Abstract
Aims
This study aimed to identify key factors with the greatest influence on glycaemic outcomes in young individuals with type 1 diabetes (T1D) and very elevated glycaemia after 3 months of automated insulin delivery (AID).
Materials and Methods
Data were combined and analysed from two separate and previously published studies with similar inclusion criteria assessing AID (MiniMed 780G) efficacy among young individuals naïve to AID (aged 7–25 years) with glycated haemoglobin A1c (HbA1c) ≥69 mmol/mol (≥8.5%). Univariate and multivariate linear models were performed to explore factors leading to the greatest improvements in HbA1c and time in range 3.9–10.0 mmol/L (70–180 mg/dL; TIR).
Results
A total of 99 young individuals (aged 17.3 ± 4.2 years; baseline HbA1c 92 ± 21 mmol/mol [10.6% ± 1.9%]) were included. After 3 months of AID use, HbA1c improved to 65 ± 16 mmol/mol (8.1% ± 1.5%) (−27 ± 23 mmol/mol; −2.5% ± 2.1% change), and TIR improved from 24.2% ± 13.5% to 58.4% ± 15.4% (p both <0.001). In the multivariate analysis, two key factors for both HbA1c and TIR improvement were identified: high baseline HbA1c (>100 mmol/mol [>11.0%]) and high time in automation mode (>80%), which led to decreased HbA1c by 27.0 mmol/mol (2.4%) and 14.2 mmol/mol (1.3%) and increased TIR by 6.1% and 11.1% (p all <0.05) respectively. Meal announcement frequency >3 times/day and glucose target of 5.5 mmol/L (100 mg/dL) also led to significant increases in TIR. No other factors, including age, prior use of multiple daily injection, ethnicity, gender and optimal active insulin time 2 h, contributed to statistically significant HbA1c or TIR improvement.
Conclusions
In young individuals naive to AID, those with the highest baseline HbA1c and high percentage time in automation experience the greatest benefits after initiation of AID. Sociodemographic background and carbohydrate counting adherence/knowledge should not prevent or delay access to AID technology (ACTRN12621000556842 and ACTRN12622001454763).
Keywords: automated insulin delivery, children and adolescents, type 1 diabetes mellitus
1. INTRODUCTION
Maintaining glucose levels in target for individuals with type 1 diabetes (T1D) is difficult particularly for children and young adults who face considerable challenges with disease and management burden. 1 , 2 Consequently, less than a fifth of these young individuals achieve glycaemic targets known to significantly reduce the risk of developing acute and chronic diabetes complications. This highlights an unmet need that requires improved methods to achieve these essential health outcomes. 3 , 4
Automated insulin delivery (AID), utilising a control algorithm (integrated with continuous glucose monitoring and insulin pumps) that automatically adjusts insulin delivery based on real‐time glucose levels and thus minimising burdens around insulin calculation and administration, has been demonstrated to improve glycaemia and quality of life among individuals with T1D. 5 Unfortunately, young individuals with markedly elevated glycaemia have largely been excluded from past pivotal clinical trials. 6 , 7 , 8 , 9 , 10 Recently, in both a single‐arm study and a 13‐week randomised controlled trial involving ethnically diverse children and young adults with T1D and very elevated glycaemia, we have demonstrated significant and sustained improvements in glycaemic outcomes following AID use, with no increase in safety concerns. 11 , 12 , 13 This evidence highlights that attention is needed to shift from whether AID systems improve glycaemia in these young individuals to how individual users, caregivers or healthcare professionals can improve glycaemic outcomes further to maximise effectiveness.
To date, no analysis of factors predictive of glycaemic improvements among children and young adults with markedly elevated glycaemia using AID has been performed. Therefore, the aims of this study were to determine the impact of various socio‐demographics, system characteristics and meal announcement factors on glycaemia for young individuals with markedly elevated glycaemia using the MiniMed™ 780G system.
2. METHODS
This study analysed data pooled from two separate and published studies conducted by our team assessing the efficacy and safety of the MiniMed™ 780G system in children and young adults with markedly elevated HbA1c of ≥69 mmol/mol (≥8.5%). These included (1) a prospective, single‐arm, dual‐centre study comprised of a 3‐month period of AID use 12 ; (2) the CO‐PILOT trial, which comprised a prospective, multicentre, parallel‐group, open‐label randomised controlled superiority trial comparing AID with usual standard care for 3 months. Additionally, data were included from the 3‐month extension phase of the CO‐PILOT trial, where control participants crossed over to use AID. 11 In both trials, the 3‐month AID use was preceded by a 2‐week baseline data collection period. Protocols and primary outcomes have been reported previously. 11 , 12 , 14 Both trials were approved by the Health and Disability Ethics Committees (Wellington, New Zealand; 21/STH/33 and 2022 FULL 13508) and registered in the Australian New Zealand Clinical Trials Registry (ACTRN12621000556842 and ACTRN12622001454763). Written informed assent/consent was provided by participants and by parents/guardians as appropriate.
