Abstract
Introduction
Despite druggable events to be present in 80 % of neuroblastomapatients within the Princess Máxima Center precision medicine program 'iTHER', clinical uptake of treatment recommendations has been low, and the clinical impact for individual patients remains hard to predict. This stresses the need for a method integrating genomics and transcriptomics with functional approaches into therapeutic decision making.
Methods
We aimed to launch an online repository integrating genomics and transcriptomics with high-throughput drug screening (HTS) of nineteen commonly used neuroblastoma cell lines and fifteen neuroblastoma patient-derived organoids (NBL-PDOs). Cell lines, NBL-PDOs and their parental tumors were characterized utilizing (lc)WGS, WES and RNAseq. Cells were exposed to ∼200 compounds. Results were transferred to the R2 visualization platform.
Results
A powerful reference set of cell lines is available, reflecting distinct known pharmacologic vulnerabilities. HTS identified additional therapeutic vulnerabilities, such as a striking correlation between a positive mesenchymal signature and sensitivity to BCL2-inhibitor venetoclax. Finally, we explored personalized drug sensitivities within iTHER, demonstrating HTS can support genomic and transcriptomic results, thereby strengthening the rationale for clinical uptake.
Conclusion
We established a dynamic publicly available dataset with detailed genomic, transcriptomic, and pharmacological annotation of classical neuroblastoma cell lines as well as novel sharable NBL-PDOs, representing the heterogeneous landscape of neuroblastoma. We anticipate that in vitro drug screening will be complementary to genomic-guided precision medicine by supporting clinical decision making, thereby improving prognosis for all neuroblastoma patients in the future.
Keywords: Neuroblastoma, Organoid, High-throughput drug screening, Precision medicine, Molecular biology, Next-generation sequencing, Molecular targeted therapy, Cancer, Child, Adolescent
Highlights
-
•
A living neuroblastoma patient-derived organoid (NBL-PDO) biobank was established.
-
•
Genomic and drug sensitivity data are available in R2, an online repository.
-
•
NBL-PDOs retain features of their parental tumor, demonstrating potential use as 3D-avatars.
-
•
Personalized drug sensitivities demonstrate the potential of functional drug testing.
-
•
Collaborative studies aim to correlate drug sensitivity with clinical response.
1. Introduction
Despite aggressive multimodal approaches, overall survival of children with high-risk neuroblastoma (HR-NBL) is limited to about 50 % of children, and effective treatments are lacking in refractory or relapsed disease [1], [2], [3], [4], [5], [6], [7], [8], [9], [10]. Tumors arise from sympathoadrenal progenitor cells and occur along the neural crest. Distinct cell states from mesenchymal to adrenal arise from epigenetic reprogramming [10], [11], [12]. Comprehensive molecular profiling studies have provided insights into the landscape of somatic alterations in newly diagnosed and relapsed neuroblastoma [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]. Spatial and temporal intratumor heterogeneity is reported, underpinning the importance of sequential and in-depth comprehensive molecular analysis to guide clinical decision making [24], [30], [31].
Over the last decade, several large-scale pediatric precision oncology programs have been initiated, investigating the potential of molecular-driven precision medicine [32], [33], [34], [35], [36], [37], [38], [39], [40]. In fact, clinical benefit of applied targeted therapies has been demonstrated for selected subgroups [34], [37], [40], [41]. However, allocation to matched treatments is limited to 12–33 % due to absence of targets in a subset of patients, in addition to lack of drug access, or the rapidly deteriorating clinical condition of the patient [32], [34], [40], [41], [42]. Furthermore, failure rate of molecularly matched treatments remains high and hard to predict. Drug development is hampered by the fact that childhood cancer remains a rare disease and thus depends on adult cancer indications and their potential market to drive innovation.[32], [43], [44].
To complement state-of-the art individualized genomic approaches, functional testing of drug sensitivities in patient-derived models is being explored [45], [46], [47], [48], [49]. Current methods include high-throughput drug testing of short-term as well as in vitro and in vivo expanded cultures [45], [49].Developing patient-derived xenograft (PDX) models requires time and resources. Recent advances in tissue engineering and cell culture techniques enabled the generation of a variety of methods to conduct in vitro drug testing including three-dimensional patient-derived organoids (3D-PDOs), organoids-on-a-chip, and micro-organospheres (MOS) [46], [47], [48], [50], [51]. PDOs recapitulate the phenotypic and genotypic characteristics of the native tumor, and hold promise to predict drug response in a personalized fashion [46], [47], [52], [53], [54], [55], [56], [57], [58]. However, established biobanks for pediatric cancer subtypes integrating molecular with drug sensitivity data are rare, and have thus far only been reported for renal tumors and rhabdomyosarcoma [59], [60].
In our study, we created a publicly available repository relating in vitro drug sensitivity to tumor genomics and transcriptomics of neuroblastoma cell lines and 3D patient-derived neuroblastoma organoid models (NBL-PDOs), accessible through the R2 Genomics Analysis and Visualization Platform (http://r2platform.com/pmc_nb_drugs/). In the first part, we present a reference dataset of in vitro drug sensitivity profiling (DSP) based on high-throughput screening (HTS) on a panel of nineteen commonly used neuroblastoma cell lines, integrated with molecular data from Whole Genome Sequencing (WGS) and bulk mRNA-sequencing (RNA-Seq), generated in parallel. In the second part, we describe the establishment of nine 3D NBL-PDOs, based on a novel culture protocol. These NBL-PDOs retain histologic and genomic features of the original tumor, as demonstrated by comprehensive molecular profiling. HTS of these NBL-PDOs, in addition to six previously established models [61], confirms known correlations between molecular targets and drug sensitivity, and reveals the opportunity to expand therapeutic options in a subset of children with neuroblastoma.
2. Methods
2.1. Cell lines: culturing and comprehensive molecular profiling
In this study we used a panel of nineteen neuroblastoma cell lines obtained from the Children’s Oncology Group (COG) Cell Culture and Xenograft Repository at Texas Tech University Health Sciences Center (www.COGcell.org), the American Type Culture Collection (Manassas, VA), or the Children’s Hospital of Philadelphia (CHOP) cell line bank, or via historic collaborations. All cell cultures were performed at the Princess Máxima Center as reported in Supplemental data Extended methods within a ML-1 certified laboratory. Cells were cultured in medium with supplementation, grown at 37°C and 5 % CO2. Cultures were tested for Mycoplasma infection every six weeks, and Short Tandem Repeat (STR) profiles were performed routinely for cell line verification. Total DNA and RNA were isolated and processed at the time of High-Throughput Screening. Whole Genome Sequencing (WGS) data were generated utilizing an in-house developed ‘No-control workflow’ pipeline at DKFZ, which was designed for samples without a germline reference (i.e., cell lines). RNA sequencing data was analyzed at the Princess Máxima Center as previously described [62].
2.2. Patient-derived neuroblastoma organoids: tumor sample acquisition, organoid culturing, and comprehensive molecular profiling
Freshly obtained neuroblastoma tissue was collected as part of either the iTHER study (n=7) [40], or the biobank initiative (n=2) of the Princess Máxima Center for Pediatric Oncology, Utrecht, Netherlands. Ethics approval was granted for both. All patients and/or their legal representatives signed informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
Tumor and germline control samples were processed as per standard of care. Only samples from primary diagnosis or relapse with a minimum of 50 % tumor content proceeded for culturing and characterization as per Supplemental data Extended methods. In addition, six previously generated patient-derived models were included [61]. Total DNA and RNA was isolated using the AllPrep DNA/RNA/Protein Mini Kit (Qiagen) according to standard protocol on the QiaCube (Qiagen). Comprehensive molecular profiling was performed and kindly provided by the INFORM program utilizing low-coverage Whole Genome Sequencing (lcWGS), Whole Exome Sequencing (WES), and mRNA sequencing (RNA-Seq) [33], [41], or according to the iTHER pipeline at the Máxima, applying WES and RNA-Seq [40], [62].
