Abstract
Background
Chromatin modified protein 4C (CHMP4C) is a charged polyvesicular protein (CHMP) that is involved in the composition of the endosomal sorting complex (ESCRT-III) required for transport III and promotes the necessary separation of daughter cells. CHMP4C involved in a wide variety of tumor progress, such as prostate cancer, cervical cancer and lung squamous cell carcinoma. However, the value of CHMP4C in lung adenocarcinoma has not been explored.
Methods
RNA-seq data and lung adenocarcinoma clinical information and corresponding pan-cancer were extracted from The Cancer Genome Atlas (TCGA) database to analyze CHMP4C expression and survival prognosis. The differential expression of CHMP4C was analyzed using the Human Protein Atlas (HPA) database. Clinical samples were collected to verify the differential expression of CHMP4C between lung adenocarcinoma and normal lung tissues via immunohistochemical (IHC) staining, qRT‒PCR and Western blotting. Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of CHMP4C-related genes were performed. The correlation between CHMP4C and chemosensitivity was analyzed in the TCGA database. Then, qRT‒PCR, western blotting, transwell assays, cell proliferation assays, colony formation assays, wound healing assays, and cell cycle analysis were used to verify the possible regulatory mechanism involved. Molecular docking was used to predict small molecule compounds with potential roles in the treatment of lung adenocarcinoma.
Results
TIMER2.0 database analysis revealed that CHMP4C was differentially expressed in different tumors.Compared with that in healthy lung tissue, CHMP4C was significantly upregulated in lung adenocarcinoma tissue, and subsequent in vitro survival analysis revealed that CHMP4C expression has significant clinical prognostic value in lung adenocarcinoma. Enrichment analysis revealed that CHMP4C was mainly related to cell proliferation, cell migration, and the PI3K-Akt signaling pathway, etc. Overexpression of CHMP4C was associated with sensitivity to chemotherapy. Knocking down CHMP4C can significantly inhibit the proliferation, migration and invasion of lung adenocarcinoma cells and prolong the G0/G1 phase of the cell cycle. Molecular docking predicts 10 key drugs that may be used for the treatment of lung adenocarcinoma.
Conclusions
CHMP4C is highly expressed in a variety of tumors. We demonstrated that CHMP4C expression may be associated with the occurrence, development, prognosis and chemotherapy sensitivity in patients with lung adenocarcinoma. These findings may open up new research directions and development opportunities for the treatment of lung adenocarcinoma.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-01986-6.
Keywords: Lung adenocarcinoma, CHMP4C, Prognostic value, Biomarker, Drug sensitivity
Introduction
According to the 2019 Global Cancer Burden Report, the disease burden of cancer is second only to cardiovascular disease, and lung cancer is the second most common cancer with the highest mortality rate [1]. Lung cancer is also an extremely aggressive and highly prevalent disease worldwide, second only to female breast cancer [2, 3]. Among them, Lung adenocarcinoma (LUAD) is the most common and aggressive type from lung cancer[4]. According to China's national statistics, approximately 631,000 people die of lung cancer each year [5]. This is closely related to the fact that lung cancer is often diagnosed at an advanced stage [6]. This is despite important advances in treatment and diagnosis over the past 10 years [7]. The diagnostic challenges are related to the development of personalized treatments and the molecular and precise histological features of lung cancer [8]. At present, the main treatment methods for lung cancer include radiotherapy, chemotherapy, targeted therapy, immunotherapy and surgery [9]. However, despite various treatments, patients with LUAD have a poor prognosis [10, 11]. The preponderance of patients with LUAD are confronted with a deficiency of sensitive biomarkers. Therefore, the discovery of new diagnostic and therapeutic biomarkers may be a breakthrough point in the treatment of this disease.
Chromatin modified protein 4C (CHMP4C) belongs to the chromatin modified protein (CHMP) family. It is expressed in the nucleus and cytoplasm and is a subunit of the endosome sorting complex required for transport. It is expressed in the nucleus and cytoplasm and is a subunit of the endosomal sorting complex required for transport [12, 13]. CHMP4C transports the endosomal sorting complex required for cell division in daughter cells [14, 15], and plays a very important role in many processes, such as the pathogenesis of cancer and the progression of extracellular vesicle formation [16]. CHMP4C has been shown to play a regulatory role in many cancers, including prostate cancer [17], ovarian cancer [18], and lung squamous cell carcinoma [19]. This indicates that CHMP4C plays a crucial role in the occurrence and development of tumors, and can better predict the prognosis of patients in a variety of tumors. However, the role of CHMP4C in LUAD has rarely been described. Therefore, we selected CHMP4C for further study. We aimed to verify the correlation between CHMP4C expression and the survival rate of patients with LUAD and to explore whether the regulation of CHMP4C expression affects the biological behavior and regulatory pathways of LUAD cells to identify new biomarkers for predicting the occurrence and development of LUAD.
Materials and methods
Clinical specimens
The patient (diagnosed with lung adenocarcinoma) samples (n = 6) used for Western blotting, immunohistochemistry and qRT‒PCR were surgical specimens from thoracic surgery patients at the First Affiliated Hospital of Nanchang University, and all specimens were collected with the informed consent of the patients. The specific steps of specimen collection are as follows: First, the specimen is observed to confirm the location and scope of the tumor, and attention is given to the identification of the surrounding tissue and necrotic tissue. The specimens were cut within 10 min after separation from the body, and the cancer and adjacent tissues were cut into small pieces with a diameter of approximately 1 cm, which were put into a numbered sterile lyophilized tube and quickly stored in a liquid nitrogen tank.
Data collection and processing
RNA-seq data and corresponding clinical information were retrieved from Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/gds) databases (Tumor n = 83, Normal n = 83) and The Cancer Genome Atlas (TCGA, http://cancergenome.nih.gov) database (Tumor n = 483, Normal n = 347). RNA-seq data for LUAD and clinical information were extracted for subsequent R (v4.0.3) software package analysis. p-value < 0.05 was considered statistically significant.
Pan-cancer analysis
TIMER2.0 (http://timer.cistrome.org/) is an integrated resource for the systematic analysis of different kinds of cancer immune infiltrates[20]. In this study, we investigated differences in the expression of CHMP4C between tumors and neighboring normal tissues.
UALCAN analysis
UALCAN (http://ualcan.path.uab.edu), which is based on the TCGA database, is a comprehensive cancer data interactive website [21]. In this study, a comprehensive comparative analysis of gene expression was performed using the UALCAN database. Gene expression patterns were compared between tumor and normal tissues, different tumor stages, and other clinicopathological features.
HPA analysis
The Human Protein Atlas (HPA) (http://www.proteinatlas.org) downloaded LUAD samples of normal tissue and tumor tissue from immunohistochemistry (IHC) images were analyzed [22].
Survival analysis
Kaplan–Meier survival curves, including overall survival (OS), disease-free interval (DFI), and progression-free interval (PFI), were plotted using the Kaplan–Meier Plotter (http://kmplot.com). The Kaplan–Meier Plotter database is a widely recognized public database for analyzing gene expression and disease prognosis.