3. PARTICIPANTS
Key eligibility criteria in both trials were T1D as per American Diabetes Association classification 15 for ≥1 year, HbA1c of greater than or equal to 69 mmol/mol (8.5%) and no previous use of AID technology. In the single‐arm study, only young individuals aged 13–25 years (inclusive) on multiple daily injection (MDI) were included. In the CO‐PILOT trial, criteria had expanded to participants aged 7–25 years (inclusive), on MDI or continuous subcutaneous insulin infusion (CSII).
4. STUDY PROCEDURES
Baseline data collection of demographics, HbA1c and 2 weeks of baseline glycaemia using blinded continuous glucose monitoring (CGM; Guardian™ 3 and Guardian™ 4 platform, Medtronic, San Fransisco, California) occurred in both trials. Demographics included age, gender, ethnicity and address used to assess socioeconomic deprivation using the geographical area–based New Zealand Index of Deprivation 2018. 16 Weight and height were measured using calibrated instruments. For each participant, HbA1c was measured by the same method at all time points, either a calibrated point‐of‐care device (either DCA Vantage Analyser, Siemens Healthcare Diagnostics, or Cobas B 101, Roche Diagnostics) or performed by a formal diagnostic laboratory if baseline values were above 130 mmol/mol (14%).
The AID system investigated in both trials was the MiniMed 780G with the Guardian™ 3 (single‐arm) and Guardian™ 4 CGM system (CO‐PILOT). All participants were trained in the use of this system and transitioned from their previous insulin modality to automation mode following the same 72‐h rapid onboarding protocol. 12 Device settings were customised, and SmartGuard™ (automation mode) was activated with the following settings: target set at 100 mg/dL (5.5 mmol/L) (allowing for 110 mg/dL [6.1 mmol/L] or 120 mg/dL [6.7 mmol/L] at investigator's discretion); automated correction on; active insulin time of 2.5 h which was adjusted to 2.0 h within 1 week of SmartGuard use if no safety concerns.
Meal announcements were encouraged in both trials. All system and CGM data were automatically uploaded to CareLink™ Clinical Therapy Management Software. Schedules for remote participant contacts in both trials were similar: daily during week 1 of AID use, then weekly for 4–6 weeks, and then once per month.
5. STATISTICAL ANALYSIS
Glycaemic data were analysed according to standardised CGM metrics. 17 Factors that were evaluated for association with glycaemia from baseline to after 3 months of AID use included (1) baseline demographics (age, gender, ethnicity, deprivation index, prior insulin therapy, glucose monitoring method and study origin/arm [control/intervention]); (2) system use characteristics in the final 2 weeks of AID use (time in automation mode [defined by percentage of time that SmartGuard was active]; time of sensor wear), most frequently used active insulin time and glucose target throughout 3 months; (3) metrics of meal announcements (number of announced meals and announced grams of carbohydrates).
General linear models were used to estimate between‐group differences in HbA1c and TIR change with 95% confidence intervals (CI) by adjusting univariate estimates alone and then in a multivariate model including factors with unadjusted p‐values <0.2 and variables of interest. Where between factor correlation >0.6 occurred, only one of the highly correlated factors was included based on clinical priority to avoid multicollinearity. p‐values from hypothesis tests were adjusted using the Bonferroni post hoc test, and 95% CI from regression models were adjusted. Backward selection was then used to achieve a parsimonious model containing only significant factors (p <0.05). Analysis was conducted using the SPSS 26.0 and R statistical Software (v4.2.1; R Core Team 2021). All p‐values are two‐sided. An alpha of 0.05 was considered statistically significant.
6. RESULTS
As shown in Table 1, a total of 99 participants were included in this analysis (one of the original 100 participants was excluded due to withdrawal from the CO‐PILOT trial before ever using AID). Table 1 depicts participant baseline characteristics. This was a relatively technology naïve cohort prior to AID initiation with MDI the predominant method of insulin delivery (87.9%) and the majority (60.6%) using capillary blood glucose monitoring. Mean HbA1c at baseline was 92 ± 21 mmol/mol (10.6% ± 1.9%).
TABLE 1.
