Abstract
Amylin, also known as islet amyloid polypeptide (IAPP), is a pancreatic β‐cell peptide hormone involved in satiation and control food intake. It is also produced in smaller quantities by neurons, the gastrointestinal tract, and spinal ganglia. Numerous studies have revealed that patients with type 2 diabetes mellitus (T2DM) and cognitive deficits exhibit IAPP deposits in the pancreas, brain, and blood vessels. IAPP has also been shown to exert neuroprotective effects against Alzheimer's disease (AD) and cognitive impairments. The objective of this review paper is to provide recent information about the pathophysiological roles of IAPP in metabolic and in neurological disorders, and its potential as a druggable target. We have reviewed preclinical and clinical human and animal research studies of IAPP. We discuss the IAPP structure, its receptors, and its physiological functions in metabolism, satiation, adiposity, obesity, and in the brain. Then we discuss its role in metabolic and neurological disorders like diabetes, obesity, bone disorder, neurodegeneration, cerebrovascular disorders, depression, alcohol use disorder, epilepsy, and in ovarian cysts. Overall, this review provides information on the progress of research into the roles of IAPP and its receptor in food intake, energy homeostasis, glucose regulation, satiation, and its role in metabolic and neurological disorders making it a potential target for therapeutic approaches. This review also suggests that the utilization of rodents overexpressing human IAPP in neurodegeneration models may unearth some significant therapeutic potentials for neurological disorders.
Keywords: amylin (IAPP), amyloid aggregates, calcitonin, neurodegeneration, satiation, type 2 diabetes
The dual roles of amylin in physiology and pathology. Under normal physiological conditions, amylin regulates food intake, gastric emptying, bone metabolism, and exhibits neuroprotective effects in the brain. On the right side, the pathogenesis of amylin is shown in conditions such as T2DM and neurodegenerative diseases like AD. Human amylin aggregates to form toxic amyloid fibrils and β‐sheets, leading to the formation of amyloid plaques in the brain and gut, which contribute to disease pathology.

1. INTRODUCTION
Amylin, also known as islet amyloid polypeptide (IAPP), deposited in the pancreatic islets of diabetic patients, was first discovered as an “islet hyalinization” in 1900. 1 It wasn't until 1943, 2 with further confirmation in 1961, 3 that this hyalinization was described as amyloid, and similar findings were later reported in diabetic cats in 1981. 4 , 5 A significant discovery was made in 1986 with the identification of the primary component of this amyloid as a peptide initially termed insulinoma amyloid peptide (IAP), later known as islet amyloid polypeptide or IAPP. 6 IAPP is a neuroendocrine hormone with anorexigenic properties and is secreted together with insulin by the β‐cells of the pancreas. 7 IAPP is centrally produced in the medial preoptic area, arcuate nucleus, and area postrema areas of the lactating rat brain, 8 and IAPP gene is expressed in other tissues such as stomach and spinal ganglia in lesser amounts. 9 IAPP is derived from an 89 residue pre‐pro‐IAPP protein, processed into a 67 residue pro‐IAPP, and subsequently to a mature biologically active 37 amino acid protein monomer through cleavage by prohormone convertase 1/3 (PC1/3), prohormone convertase 2 (PC2), 7 , 9 and carboxypeptidase‐E (Figure 1A–C). 10 Mature human IAPP (hIAPP) has a molecular mass of around 4 kilodaltons, characterized by an intramolecular disulfide bond linking the cysteine residues at positions 2 and 7 and an amide moiety at the c‐terminus (Figure 1C,D). 11 IAPP shares sequence similarity with calcitonin (CT) peptide family—calcitonin, CT gene‐related peptide (CGRPα and CGRPβ), adrenomedullin (AM), and adrenomedullin 2 (AM2). 11 , 12
FIGURE 1.

Amino acid sequences and processing of the human Amylin (hIAPP), its domains and 3D crystal structure: (A) The 89 amino acid pre‐pro‐hIAPP having the signal peptide from residue 1 to residue 22 is cleaved. (B) The 67 residue pro‐hIAPP peptide cleaved by PC2, PC1/2 and CPE to a mature 37 residue hIAPP. (C) Mature hIAPP with a disulfide bond (green) between cysteine 2 and cysteine 7, amphipathic α‐helix and C‐terminal helix amidation (based on Westermark et al., 2011 15 ). (D) 3D crystal structure of hIAPP (PDB: 5MGQ) showing the disulfide bond (yellow) and its spatial orientation as a U‐shaped protein. Residues 1–7 form a loop outside the U‐shape, residues 8–18 form the n‐terminal helix, residues 18–22 form a loop and residues 23–35 form the C‐terminal helix.
The transcription and expression of IAPP and insulin are regulated by shared promoter regions, and these hormones are concurrently released from islet cells' secretory granules in a consistent IAPP to insulin ratio of about 1:100. 7 , 13 Stimulants of this joint secretion include glucose, fatty acids and arginine, and during fasting, 7 and the plasma concentrations of IAPP in rats are between 3 and 5 picomolar, rising to approximately 20 picomolar after elevation in blood sugar level. 14 The metabolism of IAPP involves both renal excretion and proteolytic degradation, resulting in various metabolites. 11 Notably, a des‐Lys‐1 variant retains physiological activity, whereas other proteolytic byproducts are less likely to yield bioactive peptides. 15
Members of the CT peptide family interact with G‐protein‐coupled receptors that have seven transmembrane domains (Figure 2A). Although IAPP is a hormone, the identification of specific receptors for it proved elusive for a long time, despite the detection of specific binding sites, especially in the brain and renal cortex. 15 This hurdle was resolved with the discovery of three distinct receptor activity‐modifying proteins (RAMPs), which are single‐domain proteins. 16 These proteins are not receptors themselves; however, they form complexes with CT receptors and modify their ligand affinity 17 (Figure 2A). When a CT receptor binds to one of the RAMPs, it may alter the receptor's affinity, and certain combinations result in high‐affinity receptors for IAPP. 18 , 19 We also checked the IAPP interactions with other proteins using the STRING database (https://string‐db.org/) and found that IAPP binds to RAMPs and other proteins (Figure 2B). This mechanism, unique to the CT family, allows for the formation of three IAPP receptors. The roles of RAMPs are multifaceted; they are involved in the transport of receptor proteins to the cell surface, affect glycosylation, and modulation of signaling pathways. 16 , 20
FIGURE 2.

Illustration of the CT and IAPP receptor and reported and predicted protein interactions of hIAPP. (A) Receptor activity‐modifying protein 1–3 (RAMP1‐3) for complex with a CT receptor, a seven‐pass transmembrane receptor, making it IAPP receptor1‐3 (based on Hay et al., 2015 9 ). (B) Representative illustration of the protein–protein interaction network of hIAPP. The 10 most common protein interactions of hIAPP were generated using the STRING database (https://string‐db.org/). The number of lines represents the relative interaction.
