Abstract
This study was designed to validate a novel animal model of depression by testing the curative effects of the atypical antidepressant mianserin. In this paradigm, the hedonic state of rats was assessed using an intracranial self-stimulation (ICSS) procedure. The ICSS threshold was determined before, during and after a 38-day period of exposure to a variety of intermittent, unpredictable, mild stressors. After 11 days of this regimen, the ICSS threshold was significantly higher in the stressed rats, suggesting a gradual decrease of sensitivity to reward. This "anhedonia" lasted throughout the stress regimen and progressively diminished over a 20-day period after stress was terminated. When stressed animals exhibiting anhedonia were treated with mianserin, the stress-induced increase in the ICSS threshold was gradually reversed over ten days of treatment. These results provide further support for the value of this anhedonia paradigm in modelling an important aspect of human depressive disorders.
Full text
PDF

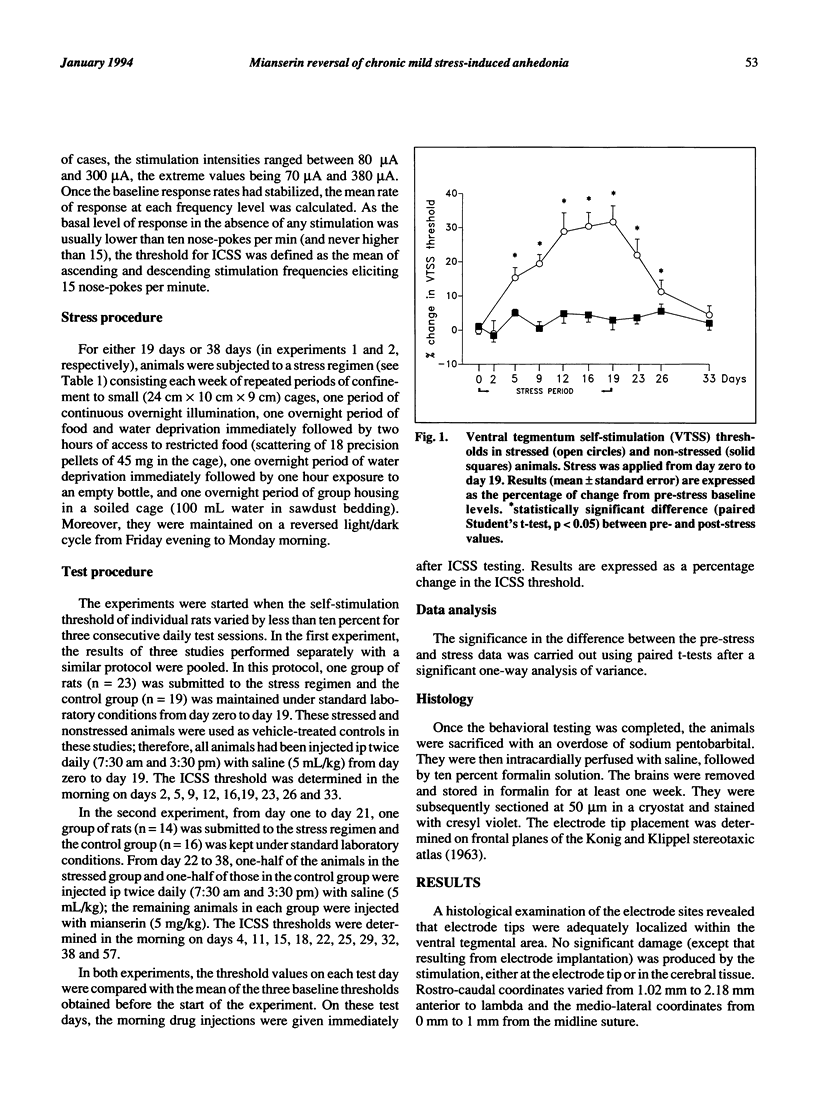
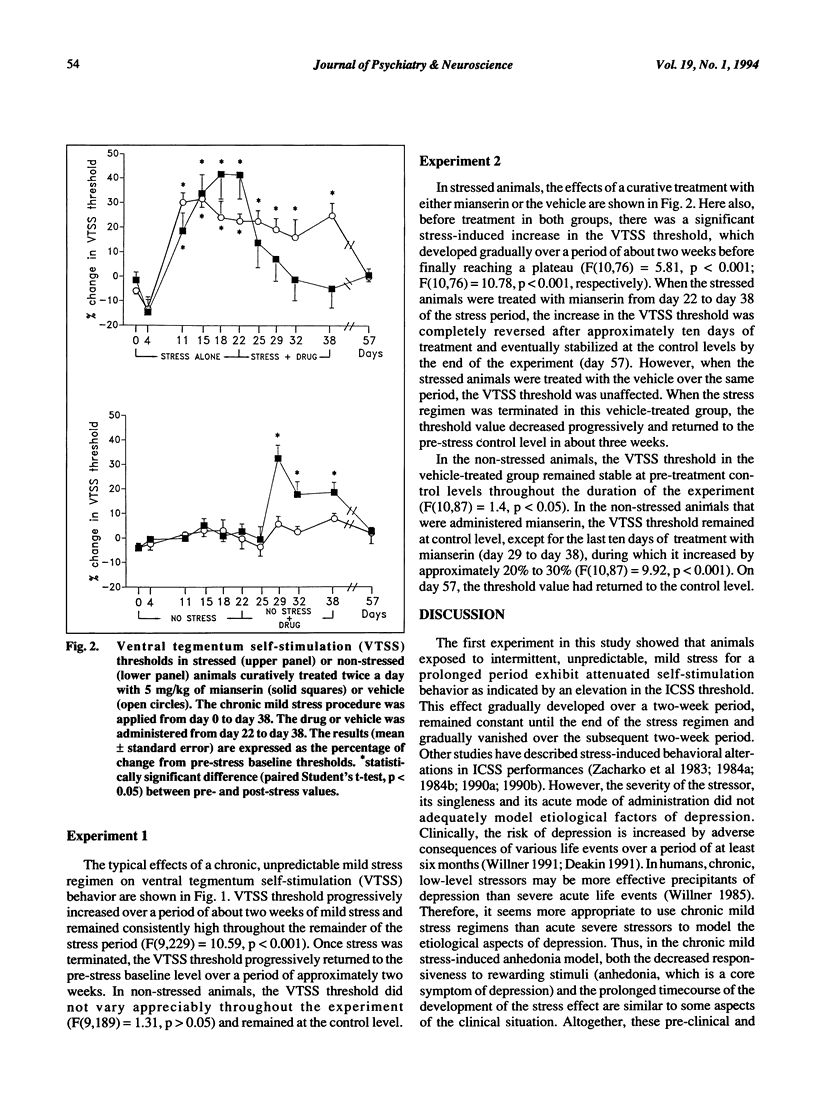


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiskal H. S. Interaction of biologic and psychologic factors in the origin of depressive disorders. Acta Psychiatr Scand Suppl. 1985;319:131–139. doi: 10.1111/j.1600-0447.1985.tb08529.x. [DOI] [PubMed] [Google Scholar]
- Akiskal H. S., McKinney W. T., Jr Overview of recent research in depression. Integration of ten conceptual models into a comprehensive clinical frame. Arch Gen Psychiatry. 1975 Mar;32(3):285–305. doi: 10.1001/archpsyc.1975.01760210019001. [DOI] [PubMed] [Google Scholar]
- Fibiger H. C. The neurobiological substrates of depression in Parkinson's disease: a hypothesis. Can J Neurol Sci. 1984 Feb;11(1 Suppl):105–107. doi: 10.1017/s0317167100046230. [DOI] [PubMed] [Google Scholar]
- Hall F. S., Stellar J. R., Kelley A. E. Acute and chronic desipramine treatment effects on rewarding electrical stimulation of the lateral hypothalamus. Pharmacol Biochem Behav. 1990 Oct;37(2):277–281. doi: 10.1016/0091-3057(90)90334-e. [DOI] [PubMed] [Google Scholar]