Enrichment analysis
We used Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Gene Set Enrichment Analysis (GSEA) analyses to predict the biological pathways and molecular functions associated with CHMP4C in LUAD. LUAD data extracted from TCGA based on CHMP4C expression were processed and divided into high-expression and low-expression groups. Differential expression analysis was performed between the two groups, and a volcano plot was drawn. Enrichment analysis was performed on the differentially expressed genes (DEGs) [23]. All these analyses were conducted using the R (v4.0.3) software packages ggplot2 and pheatmap.
Drug sensitivity analysis
We used the “pRRophetic” R package and the ridge regression method to predict drug response and estimate the half-maximal inhibitory concentration (IC50) to analyze targeted drug sensitivity in LUAD patients [24]. The data were obtained from the Genomics of Cancer Drug Sensitivity Genetics (GDSC) database (https://www.cancerrxgene.org/).
Molecular docking
To establish connections between genes, target compounds, and diseases based on gene expression profiles, we used the Clue platform [25]. By submitting differentially expressed genes (upregulated and downregulated) between the high-expression and low-expression groups to the Clue platform, we identified several potential therapeutic drugs that could improve cancer progression.
Cell culture
The human LUAD cell lines BEAS-2B, A549, NCI-H827, NCI-H1975, SPC-A-1, NCI-H460, NCI-H1299, PC9, NCI-292, and NCI-H1975 were obtained from the Chinese Academy of Sciences. The NCI-H827, NCI-H1975, SPC-A-1, NCI-H460, NCI-H1299, PC9, and NCI-H292 LUAD cell lines were grown in RPMI-1640 medium (Gibco, USA). The BEAS-2B and A549 cell lines were grown in DMEM (Gibco, USA). All media were supplemented with 10% fetal bovine serum (ExCell, China) and 1% penicillin/streptomycin (Biotech, China). The cells were cultured in an environment at 37 °C and a CO2 concentration of 5%.
Immunohistochemical (IHC) staining
Tissue sections were prepared from patient samples and immunohistochemically stained with an anti-CHMP4C antibody (Origene, USA) to assess CHMP4C expression in the tissues. The procedure was as follows: tissue section dewaxing, rehydration, and antigen repair. Subsequently, endogenous peroxidase activity was blocked with 6% hydrogen peroxide. The slides were washed three times with PBS and incubated with anti-CHMP4C antibodies at 4 °C overnight. After three washes with PBS, the membrane was incubated with a secondary antibody conjugated to horseradish peroxidase for 1 h. Nuclei were stained with hematoxylin solution.
Western blotting
Cell samples and extracted tissue samples were directly treated with sample buffer containing Tris–HCl (pH 6.8), 4% SDS, 0.006% bromophenol blue, 10% glycerol, and 2% β-mercaptoethanol. Western blotting is an immunoassay technique mainly used to qualitatively and quantitatively analyze the expression level of target proteins in cells or tissues. After heating at 100 °C for 10 min, the same amount of protein was separated by SDS‒PAGE. The proteins were then transferred to nitrocellulose membranes. Transfer occurred at 4 °C at a constant voltage of 110 V for 1 h. The membranes were clipped according to the molecular weight of the target protein. The membrane was then incubated overnight at 4 °C with the following primary antibodies: anti-CHMP4C (1:500; Origene, USA), anti-CDK2 (1:1000; ZENbio, China), anti-β-actin (1:10,000; Proteintech, China) and anti-cyclin D1 (1:1000, ZENbio, China). After incubation at 4 °C overnight, the blot was washed three times for 10 min each with TBS containing 0.1% Tween 20. The blot was then incubated at room temperature for 1 h with the following secondary antibodies: goat anti-rabbit polyclonal antibody for CHMP4C, CDK2, and cyclin D1 at a 1:3000 dilution and goat anti-mouse polyclonal antibody for β-actin at a 1:5000 dilution. The blot was then washed with the same buffer as above three times for 10 min each and incubated in an enhanced chemiluminescence detection reagent (YEASEN, China) for 1 min. The blots were then imaged with a Gel Doc XR + (Bio-Rad) imaging system.
Cell cycle assay
Trypsin was used to digest the cells to collect the samples, which were then immobilized overnight with 75% ethanol in the dark at − 20 °C. The samples were then stained with propidium iodide and RNaseA (UElandy, China) for 30 min. The cell cycle distribution of each group was detected by cyclic flow cytometry. The data were analyzed using FlowJo 7.6.
RNA interference
For interference, we purchased small interfering RNA (siRNA) and negative control RNA targeting the CHMP4C gene from GenePharma (Shanghai, China). These siRNAs and negative control RNA were transfected into NCI-H1299 and NCI-H292 cells using SuperFectin™ II In Vitro siRNA Transfection Reagent (PUFEI BIOTECH, China). The cell confluence was 50–60%. The sequences of the RNAs that disrupted the CHMP4C gene are shown in Supplementary Table S1.
Quantitative Real-time PCR
According to the manufacturer's instructions, RNA was extracted from cells using TRIzol universal RNA reagent (TIANGEN, China). For cDNA synthesis, total RNA (1 µg) was reverse-transcribed using the PrimeScript™ RT Reagent Kit and gDNA Eraser (Takara, Japan). The complementary DNA (cDNA) obtained by reverse transcription was diluted tenfold with RNase-free distilled water. Quantification was performed using TB Green Premix Ex Taq II (Tli RNaseH Plus) (Takara, Japan) and an ABI7500 System (Thermo Fisher, USA). qRT-PCR measures mRNA levels of specific genes by using fluorescently labeled probes that monitor fluorescence signals during polymerase chain reaction in real time. The analysis was repeated three times for each specimen from each target. GAPDH expression was used as a reference. The primer sequences are shown in Supplementary Table S2.
Cell proliferation assay
A total of 4000 cells were inoculated in each well of a 24-well plate, and 0.5 mL of culture medium containing 10% FBS was added to each well. After incubation for 8–12 h, when the cells were attached to the wall, the 24-well plate was removed, and the data were recorded as the 0 day group. The data at 48 h, 96 h and 144 h were recorded as the 2 day group, 4 day group and 6 day group, respectively. At room temperature, the cells were fixed with 500 μL of 4% paraformaldehyde for 15 min, and the cells were stained with 200 μL of 1% crystal violet dye in each well at room temperature for 1 min. The cells were gently washed with running water until the fluid in the pores became clear and transparent. The culture medium was changed every other day, 24-well plates were collected at the time points specified in the experiment (days 2, 4, and 6), and the cells in the culture plates were treated according to the above experimental methods. After drying all the 24-well plates, 200 μL of 10% acetic acid solution was added to each well of the 24-well plates. The acetic acid solution with dissolved crystal violet in the pore plate was transferred to a 96-well plate, and the absorbance was measured at a wavelength of 595 nm to determine cell proliferation.
