p = 0.003), serum albumin (HR = 0.945, 95% Cl = 0.923–0.967, p < 0.001), blood sodium (HR = 0.973, 95% Cl = 0.954–0.992, p = 0.002), serum urea (HR = 0.974, 95% Cl = 0.953–0.994, p = 0.025), and baseline nDEI (HR = 0.980, 95% Cl = 0.964–0.996, p = 0.017) were significantly associated with mortality. Baseline DPI, BMI and time average nDEI were not related to PD patients’ survival. When classified baseline nDEI into 4 groups (< 25 kcal/kg/day, 25-29.99 kcal/kg/day, 30-34.99 kcal/kg/day, and ≥ 35 kcal/kg/day), the univariate and multivariate Cox proportional hazard analysis showed that the patients with nDEI 30-34.99 kcal/kg/day had the lowest mortality risk (using the DEI < 25 kcal/kg/day group as reference, p < 0.05).
Conclusion
Our study revealed that DEI 30-34.99 kcal/kg/day might be beneficial to the long-term outcome for the Chinese PD population.
Clinical trial number
Not applicable.
Keywords: Peritoneal dialysis, Mortality, Dietary energy intake, Cohort study
Introduction
Chronic kidney disease (CKD) is a major burden throughout the world. In China, although studies in recent years found that the prevalence of CKD showed a minor downward trend with effective intervention and examination [1], the number of CKD patients may still reach 150 million [2]. For patients with end-stage renal disease (ESRD), if kidney transplantation is not available, dialysis becomes the necessary option to maintain their life. Although the percentage of peritoneal dialysis (PD) was much lower than hemodialysis (HD) all over the world [3], the number of patients receiving PD were still gradually increasing in China, the United States, Singapore and other places [3, 4]. The latest data from Chinese National Renal Data System (CNRDS) reported that there were more than 150 thousand patients on PD in Mainland China until the end of 2023 [5].
Nutritional status was a key factor affecting the survival of PD patients [6, 7]. Previous studies reported that the prevalence of protein-energy wasting (PEW) in patients with continuous ambulatory peritoneal dialysis (CAPD) was about 45-52.9% [6, 7]. PEW was common in dialysis patients and had become an important risk factor for mortality [8–10]. Adequate dietary energy and protein intake are essential for maintaining good nutritional status. The relationship between dietary protein intake (DPI) and survival of PD patients had been explored in several previous studies [11–13]. However, the influence of dietary energy intake (DEI) on survival of PD patients remains inconclusive.
In the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines for nutrition in CKD (2020 update), guideline 3 states that, " In adults with CKD 1-5D (1 C) or post-transplantation (OPINION) who are metabolically stable, we recommend prescribing an energy intake of 25–35 kcal/kg body weight per day based on age, sex, level of physical activity, body composition, weight status goals, CKD stage, and concurrent illness or presence of inflammation to maintain normal nutritional status [14]. Although the evidence level for this point was acceptable (1c), most data came from non-dialysis or hemodialysis patients, so whether it is applicable to PD patients remains questionable. Although PD and HD have similar patient populations, the energy metabolism of patients on PD or HD will be different due to the different dialysate components and treatment mechanism. In addition, most of the relevant evidence in K/DOQI came from western populations, and there were few studies in Chinese populations [14]. The conclusions given by K/DOQI may not be appropriate to the Chinese population. According to Chinese Clinical Practice Guidelines for Nutrition in CKD (2021 edition), the DEI of PD patients should be 35 kcal/kg/d, which is quite different from the recommendation raised by K/DOQI guideline, but the evidence level is still relatively low (2D) [15]. More high-quality studies are needed to explore the DEI recommendation in Chinese PD patients. Therefore, we conducted this study to analyze the relationship between DEI and patients’ outcome and explore the appropriate DEI level in PD patients through a single center long-term follow-up cohort.
Methods
Subjects and follow-up
It was a retrospective cohort study. The patients who started peritoneal dialysis at Peking University Third Hospital from January 2006 to June 2021 were included in this study, and all participants were followed up until June 2023. The inclusion criteria were: patients (1) had been on PD for more than 6 months; (2) were 18 years or above. Patients with multiple organ failure or malignancy were excluded. The demographic data, including gender, age, primary disease of ESRD, body weight, height, presence of diabetes mellitus (DM), and Charlson index were collected [16]. All subjects were followed up until death, transfer to HD, kidney transplantation, or the end of the study. All-cause mortality was recorded as outcome events. The study was approved by the Medical Ethical Committee of Peking University Third Hospital (approval number: IRB 2024-096-03). Individual informed consent was waived by IRB given the study was a retrospective, non-interventional design and using a deidentified dataset.
Dietary variables
We suggested at least 0.8 g/kg of dietary protein and 25 kcal/kg of dietary energy based on previous study and European Best Practice Guideline [14, 17]. During the follow-up, all patients visited the PD clinic monthly. Three-day dietary records were collected at the sixth month of dialysis as baseline dietary intake data and subsequently every one to three months. The dietary intake collection process was the same as our previous study described [13]. Daily baseline DPI and DEI were normalized as nDPI & nDEI by ideal body weight (IBW), which was defined as body height (cm) minus 105 (modified Broca method) [18].
The time-averaged calculations of nDEI were conducted based on the measurements taken over a 6-month period. The detailed methodology can be described as follows. Patients’ follow-up records were segmented into intervals of 6 months. Within each 6-month interval, the data for all patient visits were averaged to create a single average record for that 6-month period. Depending on the varying follow-up times, there might be multiple ”6-month average” follow-up records available, represented as ’n’ records. To obtain the overall average follow-up record, the data from these ’n’ records were averaged together. When calculating the average, any corresponding records with missing data were excluded from the calculation.
Dialysis adequacy and nitrogen balance at baseline
Patients’ 24-hour urine and dialysate samples were collected to measure urea and creatinine levels one day before the clinic visit. Using standard methods, we calculated weekly total Kt/V urea, creatinine clearance, and residual renal function (RRF). The urea nitrogen appearance (UNA) was defined as the urea nitrogen output in urine and dialysate, and then the total nitrogen appearance (TNA, the nitrogen output in urine, feces, and dialysate) was calculated using the Bergstrom formula [19] or new formulae which was reported in our previous study [20]. NB (nitrogen balance) = NI -TNA = DPI/6.25-TNA.
Blood chemistries
The blood test results were also collected at baseline, such as blood urea nitrogen, creatinine, hemoglobin, albumin, phosphate, potassium, and sodium etc.