IAPP research was initially focused on two primary roles: its function as a soluble, monomeric hormone that regulates glucose levels in the blood and its contribution to the formation of amyloid plaques that impair the pancreas leading to certain types of diabetes. 7 Recent studies have shown that IAPP plays an important role in gut–brain signaling. This review summarizes the IAPP physiology and its role in both the etiology of, and as a therapeutic target for metabolic and neurological disorders.
2. PHYSIOLOGICAL ROLES OF IAPP
IAPP is co‐secreted with insulin and plays a crucial role in regulating blood glucose levels, food intake, and body mass. 11 IAPP is known to affect satiation, inhibit glucagon secretion after meals, slow gastric emptying, and reduce digestive enzyme secretion via both peripheral and central mechanisms. 9 , 11 After its secretion after food intake, rat IAPP has a brief half‐life in plasma of about 13 min. 21 Originally thought to be produced only in the pancreas, it was subsequently discovered that IAPP is also synthesized in other areas of the gastrointestinal tract and spinal ganglia. 22 , 23 Recent research has further identified the presence of IAPP mRNA and protein in specific brain regions. 23 Notably, IAPP expression was found to increase significantly in the medial preoptic area of rats and was also prominently expressed alongside prodynorphin in the neurons of the lateral hypothalamic area. This latter finding indicated that IAPP mRNA levels varied significantly depending on factors such as sex, diet, and leptin levels. 23 , 24
The effect of IAPP on satiation is well‐researched, particularly through its activation of noradrenergic neurons in the glucose‐sensitive area postrema of the medulla oblongata. 23 Additionally, IAPP stimulation of neurons in area postrema results into neuroaxis activation and sends signals to forebrain including in the nucleus tractus solitarius and the lateral parabrachial nucleus and amygdala, which are key secondary sites for IAPP's action. These sites transmit signals to the lateral hypothalamus and other areas, influencing the sensation of satiation. 23 , 25 Experiments have shown that acute peripheral administration of IAPP significantly reduces food intake and thus bioenergetic status, an effect that is dose‐dependent and can be inhibited by IAPP receptor antagonists. 7 , 11 Furthermore, IAPP, directly and indirectly, suppresses glucagon secretion from pancreatic α‐cells, 26 , 27 reduces hepatic glucose release after eating, and slows gastric emptying and pancreatic enzyme secretion to lower peak blood glucose levels. 11
The concept of “homeostatic eating control” differentiates between two types of signals: adiposity (“tonic”) signals, which are related to long‐term body fat regulation, and meal‐associated (“episodic”) signals that respond to food intake. 28 IAPP has been found to potentially enhance leptin sensitivity in obesity, a condition where leptin, a hormone signaling triglyceride from adipose tissue, often becomes less effective. Experiments in rats have shown that combined administration of leptin and IAPP can lead to weight loss, and diminishes obesity. 29 , 30 Additionally, IAPP has been observed to increase energy expenditure in animal studies. 31 , 32
3. IAPP IN DISEASE STATES
Beyond its function in energy and glucose regulation, hIAPP has the propensity to self‐aggregate and is crucial in the development of various diseases. 12 , 28 In its monomeric form, IAPP is soluble and unfolded; however, it can form oligomers that exhibit a misfolded α‐helix structure, which can form into β‐sheet‐rich fibrils (Figure 3A–C). These fibrils form amyloid deposits that can be toxic in tissues where IAPP is expressed. 11 , 12 Amyloid refers to a particular state of protein aggregation characterized by molecules arranged in a β‐sheet conformation. These molecules are primarily linked through hydrogen bonds, among other types of bonds. 33 , 34 This aggregation forms thin, stable fibrils approximately 10 nanometers in width, with β‐strands aligned perpendicular to the fibril axis known as IAPP‐β amyloid (Aβ). In humans, over 25 proteins are known to form amyloid fibrils, and it is expected that more will be identified in future research. 35 , 36 IAPP amyloid formation occurs exclusively in the islets of pancreas of humans, cats, and primates but it does not occur in rodents. This is attributed to the presence of an amyloidogenic region within the IAPP amino acid sequence of these animals, which is critical for the formation and toxicity of the amyloids. 36 , 37 , 38 , 39 Below, we will discuss the role of IAPP in the pathogenesis of various disease conditions.
FIGURE 3.
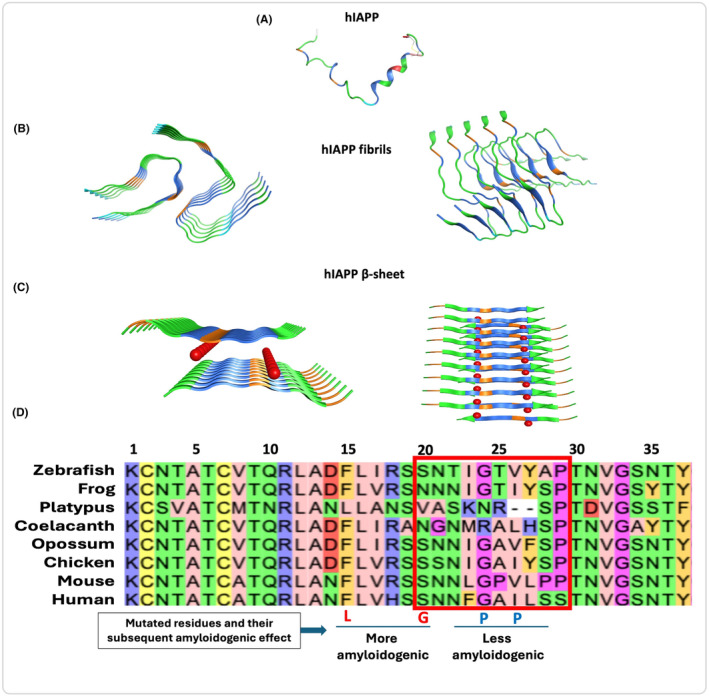
3D illustration of the hIAPP aggregate formation and multiple sequence alignment for orthologues of the IAPP protein: (A) Mature hIAPP as a monomer (PDB: 5MGQ). (B) Amyloid fibrils formation showing hIAPP aggregates without any solvent interactions in between (PDB: 7 M61). (C) β‐sheets showing hIAPP stacked with each other and having water interactions (Red balls) apart from their hydrogen bonds (PDB: 5KNZ). (D) Sequence alignment of the IAPP protein showing conservation of the cysteine 2 and 7 across vertebrates and invertebrates (based on Jeong et al., 2015 41 ). Sequences shown are for human (Uniprot: P10997), mouse(Uniprot: P12968), chicken (Uniprot: A0A8V0YAM5), opossum (Uniprot: A0A5F8H6U1), coelacanth (Uniprot: H3AWW1), platypus (Uniprot: A0A6I8PDA3), frog (Uniprot: A0A1L8GQE1), and zebrafish (Uniprot: A0A286YA88). F15L and S20G are highly amyloidogenic while the G24P and I16P mutations have less amyloidogenicity as compared to wild type hIAPP.