Participant demographics.
| Total | |
|---|---|
| n | 99 |
| Age at AID initiation, mean (SD) years | 17.3 (4.2) |
| Age group, n (%) | |
| 7–15 years | 44 (44.4) |
| 16–25 years | 55 (55.6) |
| Gender, n (%) | |
| Male | 58 (58.6) |
| Female | 40 (40.4) |
| Non‐binary | 1 (1.0) |
| Ethnicity a , n (%) | |
| New Zealand European/European | 65 (65.7) |
| Māori | 20 (20.2) |
| Pacific | 13 (13.1) |
| Other (Indian) | 1 (1.0) |
| New Zealand deprivation index b , n (%) | |
| Quintile 1 (least deprived) | 19 (19.2) |
| Quintile 2 | 15 (15.2) |
| Quintile 3 | 24 (24.2) |
| Quintile 4 | 19 (19.2) |
| Quintile 5 (most deprived) | 22 (22.2) |
| Diabetes characteristics | |
| Time since diagnosis, median (25th, 75th percentile) years | 7.4 (4.2, 10.4) |
| Prior glucose monitoring c , n (%) | |
| Self‐monitoring of capillary blood glucose | 60 (60.6) |
| Real‐time continuous glucose monitor (Dexcom G6, Dexcom G7) | 4 (4.0) |
| Intermittently scanned continuous glucose monitor (FreeStyle Libre, FreeStyle Libre 2) | 35 (35.4) |
| Insulin therapy, n (%) | |
| Multiple daily injections | 87 (87.9) |
| Continuous subcutaneous insulin infusion | 12 (12.1) |
| Baseline HbA1c, mmol/mol | 92 (21) |
| Baseline HbA1c, % | 10.6 (1.9) |
Abbreviations: AID, automated insulin delivery; HbA1c, HbA1c, glycated haemoglobin A1c.
Participants could choose multiple ethnicities; however, they were assigned to a single ethnic group for statistical evaluation with the list prioritized in the standardized order of Māori, Pacific Islander, Other and European.
Area‐based index of socioeconomic deprivation, in which the first quintile represents the 20% least deprived and the fifth quintile the 20% most deprived areas of the country.
Continuous glucose monitoring systems were not funded during these two trials.
6.1. Glycaemic outcomes after 3 months of AID use
After 3 months of AID use, HbA1c improvement was observed in 91.8% of participants (Tables S1 and S2). An overall mean change of −27 ± 23 mmol/mol (−2.5% ± 2.1% points) was seen, from 92 ± 21 mmol/mol (10.6% ± 1.9%) at baseline to 65 ± 16 mmol/mol (8.1% ± 1.5%) after 3 months (p <0.001) (Figure 1, Table S1). Percentage of time spent in range (TIR) 3.9–10.0 mmol/L (70–180 mg/dL) and time spent in tight range (TITR) 3.9–7.8 mmol/L (70–140 mg/dL) both significantly increased by 34.2% ± 17.3% points (p <0.001) and 23.0% ± 14.9% points (p <0.001) respectively. These improvements equated to an additional 8.2 and 5.5 h per day spent in the respective target ranges. Percentage of time spent in the hypoglycaemic and hyperglycaemic ranges also improved.
FIGURE 1.
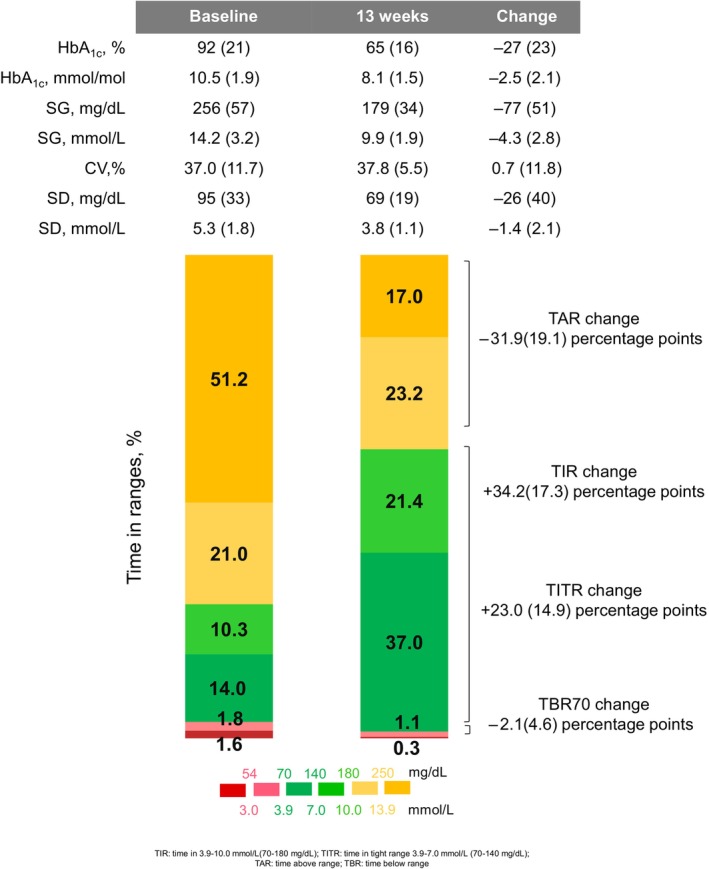
Glycaemic outcomes from baseline and after 3 months of automated insulin delivery (AID) use. Data are mean (SD) and represent the 14‐day average of baseline, 12–13 weeks of AID data. CV, coefficient of variation; HbA1c, glycated haemoglobin A1c; SG, sensor glucose; TAR, Time above range; TBR, time below range; TIR, time in range 3.9–10.0 mmol/L (70–180 mg/dL); TITR, Time in tight range 3.9–7.8 mmol/L (70–140 mg/dL).