Statistical analyses
Continuous variables were reported as either mean ± standard deviation (SD) or median (interquartile range, IQR) based on their distribution. Categorical data were presented as proportions. Patient follow-up time was computed as the time of starting PD to the date of outcome events (all-cause mortality) appeared, or being censored (transplantation, transfer to HD or other centers, or the end of the study). The one-way ANOVA, non-parametric test or Chi-square test were used to analyze the baseline parameter difference among different baseline nDEI groups based on different variable categories.
The Cox proportional hazards model of univariate and multivariate analysis was used to evaluate the association between baseline nDEI, time average nDEI and survival as well as to estimate the Hazard ratios (HR) with 95% Confidence Interval (CI). Multivariate Cox’s regression analysis (using Backward Wald method to select variable) included all the significant variables from the univariate analysis (p < 0.1) and the variables concerned by the literature (like Kt/V and eGFR) [21, 22]. For variables which had obvious collinearity, the one with lower p value was included in the multivariate analysis. Potential confounders included patients’ baseline demographic data, data of dietary intake, dialysis adequacy, residual kidney function, laboratory tests and nitrogen balance.
The hypotheses of proportional risk models of the baseline nDEI groups (nDEI < 25 kcal/kg/day; 25-29.99 kcal/kg/day; 30-34.99 kcal/kg/day; ≥35 kcal/kg/day) and serum sodium groups (≤ 137 mmol/L, 137.1–139 mmol/L, 139.1–141 mmol/L and ≥ 141.1 mmo/L) on mortality were tested by Graphical analysis using a log minus log plot (LML plot) [23]. The Kaplan-Meier analysis was used to generate survival curves. The log-rank test was used to evaluate the difference in survival rate according to baseline nDEI and serum sodium groups. Multivariate Cox’s regression analysis was used to analyze the independent risk of baseline nDEI groups on mortality adjusting the variables which were distributed differently among the four nDEI groups. As sensitivity analysis, the same multivariate Cox’s regression analysis process was implemented to test the influence of baseline nDEI groups on other outcomes, such as transfer to HD, and combination of mortality & transfer to HD.
All of the reported p-values were two-tailed, and statistical significance was set at 0.05. Statistical analyses were performed using the SPSS software package (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
Results
Characteristics of the study population
A total of 1257 patients who started PD therapy in our center from January 2006 to June 2021 were initially followed up. Within half a year of dialysis, 136 dialysis patients withdrew from PD. Three patients had complete recovery of kidney function, 324 patients could not be followed-up regularly due to living far away from the PD center, suffering from cancer or other reasons. Finally, 794 patients were included in this analysis (see Fig. 1), 424 males and 370 females, with a mean age of 58.87 ± 16.02 years, 45 receiving automated peritoneal dialysis (APD) and 749 on CAPD. According to the etiology of kidney disease, the percentage of diabetic kidney disease (DKD), chronic glomerulonephritis (CGN), hypertensive nephropathy (HTN), chronic interstitial nephritis (CIN), polycystic kidney disease and other causes was 35%, 31.1%, 13.4%, 8.9%, 3.5%, and 8.1%, respectively. There were 343 patients (43.2%) who had diabetes.
Fig. 1.
Flow chart of patients screened and excluded
The median follow-up duration was 46.58 (27.38, 78.52) months in the overall cohort. The 1-year, 2-year, 3-year, 5-year survival rates were 96.7%, 87.3%, 78.6% and 66.0%, respectively. At the end of the study as of June 2023, 148 patients were still on PD, 412 died, 91 had transferred to HD, 44 had undergone kidney transplantation, 99 had transferred to other hospitals, and the causes of death were cardiovascular diseases (159 cases, 38.6%), systemic infections (105 cases, 25.5%), multiple organ failure (55 cases, 13.3%), malignant tumors (22 cases, 5.3%) and other causes (71 cases, 17.2%).
The baseline dietary intake, nutritional status, and nitrogen balance of the patients were shown in Table 1. The time average nDEI was 24.87 ± 4.74 kcal/kg/day.
Table 1.
Baseline dietary energy intake, nutritional parameters, and dialysis adequacy of PD patients
| Variables | Data* |
|---|---|
| height, cm | 164.04 8.57 8.57 |
| body weight, kg | 63.41 12.41 12.41 |
| BMI, kg/m2 | 23.20 3.64 3.64 |
| Charlson index | 6(4,7) |
| nDPI, g/kg/day | 0.86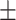 0.25 0.25 |
| nDEI, kcal/kg/day | 25.46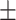 6.72 6.72 |
| Fat intake, g/d | 54.85(43.49,68.77) |
| Carbohydrate intake, g/d | 195.72(156.58,237.44) |
| Alb, g/l | 37.17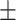 4.65 4.65 |
| Dialysis dose, ml | 6000(6000,8000) |
| ultrafiltration, ml | 350(102.5,649) |
| urine volume, ml | 550(250,992.5) |
| total fluid removal, ml | 971(700,1305) |
| Kt/V | 1.98(1.63,2.41) |
| Tccr, (L/w) | 60.31(48.29,76.61) |
| eGFR, ml/min | 2.45(1.12,3.99) |
| Nitrogen intake, g/d | 8.05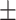 2.45 2.45 |
| TNA, g/d (Bergstrom) | 9.58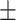 2.11 2.11 |
| TNA, g/d (New formula) | 8.05 2.17 2.17 |
| NB, g/d (Bergstrom) | -1.52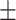 2.67 2.67 |
| NB, g/d (New formula) | 0.01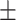 2.71 2.71 |
| Hb, g/l | 116.07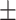 19.25 19.25 |
| Serum urea, mmol/l | 20.37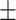 5.82 5.82 |
Serum, creatinine, 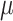 mmol/l mmol/l |
721.38 255.13 255.13 |
| Serum calcium, mmol/l | 2.26 0.28 0.28 |
| Serum phosphate, mmol/l | 1.52 0.40 0.40 |
| Serum potassium, mmol/l | 4.31 0.73 0.73 |
| Serum sodium, mmol/l | 139.99 5.40 5.40 |
| CO2CP, mmol/l | 26.58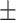 3.65 3.65 |
*: mean sd for normalized distribution parameters, median(P25, P75) for non-normalized distribution parameters
sd for normalized distribution parameters, median(P25, P75) for non-normalized distribution parameters
PD: peritoneal dialysis; BMI: body mass index; nDPI: normalized dietary protein intake; nDEI: normalized dietary energy intake; Alb: albumin; Tccr: total clearance rate of creatinine; eGFR: estimated Glomerular filtration rate; TNA: total nitrogen appearance; NB: nitrogen balance; Hb: hemoglobin; CO2CP: carbon dioxide combining power
The effect of parameters on survival
In the univariate Cox regression model, age, Charlson index, diabetes, urine volume, ultrafiltration, total fluid removal, serum urea, serum creatinine, serum potassium, serum sodium, albumin, fat intake, carbohydrate intake, DPI, DEI, baseline and time average nDEI, TNA and NB were all associated with mortality (see Table 2). Considering collinearity between variables as well as variables concerned in the literature, age, diabetes, urine volume, ultrafiltration, Kt/V, serum urea, serum creatinine, serum phosphate, serum potassium, serum sodium, albumin, fat intake, DPI, baseline nDEI, time average nDEI, and TNA were included in the multivariate Cox proportional hazard analysis. Charlson index, total fluid removal, eGFR, carbohydrate intake and DEI were excluded from the analysis. After multivariate Cox proportional hazard analysis, age, diabetes, serum urea, serum albumin, serum sodium and baseline nDEI were independently associated with patients’ mortality, see Table 2 for detail information. Baseline DPI and BMI were not related to PD patients’ mortality.