IAPP has an amyloidogenic core region between residues 20 and 29 (Figure 3D). We performed sequence alignment for orthologues of the IAPP protein showing conservation of the cysteine 2 and 7 that form a disulfide bridge. Also, the N‐terminal and C‐terminal residues are mostly conserved as compared to the amyloidogenic region. Interestingly, the rodent residues 24, 25,26, 28, and 29 are different from the hIAPP making it non‐amyloidogenic. 40 , 41 , 42 Furthermore, F15L and S20G mutations make it more amyloidogenic, on the contrary, G24P and I26P make it less amyloidogenic as compared to the wild‐type IAPP. 43 , 44 The reported mutations Q43R and S53G in pre‐pro‐hIAPP or Q10R and S20G in hIAPP are associated with diabetes based on the HGMD database (https://www.hgmd.cf.ac.uk/).
3.1. IAPP IN METABOLIC DISORDERS
3.1.1. Diabetes mellitus
The pathogenesis of diabetes mellitus (DM) is a complex multifactorial condition currently under intense ongoing research. 45 , 46 Two primary factors contribute to this disease; insulin resistance which leads to its reduced effectiveness and higher demand 47 , 48 and ultimately causes type 2 diabetes mellitus (T2DM), and pancreatic β‐cell failure, characterized by a reduction in both β‐cell mass and function 48 and leads to type 1 diabetes mellitus (T1DM). T1DM is in fact a state of hypoamylinemia similar to hypoinsulinemia. 7 IAPP is the major pathological hallmark of T2DM which we will discuss in detail later. Studies on the function of islets from individuals with T2DM are limited, but findings suggest that these islets show reduced insulin secretion compared to those from healthy controls and do not normalize glucose levels post‐transplantation; however, their glucagon response remains normal. 49
In DM, increased demands on the pancreatic β‐cells lead to elevated secretion of both insulin and IAPP, with IAPP levels notably higher during fasting (40 pM) and after meals (90 pM). As the disease progresses, IAPP secretion diminishes due to β‐cell exhaustion. 21 , 27 This persistent elevation in IAPP production can cause processing abnormalities, leading to amyloid deposition both intracellularly and extracellularly as the disease advances and β‐cells deteriorate. 28 Indeed, amyloid is detected in over 90% of T2DM pancreas, the cytotoxicity of hIAPP aggregates, believed to contribute to β‐cell loss, is linked to mechanisms such as overload of endoplasmic reticulum correction mechanisms, disruption of intracellular transport, damage to organelle membranes (e.g., mitochondria), and activation of inflammasomes. 11 , 28
Moreover, IAPP is considered to be amyloidogenic and has been shown to interact with lipid membranes in pathogenic metabolic pathways. 50 Genetic mutations such as S20G (Figure 3D) in the human IAPP (hIAPP) gene are associated with early onset or a more severe T2DM. It also increases hIAPP hydrophobicity and amyloidogenic properties and enhances its fibrillogenic potential. 51 , 52 This mutation results in information of amyloid fibrils at around twice the rate of the wild‐type protein leading to significantly higher aggregation and higher cytotoxicity. Aromatic amino acid residue F15 in IAPP is hydrophobic and plays a crucial role in the amyloidogenic pathway. 42 A study highlighted that an F15L mutation alters the protein's α‐helix and β‐sheet structures leading to rapid Aβ formation. 53
Due to the natural tendency of hIAPP to aggregate, researchers studied an analog that could mimic IAPP's physiological functions without its inherent aggregation properties. Pramlintide emerged as the leading candidate and was patented in 1997. Marketed as Symlin®, it became the first peptide‐based antidiabetic drug since insulin's discovery in 1921. 54 , 55 , 56 , 57 While no other IAPP‐based drugs have been approved since then, several new analogs are showing promise as potential treatments for obesity and diabetes. 7
3.1.2. Obesity
In obesity, there is a well‐documented tendency for resistance to develop against the appetite‐suppressing effects of both leptin and insulin as well as hormones related to food signaling like CCK and GLP‐1. 58 , 59 This leptin resistance is often shown by a decrease in the activation of phosphorylated transducer and activator of transcription‐3 (pSTAT3). pSTAT3 helps in mediating the leptin regulatory effects on eating. 60 The reduction in pSTAT3 activity within leptin‐sensitive neurons is associated with a reduced inhibitory effect on eating. Additionally, obesity may also hinder the transport of insulin and leptin into the brain by compromising the integrity of the blood–brain barrier. 61 Furthermore, an increase in leptin levels, which is a consequence of reduced sensitivity to the hormone in obesity, tends to further diminish the effectiveness of leptin's action on appetite regulation. 62
In studies involving rodents, IAPP treatment has been shown to exert a significant reduction in body weight relative to control vehicle‐administered animals. Although IAPP‐treated rodents might still gain some absolute body weight, notwithstanding less than the controls. 9 , 63 These findings may raise concerns about the clinical relevance of IAPP's effects. However, it is important to note a fundamental difference between these animal models and humans; unlike humans, most strains of rodents tend to gain weight continuously throughout their lives. 9
3.1.3. Gestational diabetes mellitus
Gestational diabetes mellitus (GDM) is associated with pregnancy and the inability to produce enough insulin to overcome pregnancy‐induced insulin resistance leading to hyperglycemia. While IAPP levels normally rise during pregnancy, GDM does not elevate the IAPP secretion. 64 Studies have shown that mid‐gestation rats respond normally to IAPP‐induced satiety which suggests IAPP resistance does not develop during pregnancy. 65 , 66 This indicates that IAPP changes may not contribute to GDM pathogenesis. However, a recent study using pregnant transgenic mice expressing hIAPP displayed GDM‐like symptoms, proposing that hIAPP aggregation could potentially influence GDM or increase the risk of type 2 diabetes post‐pregnancy. 67 This suggests a new rodent model for studying GDM and indicates a possible role for hIAPP aggregation in pregnant women. A GDM model in which the hIAPP is aggregated or factors that leads to hIAPP aggregations will provide more knowledge and a possible target for GDM therapeutics.
3.1.4.
