ABSTRACT
Comparative approaches in animal gut microbiome research have revealed patterns of phylosymbiosis, dietary and physiological convergences, and environment–host interactions. However, most large‐scale comparative studies, especially those that are highly cited, have focused on mammals, and efforts to integrate comparative approaches with existing ecological frameworks are lacking. While mammals serve as useful model organisms, developing generalised principles of how animal gut microbiomes are shaped and how these microbiomes interact bidirectionally with host ecology and evolution requires a more complete sampling of the animal kingdom. Here, we provide an overview of what past comparative studies have taught us about the gut microbiome, and how community ecology theory may help resolve certain contradictions in comparative gut microbiome research. We explore whether certain hypotheses are supported across clades, and how the disproportionate focus on mammals has introduced potential bias into gut microbiome theory. We then introduce a methodological solution by which public gut microbiome data of understudied hosts can be compiled and analysed in a comparative context. Our aggregation and analysis of 179 studies shows that generating data sets with rich host diversity is possible with public data and that key gut microbes associated with mammals are widespread across the animal kingdom. We also show the effects that sample size and taxonomic rank have on comparative gut microbiome studies and that results of multivariate analyses can vary significantly with these two parameters. While challenges remain in developing a universal model of the animal gut microbiome, we show that existing ecological frameworks can help bring us one step closer to integrating the gut microbiome into animal ecology and evolution.
Keywords: gut microbiome, comparative, ecology, host–microbe interactions, evolution
I. INTRODUCTION
Gut microbiomes are critical components of animal physiology and survival (Kinross, Darzi & Nicholson, 2011; Gibson et al., 2019; Worsley et al., 2021; Comizzoli et al., 2021). As a result, comparative gut microbiome studies are integral to understanding animal ecology and evolution. The beginning of comparative gut microbiome approaches is best highlighted by Ley et al. (2008) which leveraged 16S high‐throughput sequencing to reveal host phylogeny, diet, and environment as important host factors shaping mammalian gut microbiomes. Later studies further highlighted the role of host diet and phylogeny (Muegge et al., 2011; Groussin et al., 2017; Rojas et al., 2021) in shaping mammal gut microbiomes, however, the auto‐correlative nature between these two variables made them difficult to tease apart (Amato et al., 2018; Groussin, Mazel & Alm, 2020). Thus, uncovering generalizable ‘rules’ that govern animal gut microbiomes remains a difficult task.
Comparative gut microbiome research also revealed that results of comparative analyses are highly host dependent. For example, in primates, host phylogeny has a greater effect than host diet (Comizzoli et al., 2021; Gibson et al., 2019; Kinross et al., 2011; Worsley et al., 2021) in explaining gut microbiome diversity but the opposite is true for other mammals (Muegge et al., 2011; Youngblut et al., 2019). In fish, diet largely shapes surgeonfish (Acanthuridae) gut microbiomes (Miyake, Ngugi & Stingl, 2015) while the environment outweighs diet in a large number of fish species from the South China Sea (Kim et al., 2021). At a broader scale, phylosymbiosis (the degree to which gut microbiome compositions are specific to their host) is putatively higher in mammals than other vertebrate classes and also varies greatly across mammalian orders (Youngblut et al., 2019). By contrast, flight adaptation, rather than phylosymbiosis, may have determined gut microbiome composition in birds and bats (Song et al., 2020). These contrasting findings are pervasive across the animal kingdom, highlighting a potential phylogenetic dependency of how host factors determine gut microbiome composition and diversity and a need for more clade‐specific data.
Despite such contradictions, most comparative studies do show that gut microbiomes are highly multivariable – influenced by many host factors, such as diet (Muegge et al., 2011), gut anatomy (Amato et al., 2018), immune factors (Shi et al., 2017), and social behaviour (Song et al., 2013). What is not so obvious is how the effects of these host factors change in magnitude across different clades of hosts. Moreover, the extent to how microbes shape animal health and survival is unclear, particularly in non‐mammalian clades. For example, in humans and mice, Akkermansia muciniphila relative abundance is positively associated with high‐fibre diets and exercise and negatively associated with inflammation and disease (Geerlings et al., 2018; Naito, Uchiyama & Takagi, 2018). Fecalibacterium prausnitzii, another human‐associated microbe, has been implicated in a number of physiological processes involved in human health and disease (Cao, Shen & Ran, 2014; Lopez‐Siles et al., 2017; Parsaei et al., 2021) However, it is unclear whether such associations extend to other animal clades, including non‐mammals. Comparing host–microbe associations found in humans to other animal hosts can help us build a more accurate host–microbe model to help uncover the possible origins of these vital symbiotic relationships.
II. MAMMALIAN GUT MICROBIOME DATA MAY BIAS GUT MICROBIOME THEORY
Mammals are disproportionately studied in the gut microbiome literature relative to other clades (Fig. 1). When considering the diversity of clades such as fish (~29,000 species), reptiles (~12,000), birds (~10,000 species), and amphibians (~8000 species), compared to mammals (~5000 species), it is clear that the gut microbiome of the animal kingdom has not been sampled evenly (Colston & Jackson, 2016). Thus, most gut microbiome theory developed to date is based largely on mammal‐focused studies, potentially biasing the framework we use to construct models that attempt to explain the origins and functions of the gut microbiome. Moreover, a large majority of these studies are on humans and laboratory animals (compare upper and lower panels of Fig. 1). When considering only non‐comparative (i.e. single‐species) studies that sampled wild hosts, the numbers of studies between mammals and other taxa are more proportionate. There is thus reasonable taxon coverage in the existing literature and there is potential to analyse such data in a large‐scale comparative approach [see Hoffbeck et al. (2023) and Yang et al. (2022)]. Despite the availability of non‐mammalian gut microbiome studies, those that are highly cited tend to include only mammal‐centric data sets (Ley et al., 2008; Muegge et al., 2011; Amato et al., 2018; Youngblut et al., 2019), creating a bias may have influenced our efforts to understand the animal gut microbiome.
Fig. 1.

Number of gut microbiome studies across different host clades, and whether a study was comparative or not. Studies were collected in September 2023 using the Scopus document search tool. We counted only published journal articles and excluded all other article types (including reviews). The top row includes studies with human subjects and laboratory animals (w/ humans & lab animals) while the bottom row does not include these studies (w/o humans or lab animals). Both 16S and metagenomic studies are included.
One example of such bias is in investigation of the importance of diet in shaping gut microbiome diversity. Host diet is often found to be an important driver of gut microbiome diversity in animal hosts. However, many studies that identified a large diet effect, did so with mammalian data sets (Ley et al., 2008; Muegge et al., 2011; Groussin et al., 2017; Youngblut et al., 2019). Moreover, significant effects of host diet have been repeatedly established in comparisons of mammalian carnivores and herbivores, which largely belong to two phylogenetically distinct orders (Carnivora and Artiodactyla), making it difficult to tease apart the effects of diet from those of host phylogeny. While the importance of host diet has been established in other animals (Hong et al., 2011; Miyake et al., 2015), there are also cases where no such effect was found [e.g. in primates (Amato et al., 2018) and the giant panda Ailuropoda melanoleuca (Wei, Wang & Wu, 2015; Guo et al., 2018)]. Thus, an overrepresentation of studies on carnivores and artiodactyls, which have very different gut anatomies and feeding adaptations (Muegge et al., 2011), may have skewed current views of the importance of diet in shaping gut microbiomes. It is more likely that the relative importance of different factors shaping animal gut microbiomes will vary greatly from clade to clade (Fig. 2). Diet may well be an important driver of gut microbiome diversity, however comparisons of a wider range of hosts with different dietary adaptations across the animal kingdom are needed.
Fig. 2.
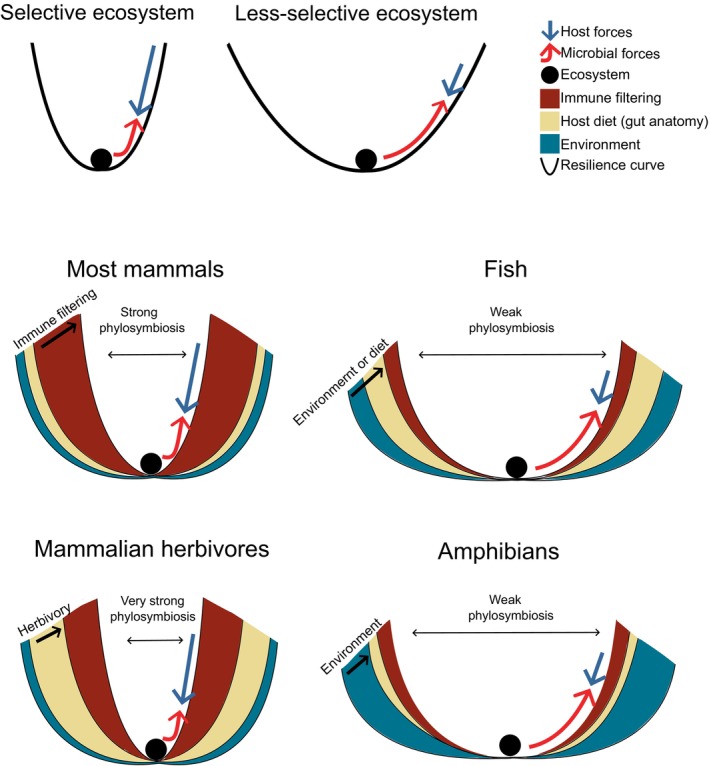
An ecosystem selection ‘ball and cup’ model of the animal gut microbiome that accounts for the multivariate shaping of gut microbiome composition across different host clades. A narrow resilience curve (black line on top left) results in a selective ecosystem due to strong host selective pressures such as immune filtering (blue arrow), whereas a wider curve (top right) results in a less‐selective ecosystem due to weaker host selective pressures and stronger microbial forces. The red arrow denotes stochastic microbial forces such as dispersal, genetic drift, and immigration. Each shaded colour under the resilience curve denotes a host factor that contributes to the width of the curve. We provide four example curves with varying widths across four groups of hosts. The width of the resilience curves is a proxy for the strength of phylosymbiosis within a given clade of hosts. The model predicts that tighter curves limit microbial forces due to highly selective host forces while wider curves allow the opposite to occur due to less‐selective host forces.
III. ESTABLISHING A COMPREHENSIVE GUT MICROBIOME THEORY
Various ecological theories have been applied to animal gut microbiomes. Currently, much gut microbiome ecological theory stems from human models (McDonald, Marchesi & Koskella, 2020; Wolff, Shoemaker & Garud, 2020), and unifying theories that account for hosts across the animal kingdom are lacking (Koskella, Hall & Metcalf, 2017). For example, many theoretical gut microbiome studies assume there is coevolution between gut microbes and their hosts (Zeng et al., 2015; Ma, 2021). Ma (2021, p. 1) states that ‘Animal (human) gut microbiomes have been coevolving with their hosts for many millions of years’. While coevolution of gut microbes and their animal hosts may indeed take place, the supporting evidence stems largely from work on hominid hosts (Moeller et al., 2014, 2016; Sanders et al., 2023). Recently, the concept of host–microbiome coevolution has been questioned (Groussin et al., 2020) and models that highlight ecology rather than evolution may describe better the variation within animal gut microbiomes (Colston & Jackson, 2016). In this section, we provide a summary of ecological models that have been applied to gut microbiomes and provide an updated framework that places these models in a more comprehensive light, using metacommunity theory. For clarity, we divide these models into top‐down host‐imposed forces and bottom‐up microbial forces.
A variety of ecological models have been proposed that focus on host factors that shape microbe community assembly. Here, we refer to these as top‐down host‐imposed forces, highlighting the host's ability to shape and facilitate gut microbiome community assembly. Habitat filtering and niche‐based selection have been discussed extensively as ecological frameworks that can be applied to host modulation of microbiomes (Weiher, Clarke & Keddy, 1998; Fierer et al., 2012; Costello et al., 2012). Habitat filtering (in contrast to niche differentiation) describes the process by which species with similar functional niches coexist in similar environments (Keddy, 1992; Díaz, Cabido & Casanoves, 1998; Yang et al., 2019). Habitat filtering can explain microbiome variation across different habitats within a given host. For example, even after controlling for phylogeny, coexisting microbes are more likely to share similar metabolic functions within the same environment (Levy & Borenstein, 2013). In the context of gut microbiomes, the host can be viewed as the habitat or environment, where various parameters such as pH, temperature, and immune function can select or filter for certain microbes. As a result, the gut microbiome is a very different community compared to microbial communities on other body sites (e.g. the skin microbiome) (Dekaboruah et al., 2020), and this differentiation has been documented across a wide variety of animal hosts (Grice & Segre, 2011; Sylvain et al., 2020; Degregori, Casey & Barber, 2021). However, gut microbiome composition and diversity can vary greatly between different host species (Youngblut et al., 2019; Song et al., 2020) and determining whether this variability is due to variation in top‐down host‐imposed forces requires further investigation.
While host‐oriented approaches can be used to model gut microbiome community composition and diversity well on broad scales, they do not account fully for the significant stochasticity observed in gut microbiome communities, particularly among individuals of the same host species. Neutral models assuming stochastic processes such as immigration (e.g. microbes colonising the gut), extinction, genetic drift, and speciation (Hubbell, 2001), have been proposed as possible frameworks to understand microbial community assembly within the gut from the perspective of bottom‐up microbial forces (Costello et al., 2012). However, while neutral models can accurately describe gut microbiome variability in theory (Zeng et al., 2015), they can fail when applied to actual gut microbiome data sets (Li & Ma, 2016). More deterministic processes such as niche partitioning (Yu et al., 2024), may also explain microbial variability in gut microbiomes, but efforts to test niche partitioning in gut microbiomes are lacking.
Specific microbe–microbe interactions such as cooperation, mutualism, and competition are also significant determinants of microbiome composition in the gut (Coyte & Rakoff‐Nahoum, 2019). For example, Segura Munoz et al. (2022) showed that Akkermansia muciniphila and Bacteroides vulgatus exhibited significantly different levels of competitive behaviour and species exclusion within the mouse gut. However, in zebrafish (Danio rerio), the effects of microbe–microbe interactions appear to diminish with increasing gut microbiome complexity (Sundarraman et al., 2020). Microbes can also exhibit cooperative and mutualistic behaviours within the gut, such as the cross‐feeding enzyme system between Bacteroides vulgatus and B. ovatus (Coyte & Rakoff‐Nahoum, 2019) or between Lactobacillus plantarum and various gut microbes (Heinken & Thiele, 2015). Such cooperative behaviours lead to a network of syntrophic relationships among microbial species within the gut (Culp & Goodman, 2023). Thus, microbe–microbe interactions may be as important as host‐imposed forces in controlling gut microbiome community assembly and diversity.