Table 2.
Univariate Cox regression analysis of the impact of data on mortality
| Variables | Univariate Cox regression analysis | Multivariate Cox regression analysis* | ||
|---|---|---|---|---|
| HR(95%Cl) | P value | HR(95%Cl)# | P value | |
| Age, years | 1.062[1.053–1.071] | < 0.001 | 1.056[1.047,1.065] | < 0.001 |
| Gender | 1.012[0.834–1.229] | 0.902 | ||
| Charlson index, points | 1.580[1.489–1.677] | < 0.001 | not included | |
| Diabetes | 1.721[1.416–2.092] | < 0.001 | 1.364[1.114,1.671] | 0.003 |
| BMI | 0.984[0.958–1.010] | 0.225 | ||
| urine volume, ml | 1.000[0.999-1.000] | 0.001 | 1.000[1.0001.000] | 0.747 |
| Dialysis dose, ml | 1.000[1.000–1.000] | 0.864 | ||
| ultrafiltration, ml | 1.278[1.006–1.625] | 0.045 | 1.000[1.000,1.000] | 0.363 |
| total fluid removal, ml | 1.000[1.000–1.000] | 0.085 | not included | |
| Kt/V | 1.061[0.898–1.252] | 0.487 | 0.872[0.717,1.060] | 0.169 |
| Tccr, L/w | 0.999[0.995–1.003] | 0.541 | ||
| eGFR, ml/min | 1.007[0.966–1.049] | 0.757 | not included | |
| Hb, g/l | 1.000[0.995–1.006] | 0.952 | ||
| Serum urea, mmol/l | 0.952[0.935–0.970] | < 0.001 | 0.974[0.953,0.994] | 0.012 |
| Serum creatinine, umol/l | 0.998[0.998–0.999] | < 0.001 | 1.000[0.999,1.000] | 0.223 |
| Serum calcium, mmol/l | 0.994[0.711–1.391] | 0.973 | ||
| Serum phosphate, mmol/l | 0.798[0.618–1.031] | 0.084 | 1.309[0.983,1.743] | 0.065 |
| Serum potassium, mmol/l | 0.729[0.631–0.842] | < 0.001 | 0.918[0.792,1.065] | 0.260 |
| Serum sodium, mmol/l | 0.977[0.966–0.988] | < 0.001 | 0.973[0.954,0.992] | 0.005 |
| CO2CP, mmol/l | 0.997[0.969–1.024] | 0.807 | ||
| Alb, g/l | 0.913[0.895–0.931] | < 0.001 | 0.945[0.923,0.967] | < 0.001 |
| Fat intake, g/d | 0.995[0.990-1.000] | 0.048 | 0.999[0.992,1.007] | 0.886 |
| Carbohydrate intake, g/d | 0.995[0.993–0.997] | < 0.001 | not included | |
| DPI, g/day | 0.992[0.985–0.998] | 0.017 | 1.002[0.992,1.012] | 0.689 |
| nDPI, g/kg/day | 0.721[0.474–1.097] | 0.127 | ||
| DEI, kcal/day | 0.999[0.999-1.000] | < 0.001 | not included | |
| nDEI, kcal/kg/day | 0.966[0.951–0.981] | < 0.001 | 0.980[0.964,0.996] | 0.017 |
| Time average nDEI, kcal/kg/day | 0.950[0.929,0.971] | < 0.001 | 0.990[0.966,1.014] | 0.410 |
| TNA, g/d (Bergstrom) | 0.874[0.831–0.918] | < 0.001 | 0.984[0.892,1.085] | 0.745 |
| TNA, g/d (New formula) | 0.877[0.836–0.920] | < 0.001 | not included | |
| NB, g/d (Bergstrom) | 1.042[1.005–1.081] | 0.027 | not included | |
| NB, g/d (New formula) | 1.044[1.007–1.082] | 0.020 | not included | |
Note BMI, body mass index; Tccr: total clearance rate of creatinine; eGFR: estimated Glomerular filtration rate; Hb: hemoglobin; CO2CP: carbon dioxide combining power; Alb: albumin; DPI: dietary protein intake; nDPI: normalized dietary protein intake; DEI: dietary energy intake; nDEI: normalized dietary energy intake; TNA: total nitrogen appearance; NB, nitrogen balance
*Multivariate Cox’s regression analysis (using Backward Wald method to select variable) included all the variables which showed significance [p < 0.1, including age, diabetes, urine volume, ultrafiltration, serum urea, serum creatinine, serum phosphate, serum potassium, serum sodium, Alb, DPI, nDEI, fat intake, and TNA(Bergstrom)] from the univariate analysis. For variables which had obvious collinearity, the one with lower P value was included in the multivariate analysis
#For the variable removed from the model, the P value and HR of the last step were reported
If patients were divided into four quartiles based on blood sodium level (≤ 137 mmol/L, 137.1–139 mmol/L, 139.1–141 mmol/L and ≥ 141.1 mmo/L), only patients having the lowest blood sodium (≤ 137 mmol/l) had poorer outcome as compared with the others (see Fig. 2).
Fig. 2.
