Abstract
Real-world data on venous thromboembolism (VTE) in Japanese patients with gynecological cancer are lacking. The GOTIC-VTE trial aimed to evaluate the frequency of VTE-associated events and risk factors at the time of cancer diagnosis and during 1-year follow-up. From July 2017 to February 2019, patients with endometrial, cervical, ovarian, tubal, or peritoneal cancer who underwent VTE screening within 2 months before registration, were enrolled. Of the 1008 patients enrolled, 881 were included in the analysis set, 51 (5.8%) had VTE at the time of cancer diagnosis (baseline), 7 (0.8%) had symptomatic VTE, and the majority had asymptomatic VTE (n = 44; 5.0%). Patients with ovarian, tubal, or peritoneal cancer had a higher incidence of VTE (13.7%) than those with other cancer types. During the 1-year follow-up, 0.9% (n = 8) of the patients had symptomatic VTE, 3.5% (n = 31) had composite VTE (symptomatic VTE and incidental VTE requiring treatment), 0.2% (n = 2) had bleeding events, and 4.3% (n = 38) had all-cause death, all of which were significantly higher in the VTE group at baseline. In the multivariate analysis, chemotherapy was an independent risk factor for composite VTE during the 1-year follow-up (hazard ratio 3.85, 95% confidence interval 1.39–13.63, p = 0.018). Among gynecological cancers, VTE incidence is particularly high in ovarian, tubal, or peritoneal cancer, and patients undergoing chemotherapy should be cautioned against VTE occurrence during treatment.
The GOTIC-VTE trial Unique identifier, jRCTs031180124; Registration date, April 06, 2017.
Graphical Abstract
Incidence and risk factors for venous thromboembolism in gynecological cancer: the GOTIC-VTE trial.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11239-024-03055-1.
Keywords: Chemotherapy, Deep vein thrombosis, Gynecologic neoplasms, Pulmonary embolism, Venous thromboembolism
Highlights
The GOTIC-VTE trial was a prospective observational study aimed at evaluating the frequency of VTE-related events and risk factors at the time of cancer diagnosis and during a 1-year follow-up.
The analysis included 881 patients with gynecological cancer.
Fifty-one patients (5.8%) had VTE at the time of cancer diagnosis, with ovarian, tubal, or peritoneal cancer showing a higher incidence of VTE (13.7%).
Chemotherapy was identified as an independent risk factor for composite VTE during the 1-year follow-up.
Future goal is to develop an original score that can predict the risk of VTE in Japanese patients with gynecological cancer.
Introduction
Venous thromboembolism (VTE) involves pulmonary embolism (PE) and deep vein thrombosis (DVT). PE is a life-threatening cardiovascular disease. Risk factors for VTE include age, obesity, pregnancy and delivery, long-term bed rest, surgery, and trauma of which malignancy is the most relevant [1, 2]. In fact, patients with cancer are 4–7 times more at risk of developing VTE than patients without cancer [3, 4]. In addition to individual patient factors, such as comorbidity and activity, tumor associated factors, such as stage and histology, and treatment associated factors, such as surgery and chemotherapy, are risk factors for VTE in patients with cancer [5], requiring strict VTE prevention and management in cancer treatment settings.
The incidence of VTE depends on the cancer type; hematologic malignancies and pancreatic, lung, and ovarian cancers have a high risk of VTE, whereas breast and prostate cancers have a relatively low risk [6]. The incidence of VTE at the time of gynecological cancer diagnosis in retrospective studies was 0.5–7.3% for cervical cancer [7–10], 0.3–14.6% for endometrial cancer [8–12], and 4.1–27.0% for ovarian cancer [8–11]. Prospective, large-scale studies of cancer-associated thrombosis and data are lacking in Japan. Recently, a multicenter, prospective, cooperative study (Cancer-VTE Registry) was conducted in Japan to evaluate the occurrence of VTE events during the 1-year follow-up period from the time of cancer diagnosis in 10,000 patients with colorectal, lung, gastric, breast, pancreatic, and gynecological cancers [13–19]. This study showed differences in the occurrence of VTE according to cancer type, and found that stage is a dominant risk factor for VTE [13, 14].
The GOTIC-VTE trial (jRCTs031180124) was an investigator-initiated study conducted independently as a gynecological cancer part of the Cancer-VTE Registry. Data collected were submitted to the Cancer-VTE Registry for integrated analysis [13, 14]. Our analysis focused on patients with gynecological cancer and was independent of the Cancer-VTE Registry.
Materials and methods
Participants
Inclusion criteria were as follows: patients (1) aged ≥ 20 years; (2) with primary or recurrent gynecological cancers, including endometrial, cervical, ovarian, tubal, or peritoneal; (3) planning to start cancer treatment, such as surgery, chemotherapy, radiotherapy, hormonal therapy, molecular targeted therapy, or immunotherapy; (4) who underwent VTE screening by lower extremity venous ultrasound or computed tomography (CT) scan 2 months prior to registration, unless D-dimer was ≤ 1.2 μg/mL considered non-VTE [20]; (5) at clinical stage I–IV; (6) with life expectancy ≥ 6 months after enrollment; (7) whose Eastern Cooperative Oncology Group performance status (ECOG PS) was 0–2; and (8) who have given written informed consent. Exclusion criteria were as follows: patients (1) with active double cancer and (2) judged ineligible by the investigator on the basis of inability to be followed up because of reasons such as planned relocation.