To account for both microbe and host‐imposed forces, we propose a metacommunity theory approach to animal gut microbiomes. Metacommunity theory attempts to consolidate niche‐based selection and neutral theory into a unifying framework (Fierer et al., 2012). Here, we take a similar but slightly modified approach that assumes that both top‐down selection imposed by the environment (i.e. the host) and bottom‐up microbial forces occurring within the microbial community work simultaneously to shape gut microbiome composition (Fig. 2). In our proposed model, these two opposing forces vary among different host clades. Gut microbes likely undergo varying levels of selection imposed by different host factors, leading to co‐diversification or phylosymbiosis. Because mammals putatively impose the strongest selection on their gut microbiomes (Mallott & Amato, 2021), mammalian gut microbiomes may particularly exhibit patterns that mimic host–microbe coevolution in contrast to other hosts. Simultaneously, stochastic microbial forces, such as dispersal, horizontal gene transfer, and rapid evolution, counteract host selection, and may dominate the shaping of gut microbiomes in hosts with weaker selection. Building on this framework, future studies should investigate whether stochastic microbial forces are more pronounced in non‐mammalian hosts with weaker host selection on their gut microbiomes and whether the opposite is true in mammalian hosts.
IV. USE OF HOST PHYLOGENY AS AN ALL‐ENCOMPASSING FACTOR
Host phylogeny, sometimes used interchangeably with phylosymbiosis, can conveniently help researchers capture the complexity of host species differences into a single variable for analysis. However, finding consistent patterns between animal gut microbiome diversity and host phylogeny has proved difficult (Mallott & Amato, 2021). For example, among vertebrates, mammals have the highest levels of phylosymbiosis, with artiodactyls showing the highest within‐taxon gut microbiome similarity (Song et al., 2020), but the extent to which this is confounded by their herbivorous diet remains unclear (Muegge et al., 2011). Host phylogeny has been shown partly to shape fish gut microbiomes (Sullam et al., 2012), but contradictory examples exist (Givens et al., 2015; Miyake et al., 2015; Kim et al., 2021). It has been proposed that mammal gut microbiomes may exhibit the strongest phylosymbiosis as a result of their complex immune systems (Woodhams et al., 2020; Mallott & Amato, 2021), and host phylogeny can greatly outweigh host ecology in shaping gut microbiome diversity in different mammalian clades (Wei et al., 2015; Amato et al., 2018). More diverse sampling could help us understand why phylosymbiosis appears to be strongest in mammals compared to other animal clades.
Furthermore, while terms such as ‘phylosymbiosis’ and ‘host phylogeny’, can be useful in describing gut microbiome variation across different hosts, it can be misleading in terms of how gut microbiomes fit into animal ecology and evolution. Comparative studies often separate potential host factors for analysis into broad categories such as host phylogeny, host diet, and host environment (Ley et al., 2008; Sullam et al., 2012; Groussin et al., 2017; Youngblut et al., 2019). However, they may be intercorrelated: host diet, for example, is an evolutionary trait that is correlated with phylogeny (Román‐Palacios, Scholl & Wiens, 2019). Thus, a study claiming a greater effect of host diet than of host phylogeny is actually arguing that one defined evolutionary trait (diet) is outweighing all other evolutionary traits of that host (phylogeny). In physiological terms, host diet outweighing host phylogeny can be seen as digestive anatomy outweighing the sum of all non‐dietary physiology such as immune function, thermoregulation, liver metabolism, and many other physiological functions. Delsuc et al. (2014), for example found the effects of insectivory outweighed host phylogeny in determining the microbiome of certain myrmecophagous mammals. Because insectivory evolved independently multiple times in mammals, convergence of their gut microbiome highlights the impact of their recent dietary evolution over their other biological traits. By contrast, Amato et al. (2018) found that the effects of host phylogeny outweighed those of host diet in the gut microbiome of primates, and attributed the correlation with host phylogeny to host physiology. While host phylogeny can serve as a useful proxy, more comprehensive physiological sampling of the host could help researchers pinpoint specific physiological variables that drive gut microbiome variability.
V. ENVIRONMENTAL IMPACTS VARY ACROSS HOST CLADES
Different biomes have very distinct microbial compositions (Thompson et al., 2017), and it is thus possible that gut microbiomes reflect this diversity. However, given the differences in temperature, pH, nutrient levels, and oxygen content in the gut compared to the external environment, it is also possible that gut microbiomes may not reflect the external environment at all. Many contradictory results exist in the literature showing the environment to have either a large or minimal role in shaping animal gut microbiomes. For example, Kim et al. (2021) found that saltwater and freshwater fish from the South China Sea region exhibited distinct gut microbiome compositions. The effect of host environment outweighed other investigated factors such as host phylogeny and diet. Similar findings have been reported for reptiles (Hoffbeck et al., 2023; Moeller et al., 2020; Vasconcelos et al., 2023) and amphibians (Bletz et al., 2016). By contrast, comparative studies on mammals often find little effect of environment (Youngblut et al., 2019; Song et al., 2020). Some environment‐related effects have been identified in single‐species studies on mammals (Song et al., 2013; Degregori et al., 2023) or in comparisons of closely related species (Grieneisen et al., 2019), perhaps due to isolation of the effects of the environment from phylogeny. It appears that environmental impacts on animal gut microbiomes are stronger in clades with lower levels of phylosymbiosis such as fish, reptiles, and amphibians (Fig. 2), while in mammals, any effects of host environment may be masked by other factors such as host physiology. Additional comparative research is needed to confirm this hypothesis.
Multiple challenges exist that limit our ability to isolate the effects of environment on animal gut microbiome diversity and function. Obtaining wild samples, for example, can be difficult and costly, and so researchers often resort to sampling captive hosts (Ley et al., 2008; Hale et al., 2018; Gibson et al., 2019), whose gut microbiomes are unlikely to be representative of their wild counterparts (Clayton et al., 2016; McKenzie et al., 2017; Alberdi, Martin Bideguren & Aizpurua, 2021) and may reflect the microbiome of the captivity environment (Clayton et al., 2016; Dehler, Secombes & Martin, 2017). When researchers can access wild hosts, the challenge of an effective study design arises, where sufficient variation in host habitats must be sampled to analyse properly the effect of host environment. Moreover, to isolate an effect of environment from that of other factors such as host phylogeny and ecology, an effective study design must include not only a variety of habitats, but also a variety of host species with varying biological and ecological traits.
VI. DETERMINING SAMPLE SIZE IN COMPARATIVE GUT MICROBIOME RESEARCH
In addition to the challenges of sampling diverse environments, comparative gut microbiome studies also face the costs associated with sample collection and sequencing. One recent estimate found the average cost of a 16S sequencing workflow to be around $29 per sample (Lao, 2022), if a developed laboratory pipeline is in place. For new laboratories, equipment and field collection costs could increase this to in excess of $50–100 per sample. Thus, comparative microbiome studies often face a challenging trade‐off due to budget limitations: choosing either a large number of host species with a low number of replicates, or more replicates with fewer host species. In addition, targeting wild hosts over captive hosts can incur significantly higher costs, require additional expertise, and involve travel to multiple remote locations to collect samples with approved permits. Thus, it may be the case that a comparative gut microbiome study is not possible within a given budget.
To investigate how many replicate samples per host species are needed, we aggregated data from 179 gut microbiome studies (see online Supporting Information, Appendix S1, for methods; Table S1 for search results, Data S1 for the list of included studies, and Fig. S1 for sample counts across host clades) and randomly pooled these into data sets containing different numbers of samples sizes per host species (Fig. 3). We found that the influence of phylogeny was much larger for low sample sizes, with the largest effect at a sample size of one, and a more consistent effect after about five replicate samples per host species. A batch effect also may be present due to combining studies (‘study’ in Fig. 3), but we instead focus on how the results change across sample sizes rather than the actual effect sizes for each taxonomic rank.
Fig. 3.
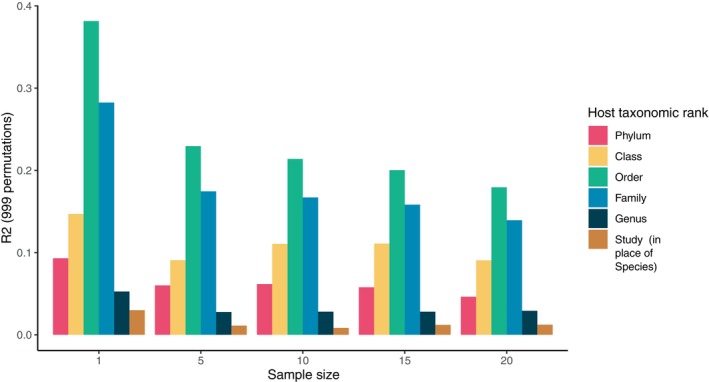
Results of Adonis analysis of the effects of sample size per host species on the calculated influence of taxonomic rank on animal gut microbiomes. Samples from each host were randomly subsampled at 999 permutations; the R2 value plotted is the mean from all permutations. Error bars are too small to show on the graph but were all <0.001. Colours denote host taxonomic ranks which were treated as separate factors. Because Study and Species are nearly identical (most studies had a unique host species), study, as a factor, is included to explore the batch effect after taking taxonomic rank into account. We rarefied the data to 1000 reads and used unweighted UniFrac distance matrices as inputs for the Adonis test.
Gut microbiome studies often utilise public data sets from other comparative studies in phylogenetic comparisons with their study species (Delsuc et al., 2014; Youngblut et al., 2019; Song et al., 2020; Sanders et al., 2023). Such comparisons can enable researchers to identify convergences between distantly related hosts (Muegge et al., 2011; Delsuc et al., 2014; Song et al., 2020; Degregori et al. 2024) or to identify elements of the gut microbiome that follow host evolution (Moeller et al., 2014; Groussin et al., 2017). However, the majority of comparative studies have been focused on mammals, and comprehensive data sets of other clades are lacking (Table S2). While such data sets are emerging, such as for fish and birds (Song et al., 2020; Kim et al., 2021; Minich et al., 2022), data sets that span the entire animal kingdom are not yet available (although see Yang et al., 2022). An easily filterable comparative data set of gut microbiomes across the animal kingdom would provide researchers with the ability to conduct robust evolutionary and ecological analyses.
As shown in Fig. 1, when excluding studies on humans and laboratory animals, there is a reasonably comparable number of single‐species studies, across various vertebrate and invertebrate clades. Combining results from single‐species studies to curate a comparative data set can serve as a low‐cost alternative approach for researchers compared with the high costs of generating an original comparative data set. While prone to individual laboratory processing biases, studies can be combined on the basis of using identical primers such as the commonly used V3‐V4 (341F‐806R) and V4 (515F‐806R) primers to minimise such bias. To show the analytical power of such an approach, we used our database of 179 gut microbiome studies (see Data S1) to examine the prevalence of two commonly studied microbes, Akkermansia muciniphila and Faecalibacterium prausnitzii, across the animal kingdom (Fig. 4). While both microbes are of interest in human biomedical studies (Naito et al., 2018; Parsaei et al., 2021), Fig. 4 shows that, among vertebrates, A. muciniphila appears to be widespread across vertebrate gut microbiomes while F. prausnitzii is present most often in mammalian hosts. This type of broad‐scale analysis could guide researchers to focus their efforts on particular host species of interest and their closest relatives.
Fig. 4.

An annotated phylogeny of the presence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the gut microbiomes of hosts from 179 compiled studies. Orange and blue colours at the tips denote the presence of these gut microbes. Each tree tip represents a host species included in our database.
VII. BEST PRACTICES AND FUTURE DIRECTIONS
Comparative analyses of the gut microbiome can utilise 16S data, metabolomic data, transcriptomic data, metagenomic data, or even proteomic data (Knight et al., 2018). These data can then be compared against a range of host variables, including ecological data, host phylogeny, biometric data or physiological measurements (Gomez et al., 2015; Amato et al., 2018; Youngblut et al., 2019). Thus, comparative gut microbiome studies vary greatly in their methodologies and study designs.
Moving forward, general guidelines are needed for future comparative gut microbiome research to improve standardisation. While any comparative study has its own specific goals and limitations, standardising methodology and study design could greatly improve our ability to investigate more general aspects of how gut microbiomes fit into animal ecology and evolution. Without such standardisation we are forced to limit any conclusions to specific studies and the specific methodology they employed. We therefore provide the following recommended guidelines for future comparative work.
(1). Include publicly available data in comparative studies
The increasing availability of public gut microbiome data from hosts across the animal kingdom provides an opportunity for researchers to perform comparative analyses of the gut microbiomes of their target host(s). For example, studies that only compare two host species, such as yaks and pikas (Fu et al., 2020) or two different amphipods (Cheng et al., 2019), could utilise such supplementary data to investigate whether differences in gut microbiomes between two hosts are driven by host phylogeny, diet, or environment. As noted in Section VI, studies using publicly available data will have to consider primer bias, and ideally focus on results from studies with the same primer (Darwish et al., 2021).
(2). Collect a minimum of five samples per host species
Comparative gut microbiome studies vary widely in how many host species are targeted and how many samples are collected per host. Some studies include many host species but only collect one sample per host (Ley et al., 2008; Levin et al., 2021), while at the other extreme a study may obtain many samples per host for only one or a few host species (Zhang, Ren & Gong, 2019; Vasconcelos et al., 2023). Combining the results of such studies can be difficult. Moreover, as shown in Fig. 3, undersampling could lead to overestimations of impacts of host phylogeny on animal gut microbiomes. We recommend sampling a minimum of five samples per host species. Additional samples should be allocated towards further host diversity as we show that potential source of bias is lower for sample sizes of five or greater. However, in some cases this minimum may be insufficient. Comparative analyses focused on a specific variable should perform a power analysis to ensure an adequate sample size (Rahman et al., 2023). There is a lack of statistical tools to help scientists determine the appropriate adequate amount of host diversity for comparative analyses. While we suggest that a sample size of 5 per host species is adequate for some analyses, such as Adonis, further tools to determine the appropriate sampling level at higher taxonomic ranks, such as host family or phylum, or in studies using specialised statistical models, are needed.