2.3. High-throughput drug screening
High-throughput drug screens were conducted in duplicate in collaboration with the high-throughput screening facility of the Princess Máxima Center, as reported in Supplemental data Extended methods. In summary, cells were seeded in a 384-well plate and treated with a ten-fold dilution series of ∼200 cytostatic and targeted drugs on a library plate (0.1 nM to 10 µM). Readouts were performed at T0 and T72 (cell lines) or T120 (NBL-PDOs), respectively. Viability was calculated per drug and concentration, in relation to DMSO-treated cells (100 % viability) and medium-only controls without cells (0 % viability). Quality control included assessment of cell growth and variability between duplicates. Curve fitting was done using the drc R package using a four-parameter log-logistic function [63]. Based on the fitted curves two different metrics were calculated. The Area Under the Curve (AUC), calculated by determining the definite integral of the dose-response curve, and the concentration of the drug needed to achieve a 50 % reduction in cell viability (IC50).
3. Results
3.1. Establishment of a repository of neuroblastoma cell lines with detailed genetic and pharmacological annotation
High-throughput drug screening was performed in duplicate for nineteen commonly used neuroblastoma cell lines (Fig. 1B). Supplementary Table 1 summarizes the details as included in this study.
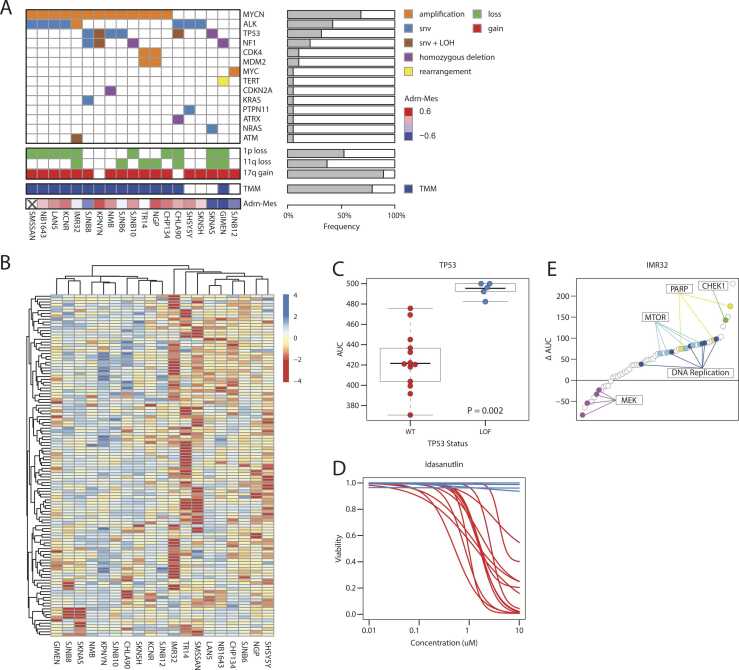
Fig. 1.
A repository of neuroblastoma cell lines with detailed genetic and pharmacological annotation. (a) Oncoplot showing the genetic aberrations, TMM and adrenergic-mesenchymal score found in the panel of 19 neuroblastoma cell lines as detected by WGS and RNA-Seq (snv = single nucleotide variant, LOH = Loss Of Heterozygosity). (b) Heatmap showing the compound response profiles (AUC values) for the panel of 19 neuroblastoma cell lines. Only compounds are shown measured for all 19 cell lines and with at least one AUC below 450. AUC values are standardized by row. (c) Boxplots showing the response of the cell lines to idasanutlin treatment separated by TP53 status (WT = Wild Type; LOF = Loss Of Function). P value was determined with the Wilcoxon rank-sum test. (d) Fitted curves for the idasanutlin response of the panel of cell lines. The red curves indicate a TP53 wild type status, the blue curves a TP53 loss-of-function. (e) Plot showing the difference between the mean AUC of the cell line panel (excluding IMR32) and IMR32 (ΔAUC). A positive ΔAUC indicates an above average sensitivity of IMR32, a negative value a higher resistance. A selection of common targets is highlighted. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In parallel, neuroblastoma cell lines were characterized by whole-genome and bulk RNA sequencing (Fig. 1A). High-throughput drug sensitivity profiling reflected distinct known pharmacologic vulnerabilities linked to specific genomic patterns. For instance, TP53-inactivated cell lines were drug-resistant to MDM2-inhibitor idasanutlin (Fig. 1C and D). Also, remarkable sensitivity to PARP- and CHEK1-inhibitors is demonstrated in the DNA-damage-response (DDR) defective cell line IMR-32, carrying MYCN-amplification, ATM p.V2716A SNV and loss of 11q (Fig. 1E). Moreover, we observed a distinct drug resistance phenotype (Fig. 1B & Supplementary Figure 1A) that was strongly correlated with high expression of the mesenchymal gene signature and interferon alpha and gamma response genes (Supplementary Figure 1B). Additionally, we identified genetic aberrations associated with distinct patterns of drug sensitivity, for example in MYCN-amplified versus MYCN-non-amplified cell lines (Supplementary Figure 1C) but also for other well-known genetic drivers of neuroblastoma (Supplementary Figure 1D). We also found that expression of specific gene sets is correlated with response to specific compounds (Supplementary Figure 1E). In some cases, these associations are shared by multiple gene sets (e.g. cobimetinib), but we also identified more specific associations, such as high expression of apical surface genes and resistance to temozolomide.
In conclusion, we generated a robust reference dataset of neuroblastoma cell lines with functional characterization of drug-sensitivity integrated with genomic and transcriptomic data, providing a valuable resource for the acceleration of neuroblastoma cancer research.
3.2. Establishment of a living neuroblastoma patient-derived organoid biobank
Next, we established a neuroblastoma organoid biobank by culturing nine novel 3D patient-derived neuroblastoma organoid models of tumors of eight males and one female, aged 5–134 months at diagnosis. Clinical and genomic characteristics of the patients and their native tumor are summarized in Supplementary Table 2. Eight children were diagnosed with INRG stage M neuroblastoma; one progressed from MS to stage M. Eight samples were obtained at relapse; one at primary diagnosis prior to initiating chemotherapy. One organoid was derived from bone marrow cells and harvested postmortem (no genomic data available from the patient’s sample). Segmental chromosomal aberrations (SCA) were observed in all but one tumor, including loss of 1p (87.5 %) and 11q (25.0 %) as well as gain of 17q (62.5 %) (Fig. 2A). MYCN-amplification was the most frequently occurring event, detected in six out of eight samples. Other aberrations include SNVs in ALK, TP53, NRAS, FGFR1, and SMARCA4; a frameshift mutation in NF1, as well as amplification of CCND1, reflecting the heterogeneous landscape of neuroblastoma tumors.