- Klein D. F. Endogenomorphic depression. A conceptual and terminological revision. Arch Gen Psychiatry. 1974 Oct;31(4):447–454. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- Markou A., Koob G. F. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991 Jan;4(1):17–26. [PubMed] [Google Scholar]
- Moreau J. L., Jenck F., Martin J. R., Mortas P., Haefely W. E. Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behavior in rats. Eur Neuropsychopharmacol. 1992 Mar;2(1):43–49. doi: 10.1016/0924-977x(92)90035-7. [DOI] [PubMed] [Google Scholar]
- Moreau J. L., Jenck F., Martin J. R., Mortas P., Haefely W. Effects of moclobemide, a new generation reversible Mao-A inhibitor, in a novel animal model of depression. Pharmacopsychiatry. 1993 Jan;26(1):30–33. doi: 10.1055/s-2007-1014338. [DOI] [PubMed] [Google Scholar]
- Muscat R., Papp M., Willner P. Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology (Berl) 1992;109(4):433–438. doi: 10.1007/BF02247719. [DOI] [PubMed] [Google Scholar]
- Muscat R., Sampson D., Willner P. Dopaminergic mechanism of imipramine action in an animal model of depression. Biol Psychiatry. 1990 Aug 1;28(3):223–230. doi: 10.1016/0006-3223(90)90577-o. [DOI] [PubMed] [Google Scholar]
- Papp M., Muscat R., Willner P. Subsensitivity to rewarding and locomotor stimulant effects of a dopamine agonist following chronic mild stress. Psychopharmacology (Berl) 1993;110(1-2):152–158. doi: 10.1007/BF02246965. [DOI] [PubMed] [Google Scholar]
- Papp M., Willner P., Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104(2):255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Phillips A. G. Brain reward circuitry: a case for separate systems. Brain Res Bull. 1984 Feb;12(2):195–201. doi: 10.1016/0361-9230(84)90189-8. [DOI] [PubMed] [Google Scholar]
- Sampson D., Willner P., Muscat R. Reversal of antidepressant action by dopamine antagonists in an animal model of depression. Psychopharmacology (Berl) 1991;104(4):491–495. doi: 10.1007/BF02245655. [DOI] [PubMed] [Google Scholar]