(3). Accurately quantify diet
Comparative gut microbiome studies often group hosts into broad diet categories for analysis (Ley et al., 2008; Muegge et al., 2011; Groussin et al., 2017). However, more accurate measures of diet composition, such as diet metabarcoding (Kartzinel et al., 2019) or survey data (Song et al., 2020), would allow researchers to analyse better how diet impacts animal gut microbiomes across host species. Moreover, categories such as ‘herbivory’ and ‘carnivory’ in mammals, for example, may be significantly correlated with host phylogeny (Ley et al., 2008; Groussin et al., 2020) and may also differ in meaning depending on the host clade of interest. Fish and mammal carnivores, for example, may differ greatly in their respective feeding strategies (Román‐Palacios et al., 2019), but such differences are ignored by coarse diet categories. We recommend that comparative studies should use quantifiable measures of host diet instead of broad categories.
(4). Collect host physiological data
In humans and mouse models, gut microbiomes are significantly associated with many aspects of host physiology (Besten et al., 2013; Barouei et al., 2017; Thiele et al., 2020), but the extent to which this is true in other taxa remains unclear. For instance, it has been posited that immune factors may play a large role in shaping gut microbiome composition across vertebrates (Woodhams et al., 2020; Mallott & Amato, 2021), but comparative approaches that also sample host physiology, such as red or white blood cell counts, across a diverse set of host species are lacking. Sampling a range of physiological variables, such as the host metabolome, immune factors, or levels of cortisol and testosterone, could provide a more complete picture of how animal physiology shapes the gut microbiome and how this varies across the animal kingdom. Authors then will be able to make stronger inferences on the differences underlying gut microbiome diversity among species instead of resorting to host phylogeny as the underlying mechanism.
(5). Moving beyond host phylogeny as a host factor
Comparative studies often cite host phylogeny as a factor determining differences in gut microbiomes among animal species. As described previously, host phylogeny can serve as a useful tool to investigate how differences in gut microbiomes correlate with host evolution. However, treating host phylogeny, host diet, and host environment as separate factors can be problematic considering that host diet also is an evolved trait and all animals will have evolved adaptations to their environment. We encourage researchers, when possible, to collect physiological data to supplement the use of host phylogeny in analyses of how gut microbiomes are correlated with host evolution.
VIII. CONCLUSIONS
-
(1)
Comparative work on animal gut microbiomes has shown that ecological and evolutionary factors can shape gut microbial communities, and that these effects are host dependent and vary among taxa. The underlying mechanisms are poorly understood due to the lack of comprehensive meta‐analyses on comparative data sets, including data sets with non‐mammalian hosts. Our proposed ecosystem‐selection framework accounts for the stronger phylosymbiosis and larger core microbiome we see in mammals while predicting weaker phylosymbiosis in other clades.
-
(2)
To test this hypothesis and other predictions, data sets that are rich in host diversity and span the entire animal kingdom are needed. Generating such data with standardised field and laboratory practices will be critical and quantifying host physiology in addition to host phylogeny can help us delineate the complex network of host factors that shape gut microbiome diversity.
-
(3)
We show that the aggregation of individual studies into large‐scale data sets is possible and can be used to investigate broad questions concerning the relationship between gut microbiomes and animal ecology and evolution. Future efforts should seek to combine 16S, metagenomic, and metabolomic data sets, with rich host biological and ecological data, to enable a more detailed understanding of both the diversity and function of animal gut microbiomes.
AUTHOR CONTRIBUTIONS
S. D. and K. R. A. conceived the study. X. W. led data collection and curation. A. K., N. S., S. M., A. M., K. E., S. J., E. S., E. K., K. S., Z. H., S. D., R. K., and K. R. A aided in data collection. S. D., N. S., A. K., and A. M. developed code for analyses. S. D. carried out analyses and manuscript writing. K. R. A. and R. K. provided computational resources and guidance. All authors provided feedback on the analyses and editing of the manuscript.
Supporting information
Appendix S1. Supplementary methods.
Table S1. Web of Science query results.
Table S2. Overview of large‐scale comparative gut microbiome studies (>30 host species).
Fig. S1. Number of samples aggregated across 179 gut microbiome studies spanning 15 host classes.
Data S1. Sample and study metadata.
ACKNOWLEDGEMENTS
We would like to thank all the members of the Gut Microbiome Tree of Life project that aided in aggregating the data used in this study. S. D. was funded by the National Science Foundation's Postdoctoral Research in Biology Fellowship during the study. The authors declare no competing interests. This study included multiple members from underrepresented minority backgrounds and first‐generation college students. We strongly advocate for furthering the inclusion and equity of the STEM community through collaborative research projects that include scientists at all career stages and backgrounds.
DATA AVAILABILITY STATEMENT
All metadata used in this study along with a list of compiled studies are available in the supporting information and at https://github.com/samd1993/GutMicrobiomeTreeOfLife as an excel spreadsheet named: ‘Degregori_etal_Comparative_Review_metadata_Oct20_24.xlsx’ and a text file of related scripts named: ‘Degregori_etal_Comparative_Review_DataScripts.txt’. The sequence table used for Figs 3 and 4 is named ‘N30GMTOLsolo_table.biom’ and is also uploaded to GitHub. The package q2sra is available at https://pypi.org/project/q2sra/.
REFERENCES
References identified with an asterisk (*) are cited only within the online Supporting Information.
- * Abdelrhman, K. F. A. , Bacci, G. , Mancusi, C. , Mengoni, A. , Serena, F. & Ugolini, A. (2016). A first insight into the gut microbiota of the sea turtle Caretta caretta . Frontiers in Microbiology 7, 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Akhremchuk, K. , Skapavets, K. , Akhremchuk, A. , Kirsanava, N. , Sidarenka, A. & Valentovich, L. (2022). Gut microbiome of healthy people and patients with hematological malignancies in Belarus. Microbiology Independent Research Journal (MIR Journal) 9, 18–30. [Google Scholar]
- Alberdi, A. , Martin Bideguren, G. & Aizpurua, O. (2021). Diversity and compositional changes in the gut microbiota of wild and captive vertebrates: a meta‐analysis. Scientific Reports 11, 2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato, K. R. , Sanders, G. , Song, S. J. , Nute, M. , Metcalf, J. L. , Thompson, L. R. , Morton, J. T. , Amir, A. , McKenzie, V. J. , Humphrey, G. , Gogul, G. , Gaffney, J. , Baden, A. L. , Britton, G. A. O. , Cuozzo, G. F. P. , et al. (2018). Evolutionary trends in host physiology outweigh dietary niche in structuring primate gut microbiomes. The ISME Journal 13, 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Angelakis, E. , Yasir, M. , Bachar, D. , Azhar, E. I. , Lagier, J.‐C. , Bibi, F. , Jiman‐Fatani, A. A. , Alawi, M. , Bakarman, M. A. , Robert, C. & Raoult, D. (2016). Gut microbiome and dietary patterns in different Saudi populations and monkeys. Scientific Reports 6, 32191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Aronson, H. S. , Zellmer, A. J. & Goffredi, S. K. (2017). The specific and exclusive microbiome of the deep‐sea bone‐eating snail, Rubyspira osteovora . FEMS Microbiology Ecology 93, fiw250. [DOI] [PubMed] [Google Scholar]
- * Bai, S. , Zhang, P. , Zhang, X. , Yang, Z. & Li, S. (2022). Gut microbial characterization of melon‐headed whales (Peponocephala electra) stranded in China. Microorganisms 10, 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Baldo, L. , Tavecchia, G. , Rotger, A. , Igual, J. M. & Riera, J. L. (2023). Insular holobionts: persistence and seasonal plasticity of the Balearic wall lizard (Podarcis lilfordi) gut microbiota. PeerJ 11, e14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouei, J. , Bendiks, Z. , Martinic, A. , Mishchuk, D. , Heeney, D. , Hsieh, Y.‐H. , Kieffer, D. , Zaragoza, J. , Martin, R. , Slupsky, C. & Marco, M. L. (2017). Microbiota, metabolome, and immune alterations in obese mice fed a high‐fat diet containing type 2 resistant starch. Molecular Nutrition & Food Research 61, 1700184. [DOI] [PubMed] [Google Scholar]
- * Berlow, M. , Phillips, J. N. & Derryberry, E. P. (2021). Effects of urbanization and landscape on gut microbiomes in white‐crowned sparrows. Microbial Ecology 81, 253–266. [DOI] [PubMed] [Google Scholar]
- Bletz, M. C. , Goedbloed, D. J. , Sanchez, E. , Reinhardt, T. , Tebbe, C. C. , Bhuju, S. , Geffers, R. , Jarek, M. , Vences, M. & Steinfartz, S. (2016). Amphibian gut microbiota shifts differentially in community structure but converges on habitat‐specific predicted functions. Nature Communications 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Bolyen, E. , Rideout, J. R. , Dillon, M. R. , Bokulich, N. A. , Abnet, C. C. , Al‐Ghalith, G. A. , Alexander, H. , Alm, E. J. , Arumugam, M. , Asnicar, F. , Bai, Y. , Bisanz, J. E. , Bittinger, K. , Brejnrod, A. , Brislawn, C. J. , et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnology 37, 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Bornbusch, S. L. , Clarke, T. A. , Hobilalaina, S. , Reseva, H. S. , LaFleur, M. & Drea, C. M. (2022). Microbial rewilding in the gut microbiomes of captive ring‐tailed lemurs (Lemur catta) in Madagascar. Scientific Reports 12, 22388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Budd, K. , Gunn, J. C. , Finch, T. , Klymus, K. , Sitati, N. & Eggert, L. S. (2020). Effects of diet, habitat, and phylogeny on the fecal microbiome of wild African savanna (Loxodonta africana) and forest elephants (L. cyclotis). Ecology and Evolution 10, 5637–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Bunker, M. E. , Arnold, A. E. & Weiss, S. L. (2022). Wild microbiomes of striped plateau lizards vary with reproductive season, sex, and body size. Scientific Reports 12, 20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Burke, C. , Burnard, D. , Polkinghorne, A. , Webb, J. & Huston, W. M. (2018). Cloacal and ocular microbiota of the endangered Australian Northern quoll. Microorganisms 6, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Campbell, T. P. , Sun, X. , Patel, V. H. , Sanz, C. , Morgan, D. & Dantas, G. (2020). The microbiome and resistome of chimpanzees, gorillas, and humans across host lifestyle and geography. The ISME Journal 14, 1584–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Campos, P. , Guivernau, M. , Prenafeta‐Boldú, F. X. & Cardona, L. (2018). Fast acquisition of a polysaccharide fermenting gut microbiome by juvenile green turtles Chelonia mydas after settlement in coastal habitats. Microbiome 6, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. , Shen, J. & Ran, Z. H. (2014). Association between Faecalibacterium prausnitzii reduction and inflammatory bowel disease: a meta‐analysis and systematic review of the literature. Gastroenterology Research and Practice 2014, e872725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Chakraborty, A. , Ashraf, M. Z. , Modlinger, R. , Synek, J. , Schlyter, F. & Roy, A. (2020). Unravelling the gut bacteriome of Ips (Coleoptera: Curculionidae: Scolytinae): identifying core bacterial assemblage and their ecological relevance. Scientific Reports 10, 18572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Chen, C. , Zhang, J. , Tan, H. , Fu, Z. & Wang, X. (2022). Characterization of the gut microbiome in the beet armyworm Spodoptera exigua in response to the short‐term thermal stress. Journal of Asia‐Pacific Entomology 25, 101863. [Google Scholar]
- * Chen, L. , Li, S. , Xiao, Q. , Lin, Y. , Li, X. , Qu, Y. , Wu, G. & Li, H. (2021. a). Composition and diversity of gut microbiota in Pomacea canaliculata in sexes and between developmental stages. BMC Microbiology 21, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Chen, X. , Fang, S. , Wei, L. & Zhong, Q. (2019). Systematic evaluation of the gut microbiome of swamp eel (Monopterus albus) by 16S rRNA gene sequencing. PeerJ 7, e8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Chen, X. , Lu, D. , Li, Z. , Yue, W. , Wang, J. , Jiang, X. , Han, H. & Wang, C. (2021. b). Plant and animal‐type feedstuff shape the gut microbiota and metabolic processes of the Chinese mitten crab Eriocheir sinensis . Frontiers in Veterinary Science 8, 589624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, X. , Wang, Y. , Li, J. , Yan, G. & He, L. (2019). Comparative analysis of the gut microbial communities between two dominant amphipods from the Challenger Deep, Mariana Trench. Deep Sea Research Part I: Oceanographic Research Papers 151, 103081. [Google Scholar]
- * Chouaia, B. , Goda, N. , Mazza, G. , Alali, S. , Florian, F. , Gionechetti, F. , Callegari, M. , Gonella, E. , Magoga, G. , Fusi, M. , Crotti, E. , Daffonchio, D. , Alma, A. , Paoli, F. , Roversi, P. F. , et al. (2019). Developmental stages and gut microenvironments influence gut microbiota dynamics in the invasive beetle Popillia japonica Newman (Coleoptera: Scarabaeidae). Environmental Microbiology 21, 4343–4359. [DOI] [PubMed] [Google Scholar]
- * Cini, A. , Meriggi, N. , Bacci, G. , Cappa, F. , Vitali, F. , Cavalieri, D. & Cervo, R. (2020). Gut microbial composition in different castes and developmental stages of the invasive hornet Vespa velutina nigrithorax . Science of the Total Environment 745, 140873. [DOI] [PubMed] [Google Scholar]