VTE diagnosis and classification
DVT was diagnosed if venous ultrasound showed thrombus, incompressibility abnormalities, or perfusion defects in the deep veins. DVT was also diagnosed if thrombus was detected on CT angiography of the lower extremities. PE was diagnosed by CT angiography, pulmonary angiography, pulmonary ventilation, or perfusion scintigraphy and classified as collapse with cardiac arrest, massive, submassive, or nonmassive type [21].
Outcome
The outcomes of this study included VTE incidence at baseline and risk factors in patients with gynecological cancer, and the cumulative incidence of symptomatic VTE, incidental VTE requiring treatment, composite VTE (symptomatic VTE and incidental VTE requiring treatment), bleeding (major bleeding or clinically relevant nonmajor bleeding), cerebral infarction/transient ischemic attack (TIA)/systemic embolic events (SEE), and all-cause death during 1-year follow-up. Newly occurring events during the follow-up period were recorded, and incidental VTE appearing at the same site as at baseline was not counted twice during the follow-up period. Incidental VTE without anticoagulant treatment was excluded as an event. All events were judged by an independent committee that included a VTE specialist.
Statistical analyses
Continuous variables were described as mean values and standard deviations and categorical variables as n (%). Comparisons between the two groups for categorical variables were performed using the chi-square test; time-to-event rates were estimated using the cumulative number of events. The log-rank test was used to compare groups according to the incidence of VTE at baseline. Univariate logistic regression analysis was used to investigate factors related to the incidence of VTE at baseline. Univariate and multivariate logistic regression analyses were used to investigate factors related to composite VTE during follow-up period. For multivariate analysis, exploratory factors were selected as clinically important or strongly expected to be relevant rather than using a statistical procedure, such as a stepwise method. These factors included cancer stage (I–II and III–IV), histological type (clear cell, other), ECOG PS (0 and 1–2), surgery, chemotherapy, bevacizumab, and VTE at baseline. A p-value < 0.05 was regarded statistically significant. SAS statistical software (version 9.3) and R software (version 4.0.2) were used for data analysis.
Results
Patient characteristics
Between July 2017 and February 2019, 1008 patients with endometrial cancer, cervical cancer, ovarian, tubal, or peritoneal cancer were enrolled in the GOTIC-VTE trial at 16 institutions in Japan (Table S1). In total, 881 patients were included in the analysis set, excluded for criteria violation in 122 patients (most were postoperatively diagnosed as benign or borderline malignancies) and withdrawal of consent in 5 (Fig. 1). Table S2 shows the baseline characteristics. Endometrial cancer was the most common (n = 412; 46.8%), followed by cervical cancer (n = 236; 26.8%), and ovarian, tubal, or peritoneal cancer (n = 233; 26.4%). Mean patient age was 57.4 years and 67.2% had stage I disease. There were 839 (95.2%) primary cases and 42 (4.8%) recurrences. Regarding cancer treatment, 84.4% of the patients had surgery, 55.6% had chemotherapy, and 14.5% had radiotherapy. For approximately 25% of the cases, the D-dimer level was > 1.2 µg/mL.
Fig. 1.
Patient flowchart. Of the 1008 patients registered, 881 were included in the analysis set, 122 were excluded for violation of inclusion and exclusion criteria, and 5 withdrew consent
VTE incidence at baseline
Table S3 shows the incidence of VTE at baseline. Among the 881 patients, 51 (5.8%) showed VTE at baseline, 7 (0.8%) had symptomatic VTE, and the majority had asymptomatic VTE (n = 44; 5.0%). DVT alone accounted for 34 (3.9%), followed by concomitant DVT and PE for 9 (1.0%), and PE alone for 8 (0.9%). Table S4 shows details of VTE at baseline. Of the DVTs, 5 (0.6%) were of proximal type and 38 (4.3%) of distal type. Most PE were nonmassive (88.2%; 15/17). VTE detected at baseline was treated with direct oral anticoagulant or warfarin in 32/51 (62.7%) cases. Recurrent cancer accounted for 4.8% (n = 42) of all patients, and at baseline, only one (2.4%) of them had VTE, an asymptomatic distal DVT.
Table 1 shows the univariate analysis of associated factors for VTE at baseline. Associated factors were clinical stages III–IV, cancer type (ovarian, tubal, or peritoneal cancer), ECOG PS 1–2, history of VTE, platelet count ≥ 350 × 109/L, Hb < 10 g/dL, white blood cell (WBC) count > 11 × 109/L, and D-dimer > 1.2 µg/mL.
Table 1.