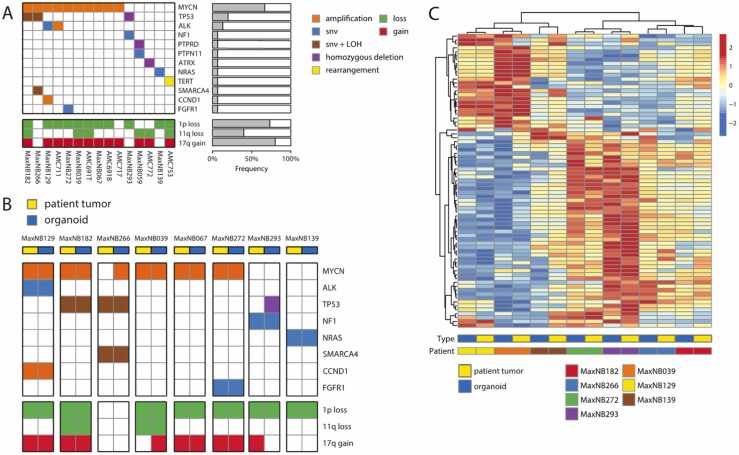
Fig. 2.
Patient-derived neuroblastoma organoids resemble their parental tumor. (a) Oncoplot showing the genetic aberrations found in the panel of nine novel as well as six previously established neuroblastoma organoids, as detected by lcWGS and/or WES (snv = single nucleotide variant, LOH = Loss Of Heterozygosity). (b) Oncoplot showing the genetic aberrations of eight novel neuroblastoma organoids and their parental tumors. Aberrations were based on lcWGS and/or WES. (c) Heatmap showing the clustered expression profiles of seven neuroblastoma organoids and their parental tumors (patient tumor data for MaxNB067 was not available). The 75 genes with the highest standard deviation were included. Distance measure is ‘correlation’. The colored bars below the heatmap indicate the type of sample (blue = organoid, yellow=patient tumor) and the patient identifier. Batch correction was applied to account for differences in platform and sample type (see Methods). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To establish NBL-PDOs, tumor tissue was minced and enzymatically digested, subsequently placed in optimized culture medium, and passaged, as described in Supplemental data Extended methods. After successful in vitro expansion, we characterized these novel organoids by genomic and transcriptomic analysis. Genomic similarity of NBL-PDOs to their tumor tissue counterparts was verified by comparing tumor driving events in the parental tumor with the matching organoid, utilizing WES and/or low coverage-WGS data (Fig. 2B). SCAs remained identical for all 1p and 11q loss events. Gain of 17q differed in 2 models: one NBL-PDO gained 17q (MaxNB039) while one PDO lacked the 17q gain which was present in the paternal tumor (MaxNB293). Other known tumor driving events were mostly re-capitulated in the NBL-PDO, including MYCN-amplification and TP53-loss. In two cases however, we detected a known driver aberration in the NBL-PDO that was not present in the parental tumor. Presumably, these organoids were established from a subclone not detected in the patient’s tumor. This illustrates the importance of longitudinal molecular analyses, especially since tumor treatment might select for more resistant subclones. Finally, seven organoid-tumor pairs with available RNA-Seq profiles, clustered together using unsupervised hierarchical clustering (Fig. 2C), demonstrating the potential of tumor-derived organoids as 3D-avatars on genomic as well as transcriptomic level.
A point that is underlined by the distinct subdivision into two main groups, which are characterised by differential regulation of, among others, genes of the adrenergic signature and E2F targets genes (Fig. 2D).
All established NBL-PDOs are available upon request.
3.3. High-throughput drug screening reflects established drug sensitivities and identifies additional therapeutic vulnerabilities in vitro
After successful long-term propagation, the nine novel NBL-PDOs and six models previously established by our group were exposed to the same drug library used for the neuroblastoma cell lines (Fig. 3A; Supplementary Table 3) [64]. Again, known correlations between molecular targets and drug sensitivities were confirmed, as demonstrated by sensitivity to idasanutlin treatment in TP53-wild type neuroblastoma models versus resistance in models without functional TP53 (Fig. 3B and C). We also observed a positive correlation of venetoclax sensitivity with expression of its direct target BCL2, supporting BCL2 as a robust biomarker, as reported previously (Fig. 3D) [65], [66]. Comparing BCL2 expression between patients and organoids shows a conservation of these expression levels, confirming the clinical relevance of these models (Fig. 3E). Strikingly, we also observed a strong positive correlation between venetoclax sensitivity and high expression of genes that are part of the neuroblastoma mesenchymal signature. This suggests venetoclax might be particularly effective against tumor cells with a mesenchymal phenotype (Fig. 3F and G), which are deemed to be more chemo-resistant in vitro [18], [67]. In addition to previously reported correlations, we also identified new associations of genetic driver events with compound sensitivities, but also with specific expression phenotypes of our NBL-PDO models (Supplementary Figure 2A & 2B).
Fig. 3.
Patient-derived neuroblastoma organoids resemble their parental tumor. (a) Heatmap showing the compound response profiles (AUC values) of the panel of 15 neuroblastoma organoids. Only compounds are shown measured for all 15 organoids, with at least one AUC below 450 and not flagged previously (see Methods). AUC values are standarised row-wise. (b) Boxplots showing the response of the organoids to idasanutlin treatment separated by TP53 status (WT = Wild Type; LOF = Loss Of Function). P value was determined with the Wilcoxon rank-sum test. (c) Fitted curves for the idasanutlin response of the panel of organoids. The red curves indicate a TP53 wild type status, the blue curves a TP53 loss-of-function. (d) Plot showing the correlation between BCL2 gene expression of the organoids and their sensitivity to the BCL2 inhibitor Venetoclax. AUC values on the x-axis decrease from left to right. (e) Plot showing the correlation of BCL2 gene expression in organoids and their matching patient tumor, where available (n = 7). (f) Top 5 results of the GSEA testing the association of RNA expression gene sets with the sensitivity to Venetoclax treatment (AUC). Values shown are the −10 log of the adjusted P values. Red indicates a positive correlation with venetoclax sensitivity, blue a negative correlation. (g) Heatmap showing the expression of genes included in the neuroblastoma mesenchymal and adrenergic signatures (van Groningen et al.). Only genes are included with a significant correlation (limma, adjusted P value < 0.05) with the venetoclax sensitivity. The organoids (columns) are ordered by venetoclax sensitivity. The bar on the left side of the heatmap indicates gene set membership (red = mesenchymal, blue = adrenergic). Gene expression values are standarised row-wise. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Exploring high-throughput drug screening in patient-derived neuroblastoma organoids in the precision medicine program “iTHER”
In addition, we prospectively explored the potential of high-throughput drug screening for personalized treatment of neuroblastoma patients within The Individualized Therapies for Children with cancer program 'iTHER', a clinically implemented precision medicine program at the Princess Máxima Center in the Netherlands [40]. We hypothesized that the additional value of HTS to molecular analyses only, could be threefold. Firstly, in vitro drug sensitivity can support therapeutic recommendations suggested by genomic and transcriptomic results, thereby strengthening the rationale for clinical uptake. Secondly, in vitro insensitivity to drugs suggested by molecular analyses may avoid ineffective and thus unnecessary treatments. Lastly, novel treatment options without known molecular biomarkers may be identified.
To investigate correlation between drug screening results and presumed clinically relevant and druggable genomic events, we analyzed three NBL-PDOs derived from tumors which were identified to harbor driving genomic events in ALK, NRAS, and NF1 of very high or high priority, based on a previously published seven-scale algorithm [33], [40]. Of these molecular targets known to confer drug sensitivity in neuroblastoma, clinically achievable IC50 values confirmed in vitro sensitivity (Fig. 4). Crizotinib sensitivity correlated with the ALK F1174L mutation in patient MaxNB129; sensitivity to MEK-inhibitor cobimetinib was demonstrated in patient MaxNB139 harboring NRAS Q61R mutation; in patient MaxNB272 harboring FGFR1 mutation in vitro sensitivity to ponatinib was confirmed; and MEK-inhibitor trametinib sensitivity in patient MaxNB293 correlated with loss-of-function of NF1 through R156X mutation and Loss of Heterozygosity (LOH). These findings might support clinical decision making for targeted treatment.