- Clayton, J. B. , Vangay, P. , Huang, H. , Ward, T. , Hillmann, B. M. , Al‐Ghalith, G. A. , Travis, D. A. , Long, H. T. , Tuan, B. V. , Minh, V. V. , Cabana, F. , Nadler, T. , Toddes, B. , Murphy, T. , Glander, K. E. , et al. (2016). Captivity humanizes the primate microbiome. Proceedings of the National Academy of Sciences 113, 10376–10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colston, T. J. & Jackson, C. R. (2016). Microbiome evolution along divergent branches of the vertebrate tree of life: what is known and unknown. Molecular Ecology 16, 3776–3800. [DOI] [PubMed] [Google Scholar]
- Comizzoli, P. , Power, M. L. , Bornbusch, S. L. & Muletz‐Wolz, C. R. (2021). Interactions between reproductive biology and microbiomes in wild animal species. Animal Microbiome 3, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Cornejo‐Granados, F. , Lopez‐Zavala, A. A. , Gallardo‐Becerra, L. , Mendoza‐Vargas, A. , Sánchez, F. , Vichido, R. , Brieba, L. G. , Viana, M. T. , Sotelo‐Mundo, R. R. & Ochoa‐Leyva, A. (2017). Microbiome of Pacific whiteleg shrimp reveals differential bacterial community composition between Wild, Aquacultured and AHPND/EMS outbreak conditions. Scientific Reports 7, 11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello, E. K. , Stagaman, K. , Dethlefsen, L. , Bohannan, B. J. M. & Relman, D. A. (2012). The application of ecological theory towards an understanding of the human microbiome. Science (New York, N.Y.) 336, 1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Couch, C. E. , Neal, W. T. , Herron, C. L. , Kent, M. L. , Schreck, C. B. & Peterson, J. T. (2023). Gut microbiome composition associates with corticosteroid treatment, morbidity, and senescence in Chinook salmon (Oncorhynchus tshawytscha). Scientific Reports 13, 2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Couch, C. E. , Stagaman, K. , Spaan, R. S. , Combrink, H. J. , Sharpton, T. J. , Beechler, B. R. & Jolles, A. E. (2021). Diet and gut microbiome enterotype are associated at the population level in African buffalo. Nature Communications 12, 2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyte, K. Z. & Rakoff‐Nahoum, S. (2019). Understanding competition and cooperation within the mammalian gut microbiome. Current Biology 29, R538–R544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp, E. J. & Goodman, A. L. (2023). Cross‐feeding in the gut microbiome: ecology and mechanisms. Cell Host & Microbe 31, 485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Danckert, N. P. , Wilson, N. , Phan‐Thien, K.‐Y. & Stone, D. A. J. (2021). The intestinal microbiome of Australian abalone, Haliotis laevigata and Haliotis laevigata × Haliotis rubra, over a 1‐year period in aquaculture. Aquaculture 534, 736245. [Google Scholar]
- Darwish, N. , Shao, J. , Schreier, L. L. & Proszkowiec‐Weglarz, M. (2021). Choice of 16S ribosomal RNA primers affects the microbiome analysis in chicken ceca. Scientific Reports 11, 11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * De Cock, M. , Virgilio, M. , Vandamme, P. , Augustinos, A. , Bourtzis, K. , Willems, A. & De Meyer, M. (2019). Impact of sample preservation and manipulation on insect gut microbiome profiling. A test case with fruit flies (Diptera, Tephritidae). Frontiers in Microbiology 10, 2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degregori, S. , Casey, J. M. & Barber, P. H. (2021). Nutrient pollution alters the gut microbiome of a territorial reef fish. Marine Pollution Bulletin 169, 112522. [DOI] [PubMed] [Google Scholar]
- Degregori, S. , Johnson, G. C. , Barber, P. H. & Blumstein, D. T. (2023). Firmicutes and Bacteroidetes contribute to mass gain variation in female obligate hibernators. Journal of Mammalogy 105, 2–12. [Google Scholar]
- Degregori, S. , Schiettekatte, N. M. D. , Casey, J. M. , Brandl, S. J. , Mercière, A. , Amato, K. R. , Mazel, F. , Parravicini, V. & Barber, P. H. (2024). Host diet drives gut microbiome convergence between coral reef fishes and mammals. Molecular Ecology 33, e17520. [DOI] [PubMed] [Google Scholar]
- Dehler, C. E. , Secombes, C. J. & Martin, S. A. M. (2017). Environmental and physiological factors shape the gut microbiota of Atlantic salmon parr (Salmo salar L.). Aquaculture 467, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekaboruah, E. , Suryavanshi, M. V. , Chettri, D. & Verma, A. K. (2020). Human microbiome: an academic update on human body site specific surveillance and its possible role. Archives of Microbiology 202, 2147–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsuc, F. , Metcalf, J. L. , Wegener Parfrey, L. , Song, S. J. , González, A. & Knight, R. (2014). Convergence of gut microbiomes in myrmecophagous mammals. Molecular Ecology 23, 1301–1317. [DOI] [PubMed] [Google Scholar]
- den Besten, G. , van Eunen, K. , Groen, A. K. , Venema, K. , Reijngoud, D.‐J. & Bakker, B. M. (2013). The role of short‐chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research 54, 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Dewar, M. L. , Arnould, J. P. Y. , Allnutt, T. R. , Crowley, T. , Krause, L. , Reynolds, J. , Dann, P. & Smith, S. C. (2017). Microbiota of little penguins and short‐tailed shearwaters during development. PLoS One 12, e0183117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz, S. , Cabido, M. & Casanoves, F. (1998). Plant functional traits and environmental filters at a regional scale. Journal of Vegetation Science 9, 113–122. [Google Scholar]
- * Eliades, S. J. , Brown, J. C. , Colston, T. J. , Fisher, R. N. , Niukula, J. B. , Gray, K. , Vadada, J. , Rasalato, S. & Siler, C. D. (2021). Gut microbial ecology of the Critically Endangered Fijian crested iguana (Brachylophus vitiensis): effects of captivity status and host reintroduction on endogenous microbiomes. Ecology and Evolution 11, 4731–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Escalas, A. , Auguet, J.‐C. , Avouac, A. , Seguin, R. , Gradel, A. , Borrossi, L. & Villéger, S. (2021). Ecological specialization within a carnivorous fish family is supported by a herbivorous microbiome shaped by a combination of gut traits and specific diet. Frontiers in Marine Science 8, 622883. [Google Scholar]
- Fierer, N. , Ferrenberg, S. , Flores, G. E. , González, A. , Kueneman, J. , Legg, T. , Lynch, R. C. , McDonald, D. , Mihaljevic, J. R. , O'Neill, S. P. , Rhodes, M. E. , Song, S. J. & Walters, W. A. (2012). From animalcules to an ecosystem: application of ecological concepts to the human microbiome. Annual Review of Ecology, Evolution, and Systematics 43, 137–155. [Google Scholar]
- * Fontaine, S. S. & Kohl, K. D. (2020). Gut microbiota of invasive bullfrog tadpoles responds more rapidly to temperature than a noninvasive congener. Molecular Ecology 29, 2449–2462. [DOI] [PubMed] [Google Scholar]
- * Fontaine, S. S. , Mineo, P. M. & Kohl, K. D. (2021). Changes in the gut microbial community of the eastern newt (Notophthalmus viridescens) across its three distinct life stages. FEMS Microbiology Ecology 97, fiab021. [DOI] [PubMed] [Google Scholar]
- Fu, H. , Zhang, L. , Fan, C. , Liu, C. , Li, W. , Cheng, Q. , Zhao, X. , Jia, S. & Zhang, Y. (2020). Environment and host species identity shape gut microbiota diversity in sympatric herbivorous mammals. Microbial Biotechnology 14, 1300–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Fujio‐Vejar, S. , Vasquez, Y. , Morales, P. , Magne, F. , Vera‐Wolf, P. , Ugalde, J. A. , Navarrete, P. & Gotteland, M. (2017). The gut microbiota of healthy Chilean subjects reveals a high abundance of the Phylum Verrucomicrobia. Frontiers in Microbiology 8, 1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Gaulke, C. A. , Martins, M. L. , Watral, V. G. , Humphreys, I. R. , Spagnoli, S. T. , Kent, M. L. & Sharpton, T. J. (2019). A longitudinal assessment of host‐microbe‐parasite interactions resolves the zebrafish gut microbiome's link to Pseudocapillaria tomentosa infection and pathology. Microbiome 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings, S. Y. , Kostopoulos, I. , de Vos, W. M. & Belzer, C. (2018). Akkermansia muciniphila in the human gastrointestinal tract: when, where, and how? Microorganisms 6, 6030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, K. M. , Nguyen, B. N. , Neumann, L. M. , Miller, M. , Buss, P. , Daniels, S. , Ahn, M. J. , Crandall, K. A. & Pukazhenthi, B. (2019). Gut microbiome differences between wild and captive black rhinoceros – implications for rhino health. Scientific Reports 9, 7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Gillingham, M. A. F. , Borghesi, F. , Montero, B. K. , Migani, F. , Béchet, A. , Rendón‐Martos, M. , Amat, J. A. , Dinelli, E. & Sommer, S. (2021). Bioaccumulation of trace elements affects chick body condition and gut microbiome in greater flamingos. The Science of the Total Environment 761, 143250. [DOI] [PubMed] [Google Scholar]
- Givens, C. , Ransom, B. , Bano, N. & Hollibaugh, J. (2015). Comparison of the gut microbiomes of 12 bony fish and 3 shark species. Marine Ecology Progress Series 518, 209–223. [Google Scholar]
- * Gobet, A. , Mest, L. , Perennou, M. , Dittami, S. M. , Caralp, C. , Coulombet, C. , Huchette, S. , Roussel, S. , Michel, G. & Leblanc, C. (2018). Seasonal and algal diet‐driven patterns of the digestive microbiota of the European abalone Haliotis tuberculata, a generalist marine herbivore. Microbiome 6, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, A. , Petrzelkova, K. , Yeoman, C. J. , Vlckova, K. , Mrázek, J. , Koppova, I. , Carbonero, F. , Ulanov, A. , Modry, D. , Todd, A. , Torralba, M. , Nelson, K. E. , Gaskins, H. R. , Wilson, B. , Stumpf, R. M. , et al. (2015). Gut microbiome composition and metabolomic profiles of wild western lowland gorillas (Gorilla gorilla gorilla) reflect host ecology. Molecular Ecology 24, 2551–2565. [DOI] [PubMed] [Google Scholar]
- * Gong, X. , Chen, T.‐W. , Zhang, L. , Pižl, V. , Tajovský, K. & Devetter, M. (2022). Gut microbiome reflect adaptation of earthworms to cave and surface environments. Animal Microbiome 4, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * González‐Serrano, F. , Pérez‐Cobas, A. E. , Rosas, T. , Baixeras, J. , Latorre, A. & Moya, A. (2020. a). The gut microbiota composition of the moth Brithys crini reflects insect metamorphosis. Microbial Ecology 79, 960–970. [DOI] [PubMed] [Google Scholar]
- Grice, E. A. & Segre, J. A. (2011). The skin microbiome. Nature Reviews Microbiology 9, 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieneisen, L. E. , Charpentier, M. J. E. , Alberts, S. C. , Blekhman, R. , Bradburd, G. , Tung, J. & Archie, E. A. (2019). Genes, geology and germs: gut microbiota across a primate hybrid zone are explained by site soil properties, not host species. Proceedings of the Royal Society B: Biological Sciences 286, 20190431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Griffin, T. W. , Baer, J. G. & Ward, J. E. (2021). Direct comparison of fecal and gut microbiota in the blue mussel (Mytilus edulis) discourages fecal sampling as a proxy for resident gut community. Microbial Ecology 81, 180–192. [DOI] [PubMed] [Google Scholar]
- Groussin, M. , Mazel, F. & Alm, E. J. (2020). Co‐evolution and co‐speciation of host‐gut bacteria systems. Cell Host and Microbe 28, 12–22. [DOI] [PubMed] [Google Scholar]
- Groussin, M. , Mazel, F. , Sanders, J. G. , Smillie, C. S. , Lavergne, S. , Thuiller, W. & Alm, E. J. (2017). Unraveling the processes shaping mammalian gut microbiomes over evolutionary time. Nature Communications 8, 14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Guerrero, B. E. , Soria, M. , Salvador, R. , Ceja‐Navarro, J. A. , Campos, E. , Brodie, E. L. & Talia, P. (2016). Effect of different lignocellulosic diets on bacterial microbiota and hydrolytic enzyme activities in the gut of the cotton boll weevil (Anthonomus grandis). Frontiers in Microbiology 7, 2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W. , Mishra, S. , Zhao, J. , Tang, J. , Zeng, B. , Kong, F. , Ning, R. , Li, M. , Zhang, H. , Zeng, Y. , Tian, Y. , Zhong, Y. , Luo, H. , Liu, Y. , Yang, J. , et al. (2018). Metagenomic study suggests that the gut microbiota of the giant panda (Ailuropoda melanoleuca) may not be specialised for fiber fermentation. Frontiers in Microbiology 9, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Haji, D. , Vailionis, J. , Stukel, M. , Gordon, E. , Lemmon, E. M. , Lemmon, A. R. & Simon, C. (2022). Lack of host phylogenetic structure in the gut bacterial communities of New Zealand cicadas and their interspecific hybrids. Scientific Reports 12, 20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Hakim, J. A. , Schram, J. B. , Galloway, A. W. E. , Morrow, C. D. , Crowley, M. R. , Watts, S. A. & Bej, A. K. (2019). The purple sea urchin Strongylocentrotus purpuratus demonstrates a compartmentalization of gut bacterial microbiota, predictive functional attributes, and taxonomic co‐occurrence. Microorganisms 7, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale, V. L. , Tan, C. L. , Niu, K. , Yang, Y. , Knight, R. , Zhang, Q. , Cui, D. & Amato, K. R. (2018). Diet versus phylogeny: a comparison of gut microbiota in captive colobine monkey species. Microbial Ecology 75, 515–527. [DOI] [PubMed] [Google Scholar]
- * Härer, A. , Torres‐Dowdall, J. , Rometsch, S. J. , Yohannes, E. , Machado‐Schiaffino, G. & Meyer, A. (2020). Parallel and non‐parallel changes of the gut microbiota during trophic diversification in repeated young adaptive radiations of sympatric cichlid fish. Microbiome 8, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * He, B. , Chen, X. , Yang, H. & Cernava, T. (2021). Microbiome structure of the aphid Myzus persicae (Sulzer) is shaped by different Solanaceae plant diets. Frontiers in Microbiology 12, 667257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinken, A. & Thiele, I. (2015). Anoxic conditions promote species‐specific mutualism between gut microbes in silico. Applied and Environmental Microbiology 81, 4049–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Herder, E. A. , Spence, A. R. , Tingley, M. W. & Hird, S. M. (2021). Elevation correlates with significant changes in relative abundance in hummingbird fecal microbiota, but composition changes little. Frontiers in Ecology and Evolution 8, 597756. [Google Scholar]