The Kaplan–Meier survival curves in PD patients according to the baseline Serum sodium level. PD: peritoneal dialysis. All the subjects were classified into four groups according to baseline Serum sodium, i.e., 137mmol/L, 137.1-139mmol/L, 139.1-141mmol/L and ≥ 141.1mmo/L. As shown in Fig. 2, patients with lowest Serum sodium (
137mmol/L, 137.1-139mmol/L, 139.1-141mmol/L and ≥ 141.1mmo/L. As shown in Fig. 2, patients with lowest Serum sodium ( 137mmol/L) had lower survival compared with patients in the other three groups (p < 0.05). There was no significant difference in survival among the other 3 groups (Serum sodium 137.1–139, 139.1–141, ≥ 141.1 mmol/l; p> 0.05)
137mmol/L) had lower survival compared with patients in the other three groups (p < 0.05). There was no significant difference in survival among the other 3 groups (Serum sodium 137.1–139, 139.1–141, ≥ 141.1 mmol/l; p> 0.05)
The effect of longitudinal nDEI on survival
In the univariate Cox regression model, time average nDEI was associated with mortality (HR = 0.950, 95%CI 0.929–0.971, P < 0.001). Since the correlation between time average nDEI and baseline nDEI was low (r = 0.38), we placed both time average nDEI and baseline nDEI into multivariate Cox proportional hazard analysis. And the time average nDEI was removed from the model after adjusting other covariates (Table 2).
Baseline nDEI level as a categorical variable
All the subjects were classified into four groups according to baseline nDEI, i.e., < 25 kcal/kg/day, 25-29.99 kcal/kg/day, 30-34.99 kcal/kg/day and ≥ 35 kcal/kg/day. There were differences in age, diabetes percentage, nDPI, fat intake, carbohydrate intake, nitrogen intake, NB and albumin among the patients in the four groups (p < 0.05). Only patients with nDEI ≥ 35 kcal/kg/day could achieve positive nitrogen balance if using the Bergstrom formula to estimate TNA. However, if using the new formula, the patients in three groups (25-29.99 kcal/kg/day, 30-34.99 kcal/kg/day and ≥ 35 kcal/kg/day) could get positive nitrogen balance. Detail information is shown in Table 3.
Table 3.
Comparison of baseline parameters among patients in different nDEI groups
| Variables | Baseline nDEI level | F/Z/x2 | p | |||
|---|---|---|---|---|---|---|
| < 25 kcal /kg/day* (n = 404) |
25-29.99 kcal/kg/day* (n = 217) |
30-34.99 kcal/kg/day* (n = 107) |
≥ 35 kcal /kg/day* (n = 66) |
|||
| age, years | 58.56 16.48 16.48 |
61.86 15.15# 15.15#
|
56.57 15.67& 15.67&
|
54.65 16.21& 16.21&
|
4.784 | 0.003 |
| Male(%) | 223(55.2%) | 119(54.8%) | 50(46.7%) | 32(48.5%) | 3.253 | 0.354 |
| Diabetes(%) | 197(48.8%) | 84(38.7%) | 44(41.1%) | 18(27.3%) | 14.21 | 0.003 |
| Charlson index | 6(4,7) | 6(4,7) | 5(3,7) | 4(3,7) | 2.074 | 0.557 |
| BMI | 23.72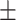 3.79 3.79 |
23.10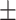 3.57 3.57 |
23.04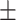 3.27 3.27 |
23.88 3.46 3.46 |
2.149 | 0.093 |
| nDPI, g/kg/day | 0.72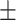 0.18 0.18 |
0.91 0.16# 0.16#
|
1.05 0.18#& 0.18#&
|
1.23 0.24#&^ 0.24#&^
|
232.435 | < 0.001 |
| Fat intake, g/d | 47.04(36.69,55.51) | 60.18(51.16,71.66) # | 69.30(57.57,83.92) #& | 78.40(58.20,100.37) #& | 158.516 | < 0.001 |
| Carbohydrate intake, g/d | 165.32(135.56,196.48) | 212.08(175.67,246.47) # | 244.21(211.00,289.56) #& | 288.99(236.86,368.42) #&^ | 222.752 | < 0.001 |
| Alb, g/l | 36.98 4.61 4.61 |
36.80 4.43 4.43 |
37.29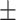 4.58 4.58 |
39.27 5.26#&^ 5.26#&^
|
5.243 | 0.001 |
| Dialysis dose, ml | 6000(6000,8000) | 6060(6000,8000) | 6000(6000,8000) | 6000(5000,8000) | 1.073 | 0.784 |
| ultrafiltration, ml | 350(102.5,650) | 350(100,605) | 300(100,600) | 425(200,740) | 6.255 | 0.100 |
| urine volume, ml | 500(200,925) | 550(255,1000) | 575(350,900) | 650(210,1000) | 2.120 | 0.548 |
| total fluid removal, ml | 950(700,1296.5) | 1000(687.5,1350) | 968(700,1299) | 1115(840,1450) | 2.384 | 0.497 |
| Kt/V | 1.93(1.55,2.35) | 1.96(1.66,2.41) | 2.02(1.66,2.53) | 2.06(1.79,2.47) | 7.459 | 0.059 |
| TCcr, (L/w) | 59.03(47.50,74.88) | 60.90(49.42,75.75) | 62.47(49.27,82.67) | 66.20(49.56,83.00) | 5.901 | 0.117 |
| eGFR, ml/min | 2.36(0.97,3.84) | 2.46(1.20,4.04) | 2.40(1.24,4.53) | 3.11(1.37,4.93) | 4.526 | 0.210 |
| Nitrogen intake, g/d | 6.91 1.99 1.99 |
8.57 1.82# 1.82#
|
9.67 2.34#& 2.34#&
|
10.80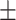 2.85#&^ 2.85#&^
|
105.157 | < 0.001 |
| TNA, g/d (Bergstrom) | 9.44 2.05 2.05 |
9.61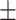 2.18 2.18 |
9.78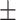 2.25 2.25 |
9.94 2.08 2.08 |
1.532 | 0.205 |
| TNA, g/d (New formula) | 7.90 2.12 2.12 |
8.08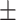 2.25 2.25 |
8.25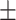 2.32 2.32 |
8.42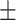 2.15 2.15 |
1.532 | 0.205 |
| NB, g/d (Bergstrom) | -2.53 2.24 2.24 |
-1.05 2.43# 2.43#
|
-0.11 2.75#& 2.75#&
|
0.86 2.94#&^ 2.94#&^
|
59.107 | < 0.001 |
| NB, g/d (New formula) | -1.00 2.28 2.28 |
0.48 2.48# 2.48#
|
1.42 2.79#& 2.79#&
|
2.38 2.96#&^ 2.96#&^
|
59.769 | < 0.001 |
| Hb, g/l | 115.68 20.29 20.29 |
117.55 18.95 18.95 |
114.95 17.31 17.31 |
115.52 15.98 15.98 |
0.622 | 0.601 |
| Serum urea, mmol/l | 20.13 6.08 6.08 |
20.45 5.64 5.64 |
21.13 5.57 5.57 |
20.38 5.03 5.03 |
0.858 | 0.463 |
Serum creatinine,  mmol/l mmol/l |
735.12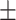 262.83 262.83 |
707.49 239.55 239.55 |
717.50 275.34 275.34 |
697.05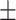 218.91 218.91 |
0.812 | 0.487 |
| Serum calcium, mmol/l | 2.25 0.28 0.28 |
2.28 0.28 0.28 |
2.26 0.24 0.24 |
2.25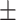 0.34 0.34 |
0.536 | 0.658 |
| Serum phosphate, mmol/l | 1.53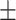 0.41 0.41 |
1.52 0.39 0.39 |
1.51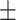 0.43 0.43 |
1.51 0.40 0.40 |
0.068 | 0.977 |
| Serum potassium, mmol/l | 4.27 0.77 0.77 |
4.36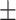 0.71 0.71 |