3.1.4.1. Bone metabolism disorders
It has been reported that IAPP may affect bone formation and resorption, 68 and aberrant IAPP and adiponectin serum levels have been associated with bone metabolism disorders. 69 Furthermore, IAPP inactivation in CTR receptor‐deficient rodents displayed abnormalities in bone, suggesting that IAPP regulates bone metabolism. 68
3.1.4.2. Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is a common endocrine disorder of women with a prevalence of 8%–13%. 70 PCOS is associated with reproductive, metabolic, and psychological features. 70 PCOS is associated with high IAPP secretion, and hyperamylasemia may be associated with the development of metabolic disorders in women with PCOS. 71
3.1.4.3. Cardiac dysfunction
Effects of IAPP on the cardiovascular system are primarily thought to be mediated through the activation of CGRP receptors, which are abundantly expressed in the vasculature. 72 These effects are considered largely pharmacological, as they require high plasma concentrations of IAPP to be observed and an intravenous injection of IAPP results in significant vasodilation that lowers arterial blood pressure along with transient increases in heart rate. 73 These vascular effects are likely mediated by CGRP receptors in the heart 74 ; however, a higher plasma concentration of IAPP is necessary to cause these effects as these receptors have low potency. Moreover, studies have revealed a 25‐fold increase in IAPP expression in the preoptic area of the hypothalamus in lactating rats. 23 , 75 , 76
It has been reported that hyperamylinemia contributes to the cardiac dysfunction in obese patients and rats expressing hIAPP. Hyperamylinemia leads to IAPP deposition in the heart, resulting in structural and functional changes in cardiac myocytes through transient Ca2+ followed by Ca2+‐dependent Ca2+/calmodulin‐dependent protein kinase II (CaMKII) pathway. 77
This research provides comprehensive insights into the multifactorial pathogenesis of DM, highlighting the roles of insulin resistance, pancreatic β‐cell failure, and IAPP amyloid accumulation, particularly in T2DM. It underscores the importance of IAPP and its analogs like pramlintide in therapeutic applications, with promising results shown in obesity and GDM. Additionally, the study touches on the potential of IAPP in addressing bone metabolism disorders and PCOS, suggesting new avenues for treatment and further research. On the contrary, these findings rely heavily on animal model studies, which may not fully translate to human conditions. The complexity of DM pathogenesis and the role of IAPP requires further research to clarify mechanisms and therapeutic efficacy in humans. The potential side effects and long‐term impacts of IAPP‐based treatments remain underexplored. Further, the link between hIAPP aggregation and metabolic conditions like GDM and PCOS needs more robust evidence to establish causality and therapeutic relevance.
3.2. IAPP IN NEUROLOGICAL DISORDERS
3.2.1. Alzheimer's disease
Metabolic disorders and aging can lead to the cerebrovascular accumulation of IAPP amyloid, also called the second amyloid in Alzheimer's disease (AD), which affects the cerebral blood vessels and alters tissue structure. Additionally, the formation of IAPP amyloid in the walls of cerebral blood vessels may hinder the removal of Aβ from the brain, thereby playing a role in the development of AD. 78
Recent studies indicate potential therapeutic benefits of IAPP and its analog pramlintide in AD 79 , 80 are often linked with metabolic disorders such as T2DM. 28 Notably, individuals with AD and mild cognitive impairment exhibit lower IAPP levels compared to those without cognitive deficits. 79 This observation prompted research into the effects of chronic pramlintide infusions in rodent models of AD. In the senescence‐accelerated prone mouse, a model for sporadic AD, pramlintide treatment improved memory and cognition, evidenced by enhanced performance in the novel object recognition task. This improvement was linked with increased synaptic markers and reduced neuroinflammation and oxidative stress markers in the hippocampus. 79 Similarly, treatment with IAPP and pramlintide, an IAPP analog, in a transgenic AD model enhanced learning and memory in the Y‐maze and Morris water maze tests, along with a decrease in amyloid burden and brain Aβ levels. These findings suggest that IAPP and pramlintide may promote the clearance of Aβ from the brain to the blood, possibly via effects on cerebral vasculature. 4 Such results point to IAPP and pramlintide as promising candidates for AD treatment, although further research is necessary to further examine their roles in the disease.
3.2.2. Parkinson's disease
The link between DM and Parkinson's disease (PD) risk is still much debated. Population‐based cohort studies have suggested an increased risk, while case–control studies often show no elevated risk, underscoring the need for further research to clarify this relationship. 81 , 82 Sanchez‐Gomez et al. 83 reported a study cohort consisting of 76 patients with PD and 39 control subjects and found no significant differences in fasting glucose, glycated hemoglobin, or HOMA‐IR scores between the PD patients and control group. However, the hIAPP/insulin ratio was higher in PD patients, and the plasma level of hIAPP was higher in older patients. A study has reported that Enterobacteriaceae, prevalent in the gut, can produce functional amyloid proteins known as Curli. These Curli proteins contribute to the formation of α‐synuclein (αSyn) in both the gut and the brain. The expression of Curli is necessary for E. coli to worsen αSyn‐induced behavioral impairments, including impairments in the intestines and motor functions. 84 Another in vitro study has revealed that pro‐IAPP amyloids hinder the formation of αSyn, while IAPP amyloid enhances αSyn aggregate formation. Conversely, αSyn aggregates promote amyloid formation by pro‐IAPP but inhibit IAPP amyloid formation. Additionally, when hIAPP and αSyn monomers are mixed, they co‐aggregate faster than either protein alone. This network of interactions indicates that the presence of IAPP can accelerate αSyn amyloid formation, which may explain the higher susceptibility of T2DM patients to developing PD. 85
3.2.3. Cerebrovascular dementia
Vascular dementia (VD), like AD, is a prominent cause of dementia. 86 The pathology of VD involves both structural and functional changes to blood vessels, impacting oxygen and nutrient delivery to the brain. 87 Risk factors for VD include hypertension, diabetes, obesity, and smoking, with VD and cognitive decline progressively seen as complications of diabetes. 88 Individuals with T2DM or prediabetics still face up to a 2‐fold higher risk of developing VD and dementia compared to those without metabolic disorders. 86 Studies recently indicated that hIAPP aggregates in the small vessels of the brains of patients with dementia and T2DM, mixed IAPP‐β amyloid (Aβ) plaques in the brains of patients exhibiting pathological signs of AD. These findings suggest that imbalances in IAPP could play a pathogenic role in the onset and progression of VD. 78 , 89 , 90
3.2.4. Depression
A study has reported that Aβ‐induced depression is mediated through the CTR receptor, and its inhibition might enhance this phenomenon. 91 Another study reported that AC187, a potent IAPP receptor antagonist, substantially abrogated the antidepressant effects of sCT (salmon calcitonin). This suggests that sCT likely alleviates depressive‐like symptoms through the IAPP signaling pathway. Based on these observations, sCT could be viewed as a promising therapeutic option for treating depressive disorders in future studies. 92
3.2.5. Alcohol use disorder (AUD)
Alcohol use disorder (AUD) is a widespread chronic condition with significant health, psychological, economic, and social impacts. IAPP, primarily produced alongside insulin after eating, is known to curb hedonic eating and enhance satiety, and is found in the cerebrospinal fluid. 90 Research in rodents indicates that IAPP might also influence the reward responses associated with substances like alcohol. sCT has been shown to decrease food intake by acting on central IAPP receptors. Studies using male mice have demonstrated that intraperitoneal injections of sCT can prevent the dopamine release typically triggered by alcohol. AM1213, a more selective AMYR agonist than sCT, was administered daily to both female and male rats and resulted in a reduction in alcohol consumption, although this effect was only observed during the first 2 days of treatment. 92 , 93 , 94 , 95
3.3. Epilepsy
A study investigated the serum IAPP concentration in epilepsy. The study comprised 45 epileptic patients and 60 control subjects. They found a statistically significant difference in IAPP serum level, with serum IAPP levels for epileptic patients higher than those of the control subjects. The pathological effect of Aβ aggregates is based on the toxic soluble oligomers that disrupt Ca2+ homeostasis and compromise the viability of various cells, including astrocytes and neurons. 96
This highlights the potential therapeutic benefits of IAPP and its analog pramlintide in AD, PD, VD, depression, and AUD. IAPP in animal models show improvements in memory, cognition, and reduction in pathological protein aggregates, and may play a role in mitigating depressive symptoms and reducing alcohol consumption. On the other hand, these findings are largely based on animal models, with limited human data available. The mechanisms through which IAPP and pramlintide exert their beneficial effects are complex and not fully understood, and require further research. Additionally, the link between diabetes, metabolic disorders, and neurodegenerative diseases such as AD and PD remains much debated and requires more research.