- * Hird, S. M. , Sánchez, C. , Carstens, B. C. & Brumfield, R. T. (2015). Comparative gut microbiota of 59 Neotropical bird species. Frontiers in Microbiology 6, 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Hoang, H. T. , Le, D. H. , Le, T. T. H. , Nguyen, T. T. N. , Chu, H. H. & Nguyen, N. T. (2021). Metagenomic 16S rDNA amplicon data of microbial diversity of guts in Vietnamese humans with type 2 diabetes and nondiabetic adults. Data in Brief 34, 106690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffbeck, C. , Middleton, D. M. R. L. , Nelson, N. J. & Taylor, M. W. (2023). 16S rRNA gene‐based meta‐analysis of the reptile gut microbiota reveals environmental effects, host influences and a limited core microbiota. Molecular Ecology 32, 6044–6058. [DOI] [PubMed] [Google Scholar]
- * Holt, C. C. , van der Giezen, M. , Daniels, C. L. , Stentiford, G. D. & Bass, D. (2020). Spatial and temporal axes impact ecology of the gut microbiome in juvenile European lobster (Homarus gammarus). The ISME Journal 14, 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, P.‐Y. , Wheeler, E. , Ko Cann, I. & Mackie, R. I. (2011). Phylogenetic analysis of the fecal microbial community in herbivorous land and marine iguanas of the Galápagos Islands using 16S rRNA‐based pyrosequencing. The ISME Journal 5, 1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Hu, Y. , Xie, H. , Gao, M. , Huang, P. , Zhou, H. , Ma, Y. , Zhou, M. , Liang, J. , Yang, J. & Lv, Z. (2020). Dynamic of composition and diversity of gut microbiota in Triatoma rubrofasciata in different developmental stages and environmental conditions. Frontiers in Cellular and Infection Microbiology 10, 587708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell, S. P. (2001). The Unified Neutral Theory of Biodiversity and Biogeography. Princeton University Press, Princeton, NJ. [Google Scholar]
- * Huyben, D. , Sun, L. , Moccia, R. , Kiessling, A. , Dicksved, J. & Lundh, T. (2018). Dietary live yeast and increased water temperature influence the gut microbiota of rainbow trout. Journal of Applied Microbiology 124, 1377–1392. [DOI] [PubMed] [Google Scholar]
- * Ibáñez, A. , Bletz, M. C. , Quezada, G. , Geffers, R. , Jarek, M. , Vences, M. & Steinfartz, S. (2021). No impact of a short‐term climatic ‘El Niño’ fluctuation on gut microbial diversity in populations of the Galápagos marine iguana (Amblyrhynchus cristatus). The Science of Nature 108, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Iwatsuki, T. , Kanazawa, T. , Ogasawara, T. , Hosotani, K. , Tsuchiya, K. , Watanabe, S. , Suzuki, T. , Moriuchi, R. , Kanesaki, Y. & Dohra, H. (2021). 16S rRNA gene amplicon sequencing of gut microbiota in three species of deep‐sea fish in Suruga Bay, Japan. Microbiology Resource Announcements 10, e01260–20. 10.1128/mra.01260-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Jeon, J. , Rahman, M.‐M. , Han, C. , Shin, J. , Sa, K. J. & Kim, J. (2023). Spodoptera frugiperda (Lepidoptera: Noctuidae) life table comparisons and gut microbiome analysis reared on corn varieties. Insects 14, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Jiang, H.‐Y. , Ma, J.‐E. , Li, J. , Zhang, X.‐J. , Li, L.‐M. , He, N. , Liu, H.‐Y. , Luo, S.‐Y. , Wu, Z.‐J. , Han, R.‐C. & Chen, J.‐P. (2017). Diets alter the gut microbiome of crocodile lizards. Frontiers in Microbiology 8, 2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Jones, J. , DiBattista, J. D. , Stat, M. , Bunce, M. , Boyce, M. C. , Fairclough, D. V. , Travers, M. J. & Huggett, M. J. (2018). The microbiome of the gastrointestinal tract of a range‐shifting marine herbivorous fish. Frontiers in Microbiology 9, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Jonge, D. N. , Carlsen, B. , Christensen, M. H. , Pertoldi, C. & Nielsen, J. L. (2022). The gut microbiome of 54 mammalian species. Frontiers in Microbiology 13, 886252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Kaczmarczyk, A. , Kucharczyk, H. , Kucharczyk, M. , Kapusta, P. , Sell, J. & Zielińska, S. (2018). First insight into microbiome profile of fungivorous thrips Hoplothrips carpathicus (Insecta: Thysanoptera) at different developmental stages: molecular evidence of Wolbachia endosymbiosis. Scientific Reports 8, 14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Kakumanu, M. L. , Maritz, J. M. , Carlton, J. M. & Schal, C. (2018). Overlapping community compositions of gut and fecal microbiomes in lab‐reared and field‐collected German cockroaches. Applied and Environmental Microbiology 84, e01037‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Kang, W. , Kim, P. S. , Tak, E. J. , Sung, H. , Shin, N.‐R. , Hyun, D.‐W. , Whon, T. W. , Kim, H. S. , Lee, J.‐Y. , Yun, J.‐H. , Jung, M.‐J. & Bae, J.‐W. (2022). Host phylogeny, habitat, and diet are main drivers of the cephalopod and mollusk gut microbiome. Animal Microbiome 4, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartzinel, T. R. , Hsing, J. C. , Musili, P. M. , Brown, B. R. P. & Pringle, R. M. (2019). Covariation of diet and gut microbiome in African megafauna. Proceedings of the National Academy of Sciences of the United States of America 166, 23588–23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Katoh, K. , Misawa, K. , Kuma, K. & Miyata, T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30, 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keddy, P. A. (1992). Assembly and response rules: two goals for predictive community ecology. Journal of Vegetation Science 3, 157–164. [Google Scholar]
- * Keiz, K. , Ulrich, S. , Wenderlein, J. , Keferloher, P. , Wiesinger, A. , Neuhaus, K. , Lagkouvardos, I. , Wedekind, H. & Straubinger, R. K. (2023). The development of the bacterial community of brown trout (Salmo trutta) during ontogeny. Microorganisms 11, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Kešnerová, L. , Emery, O. , Troilo, M. , Liberti, J. , Erkosar, B. & Engel, P. (2020). Gut microbiota structure differs between honeybees in winter and summer. The ISME Journal 14, 801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Khanal, P. , Pandey, D. , Næss, G. , Cabrita, A. R. J. , Fonseca, A. J. M. , Maia, M. R. G. , Timilsina, B. , Veldkamp, T. , Sapkota, R. & Overrein, H. (2023). Yellow mealworms (Tenebrio molitor) as an alternative animal feed source: a comprehensive characterization of nutritional values and the larval gut microbiome. Journal of Cleaner Production 389, 136104. [Google Scholar]
- * Kim, C. , Kim, J.‐M. , Choi, H. , Choi, Y.‐S. , Jin, B.‐R. , Lee, K.‐S. & Choi, K. (2022). Analysis of the gut microbiome of susceptible and resistant honeybees (Apis cerana) against sacbrood virus disease. Journal of Applied Entomology 146, 1078–1086. [Google Scholar]
- * Kim, M. , Cho, H. & Lee, W. Y. (2020). Distinct gut microbiotas between southern elephant seals and Weddell seals of Antarctica. Journal of Microbiology 58, 1018–1026. [DOI] [PubMed] [Google Scholar]
- Kim, P. S. , Shin, N.‐R. , Lee, J.‐B. , Kim, M.‐S. , Whon, T. W. , Hyun, D.‐W. , Yun, J.‐H. , Jung, M.‐J. , Kim, J. Y. & Bae, J.‐W. (2021). Host habitat is the major determinant of the gut microbiome of fish. Microbiome 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinross, J. M. , Darzi, A. W. & Nicholson, J. K. (2011). Gut microbiome‐host interactions in health and disease. Genome Medicine 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Klammsteiner, T. , Walter, A. , Bogataj, T. , Heussler, C. D. , Stres, B. , Steiner, F. M. , Schlick‐Steiner, B. C. , Arthofer, W. & Insam, H. (2020). The core gut microbiome of black soldier fly (Hermetia illucens) larvae raised on low‐bioburden diets. Frontiers in Microbiology 11, 993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, R. , Vrbanac, A. , Taylor, B. C. , Aksenov, A. , Callewaert, C. , Debelius, J. , Gonzalez, A. , Kosciolek, T. , McCall, L.‐I. , McDonald, D. , Melnik, A. V. , Morton, J. T. , Navas, J. , Quinn, R. A. , Sanders, J. G. , et al. (2018). Best practices for analysing microbiomes. Nature Reviews. Microbiology 16, 410–422. [DOI] [PubMed] [Google Scholar]
- Koskella, B. , Hall, L. J. & Metcalf, C. J. E. (2017). The microbiome beyond the horizon of ecological and evolutionary theory. Nature Ecology & Evolution 1, 1606–1615. [DOI] [PubMed] [Google Scholar]
- Lao, H.‐Y. (2022). The clinical utility of two high‐throughput 16S rRNA gene sequencing workflows for taxonomic assignment of unidentifiable bacterial pathogens in matrix‐assisted laser desorption ionization–time of flight mass spectrometry. Journal of Clinical Microbiology 60, e0176921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Lavy, O. , Gophna, U. , Gefen, E. & Ayali, A. (2019). The effect of density‐dependent phase on the locust gut bacterial composition. Frontiers in Microbiology 9, 3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Lawrence, S. D. , Novak, N. G. , Shao, J. , Ghosh, S. K. B. & Blackburn, M. B. (2020). Cabbage looper (Trichoplusia ni Hübner) labial glands contain unique bacterial flora in contrast with their alimentary canal, mandibular glands, and Malpighian tubules. MicrobiologyOpen 9, e994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Lawson, L. A. , Atkinson, C. L. & Jackson, C. R. (2022). The gut bacterial microbiome of the Threeridge mussel, Amblema plicata, varies between rivers but shows a consistent core community. Freshwater Biology 67, 1125–1136. [Google Scholar]
- * Le, D. , Nguyen, P. , Nguyen, D. , Dierckens, K. , Boon, N. , Lacoere, T. , Kerckhof, F.‐M. , De Vrieze, J. , Vadstein, O. & Bossier, P. (2020). Gut microbiota of migrating wild rabbit fish (Siganus guttatus) larvae have low spatial and temporal variability. Microbial Ecology 79, 539–551. [DOI] [PubMed] [Google Scholar]
- Levin, D. , Raab, N. , Pinto, Y. , Rothschild, D. , Zanir, G. , Godneva, A. , Mellul, N. , Futorian, D. , Gal, D. , Leviatan, S. , Zeevi, D. , Bachelet, I. & Segal, E. (2021). Diversity and functional landscapes in the microbiota of animals in the wild. Science (New York, N.Y.) 372, eabb5352. [DOI] [PubMed] [Google Scholar]
- Levy, R. & Borenstein, E. (2013). Metabolic modeling of species interaction in the human microbiome elucidates community‐level assembly rules. Proceedings of the National Academy of Sciences 110, 12804–12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley, R. E. , Hamady, M. , Lozupone, C. , Turnbaugh, P. J. , Ramey, R. R. , Bircher, J. S. , Schlegel, M. L. , Tucker, T. A. , Schrenzel, M. D. , Knight, R. & Gordon, J. I. (2008). Evolution of mammals and their gut microbes. Science (New York, N.Y.) 320, 1647–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Li, D. , Miao, J. , Pan, L. , Zhou, Y. , Gao, Z. , Yang, Y. , Xu, R. & Zhang, X. (2021). Impacts of benzo(a)pyrene exposure on scallop (Chlamys farreri) gut health and gut microbiota composition. Science of the Total Environment 799, 149471. [DOI] [PubMed] [Google Scholar]
- * Li, J. , Chen, S. , Wu, P. , Zhu, C. , Hu, R. , Li, T. & Guo, Y. (2023). Insights into the relationship between intestinal microbiota of the aquaculture worm Sipunculus nudus and surrounding sediments. Fishes 8, 32. [Google Scholar]
- Li, L. & Ma, Z. (2016). Testing the neutral theory of biodiversity with human microbiome datasets. Scientific Reports 6, 31448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Li, M. , Jin, W. , Li, Y. , Zhao, L. , Cheng, Y. & Zhu, W. (2016). Spatial dynamics of the bacterial community structure in the gastrointestinal tract of red kangaroo (Macropus rufus). World Journal of Microbiology and Biotechnology 32, 98. [DOI] [PubMed] [Google Scholar]
- * Li, Y.‐F. , Xu, J.‐K. , Chen, Y.‐W. , Ding, W.‐Y. , Shao, A.‐Q. , Liang, X. , Zhu, Y.‐T. & Yang, J.‐L. (2019). Characterization of gut microbiome in the mussel Mytilus galloprovincialis in response to thermal stress. Frontiers in Physiology 10, 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Lim, M. Y. , Hong, S. , Kim, J.‐H. & Nam, Y.‐D. (2021). Association between gut microbiome and frailty in the older adult population in Korea. The Journals of Gerontology: Series A 76, 1362–1368. [DOI] [PubMed] [Google Scholar]
- * Littleford‐Colquhoun, B. L. , Weyrich, L. S. , Jackson, N. & Frere, C. H. (2019). City life alters the gut microbiome and stable isotope profiling of the eastern water dragon (Intellagama lesueurii). Molecular Ecology 28, 4592–4607. [DOI] [PubMed] [Google Scholar]