Colony formation assay
Cells were seeded into 6-well plates (1000 cells/well). The cells were cultured continuously for 14 days, and the medium was changed every 3 days. Finally, the cells were fixed with 4% formaldehyde, stained with 0.05% crystal violet at the specified time points, and counted by ImageJ.
Transwell assay
For the invasion assay, 80,000 cells were cultured in serum-free medium in the upper layer of the experimental plates coated with Matrigel (Biozellen, USA). Serum-containing medium (10% FBS) was added to the bottom chamber. NCI-H1299 and NCI-292 cells were cultured for 24 h. For the migration assay, serum-containing medium (10% FBS) was added to the bottom chamber, 80,000 cells were cultured in serum-containing medium (1% FBS) in the upper chamber. NCI-H1299 and NCI-H292 cells were cultured for 8 h. The cells transferred to the bottom of the membrane were fixed with 4% formaldehyde, stained with 0.05% crystal violet at the specified time points, and counted by ImageJ.
Wound healing assay
A wound was generated in a 6-well plate by scratching the surface with a 200 L pipette tip. After the wound was created (0 h and 12 h), the wounded areas were photographed under a light microscope (Olympus, Japan). The wound healing rate was calculated using the following formula: [1 (empty length 24 h-empty length 0 h/empty length 0 h)] 100%.
Statistical analysis
Million transcripts per million (TPM) values were standardized by log2 transformation (1 + TPM). Kaplan–Meier (KM) estimates and the log-rank test were used for survival analysis. All statistical analyses were performed using R software (v4.0.3). One-way ANOVA was used to compare multiple groups. For the comparison of multiple measurements made at different time points, one-way repeated-measures ANOVA was used. For all experiments, all stated replicates are biological replicates. GraphPad Prism 7 was used for statistical analysis and graphing. A p-value < 0.05 was considered statistically significant.
Results
CHMP4C was significantly upregulated in LUAD based on database analysis
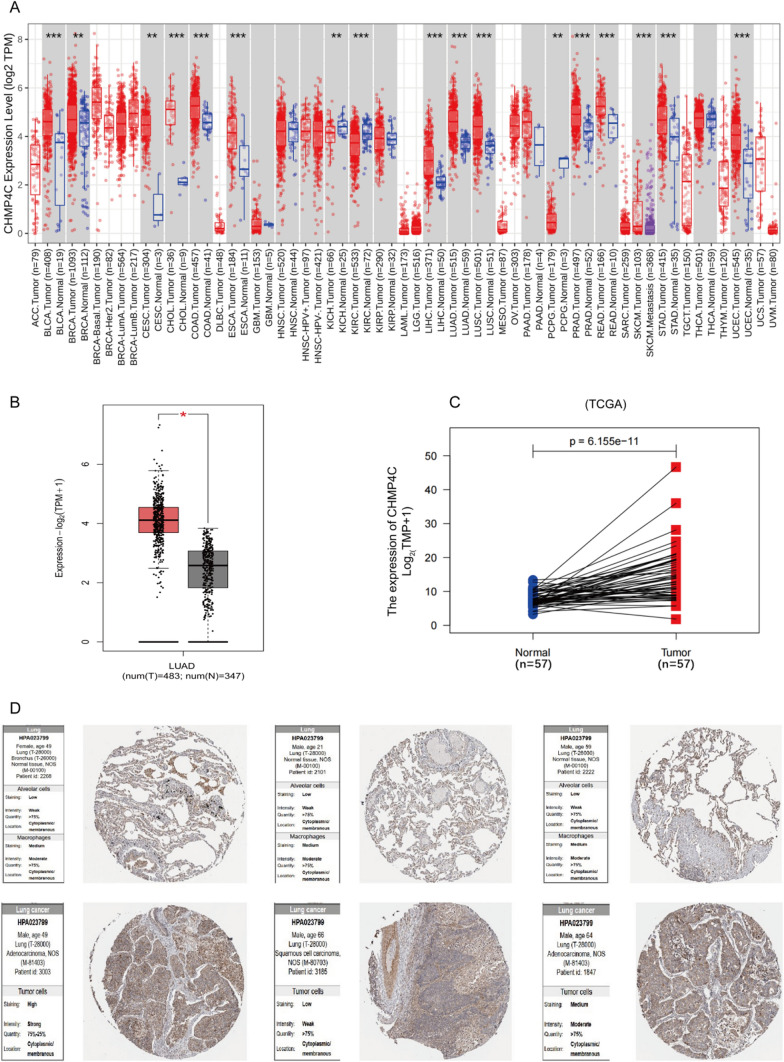
CHMP4C is highly expressed in many cancers, but whether CHMP4C expression is associated with the progression of LUAD remains unclear. First, we investigated the expression of CHMP4C in a variety of human malignancies. Differential expression analysis between tumor and adjacent normal tissues was performed with the TIMER 2.0 database, and CHMP4C was significantly increased in LUAD (Fig. 1A). In addition, according to the TCGA database and GEO data, compared with that in normal lung tissue, CHMP4C mRNA expression was significantly upregulated in LUAD tissue (Fig. 1B,C and Fig. S1A,S1B). Next, we analyzed differences in CHMP4C protein expression in the HPA database. As expected, CHMP4C protein expression in lung cancer group were significantly higher than those of normal lung tissue (Fig. 1D).
Fig. 1.
CHMP4C was upregulated in LUAD based on database analysis. A The expression levels of CHMP4C were increased in a variety of tumors according to TIMER 2.0. B The differences in the expression levels of CHMP4C between normal tissues and cancerous tissues were determined according to the TCGA database. C The mRNA expression of CHMP4C in paired LUAD tissues was compared according to the TCGA database. D CHMP4C protein expression was greater in LUAD according to the HPA database. *p < 0.05, **p < 0.01, ***p < 0.001. ns, not significant; HPA, The Human Protein Atlas
CHMP4C is significantly upregulated in LUAD tissues and cells
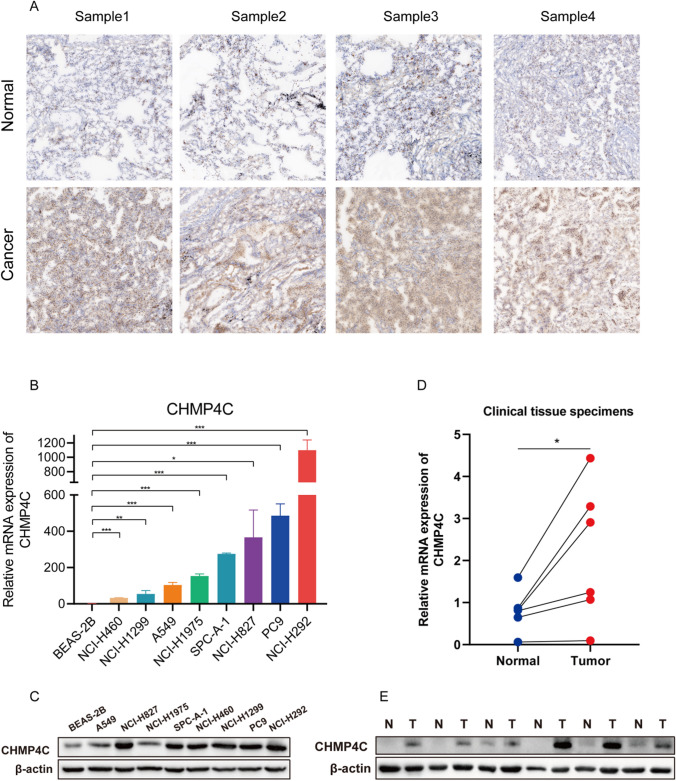
Subsequently, the expression of CHMP4C was experimentally verified in LUAD tissue samples and LUAD cell lines. Immunohistochemical staining revealed that CHMP4C expression was greater in diseased tissues than in adjacent lung tissues (Fig. 2A). qRT‒PCR and Western blotting also showed similar results (Fig. 2B, C). CHMP4C expression was significantly greater in LUAD cell lines (A549, NCI-H827, NCI-H1975, SPC-A-1, NCI-H460, NCI-H1299, PC9, and NCI-H292) than in BEAS-2B human lung epithelial cells (Fig. 2D, E). These results suggest that CHMP4C is overexpressed in patients with LUAD.
