Abstract
The status of various cholinergic markers was compared in Alzheimer's and Parkinson's diseases. Rather unexpectedly, similar decrements were observed in choline acetyltransferase (ChAT) activity and in density of muscarinic M2 and nicotinic receptors in various cortical areas in these two disorders. This may relate to the existence of important functional interactions between cholinergic and dopaminergic systems in cortical and hippocampal areas. Additionally, the parallel decrements in nicotinic and muscarinic M2 receptor subtypes, with that of ChAT activities in these disorders suggest their presynaptic location. A series of pharmacological data do in fact reveal that nicotinic receptors may act as positive autoreceptors modulating basal acetylcholine release while muscarinic M2 receptors could act as negative autoreceptors. This information may have significance for the development of new treatment strategies (for example, M2 antagonists) of disorders associated with cholinergic hypofunction.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo D. M., Lapchak P. A., Collier B., Quirion R. Characterization of N-[3H]methylcarbamylcholine binding sites and effect of N-methylcarbamylcholine on acetylcholine release in rat brain. J Neurochem. 1988 Jul;51(1):292–299. doi: 10.1111/j.1471-4159.1988.tb04869.x. [DOI] [PubMed] [Google Scholar]
- Araujo D. M., Lapchak P. A., Meaney M. J., Collier B., Quirion R. Effects of aging on nicotinic and muscarinic autoreceptor function in the rat brain: relationship to presynaptic cholinergic markers and binding sites. J Neurosci. 1990 Sep;10(9):3069–3078. doi: 10.1523/JNEUROSCI.10-09-03069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo D. M., Lapchak P. A., Regenold W., Quirion R. Characterization of [3H]AF-DX 116 binding sites in the rat brain: evidence for heterogeneity of muscarinic-M2 receptor sites. Synapse. 1989;4(2):106–114. doi: 10.1002/syn.890040204. [DOI] [PubMed] [Google Scholar]
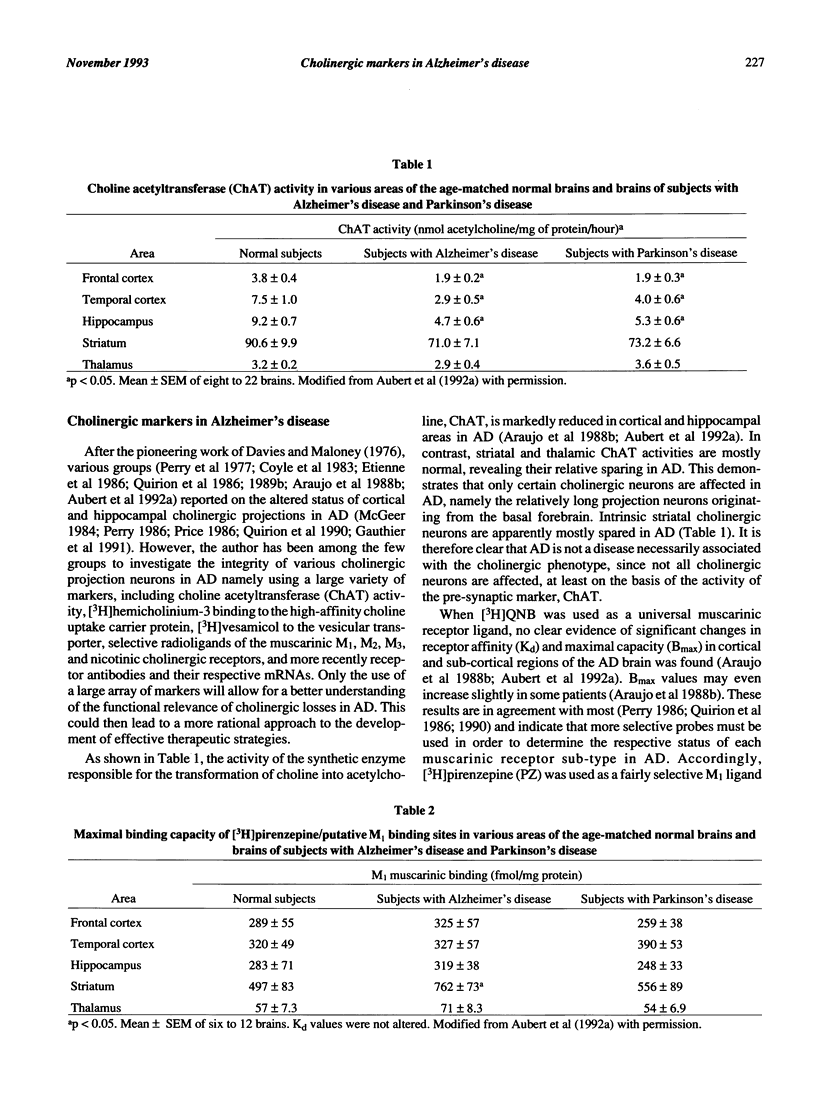
- Araujo D. M., Lapchak P. A., Robitaille Y., Gauthier S., Quirion R. Differential alteration of various cholinergic markers in cortical and subcortical regions of human brain in Alzheimer's disease. J Neurochem. 1988 Jun;50(6):1914–1923. doi: 10.1111/j.1471-4159.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
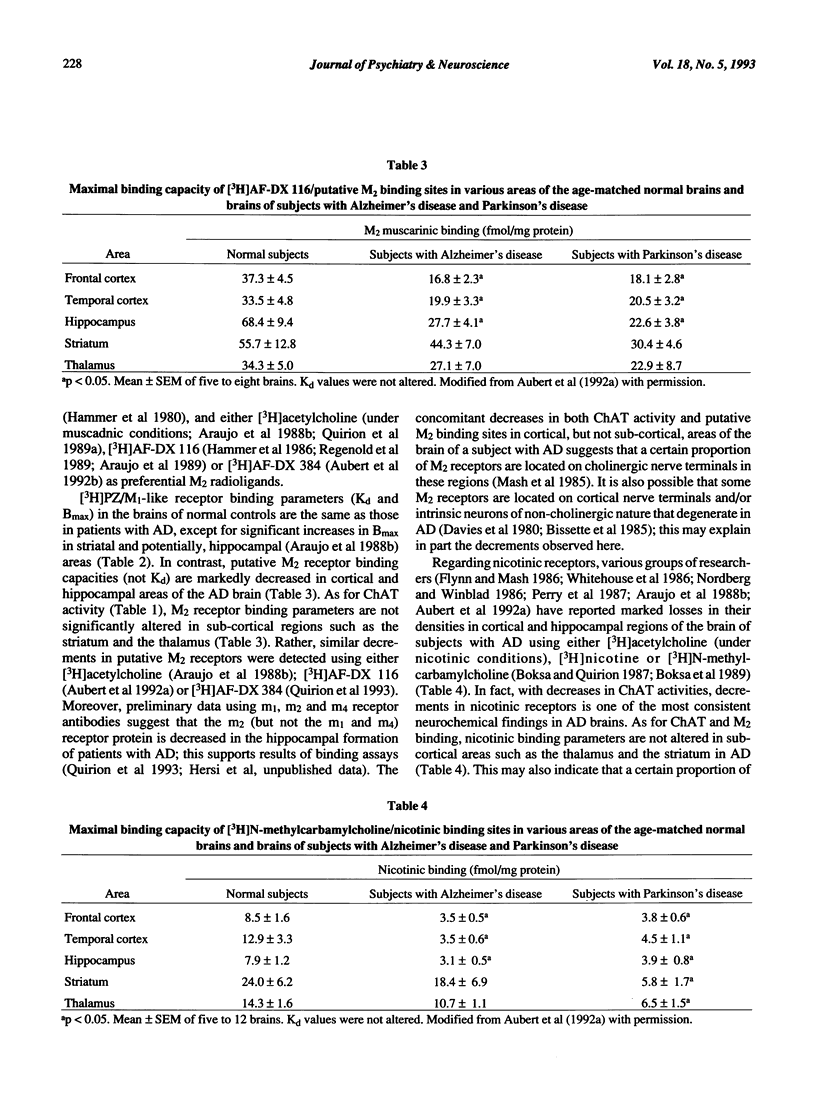
- Aubert I., Araujo D. M., Cécyre D., Robitaille Y., Gauthier S., Quirion R. Comparative alterations of nicotinic and muscarinic binding sites in Alzheimer's and Parkinson's diseases. J Neurochem. 1992 Feb;58(2):529–541. doi: 10.1111/j.1471-4159.1992.tb09752.x. [DOI] [PubMed] [Google Scholar]