4. OUTLOOK
IAPP, a neuroendocrine hormone that is co‐secreted with insulin, shares sequence similarities with other CT family peptides and affects glucose levels and satiety. IAPP and insulin are regulated by shared promoter regions and released at a consistent ratio, with IAPP levels rising after food intake. The discovery of RAMPs has advanced the understanding of IAPP. FDA‐approved pramlintide, an IAPP analog with no aggregation properties, was approved for the regulation of hyperglycemia and the treatment of T2DM. However, many more IAPP analogs must be further explored for their therapeutic potential. There is a need for research on IAPP analogs that are long‐acting as compared to pramlintide. Cagrilintide, a long‐acting IAPP agonist, along with semaglutide (CariSema) is being tested for its anti‐T2DM and anti‐obesity potentials. An important area of in‐depth research would be the use of AD rodent models expressing hIAPP, which has amyloidogenic properties as compared to the rodent IAPP. Moreover, hIAPP overexpressing AD, PD, CD, and other neurological mouse models are likely to provide a ,much useful information, and are potential therapeutic pre‐clinical models for these disease conditions.
AUTHOR CONTRIBUTIONS
Tahir Muhammad wrote the preliminary manuscript and prepared figures, Stephen F. Pastore, Katrina Good and Wai Haung Yu reviewed and edited the manuscript. John B. Vincent reviewed, edited and supervised the final draft.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest.
ACKNOWLEDGMENTS
We would like to acknowledge Dr. Stefan Kloiber, Dr. Daniel Mueller, and Dr. Victor Tang at the Centre for Addiction and Mental Health (CAMH) for their help in conceiving the idea and providing their valuable input on the abstract in the preliminary draft.
Muhammad T, Pastore SF, Good K, Yu WH, Vincent JB. The role of amylin, a gut–brain axis hormone, in metabolic and neurological disorders. FASEB BioAdvances. 2025;7:e1480. doi: 10.1096/fba.2024-00151
DATA AVAILABILITY STATEMENT
This review article presents no new data.
REFERENCES
- 1. Opie EL. The relation of diabetes mellitus to lesions of the pancreas. Hyaline degeneration of he islands of Langerhans. J Exp Med. 1901;5(5):527‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahronheim JH. The nature of the hyaline material in the pancreatic islands in diabetes mellitus. Am J Pathol. 1943;19(5):873‐882. [PMC free article] [PubMed] [Google Scholar]
- 3. Ehrlich JC, Ratner IM. Amyloidosis of the islets of Langerhans. Am J Pathol. 1961;38(1):49‐59. [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Y, Song W. Molecular links between Alzheimer's disease and diabetes mellitus. Neuroscience. 2013;250:140‐150. doi: 10.1016/j.neuroscience.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 5. Yano BL, Hayden DW, Johnson KH. Feline insular amyloid: incidence in adult cats with no clinicopathologic evidence of overt diabetes mellitus. Vet Pathol. 1981;18(3):310‐315. doi: 10.1177/030098588101800303 [DOI] [PubMed] [Google Scholar]
- 6. Westermark P, Wernstedt C, Wilander E, Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986;140(3):827‐831. doi: 10.1016/0006-291X(86)90708-4 [DOI] [PubMed] [Google Scholar]
- 7. Boyle CN, Zheng Y, Lutz TA. Mediators of amylin action in metabolic control. J Clin Med. 2022;11(8):2207. doi: 10.3390/jcm11082207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boccia L, Gamakharia S, Coester B, Whiting L, Lutz TA, Le Foll C. Amylin brain circuitry. Peptides. 2020;132:170366. doi: 10.1016/j.peptides.2020.170366 [DOI] [PubMed] [Google Scholar]
- 9. Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: pharmacology, physiology, and clinical potential. Pharmacol Rev. 2015;67(3):564‐600. doi: 10.1124/pr.115.010629 [DOI] [PubMed] [Google Scholar]
- 10. Press M, Jung T, König J, Grune T, Höhn A. Protein aggregates and proteostasis in aging: amylin and β‐cell function. Mech Ageing Dev. 2019;177:46‐54. doi: 10.1016/j.mad.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 11. Eržen S, Tonin G, Jurišić Eržen D, Klen J. Amylin, another important neuroendocrine hormone for the treatment of diabesity. Int J Mol Sci. 2024;25(3):1517. doi: 10.3390/ijms25031517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lutz TA. Creating the amylin story. Appetite. 2022;172:105965. doi: 10.1016/j.appet.2022.105965 [DOI] [PubMed] [Google Scholar]
- 13. Vardanyan R, Hruby V. Hyperglycemic and Hypoglycemic Drugs. Synthesis of Best‐Seller Drugs. Elsevier; 2016:419–458. doi: 10.1016/B978-0-12-411492-0.00026-2 [DOI] [Google Scholar]
- 14. Abedini A, Schmidt AM. Mechanisms of islet amyloidosis toxicity in type 2 diabetes. FEBS Lett. 2013;587(8):1119‐1127. doi: 10.1016/j.febslet.2013.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91(3):795‐826. doi: 10.1152/physrev.00042.2009 [DOI] [PubMed] [Google Scholar]
- 16. McLatchie LM, Fraser NJ, Main MJ, et al. RAMPs regulate the transport and ligand specificity of the calcitonin‐receptor‐like receptor. Nature. 1998;393(6683):333‐339. doi: 10.1038/30666 [DOI] [PubMed] [Google Scholar]
- 17. Young A. Receptor pharmacology. Advances in Pharmacology. Vol 52. Elsevier; 2005. 47–65. https://linkinghub.elsevier.com/retrieve/pii/S1054358905520039 [DOI] [PubMed] [Google Scholar]
- 18. Poyner DR, Sexton PM, Marshall I, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene‐related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54(2):233‐246. doi: 10.1124/pr.54.2.233 [DOI] [PubMed] [Google Scholar]
- 19. Muff R, Buhlmann N, Fischer JA, Born W. An amylin receptor is revealed following co‐transfection of a calcitonin receptor with receptor activity modifying proteins‐1 or ‐3. Endocrinology. 1999;140(6):2924‐2927. doi: 10.1210/endo.140.6.6930 [DOI] [PubMed] [Google Scholar]