Fig. 2.
CHMP4C is upregulated in LUAD tissues and cells. A The expression of CHMP4C in normal lung tissue and LUAD tissue was detected by immunohistochemistry. B, C Quantitative real-time PCR (qRT‒PCR) and western blotting analyses of CHMP4C expression in normal lung tissue and LUAD tissue. D, E Quantitative real-time PCR (qRT‒PCR) and western blotting analyses of CHMP4C expression in LUAD cell lines, including BEAS-2B, A549, NCI-H827, NCI-H1975, SPC-A-1, NCI-H460, NCI-H1299, PC9, and NCI-H292. The data are presented as the means ± SDs. *p < 0.05, **p < 0.01, ***p < 0.001
Relationships between CHMP4C expression and the clinical features and prognosis of patients with LUAD
Next, we used data from the TCGA database to explore the correlation between CHMP4C expression levels and prognosis in patients with LUAD to determine whether CHMP4C can be considered a diagnostic biomarker for LUAD. We found that upregulated CHMP4C was associated with poor overall survival (OS, defined as the time from the start of randomization until death from any cause), disease-free survival (DFI, reflecting the time between the start of randomization and the recurrence of disease or death from any cause), and progression-free survival (PFI, defined as the time from the start of randomization to progression in any aspect of tumor development or death from any cause) (Fig. 3A–C). Next, the expression of CHMP4C in LUAD patients stratified by different pathological parameters was studied using the TCGA database. In terms of the stage of LUAD, CHMP4C expression was significantly upregulated in patients with stage I/II/III/IV LUAD compared with normal controls, even though CHMP4C expression was not significantly different across clinical stages (Fig. 3D). With respect to lymph node metastasis, CHMP4C expression increased with increasing numbers of lymph node metastases (Fig. 3E).Although no significant difference was found in CHMP4C expression in the N3 samples, this may be due to the small sample size in this category (n = 2). Moreover, we found that CHMP4C expression did not significantly differ according to patient’s gender, TP53 mutation status, or patient’s race (Fig. 3F–H). In conclusion, these findings suggest that CHMP4C is indeed associated with the progression and invasion of LUAD.
Fig. 3.
CHMP4C expression is a poor predictor of prognosis in LUAD patients. A–C Relationships of CHMP4C expression with OS, DFI, and RFI in TCGA datasets. D–H Correlation of CHMP4C with D the clinical stage, E lymph node metastasis, F patient’s gender, G TP53 mutation status, H patient’s race. The data are presented as the means ± SDs. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001,ns, not significant
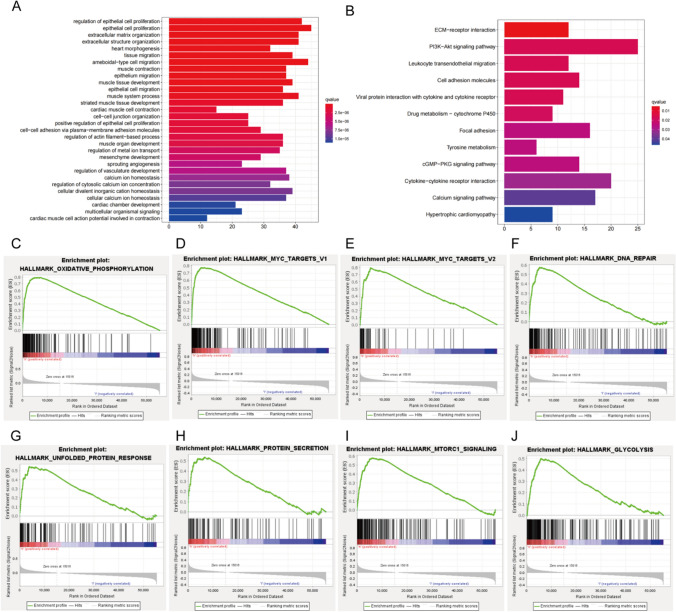
CHMP4C expression enrichment analysis
Next, we performed a grouping ANOVA for CHMP4C and generated a heatmap of 20 significantly differentially expressed genes (DEGs) (Fig. 4A and B). To investigate the functional enrichment information of CHMP4C interacting genes in greater depth, GO and KEGG functional enrichment analyses were subsequently performed for these DEGs. GO analysis showed that the identified genes were mainly involved in the regulation of cell proliferation and migration and other functions (Fig. 5A). KEGG analysis also showed that these genes in addition to participate in the regulation of cell proliferation, also participated in the PI3K—Akt signaling pathways regulating (Fig. 5B). These findings indicate the potential value of CHMP4C in promoting tumorigenesis and tumor development. In this study, we also investigated potential pathways leading to CHMP4C overexpression via GSEA. The significantly enriched pathways included Oxidative Phosphorylation, Myc Target V1, Myc Target V2, DNA Repair, Unfolded Protein Response, Protein Transcription, mTORC1 Signaling and Glycolysis (Fig. 5C–J).
Fig. 4.
CHMP4C expression enrichment analysis. A Volcano plot of differentially expressed genes (DEGs) (|log2-fold change|> 1.0 and adjusted P < 0.05). B Heatmap of 20 significantly differentially expressed genes (DEGs) (|log2-fold change|> 1.5 and adjusted p < 0.05). *p < 0.05, **p < 0.01, ***p < 0.001; “ns” indicates that the data are not significant
Fig. 5.