- Aubert I., Cécyre D., Gauthier S., Quirion R. Characterization and autoradiographic distribution of [3H]AF-DX 384 binding to putative muscarinic M2 receptors in the rat brain. Eur J Pharmacol. 1992 Jul 7;217(2-3):173–184. doi: 10.1016/0014-2999(92)90843-s. [DOI] [PubMed] [Google Scholar]
- Ball M. J. Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia. A quantitative study. Acta Neuropathol. 1977 Feb 28;37(2):111–118. doi: 10.1007/BF00692056. [DOI] [PubMed] [Google Scholar]
- Beani L., Bianchi C., Nilsson L., Nordberg A., Romanelli L., Sivilotti L. The effect of nicotine and cytisine on 3H-acetylcholine release from cortical slices of guinea-pig brain. Naunyn Schmiedebergs Arch Pharmacol. 1985 Nov;331(2-3):293–296. doi: 10.1007/BF00634252. [DOI] [PubMed] [Google Scholar]
- Bissette G., Reynolds G. P., Kilts C. D., Widerlöv E., Nemeroff C. B. Corticotropin-releasing factor-like immunoreactivity in senile dementia of the Alzheimer type. Reduced cortical and striatal concentrations. JAMA. 1985 Dec 6;254(21):3067–3069. [PubMed] [Google Scholar]
- Boksa P., Quik M., Mitchell J. B., Collier B., O'Neil W., Quirion R. Pharmacological activity of N-methyl-carbamylcholine, a novel acetylcholine receptor agonist with selectivity for nicotinic receptors. Eur J Pharmacol. 1989 Nov 28;173(1):93–108. doi: 10.1016/0014-2999(89)90012-5. [DOI] [PubMed] [Google Scholar]
- Boksa P., Quirion R. [3H]N-methyl-carbamylcholine, a new radioligand specific for nicotinic acetylcholine receptors in brain. Eur J Pharmacol. 1987 Jul 23;139(3):323–333. doi: 10.1016/0014-2999(87)90590-5. [DOI] [PubMed] [Google Scholar]
- Buxbaum J. D., Oishi M., Chen H. I., Pinkas-Kramarski R., Jaffe E. A., Gandy S. E., Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. T., Price D. L., DeLong M. R. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983 Mar 11;219(4589):1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Davies P., Katzman R., Terry R. D. Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer disease and Alzheimer senile dementa. Nature. 1980 Nov 20;288(5788):279–280. doi: 10.1038/288279a0. [DOI] [PubMed] [Google Scholar]
- Davies P., Maloney A. J. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976 Dec 25;2(8000):1403–1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Davis K. L., Thal L. J., Gamzu E. R., Davis C. S., Woolson R. F., Gracon S. I., Drachman D. A., Schneider L. S., Whitehouse P. J., Hoover T. M. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer's disease. The Tacrine Collaborative Study Group. N Engl J Med. 1992 Oct 29;327(18):1253–1259. doi: 10.1056/NEJM199210293271801. [DOI] [PubMed] [Google Scholar]
- Day J., Fibiger H. C. Dopaminergic regulation of cortical acetylcholine release. Synapse. 1992 Dec;12(4):281–286. doi: 10.1002/syn.890120405. [DOI] [PubMed] [Google Scholar]
- De Sarno P., Giacobini E. Modulation of acetylcholine release by nicotinic receptors in the rat brain. J Neurosci Res. 1989 Feb;22(2):194–200. doi: 10.1002/jnr.490220213. [DOI] [PubMed] [Google Scholar]