- * Liu, H. , Tan, K. S. , Zhang, X. , Zhang, H. , Cheng, D. , Ting, Y. , Li, S. , Ma, H. & Zheng, H. (2020). Comparison of gut microbiota between golden and brown noble scallop Chlamys nobilis and its association with carotenoids. Frontiers in Microbiology 11, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Liu, X. , Liu, J. , Xiong, K. , Zhang, C. , Fang, J. K.‐H. , Song, J. , Tai, Z. , Zhu, Q. , Hu, M. & Wang, Y. (2022). Effects of ocean acidification on molting, oxidative stress, and gut microbiota in juvenile horseshoe crab Tachypleus tridentatus . Frontiers in Physiology 12, 813582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Siles, M. , Duncan, S. H. , Garcia‐Gil, L. J. & Martinez‐Medina, M. (2017). Faecalibacterium prausnitzii: from microbiology to diagnostics and prognostics. The ISME Journal 11, 841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Lyu, T. , Zhu, J. , Yang, X. , Yang, W. & Zheng, Z. (2022). Responses of gut microbial community composition and function of the freshwater gastropod Bellamya aeruginosa to cyanobacterial bloom. Frontiers in Microbiology 13, 906278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Ma, Y. , He, H. , Zhao, H. , Xian, Y. , Guo, H. , Liu, B. & Xue, K. (2021). Microbiome diversity of cotton aphids (Aphis gossypii) is associated with host alternation. Scientific Reports 11, 5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Z. (2021). Cross‐scale analyses of animal and human gut microbiome assemblies from metacommunity to global landscape. mSystems 6, 00633–21. 10.1128/msystems.00633-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Magagnoli, S. , Alberoni, D. , Baffoni, L. , Martini, A. , Marini, F. , Di Gioia, D. , Mazzon, M. , Marzadori, C. , Campanelli, G. & Burgio, G. (2022). The ground beetle Pseudoophonus rufipes gut microbiome is influenced by the farm management system. Scientific Reports 12, 22638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallott, E. K. & Amato, K. R. (2021). Host specificity of the gut microbiome. Nature Reviews Microbiology 19, 639–653. [DOI] [PubMed] [Google Scholar]
- * Maltseva, A. L. , Varfolomeeva, M. A. , Gafarova, E. R. , Panova, M. A. Z. , Mikhailova, N. A. & Granovitch, A. I. (2021). Divergence together with microbes: a comparative study of the associated microbiomes in the closely related Littorina species. PLoS One 16, e0260792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Manor, O. , Dai, C. L. , Kornilov, S. A. , Smith, B. , Price, N. D. , Lovejoy, J. C. , Gibbons, S. M. & Magis, A. T. (2020). Health and disease markers correlate with gut microbiome composition across thousands of people. Nature Communications 11, 5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Marín‐Miret, J. , González‐Serrano, F. , Rosas, T. , Baixeras, J. , Latorre, A. , Pérez‐Cobas, A. E. & Moya, A. (2022). Temporal variations shape the gut microbiome ecology of the moth Brithys crini . Environmental Microbiology 24, 3939–3953. [DOI] [PubMed] [Google Scholar]
- * Mason, C. J. , Hoover, K. & Felton, G. W. (2021). Effects of maize (Zea mays) genotypes and microbial sources in shaping fall armyworm (Spodoptera frugiperda) gut bacterial communities. Scientific Reports 11, 4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Mathai, P. P. , Byappanahalli, M. N. , Johnson, N. S. & Sadowsky, M. J. (2021). Gut microbiota associated with different sea lamprey (Petromyzon marinus) life stages. Frontiers in Microbiology 12, 706683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Mays, Z. , Hunter, A. , Campbell, L. G. & Carlos‐Shanley, C. (2021). The effects of captivity on the microbiome of the endangered Comal Springs riffle beetle (Heterelmis comalensis). FEMS Microbiology Letters 368, fnab121. [DOI] [PubMed] [Google Scholar]
- * McCauley, M. , German, D. P. , Lujan, N. K. & Jackson, C. R. (2020). Gut microbiomes of sympatric Amazonian wood‐eating catfishes (Loricariidae) reflect host identity and little role in wood digestion. Ecology and Evolution 10, 7117–7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * McClure, E. A. , Nelson, M. C. , Lin, A. & Graf, J. (2021). Macrobdella decora: Old World leech gut microbial community structure conserved in a New World Leech. Applied and Environmental Microbiology 87, e02082‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * McDonald, D. , Jiang, Y. , Balaban, M. , Cantrell, K. , Zhu, Q. , Gonzalez, A. , Morton, J. T. , Nicolaou, G. , Parks, D. H. , Karst, S. M. , Albertsen, M. , Hugenholtz, P. , DeSantis, T. , Song, S. J. , Bartko, A. , et al. (2023). Greengenes2 unifies microbial data in a single reference tree. Nature Biotechnology 42, 715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, J. E. , Marchesi, J. R. & Koskella, B. (2020). Application of ecological and evolutionary theory to microbiome community dynamics across systems. Proceedings of the Royal Society B: Biological Sciences 287, 20202886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie, V. J. , Song, S. J. , Delsuc, F. , Prest, T. L. , Oliverio, A. M. , Korpita, T. M. , Alexiev, A. , Amato, K. R. , Metcalf, J. L. , Kowalewski, M. , Avenant, N. L. , Link, A. , Di Fiore, A. , Seguin‐Orlando, A. , Feh, C. , et al. (2017). The effects of captivity on the mammalian gut microbiome. Integrative and Comparative Biology 57, 690–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Mendoza‐Guido, B. , Rodríguez‐Hernández, N. , Ivens, A. B. F. , von Beeren, C. , Murillo‐Cruz, C. , Zuniga‐Chaves, I. , Łukasik, P. , Sanchez, E. , Kronauer, D. J. C. & Pinto‐Tomás, A. A. (2023). Low diversity and host specificity in the gut microbiome community of Eciton army ants (Hymenoptera: Formicidae: Dorylinae) in a Costa Rican rainforest. Myrmecological News 33, 19–34. [Google Scholar]
- *Wasimuddin, Menke, S. , Melzheimer, J. , Thalwitzer, S. , Heinrich, S. , Wachter, B. & Sommer, S. (2017). Gut microbiomes of free‐ranging and captive Namibian cheetahs: diversity, putative functions and occurrence of potential pathogens. Molecular Ecology 26, 5515–5527. [DOI] [PubMed] [Google Scholar]
- * Meriggi, N. , Di Paola, M. , Vitali, F. , Rivero, D. , Cappa, F. , Turillazzi, F. , Gori, A. , Dapporto, L. , Beani, L. , Turillazzi, S. & Cavalieri, D. (2019). Saccharomyces cerevisiae induces immune enhancing and shapes gut microbiota in social wasps. Frontiers in Microbiology 10, 2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Milani, C. , Alessandri, G. , Mancabelli, L. , Mangifesta, M. , Lugli, G. A. , Viappiani, A. , Longhi, G. , Anzalone, R. , Duranti, S. , Turroni, F. , Ossiprandi, M. C. , van Sinderen, D. & Ventura, M. (2020). Multi‐omics approaches to decipher the impact of diet and host physiology on the mammalian gut microbiome. Applied and Environmental Microbiology 86, e01864‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minich, J. J. , Härer, A. , Vechinski, J. , Frable, B. W. , Skelton, Z. R. , Kunselman, E. , Shane, M. A. , Perry, D. S. , Gonzalez, A. , McDonald, D. , Knight, R. , Michael, T. P. & Allen, E. E. (2022). Host biology, ecology and the environment influence microbial biomass and diversity in 101 marine fish species. Nature Communications 13, 6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake, S. , Ngugi, D. K. & Stingl, U. (2015). Diet strongly influences the gut microbiota of surgeonfishes. Molecular Ecology 24, 656–672. [DOI] [PubMed] [Google Scholar]
- Moeller, A. H. , Caro‐Quintero, A. , Mjungu, D. , Georgiev, A. V. , Lonsdorf, E. V. , Muller, M. N. , Pusey, A. E. , Peeters, M. , Hahn, B. H. & Ochman, H. (2016). Cospeciation of gut microbiota with hominids. Science 353, 380–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller, A. H. , Ivey, K. , Cornwall, M. B. , Herr, K. , Rede, J. , Taylor, E. N. & Gunderson, A. R. (2020). The lizard gut microbiome changes with temperature and is associated with heat tolerance. Applied and Environmental Microbiology 86, e01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller, A. H. , Li, Y. , Mpoudi Ngole, E. , Ahuka‐Mundeke, S. , Lonsdorf, E. V. , Pusey, A. E. , Peeters, M. , Hahn, B. H. & Ochman, H. (2014). Rapid changes in the gut microbiome during human evolution. Proceedings of the National Academy of Sciences 111, 16431–16435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Monteiro, H. F. , Zhou, Z. , Gomes, M. S. , Peixoto, P. M. G. , Bonsaglia, E. C. R. , Canisso, I. F. , Weimer, B. C. & Lima, F. S. (2022). Rumen and lower gut microbiomes relationship with feed efficiency and production traits throughout the lactation of Holstein dairy cows. Scientific Reports 12, 4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Montoya‐Ciriaco, N. , Gómez‐Acata, S. , Muñoz‐Arenas, L. C. , Dendooven, L. , Estrada‐Torres, A. , Díaz de la Vega‐Pérez, A. H. & Navarro‐Noya, Y. E. (2020). Dietary effects on gut microbiota of the mesquite lizard Sceloporus grammicus (Wiegmann, 1828) across different altitudes. Microbiome 8, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muegge, B. D. , Kuczynski, J. , Knights, D. , Clemente, J. C. , González, A. , Fontana, L. , Henrissat, B. , Knight, R. & Gordon, J. I. (2011). Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 332, 970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Muhammad, A. , Fang, Y. , Hou, Y. & Shi, Z. (2017). The gut entomotype of red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae) and their effect on host nutrition metabolism. Frontiers in Microbiology 8, 2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Muturi, E. J. , Dunlap, C. , Ramirez, J. L. , Rooney, A. P. & Kim, C.‐H. (2019). Host blood‐meal source has a strong impact on gut microbiota of Aedes aegypti . FEMS Microbiology Ecology 95, fiy213. [DOI] [PubMed] [Google Scholar]
- Naito, Y. , Uchiyama, K. & Takagi, T. (2018). A next generation beneficial microbe: Akkermansia muciniphila . Journal of Clinical Biochemistry and Nutrition 63, 33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Nakagawa, S. , Saito, H. , Tame, A. , Hirai, M. , Yamaguchi, H. , Sunata, T. , Aida, M. , Muto, H. , Sawayama, S. & Takaki, Y. (2017). Microbiota in the coelomic fluid of two common coastal starfish species and characterization of an abundant Helicobacter‐related taxon. Scientific Reports 7, 8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Nielsen, S. , Walburn, J. W. , Vergés, A. , Thomas, T. & Egan, S. (2017). Microbiome patterns across the gastrointestinal tract of the rabbitfish Siganus fuscescens . PeerJ 5, e3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Ning, Y. , Qi, J. , Dobbins, M. T. , Liang, X. , Wang, J. , Chen, S. , Ma, J. & Jiang, G. (2020). Comparative analysis of microbial community structure and function in the gut of wild and captive Amur tiger. Frontiers in Microbiology 11, 11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Nones, S. , Simões, F. , Trindade, C. S. , Matos, J. & Sousa, E. (2021). Microbiome associated with the mycangia of female and male adults of the ambrosia beetle Platypus cylindrus Fab. (Coleoptera: Curculionidae). Insects 12, 881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Nyman, A. , Huyben, D. , Lundh, T. & Dicksved, J. (2017). Effects of microbe‐ and mussel‐based diets on the gut microbiota in Arctic charr (Salvelinus alpinus). Aquaculture Reports 5, 34–40. [Google Scholar]
- * Ojha, A. , Sinha, D. K. , Padmakumari, A. P. , Bentur, J. S. & Nair, S. (2017). Bacterial community structure in the Asian rice gall midge reveals a varied microbiome rich in proteobacteria. Scientific Reports 7, 9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Okamoto, Y. , Ichinohe, N. , Woo, C. , Han, S.‐Y. , Kim, H.‐H. , Ito, S. , Nakamura, C. , Kumura, J. , Nagaoka, K. & Yamamoto, N. (2021). Contrasting gut microbiota in captive Eurasian otters (Lutra lutra) by age. Archives of Microbiology 203, 5405–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Ooi, M. C. , Goulden, E. F. , Smith, G. G. , Nowak, B. F. & Bridle, A. R. (2017). Developmental and gut‐related changes to microbiomes of the cultured juvenile spiny lobster Panulirus ornatus . FEMS Microbiology Ecology 93, fix159. [DOI] [PubMed] [Google Scholar]
- * Pagán‐Jiménez, M. , Ruiz‐Calderón, J. F. , Dominguez‐Bello, M. G. & García‐Arrarás, J. E. (2019). Characterization of the intestinal microbiota of the sea cucumber Holothuria glaberrima . PLoS One 14, e0208011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Pan, Y. , Qian, J. , Ma, X. , Huang, W. , Fang, J. K.‐H. , Arif, I. , Wang, Y. , Shang, Y. & Hu, M. (2023). Response of moulting genes and gut microbiome to nano‐plastics and copper in juvenile horseshoe crab Tachypleus tridentatus . Marine Environmental Research 191, 106128. [DOI] [PubMed] [Google Scholar]
- * Parata, L. , Nielsen, S. , Xing, X. , Thomas, T. , Egan, S. & Vergés, A. (2020). Age, gut location and diet impact the gut microbiome of a tropical herbivorous surgeonfish. FEMS Microbiology Ecology 96, fiz179. [DOI] [PubMed] [Google Scholar]
