Abstract
Human epidermal growth factor receptor 2 (HER2) is a critical therapeutic target for HER2-positive or HER2-dependent cancers. While several HER2 kinase inhibitors have been identified, achieving high selectivity for HER2 over EGFR remains a significant challenge. In this study, we aimed to develop HER2-selective inhibitors with enhanced cellular activity. To improve the limited cellular activity of derivatives with a quinoline moiety against HER2, we synthesized a novel series of derivatives by bioisosteric replacement. These derivatives demonstrated significantly improved selectivity for HER2 over EGFR, with a 7- to 12-fold enhancement compared to lapatinib in kinase assays. Furthermore, they exhibited enhanced cellular activity, leading to improved anti-proliferative effects against HER2-dependent SKBR3 cells. Notably, the representative compound 14f demonstrated more potent inhibition of HER2 phosphorylation at the cellular level compared to lapatinib. Additionally, compound 14f exhibited high HER2 selectivity, significantly inhibited colony formation in SKBR3 cells, and displayed good metabolic stability. These findings suggest the potential of these compounds as novel therapeutic candidates for HER2-positive cancers.
The bioisosteric analogs exhibited improved cellular activity and microsomal stability, and greater HER2 phosphorylation inhibition than lapatinib.
Introduction
Epidermal growth factor receptor 2 (HER2), also known as ERBB2, is a member of the ERBB family, which includes EGFR (or ERBB1), ERBB3, and ERBB4. Among them, EGFR and HER2 have been established as therapeutic targets for cancer. Amplification of ERBB signaling promotes cancer cell proliferation, survival, and metastasis.1 Blocking HER2 signaling is a key strategy for the treatment of HER2 positive cancers, such as breast cancer.1 Anti-HER2 antibodies including trastuzumab block the dimerization of HER2 to inhibit tumor growth. However, acquired resistance to antibody therapy has been observed in many patients. A potential resistance mechanism may occur because of the presence of kinase-active truncated forms of HER2 which trastuzumab does not bind.2 In this case, HER2 tyrosine kinase inhibitors are thought to have an advantage over antibodies such as trastuzumab.3,4 Small molecules targeting the tyrosine kinase domain of HER2 have been developed (Fig. 1).5,6 Lapatinib, however, exhibits strong inhibitory activity against EGFR which leads to adverse effects, including the most common rash and diarrhoea.7,8 Great efforts have been made to identify selective inhibitors for HER2 over EGFR. The HER2 selective inhibitor, tucatinib, was approved by the US Food and Drug Administration in 2020. Treatment with tucatinib in combination with trastuzumab and capecitabine improved the duration of progression-free survival and overall survival in patients with HER2-positive metastatic breast cancer.9 Furthermore, tucatinib exhibited clinically significant antitumor activity and tolerable toxicities in patients with HER2-positive metastatic biliary tract cancer10 and metastatic colorectal cancer.11 In HER2 targeted therapy, the combination of trastuzumab and tucatinib exhibited even better clinical benefit than trastuzumab plus concurrent inhibition of HER2/EGFR using lapatinib.12 Therefore, HER2 selective inhibitors combined with other anti-cancer drugs could be useful in treating various HER2 positive cancers. Therefore, novel HER2-selective inhibitors with diverse profiles are needed to expand treatment options for cancer. We previously reported potent HER2 inhibitors derived from EGFR inhibitor, erlotinib, including compound MK01, with high selectivity for HER2 over EGFR.13 However, the reported HER2 inhibitors including MK01 displayed poor cellular activity, with IC50 values against SKBR3 more than 10-fold higher than that of lapatinib. In addition, there was a large difference in potency between enzymatic kinase assay and cell-based cytotoxicity. To address this discrepancy and thereby enhance cellular activity, we, herein, describe the optimization of novel HER2 inhibitors with selectivity for HER2 over EGFR.
Fig. 1. Structures of reported HER2 inhibitors. Lapatinib is a dual inhibitor for HER2 and EGFR. CP724714, tucatinib and MK01 are highly selective for HER2 over EGFR.
Results and discussion
As shown in Scheme 1, compounds 2a–f were synthesized by the SNAr reaction of 1-fluoro-4-nitrobenzenes 1 with hydroxyquinolines in excess amount of K2CO3 under microwave irradiation. Nitro compounds 2a–f were reduced with Fe and NH4Cl at 80 °C and then the resulting amines were coupled with 4-chloro-6-quinazolinol to provide the following 3. O-Alkylation of the 6-oxyquinazoline intermediate 3 with 4-(2-iodoethyl)morpholine yielded 4. Reduction of 2 and subsequent coupling reaction with intermediate 8 yielded 9a–d. Intermediate 8 was obtained from 4,6-dihydorxyquinazolines 5 by acetylation, chlorination, deacetylation and Mitsunobu reaction.
Scheme 1. Synthesis of 4 and 9a–9d. Reagents and conditions: (a) ArOH, K2CO3, DMF, microwave, 100–130 °C, 20–60 min, 37–95%; (b) i) Fe, NH4Cl, EtOH/H2O, 80 °C, 3 h; ii) 4-chloro-6-quinazolinol, i-PrOH, 80 °C, 6 h, 91% over 2 steps. (c) 4-(2-Iodoethyl)morpholine, K2CO3, DMF, 80 °C, 66% (d) Fe, NH4Cl, EtOH/H2O, 80 °C, 3 h; (e) 8, i-PrOH, 80 °C, 6 h, 12–91% over 2 steps. (f) Ac2O, pyridine, 100 °C, 2 h; (g) SOCl2, DMF, toluene, 100 °C, 2 h, 64% over 2 steps (h) NH3 in MeOH, RT, 30 min, 33%; (i) PPh3, DEAD, DCM, 2 h, RT, 79%.