Fig. 4.
Neuroblastoma organoids representing clinically relevant molecular targets and their respective in vitro drug sensitivities. Every neuroblastoma model is depicted microscopically, as well as its circos plot (also available on (http://r2platform.com/pmc_nb_drugs/). Subsequent target matching selected drug sensitivities are demonstrated, as well as unexpected in vitro insensitivities. The dotted line represents maximum achievable plasma concentration.
Remarkedly, predicted drug sensitivity that could not be confirmed by drug screening includes lorlatinib in patient sample MaxNB129 harboring the ALK F1174L mutation [68]. Similarly, no clinically relevant IC50 values were achieved for MEK inhibitors trametinib and selumetinib in patient sample MaxNB139 harboring the NRAS Q61R mutation. This illustrates the importance of personalized approaches and suggests drug screening might potentially also be utilized to establish the best drug in case of multiple candidates hitting the same target, thereby further reducing the clinical use of ineffective therapies.
We also explored whether therapeutic options could be expanded beyond those identified by molecular profiling by analyzing drug sensitivity profiles in depth, unguided by prior molecular knowledge. That this is a potentially useful approach is confirmed by several patient samples with a striking sensitivity to ponatinib, a multi-tyrosine kinase inhibitor. Interestingly, the tumors exhibited overexpression of proto-oncogenes KIT or RET, an event of intermediate priority (score four out of seven based on the current prioritization algorithm). This illustrates the potential of utilizing drug sensitivity in addition to gene expression measured by RNA-Seq, which is currently not recognized as molecular biomarker within clinical trials to select patients for targeted treatments.
We then proceeded to examine the correlation between in vitro drug sensitivity and clinical response retrospectively. Of the patients included with a successful NBL-PDO, one was assigned to a molecularly matched treatment (MaxNB182). The patient received monotherapy with cabozantinib (40 mg/m2) to target an intermediate-priority event of RET overexpression, but treatment was discontinued after 8 weeks due to progressive disease. This lack of clinical efficacy was reflected by insufficient in vitro sensitivity of the MaxNB182 organoid to cabozantinib, with IC50 values above the clinically relevant range (Supplementary Figure 3). In contrast, the ponatinib response did suggest a potentially relevant clinical efficacy in vitro for the same organoid. Unfortunately, these results were not yet available at the time of clinical decision making.
In conclusion, drug screens can potentially support clinical decision making by identifying patient-specific drug sensitivities and reveal the opportunity to expand therapeutic options in a subset of children with neuroblastoma.
3.5. Data analysis and visualization
To increase the utility of the genomic characterization and drug sensitivity of cell lines and NBL-PDOs as a resource, data were transferred to the R2 Genomics Analysis and Visualization platform (http://r2platform.com/pmc_nb_drugs/), where a comprehensive suite of visualizations and analysis options was implemented (Supplementary Figure 4). Specific tiles provide access to high-throughput drug screening data and genomic profiles per sample, and in addition cohort-based analyses can be readily performed. This web-based application serves as tumor board platform for the iTHER study [40], and is extensively used by the pediatric oncology community to integrate genomic and clinical data as well as in vitro and in vivo model systems and drug sensitivity profiles, including the “Innovative Therapies for Children with Cancer Pediatric Preclinical Proof-of-concept Platform” (ITCC-P4; https://www.itccp4.eu/).
4. Discussion
In the past decade, tailored treatment of neuroblastoma patients has evolved based on clinical and biological prognostic factors. However, a significant unmet need remains for children with high-risk, relapsed, or refractory disease. Translating cancer genomic data into therapeutic prospects remains challenging, yet essential to support novel curative approaches.
In our study, we created a dynamic publicly available dataset comprised of classical neuroblastoma cell lines as well as novel patient-derived organoids with detailed genomic, transcriptomic, and pharmacological information. Our novel NBL-PDOs display overall genetic stability and retain the molecular characteristics of the parental tumor they were derived from, allowing the use of high-throughput screening approaches. Although immortalized cell lines in general are easy to propagate and manipulate at low cost, they frequently do not resemble the originating tumor due to long-term growth in artificial culture conditions, thus possessing only limited predictive value. By generating in depth genomic and transcriptomic data together with high-throughput drug sensitivity data, these challenges regarding genotype–drug sensitivity associations might be alleviated. Given the genomic complexity of neuroblastoma however the current dataset is too small to statistically prove association or correlate the drug sensitivity to certain genomic biomarkers and thus only allows to validate the already known associations We’re aiming to expand this repository prospectively to support clinically relevant development of promising therapeutic strategies for children with neuroblastoma.
The successful development of NBL-PDOs harbors unique opportunities to contribute to crucial advancements in neuroblastoma. Firstly, establishing a “living” biobank of preclinical models reflecting the genomic heterogeneity in neuroblastoma is essential to study the biology and support biomarker-driven preclinical studies, since many anti-cancer agents entering clinical trial fail to acquire regulatory approval due to insufficient safety or inefficacy [69], [70]. NBL-PDOs are generated faster and at a lower cost compared to patient-derived xenografts and can contribute to reduction of use of animal models in preclinical and translational research. Secondly, NBL-PDOs might reveal personalized drug sensitivities, thereby supporting clinical decision making. Although potentially druggable events are reported in a majority of pediatric patients, the application of molecularly matched treatments is still limited, and clinical efficacy remains hard to predict [34], [37], [40], [41], [71]. Recently, the Australian ZERO Precision Childhood Cancer Program as well as the German-led international INFORM program reported the first pediatric precision oncology studies integrating comprehensive molecular profiling with in vitro, in ZERO additionally combined with in vivo drug efficacy testing [45], [49]. Both programs observed differential drug sensitivity as predicted by molecular events, e.g., ALK or TP53 status. ZERO also demonstrated a strong correlation between in vitro drug sensitivities and PDX results, and the clinical responses in patients. Striking parallels between clinical course and in vitro sensitivities were also observed for selected cases in INFORM. In our dataset, high-throughput drug screening also confirms established drug sensitivities, but correlation to clinical efficacy is limited due to lack of data. Future prospective research might support individual therapeutic approaches, for instance selecting the optimal ALK inhibitor based on functional drug testing in patients with ALK activation. In addition, compounds ineffective in vitro might be avoided, and the proportion of patients with viable treatment options might be expanded.
However, several challenges remain regarding the establishment of PDOs, analysis and interpretation of in vitro data, and the implementation of in vitro drug testing to support clinical decision making. Firstly, culture success rate strongly depends on the quality and quantity of fresh tumor material, which is obviously limited due to minimally invasive procedures used in children. Of 46 eligible fresh samples, 31 samples across all tumor types were subjected to in vitro expansion within the ZERO pilot, and seven were successfully expanded to proceed to HTS. INFORM included 132 fresh samples, and 69 were included after quality control. Also, the time needed to establish long-term cultures (ZERO: 4.5 months) might hamper clinical implementation, as patient treatment cannot be delayed. Alternatively, upfront drug screening is piloted, as reported by INFORM, reducing median turnaround time to 7 days. Again, tumor tissue availability is the most important limiting factor: of the 46 fresh samples included, adequate cell numbers allowing upfront HTS were available in only three cases [45]. Furthermore, intratumoral heterogeneity as well as evolution of genetically distinct drug-resistant subclones is likely not represented in a single biopsy, indicating a need for resampling at relapse and an opportunity for liquid biopsies, in particular of the bone marrow niche in neuroblastoma [24], [72], [73].