- * Pardesi, B. , Roberton, A. M. , Lee, K. C. , Angert, E. R. , Rosendale, D. I. , Boycheva, S. , White, W. L. & Clements, K. D. (2022). Distinct microbiota composition and fermentation products indicate functional compartmentalization in the hindgut of a marine herbivorous fish. Molecular Ecology 31, 2494–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Parris, D. J. , Morgan, M. M. & Stewart, F. J. (2019). Feeding rapidly alters microbiome composition and gene transcription in the clownfish gut. Applied and Environmental Microbiology 85, e02479‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsaei, M. , Sarafraz, N. , Moaddab, S. Y. & Ebrahimzadeh Leylabadlo, H. (2021). The importance of Faecalibacterium prausnitzii in human health and diseases. New Microbes and New Infections 43, 100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Perry, T. , West, E. , Eisenhofer, R. , Stenhouse, A. , Wilson, I. , Laming, B. , Rismiller, P. , Shaw, M. & Grützner, F. (2022). Characterising the gut microbiomes in wild and captive short‐beaked echidnas reveals diet‐associated changes. Frontiers in Microbiology 13, 687115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Phalnikar, K. , Kunte, K. & Agashe, D. (2019). Disrupting butterfly caterpillar microbiomes does not impact their survival and development. Proceedings of the Royal Society B: Biological Sciences 286, 20192438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Pierce, M. L. & Ward, J. E. (2019). Gut microbiomes of the eastern oyster (Crassostrea virginica) and the blue mussel (Mytilus edulis): temporal variation and the influence of marine aggregate‐associated microbial communities. mSphere 4, 00730–19. 10.1128/msphere.00730-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Pires, E. S. , Hardoim, C. C. P. , Miranda, K. R. , Secco, D. A. , Lobo, L. A. , de Carvalho, D. P. , Han, J. , Borchers, C. H. , Ferreira, R. B. R. , Salles, J. F. , Domingues, R. M. C. P. & Antunes, L. C. M. (2019). The gut microbiome and metabolome of two riparian communities in the Amazon. Frontiers in Microbiology 10, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Plummer, E. , Bulach, D. , Carter, G. & Albert, M. J. (2020). Gut microbiome of native Arab Kuwaitis. Gut Pathogens 12, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Pratte, Z. A. , Perry, C. , Dove, A. D. M. , Hoopes, L. A. , Ritchie, K. B. , Hueter, R. E. , Fischer, C. , Newton, A. L. & Stewart, F. J. (2022). Microbiome structure in large pelagic sharks with distinct feeding ecologies. Animal Microbiome 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Raethong, N. , Nakphaichit, M. , Suratannon, N. , Sathitkowitchai, W. , Weerapakorn, W. , Keawsompong, S. & Vongsangnak, W. (2021). Analysis of human gut microbiome: taxonomy and metabolic functions in Thai adults. Genes 12, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, G. , McDonald, D. , Gonzalez, A. , Vázquez‐Baeza, Y. , Jiang, L. , Casals‐Pascual, C. , Hakim, D. , Dilmore, A. H. , Nowinski, B. , Peddada, S. & Knight, R. (2023). Determination of effect sizes for power analysis for microbiome studies using large microbiome databases. Genes 14, 1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Reich, I. , Ijaz, U. Z. , Gormally, M. & Smith, C. J. (2018). 16S rRNA sequencing reveals likely beneficial core microbes within faecal samples of the EU protected slug Geomalacus maculosus . Scientific Reports 8, 10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Rohrer, S. D. , Jiménez‐Uzcátegui, G. , Parker, P. G. & Chubiz, L. M. (2023). Composition and function of the Galapagos penguin gut microbiome vary with age, location, and a putative bacterial pathogen. Scientific Reports 13, 5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas, C. A. , Ramírez‐Barahona, S. , Holekamp, K. E. & Theis, K. R. (2021). Host phylogeny and host ecology structure the mammalian gut microbiota at different taxonomic scales. Animal Microbiome 3, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román‐Palacios, C. , Scholl, J. P. & Wiens, J. J. (2019). Evolution of diet across the animal tree of life. Evolution Letters 3, 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Rothenberg, S. E. , Sweitzer, D. N. , Rackerby, B. R. , Couch, C. E. , Cohen, L. A. , Broughton, H. M. , Steingass, S. M. & Beechler, B. R. (2021). Fecal methylmercury correlates with gut microbiota taxa in Pacific walruses (Odobenus rosmarus divergens). Frontiers in Microbiology 12, 648685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Rubanov, A. , Russell, K. A. , Rothman, J. A. , Nieh, J. C. & McFrederick, Q. S. (2019). Intensity of Nosema ceranae infection is associated with specific honey bee gut bacteria and weakly associated with gut microbiome structure. Scientific Reports 9, 3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Ruiz Barrionuevo, J. M. , Vilanova‐Cuevas, B. , Alvarez, A. , Martín, E. , Malizia, A. , Galindo‐Cardona, A. , de Cristóbal, R. E. , Occhionero, M. A. , Chalup, A. , Monmany‐Garzia, A. C. & Godoy‐Vitorino, F. (2022). The bacterial and fungal gut microbiota of the greater wax moth, Galleria mellonella L. consuming polyethylene and polystyrene. Frontiers in Microbiology 13, 918861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, J. G. , Sprockett, D. D. , Li, Y. , Mjungu, D. , Lonsdorf, E. V. , Ndjango, J.‐B. N. , Georgiev, A. V. , Hart, J. A. , Sanz, C. M. , Morgan, D. B. , Peeters, M. , Hahn, B. H. & Moeller, A. H. (2023). Widespread extinctions of co‐diversified primate gut bacterial symbionts from humans. Nature Microbiology 8, 1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Santos, B. , Bletz, M. C. , Sabino‐Pinto, J. , Cocca, W. , Fidy, J. F. S. , Freeman, K. L. , Kuenzel, S. , Ndriantsoa, S. , Noel, J. , Rakotonanahary, T. , Vences, M. & Crottini, A. (2021). Characterization of the microbiome of the invasive Asian toad in Madagascar across the expansion range and comparison with a native co‐occurring species. PeerJ 9, e11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Sapkota, R. , Santos, S. , Farias, P. , Krogh, P. H. & Winding, A. (2020). Insights into the earthworm gut multi‐kingdom microbial communities. Science of the Total Environment 727, 138301. [DOI] [PubMed] [Google Scholar]
- * Sapkota, R. & Scharf, M. E. (2022). Intercolony comparisons of gut microbiome composition from lab reared eastern subterranean termites (Blattodea: Rhinotermitidae). Journal of Insect Science 22, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Scalvenzi, T. , Clavereau, I. , Bourge, M. & Pollet, N. (2021). Gut microbial ecology of Xenopus tadpoles across life stages. Peer Community Journal 1, e41. [Google Scholar]
- * Schaan, A. P. , Sarquis, D. , Cavalcante, G. C. , Magalhães, L. , Sacuena, E. R. P. , Costa, J. , Fonseca, D. , Mello, V. J. , Guerreiro, J. F. & Ribeiro‐dos‐Santos, Â. (2021). The structure of Brazilian Amazonian gut microbiomes in the process of urbanisation. npj Biofilms and Microbiomes 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Schmidt, E. , Mykytczuk, N. & Schulte‐Hostedde, A. I. (2019). Effects of the captive and wild environment on diversity of the gut microbiome of deer mice (Peromyscus maniculatus). The ISME Journal 13, 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Segers, F. H. I. D. , Kaltenpoth, M. & Foitzik, S. (2019). Abdominal microbial communities in ants depend on colony membership rather than caste and are linked to colony productivity. Ecology and Evolution 9, 13450–13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura Munoz, R. R. , Mantz, S. , Martínez, I. , Li, F. , Schmaltz, R. J. , Pudlo, N. A. , Urs, K. , Martens, E. C. , Walter, J. & Ramer‐Tait, A. E. (2022). Experimental evaluation of ecological principles to understand and modulate the outcome of bacterial strain competition in gut microbiomes. The ISME Journal 16, 1594–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Sehli, S. , Idrissi Azami, A. , Dini, N. , Habib, N. , Chaouni, B. , Hamdi, S. , Al Idrissi, N. , Amzazi, S. , Allali, I. , Nejjari, C. & Ghazal, H. (2022). Gut microbiome 16S rRNA gene amplicon taxonomic profiling of hospitalized Moroccan COVID‐19 patients. Microbiology Resource Announcements 11, e00256‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Sevellec, M. , Derome, N. & Bernatchez, L. (2018). Holobionts and ecological speciation: the intestinal microbiota of lake whitefish species pairs. Microbiome 6, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Shelomi, M. & Chen, M.‐J. (2020). Culturing‐enriched metabarcoding analysis of the Oryctes rhinoceros gut microbiome. Insects 11, 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, N. , Li, N. , Duan, X. & Niu, H. (2017). Interaction between the gut microbiome and mucosal immune system. Military Medical Research 4, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Silva, H. , Oliveira, T. M. P. , Sabino, E. C. , Alonso, D. P. & Sallum, M. A. M. (2022). Bacterial diversity in Haemagogus leucocelaenus (Diptera: Culicidae) from Vale do Ribeira, São Paulo, Brazil. BMC Microbiology 22, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Skrodenytė‐Arbačiauskienė, V. , Virbickas, T. , Lukša, J. , Servienė, E. , Blažytė‐Čereškienė, L. & Kesminas, V. (2022). Gut microbiome of wild Baltic salmon (Salmo salar L.) Parr. Microbial Ecology 84, 1294–1298. [DOI] [PubMed] [Google Scholar]
- * Smith, C. C. , Srygley, R. B. , Healy, F. , Swaminath, K. & Mueller, U. G. (2017). Spatial structure of the Mormon cricket gut microbiome and its predicted contribution to nutrition and immune function. Frontiers in Microbiology 8, 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Smith, S. N. , Colston, T. J. & Siler, C. D. (2021). Venomous snakes reveal ecological and phylogenetic factors influencing variation in gut and oral microbiomes. Frontiers in Microbiology 12, 657754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Smits, S. A. , Leach, J. , Sonnenburg, E. D. , Gonzalez, C. G. , Lichtman, J. S. , Reid, G. , Knight, R. , Manjurano, A. , Changalucha, J. , Elias, J. E. , Dominguez‐Bello, M. G. & Sonnenburg, J. L. (2017). Seasonal cycling in the gut microbiome of the Hadza hunter‐gatherers of Tanzania. Science 357, 802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, S. J. , Lauber, C. , Costello, E. K. , Lozupone, C. A. , Humphrey, G. , Berg‐Lyons, D. , Gregory Caporaso, J. , Knights, D. , Clemente, J. C. , Nakielny, S. , Gordon, J. I. , Fierer, N. & Knight, R. (2013). Cohabiting family members share microbiota with one another and with their dogs. eLife 2, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, S. J. , Sanders, J. G. , Delsuc, F. , Metcalf, J. , Amato, K. , Taylor, M. W. , Mazel, F. , Lutz, H. L. , Winker, K. , Graves, G. R. , Humphrey, G. , Gilbert, J. A. , Hackett, S. J. , White, K. P. , Skeen, H. R. , et al. (2020). Comparative analyses of vertebrate gut microbiomes reveal convergence between birds and bats. MBio 11, 2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Stoffel, M. A. , Acevedo‐Whitehouse, K. , Morales‐Durán, N. , Grosser, S. , Chakarov, N. , Krüger, O. , Nichols, H. J. , Elorriaga‐Verplancken, F. R. & Hoffman, J. I. (2020). Early sexual dimorphism in the developing gut microbiome of northern elephant seals. Molecular Ecology 29, 2109–2122. [DOI] [PubMed] [Google Scholar]
- * Suárez‐Moo, P. , Cruz‐Rosales, M. , Ibarra‐Laclette, E. , Desgarennes, D. , Huerta, C. & Lamelas, A. (2020). Diversity and composition of the gut microbiota in the developmental stages of the dung beetle Copris incertus Say (Coleoptera, Scarabaeidae). Frontiers in Microbiology 11, 1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Suenami, S. , Konishi Nobu, M. & Miyazaki, R. (2019). Community analysis of gut microbiota in hornets, the largest eusocial wasps, Vespa mandarinia and V. simillima . Scientific Reports 9, 9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullam, K. E. , Essinger, S. D. , Lozupone, C. A. , O'Connor, M. P. , Rosen, G. L. , Knight, R. , Kilham, S. S. & Russell, J. A. (2012). Environmental and ecological factors that shape the gut bacterial communities of fish: a meta‐analysis. Molecular Ecology 21, 3363–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundarraman, D. , Hay, E. A. , Martins, D. M. , Shields, D. S. , Pettinari, N. L. & Parthasarathy, R. (2020). Higher‐order interactions dampen pairwise competition in the zebrafish gut microbiome. MBio 11, 01667–20. 10.1128/mbio.01667-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvain, F.‐É. , Holland, A. , Bouslama, S. , Audet‐Gilbert, É. , Lavoie, C. , Val, A. L. & Derome, N. (2020). Fish skin and gut microbiomes show contrasting signatures of host species and habitat. Applied and Environmental Microbiology 86, e00789‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Tang, G.‐S. , Liang, X.‐X. , Yang, M.‐Y. , Wang, T.‐T. , Chen, J.‐P. , Du, W.‐G. , Li, H. & Sun, B.‐J. (2020). Captivity influences gut microbiota in crocodile lizards (Shinisaurus crocodilurus). Frontiers in Microbiology 11, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Tang, K.‐Y. , Wang, Z.‐W. , Wan, Q.‐H. & Fang, S.‐G. (2019). Metagenomics reveals seasonal functional adaptation of the gut microbiome to host feeding and fasting in the Chinese alligator. Frontiers in Microbiology 10, 2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Tang, S. , Li, Y. , Huang, C. , Yan, S. , Li, Y. , Chen, Z. & Wu, Z. (2022). Comparison of gut microbiota diversity between captive and wild Tokay gecko (Gekko gecko). Frontiers in Microbiology 13, 897923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Terova, G. , Rimoldi, S. , Ascione, C. , Gini, E. , Ceccotti, C. & Gasco, L. (2019). Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Reviews in Fish Biology and Fisheries 29, 465–486. [Google Scholar]
- Thiele, I. , Sahoo, S. , Heinken, A. , Hertel, J. , Heirendt, L. , Aurich, M. K. & Fleming, R. M. (2020). Personalized whole‐body models integrate metabolism, physiology, and the gut microbiome. Molecular Systems Biology 16, e8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, L. R. , Sanders, J. G. , McDonald, D. , Amir, A. , Ladau, J. , Locey, K. J. , Prill, R. J. , Tripathi, A. , Gibbons, S. M. , Ackermann, G. , Navas‐Molina, J. A. , Janssen, S. , Kopylova, E. , Vázquez‐Baeza, Y. , González, A. , et al. (2017). A communal catalogue reveals Earth's multiscale microbial diversity. Nature 551, 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Tinker, K. A. & Ottesen, E. A. (2016). The core gut microbiome of the American cockroach, Periplaneta americana, is stable and resilient to dietary shifts. Applied and Environmental Microbiology 82, 6603–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Tinker, K. A. & Ottesen, E. A. (2018). The hindgut microbiota of praying mantids is highly variable and includes both prey‐associated and host‐specific microbes. PLoS One 13, e0208917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Tong, Q. , Cui, L.‐Y. , Hu, Z.‐F. , Du, X.‐P. , Abid, H. M. & Wang, H.‐B. (2020). Environmental and host factors shaping the gut microbiota diversity of brown frog Rana dybowskii . Science of the Total Environment 741, 140142. [DOI] [PubMed] [Google Scholar]
- * Trevelline, B. K. , MacLeod, K. J. , Langkilde, T. & Kohl, K. D. (2019). Gestation alters the gut microbiota of an oviparous lizard. FEMS Microbiology Ecology 95, fiz086. [DOI] [PubMed] [Google Scholar]
