Abstract
Aeromonas inhabit diverse aquatic habitats and are recognized as both opportunistic and primary pathogens of fish and humans. This study delineates the biochemical and gyrB sequence-based molecular identification of 14 Aeromonas strains isolated from aquatic environments in Kerala, India, identifying them as A. dhakensis (50%), A. hydrophila (28.6%), and A. jandaei (21.4%). These strains exhibit a high prevalence of virulence genes (act, flaA, ser, gcat, lip, and ela) implicated in pathogenesis in both fish and humans. These findings underline the emergence of A. dhakensis, often misidentified as A. hydrophila, as a potential pathogen, highlighting the necessity for comprehensive identification methods. Significantly, all strains demonstrated beta-hemolysis and moderate to strong biofilm formation, enhancing their infectivity potential. Moreover, all isolates exhibited multidrug resistance, with a multiple antimicrobial resistance (MAR) index ranging from 0.39 to 0.56, and a significant presence of class 1 (500–1100 bp) and class 2 (250–700 bp) integrons, indicating their potential risk to both fish and human populations. Our results underscore the role of aquatic environment as a repository for virulent and multidrug-resistant Aeromonas spp., emphasizing the imperative for prudent antimicrobial usage and regular monitoring of antimicrobial resistance (AMR) in these environments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-024-01601-w.
Keywords: Aeromoniasis, Water bodies, Virulent determinants, Antibiotic resistance, Biofilm, Integrons
Introduction
The genus Aeromonas, comprising 36 gram-negative aquatic species, represents a diverse group with notable implications for aquatic ecosystems and public health. These species are significant fish pathogens, causing diseases like haemorrhagic septicemia and furunculosis, leading to large-scale outbreaks in aquaculture farms [1]. Additionally, they are recognized as emerging opportunistic pathogens, transitioning from environmental sources to humans and causing various intestinal and extra-intestinal infections [2]. Aeromonas plays a critical role in the One Health concept, being associated with economic losses in aquaculture, the spread of antibiotic resistance genes, and infections related to leech therapy in humans [3].
Aeromonas species are ubiquitous, found in aquatic environments, soil, and food, posing significant public health challenges [4]. Among them, A. hydrophila, A. dhakensis, A. caviae, and A. veronii (biovar veronii and sobria) are responsible for over 95% of human intestinal infections caused by Aeromonas [5]. However, A. dhakensis is often misidentified as A. hydrophila, leading to an underestimation of its cases [6, 7]. Research shows that A. dhakensis is predominant in tropical and subtropical regions and exhibits greater virulence than A. veronii, A. caviae, and A. hydrophila [5, 8]. Recently, virulent and antibiotic-resistant A. dhakensis strains have been increasingly reported from various environmental sources, causing numerous infections in fish and humans [6, 9]. Species-level identification of Aeromonas is complex and challenging due to the lack of standardized phenotypic tests, discrepancies between phenotypic and genotypic methods, genetic heterogeneity, and the increasing number of new species [4]. Recent studies have utilized housekeeping genes (gyrB, rpoD, and rpoB) as molecular markers for precise species-level identification and to establish phylogenetic relationships [2, 6, 10].
The virulence potential of Aeromonas is complex, dependent on host susceptibility, and not fully understood [3]. Various virulence factors, including cell-associated structural components, extracellular products, toxins, and biofilm formation, enable Aeromonas cells to colonize and destroy host cells [11]. In recent years, antibiotic resistance among Aeromonas strains has become a significant public health concern, particularly in developing countries where the use of antibiotics is often unregulated and uncontrolled [5]. Pathogens affecting humans, animals, and the environment face the dual challenges of increased virulence determinants and growing antimicrobial resistance. Therefore, this study aimed to identify Aeromonas strains, isolated from aquatic environments of Kerala, India and examine their pathogenicity, biofilm formation, and resistance potential.
Materials and methods
Aeromonas cultures and biochemical identification
Fourteen presumptive Aeromonas strains, previously isolated from both healthy and moribund finfish exhibiting symptoms such as exophthalmia, abdominal distention, haemorrhagic spots, necrotic fin rot, and ragged fins, were collected from aquaculture farms and river water samples in Ernakulam and Thrissur, Kerala, India (Suppl Table 1). The affected animals, including species such as Catla (Catla catla), Rohu (Labeo rohita), and Gourami (family Osphronemidae), were brought to the laboratory by farmers. These farms utilized traditional intensive freshwater aquaculture ponds, either for food fish production or for rearing ornamental fish. The isolates were identified to the species level using comprehensive biochemical tests [12–14]. The strains’ hemolytic activity was assessed on blood agar plates (HiMedia, India) incubated at 30 °C for 18 h [15].