- 20. Morfis M, Tilakaratne N, Furness SGB, et al. Receptor activity‐modifying proteins differentially modulate the G protein‐coupling efficiency of amylin receptors. Endocrinology. 2008;149(11):5423‐5431. doi: 10.1210/en.2007-1735 [DOI] [PubMed] [Google Scholar]
- 21. Pittner RA, Albrandt K, Beaumont K, et al. Molecular physiology of amylin. J Cell Biochem. 1994;55(S1994A):19‐28. doi: 10.1002/jcb.240550004 [DOI] [PubMed] [Google Scholar]
- 22. Ferrier GJ, Pierson AM, Jones PM, Bloom SR, Girgis SI, Legon S. Expression of the rat amylin (IAPP/DAP) gene. J Mol Endocrinol. 1989;3(1):R1‐R4. doi: 10.1677/jme.0.003r001 [DOI] [PubMed] [Google Scholar]
- 23. Dobolyi A. Central amylin expression and its induction in rat dams. J Neurochem. 2009;111(6):1490‐1500. doi: 10.1111/j.1471-4159.2009.06422.x [DOI] [PubMed] [Google Scholar]
- 24. Li Z, Kelly L, Gergi I, et al. Hypothalamic amylin acts in concert with leptin to regulate food intake. Cell Metab. 2015;22(6):1059‐1067. doi: 10.1016/j.cmet.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 25. Abegg K, Hermann A, Boyle CN, Bouret SG, Lutz TA, Riediger T. Involvement of amylin and leptin in the development of projections from the area Postrema to the nucleus of the solitary tract. Front Endocrinol. 2017;8:324. doi: 10.3389/fendo.2017.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gedulin BR, Rink TJ, Young AA. Dose‐response for glucagonostatic effect of amylin in rats. Metabolism. 1997;46(1):67‐70. doi: 10.1016/S0026-0495(97)90170-0 [DOI] [PubMed] [Google Scholar]
- 27. Scherbaum WA. The role of amylin in the physiology of glycemic control. Exp Clin Endocrinol Diabetes. 1998;106(2):97‐102. doi: 10.1055/s-0029-1211958 [DOI] [PubMed] [Google Scholar]
- 28. Lutz TA, Meyer U. Amylin at the interface between metabolic and neurodegenerative disorders. Front Neurosci. 2015;9:216. doi: 10.3389/fnins.2015.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roth JD, Roland BL, Cole RL, et al. Leptin responsiveness restored by amylin agonism in diet‐induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci. 2008;105(20):7257‐7262. doi: 10.1073/pnas.0706473105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trevaskis JL, Wittmer C, Athanacio J, Griffin PS, Parkes DG, Roth JD. Amylin/leptin synergy is absent in extreme obesity and not restored by calorie restriction‐induced weight loss in rats. Obes Sci Pract. 2016;2(4):385‐391. doi: 10.1002/osp4.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wielinga PY, Lowenstein C, Muff S, Munz M, Woods SC, Lutz TA. Central amylin acts as an adiposity signal to control body weight and energy expenditure. Physiol Behav. 2010;101(1):45‐52. doi: 10.1016/j.physbeh.2010.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lutz TA. Control of energy homeostasis by amylin. Cell Mol Life Sci. 2012;69(12):1947‐1965. doi: 10.1007/s00018-011-0905-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rochet JC, Lansbury PT. Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol. 2000;10(1):60‐68. doi: 10.1016/S0959-440X(99)00049-4 [DOI] [PubMed] [Google Scholar]
- 34. Westermark P, Benson MD, Buxbaum JN, et al. A primer of amyloid nomenclature. Amyloid. 2007;14(3):179‐183. doi: 10.1080/13506120701460923 [DOI] [PubMed] [Google Scholar]
- 35. Westermark P. Aspects on human amyloid forms and their fibril polypeptides. FEBS J. 2005;272(23):5942‐5949. doi: 10.1111/j.1742-4658.2005.05024.x [DOI] [PubMed] [Google Scholar]
- 36. Betsholtz C, Christmansson L, Engström U, et al. Sequence divergence in a specific region of islet amyloid polypeptide (IAPP) explains differences in islet amyloid formation between species. FEBS Lett. 1989;251(1–2):261‐264. doi: 10.1016/0014-5793(89)81467-X [DOI] [PubMed] [Google Scholar]
- 37. Höppener JWM, Jansz HS, Oosterwijk C, et al. Molecular physiology of the islet amyloid polypeptide (IAPP)/amylin gene in man, rat, and transgenic mice. J Cell Biochem. 1994;55(S1994A):39‐53. doi: 10.1002/jcb.240550006 [DOI] [PubMed] [Google Scholar]
- 38. Moriarty DF, Raleigh DP. Effects of sequential proline substitutions on amyloid formation by human amylin20‐29. Biochemistry. 1999;38(6):1811‐1818. doi: 10.1021/bi981658g [DOI] [PubMed] [Google Scholar]
- 39. Matveyenko AV, Butler PC. Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes. ILAR J. 2006;47(3):225‐233. doi: 10.1093/ilar.47.3.225 [DOI] [PubMed] [Google Scholar]
- 40. Chuang CL, Hay DL. Amylin. In: Offermanns S, Rosenthal W, eds. Encyclopedia of Molecular Pharmacology. Springer International Publishing; 2021:97–102. doi: 10.1007/978-3-030-57401-7_10021 [DOI] [Google Scholar]
- 41. Jeong HR, An SSA. Causative factors for formation of toxic islet amyloid polypeptide oligomer in type 2 diabetes mellitus. Clin Interv Aging. 2015;10:1873‐1879. doi: 10.2147/CIA.S95297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marek P, Abedini A, Song B, et al. Aromatic interactions are not required for amyloid fibril formation by islet amyloid polypeptide but do influence the rate of fibril formation and fibril morphology. Biochemistry. 2007;46(11):3255‐3261. doi: 10.1021/bi0621967 [DOI] [PubMed] [Google Scholar]
- 43. Abedini A, Meng F, Raleigh DP. A single‐point mutation converts the highly Amyloidogenic human islet amyloid polypeptide into a potent fibrillization inhibitor. J Am Chem Soc. 2007;129(37):11300‐11301. doi: 10.1021/ja072157y [DOI] [PubMed] [Google Scholar]
- 44. Miller C, Zerze GH, Mittal J. Molecular simulations indicate marked differences in the structure of amylin mutants, correlated with known aggregation propensity. J Phys Chem B. 2013;117(50):16066‐16075. doi: 10.1021/jp409755y [DOI] [PubMed] [Google Scholar]
- 45. Federici M, Hribal M, Perego L, et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50(6):1290‐1301. doi: 10.2337/diabetes.50.6.1290 [DOI] [PubMed] [Google Scholar]
- 46. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85‐96. doi: 10.1038/nrm1837 [DOI] [PubMed] [Google Scholar]