7. FACTORS PREDICTING GLYCAEMIC IMPROVEMENTS
7.1. Baseline diabetes management characteristics
Larger glycaemic improvements were observed in participants with baseline HbA1c >100 mmol/mol (>11.0%) when compared with a lower baseline HbA1c group (69–100 mmol/mol [8.5%–11.0%]) (Table S2), with HbA1c decreasing from 116 ± 16 mmol/mol (12.7% ± 1.5%) to 70 ± 23 mmol/mol (8.5% ± 2.1%) and TIR increasing from 15.0% ± 12.7% to 54.6% ± 19.4% (p both <0.001). Participants using MDI at baseline observed larger HbA1c improvements than those using CSII. In the multivariate model including all variables of interest or using backward selection (Figure 2, Table 2), higher baseline HbA1c of >100 mmol/mol (>11.0%) was still associated with greater improvements, with HbA1c decreasing by 27.0 mmol/mol (2.4% points; p <0.001) and TIR improving more by 6.7% points (p = 0.04). Glucose monitoring and insulin delivery methods were no longer associated with any improvement (p >0.05) in the multivariate analysis.
FIGURE 2.
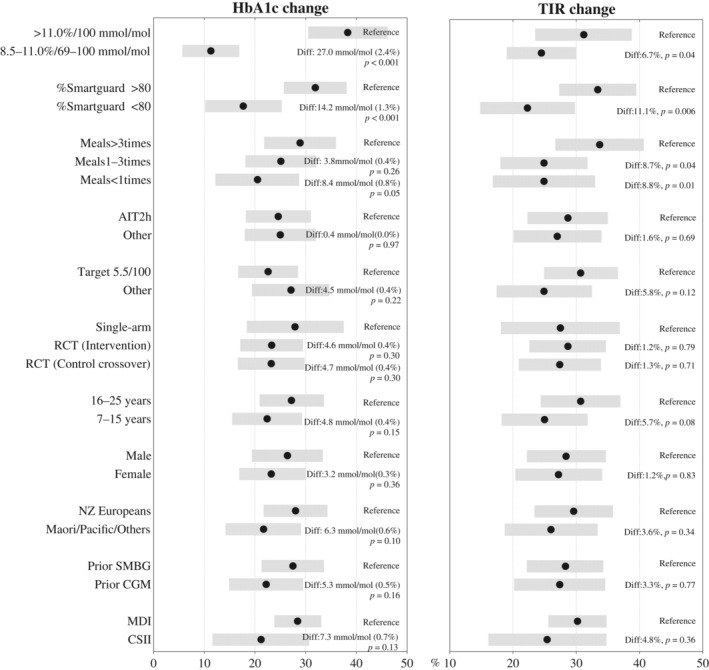
Point range plots showing the estimated mean change in HbA1c, glycated haemoglobin A1c (HbA1c) (in mmol/mol; left panel), and time in range (TIR) 3.9–10.0 mmol/L (70–180 mg/dL; right panel) after 3 months of automated insulin delivery use. Black dots with grey bands represent estimated mean change with 95% confidence intervals. Within each panel, pairwise comparisons of groups were calculated from multivariate analysis after adjusting for all other covariates in the figure.
TABLE 2.
Multivariate model with backward selection for changes in glycated haemoglobin A1c (HbA1c) and TIR post‐automated insulin delivery using significant factors.
| Unstandardized coefficients | Std. error | p‐values | |
|---|---|---|---|
| Model for HbA1c change | |||
| Baseline HbA1c >100 mmol/mol (11.0%) | 28.651 | 3.492 | <0.001 |
| Time in automation >80% | 8.940 | 1.790 | <0.001 |
| Meals >3 times per day | 4.411 | 2.171 | 0.045 |
| Model for TIR change | |||
| Baseline HbA1c >100 mmol/mol (11.0%) | 8.817 | 3.403 | 0.011 |
| Time in automation >80% | 6.782 | 1.745 | <0.001 |
| Meals >3 times per day | 5.141 | 2.101 | 0.016 |
| Glucose target at 5.5 mmol/L (100 mg/dL) | 6.887 | 3.384 | 0.045 |
Abbreviation: TIR, time in range 3.9–10.0 mmol/L (70–180 mg/dL).