Another challenge includes the lack of consensus on translating in vitro responses into drug hits and subsequent treatment advice. In our study, we employed single dosing of drugs in different concentrations and explored AUC, AUC z-scores and IC50 values, taking published achievable plasma levels into account [74]. Others utilized a z-score outlier approach (ZERO), or quantitative Drug Sensitivity Score (Institute for Molecular Medicine Finland) [75]. Importantly, monotherapy is not curative in hard-to-treat neuroblastoma, and drug sensitivity testing should aim to test combination strategies, including combining novel therapeutics [70]. Finally, future directions will need to include generating novel culture systems to study immunotherapeutics, for example in organ-on-chip short-term spheroid cultures with accompanying microfluidic culture [48].
5. Conclusion
In conclusion, we established a publicly available dataset with detailed genomic, transcriptomic, and pharmacological annotation of classical neuroblastoma cell lines as well as novel patient-derived neuroblastoma organoids representing the heterogeneous genomic and phenotypical landscape of neuroblastoma. All established models are available upon request, and reported data are accessible through the user-friendly R2 Genomics Analysis and Visualization platform (http://r2platform.com/pmc_nb_drugs/). We explored integrating genomic, transcriptomic, and in vitro drug sensitivity testing for neuroblastoma patients within a precision medicine program, demonstrating the potential to tailor therapeutic options for children with neuroblastoma. International collaborations are urgently needed to optimize and standardize in vitro drug sensitivity combination testing and evaluate clinical responses.
We anticipate that in vitro drug screening will be complementary to genomic-guided precision medicine by providing additional evidence for clinical decision making, improving prognosis for all neuroblastoma patients in the future.
Funding
We are grateful to all funders.
The iTHER study was supported by grants from ZonMw (project number 848101004) and the Dutch Foundation Children Cancer-free (KiKa Core funding). This work is part of the research program Vernieuwingsimpuls Vidi (Combining targeted compounds in neuroblastoma tumors; is two better than one?) with project number 91716482, which is partly financed by the Dutch Research Council (NWO). This project also received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program, grant agreement No 716079 Predict, as well as grant agreement No 826121 iPC. Jette Bakhuizen, Roland Kuiper and Marjolijn Jongmans were supported by a grant from the Dutch Foundation Children Cancer-free (KiKa, project number 355).
The INFORM program is financially supported by the German Cancer Research Center (DKFZ), the German Cancer Consortium (DKTK), the German Federal Ministry of Education and Research (BMBF), the German Federal Ministry of Health (BMG), the Ministry of Science, Research and the Arts of the State of Baden-Württemberg (MWK BW); the German Cancer Aid (DKH), the German Childhood Cancer Foundation (DKS), RTL Television, the aid organization BILD hilft e.V. (Ein Herz für Kinder) and the generous private donation of the Scheu family.
Part of the study was supported in the framework of ERA PerMed (ERAPERMED2018-121, COMPASS) as well as EraCoSysMed INFER-NB ID-Nr 29.
CRediT authorship contribution statement
Jaeger Natalie: Data curation. Ober Kimberley: Investigation, Formal analysis, Data curation. Witt Olaf: Data curation. Zwijnenburg Danny A.: Software. Van Eijkelenburg Natasha K.A.: Investigation. van der Hoek Jessica J.F.: Formal analysis. Dierselhuis Miranda: Investigation. Keller Kaylee K.M.: Investigation, Formal analysis. Tytgat Godelieve A.M.: Investigation. Vernooij Lindy: Investigation, Formal analysis. Wijnen Marc H.W.: Investigation. Schild Linda G.: Investigation, Formal analysis. van Noesel Max M.: Investigation. Looze Eleonora: Formal analysis, Data curation. de Krijger Ronald R.: Data curation. Ebus Marli E.: Methodology, Formal analysis, Conceptualization. Essing Anke H.W.: Methodology, Formal analysis. de Vree Paula: Project administration. Eising Selma: Methodology, Formal analysis, Data curation. Koster Jan: Supervision, Software, Methodology, Formal analysis, Data curation. Dolman M. Emmy M.: Writing – review & editing, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Tas Michelle L.: Investigation. Matser Yvette A.H.: Investigation. Molenaar Jan J.: Writing – review & editing, Supervision, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Wienke Judith: Supervision, Methodology, Formal analysis. Volckmann Richard: Software. Tops Bastiaan B.J.: Investigation. Kester Lennart A.: Investigation. Badloe Shashi: Data curation. Hehir-Kwa Jayne Y.: Data curation. Langenberg Karin P.S.: Writing – review & editing, Writing – original draft, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Kemmeren Patrick: Data curation. van Hooff Sander R.: Writing – review & editing, Software, Methodology, Data curation, Conceptualization. Goemans Bianca F.: Project administration, Methodology. Koopmans Bianca: Project administration, Methodology, Data curation. Strijker Josephine G.M.: Formal analysis, Data curation. Kholosy Waleed M.: Formal analysis, Data curation, Conceptualization. Zwaan C. Michel: Resources, Funding acquisition. Oehme Ina: Methodology, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are extremely grateful to all patients and their families who participated in this trial.
We thank the treating clinical teams, surgery and interventional radiology departments, the pathology department of UMC Utrecht (Petra van der Weide and Marja van Blokland), the laboratory for childhood cancer pathology of the Princess Máxima Center (especially Arie Maat, Eric Strengman and Marc van Tuil), as well as the biobanking initiative. We are grateful for the support of the Trial and Data center, particularly clinical trial managers Miriam Stumpf and Yvonne Ruchti, research nurses Jenny Smink, Margot Geerdink, and their colleagues, as well as central data manager Ria Koolma and local data manager Saskia Rasser. The Omics IT and Data Management Core Facility of DKFZ is acknowledged for their sincere contribution, in particular Lena Weiser for dedicated contribution in the bioinformatics processing and data transfers.
We are thankful to all clinicians and researchers who contributed to the valuable discussions at the Molecular Tumor Board.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ejca.2025.115275.
Contributor Information
Karin P.S. Langenberg, Email: k.p.s.langenberg@prinsesmaximacentrum.nl.
Sander R. van Hooff, Email: s.r.vanhooff-3@prinsesmaximacentrum.nl.
Bianca Koopmans, Email: B.Koopmans@prinsesmaximacentrum.nl.
Josephine G.M. Strijker, Email: J.G.M.Strijker@prinsesmaximacentrum.nl.
Waleed M. Kholosy, Email: w.kholosy@amsterdamumc.nl.
Kimberley Ober, Email: k.ober@erasmusmc.nl.
Danny A. Zwijnenburg, Email: dannyzwijnenburg@amsterdamumc.nl.
Kaylee M. Keller, Email: k.keller@nki.nl.
Linda G. Schild, Email: g.g.schild@prinsesmaximacentrum.nl.
Eleonora J. Looze, Email: E.J.Looze@prinsesmaximacentrum.nl.
Yvette A.H. Matser, Email: Y.A.H.Matser@prinsesmaximacentrum.nl.
Judith Wienke, Email: J.Wienke-4@prinsesmaximacentrum.nl.