CHMP4C expression enrichment analysis. A, B GO and KEGG enrichment analysis of differentially expressed genes (DEGs) in CHMP4C samples with high and low expression. C–J Gene set enrichment plots of genes related to C oxidative phosphorylation, D Myc Target V1, E Myc Target V2, F DNA repair, G unfolded protein response, H protein selection, I mTORC1 signaling, and J glycolysis associated with high CHMP4C expression according to GSEA
CHMP4C expression is associated with chemotherapy sensitivity in LUAD
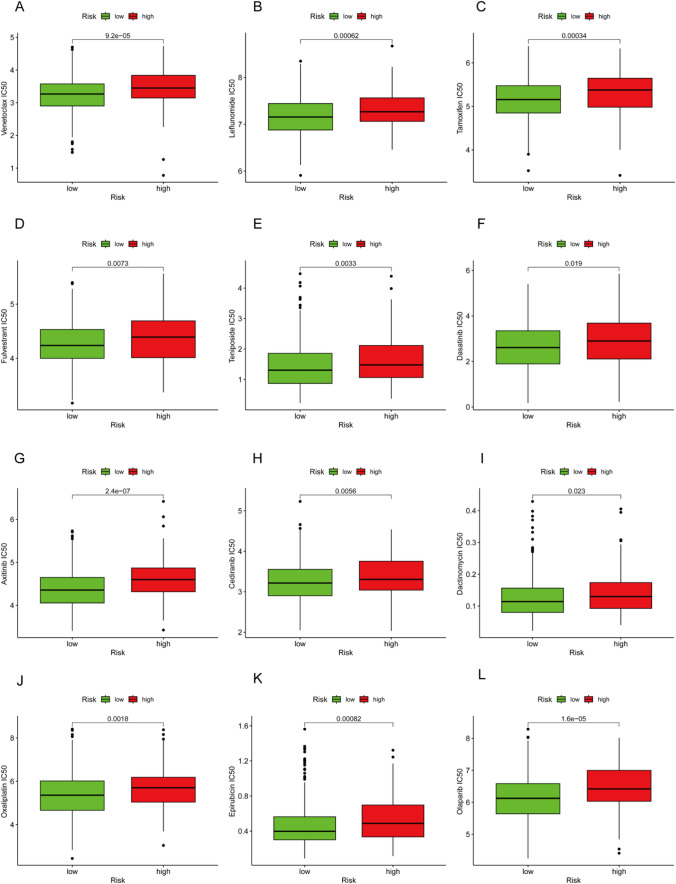
Next, we want to explore CHMP4C high expression in LUAD is associated with clinical drug sensitivity. The effect of CHMP4C-expressing LUAD patients on sensitivity to clinical antitumor drugs was evaluated using the pRRophetic algorithm and the maximum half-maximal inhibitory concentration available in the Cancer Drug Sensitivity Genomics (GDSC) database. The results showed that in the group overexpressing CHMP4C, 12 chemotherapeutic agents had significant effects (Fig. 6A–L), especially acitinib (p = 2.4*10-7) and olaparib (p = 1.6*10-5). According to these results, the drug sensitivity of these small molecule compounds decreases when CHMP4C is highly expressed. This finding is very important and may provide theoretical guidance for drug selection in LUAD patients and provide new ideas for clinical diagnosis and treatment, but further exploration and experimental verification are needed.
Fig. 6.
CHMP4C expression predicts chemotherapy sensitivity of LUAD. A‒L Box plots showing the chemosensitivity of LUAD cells to A venetoclax, B leflunomide, C tamoxifen, D fulvestrant, E teniposide, F dasatinib, G axitinib, H cediranib, I dactinomycin, J oxaliplatin, K epirubicin, and L olaparib to high and low CHMP4C expression
CHMP4C knockdown inhibited the proliferation, migration and invasion of LUAD cells
In order to clarify the biological effect of CHMP4C in the progression and prognosis of LUAD biology effect,We used NCI-H1299 and NCI-H292 for our experiments. First, to achieve CHMP4C knockdown in cells, we transfected si-CHMJP4C#NC, si-CHMP4C#1 and si-CHMP4C#2 into NCI-H1299 and NCI-H292 cells. After transfection for 48 h, qRT‒PCR and western blotting were used to detect the mRNA and protein levels of CHMP4C in the transfected cells. Transfection of si-CHMP4C#1 or si-CHMP4C#2 significantly decreased the mRNA and protein levels of CHMP4C in NCI-H1299 and NCI-H292 cells (Fig. 7A–D). Next, we performed cell function experiments to investigate whether CHMP4C induces malignancy in LUAD cells. The results of the cell proliferation assay showed that CHMP4C knockdown decreased the proliferation of NCI-H1299 and NCI-H292 cells compared with that in the control group (Fig. 7E, F). Similar conclusions were confirmed in the colony formation assay, where we found a significant reduction in the number of si-CHMP4C clones compared to controls (Fig. 7G). In the wound healing assay, CHMP4C knockdown further reduced the migration distance of NCI-H1299 and NCI-H292 cells (Fig. 7I–L). In the migration and invasion assays, there were fewer migrating and invading cells in the NCI-H1299 and NCI-H292 cells transfected with si-CHMP4C#1 or si-CHMP4C#2 than in the corresponding control cells (Fig. 7M–P). These results indicate that CHMP4C knockdown can successfully inhibit the proliferation, migration and invasion of cancer cells in vitro. In conclusion, our results suggest that CHMP4C is a positive factor for the proliferation and invasion of LUAD cells.
Fig. 7.
CHMP4C knockdown inhibited the proliferation, migration and invasion of LUAD cells. A–D After transfection, qRT‒PCR and Western blotting were used to measure the expression of CHMP4C in NCI-H1299 and NCI-H292 cells. E, F The results of the cell proliferation assay showed that the downregulation of CHMP4C significantly inhibited the proliferation of NCI-H1299 and NCI-H292 cells. G, H The plate colony formation assay results showed that CHMP4C downregulation inhibited the colony formation ability of NCI-H1299 and NCI-H292 cells. I–L Wound healing assays showed that CHMP4C downregulation significantly slowed the wound healing rate of NCI-H1299 and NCI-H292 cells. M–P Transwell assays showed that CHMP4C downregulation significantly reduced the invasion and migration ability of NCI-H1299 and NCI-H292 cells. The data are presented as the means ± SDs. *p < 0.05, **p < 0.01, ***p < 0.001
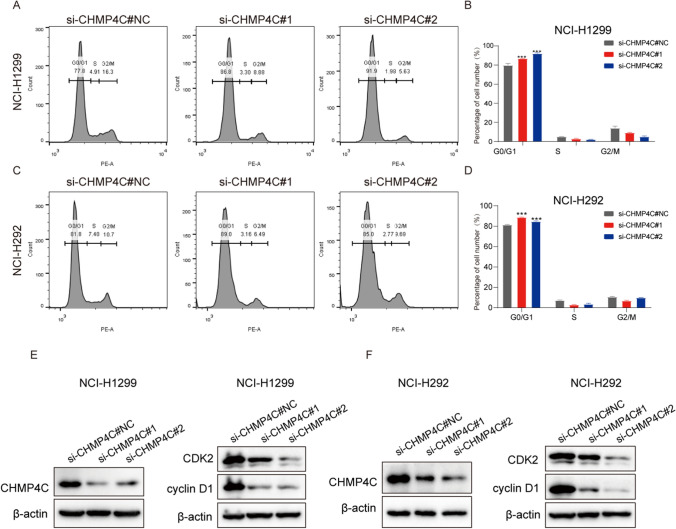
CHMP4C controls the LUAD cell cycle in vitro
Chromatin-modified protein 4C (CHMP4C) belongs to the chromatin-modified protein (CHMP) family. We hypothesized that CHMP4C promotes the development of LUAD by regulating the cell cycle. To test this hypothesis, we analyzed the cell cycle distribution using flow cytometry and found that the proportion of NCI-H1299 and NCI-H292 cells in the G0/G1 phase was prolonged after CHMP4C knockdown (Fig. 8A–D). In addition, CHMP4C expression was positively correlated with the expression of CDK2 and cyclin D1, key cell cycle proteins that regulate G0/G1 (Fig. 8E, F).