Molecular Identification and phylogenetic analysis
All biochemically identified Aeromonas species were confirmed through gyrB (~ 1100 bp) gene sequencing, following the primers and procedures of Soler et al. [16]. The amplified products were purified, sequenced, and compared against GenBank database entries using the BLASTN program (www.ncbi.nih.gov/BLAST/). These sequences were then uploaded to GenBank (Accession numbers: OQ743456-64, OQ789562-65, and OQ920270). The gyrB gene sequences were aligned, and sequence similarities were estimated for a 920 base segment (positions 852,606–853,528, according to E. coli ATCC 25922 numbering, NZ_CP009072). A phylogenetic tree was constructed using the UPGMA method and Kimura two-parameter model in MEGA 11 [17].
Virulence genes
Virulence genes, including aerolysin (aer), cytotoxic enterotoxin (act), hemolysin (hly), lipase (lip), elastase (ahyB), glycerophospholipid cholesterol acyltransferase (gcat), cytotonic enterotoxins (ast, alt), serine protease (ser), polar flagella (fla), and lateral flagella (lafA), were amplified from chromosomal DNA using previously described primers and PCR conditions [18, 19].
Biofilm formation and analysis
Biofilm formation was evaluated by culturing various Aeromonas strains in TSB within 96-well flat-bottomed microtiter plates (Falcon, BD Biosciences, NJ, USA) and quantified using a modified crystal violet (CV) assay [19]. The biofilm-forming ability was classified using the specific biofilm formation (SBF) index, calculated for each isolate by normalizing biofilm accumulation (OD570nm) relative to cell growth (OD600nm) with the formula SBF = (B - NC)/G, where B represents the OD570nm of the attached and stained bacteria, NC is the OD570nm of the control wells containing bacteria-free medium, and G is the OD600nm of cell growth in the medium [20]. The extent of biofilm formation was categorized into three groups: no biofilm (SBF < 0.1), weak (0.1 ≤ SBF ≤ 0.5), moderate (0.5 ≤ SBF ≤ 1), or strong (SBF > 1) [21].
Antimicrobial susceptibility and integron analysis
The antimicrobial resistance patterns of these strains were determined against 18 commonly used antibiotics from 11 classes, using the disc diffusion method on Mueller-Hinton Agar, as specified by the Clinical and Laboratory Standards Institute (CLSI) [22]. Resistance profiles were assigned based on zone diameters according to CLSI breakpoints [23]. Multi-drug resistance and multiple antibiotic resistance (MAR) indices were calculated following Magiorakos et al. [24] and Krumperman [25], respectively. The MAR index for a single isolate is calculated as the ratio of a to b, where a is the number of antibiotics the isolate is resistant to, and b is the total number of antibiotics it has been exposed to. The presence of class 1 and class 2 integrons was assessed under conditions described by Nagar et al. [19].
Results and discussion
Identification of Aeromonas from aquatic environment
Fourteen presumptive Aeromonas isolates were identified at the genus level based on biochemical tests (positive oxidase, nitrate reductase reactions, catalase; fermentation of D-glucose and trehalose; utilization of dulcitol, D-arabitol, erythritol and xylose; and resistance to vibriostatic agent O/129), as described by Abbott et al. [12] and Nagar et al. [10]. Based on the additional biochemical characteristics: aesculin hydrolysis, and acid production from L-arabinose, urocanic acid, salicin, sucrose, D-cellobiose and L-fucose [13, 14], they were further identified up to the species level (Table 1).
Table 1.