- 47. Kahn SE. The relative contributions of insulin resistance and beta‐cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46(1):3‐19. doi: 10.1007/s00125-002-1009-0 [DOI] [PubMed] [Google Scholar]
- 48. Kahn SE. The importance of the β‐cell in the pathogenesis of type 2 diabetes mellitus1. Am J Med. 2000;108(6, Supplement 1):2‐8. doi: 10.1016/S0002-9343(00)00336-3 [DOI] [PubMed] [Google Scholar]
- 49. Deng S, Vatamaniuk M, Huang X, et al. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes. 2004;53(3):624‐632. doi: 10.2337/diabetes.53.3.624 [DOI] [PubMed] [Google Scholar]
- 50. Jayasinghe SA, Langen R. Lipid membranes modulate the structure of islet amyloid polypeptide. Biochemistry. 2005;44(36):12113‐12119. doi: 10.1021/bi050840w [DOI] [PubMed] [Google Scholar]
- 51. Sakagashira S, Sanke T, Hanabusa T, et al. Missense mutation of amylin gene (S20G) in Japanese NIDDM patients. Diabetes. 1996;45(9):1279‐1281. doi: 10.2337/diab.45.9.1279 [DOI] [PubMed] [Google Scholar]
- 52. Sakagashira S, Hiddinga HJ, Tateishi K, et al. S20G mutant amylin exhibits increased in vitro Amyloidogenicity and increased intracellular cytotoxicity compared to wild‐type amylin. Am J Pathol. 2000;157(6):2101‐2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tu LH, Raleigh DP. Role of aromatic interactions in amyloid formation by islet amyloid polypeptide. Biochemistry. 2013;52(2):333‐342. doi: 10.1021/bi3014278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dehestani B, Stratford NR, le Roux CW. Amylin as a future obesity treatment. J Obes Metab Syndr. 2021;30(4):320‐325. doi: 10.7570/jomes21071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mathiesen DS, Bagger JI, Knop FK. Long‐acting amylin analogues for the management of obesity. Curr Opin Endocrinol Diabetes Obes. 2022;29(2):183‐190. doi: 10.1097/MED.0000000000000716 [DOI] [PubMed] [Google Scholar]
- 56. Larsen AT, Gydesen S, Sonne N, Karsdal MA, Henriksen K. The dual amylin and calcitonin receptor agonist KBP‐089 and the GLP‐1 receptor agonist liraglutide act complimentarily on body weight reduction and metabolic profile. BMC Endocr Disord. 2021;21(1):10. doi: 10.1186/s12902-020-00678-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sonne N, Larsen AT, Andreassen KV, Karsdal MA, Henriksen K. The dual amylin and calcitonin receptor agonist, KBP‐066, induces an equally potent weight loss across a broad dose range while higher doses may further improve insulin action. J Pharmacol Exp Ther. 2020;373(1):92‐102. doi: 10.1124/jpet.119.263723 [DOI] [PubMed] [Google Scholar]
- 58. Zhou Y, Rui L. Leptin signaling and leptin resistance. Front Med. 2013;7(2):207‐222. doi: 10.1007/s11684-013-0263-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Deacon CF, Ahrén B. Physiology of incretins in health and disease. Rev Diabet Stud. 2011;8(3):293‐306. doi: 10.1900/RDS.2011.8.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wauman J, Tavernier J. Leptin receptor signaling: pathways to leptin resistance. Front Biosci (Landmark Ed). 2011;16(7):2771‐2793. doi: 10.2741/3885 [DOI] [PubMed] [Google Scholar]
- 61. Banks WA. The blood‐brain barrier as a regulatory interface in the gut‐brain axes. Physiol Behav. 2006;89(4):472‐476. doi: 10.1016/j.physbeh.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 62. Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav. 2006;88(3):249‐256. doi: 10.1016/j.physbeh.2006.05.038 [DOI] [PubMed] [Google Scholar]
- 63. Roth JD, Hughes H, Kendall E, Baron AD, Anderson CM. Antiobesity effects of the β‐cell hormone amylin in diet‐induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology. 2006;147(12):5855‐5864. doi: 10.1210/en.2006-0393 [DOI] [PubMed] [Google Scholar]
- 64. Kautzky‐Willer A, Thomaseth K, Ludvik B, et al. Elevated islet amyloid pancreatic polypeptide and proinsulin in lean gestational diabetes. Diabetes. 1997;46(4):607‐614. doi: 10.2337/diab.46.4.607 [DOI] [PubMed] [Google Scholar]
- 65. Boyle CN, Le Foll C. Amylin and leptin interaction: role during pregnancy, lactation and neonatal development. Neuroscience. 2020;447:136‐147. doi: 10.1016/j.neuroscience.2019.11.034 [DOI] [PubMed] [Google Scholar]
- 66. Leuthardt AS, Bayer J, Monné Rodríguez JM, Boyle CN. Influence of high energy diet and polygenic predisposition for obesity on postpartum health in rat dams. Front Physiol. 2022;12:772707. doi: 10.3389/fphys.2021.772707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gurlo T, Kim S, Butler AE, et al. Pregnancy in human IAPP transgenic mice recapitulates beta cell stress in type 2 diabetes. Diabetologia. 2019;62(6):1000‐1010. doi: 10.1007/s00125-019-4843-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dacquin R, Davey RA, Laplace C, et al. Amylin inhibits bone resorption while the calcitonin receptor controls bone formation in vivo. J Cell Biol. 2004;164(4):509‐514. doi: 10.1083/jcb.200312135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang XJ, Bai X, Miu Y, Chen P, Yan PJ, Jiang CX. The assessment value of pathological condition of serum adiponectin and amylin in primary osteoporosis and its correlation analysis with bone metabolism indexes. J Med Biochem. 2023;42(1):86‐93. doi: 10.5937/jomb0-35877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nguo K, McGowan M, Cowan S, et al. Exploring the physiological factors relating to energy balance in women with polycystic ovary syndrome: a scoping review. Nutr Rev. 2024;nuad169. doi: 10.1093/nutrit/nuad169 [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. James S, Moralez J, Nagamani M. Increased secretion of amylin in women with polycystic ovary syndrome. Fertil Steril. 2010;94(1):211‐215. doi: 10.1016/j.fertnstert.2009.02.086 [DOI] [PubMed] [Google Scholar]