4.37 0.72 0.72 |
4.28 0.62 0.62 |
0.986 | 0.399 |
| Serum sodium, mmol/l | 138.90 3.08 3.08 |
138.82 8.98 8.98 |
139.42 3.20 3.20 |
139.48 3.24 3.24 |
0.513 | 0.673 |
| CO2CP, mmol/l | 26.72 3.51 3.51 |
26.64 3.70 3.70 |
26.10 3.99 3.99 |
26.30 3.77 3.77 |
0.979 | 0.402 |
*: mean sd for normalized distribution parameters, median(P25, P75) for non-normalized distribution parameters
sd for normalized distribution parameters, median(P25, P75) for non-normalized distribution parameters
#: there is a significant difference compared with the first group (nDEI < 25 kcal/kg/day; p < 0.05)
&: there is a significant difference compared with the second group (nDEI 25-29.99 kcal/kg/day; p < 0.05)
^: there is a significant difference compared with the third group (nDEI 30-34.99 kcal/kg/day; p < 0.05)
nDEI: normalized dietary energy intake; BMI: body mass index; nDPI: normalized dietary protein intake; Alb: albumin; TCcr: total clearance rate of creatinine; eGFR: estimated Glomerular filtration rate; TNA: total nitrogen appearance; NB: nitrogen balance; Hb: hemoglobin; CO2CP: carbon dioxide combining power
As shown in Fig. 3; Table 4, compared with the two groups with higher nDEI (30-34.99 kcal/kg/day and ≥ 35 kcal/kg/day), the two groups with lower nDEI (< 25 kcal/kg/day and 25-29.99 kcal/kg/day) had inferior survival (p < 0.05). There was no significant difference between the two groups with relatively higher nDEI (30-34.99 kcal/kg/day compared with ≥ 35 kcal/kg/day, p > 0.05) and relatively lower nDEI (< 25 kcal/kg/day versus 25-29.99 kcal/kg/day, p > 0.05).
Fig. 3.
The Kaplan–Meier survival curves in PD patients according to the baseline nDEI level PD: peritoneal dialysis; nDEI: normalized dietary energy intake. Compared with the two groups with higher DEI (30-34.99 kcal/kg/day and ≥ 35 kcal/kg/day), the two groups with lower DEI (< 25 kcal/kg/day and 25-29.99 kcal/kg/day) had lower survival (p < 0.05). There was no significant difference between the two groups with relatively high (30-34.99 kcal/kg/day versus ≥ 35 kcal/kg/day, p > 0.05) and relatively low DEI (< 25 kcal/kg/day versus 25-29.99 kcal/kg/day, p > 0.05)
Table 4.
Association between baseline nDEI groups and mortality in proportional hazards models (N = 749)
| No. of participants | No. of events |
univariable model | Multivariable model | |||
|---|---|---|---|---|---|---|
| HR | p | HR | p | |||
| nDEI group | 0.003 | 0.038 | ||||
| < 25 kcal/kg/day | 404 | 222 | 1.00(ref) | 1.00(ref) | ||
| 25-29.99 kcal/kg/day | 217 | 119 | 0.870[0.696,1.087] | 0.221 | 0.839[0.669,1.052] | 0.128 |
| 30-34.99 kcal/kg/day | 107 | 42 | 0.567[0.408,0.789] | < 0.001 | 0.632[0.455,0.877] | 0.006 |
| ≥ 35 kcal/kg/day | 66 | 29 | 0.655[0.445,0.965] | 0.032 | 0.956[0.642,1.424] | 0.826 |
Note Multivariable model was adjusted for age, diabetes, BMI, nDPI, fat intake, Alb, ultrafiltration, Kt/V, eGFR. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were calculated by proportional hazards model
As shown in Table 3, there were differences on age, diabetes, BMI, nDPI, fat intake, albumin, ultrafiltration, Kt/V, and eGFR among the four nDEI groups. After adjusting these covariates, the baseline nDEI group was still associated with mortality (p = 0.038). Table 4 shows that the third group of patients with nDEI 30-34.99 kcal/kg/day had the lowest risk in both univariate and multivariate Cox proportional risk models (HR = 0.567 vs. 0.632, respectively). However, if using other outcome events, such as transfer to HD, and combination of mortality & transfer to HD, the associations between baseline nDEI group and these outcomes were not significant (Table 5).
Table 5.
Association between baseline nDEI groups and different ending in proportional hazards models (N = 749)
| baseline nDEI groups |
proportional hazards model of death | proportional hazards model of transfer to HD | proportional hazards model of death and transfer to HD | |||
|---|---|---|---|---|---|---|
| No. of events |
Multivariable model | No. of events |
Multivariable model | No. of events |
Multivariable model | |
| < 25 kcal/kg/day | 222 | 1.00(ref) | 49 | 1.00(ref) | 271 | 1.00(ref) |
| 25-29.99 kcal/kg/day | 119 | 0.839[0.669,1.052] | 16 | 0.672[0.370,1.219] | 135 | 0.855[0.677,1.080] |
| 30-34.99 kcal/kg/day | 42 | 0.632[0.455,0.877] | 16 | 1.268[0.653,2.465] | 58 | 0.793[0.573,1.098] |
| ≥ 35 kcal/kg/day | 29 | 0.956[0.642,1.424] | 10 | 1.512[0.600,3.809] | 39 | 1.177[0.767,1.807] |
*Multivariable model was fully adjusted for the same covariates in the Table 4
#Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were calculated by proportional hazards model
Discussion
In this single-center long-term follow-up PD cohort, we found a higher baseline level of nDEI was significantly associated with a lower risk of death (HR = 0.980; 95% CI = 0.964–0.996, p = 0.017) even after adjusted by age, diabetes, urine volume, ultrafiltration, Kt/V, serum urea, serum creatinine,serum phosphate, serum potassium, serum sodium, albumin and DPI by multivariate Cox proportional hazard analysis. According to the multivariate Cox regression analysis, old age, having diabetes, hypoalbuminemia, a lower level of blood sodium and urea were also significantly associated with inferior survival. Baseline BMI was not related to PD patients’ survival. Higher longitudinal time average nDEI was also related to better survival in univariate Cox proportional hazard analysis, but the association disappeared after adjusting other parameters in multivariate Cox proportional hazard analysis. It indicated that the time average nDEI was less sensitive than baseline one to predict mortality. In this study, the baseline data was collected at the sixth month of dialysis when most of the patients had improved from uremia, while the time-average dietary intake may be influenced by decreased residual renal function or new complication(s) over time, all of these factors may explain why the baseline nDEI was associated with mortality rather than time-average nDEI.