Fig. 8.
CHMP4C regulates the LUAD cell cycle. A–D Knockdown of CHMP4C prolonged the proportion of NCI-H1299 and NCI-H292 cells in the G0/G1 phase. E, F Western blotting showing the expression of CHMP4C, CDK2 and cyclin D1 in NCI-H1299 and NCI-H292 cells
Molecular docking
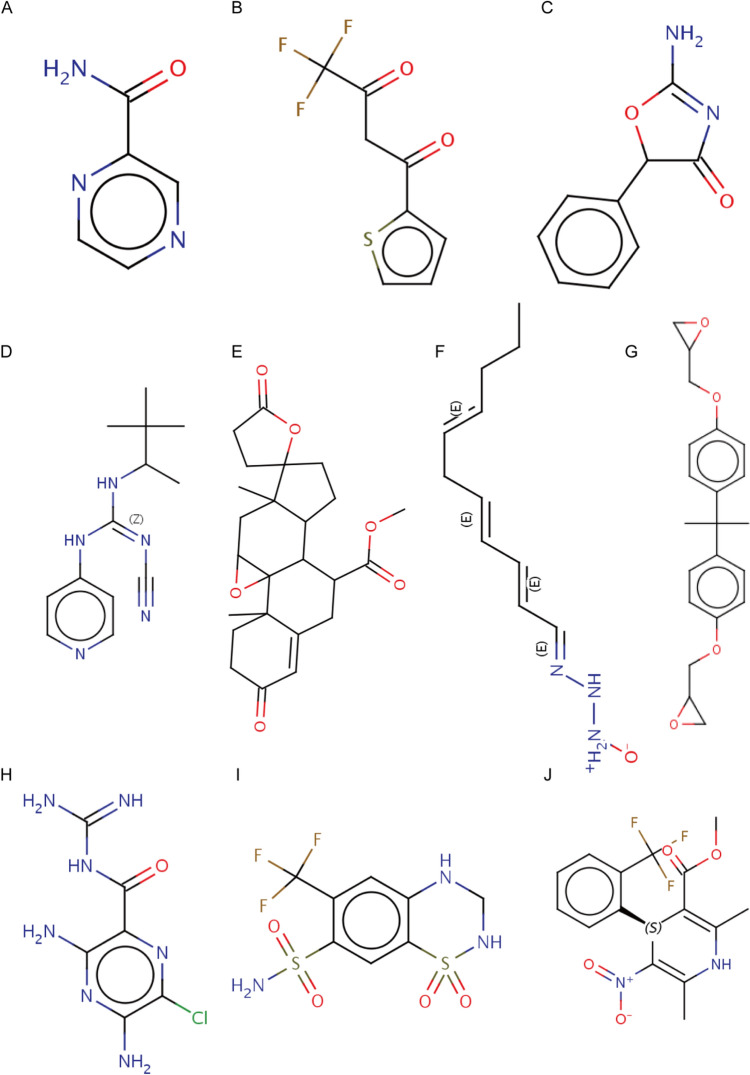
Next, We uploaded the list of DEGs between the high- and low-expression groups of CHMP4C to the Clue platform to predict 10 small-molecule compounds with potential roles in LUAD treatment and molecularly linked 10 key drugs (pyrazinamid, thenoyltrifluoroacetone, amiloride, BAY-K8644, bisphenol-a, eplerenone, hydroflumethiazide, pemoline, pinacidil, and triacsin-c) to CHMP4C (Fig. 9A–J). Molecular docking is a key approach for structure-based drug design and screening because it helps determine the optimal conformation of the interaction between a drug and its target molecule [25]. Similarity was evaluated by a score ranging from -100 to 100, and the results were ranked in descending order from highest to lowest. The closer the score was to 100, the more similar the gene list was to the small molecule processing record. The closer the value is to -100, the more opposite the gene list is to the small molecule processing record.
Fig. 9.
Molecular Docking. A–J 3D structures of small molecule drugs, including A pyrazinamid, B thenoyltrifluoroacetone, C amiloride, D BAY-K8644, E bisphenol-a, F eplerenone, G hydroflumethiazide, H pemoline, I pinacidil, J triacsin-c
Discussion
Lung cancer is the most common cancer and the leading cause of cancer-related deaths worldwide, with an estimated 2.2 million new cases (11.4%) and nearly 1.83 million deaths (18.0%) in 2020 [26, 27]. The challenge posed by lung cancer treatment has resulted in impressive scientific advances in the understanding of molecular pathways related to tumor progression and innovative treatments [28]. Therefore, the current therapeutic outlook for NSCLC is very complex and closely related to the molecular characteristics of each specific tumor [8]. This concept is extremely important because it means that the development of NSCLC treatment goes hand in hand with the development of molecular biology and the affirmation of precision medicine-based approaches [29]. Although the current treatments for lung cancer include radiotherapy, chemotherapy, targeted therapy, immunotherapy and surgery, the prognosis of patients receiving these treatments is still very poor [30, 31]; therefore, finding effective therapeutic targets is urgently needed. In this study, we found that CHMP4C expression was elevated in both LUAD tissue and cell lines. High CHMP4C expression leads to poor prognosis in patients with LUAD and is associated with the stage of LUAD. Subsequently, we demonstrated through functional experiments that CHMP4C promotes the proliferation, migration and invasion of LUAD cells. The role and prognostic value of CHMP4C in different types of cancer may differ, and its mechanism needs to be further studied.