Univariate analysis of associated factors for VTE incidence at baseline
| Items | Category | n | Events, n (%) | Univariate | ||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | ||||
| Age, years | < 65 | 601 | 30 (5.0) | – | – | – |
| ≥ 65 | 280 | 21 (7.5) | 1.54 | 0.857–2.733 | 0.1403 | |
| BMI, kg/m2 | < 18.5 | 76 | 3 (3.9) | – | – | – |
| 18.5 to < 25 | 534 | 35 (6.6) | 1.71 | 0.595–7.207 | 0.3842 | |
| ≥ 25 | 269 | 13 (4.8) | 1.24 | 0.386–5.494 | 0.7462 | |
| Cancer occurrence | Primary | 839 | 50 (6.0) | – | – | – |
| Recurrence | 42 | 1 (2.4) | 0.38 | 0.021–1.828 | 0.3504 | |
| Cancer stage | I–II | 668 | 22 (3.3) | – | – | – |
| III–IV | 213 | 29 (13.7) | 4.63 | 2.606–8.33 | < 0.0001 | |
| Cancer type | Cervical | 236 | 8 (3.4) | – | – | – |
| Endometrial | 412 | 11 (2.7) | 0.78 | 0.312–2.046 | 0.602 | |
| Ovary, Fallopian tube, Peritoneal | 233 | 32 (13.7) | 4.54 | 2.142–10.785 | 0.0002 | |
| Histology | Others | 845 | 47 (5.6) | – | – | – |
| Clear cell | 36 | 4 (11.1) | 2.12 | 0.613–5.631 | 0.1721 | |
| ECOG PS | 0 | 797 | 37 (4.6) | – | – | – |
| 1–2 | 84 | 14 (16.7) | 4.11 | 2.06–7.813 | < 0.0001 | |
| History of smoking | Never | 675 | 44 (6.5) | – | – | – |
| Previous | 136 | 5 (3.7) | 0.55 | 0.187–1.284 | 0.2108 | |
| Current | 70 | 2 (2.9) | 0.42 | 0.068–1.409 | 0.2397 | |
| History of VTE | No | 879 | 50 (5.7) | – | – | – |
| Yes | 2 | 1 (50.0) | 16.58 | 1.022–268.986 | 0.0482 | |
| Bed rest for 4 days or more | No | 878 | 51 (5.8) | – | – | – |
| Yes | 3 | 0 (0) | NA | NA | NA | |
| Platelet count, × 109/L | < 350 | 684 | 28 (4.1) | – | – | – |
| ≥ 350 | 181 | 22 (12.2) | 3.24 | 1.791–5.804 | < 0.0001 | |
| Hb, g/dL | ≥ 10 | 809 | 42 (5.2) | – | – | – |
| < 10 | 56 | 8 (14.3) | 3.04 | 1.267–6.539 | 0.0071 | |
| WBC count, × 109/L | ≤ 11 | 831 | 45 (5.4) | – | – | – |
| > 11 | 34 | 5 (14.7) | 3.01 | 0.989–7.544 | 0.03 | |
| D-dimer, μg/mL | ≤ 1.2 | 591 | 7 (1.2) | – | – | – |
| > 1.2 | 202 | 44 (21.8) | 23.23 | 10.93–57.358 | < 0.0001 | |
BMI body mass index, ECOG PS Eastern Cooperative Oncology Group performance status, Hb haemoglobin, OR odds ratio, SD standard deviation, VTE venous thromboembolism, WBC white blood cell
Events during follow-up
Median follow-up duration was 363 days (interquartile range: 265–386). Table S5 shows events during follow-up. Symptomatic VTE occurred in 0.9%, incidental VTE requiring treatment in 3.1%, composite VTE in 3.5%, bleeding in 0.2%, cerebral infarction/TIA/SEE in 0.7%, and all-cause death in 4.3%. In addition, only one VTE event (2.4%) occurred during follow-up in patients with recurrent cancer, and it was an incidental VTE requiring treatment. In the 27 cases of incidental VTE requiring treatment, the most common reason for diagnosis was image examination owing to elevated D-dimer (48.1%, 13/27), followed by incidental VTE detection on CT for cancer evaluation (37%, 10/27).
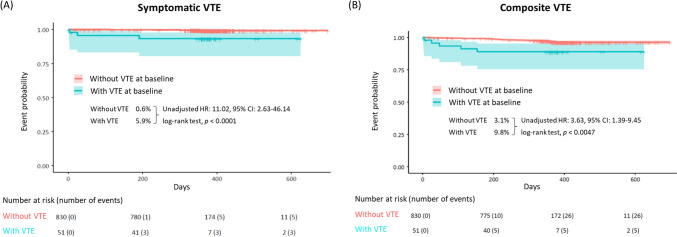
Figure 2 shows the 1-year cumulative incidence of symptomatic VTE and composite VTE based on VTE at baseline. Compared to without VTE at baseline, the occurrence of VTE at baseline was associated with higher rates of symptomatic VTE (unadjusted hazard ratio [HR] = 11.02, 95% confidence interval [CI]: 2.63–46.14, log-rank test p < 0.0001), composite VTE (unadjusted HR = 3.63, 95% CI: 1.39–9.45, log-rank test p = 0.0047). Figure 3 shows the cumulative incidence of incidental VTE requiring treatment, bleeding, cerebral infarction/TIA/SEE, and all-cause death. Compared to without VTE at baseline, the occurrence of VTE at baseline was significantly associated with higher bleeding rate, cerebral infarction/TIA/SEE, and all-cause mortality. The incidence of incidental VTE requiring treatment increased with HR 2.32 (95% CI: 0.70–7.72) with VTE at baseline but not statistically significant (log-rank test p = 0.16).