- * Vasco, K. , Guevara, N. , Mosquera, J. , Zapata, S. & Zhang, L. (2022). Characterization of the gut microbiome and resistome of Galapagos marine iguanas (Amblyrhynchus cristatus) from uninhabited islands. Animal Microbiome 4, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos, D. S. , Harris, D. J. , Damas‐Moreira, I. , Pereira, A. & Xavier, R. (2023). Factors shaping the gut microbiome of five species of lizards from different habitats. PeerJ 11, e15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Vasemägi, A. , Visse, M. & Kisand, V. (2017). Effect of environmental factors and an emerging parasitic disease on gut microbiome of wild salmonid fish. mSphere 2, 00418–17. 10.1128/msphere.00418-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Vernier, C. L. , Chin, I. M. , Adu‐Oppong, B. , Krupp, J. J. , Levine, J. , Dantas, G. & Ben‐Shahar, Y. (2020). The gut microbiome defines social group membership in honey bee colonies. Science Advances 6, eabd3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Videvall, E. , Strandh, M. , Engelbrecht, A. , Cloete, S. & Cornwallis, C. K. (2018). Measuring the gut microbiome in birds: comparison of faecal and cloacal sampling. Molecular Ecology Resources 18, 424–434. [DOI] [PubMed] [Google Scholar]
- * Wagener, C. , du Plessis, M. & Measey, J. (2022). Invasive amphibian gut microbiota and functions shift differentially in an expanding population but remain conserved across established populations. Microbial Ecology 84, 1042–1054. [DOI] [PubMed] [Google Scholar]
- * Walker, D. M. , Hill, A. J. , Albecker, M. A. , McCoy, M. W. , Grisnik, M. , Romer, A. , Grajal‐Puche, A. , Camp, C. , Kelehear, C. , Wooten, J. , Rheubert, J. & Graham, S. P. (2020). Variation in the slimy salamander (Plethodon spp.) skin and gut‐microbial assemblages is explained by geographic distance and host affinity. Microbial Ecology 79, 985–997. [DOI] [PubMed] [Google Scholar]
- * Walter, J. M. , Bagi, A. & Pampanin, D. M. (2019). Insights into the potential of the Atlantic cod gut microbiome as biomarker of oil contamination in the marine environment. Microorganisms 7, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Wang, F. , Liu, Y. , Li, G. , Yang, X. & Gao, Q. (2021. a). 16S rRNA gene amplicon sequencing of gut microbiota from naked carp (Gymnocypris przewalskii) in Qinghai Lake, China. Microbiology Resource Announcements 10, e00374‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Wang, H.‐T. , Zhu, D. , Li, G. , Zheng, F. , Ding, J. , O'Connor, P. J. , Zhu, Y.‐G. & Xue, X.‐M. (2019). Effects of arsenic on gut microbiota and its biotransformation genes in earthworm Metaphire sieboldi . Environmental Science & Technology 53, 3841–3849. [DOI] [PubMed] [Google Scholar]
- * Wang, J.‐M. , Bai, J. , Zheng, F.‐Y. , Ling, Y. , Li, X. , Wang, J. , Zhi, Y.‐C. & Li, X.‐J. (2020). Diversity of the gut microbiome in three grasshopper species using 16S rRNA and determination of cellulose digestibility. PeerJ 8, e10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Wang, S.‐T. , Meng, X.‐Z. , Dai, Y.‐F. , Zhang, J.‐H. , Shen, Y. , Xu, X.‐Y. , Wang, R.‐Q. & Li, J.‐L. (2021. c). Characterization of the intestinal digesta and mucosal microbiome of the grass carp (Ctenopharyngodon della). Comparative Biochemistry and Physiology Part D: Genomics and Proteomics 37, 100789. [DOI] [PubMed] [Google Scholar]
- * Wang, W. , Gao, X. , Zheng, S. , Lancuo, Z. , Li, Y. , Zhu, L. , Hou, J. , Hai, J. , Long, X. , Chen, H. , Druzyaka, A. & Sharshov, K. (2021). The gut microbiome and metabolome of Himalayan Griffons (Gyps himalayensis): insights into the adaptation to carrion‐feeding habits in avian scavengers. Avian Research 12, 52. [Google Scholar]
- * Wang, Y. , Smith, H. K. , Goossens, E. , Hertzog, L. , Bletz, M. C. , Bonte, D. , Verheyen, K. , Lens, L. , Vences, M. , Pasmans, F. & Martel, A. (2021. b). Diet diversity and environment determine the intestinal microbiome and bacterial pathogen load of fire salamanders. Scientific Reports 11, 20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Wasimuddin, B. S. D. , Tschapka, M. , Page, R. , Rasche, A. , Corman, V. M. , Drosten, C. & Sommer, S. (2018). Astrovirus infections induce age‐dependent dysbiosis in gut microbiomes of bats. The ISME Journal 12, 2883–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, F. , Wang, X. & Wu, Q. (2015). The giant panda gut microbiome. Trends in Microbiology 11, 180–452. [DOI] [PubMed] [Google Scholar]
- * Wei, H. , Li, X. , Tang, L. , Yao, H. , Ren, Z. , Wang, C. , Mu, C. , Shi, C. & Wang, H. (2020). 16S rRNA gene sequencing reveals the relationship between gut microbiota and ovarian development in the swimming crab Portunus trituberculatus . Chemosphere 254, 126891. [DOI] [PubMed] [Google Scholar]
- * Wei, H. , Wang, H. , Tang, L. , Mu, C. , Ye, C. , Chen, L. & Wang, C. (2019). High‐throughput sequencing reveals the core gut microbiota of the mud crab (Scylla paramamosain) in different coastal regions of southern China. BMC Genomics 20, 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Wei, S. S. , Yen, C. M. , Marshall, I. P. G. , Hamid, H. A. , Kamal, S. S. , Nielsen, D. S. & Ahmad, H. F. (2022). Gut microbiome and metabolome of sea cucumber (Stichopus ocellatus) as putative markers for monitoring the marine sediment pollution in Pahang, Malaysia. Marine Pollution Bulletin 182, 114022. [DOI] [PubMed] [Google Scholar]
- Weiher, E. , Clarke, G. D. P. & Keddy, P. A. (1998). Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos 81, 309–322. [Google Scholar]
- * Weng, F. C.‐H. , Yang, Y.‐J. & Wang, D. (2016). Functional analysis for gut microbes of the brown tree frog (Polypedates megacephalus) in artificial hibernation. BMC Genomics 17, 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Wiebler, J. M. , Kohl, K. D. , Lee, R. E. & Costanzo, J. P. (2018). Urea hydrolysis by gut bacteria in a hibernating frog: evidence for urea‐nitrogen recycling in Amphibia. Proceedings of the Royal Society B: Biological Sciences 285, 20180241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Williams, C. E. , Kueneman, J. G. , Nicholson, D. J. , Rosso, A. A. , Folfas, E. , Casement, B. , Gallegos‐Koyner, M. A. , Neel, L. K. , Curlis, J. D. , McMillan, W. O. , Cox, C. L. & Logan, M. L. (2022). Sustained drought, but not short‐term warming, alters the gut microbiomes of wild anolis lizards. Applied and Environmental Microbiology 88, e00530‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff, R. , Shoemaker, W. & Garud, N. (2020). Ecological stability emerges at the level of strains in the human gut microbiome. MBio 14, e0250222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams, D. C. , Bletz, M. C. , Becker, C. G. , Bender, H. A. , Buitrago‐Rosas, D. , Diebboll, H. , Huynh, R. , Kearns, P. J. , Kueneman, J. , Kurosawa, E. , Labumbard, B. C. , Lyons, C. , McNally, K. , Schliep, K. , Shankar, N. , et al. (2020). Host‐associated microbiomes are predicted by immune system complexity and climate. Genome Biology 21, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley, S. F. , Davies, C. S. , Mannarelli, M.‐E. , Hutchings, M. I. , Komdeur, J. , Burke, T. , Dugdale, H. L. & Richardson, D. S. (2021). Gut microbiome composition, not alpha diversity, is associated with survival in a natural vertebrate population. Animal Microbiome 3, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Wu, Y.‐Y. , Cheng, C.‐X. , Yang, L. , Ye, Q.‐Q. , Li, W.‐H. & Jiang, J.‐Y. (2022). Characterization of gut microbiome in the mud snail Cipangopaludina cathayensis in response to high‐temperature stress. Animals 12, 2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Xiao, Q. , Wang, L. , Chen, S.‐Q. , Zheng, C.‐Y. , Lu, Y.‐Y. & Xu, Y.‐J. (2023). Gut microbiome composition of the fire ant Solenopsis invicta: an integrated analysis of host genotype and geographical distribution. Microbiology Spectrum 11, e03585‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Yanez‐Montalvo, A. , Gaona, O. , Águila, B. , Arias‐Domínguez, N. , Falcón, L. I. & Pérez‐Flores, J. (2021). Tapirus bairdii‐associated fecal microbiome from a critical conservation area: Calakmul, México. Current Microbiology 78, 2648–2659. [DOI] [PubMed] [Google Scholar]
- Yang, J. , Park, J. , Jung, Y. & Chun, J. (2022). AMDB: a database of animal gut microbial communities with manually curated metadata. Nucleic Acids Research 50, D729–D735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Wang, Y. , Cui, X. , Xue, K. , Zhang, Y. & Yu, Z. (2019). Habitat filtering shapes the differential structure of microbial communities in the Xilingol grassland. Scientific Reports 9, 19326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblut, N. , Reischer, G. H. , Walters, W. , Schuster, N. , Walzer, C. , Stalder, G. , Ley, R. E. & Farnleitner, A. H. (2019). Host diet and evolutionary history explain different aspects of gut microbiome diversity among vertebrate clades. Nature Communications 10, 2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Lee, J. Y. Y. , Tang, S. N. & Lee, P. K. H. (2024). Niche differentiation in microbial communities with stable genomic traits over time in engineered systems. The ISME Journal 18, wrae042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Q. , Sukumaran, J. , Wu, S. & Rodrigo, A. (2015). Neutral models of microbiome evolution. PLoS Computational Biology 11, e1004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B. , Ren, J. & Gong, X. (2019). Comparative analysis and characterization of the gut microbiota of four farmed snakes from southern China. PeerJ 7, e6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zhang, F. , Xu, N. , Wang, W. , Yu, Y. & Wu, S. (2021. a). The gut microbiome of the Sunda pangolin (Manis javanica) reveals its adaptation to specialized myrmecophagy. PeerJ 9, e11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zhang, L. , Yang, F. , Li, N. & Dayananda, B. (2021. b). Environment‐dependent variation in gut microbiota of an oviparous lizard (Calotes versicolor). Animals 11, 2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zhang, M. , Zhang, X. , Tran, N. T. , Sun, Z. , Zhang, X. , Ye, H. , Zhang, Y. , Ma, H. , Aweya, J. J. & Li, S. (2021. c). Molting alters the microbiome, immune response, and digestive enzyme activity in mud crab (Scylla paramamosain). mSystems 6, e00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zhang, N. , Song, C. , Wang, M. , Liu, Y. , Hui, M. & Cui, Z. (2017). Diversity and characterization of bacteria associated with the deep‐sea hydrothermal vent crab Austinograea sp. comparing with those of two shallow‐water crabs by 16S ribosomal DNA analysis. PLoS One 12, e0187842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zhang, Z. , Zhu, Q. , Chen, J. , Khattak, R. H. , Li, Z. , Teng, L. & Liu, Z. (2022). Insights into the composition of gut microbiota in response to environmental temperature: the case of the Mongolia racerunner (Eremias argus). Global Ecology and Conservation 36, e02125. [Google Scholar]
- * Zhong, H. , Zhang, J. , Li, F. & Chen, J. (2021). Gut microbial communities associated with phenotypically divergent populations of the striped stem borer Chilo suppressalis (Walker, 1863). Scientific Reports 11, 15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zhong, J. , Guo, K. , Liao, Z.‐L. , Hu, S.‐C. , Du, Y. & Ji, X. (2022). Comparative analysis of the gut microbiota composition between two sea snakes, Hydrophis curtus, and Hydrophis cyanocinctus . Coral Reefs 41, 53–62. [Google Scholar]
- * Zhou, L. , Chen, C. & Wang, X. (2022. a). Gut bacterial diversity and community structure of Spodoptera exigua (Lepidoptera: Noctuidae) in the Welsh onion‐producing areas of North China. Journal of Economic Entomology 115, 1102–1114. [DOI] [PubMed] [Google Scholar]
- * Zhou, Z. , Huang, H. & Che, X. (2022. b). Insects bacterial communities in the feces of laboratory reared Gampsocleis gratiosa (Orthoptera: Tettigoniidae) across different developmental stages and sexes. Insects 13, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zhu, J. , Li, H. , Jing, Z. Z. , Zheng, W. , Luo, Y. R. , Chen, S. X. & Guo, F. (2022. b). Robust host source tracking building on the divergent and non‐stochastic assembly of gut microbiomes in wild and farmed large yellow croaker. Microbiome 10, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zhu, L. , Zhu, W. , Zhao, T. , Chen, H. , Zhao, C. , Xu, L. , Chang, Q. & Jiang, J. (2021). Environmental temperatures affect the gastrointestinal microbes of the Chinese giant salamander. Frontiers in Microbiology 12, 543767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zhu, Y.‐X. , Chang, Y.‐W. , Wen, T. , Yang, R. , Wang, Y.‐C. , Wang, X.‐Y. , Lu, M.‐X. & Du, Y.‐Z. (2022. a). Species identity dominates over environment in driving bacterial community assembly in wild invasive leaf miners. Microbiology Spectrum 10, e00266‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zoqratt, M. Z. H. , Eng, W. W. H. , Thai, B. T. , Austin, C. M. & Gan, H. M. (2018). Microbiome analysis of Pacific white shrimp gut and rearing water from Malaysia and Vietnam: implications for aquaculture research and management. PeerJ 6, e5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zuo, Y. , Xie, W. , Pang, Y. , Li, T. , Li, Q. & Li, Y. (2017). Bacterial community composition in the gut content of Lampetra japonica revealed by 16S rRNA gene pyrosequencing. PLoS One 12, e0188919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary methods.
Table S1. Web of Science query results.
Table S2. Overview of large‐scale comparative gut microbiome studies (>30 host species).
Fig. S1. Number of samples aggregated across 179 gut microbiome studies spanning 15 host classes.
Data S1. Sample and study metadata.
Data Availability Statement
All metadata used in this study along with a list of compiled studies are available in the supporting information and at https://github.com/samd1993/GutMicrobiomeTreeOfLife as an excel spreadsheet named: ‘Degregori_etal_Comparative_Review_metadata_Oct20_24.xlsx’ and a text file of related scripts named: ‘Degregori_etal_Comparative_Review_DataScripts.txt’. The sequence table used for Figs 3 and 4 is named ‘N30GMTOLsolo_table.biom’ and is also uploaded to GitHub. The package q2sra is available at https://pypi.org/project/q2sra/.