Phenotypic and genetic identification, and integrons of 14 Aeromonas strains isolated from aquatic environment of Kerala
| Strain | Source | Taxonomic identification (species name) based on | NCBI Acc. No. | Class 1 integron (bp) | Class 2 integron (bp) | |
|---|---|---|---|---|---|---|
| Biochemical tests | gyrB gene | |||||
| A1 | River_ Ernakulam | A. dhakensis | A. dhakensis | OQ743456 | 650, 1100 | 650 |
| A2 | River_ Ernakulam | A. dhakensis | A. dhakensis | OQ743457 | 650, 1100 | - |
| A3 | River_ Ernakulam | A. hydrophila | A. hydrophila | OQ920270 | - | - |
| A4 | River_ Ernakulam | A. dhakensis | A. dhakensis | OQ789562 | 1100 | - |
| A5 | River_ Ernakulam | A. dhakensis | A. dhakensis | OQ789563 | 500 | 650 |
| A6 | River_Thrissur | A. dhakensis | A. dhakensis | OQ789564 | 500 | 650 |
| A7 | River_Thrissur | A. dhakensis | A. dhakensis | OQ743458 | 1100 | - |
| A8 | River_Thrissur | A. hydrophila | A. hydrophila | OQ743459 | 650 | 650 |
| A9 | River_Thrissur | A. dhakensis | A. dhakensis | OQ789565 | 550 | 700 |
| M14 | Catla, Aquaculture pond, Thrissur | A. hydrophila | A. hydrophila | OQ743460 | - | - |
| M22 | Rohu, Aquaculture pond, Thrissur | A. hydrophila | A. hydrophila | OQ743461 | - | 250 |
| N14 | Catla, Aquaculture pond, Ernakulam | A. jandaei | A. jandaei | OQ743463 | 800 | - |
| N46 | Rohu, Aquaculture pond, Ernakulam | A. jandaei | A. jandaei | OQ743464 | - | - |
| P31 | Gourami, Aquaculture pond, Ernakulam | A. jandaei | A. jandaei | OQ743462 | 850 | - |
Relying solely on morphological and biochemical characteristics for Aeromonas identification is often contentious and unreliable, leading to potential misidentifications [5]. Therefore, the gyrB gene, which has higher discriminatory power for phylogenetic analysis [16], was employed for species-level identification of the biochemically characterized Aeromonas strains. Partial gyrB gene sequences were submitted to GenBank at NCBI (Accession numbers: OQ743456-64, OQ789562-65, and OQ920270). Based on biochemical tests and gyrB gene sequences, these Aeromonas strains were identified and confirmed as A. dhakensis (50%), A. hydrophila (28.6%), and A. jandaei (21.4%). Initially misidentified as A. hydrophila in 2002, A. dhakensis was later recognized as a distinct species in 2013 [8]. Since then, A. dhakensis has been isolated from diverse sources across different countries with varying frequencies [6, 7, 9].
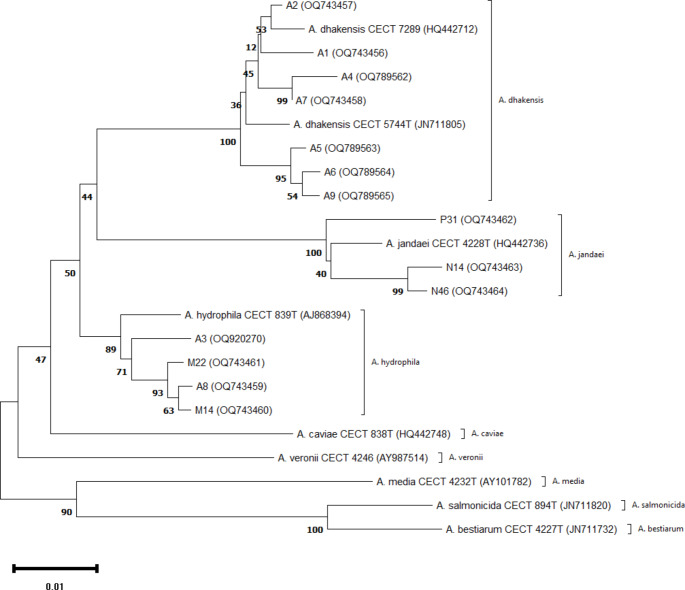
Pairwise and mean distances of aligned gyrB sequences from these strains were estimated for a continuous stretch of 920 bases (positions 852,606–853,528 according to E. coli ATCC 25922 numbering, NZ_CP009072) using ClustalW. The sequence homology among all Aeromonas strains for the gyrB gene ranged from 89.91 to 99.67%, representing 3 to 93 nucleotide variations. The mean sequence similarity, serving as a measure of discriminatory capability, was determined to be 95.0%, comparable to the 92.2% for the gyrB gene of Aeromonas strains described by Soler et al. [16]. The alignment revealed a total of 173 variable positions (18.8% of the sequenced fragment), along with a single triplet (ACA) insertion in A. salmonicida (CECT 894T) and A. bestiarum (CECT 4227T). Intra-species nucleotide substitution rates, determined by calculating mean distance within each species, ranged from 1.18% in A. hydrophila to 1.93% in A. jandaei. A phylogenetic tree constructed using these genetic matrices showed significant divergence among all Aeromonas species, with consistent clustering patterns between the investigated strains and their type or reference strains (Fig. 1).