- Doods H. N., Quirion R., Mihm G., Engel W., Rudolf K., Entzeroth M., Schiavi G. B., Ladinsky H., Bechtel W. D., Ensinger H. A. Therapeutic potential of CNS-active M2 antagonists: novel structures and pharmacology. Life Sci. 1993;52(5-6):497–503. doi: 10.1016/0024-3205(93)90307-o. [DOI] [PubMed] [Google Scholar]
- Etienne P., Robitaille Y., Wood P., Gauthier S., Nair N. P., Quirion R. Nucleus basalis neuronal loss, neuritic plaques and choline acetyltransferase activity in advanced Alzheimer's disease. Neuroscience. 1986 Dec;19(4):1279–1291. doi: 10.1016/0306-4522(86)90142-9. [DOI] [PubMed] [Google Scholar]
- Flynn D. D., Mash D. C. Characterization of L-[3H]nicotine binding in human cerebral cortex: comparison between Alzheimer's disease and the normal. J Neurochem. 1986 Dec;47(6):1948–1954. doi: 10.1111/j.1471-4159.1986.tb13113.x. [DOI] [PubMed] [Google Scholar]
- Gauthier S., Gauthier L., Bouchard R., Quirion R., Sultan S. Treatment of Alzheimer's disease: hopes and reality. Can J Neurol Sci. 1991 Aug;18(3 Suppl):439–441. doi: 10.1017/s0317167100032637. [DOI] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Hammer R., Giraldo E., Schiavi G. B., Monferini E., Ladinsky H. Binding profile of a novel cardioselective muscarine receptor antagonist, AF-DX 116, to membranes of peripheral tissues and brain in the rat. Life Sci. 1986 May 5;38(18):1653–1662. doi: 10.1016/0024-3205(86)90409-1. [DOI] [PubMed] [Google Scholar]
- Hoss W., Messer W. S., Jr, Monsma F. J., Jr, Miller M. D., Ellerbrock B. R., Scranton T., Ghodsi-Hovsepian S., Price M. A., Balan S., Mazloum Z. Biochemical and behavioral evidence for muscarinic autoreceptors in the CNS. Brain Res. 1990 May 28;517(1-2):195–201. doi: 10.1016/0006-8993(90)91026-d. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kish S. J., Distefano L. M., Dozic S., Robitaille Y., Rajput A., Deck J. H., Hornykiewicz O. [3H]vesamicol binding in human brain cholinergic deficiency disorders. Neurosci Lett. 1990 Sep 18;117(3):347–352. doi: 10.1016/0304-3940(90)90689-7. [DOI] [PubMed] [Google Scholar]
- Lange K. W., Wells F. R., Jenner P., Marsden C. D. Altered muscarinic and nicotinic receptor densities in cortical and subcortical brain regions in Parkinson's disease. J Neurochem. 1993 Jan;60(1):197–203. doi: 10.1111/j.1471-4159.1993.tb05838.x. [DOI] [PubMed] [Google Scholar]
- Lapchak P. A., Araujo D. M., Quirion R., Collier B. Binding sites for [3H]AF-DX 116 and effect of AF-DX 116 on endogenous acetylcholine release from rat brain slices. Brain Res. 1989 Sep 4;496(1-2):285–294. doi: 10.1016/0006-8993(89)91075-5. [DOI] [PubMed] [Google Scholar]