The influence of DEI on PD patients’ all-cause mortality was found in our previous longitudinal study which also reported that DEI rather than DPI was an independent risk factor for mortality of patients having PD [13], it indicated the importance of adequate energy intake for PD patients to maintain the nutritional status and achieve good outcome. Additionally, the present study further analyzed the optimal nDEI level in Chinese PD population. In univariate analysis, patients whose nDEI was higher than 30 kcal/kg/day had better prognosis. After adjusting other confounding factors, patients with nDEI of 30-34.99 kcal/kg/day had the lowest mortality risk among the four groups. In the sensitivity analysis, the baseline nDEI group was not associated with other outcome events, such as transfer to HD or the combination of mortality & transfer to HD. In present study, most of the causes for transfer to HD were catheter-related infection, catheter malfunction, hernia, and surgical operation. The competing risk was small since dietary intake had limited influence on these events.
On the other hand, in our study, the calculation of nDEI did not take into account the energy absorption from the dialysate (usually 250–300 kcal/d, about equals to 5 kcal/kg/d for a patient with 60 kg normalized body weight) [24]. If considering this part of energy intake, it may mean that the nDEI of our patients on PD should be more than 35 kcal/kg/day to ensure better survival. The result exceeds the recommendations (25–35 kcal/kg/day) provided by the K/DOQI guidelines [14, 25], and is consistent with the Chinese Clinical Practice Guidelines for Nutrition in CKD (2021 edition) [15].
Why do our Chinese PD patients have higher energy requirements? The increased energy expenditure was one of the main reasons. To maintain the patient’s energy balance, their energy intake should be equal to their energy expenditure when at a stable metabolic condition. The total energy expenditure (TEE) of the human body mainly includes three parts, named resting energy expenditure (REE), thermic effect of food and activities induced energy expenditure (AEE). In these three parts, REE can account for 60–75% of TEE in normal population [26]. Therefore, REE plays an important role in evaluating energy expenditure in patients with CKD. For the patients with CKD, many studies have found that REE generally increased [27, 28]. Although the cause of increased REE in CKD patients has not been clearly determined, many possible causes exist. For example, chronic inflammation can lead to increased REE [29], and the incidence of chronic inflammation was higher in CKD patients [30]. The excitation of the sympathetic nervous system may also be one of the reasons for the increase of REE in the dialysis patients [31]. In addition, the comorbidity of CKD patients, such as diabetes, may also be the cause of their increased REE [27, 28]. Thus, dialysis patients may need higher DEI to maintain energy balance. In present study, patients with nDEI ≥ 30 kcal/kg/day could also achieve positive nitrogen balance if using new formula (which may be more suitable for Chinese PD patients) [20] rather than the Bergstrom formula to estimate TNA. Some nutritional studies involving hemodialysis patients or non-dialysis ESRD patients also showed that DEI 35 kcal/kg/day and above was beneficial to maintain patients’ nutritional status [32, 33].
Except the higher REE level, the BMI characteristic of our study population may also be a reason for their higher energy requirements. Patients’ BMI levels in this study were lower than patients from Western countries in other studies [34]. More than 60% percent of the study population were in the normal range or underweight status in spite of using the BMI stratification criteria for Chinese population [35]. It is obvious that underweight or normal weight people need more energy intake than the overweight or obese ones.
In addition, in present study, we found that lower serum albumin levels, as well as lower serum sodium levels, were associated with poor prognosis in patients having PD. Serum albumin, a popular-used nutritional & inflammatory marker for dialysis patients, its correlation with survival of patients on PD had been fully described in previous studies [36–38]. As for blood sodium levels, Ravel VA et al. also reported that lower baseline serum sodium levels had been associated with a poorer prognosis in PD patients, although the exact sodium threshold below which higher mortality risk was observed varied across different analyses (134–140 mmol/L) [39]. The results of the present study added a flavor on this phenomenon, we found patients with serum sodium ≤ 137mmol/L had poorer outcomes as compared with the others. Hyponatremia may lead to poor prognosis of PD patients in a number of ways. Some studies have shown that low serum sodium levels may be associated with higher cardiovascular risk, central system adverse reactions, and higher risk of infection [40–42]. Additionally, low serum sodium in dialysis patients can also aggravate PEW, which leads to poor prognosis in dialysis patients [43]. However, a most recent study from a provincial HD cohort from China reported a U-shaped relationship between serum sodium and all-cause mortality in HD patients, with serum sodium ≤ 137.5 mmol/L or > 142.5 mmol/L being related to a higher risk of death [44]. The exact serum sodium range which could lead to better survival in dialysis patients still needs further studies.
Limitations
There are some limitations of this study. First, the three-day dietary recall data may underestimate the dietary intake, even though we gave all the patients and their caregivers sufficient education. However, the study used the guidelines’ recommending 3-day diet record to assess diet, which is currently a reliable assessment method with both high reliability and validity. In addition, this does not affect the conclusion of this study. If there is an underestimation of dietary intake, then PD patients may need a higher DEI to ensure a good survival than the results of the present study. Second, the study was conducted in a single center, and the results were more internally validated, which might have some limitations in terms of generalizability. Third, some potential confounding factors (including both baseline and longitudinal factors) which may affect the outcomes could not be completely excluded or analyzed. The last, the selection bias may exist due to many patients lost follow-up or withdrew from PD.
Conclusion
Our study revealed that baseline nDEI 30-34.99 kcal/kg/day was beneficial to the long-term outcome of our PD population based on the results of survival analysis. If considering the glucose absorption from the dialysate, the total nDEI may need to achieve 35 kcal/kg/day to ensure the optimal long-term outcome for our Chinese PD patients.
Acknowledgements
not applicable.