8. SYSTEM CHARACTERISTICS
During weeks 12–13, mean percentage of time in automation mode and sensor wear were 83.8% ± 21.4% and 84.7% ± 18.2% respectively. A total of 61 (62.2%) and 68 (69.4%) participants used the recommended optimal SmartGuard settings of active insulin time of 2 h and target of 5.5 mmol/L (100 mg/dL) respectively for more than 80% of the 3‐month duration. 18 Higher percentage of time in automation mode and sensor wear time were both associated with greater HbA1c and TIR improvements in the univariate analysis (p <0.001, p = 0.007; Table S2). Correlation between time in automation mode and sensor wear time was 0.92 (p <0.001; Figure S2). Reasons for SmartGuard exits per week are presented in Table S3 and Figure S1, with expired sensors (53.6%), no sensor glucose value available (50.0%) and SmartGuard max delivery (39.3%) contributing to the majority of exits.
In the multivariate analysis (Figure 2, Table 2), mean glycaemic improvement was still higher among participants with greater than 80% of time in automation mode, with HbA1c decreasing by 14.2 mmol/mol (1.3% points; p = 0.001) and TIR improving by 11.1% points, equal to 2.7 h per day more in target range (p = 0.01). In addition, after using backward selection, optimal glucose target at 5.5 mmol/L (100 mg/dL) was found to be associated with TIR improvement (p = 0.045) despite no significant differences were found between group with the strongest settings of AIT 2 h or target 5.5 mmol/L (100 mg/dL) in the univariate and multivariate analyses including all variables of interests (Figure 2, Table 2).
9. MEAL ANNOUNCEMENT/CARBOHYDRATE ENTRY
Mean number of daily meal announcements and carbohydrate amounts entered during weeks 12–13 were 2.7 ± 1.8 meals and 119 ± 97 g respectively with 41 (41.8%), 39 (40.0%) and 18 (18.2%) participants announcing daily meals >3 times, 1–3 times and <1 time respectively (Table S2). In the univariate analysis, meal announcement frequency was only associated with improvement in TIR (p = 0.03) rather than HbA1c (p = 0.37).
In the multivariate analysis (Figure 2, Table 2), participants announcing meals >3 times daily had an additional HbA1c improvement of 8.4 mmol/mol (0.8% points) compared with those announcing <1 times (p = 0.05). No difference in HbA1c improvement was seen between participants announcing meals 1–3 times daily and >3 times daily (Figure 2). TIR improvements were seen for participants announcing meals >3 times daily, improving TIR by an additional 8.7% points (p = 0.04) and 8.8% points (p = 0.01) compared with participants announcing meals 1–3 times daily and <1 times daily respectively. These improvements equated to additional 2.1 h both per day spent in target range.
10. SOCIO‐DEMOGRAPHIC CHARACTERISTICS
New Zealand European ethnicity was associated with larger glycaemic improvements in the univariate analysis (Table S1). However, in the multivariate analysis, between‐group differences were no longer significant (adjusted HbA1c difference: 6.3 mmol/mol [0.6%], p = 0.10; adjusted TIR difference: 3.6%, adjusted p = 0.34; Figure 2). In the multivariate analysis, age, gender and deprivation index were not associated with HbA1c and TIR improvement (Table S1, Figure 2).
11. DISCUSSION
In this combined analysis of children and young adults naïve to AID and with markedly elevated glycaemia, overall large glycaemic improvements were seen after implementation of the MiniMed 780G system. Benefits of using automation were largely seen irrespective of baseline socio‐demographic backgrounds, and system settings and behavioural characteristics (including carbohydrate counting). Notably, the key factors predicting overall greatest glycaemic improvement were a higher baseline HbA1c of >100 mmol/mol (>11.0%), and spending more than 80% time in automation mode. Meal announcement frequency of exceeding 3 times daily and an optimal glucose target of 5.5 mmol/L (100 mg/dL) also predicted greater improvement in TIR. In this specific population of youth, no other factors, including age, gender, ethnicity, prior insulin delivery method and an active insulin time of 2 h contributed to statistically significant HbA1c and TIR improvements.
In the past, safety and engagement have been acknowledged as major concerns limiting the promotion and uptake of diabetes technology among individuals with very high glycaemia. 19 , 20 This has contributed to lower utilisation rates of advanced diabetes technology among underserved and minority populations. 21 , 22 However, findings from this analysis underscore that higher baseline HbA1c predicted the greatest glycaemic improvements, and over 90% of individuals with very high HbA1c achieving glycaemic improvements, which were often large (>20 mmol/mol, >2.0% points). These data add to a growing body of evidence highlight that young age, diverse socio‐demographic background and prior out‐of‐target glycaemia are not barriers to successful use of advanced technology. 9 , 23 , 24 , 25 , 26 Importantly, our study suggests that gender, ethnicity, socioeconomic deprivation and current insulin delivery and glucose monitoring are also not barriers to successful use of advanced technology, indicating those previously often perceived as inappropriate for AID use in fact have the greatest to gain from this technology. More importantly, our recent qualitative data also showed transition to AHCL therapy appears to create a window of opportunity in which youth may re‐engage with diabetes management. 27 Therefore, more support is needed to ensure all individuals with diabetes particularly those with the highest burden have expedited access to initiate AID systems considering the potential glycaemic benefits.