CHMP4C is the human ESCRT-III subunit, and the endosome sorting complex (ESCRT) mechanism required for transport plays an evolutionarily conserved role in cytodynamic division, the final step in cell division in which daughter cells are physically separated. CHMP4C has been shown to function at Aurora B-dependent chromosome break checkpoints to prevent the premature breakdown of chromosomal bridges between cells and the accumulation of DNA damage. Others have shown that cells expressing CHMP4C (T232) can exhibit DNA damage because the CHMP4C (T232) allele unlocks the C-terminal helix of CHMP4C and disrupts binding to the early ESCRT factor ALIX [32]. It has also been suggested that CHMP4C (T232) may promote tumorigenesis and genomic instability through oncogene induced mitogenic stress [33]. CHMP4C is a potential drug therapeutic target for cardiac hypertrophy, which can inhibit cardiac hypertrophy by regulating the lysosomal degradation of EGFR [34], moreover, CHMP4C is a novel risk gene for hereditary dilated cardiomyopathy [35]. The aim of this study is to investigate the clinical value of CHMP4C in patients with LUAD. Using TCGA and GEO databases, we determined that CHMP4C was highly expressed in LUAD and confirmed this result at the molecular and protein levels. In addition, when CHMP4C was knocked down, the migration, invasion and proliferation of LUAD cells in vitro were significantly reduced, and CHMP4C prolonged the proportion of cells in the G0/G1 phase of the cell cycle. Cancer is characterized by the uncontrolled proliferation of tumor cells due to the abnormal activity of various cell cycle-related proteins [36]. Studies have also shown that increased cell cycle activity in cancer cells suppresses antitumor immunity. Therefore, cell cycle regulators are considered attractive targets in cancer therapy [37]. CDK4/6 inhibitors have emerged as the most promising cell cycle therapy, and there is a major effort underway to expand the reach of this paradigm [38]. In our study, we investigated the carcinogenic role of CHMP4C in the development of LUAD. Decreased CHMP4C expression led to cell cycle arrest at the G0/G1 phase. The interaction between CHMP4C and cell cycle-related molecules is the basis of cell cycle arrest, and we found that CHMP4C is positively correlated with CDK2 and cyclin D1. However, whether CHMP4C is a promising target for cell cycle inhibitors still needs further experimental verification.
These results fully indicate that CHMP4C can be used as a new marker for the diagnosis and prognosis of LUAD. In other cancers, Yong Xu et al. reported that CHMP4C was significantly upregulated in the prostate and that the expression level of CHMP4C was significantly correlated with the Gleason score and lymph node metastasis status of prostate cancer patients. In subsequent in vitro validation, CHMP4C promoted the malignant biological behavior of prostate cancer cell lines by modulating the cell cycle, This is similar to our findings. Patients with high CHMP4C expression were more sensitive to paclitaxel and 5-fluorouracil. These findings reveal that CHMP4C is a new diagnostic marker for prostate cancer and facilitates the subsequent precise treatment of prostate cancer [17]. In addition, CHMP4C regulates the proliferation of tumor cells through the cell cycle in lung squamous cell carcinoma and can be used as a potential diagnostic and prognostic biomarker for lung squamous cell carcinoma [19]. In osteosarcoma, CHMP4 knockdown prevents prevent osteosarcoma cell proliferation, migration and invasion. Similarly, in LUAD, we found that CHMP4C knockdown also inhibited the proliferation, migration, and invasion of LUAD cells. These findings may provide a new direction for osteosarcoma treatment [39]. In addition, studies by other scholars have shown that CHMP4C, a member of the pyrodeath-related gene family, can be used to predict the prognosis of patients with cervical cancer, different from our study, this study mainly discussed the role of CHMP4C in immune response and chemotaxis of inflammatory cells, enriching the cognition of CHMP4C in immune-related fields [40]. In the present study, we found that CHMP4C promoted the proliferation of LUAD cells and was highly expressed in LUAD tissues. Through data analysis, we also found that patients with high expression of CHMP4C were significantly sensitive to clinical drugs such as atinib and olaparil, which has guiding significance for clinical drug use in LUAD patients. However, we still need a large number of relevant trials and clinical cases to confirm our screening results. We need to verify the drug sensitivity of this discovery through cell and animal studies, and then proceed to clinical trials when we are sure that it meets our expectations.
CHMP4C is intrinsically involved in cell division, and there is a distinct correlation between the number of cells in a tissue or cell sample and the levels of CHMP4C. Consequently, our research aims to further elucidate the internal mechanisms by which CHMP4C regulates tumorigenesis and development. Moreover, our study suggests that CHMP4C could be a potential drug target for LUAD. However, it is crucial to acknowledge that CHMP4C expression is also detectable in normal human cells, indicating that targeting CHMP4C might have deleterious effects on non-tumor cells. Nevertheless, the expression of CHMP4C influences the sensitivity to chemotherapeutic drugs, which raises the possibility that a combination of CHMP4C targeting with chemotherapy drugs could be an effective therapeutic strategy. Yet, the association of CHMP4C with increased cell numbers complicates early detection and does not significantly enhance the efficacy of current scanning and biopsy methods. Therefore, it is of paramount importance to further investigate the expression of CHMP4C to detect early changes in samples such as blood.
In summary, our study showed that CHMP4C is highly expressed in LUAD and contributes to the poor prognosis of LUAD patients. LUAD patients can be grouped according to CHMP4C expression, and different treatment regimens are used for patients with low and high CHMP4C expression to achieve precise treatment for LUAD patients. However, our study still has some limitations. We have sparse experimental data in our research, and the effect of CHMP4C on the occurrence and development of LUAD has not been verified in animal models. Therefore, further studies are needed to determine the exact mechanisms by which CHMP4C regulates cell proliferation, migration, and invasion, prolongs the G0/G1 phase of the cell cycle, and increases sensitivity to drugs.
Conclusions
Using bioinformatics techniques, our study highlights that CHMP4C can be used as a marker for poor prognosis and a potential target for drug therapy with LUAD. By preliminatively studying the function of CHMP4C, we can better understand its role in tumor suppression and thus develop new therapeutic strategies. We also found that high expression of CHMP4C in LUAD increases sensitivity to related drugs, and further understanding of how CHMP4C regulates drug sensitivity could help develop more effective chemotherapy regimens. This makes targeting CHMP4C in combination with related sensitive drugs a promising research direction. Therefore, our study shows that CHMP4C has a potential carcinogenic effect in LUAD, and by studying the expression and function of CHMP4C in different patients, it can provide a more personalized treatment plan for LUAD patients and provide a new direction for the treatment of LUAD patients.
Supplementary Information
Acknowledgments
Bentong Yu, Jingtao Zhang, and Wan Zhang designed the research; Chuan Xu, Mingshan Liu, Yang Li, performed the bioinformatics analysis and interpreted the results; Chuan Xu and Yang Li performed the experiments, Chuan Xu, Mingshan Liu, Yang Li, Wei Zhou and Xiaoyue Peng analyzed and interpreted the results; Yang Li drafted the manuscript; Chuan Xu and Xiaoyue Peng revised the manuscript; All authors read and approved the final manuscript.