Abbreviations
- AEE
Activities induced energy expenditure
- Alb
Albumin
- BMI
Body mass index
- CAPD
Continuous ambulatory peritoneal dialysis
- CI
Confidence Interval
- CGN
Chronic glomerulonephritis
- CKD
Chronic kidney disease
- CNRDS
Chinese National Renal Data System
- CO2CP
Carbon dioxide combining power
- DEI
Dietary energy intake
- DKD
Diabetic kidney disease
- DM
Diabetes mellitus
- DPI
Dietary protein intake
- eGFR
Estimated Glomerular filtration rate
- ESRD
End-stage renal disease
- Hb
Hemoglobin
- HD
Hemodialysis
- HR
Hazard ratios
- HTN
Hypertensive nephropathy
- IBW
Ideal body weight
- IQR
Interquartile range
- K/DOQI
The National Kidney Foundation Kidney Disease Outcomes Quality Initiative
- NB
Nitrogen balance
- nDEI
Normalized dietary energy intake
- nDPI
Normalized dietary protein intake
- PD
Peritoneal dialysis
- PEW
Protein-energy wasting
- REE
Resting energy expenditure
- RKF
Residual kidney function
- SD
Standard deviation
- Tccr
Total clearance rate of creatinine
- TEE
Total energy expenditure
- TNA
Total nitrogen appearance
- UNA
Urea nitrogen appearance
Author contributions
L SX contributed to the conception and design of the study, data analysis, and drafting of the manuscript. XK Z XQ and made significant contributions to the data collection and analysis. L MY and Z XQ revised the manuscript. S CY and TW, as the corresponding authors, made substantial contributions to the conception and design, and critically important intellectual content revisions of the manuscript. All authors have read and approved the final version of the manuscript and agree with the order of the authors’ presentation.
Funding
This study was supported by the National Natural Science Foundation of China (U23A20468) and Clinical Cohort Construction Program of Peking University Third Hospital (No. BYSYDL2023004).
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Medical Ethical Committee of Peking University Third Hospital with approval number IRB 2024-096-03. Individual informed consent was waived by the IRB since the study was a retrospective, non-interventional design and using a deidentified dataset. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chun-Yan Su, Email: scybmu@126.com.
Wen Tang, Email: tanggwen@126.com.
References
- 1.Wang L, Xu X, Zhang M, Hu C, Zhang X, Li C, Nie S, Huang Z, Zhao Z, Hou FF, et al. Prevalence of chronic kidney disease in China: results from the sixth China chronic disease and risk factor surveillance. JAMA Intern Med. 2023;183(4):298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liyanage T, Toyama T, Hockham C, Ninomiya T, Perkovic V, Woodward M, Fukagawa M, Matsushita K, Praditpornsilpa K, Hooi LS et al. Prevalence of chronic kidney disease in Asia: a systematic review and analysis. BMJ Global Health 2022, 7(1). [DOI] [PMC free article] [PubMed]
- 3.Li PK, Chow KM, Van de Luijtgaarden MW, Johnson DW, Jager KJ, Mehrotra R, Naicker S, Pecoits-Filho R, Yu XQ, Lameire N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13(2):90–103. [DOI] [PubMed] [Google Scholar]
- 4.Briggs V, Davies S, Wilkie M. International variations in peritoneal Dialysis utilization and implications for practice. Am J Kidney Diseases: Official J Natl Kidney Foundation. 2019;74(1):101–10. [DOI] [PubMed] [Google Scholar]
- 5.Ministry of health. P.R.C. Chinese National Renal Data System [online]. http://www.cnrds.net/ (2023).
- 6.Chung SH, Lindholm B, Lee HB. Influence of initial nutritional status on continuous ambulatory peritoneal dialysis patient survival. Perit dialysis International: J Int Soc Perit Dialysis. 2000;20(1):19–26. [PubMed] [Google Scholar]
- 7.Hiruy AF, Opoku S, Xiong Q, Jin Q, Zhao J, Lin X, He S, Zuo X, Ying C. Nutritional predictors associated with malnutrition in continuous ambulatory peritoneal dialysis patients. Clin Nutr ESPEN. 2021;45:454–61. [DOI] [PubMed] [Google Scholar]
- 8.Han SH, Han DS. Nutrition in patients on peritoneal dialysis. Nat Rev Nephrol. 2012;8(3):163–75. [DOI] [PubMed] [Google Scholar]
- 9.Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. J Am Soc Nephrology: JASN. 1996;7(2):198–207. [DOI] [PubMed] [Google Scholar]
- 10.Kittiskulnam P, Chuengsaman P, Kanjanabuch T, Katesomboon S, Tungsanga S, Tiskajornsiri K, Praditpornsilpa K, Eiam-Ong S. Protein-Energy wasting and mortality risk prediction among peritoneal Dialysis patients. J Ren Nutrition: Official J Council Ren Nutr Natl Kidney Foundation. 2021;31(6):679–86. [DOI] [PubMed] [Google Scholar]
- 11.Dong J, Li Y, Xu Y, Xu R. Daily protein intake and survival in patients on peritoneal dialysis. Nephrol Dial Transpl. 2011;26(11):3715–21. [DOI] [PubMed] [Google Scholar]
- 12.Dong J, Wang T, Wang HY. The impact of new comorbidities on nutritional status in continuous ambulatory peritoneal dialysis patients. Blood Purif. 2006;24(5–6):517–23. [DOI] [PubMed] [Google Scholar]
- 13.Bi SH, Wang X, Tang W, Wang T, Li B, Su C. Longitudinal association between dietary protein intake and survival in peritoneal dialysis patients. Ren Fail. 2023;45(1):2182605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W, Fouque D, Friedman AN, Ghaddar S, Goldstein-Fuchs DJ, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Diseases: Official J Natl Kidney Foundation. 2020;76(3 Suppl 1):S1–107. [DOI] [PubMed] [Google Scholar]
- 15.Chinese Nephrologist Association, Expert Collaboration Group for Nutrition Treatment Guidelines of Chinese Association of Integrated Medicine Kidney Disease Committee. Chinese clinical practice guideline for nutrition in CKD (2021 edition). Chin Med J. 2021;101(8):539–59. [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 17.Dombros N, Dratwa M, Feriani M, Gokal R, Heimbürger O, Krediet R, Plum J, Rodrigues A, Selgas R, Struijk D, et al. European best practice guidelines for peritoneal dialysis. 8 nutrition in peritoneal dialysis. Nephrol dialysis Transplantation: Official Publication Eur Dialysis Transpl Association - Eur Ren Association. 2005;20(Suppl 9):ix28–33. [DOI] [PubMed] [Google Scholar]
- 18.Wu G. Practical clinical nutrition. Shanghai, China: Fudan University; 2006. [Google Scholar]