- 72. Russo AF. Calcitonin gene‐related peptide (CGRP): A new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533‐552. doi: 10.1146/annurev-pharmtox-010814-124701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bhavsar S, Watkins J, Young A. Synergy between amylin and cholecystokinin for inhibition of food intake in mice. Physiol Behav. 1998;64(4):557‐561. doi: 10.1016/S0031-9384(98)00110-3 [DOI] [PubMed] [Google Scholar]
- 74. Kaygisiz Z, Ozden H, Erkasap N, et al. Positive inotropic, positive chronotropic and coronary vasodilatory effects of rat amylin: mechanisms of amylin‐induced positive inotropy. Acta Physiol Hung. 2010;97(4):362‐374. doi: 10.1556/aphysiol.97.2010.4.2 [DOI] [PubMed] [Google Scholar]
- 75. Bell D, McDermott BJ. Activity of amylin at CGRP1‐preferring receptors coupled to positive contractile response in rat ventricular cardiomyocytes. Regul Pept. 1995;60(2):125‐133. doi: 10.1016/0167-0115(95)00120-4 [DOI] [PubMed] [Google Scholar]
- 76. Young A. Cardiovascular effects. Adv Pharmacol. 2005;52:239‐250. [DOI] [PubMed] [Google Scholar]
- 77. Despa S, Margulies KB, Chen L, et al. Hyperamylinemia contributes to cardiac dysfunction in obesity and diabetes. Circ Res. 2012;110(4):598‐608. doi: 10.1161/CIRCRESAHA.111.258285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jackson K, Barisone GA, Diaz E, Jin LW, DeCarli C, Despa F. Amylin deposition in the brain: a second amyloid in Alzheimer disease? Ann Neurol. 2013;74(4):517‐526. doi: 10.1002/ana.23956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Adler BL, Yarchoan M, Hwang HM, et al. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer's disease pathogenesis and cognition. Neurobiol Aging. 2014;35(4):793‐801. doi: 10.1016/j.neurobiolaging.2013.10.076 [DOI] [PubMed] [Google Scholar]
- 80. Zhu H, Wang X, Wallack M, et al. Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioral impairment and brain amyloid pathology in murine models of Alzheimer's disease. Mol Psychiatry. 2015;20(2):252‐262. doi: 10.1038/mp.2014.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lu L, Fu DL, Li HQ, Liu AJ, Li JH, Zheng GQ. Diabetes and risk of Parkinson's disease: An updated meta‐analysis of case‐control studies. PLoS One. 2014;9(1):e85781. doi: 10.1371/journal.pone.0085781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yue X, Li H, Yan H, Zhang P, Chang L, Li T. Risk of Parkinson disease in diabetes mellitus: An updated meta‐analysis of population‐based cohort studies. Medicine (Baltimore). 2016;95(18):e3549. doi: 10.1097/MD.0000000000003549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sánchez‐Gómez A, Alcarraz‐Vizán G, Fernández M, et al. Peripheral insulin and amylin levels in Parkinson's disease. Parkinsonism Relat Disord. 2020;79:91‐96. doi: 10.1016/j.parkreldis.2020.08.018 [DOI] [PubMed] [Google Scholar]
- 84. Sampson TR, Challis C, Jain N, et al. A gut bacterial amyloid promotes α‐synuclein aggregation and motor impairment in mice. elife. 2020;9:e53111. doi: 10.7554/eLife.53111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Horvath I, Wittung‐Stafshede P. Cross‐talk between amyloidogenic proteins in type‐2 diabetes and Parkinson's disease. Proc Natl Acad Sci USA. 2016;113(44):12473‐12477. doi: 10.1073/pnas.1610371113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ly H, Despa F. Diabetes‐related amylin dyshomeostasis: a contributing factor to cerebrovascular pathology and dementia. J Lipid Atheroscler. 2019;8(2):144‐151. doi: 10.12997/jla.2019.8.2.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844‐866. doi: 10.1016/j.neuron.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tong X, Yang Q, Ritchey MD, et al. The burden of cerebrovascular disease in the United States. Prev Chronic Dis. 2019;16:E52. doi: 10.5888/pcd16.180411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ly H, Verma N, Wu F, et al. Brain microvascular injury and white matter disease provoked by diabetes‐associated hyperamylinemia. Ann Neurol. 2017;82(2):208‐222. doi: 10.1002/ana.24992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Oskarsson ME, Paulsson JF, Schultz SW, Ingelsson M, Westermark P, Westermark GT. In vivo seeding and cross‐seeding of localized amyloidosis: a molecular link between type 2 diabetes and Alzheimer disease. Am J Pathol. 2015;185(3):834‐846. doi: 10.1016/j.ajpath.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 91. Kimura R, MacTavish D, Yang J, Westaway D, Jhamandas JH. Beta amyloid‐induced depression of hippocampal long‐term potentiation is mediated through the amylin receptor. J Neurosci. 2012;32(48):17401‐17406. doi: 10.1523/JNEUROSCI.3028-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jiang J, Ju J, Luo L, et al. Salmon calcitonin exerts an antidepressant effect by activating amylin receptors. Front Pharmacol. 2022;13:826055. doi: 10.3389/fphar.2022.826055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Boukabara S, Farokhnia M, Leggio L. Amylin in alcohol addiction: a potential new treatment target or an adjuvant to other treatments? ACS Chem Neurosci. 2024;15(8):1609‐1610. doi: 10.1021/acschemneuro.4c00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kalafateli AL, Aranäs C, Jerlhag E. Effects of sub‐chronic amylin receptor activation on alcohol‐induced locomotor stimulation and monoamine levels in mice. Psychopharmacology. 2020;237(11):3249‐3257. doi: 10.1007/s00213-020-05607-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kalafateli AL, Vallöf D, Jerlhag E. Activation of amylin receptors attenuates alcohol‐mediated behaviours in rodents. Addict Biol. 2019;24(3):388‐402. doi: 10.1111/adb.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Benlier N, Ozer G, Orhan N. Relation between serum amylin level and epilepsy. Egypt J Neurol Psychiatry Neurosurg. 2020;56(1):34. doi: 10.1186/s41983-020-00164-5 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review article presents no new data.