Another key finding is the critical role of time in automation mode for achieving glycaemic improvement. The positive correlation between time in automation mode and glycaemia has also been reported in real‐world studies. 28 , 29 However, making this point in children and young adults is vital as again it emphasises the glycaemic gains to be made but also how vital adequate support is to sustain time in automation. The benefits of multidisciplinary team support and motivational input cannot be overstated. 30 These issues were seen in first generation systems where drop off was considerably higher than the data reported here. 19 This provides reassurance that technological advancements are reducing perceived burdens with many more now persisting in system use and described for periods of out to 12 months. 11 , 12 , 13 Maintaining automation mode also depends on addressing factors leading to exits. In this analysis, SmartGuard™ exits, resulting in reduced automation time, were predominantly due to sensor‐related issues. This aligns with the strong correlation observed between automation time and sensor wear time in this analysis. Already we have seen benefits from transition from Guardian™ 3 sensors to Guardian™ 4, 31 and early data highlight additional benefits of the move to the all‐in‐one Simplera Sync system (data under review). Finally, unlike optimal glucose target of 5.5 mmol/L (100 mg/dL), the recommended optimal active insulin time seen in more traditional populations 18 , 29 did not provide significant HbA1c and/or TIR improvements in our study. We hypothesise that this is due to the greater glycaemia, sensor wear and lifestyle variability seen in this more complex population of young individuals. 32
Higher meal announcement frequency did lead to greater TIR improvement in this analysis, which also aligns with observations among most real‐world data; thus, for those who are willing to do precise carbohydrate counting, higher engagement by announcing meals appropriately is also encouraged, particularly at the start of AID use. 32 , 33 However, another key finding in this analysis is that for these young individuals with prior elevated glycaemia, carbohydrate quantity entered and meal announcement frequency only provided modest benefits to glycaemia even with announcement frequency <1/day. This is important and highlights that for young individuals, the key skills and support required to gain benefits of automation mode are around maintaining the technical skills required to wear and safely manage these systems, rather than only demonstrating extensive knowledge and skills related to carbohydrate counting and diet. This marks a shift from traditional models. Previously simplified carbohydrate counting has already been shown to be a valid option, 33 , 34 and our data in this population take this a step further and highlight that few factors should delay initiating AID. For this population of young individuals, AID should be prioritised not postponed. As AID technology continues to improve, this perspective will only be further reinforced.
Strengths of this study are the sample size focused on this priority population, including from diverse ethnic and socioeconomic backgrounds and the rapid outpatient on‐boarding protocol to automation mode and remote follow‐ups that is largely reproducible for clinic settings. This population is often underrepresented from traditional automation trials. Analysis spanning aspects of individual and system aspects is also important. Limitations include the relatively short follow‐up period of 3 months, and only inclusion of MiniMed 780G device, which limits the generalisability of these glycaemic findings to other systems.
12. CONCLUSION
In children and young adults naive to automation and with markedly elevated glycaemia, the two most important predictors of glycaemic improvement are high baseline HbA1c and high use of automation mode. This continues to highlight the message that those with the highest HbA1c significantly benefit from automation, as long as the device is worn and automation time is high. Sociodemographic background and carbohydrate counting adherence/knowledge should not prevent or delay access to AID, and prioritising equity of access to AID for all is vital.
INFORMED CONSENT STATEMENT
Informed consent/assent to participate in this study will be obtained from all patients as per the requirements of ISO 14155:2011 and Good Clinical Practices.
AUTHOR CONTRIBUTIONS
B.J.W. and M.I.d.B. conceptualised the study and acquired funding. Y.Z., A.B., V.R.M., A.P.S., E.W., C.J., M.I.d.B. and B.J.W. researched data. Y.Z., A.B., V.R.M. and M.K.G conducted formal data analysis. M.K.G, A.B. and Y.Z designed the statistical analysis plan. Y.Z. and B.J.W. wrote the first draft of the manuscript. All authors contributed to and approved the final manuscript. B.J.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
FUNDING INFORMATION
The CO‐PILOT trial was funded by the Lions New Zealand District 202F, Lloyd Morgan Lions Clubs Charitable Trust, Otago Southland Diabetes Research Trust, Lottery Grants Board (grant number R‐LHR‐2023‐215456), The Bowen Family, Spinal Cord Society of New Zealand, University of Otago Wellington and Starship Foundation (grant number SF4374). The single‐arm trial was funded by the Lottery Grants Board (grant number R‐LHR‐2021‐153881). Both trials received hardware and limited financial support from Medtronic. The study design, management, analysis and reporting for these investigator‐led trials were conducted entirely independently from Medtronic and the study funders.