Fig. 2.
Cumulative incidences of events (time-to-event analysis). A Symptomatic VTE and B Composite VTE a. P values were calculated using a log-rank test. Lightly shaded areas represent 95% CIs. aA composite of symptomatic VTE events and incidental VTE events requiring treatment. CI confidence interval, HR hazard ratio, VTE venous thromboembolism
Fig. 3.
Cumulative incidence of events (time-to-event analysis). A Incidental VTE requiring treatment, B bleeding, C cerebral infarction/TIA/SEE, and D all-cause death. P values were calculated using a log-rank test. Lightly shaded areas represent 95% Cis. CI confidence interval, HR hazard ratio, SEE systemic embolic event, TIA transient ischemic attack, VTE venous thromboembolism
Risk factors for composite VTE during follow-up
Table 2 shows univariate and multivariate analyses of factors associated with composite VTE during 1-year follow-up. Multivariate analysis showed that chemotherapy was the only independent risk factor for composite VTE during follow-up (HR: 3.85, 95% CI: 1.387–13.632; p = 0.0176). Compared with the absence of VTE at baseline, the occurrence of VTE at baseline tended to have a higher HR (2.24; 95% CI: 0.689–6.148) for composite VTE, but this difference was not statistically significant (p = 0.1419). Conversely, for surgery (0.53), HR was decreased, but the value was not statistically significant (p = 0.1171).
Table 2.
Univariate and multivariate analyses of factors for composite VTE during the follow-up period
| Items | Category | n | Events, n (%) | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||||
| Age, years | < 65 | 601 | 16 (2.7) | – | – | – | |||
| ≥ 65 | 280 | 15 (5.4) | 2.07 | 0.999–4.267 | 0.0475 | ||||
| BMI, kg/m2 | < 18.5 | 76 | 1 (1.3) | – | – | – | |||
| 18.5 to < 25 | 534 | 17 (3.2) | 2.47 | 0.495–44.759 | 0.3834 | ||||
| ≥ 25 | 269 | 13 (4.8) | 3.81 | 0.74–69.75 | 0.2008 | ||||
| Cancer occurrence | Primary | 839 | 30 (3.6) | – | – | – | |||
| Recurrence | 42 | 1 (2.4) | 0.66 | 0.036–3.192 | 0.6839 | ||||
| Cancer stage | I–II | 668 | 15 (2.2) | – | – | – | – | – | – |
| III–IV | 213 | 16 (7.5) | 3.54 | 1.71–7.349 | 0.0006 | 1.68 | 0.740–3.801 | 0.2131 | |
| Cancer type | Cervical | 236 | 8 (3.4) | – | – | ||||
| Endomtrial | 424 | 12 (2.8) | 0.86 | 0.348–2.211 | 0.7356 | ||||
| Ovary, Fallopian tube, Peritoneal | 233 | 11 (4.7) | 1.41 | 0.561–3.71 | 0.4667 | ||||
| Histology | Others | 845 | 30 (3.6) | – | – | – | – | – | – |
| Clear cell | 36 | 1 (2.8) | 0.78 | 0.043–3.791 | 0.8059 | 0.64 | 0.035–3.305 | 0.675 | |
| ECOG PS | 0 | 797 | 25 (3.1) | – | – | – | – | – | – |
| 1–2 | 84 | 6 (7.1) | 2.38 | 0.861–5.613 | 0.0656 | 1.52 | 0.526–3.805 | 0.3992 | |
| Surgery | No | 152 | 11 (7.2) | – | – | – | – | – | – |
| Yes | 729 | 20 (2.7) | 0.36 | 0.172–0.797 | 0.0085 | 0.53 | 0.242–1.211 | 0.1171 | |
| Chemotherapy | No | 391 | 4 (1.0) | – | – | – | – | – | – |
| Yes | 490 | 27 (5.5) | 5.64 | 2.183–19.21 | 0.0014 | 3.85 | 1.387–13.632 | 0.0176 | |
| Bevacizumab | No | 847 | 28 (3.3) | – | – | – | – | – | – |
| Yes | 34 | 3 (8.8) | 2.83 | 0.653–8.564 | 0.101 | 1.41 | 0.309–4.666 | 0.6066 | |
| VTE at baseline | No | 830 | 26 (3.1) | – | – | – | – | – | – |
| Yes | 51 | 5 (9.8) | 3.36 | 1.098–8.494 | 0.0177 | 2.24 | 0.689–6.148 | 0.1419 | |
BMI body mass index, ECOG PS Eastern Cooperative Oncology Group performance status, HR hazard ratio, VTE venous thromboembolism
Subgroups
Patients with gynecological cancer were analyzed in two subgroups: (1) ovarian, tubal, or peritoneal and (2) endometrial and cervical. Table S6 shows the incidence of VTE at baseline in each subgroup. The incidence of VTE at baseline was significantly higher in ovarian, tubal, or peritoneal cancer at 32/233 (13.7%) compared with 19/648 (2.9%) in endometrial and cervical cancer (p < 0.0001). Table S7 shows the occurrences of events during follow-up in each subgroup. All-cause death, including with and without VTE at baseline, was significantly lower in endometrial and cervical cancer compared with ovarian, tubal, or peritoneal cancer (HR: 0.4771, 95% CI: 0.2344–0.9911, p = 0.0368). None of the other events differed in incidence by subgroup; however, all thrombotic events, except bleeding, tended to be low in patients with endometrial and cervical cancer.