Richard Volckmann, Email: r.volckmann@amsterdamumc.nl.
Bastiaan B.J. Tops, Email: b.b.j.tops@prinsesmaximacentrum.nl.
Lennart A. Kester, Email: L.A.Kester@prinsesmaximacentrum.nl.
Shashi Badloe, Email: S.Badloe@prinsesmaximacentrum.nl.
Jayne Y. Hehir-Kwa, Email: J.Y.HehirKwa@prinsesmaximacentrum.nl.
Patrick Kemmeren, Email: p.kemmeren@prinsesmaximacentrum.nl.
Bianca F. Goemans, Email: b.f.goemans@prinsesmaximacentrum.nl.
C. Michel Zwaan, Email: c.m.zwaan@prinsesmaximacentrum.nl.
Ina Oehme, Email: i.oehme@kitz-heidelberg.de.
Nathalie Jäger, Email: n.jaeger@kitz-heidelberg.de.
Olaf Witt, Email: o.witt@kitz-heidelberg.de.
Natasha K.A. van Eijkelenburg, Email: n.k.a.vaneijkelenburg@prinsesmaximacentrum.nl.
Miranda P. Dierselhuis, Email: m.p.dierselhuis@prinsesmaximacentrum.nl.
Godelieve A.M. Tytgat, Email: G.A.M.Tytgat@prinsesmaximacentrum.nl.
Marc H.W. Wijnen, Email: m.h.w.wijnen-5@prinsesmaximacentrum.nl.
Max M. van Noesel, Email: m.m.vannoesel@prinsesmaximacentrum.nl.
Ronald R. de Krijger, Email: r.r.dekrijger-2@prinsesmaximacentrum.nl, jessicavanderhoek@live.nl.
Selma Eising, Email: selma.eising@xilis.nl.
Jan Koster, Email: jankoster@amsterdamumc.nl.
Emmy M. Dolman, Email: edolman@ccia.org.au.
Jan J. Molenaar, Email: j.j.molenaar@prinsesmaximacentrum.nl.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Data availability statement
Data generated by this study are available from the European Genome Archive (EGAS00001007160) and the European Nucleotide Archive (PRJEB54725 & PRJEB55331).
References
- 1.Moreno L., Rubie H., Varo A., Le Deley M.C., Amoroso L., Chevance A., et al. Outcome of children with relapsed or refractory neuroblastoma: a meta-analysis of ITCC/SIOPEN European phase II clinical trials. Pediatr Blood Cancer. 2017;64:25–31. doi: 10.1002/pbc.26192. [DOI] [PubMed] [Google Scholar]
- 2.London W.B., Bagatell R., Weigel B.J., Fox E., Guo D., Van Ryn C., et al. Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children's Oncology Group early-phase trials. Cancer. 2017;123:4914–4923. doi: 10.1002/cncr.30934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Niksic M., et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Galindo C., Friedrich P., Alcasabas P., Antillon F., Banavali S., Castillo L., et al. Toward the cure of all children with cancer through collaborative efforts: pediatric oncology as a global challenge. J Clin Oncol. 2015;33:3065–3073. doi: 10.1200/JCO.2014.60.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basta N.O., Halliday G.C., Makin G., Birch J., Feltbower R., Bown N., et al. Factors associated with recurrence and survival length following relapse in patients with neuroblastoma. Br J Cancer. 2016;115:1048–1057. doi: 10.1038/bjc.2016.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mody R., Yu A.L., Naranjo A., Zhang F.F., London W.B., Shulkin B.L., et al. Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: a report from the children's oncology group. J Clin Oncol. 2020;38:2160–2169. doi: 10.1200/JCO.20.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steliarova-Foucher E., Colombet M., Ries L.A.G., Moreno F., Dolya A., Bray F., et al. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol. 2017;18:719–731. doi: 10.1016/S1470-2045(17)30186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham R.M., Walton M.A., Carter P.M. The major causes of death in children and adolescents in the United States. N Engl J Med. 2018;379:2468–2475. doi: 10.1056/NEJMsr1804754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu B., Matthay K.K. Advancing therapy for neuroblastoma. Nat Rev Clin Oncol. 2022 doi: 10.1038/s41571-022-00643-z. [DOI] [PubMed] [Google Scholar]
- 10.Matthay K.K., Maris J.M., Schleiermacher G., Nakagawara A., Mackall C.L., Diller L., et al. Neuroblastoma. Nat Rev Dis Prim. 2016;2 doi: 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- 11.Kildisiute G., Kholosy W.M., Young M.D., Roberts K., Elmentaite R., van Hooff S.R., et al. Tumor to normal single-cell mRNA comparisons reveal a pan-neuroblastoma cancer cell. Sci Adv. 2021;7 doi: 10.1126/sciadv.abd3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong R., Yang R., Zhan Y., Lai H.-D., Ye C.-J., Yao X.-Y., et al. Single-cell characterization of malignant phenotypes and developmental trajectories of adrenal neuroblastoma. Cancer Cell. 2020;38:716–733. doi: 10.1016/j.ccell.2020.08.014. e6. [DOI] [PubMed] [Google Scholar]
- 13.Brady S.W., Liu Y., Ma X., Gout A.M., Hagiwara K., Zhou X., et al. Pan-neuroblastoma analysis reveals age- and signature-associated driver alterations. Nat Commun. 2020;11:5183. doi: 10.1038/s41467-020-18987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellini A., Pötschger U., Bernard V., Lapouble E., Baulande S., Ambros P.F., et al. Frequency and prognostic impact of ALK amplifications and mutations in the european neuroblastoma study group (SIOPEN) high-risk neuroblastoma trial (HR-NBL1) J Clin Oncol. 2021;39:3377–3390. doi: 10.1200/JCO.21.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgenstern D.A., Bagatell R., Cohn S.L., Hogarty M.D., Maris J.M., Moreno L., et al. The challenge of defining "ultra-high-risk" neuroblastoma. Pedia Blood Cancer. 2019;66 doi: 10.1002/pbc.27556. [DOI] [PubMed] [Google Scholar]
- 16.George S.L., Lorenzi F., King D., Hartlieb S., Campbell J., Pemberton H., et al. Therapeutic vulnerabilities in the DNA damage response for the treatment of ATRX mutant neuroblastoma. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gröbner S.N., Worst B.C., Weischenfeldt J., Buchhalter I., Kleinheinz K., Rudneva V.A., et al. The landscape of genomic alterations across childhood cancers. Nature. 2018;555:321–327. doi: 10.1038/nature25480. [DOI] [PubMed] [Google Scholar]
- 18.van Groningen T., Koster J., Valentijn L.J., Zwijnenburg D.A., Akogul N., Hasselt N.E., et al. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat Genet. 2017;49:1261–1266. doi: 10.1038/ng.3899. [DOI] [PubMed] [Google Scholar]
- 19.Eleveld T.F., Oldridge D.A., Bernard V., Koster J., Colmet Daage L., Diskin S.J., et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet. 2015;47:864–871. doi: 10.1038/ng.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valentijn L.J., Koster J., Zwijnenburg D.A., Hasselt N.E., van Sluis P., Volckmann R., et al. TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. Nat Genet. 2015;47:1411–1414. doi: 10.1038/ng.3438. [DOI] [PubMed] [Google Scholar]
- 21.Molenaar J.J., Koster J., Zwijnenburg D.A., van Sluis P., Valentijn L.J., van der Ploeg I., et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483:589–593. doi: 10.1038/nature10910. [DOI] [PubMed] [Google Scholar]
- 22.Schramm A., Köster J., Assenov Y., Althoff K., Peifer M., Mahlow E., et al. Mutational dynamics between primary and relapse neuroblastomas. Nat Genet. 2015;47:872–877. doi: 10.1038/ng.3349. [DOI] [PubMed] [Google Scholar]