Fig. 1.
Unrooted phylogenetic tree (UPGMA) of Aeromonas isolates and other known Aeromonas species based on the gyrB gene sequences. CECT numbers indicate the Spanish Type culture collection numbers of the Aeromonas reference strains. Numbers in the parenthesis represent the GenBank accession numbers. Numbers shown next to each node indicate bootstrap values (percentage of 1,000 replicates). The bar indicates a sequence divergence
This study reinforces the importance of a combined phenotypic and genotypic approach for reliable taxonomic classification of Aeromonas from diverse samples, as previously reported [2, 9]. A. dhakensis, A. hydrophila, and A. jandaei have been reported from fish and aquatic environments [11], as well as clinical and outbreak samples [6, 8]. The rising prevalence of Aeromonas species, known pathogens for both humans and aquatic animals, in aquatic environments raises concerns about increased infections in both populations, especially given the growing demand for seafood and the potential for fish-to-human transmission.
Virulence genes analysis
Virulence factors in Aeromonas strains contribute to their pathogenesis and transmission. All aquatic Aeromonas strains in this study carried the genes act (232 bp), ser (350 bp), gcat (237 bp), ahyB (540 bp), and lip (390 bp) (Table 2). The aer (252 bp) and hlyA (597 bp) genes were present in all A. dhakensis and A. hydrophila strains, but only 33.3% and none of the A. jandaei strains, respectively (Table 2). Cytotonic enterotoxins, alt and ast, were found in 78.6% and 64.3% of the strains, respectively (Table 2). Both genes were highly prevalent in A. dhakensis and A. hydrophila strains (> 70%), but only 33.3% and none of the A. jandaei strains, respectively. Structural genes for polar flagella (fla) and lateral flagella (lafA) were present in 100% and 28.6% of these Aeromonas strains, respectively (Table 2). All A. dhakensis and A. hydrophila strains harbored act, aer, hlyA, and fla genes, while ast, hlyA, and lafA genes were absent in all A. jandaei strains. Recently, Aeromonas strains with various virulence genes have been reported from water [9], aquaculture farms [2], and clinical samples [6]. In this study, all strains exhibited distinct β-hemolysis zones on blood agar plates, indicating potential pathogenicity (Table 2). Hoel et al. [26] documented β-hemolysis in 91% of food strains.
Table 2.
Distribution of genotypic virulence markers and β-hemolysis in Aeromonas strains
| Aeromonas spp. | Cytotoxic enterotoxin | Cytotonic enterotoxins | Aerolysin / haemolysin genes | Flagellar genes | Serine protease | GCAT | Lipase (390) |
Ela (540) |
β-hemolysis | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| act (232) | Heat-stable ast (331) | Heat-labile alt (442) |
aer (252) | hlyA (597) | lafA (550) | flaA (608) | ser (350) | gcat (237) | lip (390) |
ela
(540) |
||
|
A. dhakensis (n = 7) |
7 (100) | 5 (71.4) | 7 (100) | 7 (100) | 7 (100) | 2 (28.6) | 7 (100) | 7 (100) | 7 (100) | 7 (100) | 7 (100) | 7 (100) |
| A. hydrophila (n = 4) | 4 (100) | 4 (100) | 3 (75) | 4 (100) | 4 (100) | 2 (50) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 4 (100) |
|
A. jandaei (n = 3) |
3 (100) | 0 (0) | 1 (33.3) | 1 (33.3) | 0 (0) | 0 (0) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) |
| Total (n = 14) | 14 (100) | 9 (64.3) | 11 (78.6) | 12 (85.7) | 11 (78.6) | 4 (28.6) | 14 (100) | 14 (100) | 14 (100) | 14 (100) | 14 (100) | 14 (100) |
Biofilm
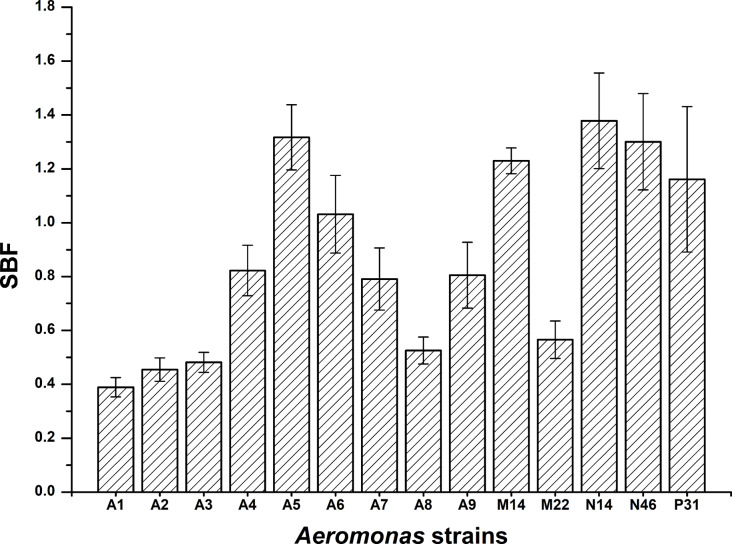