Discussion
The GOTIC-VTE trial was the first large prospective observational study to evaluate VTE incidence in Japanese patients with gynecological cancer. The VTE incidence at the time of cancer diagnosis was 5.8%, with a particularly high rate of 13.7% in ovarian, tubal, or peritoneal cancer. During the 1-year follow-up, 0.9% of the patients had symptomatic VTE and 3.5% had composite VTE. The cumulative incidence of these events was significantly higher in the group with VTE at baseline than that in the group without VTE. In the multivariate analysis, chemotherapy was an independent risk factor for composite VTE during the 1-year follow-up.
In the Cancer-VTE Registry, a prospective study of VTE incidence in various cancer types in Japan, the incidence of VTE was 5.9% among 9630 patients at diagnosis (before treatment). Pancreatic cancer had the highest incidence (8.5%), followed by gastric (6.9%), colorectal (6.4%), gynecologic (5.8%) (data provided in this study), lung (5.0%), and breast (2.0%) cancers [14]. The incidence of cancer-associated thrombosis was higher in the more advanced stages [6, 22]. In total, 67.2% of patients with gynecologic cancers registered in the Cancer-VTE Registry were stage I; only 19.3% of those with lung cancer were stage I; and stage I was excluded from the registry in other cancer types. An analysis of VTE incidence by stage showed that the rate was 5.2% for gynecologic cancer stage II (vs. 3.8% overall), 10.4% for stage III (vs. 5.3% overall), and 17.1% for stage IV (vs. 11.2% overall), indicating that gynecologic cancer has higher VTE concomitant rates than other cancer types [13].
In this analysis of the GOTIC-VTE trial, ovarian, tubal, or peritoneal cancer had a higher rate of VTE than endometrial and cervical cancers (13.7% vs. 2.9%, p < 0.0001). In the United Kingdom, 4.8% of 397 patients with ovarian cancer had VTE at diagnosis [23], and in the USA, 3.1% of 843 patients with ovarian cancer had VTE [24]. In comparison, the higher rate of VTE (13.7%) in this study was probably due to the influence of clear cell carcinoma (CCC). Studies have reported that CCC releases many tissue factors and activates coagulation, leading to the development of VTE [25, 26]. In Europe and the USA, CCC is rare; only 5.3 and 7.7% of all cases had CCC, respectively [23, 24]. In contrast, in Japan, CCC is the second most common histological type of ovarian cancer [27] and accounted for 15.5% of all ovarian cancers enrolled in this study. Univariate analysis of associated factors for VTE at baseline revealed no statistically significant differences in VTE incidence for CCC compared with other histologic findings (11.1% vs. 5.6%) but showed an odds ratio (OR) of 2.12 (95% CI: 0.31–5.63). In a retrospective analysis of 470 patients with ovarian cancer reported from Japan, the OR of VTE in CCC versus non-CCC significantly increased to 1.41 (95% CI: 1.04–1.93) [10]. Consistently, a meta-analysis of 6324 ovarian cancers showed a significantly increased OR of 2.11 (95% CI: 1.55–2.89) for CCC [28]. Therefore, Japanese patients with gynecologic cancers, especially ovarian cancer, should be considered at high risk for VTE.
Multivariate analysis showed that chemotherapy was an independent risk factor for composite VTE during the 1-year follow-up (HR: 3.85, 95% CI: 1.387–13.632). Among 26,863 Korean patients with ovarian cancer, chemotherapy was associated with the highest rate of VTE (306 per 10,000 women) compared to surgery and radiation therapy [29]. Regarding the risk of VTE with platinum agents, a key drug for gynecological cancers, a meta-analysis reported increased risk, with a relative risk (RR) of 1.67 (95% CI: 1.25–2.23) [30]. Conversely, bevacizumab was not a risk factor for composite VTE (HR: 1.41, 95% CI: 0.309–4.666). Bevacizumab is currently approved in Japan for cervical and ovarian cancer treatment. In the randomized control trial (RCT) of bevacizumab combined with chemotherapy in cervical cancer (GOG-240), thromboembolic events were significantly higher with bevacizumab (8% vs. 1%, p = 0.001) [31]. In contrast, the RCT of bevacizumab in ovarian cancer (GOG-218) showed no difference in the incidence of venous and arterial thromboembolic events [32]. Two meta-analyses showed an increased risk (RR: 1.29, 95% CI: 1.12–1.47 [33] and RR: 1.33, 95% CI: 1.13–1.56 [34]), and another two meta-analyses showed no increased risk (RR: 0.89, 95% CI: 0.66–1.20 [35] and OR: 1.14, 95% CI: 0.96–1.35 [36]). Therefore, the findings are controversial [37]. In the present study, bevacizumab was not a risk factor for VTE (HR: 1.41, 94% CI: 0.309–4.666). However, bevacizumab significantly increased the thrombotic response in a mouse model [38]; thus, caution is recommended for VTE with bevacizumab use.