- 19.Bergstrom J, Heimburger O, Lindholm B. Calculation of the protein equivalent of total nitrogen appearance from Urea appearance. Which formulas should be used? Perit Dial Int. 1998;18(5):467–73. [PubMed] [Google Scholar]
- 20.Su C, Wang T, Wang P, Lu X, Tang W. The Estimation of protein equivalents of total nitrogen in Chinese CAPD patients: an explanatory study. Ren Fail. 2022;44(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Zhang L, Ma S, Xiao J, Liu D, Ding R, Li Z, Zhao Z. Kt/V reach rate is associated with clinical outcome in incident peritoneal dialysis patients. Ren Fail. 2022;44(1):482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Susantitaphong P, Altamimi S, Ashkar M, Balk EM, Stel VS, Wright S, Jaber BL. GFR at initiation of dialysis and mortality in CKD: a meta-analysis. Am J Kidney Diseases: Official J Natl Kidney Foundation. 2012;59(6):829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.In J, Lee DK. Survival analysis: part II - applied clinical data analysis. Korean J Anesthesiol. 2019;72(5):441–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su CY, Lu XH, Tang W, Wang PY, Wang T. Peritoneal dialysis can maintain nitrogen balance with low Kt/V in anuric patients. Clin Nephrol. 2022;97(4):206–14. [DOI] [PubMed] [Google Scholar]
- 25.Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar-Zadeh K, Kuhlmann MK, Stenvinkel P, TerWee P, Teta D, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the international society of renal nutrition and metabolism. Kidney Int. 2013;84(6):1096–107. [DOI] [PubMed] [Google Scholar]
- 26.Avesani CM, Kamimura MA, Cuppari L. Energy expenditure in chronic kidney disease patients. J Ren Nutrition: Official J Council Ren Nutr Natl Kidney Foundation. 2011;21(1):27–30. [DOI] [PubMed] [Google Scholar]
- 27.Neyra R, Chen KY, Sun M, Shyr Y, Hakim RM, Ikizler TA. Increased resting energy expenditure in patients with end-stage renal disease. JPEN J Parenter Enter Nutr. 2003;27(1):36–42. [DOI] [PubMed] [Google Scholar]
- 28.Ikizler TA, Wingard RL, Sun M, Harvell J, Parker RA, Hakim RM. Increased energy expenditure in Hemodialysis patients. J Am Soc Nephrology: JASN. 1996;7(12):2646–53. [DOI] [PubMed] [Google Scholar]
- 29.Avesani CM, Draibe SA, Kamimura MA, Colugnati FA, Cuppari L. Resting energy expenditure of chronic kidney disease patients: influence of renal function and subclinical inflammation. Am J Kidney Diseases: Official J Natl Kidney Foundation. 2004;44(6):1008–16. [DOI] [PubMed] [Google Scholar]
- 30.Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM. Association of morbidity with markers of nutrition and inflammation in chronic Hemodialysis patients: a prospective study. Kidney Int. 1999;55(5):1945–51. [DOI] [PubMed] [Google Scholar]
- 31.Himmelfarb J, Holbrook D, McMonagle E, Robinson R, Nye L, Spratt D. Kt/V, nutritional parameters, serum cortisol, and insulin growth factor-1 levels and patient outcome in Hemodialysis. Am J Kidney Diseases: Official J Natl Kidney Foundation. 1994;24(3):473–9. [DOI] [PubMed] [Google Scholar]
- 32.Slomowitz LA, Monteon FJ, Grosvenor M, Laidlaw SA, Kopple JD. Effect of energy intake on nutritional status in maintenance Hemodialysis patients. Kidney Int. 1989;35(2):704–11. [DOI] [PubMed] [Google Scholar]
- 33.Kopple JD, Monteon FJ, Shaib JK. Effect of energy intake on nitrogen metabolism in nondialyzed patients with chronic renal failure. Kidney Int. 1986;29(3):734–42. [DOI] [PubMed] [Google Scholar]
- 34.Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the united States. Kidney Int. 2003;64(5):1838–44. [DOI] [PubMed] [Google Scholar]
- 35.Standardization Administration of China. WS/T 428–2013 Adult weight determination.
- 36.Hao N, Cheng BC, Yang HT, Wu CH, Lei YY, Chao MC, Wang PY, Kuo LC, Moi SH, Yang CH, et al. Time-varying serum albumin levels and all-cause mortality in prevalent peritoneal dialysis patients: a 5-year observational study. BMC Nephrol. 2019;20(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Han Q, Wang T, Tang W. Serum albumin changes and mortality risk of peritoneal dialysis patients. Int Urol Nephrol. 2020;52(3):565–71. [DOI] [PubMed] [Google Scholar]
- 38.Basol M, Goksuluk D, Sipahioglu MH, Karaagaoglu E. Effect of Serum Albumin Changes on Mortality in Patients with Peritoneal Dialysis: A Joint Modeling Approach and Personalized Dynamic Risk Predictions. Biomed Res Int 2021, 2021:6612464. [DOI] [PMC free article] [PubMed]
- 39.Ravel VA, Streja E, Mehrotra R, Sim JJ, Harley K, Ayus JC, Amin AN, Brunelli SM, Kovesdy CP, Kalantar-Zadeh K, et al. Serum sodium and mortality in a National peritoneal dialysis cohort. Nephrol dialysis Transplantation: Official Publication Eur Dialysis Transpl Association - Eur Ren Association. 2017;32(7):1224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hecking M, Karaboyas A, Saran R, Sen A, Hörl WH, Pisoni RL, Robinson BM, Sunder-Plassmann G, Port FK. Predialysis serum sodium level, dialysate sodium, and mortality in maintenance Hemodialysis patients: the Dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Diseases: Official J Natl Kidney Foundation. 2012;59(2):238–48. [DOI] [PubMed] [Google Scholar]
- 41.Hoorn EJ, Zietse R. Hyponatremia and mortality: moving beyond associations. Am J Kidney Diseases: Official J Natl Kidney Foundation. 2013;62(1):139–49. [DOI] [PubMed] [Google Scholar]
- 42.Mandai S, Kuwahara M, Kasagi Y, Kusaka K, Tanaka T, Shikuma S, Akita W, Sasaki S. Lower serum sodium level predicts higher risk of infection-related hospitalization in maintenance Hemodialysis patients: an observational cohort study. BMC Nephrol. 2013;14:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canaud B, Morena-Carrere M, Leray-Moragues H, Cristol JP. Fluid overload and tissue sodium accumulation as main drivers of protein energy malnutrition in Dialysis patients. Nutrients 2022, 14(21). [DOI] [PMC free article] [PubMed]
- 44.Chen S, Pan B, Lou X, Chen J, Zhang P. Effect of long-term serum sodium levels on the prognosis of patients on maintenance Hemodialysis. Ren Fail. 2024;46(1):2314629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.