Abbreviations
- CHMP4C
Chromatin modified protein 4C
- DFI
Disease-free survival
- GSEA
Gene set enrichment analysis
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- GO
Gene ontology
- KM
Kaplan–Meier
- LUAD
Lung adenocarcinoma
- OS
Overall survival
- PFI
Progression-free survival
- TCGA
The Cancer Genome Atlas
- TIMER
Tumor Immunity Evaluation Resource
Author contributions
Bentong Yu, Jingtao Zhang, and Wan Zhang designed the research; Chuan Xu, Mingshan Liu, Yang Li, performed the bioinformatics analysis and interpreted the results; Chuan Xu and Yang Li performed the experiments, Chuan Xu, Mingshan Liu, Yang Li, Wei Zhou and Xiaoyue Peng analyzed and interpreted the results; Yang Li drafted the manuscript; Chuan Xu and Xiaoyue Peng revised the manuscript; All authors read and approved the final manuscript.
Funding
The study was supported by the Jiangxi Provincial Natural Science Foundation (20212ACB206014), the Science and Technology Program of Health Commission of Jiangxi Province (2024ZD004), the Introduction of Training High-level Innovative Undertaking Talents in Jiangxi Province "One Thousand People Plan" (jxsg2023201025), the National Natural Science Foundation of China (82260561).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
This study was approved by the Tumor Gene Atlas Project of the First Affiliated Hospital of Nanchang University and approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University on May 1, 2022 (Approval Number. 202203005). All study involving human experiments was informed written consent from all participants and in accordance with the Helsinki Declaration of Ethics Principles for Medical Research.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chuan Xu, Mingshan Liu and Yang Li contributed equally to the article and should be considered as co-first authors.
Contributor Information
Wan Zhang, Email: WanZhang@ncu.edu.cn.
Jingtao Zhang, Email: ndyfy07806@ncu.edu.cn.
Bentong Yu, Email: yubentong@126.com.
References
- 1.Fitzmaurice C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 3.Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. 2023;20(9):624–39. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Guo L, Chen J. Rationale for lung adenocarcinoma prevention and drug development based on molecular biology during carcinogenesis. Onco Targets Ther. 2020;13:3085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu F, Wang L, Zhou C. Lung cancer in China: current and prospect. Curr Opin Oncol. 2021;33(1):40–6. [DOI] [PubMed] [Google Scholar]
- 6.Nooreldeen R, Bach H. Current and future development in lung cancer diagnosis. Int J Mol Sci. 2021;22(16):8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones GS, Baldwin DR. Recent advances in the management of lung cancer. Clin Med (Lond). 2018;18(Suppl 2):s41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Sousa V, Carvalho L. Heterogeneity in lung cancer. Pathobiology. 2018;85(1–2):96–107. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch FR, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. [DOI] [PubMed] [Google Scholar]
- 10.Rami-Porta R, Osarogiagbon RU, Asamura H. The TNM system is adequate for making treatment decisions and prognostication in lung cancer. J Thorac Oncol. 2022;17(11):1255–7. [DOI] [PubMed] [Google Scholar]
- 11.Van Damme V, et al. Clinical factors predictive of long-term survival in advanced non-small cell lung cancer. Lung Cancer. 2013;79(1):73–6. [DOI] [PubMed] [Google Scholar]
- 12.Petsalaki E, Dandoulaki M, Zachos G. The ESCRT protein Chmp4c regulates mitotic spindle checkpoint signaling. J Cell Biol. 2018;217(3):861–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petsalaki E, Zachos G. CHMP4C: a novel regulator of the mitotic spindle checkpoint. Mol Cell Oncol. 2018;5(3): e1445944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurley JH. ESCRTs are everywhere. EMBO J. 2015;34(19):2398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lata S, et al. Structure and function of ESCRT-III. Biochem Soc Trans. 2009;37(Pt 1):156–60. [DOI] [PubMed] [Google Scholar]
- 16.Juan T, Furthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol. 2018;74:66–77. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, et al. CHMP4C as a novel marker regulates prostate cancer progression through cycle pathways and contributes to immunotherapy. Front Oncol. 2023;13:1170397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gusev A, et al. A transcriptome-wide association study of high-grade serous epithelial ovarian cancer identifies new susceptibility genes and splice variants. Nat Genet. 2019;51(5):815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu B, et al. CHMP4C regulates lung squamous carcinogenesis and progression through cell cycle pathway. J Thorac Dis. 2021;13(8):4762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng L, et al. A pan-cancer analysis of SMARCA4 alterations in human cancers. Front Immunol. 2021;12: 762598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen F, et al. Pan-cancer molecular subtypes revealed by mass-spectrometry-based proteomic characterization of more than 500 human cancers. Nat Commun. 2019;10(1):5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uhlen M, et al. A pathology atlas of the human cancer transcriptome. Science. 2017. 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geeleher P, Cox N, Huang RS. pRRophetic: an R package for prediction of clinical chemotherapeutic response from tumor gene expression levels. PLoS ONE. 2014;9(9): e107468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinzi L, Rastelli G. Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci. 2019;20(18):4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bade BC, Dela CC. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. 2020;41(1):1–24. [DOI] [PubMed] [Google Scholar]
- 27.Li C, et al. Global burden and trends of lung cancer incidence and mortality. Chin Med J (Engl). 2023;136(13):1583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Maglio G, et al. The storm of NGS in NSCLC diagnostic-therapeutic pathway: How to sun the real clinical practice. Crit Rev Oncol Hematol. 2022;169: 103561. [DOI] [PubMed] [Google Scholar]
- 29.Gray ER, et al. Ultra-sensitive molecular detection of gene fusions from RNA using ASPYRE. BMC Med Genomics. 2022;15(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27(8):1345–56. [DOI] [PubMed] [Google Scholar]
- 31.Collins LG, et al. Lung cancer: diagnosis and management. Am Fam Physician. 2007;75(1):56–63. [PubMed] [Google Scholar]
- 32.Carlton JG, et al. ESCRT-III governs the Aurora B-mediated abscission checkpoint through CHMP4C. Science. 2012;336(6078):220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadler J, et al. A cancer-associated polymorphism in ESCRT-III disrupts the abscission checkpoint and promotes genome instability. Proc Natl Acad Sci U S A. 2018;115(38):E8900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu A, et al. ESCRT-III component CHMP4C attenuates cardiac hypertrophy by targeting the endo-lysosomal degradation of EGFR. Hypertension. 2023;80(12):2674–86. [DOI] [PubMed] [Google Scholar]
- 35.Zhou N, et al. Identification of CHMP4C as a new risk gene for inherited dilated cardiomyopathy. J Genet Genomics. 2022;49(2):169–72. [DOI] [PubMed] [Google Scholar]
- 36.Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Stanger BZ. Cell cycle regulation meets tumor immunosuppression. Trends Immunol. 2020;41(10):859–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingham M, Schwartz GK. Cell-cycle therapeutics come of age. J Clin Oncol. 2017;35(25):2949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao S, et al. A novel defined necroptosis-related genes prognostic signature for predicting prognosis and treatment of osteosarcoma. Biochem Genet. 2024;62(2):831–52. [DOI] [PubMed] [Google Scholar]
- 40.Hu H, et al. A pyroptosis-related gene panel for predicting the prognosis and immune microenvironment of cervical cancer. Front Oncol. 2022;12: 873725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.