In this study, univariate analysis of associated factors for VTE at baseline identified the following factors: clinical stages III–IV, cancer type (ovarian, tubal, or peritoneal), ECOG PS 1–2, history of VTE, platelet count ≥ 350 × 109/L, Hb < 10 g/dL, WBC count ≥ 11 × 109/L, and D-dimer > 1.2 µg/mL. Univariate analysis identified the following associated factors for composite VTE during the 1-year follow-up: age > 65 years, clinical stages III–IV, chemotherapy, and VTE incidence at baseline. The Khorana score was proposed in 2008 as a predictive model for VTE in a prospective study of outpatients with cancer receiving chemotherapy [39]. The Khorana score was calculated from the primary site, platelet count, Hb, WBC count, and body mass index (BMI). However, a study to validate the Khorana score for various cancer types reported that the score failed to stratify the risk of gynecologic cancers [40]. The BMI threshold used in the Khorana score is 35 kg/m2, but it is questionable whether this score can be a reasonable cutoff value for the Japanese population, considering their small stature compared with that of foreigners. Based on these findings, it is aimed to develop an original score that can predict the risk of VTE in Japanese patients with gynecologic cancer.
This study had several limitations. First, follow-up was limited to 1 year. Second, the patient selection was biased. This study excluded patients with PS ≥ 3 and life expectancy < 6 months, which prevents generalization to patients with a poor condition. Third, imaging was not performed in all patients at enrollment and was not routinely requested during follow-up unless symptoms of suspected VTE were present, which may have affected the accuracy of asymptomatic VTE detection. Finally, this was an observational study, and anticoagulation for any VTE was performed at the discretion of the physician.
Conclusions
This study provides real-world data on VTE incidence in Japanese patients with gynecologic cancer for 1 year from the time of diagnosis. This is the first prospective, large-scale validation of data in Japan, and the findings of this study may be useful to clinicians.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the patients for participating in this study. We are grateful for the support from the Global Health Research Coordinating Center of Kanagawa Institute of Science and Technology, the Gynecologic Oncology Trial and Investigation Consortium (GOTIC) study coordinating center, and GOTIC administration office.
Authors’ contribution
Yoshifumi Takahashi: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; validation; visualization; writing—original draft; writing—review and editing. Hiroyuki Fujiwara: Conceptualization; funding acquisition; investigation; methodology; project administration; supervision; writing—original draft; writing—review and editing. Kouji Yamamoto: Data curation; formal analysis; methodology; writing—review and editing. Satoshi Yamaguchi: Conceptualization; investigation; writing—review and editing. Shoji Nagao: Conceptualization; investigation; writing—review and editing. Masashi Takano: Conceptualization; investigation; writing—review and editing. Morikazu Miyamoto: Conceptualization; investigation; writing—review and editing. Kosei Hasegawa: Conceptualization; investigation; writing—review and editing. Maiko Miwa: Conceptualization; investigation; writing—review and editing. Toshiaki Yasuoka: Conceptualization; investigation; writing—review and editing. Soichi Yamashita: Conceptualization; investigation; writing—review and editing. Takashi Hirakawa: Conceptualization; investigation; writing—review and editing. Tomonori Nagai: Conceptualization; investigation; writing—review and editing. Yoshinobu Hamada: Conceptualization; investigation; writing—review and editing. Masaya Uno: Conceptualization; investigation; writing—review and editing. Mayuyo Mori-Uchino: Conceptualization; investigation; writing—review and editing. Michitaka Ohwada: Conceptualization; investigation; writing—review and editing. Akira Mitsuhashi: Conceptualization; investigation; writing—review and editing. Toyomi Satoh: Conceptualization; funding acquisition; methodology; supervision; writing—review and editing. Keiichi Fujiwara: Conceptualization; funding acquisition; investigation; methodology; project administration; supervision; writing—review and editing. Mitsuaki Suzuki: Conceptualization; funding acquisition; supervision; writing—review and editing.
Funding
The GOTIC-VTE trial was funded by Daiichi Sankyo Co., Ltd., Tokyo, Japan. Daiichi Sankyo Co., Ltd., had no role in the study design, conduct of the study, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
The data will be available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Yoshifumi Takahashi received honoraria from Daiichi Sankyo. Hiroyuki Fujiwara received honoraria from Daiichi Sankyo, AstraZeneca, and Takeda Pharmaceuticals. Toyomi Satoh received honoraria from Daiichi Sankyo. Kouji Yamamoto received a research grant from Daiichi Sankyo. Keiich Fujiwara received honoraria from Daiichi Sankyo. The other authors have no conflict of interest. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Ethical approval
This study was conducted in accordance with the principles of the Declaration of Helsinki. Approval of the research protocol by an Institutional Reviewer Board: Jichi Medical University Central Clinical Research Ethics Committee (Permission number: 20JMU001Mre).