Biofilm formation enhances bacterial survival and virulence. Aeromonas can adhere to and colonize both biotic and abiotic surfaces [1]. Biofilm formation was measured using the SBF index, which incorporates bacterial growth rate (OD600nm) for consistent categorization, as described by Naves et al. [20]. In this study, biofilm formation by 14 Aeromonas strains in TSB ranged from 0.39 to 1.38. The strains were categorized as weak (n = 3, 21.4%), moderate (n = 5, 35.7%), and strong (n = 6, 42.9%) biofilm producers (Fig. 2). All A. jandaei strains were strong biofilm producers, while A. dhakensis and A. hydrophila showed no clear pattern. Aeromonas strains from fish produced moderate to strong biofilms, whereas those from water samples were predominantly weak to moderate producers. This aligns with research by Chenia and Duma [27], showing that most Aeromonas strains from freshwater fish and seafood produce moderate to strong biofilms, while strains from estuarine and river waters typically produce weak biofilms [28]. Chen et al. [8] reported that clinical A. dhakensis strains form stronger biofilms compared to A. hydrophila strains in Taiwan.
Fig. 2.
Biofilm formation by Aeromonas strains in TSB 30 °C. Bars represent average SBF values and standard errors
Antimicrobial susceptibility
The antimicrobial susceptibility of 14 confirmed Aeromonas strains was evaluated against 18 antibiotics to create an antibiogram profile. All strains were resistant to ampicillin, cephalothin, imipenem, ampicillin/sulbactam, aztreonam, piperacillin-tazobactam, and clindamycin, but were sensitive to gentamicin, co-trimoxazole, amikacin, chloramphenicol, nitrofuran, and ciprofloxacin (Table 3). Notably, strains from water samples in Ernakulam and Thrissur showed resistance to ceftriaxone and nalidixic acid, whereas strains from fish samples were sensitive to these antibiotics. This antimicrobial resistance (AMR) pattern aligns with findings by Dubey et al. [2].
Table 3.
Percentage antimicrobial resistance of Aeromonas spp. isolated from aquatic environment of Kerala
| Antibiotic (concentration, µg) |
A. dhakensis
(n = 7) |
A. hydrophila
(n = 4) |
A. jandaei
(n = 3) |
Resistant strains (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | I | S | R | I | S | R | I | S | ||
| Ampicillin (10) | 100 | 100 | 100 | 100 | ||||||
| Ampicillin/Sulbactam (10/10) | 100 | 100 | 100 | 100 | ||||||
| Piperacillin/Tazobactam (100/10) | 100 | 100 | 100 | 100 | ||||||
| Tetracycline (30) | 71.4 | 28.6 | 25 | 75 | 100 | 42.9 | ||||
| Gentamicin (10) | 100 | 100 | 100 | - | ||||||
| Streptomycin (10) | 14.3 | 85.7 | 100 | 100 | - | |||||
| Kanamycin (30) | 14.3 | 85.7 | 100 | 100 | - | |||||
| Amikacin (30) | 100 | 100 | 100 | - | ||||||
| Trimethoprim/sulfamethoxazole (25) | 100 | 100 | 100 | - | ||||||
| Chloramphenicol (30) | 100 | 100 | 100 | - | ||||||
| Cephalothin (30) | 100 | 100 | 100 | 100 | ||||||
| Ceftriaxone (30) | 100 | 50 | 25 | 25 | 100 | 64.3 | ||||
| Imipenem (10) | 100 | 100 | 100 | 100 | ||||||
| Nitrofurantoin (30) | 100 | 100 | 100 | - | ||||||
| Aztreonam (30) | 100 | 100 | 100 | 100 | ||||||
| Ciprofloxacin (5) | 100 | 100 | 100 | - | ||||||
| Nalidixic Acid (30) | 71.4 | 28.6 | 25 | 75 | 100 | 42.9 | ||||
| Clindamycin (2) | 100 | 100 | 100 | 100 | ||||||
Aeromonas, a ubiquitous water-borne organism, easily acquires and exchanges AMR genes, making it a recognized reservoir of antibiotic resistance genes (ARGs) [3]. Water environments host diverse microbial communities and ARG reservoirs. Overuse of antibiotics and inadequate sanitation expose these communities to external ARGs, accelerating their acquisition and dissemination. In this study, 43% of strains were resistant to tetracycline, although this was lower than in recent studies by Jacobs and Chenia [29].