- Marchi M., Raiteri M. On the presence in the cerebral cortex of muscarinic receptor subtypes which differ in neuronal localization, function and pharmacological properties. J Pharmacol Exp Ther. 1985 Oct;235(1):230–233. [PubMed] [Google Scholar]
- Marx J. Alzheimer's pathology begins to yield its secrets. Science. 1993 Jan 22;259(5094):457–458. doi: 10.1126/science.8424167. [DOI] [PubMed] [Google Scholar]
- Mash D. C., Flynn D. D., Potter L. T. Loss of M2 muscarine receptors in the cerebral cortex in Alzheimer's disease and experimental cholinergic denervation. Science. 1985 May 31;228(4703):1115–1117. doi: 10.1126/science.3992249. [DOI] [PubMed] [Google Scholar]
- McGeer P. L. The 12th J. A. F. Stevenson memorial lecture. Aging, Alzheimer's disease, and the cholinergic system. Can J Physiol Pharmacol. 1984 Jul;62(7):741–754. doi: 10.1139/y84-123. [DOI] [PubMed] [Google Scholar]
- McGurk S. R., Levin E. D., Butcher L. L. Dopaminergic drugs reverse the impairment of radial-arm maze performance caused by lesions involving the cholinergic medial pathway. Neuroscience. 1992 Sep;50(1):129–135. doi: 10.1016/0306-4522(92)90387-h. [DOI] [PubMed] [Google Scholar]
- Newhouse P. A., Sunderland T., Tariot P. N., Blumhardt C. L., Weingartner H., Mellow A., Murphy D. L. Intravenous nicotine in Alzheimer's disease: a pilot study. Psychopharmacology (Berl) 1988;95(2):171–175. doi: 10.1007/BF00174504. [DOI] [PubMed] [Google Scholar]
- Nordberg A., Winblad B. Reduced number of [3H]nicotine and [3H]acetylcholine binding sites in the frontal cortex of Alzheimer brains. Neurosci Lett. 1986 Dec 3;72(1):115–119. doi: 10.1016/0304-3940(86)90629-4. [DOI] [PubMed] [Google Scholar]
- Packard M. G., Regenold W., Quirion R., White N. M. Post-training injection of the acetylcholine M2 receptor antagonist AF-DX 116 improves memory. Brain Res. 1990 Jul 30;524(1):72–76. doi: 10.1016/0006-8993(90)90493-u. [DOI] [PubMed] [Google Scholar]
- Pascual J., Fontán A., Zarranz J. J., Berciano J., Flórez J., Pazos A. High-affinity choline uptake carrier in Alzheimer's disease: implications for the cholinergic hypothesis of dementia. Brain Res. 1991 Jun 21;552(1):170–174. doi: 10.1016/0006-8993(91)90676-m. [DOI] [PubMed] [Google Scholar]
- Perry E. K., Gibson P. H., Blessed G., Perry R. H., Tomlinson B. E. Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. J Neurol Sci. 1977 Nov;34(2):247–265. doi: 10.1016/0022-510x(77)90073-9. [DOI] [PubMed] [Google Scholar]
- Perry E. K., McKeith I., Thompson P., Marshall E., Kerwin J., Jabeen S., Edwardson J. A., Ince P., Blessed G., Irving D. Topography, extent, and clinical relevance of neurochemical deficits in dementia of Lewy body type, Parkinson's disease, and Alzheimer's disease. Ann N Y Acad Sci. 1991;640:197–202. doi: 10.1111/j.1749-6632.1991.tb00217.x. [DOI] [PubMed] [Google Scholar]