- Stamford J. A., Muscat R., O'Connor J. J., Patel J., Trout S. J., Wieczorek W. J., Kruk Z. L., Willner P. Voltammetric evidence that subsensitivity to reward following chronic mild stress is associated with increased release of mesolimbic dopamine. Psychopharmacology (Berl) 1991;105(2):275–282. doi: 10.1007/BF02244322. [DOI] [PubMed] [Google Scholar]
- Tiller J. W., Schweitzer I., Maguire K. P., Davis B. Is diazepam an antidepressant? Br J Psychiatry. 1989 Oct;155:483–489. doi: 10.1192/bjp.155.4.483. [DOI] [PubMed] [Google Scholar]
- Willner P., Muscat R., Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992 Winter;16(4):525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Willner P., Towell A., Sampson D., Sophokleous S., Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Wise R. A., Bozarth M. A. Brain reward circuitry: four circuit elements "wired" in apparent series. Brain Res Bull. 1984 Feb;12(2):203–208. doi: 10.1016/0361-9230(84)90190-4. [DOI] [PubMed] [Google Scholar]
- Wise R. A. Catecholamine theories of reward: a critical review. Brain Res. 1978 Aug 25;152(2):215–247. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- Zacharko R. M., Bowers W. J., Anisman H. Responding for brain stimulation: stress and desmethylimipramine. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8(4-6):601–606. doi: 10.1016/0278-5846(84)90021-6. [DOI] [PubMed] [Google Scholar]
- Zacharko R. M., Bowers W. J., Kelley M. S., Anisman H. Prevention of stressor-induced disturbances of self-stimulation by desmethylimipramine. Brain Res. 1984 Oct 29;321(1):175–179. doi: 10.1016/0006-8993(84)90697-8. [DOI] [PubMed] [Google Scholar]
- Zacharko R. M., Bowers W. J., Kokkinidis L., Anisman H. Region-specific reductions of intracranial self-stimulation after uncontrollable stress: possible effects on reward processes. Behav Brain Res. 1983 Aug;9(2):129–141. doi: 10.1016/0166-4328(83)90123-7. [DOI] [PubMed] [Google Scholar]
- Zacharko R. M., Kasian M., MacNeil G., Anisman H. Stressor-induced behavioral alterations in intracranial self-stimulation from the ventral tegmental area: evidence for regional variations. Brain Res Bull. 1990 Oct;25(4):617–621. doi: 10.1016/0361-9230(90)90122-g. [DOI] [PubMed] [Google Scholar]