- 23.Ackermann S., Cartolano M., Hero B., Welte A., Kahlert Y., Roderwieser A., et al. A mechanistic classification of clinical phenotypes in neuroblastoma. Science. 2018;362:1165–1170. doi: 10.1126/science.aat6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmelz K., Toedling J., Huska M., Cwikla M.C., Kruetzfeldt L.M., Proba J., et al. Spatial and temporal intratumour heterogeneity has potential consequences for single biopsy-based neuroblastoma treatment decisions. Nat Commun. 2021;12:6804. doi: 10.1038/s41467-021-26870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin M.S., Naranjo A., Zhang F.F., Cohn S.L., London W.B., Gastier-Foster J.M., et al. Revised neuroblastoma risk classification system: a report from the children's oncology group. J Clin Oncol. 2021;39:3229–3241. doi: 10.1200/JCO.21.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harenza J.L., Diamond M.A., Adams R.N., Song M.M., Davidson H.L., Hart L.S., et al. Transcriptomic profiling of 39 commonly-used neuroblastoma cell lines. Sci Data. 2017;4 doi: 10.1038/sdata.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiele C.J. Kluwer Academic Publishers; Lancaster, UK: 1998. Neuroblastoma cell lines. [Google Scholar]
- 28.Upton K., Modi A., Patel K., Kendsersky N.M., Conkrite K.L., Sussman R.T., et al. Epigenomic profiling of neuroblastoma cell lines. Sci Data. 2020;7:116. doi: 10.1038/s41597-020-0458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campos Cogo S., Gradowski Farias da Costa do Nascimento T., de Almeida Brehm Pinhatti F., de França Junior N., Santos Rodrigues B., Cavalli L.R., et al. An overview of neuroblastoma cell lineage phenotypes and in vitro models. Exp Biol Med. 2020;245:1637–1647. doi: 10.1177/1535370220949237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janoueix-Lerosey I., Schleiermacher G., Michels E., Mosseri V., Ribeiro A., Lequin D., et al. Overall genomic pattern is a predictor of outcome in neuroblastoma. J Clin Oncol. 2009;27:1026–1033. doi: 10.1200/JCO.2008.16.0630. [DOI] [PubMed] [Google Scholar]
- 31.Defferrari R., Mazzocco K., Ambros I.M., Ambros P.F., Bedwell C., Beiske K., et al. Influence of segmental chromosome abnormalities on survival in children over the age of 12 months with unresectable localised peripheral neuroblastic tumours without MYCN amplification. Br J Cancer. 2015;112:290–295. doi: 10.1038/bjc.2014.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langenberg K.P.S., Looze E.J., Molenaar J.J. The landscape of pediatric precision oncology: program design, actionable alterations, and clinical trial development. Cancers. 2021;13:4324. doi: 10.3390/cancers13174324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Worst B.C., van Tilburg C.M., Balasubramanian G.P., Fiesel P., Witt R., Freitag A., et al. Next-generation personalised medicine for high-risk paediatric cancer patients - The INFORM pilot study. Eur J Cancer. 2016;65:91–101. doi: 10.1016/j.ejca.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Berlanga P., Pierron G., Lacroix L., Chicard M., Adam de Beaumais T., Marchais A., et al. The European MAPPYACTS trial: precision medicine program in pediatric and adolescent patients with recurrent malignancies. Cancer Discov. 2022;12:1266–1281. doi: 10.1158/2159-8290.CD-21-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George S.L., Izquierdo E., Campbell J., Koutroumanidou E., Proszek P., Jamal S., et al. A tailored molecular profiling programme for children with cancer to identify clinically actionable genetic alterations. Eur J Cancer. 2019;121:224–235. doi: 10.1016/j.ejca.2019.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen C.E., Laetsch T.W., Mody R., Irwin M.S., Lim M.S., Adamson P.C., et al. Target and agent prioritization for the Children's Oncology Group-National Cancer Institute Pediatric MATCH Trial. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djw274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong M., Mayoh C., Lau L.M.S., Khuong-Quang D.A., Pinese M., Kumar A., et al. Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat Med. 2020;26:1742–1753. doi: 10.1038/s41591-020-1072-4. [DOI] [PubMed] [Google Scholar]
- 38.Parsons D.W., Janeway K.A., Patton D.R., Winter C.L., Coffey B., Williams P.M., et al. Actionable tumor alterations and treatment protocol enrollment of pediatric and young adult patients with refractory cancers in the National Cancer Institute–Children's Oncology Group Pediatric MATCH Trial. J Clin Oncol. 0:JCO.21.02838. [DOI] [PMC free article] [PubMed]
- 39.Church A.J., Corson L.B., Kao P.C., Imamovic-Tuco A., Reidy D., Doan D., et al. Molecular profiling identifies targeted therapy opportunities in pediatric solid cancer. Nat Med. 2022;28:1581–1589. doi: 10.1038/s41591-022-01856-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langenberg K.P.S., Meister M.T., Bakhuizen J.J., Boer J.M., van Eijkelenburg N.K.A., Hulleman E., et al. Implementation of paediatric precision oncology into clinical practice: the individualized therapies for children with cancer program 'iTHER. Eur J Cancer. 2022;175:311–325. doi: 10.1016/j.ejca.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Tilburg C.M., Pfaff E., Pajtler K.W., Langenberg K.P.S., Fiesel P., Jones B.C., et al. The pediatric precision oncology INFORM registry: clinical outcome and benefit for patients with very high-evidence targets. Cancer Discov. 2021 doi: 10.1158/2159-8290.CD-21-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong R.L.Y., Wong M.R.E., Kuick C.H., Saffari S.E., Wong M.K., Tan S.H., et al. Integrated Genomic Profiling and Drug Screening of Patient-Derived Cultures Identifies Individualized Copy Number-Dependent Susceptibilities Involving PI3K Pathway and 17q Genes in Neuroblastoma. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.709525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Rojas T., Kearns P., Blanc P., Skolnik J., Fox E., Knox L., et al. Changing incentives to accelerate drug development for paediatric cancer. Cancer Med. n/a. [DOI] [PMC free article] [PubMed]
- 44.Moreno L., Barone G., DuBois S.G., Molenaar J., Fischer M., Schulte J., et al. Accelerating drug development for neuroblastoma: summary of the second neuroblastoma drug development strategy forum from innovative therapies for children with cancer and International Society of Paediatric Oncology Europe Neuroblastoma. Eur J Cancer. 2020;136:52–68. doi: 10.1016/j.ejca.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Lau L.M.S., Mayoh C., Xie J., Barahona P., MacKenzie K.L., Wong M., et al. In vitro and in vivo drug screens of tumor cells identify novel therapies for high-risk child cancer. EMBO Mol Med. 2022;14 doi: 10.15252/emmm.202114608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tucker E.R., George S., Angelini P., Bruna A., Chesler L. The promise of patient-derived preclinical models to accelerate the implementation of personalised medicine for children with neuroblastoma. J Pers Med. 2021;11 doi: 10.3390/jpm11040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veninga V., Voest E.E. Tumor organoids: opportunities and challenges to guide precision medicine. Cancer Cell. 2021;39:1190–1201. doi: 10.1016/j.ccell.2021.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Barroso M., Chheda M.G., Clevers H., Elez E., Kaochar S., Kopetz S.E., et al. A path to translation: how 3D patient tumor avatars enable next generation precision oncology. Cancer Cell. 2022 doi: 10.1016/j.ccell.2022.