CONFLICT OF INTEREST STATEMENT
This study was investigator designed and led. As above, funding for the two included studies were largely independent of Medtronic, with limited financial support from Medtronic provided for CO‐PILOT. The diabetes technology used in this study was provided by Medtronic. Medtronic was not involved in data analysis but was provided a copy of the manuscript for review before submission. B.J.W. and M.I.d.B. have received honorarium, expenses, and research funding from Medtronic. No other potential conflicts of interest relevant to this study were reported.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1111/dom.16210.
ETHICS STATEMENT
This trial has been approved by the New Zealand Health and Disability Ethics Committees (21/STH/33 and 2022 FULL 13508).
Supporting information
Data S1. Supporting information.
Data S2. STROBE‐checklist.
ACKNOWLEDGMENT
The authors thank the participants, and their families for taking part in this study. Open access publishing facilitated by University of Otago, as part of the Wiley ‐ University of Otago agreement via the Council of Australian University Librarians.
Zhou Y, Boucsein A, Michaels VR, et al. Predictors of glycaemic improvement in children and young adults with type 1 diabetes and very elevated HbA1c using the MiniMed 780G system. Diabetes Obes Metab. 2025;27(4):2138‐2146. doi: 10.1111/dom.16210
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. de Bock M, Codner E, Craig ME, et al. ISPAD clinical practice consensus guidelines 2022: glycemic targets and glucose monitoring for children, adolescents, and young people with diabetes. Pediatr Diabetes. 2022;23(8):1270‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maahs DM, Hermann JM, DuBose SN, et al. Contrasting the clinical care and outcomes of 2,622 children with type 1 diabetes less than 6 years of age in the United States T1D exchange and German/Austrian DPV registries. Diabetologia. 2014;57(8):1578‐1585. [DOI] [PubMed] [Google Scholar]
- 3. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016‐2018. Diabetes Technol Ther. 2019;21(2):66‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. James S, Perry L, Lowe J, Harris M, Craig ME, group As . Suboptimal glycemic control in adolescents and young adults with type 1 diabetes from 2011 to 2020 across Australia and New Zealand: data from the Australasian diabetes data network registry. Pediatr Diabetes. 2022;23(6):736‐741. [DOI] [PubMed] [Google Scholar]
- 5. Phillip M, Nimri R, Bergenstal RM, et al. Consensus recommendations for the use of automated insulin delivery Technologies in Clinical Practice. Endocr Rev. 2023;44(2):254‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collyns OJ, Meier RA, Betts ZL, et al. Improved glycemic outcomes with Medtronic MiniMed advanced hybrid closed‐loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care. 2021;44(4):969‐975. [DOI] [PubMed] [Google Scholar]
- 7. Brown SA, Kovatchev BP, Raghinaru D, et al. Six‐month randomized, multicenter trial of closed‐loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707‐1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Breton MD, Kanapka LG, Beck RW, et al. A randomized trial of closed‐loop control in children with type 1 diabetes. N Engl J Med. 2020;383(9):836‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown SA, Forlenza GP, Bode BW, et al. Multicenter trial of a tubeless, on‐body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care. 2021;44(7):1630‐1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burnside MJ, Lewis DM, Crocket HR, et al. Open‐source automated insulin delivery in type 1 diabetes. N Engl J Med. 2022;387(10):869‐881. [DOI] [PubMed] [Google Scholar]
- 11. Boucsein A, Zhou Y, Michaels V, et al. Automated insulin delivery for young people with type 1 diabetes and elevated A1c. NEJM Evid. 2024;3(10):EVIDoa2400185. [DOI] [PubMed] [Google Scholar]
- 12. Boucsein A, Watson AS, Frewen CM, et al. Impact of advanced hybrid closed loop on youth with high‐risk type 1 diabetes using multiple daily injections. Diabetes Care. 2023;46(3):628‐632. [DOI] [PubMed] [Google Scholar]
- 13. Michaels VR, Boucsein A, Watson AS, et al. Glucose and psychosocial outcomes 12 months following transition from multiple daily injections to advanced hybrid closed loop in youth with type 1 diabetes and suboptimal glycemia. Diabetes Technol Ther. 2024;26(1):40‐48. [DOI] [PubMed] [Google Scholar]
- 14. Boucsein A, Zhou Y, Haszard JJ, et al. Protocol for a prospective, multicenter, parallel‐group, open‐label randomized controlled trial comparing standard care with closed lOoP in chiLdren and yOuth with type 1 diabetes and high‐risk glycemic control: the CO‐PILOT trial. J Diabetes Metab Disord. 2024;23(1):1397‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association Professional Practice C. 2 . Diagnosis and classification of diabetes: standards of Care in Diabetes‐2024. Diabetes Care. 2024;47(Suppl 1):S20‐S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ajwani S, Blakely T, Robson B, Tobias M, Bonne M. Decades of Disparity: Ethnic Mortality Trends in New Zealand 1980–1999. Ministry of Health and University of Otago; 2003:130. [Google Scholar]