Multidrug resistance, defined as resistance to at least one antimicrobial agent from three or more different classes [24], was observed in all Aeromonas strains. Six strains (A4, A5, A6, A7, A8, and A9) resisted six antibiotic classes: penicillin and its derivatives, tetracycline, first-generation cephalosporins, penems, monobactams, and fluoroquinolones. Eight strains (A1, A2, A3, M14, M22, P31, N14, and N46) resisted four classes: penicillin and its derivatives, first-generation cephalosporins, penems, and monobactams. The prevalence of AMR microorganisms is rising and is expected to become a major public health concern.
Our findings corroborate earlier research on the emergence of multidrug-resistant Aeromonas strains in river water, fish, food, and clinical settings [5, 29, 30]. The Multiple Antibiotic Resistance (MAR) index, a valuable risk assessment tool, indicated MAR values between 0.39 and 0.56 (Suppl Table 2) for Aeromonas strains in this study, with all values exceeding 0.2. This suggests contamination from sources with frequent antimicrobial use, indicating a high-risk environment. The MAR index range observed is consistent with previous research [28, 29], further implying potential antimicrobial contamination in the surveyed aquatic environments. To control antimicrobial-resistant Aeromonas in these environments, farmers and veterinary teams should prioritize strong biosecurity measures, such as maintaining water quality and reducing animal stress, while incorporating alternatives like phage therapy, probiotics, vaccines, plant-based antimicrobials, and silver nanoparticles. Phage therapy, in particular, offers a targeted approach to combat resistance, while responsible antibiotic use, effective water management, and staff education are essential for the long-term health and sustainability of aquaculture systems.
Integron analysis
Class 1 integrons were identified in 71.4% of the Aeromonas aquatic strains, with variable region sizes ranging from 500 to 1100 bp; the remaining 4 strains showed no amplification (Table 1). These results are consistent with previous research showing 21.7% of Aeromonas strains from ornamental freshwater fish farms carrying class 1 integrons [9]. Additionally, 42.9% of strains exhibited class 2 integrons (250–700 bp) (Table 1), aligning with findings by Jacobs and Chenia [29] and Nagar et al. [19], who reported class 2 integrons in 27% of aquaculture and 50% of food strains, respectively.
Integrons, mobile genetic elements, facilitate the integration and expression of diverse gene cassettes, including antibiotic resistance genes. They play a significant role in promoting antibiotic resistance within environmental bacterial populations via horizontal gene transfer [3]. In Aeromonas strains, integrons could potentially enhance the transfer of multiple antibiotic resistance genes among environmental microorganisms.