- Perry E. K., Perry R. H., Smith C. J., Dick D. J., Candy J. M., Edwardson J. A., Fairbairn A., Blessed G. Nicotinic receptor abnormalities in Alzheimer's and Parkinson's diseases. J Neurol Neurosurg Psychiatry. 1987 Jun;50(6):806–809. doi: 10.1136/jnnp.50.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry E. K. The cholinergic hypothesis--ten years on. Br Med Bull. 1986 Jan;42(1):63–69. doi: 10.1093/oxfordjournals.bmb.a072100. [DOI] [PubMed] [Google Scholar]
- Pohorecki R., Head R., Domino E. F. Effects of selected muscarinic cholinergic antagonists on [3H]acetylcholine release from rat hippocampal slices. J Pharmacol Exp Ther. 1988 Jan;244(1):213–217. [PubMed] [Google Scholar]
- Price D. L. New perspectives on Alzheimer's disease. Annu Rev Neurosci. 1986;9:489–512. doi: 10.1146/annurev.ne.09.030186.002421. [DOI] [PubMed] [Google Scholar]
- Quirion R., Araujo D., Regenold W., Boksa P. Characterization and quantitative autoradiographic distribution of [3H]acetylcholine muscarinic receptors in mammalian brain. Apparent labelling of an M2-like receptor sub-type. Neuroscience. 1989;29(2):271–289. doi: 10.1016/0306-4522(89)90057-2. [DOI] [PubMed] [Google Scholar]
- Quirion R., Aubert I., Araujo D. M., Hersi A., Gaudreau P. Autoradiographic distribution of putative muscarinic receptor sub-types in mammalian brain. Prog Brain Res. 1993;98:85–93. doi: 10.1016/s0079-6123(08)62384-5. [DOI] [PubMed] [Google Scholar]
- Quirion R., Aubert I., Lapchak P. A., Schaum R. P., Teolis S., Gauthier S., Araujo D. M. Muscarinic receptor subtypes in human neurodegenerative disorders: focus on Alzheimer's disease. Trends Pharmacol Sci. 1989 Dec;Suppl:80–84. [PubMed] [Google Scholar]
- Quirion R., Aubert I., Robitaille Y., Gauthier S., Araujo D. M., Chabot J. G. Neurochemical deficits in pathological brain aging: specificity and possible relevance for treatment strategies. Clin Neuropharmacol. 1990;13 (Suppl 3):S73–S80. doi: 10.1097/00002826-199013003-00008. [DOI] [PubMed] [Google Scholar]
- Quirion R. Characterization and autoradiographic distribution of hemicholinium-3 high-affinity choline uptake sites in mammalian brain. Synapse. 1987;1(4):293–303. doi: 10.1002/syn.890010403. [DOI] [PubMed] [Google Scholar]
- Quirion R., Martel J. C., Robitaille Y., Etienne P., Wood P., Nair N. P., Gauthier S. Neurotransmitter and receptor deficits in senile dementia of the Alzheimer type. Can J Neurol Sci. 1986 Nov;13(4 Suppl):503–510. doi: 10.1017/s0317167100037215. [DOI] [PubMed] [Google Scholar]
- Raiteri M., Leardi R., Marchi M. Heterogeneity of presynaptic muscarinic receptors regulating neurotransmitter release in the rat brain. J Pharmacol Exp Ther. 1984 Jan;228(1):209–214. [PubMed] [Google Scholar]
- Regenold W., Araujo D. M., Quirion R. Quantitative autoradiographic distribution of [3H]AF-DX 116 muscarinic-M2 receptor binding sites in rat brain. Synapse. 1989;4(2):115–125. doi: 10.1002/syn.890040205. [DOI] [PubMed] [Google Scholar]