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peterziel H., Jamaladdin N., ElHarouni D., Gerloff X.F., Herter S., Fiesel P., et al. Drug sensitivity profiling of 3D tumor tissue cultures in the pediatric precision oncology program INFORM. NPJ Precis Oncol. 2022;6:94. doi: 10.1038/s41698-022-00335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding S., Hsu C., Wang Z., Natesh N.R., Millen R., Negrete M., et al. Patient-derived micro-organospheres enable clinical precision oncology. Cell Stem Cell. 2022;29:905–917. doi: 10.1016/j.stem.2022.04.006. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park S.E., Georgescu A., Huh D. Organoids-on-a-chip. Science. 2019;364:960–965. doi: 10.1126/science.aaw7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bleijs M., van de Wetering M., Clevers H., Drost J. Xenograft and organoid model systems in cancer research. EMBO J. 2019;38 doi: 10.15252/embj.2019101654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 54.Drost J., Clevers H. Translational applications of adult stem cell-derived organoids. Development. 2017;144:968–975. doi: 10.1242/dev.140566. [DOI] [PubMed] [Google Scholar]
- 55.Ponsioen B., Post J.B., Buissant des Amorie J.R., Laskaris D., van Ineveld R.L., Kersten S., et al. Quantifying single-cell ERK dynamics in colorectal cancer organoids reveals EGFR as an amplifier of oncogenic MAPK pathway signalling. Nat Cell Biol. 2021;23:377–390. doi: 10.1038/s41556-021-00654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vlachogiannis G., Hedayat S., Vatsiou A., Jamin Y., Fernández-Mateos J., Khan K., et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359:920–926. doi: 10.1126/science.aao2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van de Wetering M., Francies H.E., Francis J.M., Bounova G., Iorio F., Pronk A., et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bleijs M., Pleijte C., Engels S., Ringnalda F., Meyer-Wentrup F., van de Wetering M., et al. EWSR1-WT1 target genes and therapeutic options identified in a novel DSRCT in vitro model. Cancers. 2021;13 doi: 10.3390/cancers13236072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meister M.T., Groot Koerkamp M.J.A., de Souza T., Breunis W.B., Frazer-Mendelewska E., Brok M., et al. Mesenchymal tumor organoid models recapitulate rhabdomyosarcoma subtypes. bioRxiv. 2022;2022 doi: 10.15252/emmm.202216001. 01.03.474504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calandrini C., Schutgens F., Oka R., Margaritis T., Candelli T., Mathijsen L., et al. An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity. Nat Commun. 2020;11:1310. doi: 10.1038/s41467-020-15155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bate-Eya L.T., Ebus M.E., Koster J., den Hartog I.J.M., Zwijnenburg D.A., Schild L., et al. Newly-derived neuroblastoma cell lines propagated in serum-free media recapitulate the genotype and phenotype of primary neuroblastoma tumours. Eur J Cancer. 2014;50:628–637. doi: 10.1016/j.ejca.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 62.Hehir-Kwa J.Y., Koudijs M.J., Verwiel E.T.P., Kester L.A., van Tuil M., Strengman E., et al. Improved gene fusion detection in childhood cancer diagnostics using RNA sequencing. JCO Precis Oncol. 2022;6 doi: 10.1200/PO.20.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ritz C., Baty F., Streibig J.C., Gerhard D. Dose-response analysis using R. PLoS One. 2015;10 doi: 10.1371/journal.pone.0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bate-Eya L.T., Ebus M.E., Koster J., den Hartog I.J., Zwijnenburg D.A., Schild L., et al. Newly-derived neuroblastoma cell lines propagated in serum-free media recapitulate the genotype and phenotype of primary neuroblastoma tumours. Eur J Cancer. 2014;50:628–637. doi: 10.1016/j.ejca.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 65.Place A.E., Goldsmith K., Bourquin J.P., Loh M.L., Gore L., Morgenstern D.A., et al. Accelerating drug development in pediatric cancer: a novel Phase I study design of venetoclax in relapsed/refractory malignancies. Future Oncol. 2018;14:2115–2129. doi: 10.2217/fon-2018-0121. [DOI] [PubMed] [Google Scholar]
- 66.Vernooij L., Bate-Eya L.T., Alles L.K., Lee J.Y., Koopmans B., Jonus H.C., et al. High-throughput screening identifies idasanutlin as a resensitizing drug for venetoclax-resistant neuroblastoma cells. Mol Cancer Ther. 2021;20:1161–1172. doi: 10.1158/1535-7163.MCT-20-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Wezel E.M., van Zogchel L.M.J., van Wijk J., Timmerman I., Vo N.K., Zappeij-Kannegieter L., et al. Mesenchymal neuroblastoma cells are undetected by current mRNA marker panels: the development of a specific neuroblastoma mesenchymal minimal residual disease panel. JCO Precis Oncol. 2019;3 doi: 10.1200/PO.18.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Infarinato N.R., Park J.H., Krytska K., Ryles H.T., Sano R., Szigety K.M., et al. The ALK/ROS1 inhibitor PF-06463922 overcomes primary resistance to crizotinib in ALK-driven neuroblastoma. Cancer Discov. 2016;6:96–107. doi: 10.1158/2159-8290.CD-15-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong C.H., Siah K.W., Lo A.W. Estimation of clinical trial success rates and related parameters. Biostatistics. 2018;20:273–286. doi: 10.1093/biostatistics/kxx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nader J.H., Bourgeois F., Bagatell R., Moreno L., Pearson A.D.J., DuBois S.G. Systematic review of clinical drug development activities for neuroblastoma from 2011 to 2020. Pedia Blood Cancer. 2022 doi: 10.1002/pbc.30106. [DOI] [PubMed] [Google Scholar]
- 71.Parsons D.W., Janeway K.A., Patton D.R., Lee J., Coffey B., Williams P.M., et al. Factors impacting enrollment on NCI-COG Pediatric MATCH trial treatment protocols. J Clin Oncol. 2021;39:10007. [Google Scholar]
- 72.Bosse K.R., Giudice A.M., Lane M.V., McIntyre B., Schurch P.M., Pascual-Pasto G., et al. Serial profiling of circulating tumor DNA identifies dynamic evolution of clinically actionable genomic alterations in high-risk neuroblastoma. Cancer Discov. 2022 doi: 10.1158/2159-8290.CD-22-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stankunaite R., George S.L., Gallagher L., Jamal S., Shaikh R., Yuan L., et al. Circulating tumour DNA sequencing to determine therapeutic response and identify tumour heterogeneity in patients with paediatric solid tumours. Eur J Cancer. 2022;162:209–220. doi: 10.1016/j.ejca.2021.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liston D.R., Davis M. Clinically relevant concentrations of anticancer drugs: a guide for nonclinical studies. Clin Cancer Res. 2017;23:3489–3498. doi: 10.1158/1078-0432.CCR-16-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yadav B., Pemovska T., Szwajda A., Kulesskiy E., Kontro M., Karjalainen R., et al. Quantitative scoring of differential drug sensitivity for individually optimized anticancer therapies. Sci Rep. 2014;4:5193. doi: 10.1038/srep05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Data Availability Statement
Data generated by this study are available from the European Genome Archive (EGAS00001007160) and the European Nucleotide Archive (PRJEB54725 & PRJEB55331).