Conclusions
The study identified diverse Aeromonas species, including potentially pathogenic strains, in water and fish samples from Kerala, India. By combining gyrB analysis with biochemical tests, accurate species-level identification was achieved, highlighting the importance of these methods in differentiating Aeromonas species. The high occurrence of A. dhakensis raises significant concerns for India’s freshwater and aquaculture ecosystems. The presence of pathogenic Aeromonas strains in Indian aquaculture underscores the critical need for regular surveillance and stringent antibiotic controls. Their varied biofilm-forming abilities and widespread antibiotic resistance, including multidrug resistance, suggest that aquatic environments may act as reservoirs for resistant bacteria, potentially promoting the emergence of more virulent strains due to antibiotic misuse. Responsible antibiotic use is crucial for the sustainability of Indian aquaculture and the health of its ecosystems. This study focused on characterizing Aeromonas strains for therapeutic phage recovery in the treatment of aeromoniasis, revealing diverse strains with varying levels of virulence and resistance. Future work will explore bacteriophages and bacteriophage-derived proteins for their potential therapeutic applications in combating Aeromonas infections.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Dr. Shashidhar R. for his critical suggestions in improving the manuscript. We gratefully acknowledge Dr. Valérie Leclère, Université des Sciences et Technologies de Lille USTL, Villeneuve d’Ascq cedex, France for her generous gift of A. hydrophila CECT 839T, A. veronii bv. veronii CECT 4257T, A. veronii bv. sobria CECT 4246, A. dhakensis CECT 5744T, A. jandaei CECT 4228T and A. bivalvium CECT 7113T cultures.
Author contributions
VN conceptualized, designed and performed the experiments, analyzed data, and wrote and reviewed the manuscript. FA contributed in experiments and data analysis. TCJ reviewed data and manuscript. MV was responsible for conceptualization, writing and editing the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by Department of Atomic Energy.
This work was funded by Department of Atomic Energy, Government of India.
Data availability
All data included in this study are available upon request. Partial gyrB gene sequences of Aeromonas strains have been submitted to GenBank at NCBI (Accession numbers: OQ743456-64, OQ789562-65 and OQ920270).
Declarations
Consent for publication
All listed authors have approved the manuscript before submission, including the names and order of authors.
Conflict of interest
The authors have no conflict of interests to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Talagrand-Reboul E, Colston SM, Graf J, Lamy B, Jumas-Bilak E (2020) Comparative and evolutionary genomics of isolates provide insight into the pathoadaptation of Aeromonas. Genome Biol Evol 12(5):535–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubey S, Maiti B, Girisha SK et al (2021) Aeromonas species obtained from different farmed aquatic species in India and Taiwan show high phenotypic relatedness despite species diversity. BMC Res Notes 14(1):313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamy B, Baron S, Barraud O (2022) Aeromonas: the multifaceted middleman in the One Health world. Curr Opin Microbiol 65:24–32 [DOI] [PubMed] [Google Scholar]
- 4.Goncalves Pessoa RB, de Oliveira WF, Marques DSC et al (2019) The genus Aeromonas: a general approach. Microb Pathog 130:81–94 [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Bravo A, Figueras MJ (2020) An update on the genus Aeromonas: taxonomy, epidemiology, and pathogenicity. Microorganisms 8(1) [DOI] [PMC free article] [PubMed]
- 6.Lau TTV, Tan JMA, Puthucheary SD, Puah SM, Chua KH (2020) Genetic relatedness and novel sequence types of clinical Aeromonas dhakensis from Malaysia. Braz J Microbiol 51(3):909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson VI (2023) Comparative genomic analysis of Aeromonas dhakensis and Aeromonas hydrophila from diseased striped catfish fingerlings cultured in Vietnam. Front Microbiol 14:1254781Khoi LM, Hounmanou YMG, Dung TT, Phu TM, Dalsgaard A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen PL (2014) A comparative study of clinical Aeromonas dhakensis and Aeromonas hydrophila isolates in southern Taiwan: A. dhakensis is more predominant and virulent. Clin Microbiol Infect 20(7):O428–434Wu CJ, Chen CS, Tsai PJ, Tang HJ, Ko WC [DOI] [PubMed] [Google Scholar]
- 9.Dhanapala PM (2021) Kalupahana RS, Kalupahana AW, Wijesekera DPH, Kottawatta SA, Jayasekera NK, Silva-Fletcher A, Jagoda SSSdS Characterization and antimicrobial resistance of environmental and clinical Aeromonas species isolated from fresh water ornamental fish and associated farming environment in Sri Lanka. Microorganisms 9(10) [DOI] [PMC free article] [PubMed]