- 17. Battelino T, Alexander CM, Amiel SA, et al. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol. 2023;11(1):42‐57. [DOI] [PubMed] [Google Scholar]
- 18. Lombardo F, Passanisi S, Alibrandi A, et al. MiniMed 780G six‐month use in children and adolescents with type 1 diabetes: clinical targets and predictors of optimal glucose control. Diabetes Technol Ther. 2023;25(6):404‐413. [DOI] [PubMed] [Google Scholar]
- 19. Messer LH, Berget C, Vigers T, et al. Real world hybrid closed‐loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes. 2020;21(2):319‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G , Tamborlane WV, Beck RW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464‐1476. [DOI] [PubMed] [Google Scholar]
- 21. Burnside MJ, Williman JA, Davies HM, et al. Inequity in access to continuous glucose monitoring and health outcomes in paediatric diabetes, a case for national continuous glucose monitoring funding: a cross‐sectional population study of children with type 1 diabetes in New Zealand. Lancet Reg Health West Pac. 2023;31:100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hennessy LD, De Lange M, Wiltshire EJ, Jefferies C, Wheeler BJ. Youth and non‐European ethnicity are associated with increased loss of publicly funded insulin pump access in New Zealand people with type 1 diabetes. Diabet Med. 2021;38(1):e14450. [DOI] [PubMed] [Google Scholar]
- 23. Marks BE, Grundman JB, Meighan S, Monaghan M, Streisand R, Perkins A. Hybrid closed loop systems improve glycemic control and quality of life in historically minoritized youth with diabetes. Diabetes Technol Ther. 2024;26(3):167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lepore G, Borella ND, Castagna G, et al. Advanced hybrid closed‐loop system achieves and maintains recommended time in range levels for up to 2 years: predictors of best efficacy. Diabetes Technol Ther. 2024;26(1):49‐58. [DOI] [PubMed] [Google Scholar]
- 25. Passanisi S, Salzano G, Bombaci B, et al. Sustained effectiveness of an advanced hybrid closed‐loop system in a cohort of children and adolescents with type 1 diabetes: a 1‐year real‐world study. Diabetes Care. 2024;47(6):1084‐1091. [DOI] [PubMed] [Google Scholar]
- 26. Gruber N, Wittenberg A, Brener A, et al. Real‐life achievements of MiniMed 780G advanced closed‐loop system in youth with type 1 diabetes: AWeSoMe study group multicenter prospective trial. Diabetes Technol Ther. 2024;26(11):869‐880. [DOI] [PubMed] [Google Scholar]
- 27. Wong JY, Styles SE, Wiltshire EJ, et al. Experiences of adolescents and young adults with type 1 diabetes and chronically elevated glucose levels following the transition from multiple daily injections to advanced hybrid closed‐loop: a qualitative study. Diabet Med. 2025;42(1):e15449. [DOI] [PubMed] [Google Scholar]
- 28. Castaneda J, Mathieu C, Aanstoot HJ, et al. Predictors of time in target glucose range in real‐world users of the MiniMed 780G system. Diabetes Obes Metab. 2022;24(11):2212‐2221. [DOI] [PubMed] [Google Scholar]
- 29. Castaneda J, Arrieta A, van den Heuvel T, Battelino T, Cohen O. Time in tight glucose range in type 1 diabetes: predictive factors and achievable targets in real‐world users of the MiniMed 780G system. Diabetes Care. 2024;47(5):790‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schoelwer MJ, DeBoer MD, Breton MD. Use of diabetes technology in children. Diabetologia. 2024;67:2075‐2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sehgal S, De Bock M, Jones S, Frewen C, Wheeler BJ. User experiences during the transition to calibration‐free sensors with remote monitoring while using automated insulin delivery⸺A qualitative study. Front Endocrinol. 2023;14:1214975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kovatchev BP, Singh H, Mueller L, Gonder‐Frederick LA. Biobehavioral changes following transition to automated insulin delivery: a large real‐life database analysis. Diabetes Care. 2022;45(11):2636‐2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petrovski G, Campbell J, Pasha M, et al. Simplified meal announcement versus precise carbohydrate counting in adolescents with type 1 diabetes using the MiniMed 780G advanced hybrid closed loop system: a randomized controlled trial comparing glucose control. Diabetes Care. 2023;46(3):544‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Minsky N, Shalit R, Benedetti A, et al. Simplified meal management in adults using an advanced hybrid closed‐loop system. Diabetes Technol Ther. 2025;7(1):27‐33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.
Data S2. STROBE‐checklist.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