Consent to participate
All participants provided written informed consent at the time of the registration.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anderson FA Jr, Spencer FA (2003) Risk factors for venous thromboembolism. Circulation 107:I9–I16. 10.1161/01.CIR.0000078469.07362.E6 [DOI] [PubMed] [Google Scholar]
- 2.Nakamura M, Miyata T, Ozeki Y et al (2014) Current venous thromboembolism management and outcomes in Japan. Circ J 78:708–717. 10.1253/circj.cj-13-0886 [DOI] [PubMed] [Google Scholar]
- 3.Blom JW, Doggen CJ, Osanto S, Rosendaal FR (2005) Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 293:715–722. 10.1001/jama.293.6.715 [DOI] [PubMed] [Google Scholar]
- 4.Walker AJ, Card TR, West J, Crooks C, Grainge MJ (2013) Incidence of venous thromboembolism in patients with cancer—a cohort study using linked United Kingdom databases. Eur J Cancer 49:1404–1413. 10.1016/j.ejca.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 5.Ay C, Pabinger I, Cohen AT (2017) Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost 117:219–230. 10.1160/TH16-08-0615 [DOI] [PubMed] [Google Scholar]
- 6.Gade IL, Braekkan SK, Naess IA et al (2017) The impact of initial cancer stage on the incidence of venous thromboembolism: the Scandinavian Thrombosis and Cancer (STAC) Cohort. J Thromb Haemost 15:1567–1575. 10.1111/jth.13752 [DOI] [PubMed] [Google Scholar]
- 7.Satoh T, Matsumoto K, Tanaka YO et al (2013) Incidence of venous thromboembolism before treatment in cervical cancer and the impact of management on venous thromboembolism after commencement of treatment. Thromb Res 131:e127–e132. 10.1016/j.thromres.2013.01.027 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki T, Tsurimoto S, Tada T et al (2023) Venous thromboembolism in Japanese patients with gynecologic cancer. Clin Appl Thromb Hemost 29:10760296221124120. 10.1177/10760296221124121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morimoto A, Ueda Y, Yokoi T et al (2014) Perioperative venous thromboembolism in patients with gynecological malignancies: a lesson from four years of recent clinical experience. Anticancer Res 34:3589–3595 [PubMed] [Google Scholar]
- 10.Tasaka N, Minaguchi T, Hosokawa Y et al (2020) Prevalence of venous thromboembolism at pretreatment screening and associated risk factors in 2086 patients with gynecological cancer. J Obstet Gynaecol Res 46:765–773. 10.1111/jog.14233 [DOI] [PubMed] [Google Scholar]
- 11.Habu Y, Mitsuhashi A, Hanawa S et al (2021) High prevalence of pulmonary embolism prior to cancer therapies in patients with ovarian and endometrial cancers detected by contrast-enhanced CT using D-dimer as an index. J Surg Oncol 124:106–114. 10.1002/jso.26471 [DOI] [PubMed] [Google Scholar]
- 12.Satoh T, Matsumoto K, Uno K et al (2008) Silent venous thromboembolism before treatment in endometrial cancer and the risk factors. Br J Cancer 99:1034–1039. 10.1038/sj.bjc.6604658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohashi Y, Ikeda M, Kunitoh H et al (2020) Venous thromboembolism in cancer patients: report of baseline data from the multicentre, prospective Cancer-VTE Registry. Jpn J Clin Oncol 50:1246–1253. 10.1093/jjco/hyaa112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohashi Y, Ikeda M, Kunitoh H et al (2022) One-year incidence of venous thromboembolism, bleeding, and death in patients with solid tumors newly initiating cancer treatment: results from the Cancer-VTE Registry. Thromb Res 213:203–213. 10.1016/j.thromres.2021.09.012 [DOI] [PubMed] [Google Scholar]
- 15.Ikeda M, Uetake H, Yoshino T et al (2022) Incidence and risk factors for venous thromboembolism, bleeding, and death in colorectal cancer (Cancer-VTE Registry). Cancer Sci 113:3901–3911. 10.1111/cas.15527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awano N, Okano T, Kawachi R et al (2022) One-year incidences of venous thromboembolism, bleeding, and death in patients with lung cancer (Cancer-VTE Subanalysis). JTO Clin Res Rep 3:100392. 10.1016/j.jtocrr.2022.100392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshikawa T, Sano T, Terashima M et al (2023) Incidence and risk factors for venous thromboembolism in the Cancer-VTE Registry stomach cancer subcohort. Gastric Cancer 26:493–503. 10.1007/s10120-023-01378-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohsumi S, Watanabe K, Kondo N et al (2023) Venous thromboembolism in Japanese patients with breast cancer: subgroup analysis of the Cancer-VTE Registry. Breast Cancer 30:607–616. 10.1007/s12282-023-01452-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okusaka T, Saiura A, Shimada K et al (2023) Incidence and risk factors for venous thromboembolism in the Cancer-VTE Registry pancreatic cancer subcohort. J Gastroenterol 58:1261–1271. 10.1007/s00535-023-02033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomura H, Wada H, Mizuno T et al (2008) Negative predictive value of D-dimer for diagnosis of venous thromboembolism. Int J Hematol 87:250–255. 10.1007/s12185-008-0047-x [DOI] [PubMed] [Google Scholar]