- Richards M. H. Rat hippocampal muscarinic autoreceptors are similar to the M2 (cardiac) subtype: comparison with hippocampal M1, atrial M2 and ileal M3 receptors. Br J Pharmacol. 1990 Apr;99(4):753–761. doi: 10.1111/j.1476-5381.1990.tb13002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson I. W., Szerb J. C. The release of labelled acetylcholine and choline from cerebral cortical slices stimulated electrically. Br J Pharmacol. 1974 Dec;52(4):499–507. doi: 10.1111/j.1476-5381.1974.tb09717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell P. P., Winkler D. L. Nicotinic stimulation of [3H]acetylcholine release from mouse cerebral cortical synaptosomes. J Neurochem. 1984 Dec;43(6):1593–1598. doi: 10.1111/j.1471-4159.1984.tb06083.x. [DOI] [PubMed] [Google Scholar]
- Ruberg M., Mayo W., Brice A., Duyckaerts C., Hauw J. J., Simon H., LeMoal M., Agid Y. Choline acetyltransferase activity and [3H]vesamicol binding in the temporal cortex of patients with Alzheimer's disease, Parkinson's disease, and rats with basal forebrain lesions. Neuroscience. 1990;35(2):327–333. doi: 10.1016/0306-4522(90)90086-j. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Amyloid beta protein precursor and the pathogenesis of Alzheimer's disease. Cell. 1989 Aug 25;58(4):611–612. doi: 10.1016/0092-8674(89)90093-7. [DOI] [PubMed] [Google Scholar]
- Summers W. K., Majovski L. V., Marsh G. M., Tachiki K., Kling A. Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer type. N Engl J Med. 1986 Nov 13;315(20):1241–1245. doi: 10.1056/NEJM198611133152001. [DOI] [PubMed] [Google Scholar]
- Sunderland T., Tariot P. N., Newhouse P. A. Differential responsivity of mood, behavior, and cognition to cholinergic agents in elderly neuropsychiatric populations. Brain Res. 1988 Dec;472(4):371–389. doi: 10.1016/0006-8993(88)91227-9. [DOI] [PubMed] [Google Scholar]
- Szerb J. C., Hadházy P., Dudar J. D. Release of [3H]acetylcholine from rat hippocampal slices: effect of septal lesion and of graded concentrations of muscarnic agonists and antagonists. Brain Res. 1977 Jun 10;128(2):285–291. doi: 10.1016/0006-8993(77)90995-7. [DOI] [PubMed] [Google Scholar]
- Terry R. D., Katzman R. Senile dementia of the Alzheimer type. Ann Neurol. 1983 Nov;14(5):497–506. doi: 10.1002/ana.410140502. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Martino A. M., Antuono P. G., Lowenstein P. R., Coyle J. T., Price D. L., Kellar K. J. Nicotinic acetylcholine binding sites in Alzheimer's disease. Brain Res. 1986 Apr 16;371(1):146–151. doi: 10.1016/0006-8993(86)90819-x. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Brain nicotine binding sites. Hum Toxicol. 1987 Sep;6(5):343–353. doi: 10.1177/096032718700600502. [DOI] [PubMed] [Google Scholar]