- 10.Nagar V, Shashidhar R, Bandekar JR (2013) Characterization of Aeromonas strains isolated from Indian foods using rpoD gene sequencing and whole cell protein analysis. World J Microbiol Biotechnol 29(4):745–752 [DOI] [PubMed] [Google Scholar]
- 11.Majeed S, De Silva L, Kumarage PM, Heo GJ (2023) Occurrence of potential virulence determinants in Aeromonas spp. isolated from different aquatic environments. J Appl Microbiol 134(3) [DOI] [PubMed]
- 12.Abbott SL, Cheung WK, Janda JM (2003) The genus Aeromonas: biochemical characteristics, atypical reactions, and phenotypic identification schemes. J Clin Microbiol 41(6):2348–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esteve C, Alcaide E, Blasco MD (2012) Aeromonas hydrophila subsp. dhakensis isolated from feces, water and fish in Mediterranean Spain. Microbes Environ 27(4):367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pu W (2019) Guo G, Yang N, Li Q, Yin F, Wang P, Zheng J, Zeng J Three species of Aeromonas (A. dhakensis, A. hydrophila and A. jandaei) isolated from freshwater crocodiles (Crocodylus siamensis) with pneumonia and septicemia. Lett Appl Microbiol 68(3):212–218 [DOI] [PubMed]
- 15.Brenden R, Janda JM (1987) Detection, quantitation and stability of the beta haemolysin of Aeromonas spp. J Med Microbiol 24(3):247–251 [DOI] [PubMed] [Google Scholar]
- 16.Soler L (2004) Phylogenetic analysis of the genus Aeromonas based on two housekeeping genes. Int J Syst Evol Microbiol 54(Pt 5):1511–1519Yanez MA, Chacon MR, Aguilera-Arreola MG, Catalan V, Figueras MJ, Martínez-Murcia AJ [DOI] [PubMed] [Google Scholar]
- 17.Tamura K, Stecher G, Kumar S (2021) MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 38(7):3022–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagar V, Shashidhar R, Bandekar JR (2011) Prevalence, characterization, and antimicrobial resistance of Aeromonas strains from various retail food products in Mumbai, India. J Food Sci 76(7):M486–492 [DOI] [PubMed] [Google Scholar]
- 19.Nagar V, Sinha V, Bandekar JR (2015) Diverse profiles of N-acyl homoserine L-lactones, biofilm, virulence genes and integrons in food-borne Aeromonas isolates. J Food Sci 80(8):M1861–1870 [DOI] [PubMed] [Google Scholar]
- 20.Niu C, Gilbert ES (2004) Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl Environ Microbiol 70(12):6951–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jahid IK, Lee NY, Kim A, Ha SD (2013) Influence of glucose concentrations on biofilm formation, motility, exoprotease production, and quorum sensing in Aeromonas hydrophila. J Food Prot 76(2):239–247 [DOI] [PubMed] [Google Scholar]
- 22.Institute CLS (2022) Performance Standards for Antimicrobial Susceptibility Testing, CLSI supplement M100. in Clinical and Laboratory Standards Institute (Wayne, PA)
- 23.Institute CaLS (2015) Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria in CLSI guideline M45 (Wayne, PA)
- 24.Magiorakos AP (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3):268–281Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL [DOI] [PubMed] [Google Scholar]
- 25.Krumperman PH (1983) Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl Environ Microbiol 46(1):165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoel S, Vadstein O, Jakobsen AN (2017) Species distribution and prevalence of putative virulence factors in mesophilic Aeromonas spp. isolated from fresh retail sushi. Front Microbiol 8:931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chenia HY, Duma S (2017) Characterization of virulence, cell surface characteristics and biofilm-forming ability of Aeromonas spp. isolates from fish and sea water. J Fish Dis 40(3):339–350 [DOI] [PubMed] [Google Scholar]
- 28.Igbinosa IH, Beshiru A, Odjadjare EE, Ateba CN, Igbinosa EO (2017) Pathogenic potentials of Aeromonas species isolated from aquaculture and abattoir environments. Microb Pathogen 107:185–192 [DOI] [PubMed] [Google Scholar]
- 29.Jacobs L, Chenia HY (2007) Characterization of integrons and tetracycline resistance determinants in Aeromonas spp. isolated from South African aquaculture systems. Int J Food Microbiol 114(3):295–306 [DOI] [PubMed] [Google Scholar]
- 30.Stratev D, Odeyemi OA (2016) Antimicrobial resistance of Aeromonas hydrophila isolated from different food sources: a mini-review. J Infect Public Health 9(5):535–544 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data included in this study are available upon request. Partial gyrB gene sequences of Aeromonas strains have been submitted to GenBank at NCBI (Accession numbers: OQ743456-64, OQ789562-65 and OQ920270).