- 21.JCS Joint Working Group (2011) Guideline for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009). Circ J 75:1258–1281. 10.1253/circj.cj-88-0010 [DOI] [PubMed] [Google Scholar]
- 22.Timp JF, Braekkan SK, Versteeg HH et al (2013) Epidemiology of cancer-associated venous thrombosis. Blood 122:1712–1723. 10.1182/blood-2013-04-460121 [DOI] [PubMed] [Google Scholar]
- 23.Heath OM, van Beekhuizen HJ, Nama V et al (2016) Venous thromboembolism at time of diagnosis of ovarian cancer: survival differs in symptomatic and asymptomatic cases. Thromb Res 137:30–35. 10.1016/j.thromres.2015.11.030 [DOI] [PubMed] [Google Scholar]
- 24.Kumar A, Hurtt CC, Cliby WA et al (2017) Concomitant venous thromboembolism at the time of primary EOC diagnosis: perioperative outcomes and survival analyses. Gynecol Oncol 147:514–520. 10.1016/j.ygyno.2017.09.020 [DOI] [PubMed] [Google Scholar]
- 25.Abu Saadeh F, Norris L, O’Toole S et al (2013) Tumour expresion of tissue factor and tissue factor pathway inhibitor in ovarian cancer-relationship with venous thrombosis risk. Thromb Res 132:627–634. 10.1016/j.thromres.2013.09.016 [DOI] [PubMed] [Google Scholar]
- 26.Sakurai M, Matsumoto K, Gosho M et al (2017) Expression of tissue factor in epithelial ovarian carcinoma is involved in the development of venous thromboembolism. Int J Gynecol Cancer 27:37–43. 10.1097/IGC.0000000000000848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamagami W, Nagase S, Takahashi F et al (2017) Clinical statistics of gynecologic cancers in Japan. J Gynecol Oncol 28:e32. 10.3802/jgo.2017.28.e32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weeks KS, Herbach E, McDonald M et al (2020) Meta-analysis of VTE risk: ovarian cancer patients by stage, histology, cytoreduction, and ascites at diagnosis. Obstet Gynecol Int 2020:2374716. 10.1155/2020/2374716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuk JS, Lee B, Kim K et al (2021) Incidence and risk of venous thromboembolism according to primary treatment in women with ovarian cancer: a retrospective cohort study. PLoS ONE 16:e0250723. 10.1371/journal.pone.0250723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seng S, Liu Z, Chiu SK et al (2012) Risk of venous thromboembolism in patients with cancer treated with cisplatin: a systematic review and meta-analysis. J Clin Oncol 30:4416–4426. 10.1200/JCO.2012.42.4358 [DOI] [PubMed] [Google Scholar]
- 31.Tewari KS, Sill MW, Long HJ 3rd et al (2014) Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 370:734–743. 10.1056/NEJMoa1309748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger RA, Brady MF, Bookman MA et al (2011) Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473–2483. 10.1056/NEJMoa1104390 [DOI] [PubMed] [Google Scholar]
- 33.Totzeck M, Mincu RI, Rassaf T (2017) Cardiovascular adverse events in patients with cancer treated with bevacizumab: a meta-analysis of more than 20,000 patients. J Am Heart Assoc 6:e006278. 10.1161/JAHA.117.006278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nalluri SR, Chu D, Keresztes R et al (2008) Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 300:2277–2285. 10.1001/jama.2008.656 [DOI] [PubMed] [Google Scholar]
- 35.Scappaticci FA, Skillings JR, Holden SN et al (2007) Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 99:1232–1239. 10.1093/jnci/djm086 [DOI] [PubMed] [Google Scholar]
- 36.Hurwitz HI, Saltz LB, Van Cutsem E et al (2011) Venous thromboembolic events with chemotherapy plus bevacizumab: a pooled analysis of patients in randomized phase II and III studies. J Clin Oncol 29:1757–1764. 10.1200/JCO.2010.32.3220 [DOI] [PubMed] [Google Scholar]
- 37.Moik F, Ay C (2022) Venous and arterial thromboembolism in patients with cancer treated with targeted anti-cancer therapies. Thromb Res 213:S58–S65. 10.1016/j.thromres.2022.01.004 [DOI] [PubMed] [Google Scholar]
- 38.Chen N, Ren M, Li R et al (2015) Bevacizumab promotes venous thromboembolism through the induction of PAI-1 in a mouse xenograft model of human lung carcinoma. Mol Cancer 14:140. 10.1186/s12943-015-0418-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khorana AA, Kuderer NM, Culakova E et al (2008) Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 111:4902–4907. 10.1182/blood-2007-10-116327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overvad TF, Ording AG, Nielsen PB et al (2022) Validation of the Khorana score for predicting venous thromboembolism in 40 218 patients with cancer initiating chemotherapy. Blood Adv 6:2967–2976. 10.1182/bloodadvances.2021006484 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be available from the corresponding author on reasonable request.