Compounds 2a–2c were reacted with 4-chloro-6-iodoquinazoline to provide 10a–c, followed by introducing an acetylene group (11a–c) and click chemistry14 to give triazole compounds 12a–c (Scheme 2). Acetylene derivatives 13a–f were obtained from the Sonogashira coupling reaction15 of iodo compound 10c or 10d with various acetylenes (Scheme 3).
Scheme 2. Synthesis of compounds 12a–12c. Reagents and conditions: (a) Fe, NH4Cl, EtOH/H2O, 80 °C, 3 h; (b) 4-chloro-6-iodoquinazoline, i-PrOH, 80 °C, 6 h, 12–91% over 2 steps; (c) trimethylsilylacetylene, Pd(PPh3)4, CuI, DIPEA, DMF, microwave, 100 °C, 1 h; (d) TBAF, RT, 49–73% over 2 steps (e) 1-(azidomethyl)-4-fluorobenzene, sodium ascorbate, CuSO4·5H2O, H2O/i-PrOH, 80 °C, 36–94%.
Scheme 3. Synthesis of final compounds 13a–f. Reagents and conditions: (a) R2C2H, Pd(PPh3)4, CuI, DIPEA, DMF, microwave, 100 °C, 1 h, 7–63%.
Further triazole derivatives 14a–e with an isoquinoline moiety were synthesized by click chemistry of acetylene 11e with various alkylazides as described in Scheme 4. The Suzuki coupling reaction16 of 6-iodooquinazoline 10d with various aryl boronic acids yielded the desired derivatives 14f–i. The methyl derivative 14j of chloride 14f was obtained from 10c using the same procedure as that used for 14f.
Scheme 4. Synthesis of final compounds 14a–j. Reagents and conditions: (a) Pd(PPh3)4, CuI, DIPEA, DMF, microwave, 100 °C, 1 h;14 (b) TBAF, RT, 49–73% over 2 steps; (c) R4CH2N3, sodium ascorbate, CuSO4·5H2O, H2O/i-PrOH, 80 °C, 36–94%;15 (d) aryl boronic acids, Pd(OAc)2, K2CO3, Abs. EtOH, dry THF, microwave, 80 °C, 30 min, 37–68%.
We commenced with screening the derivatives of compound MK01, which exhibited high selectivity for HER2 over EGFR in kinase assays, but poor cellular activity (IC50 of 643 nM against SKBR3).13 Compounds 4, 9a–9d, bearing a common morpholinoethoxyquinazoline moiety (a) for part B, were evaluated in in vitro enzymatic kinase assays and cell-based assays using SK-BR3 (a HER2 overexpressing breast cancer cell line) and A431 (an EGFR overexpressing epidermoid carcinoma cell line) to determine the optimal structure of part A moieties. As shown in Table 1, isoquinoline derivatives 9a and 9b exhibited good inhibitory activities against HER2 kinase and proliferation of SKBR3 cells. In the case of the ethynyl quinazoline moiety (b), the isoquinoline derivative 11c showed better inhibitory activity against SKBR3 cells than the quinoline derivatives. Notably, isoquinoline 14a with a triazole moiety (c) displayed excellent activity in SKBR3 cells with an IC50 value of 103 nM while maintaining HER2 kinase activity. The differential activity for SKBR3 over A431 was interesting. Furthermore, unlike lapatinib, which is more potent against EGFR, 14a showed comparable inhibitory activity against both EGFR and HER2 at 0.1 μM, although it showed weak activity. Therefore, isoquinoline derivatives exhibited great potential and have been further explored. Extended derivatives of 11c were synthesized and evaluated (Table 2). Morpholino derivative 13a exhibited greater potency in both kinases assays and cell-based assays compared to piperidine (13b), pyrrolidine (13c), and γ-lactam (13e) derivatives, suggesting the importance of terminal heteroatoms for strong target binding. The acetyl piperazine compound (13d) showed almost equipotency to 13a. Interestingly, replacing the methyl group of 13a with chloride (13f) further improved the inhibitory activity against HER2 and anti-proliferative activity in cells, exceeding the potency of lapatinib against HER2.
Table 1. Activity of quinoline and isoquinoline derivatives in in vitro kinase and cell-based assays.

| ||||||
|---|---|---|---|---|---|---|
| Compound | Structures | % inhibition | IC50 (nM) | |||
| EGFR | HER2 | SKBR3 | ||||
| 1 μM | 0.1 μM | 1 μM | 0.1 μM | |||
| 4 | a–e | 90 | 59 | 92 | 62 | 1358 |
| 9a | a–f | 91 | 56 | 92 | 55 | 235 |
| 9b | a–g | 98 | 85 | 95 | 58 | 125 |
| 9c | a–h | 76 | 32 | 80 | 40 | 3199 |
| 9d | a–i | 59 | 22 | 73 | 30 | 4527 |
| 11a | b–d | 69 | 16 | 91 | 50 | 1175 |
| 11b | b–e | 87 | 33 | 86 | 32 | 725 |
| 11c | b–f | 84 | 43 | 77 | 33 | 553 |
| 12a | c–d | 68 | 10 | 92 | 56 | 1389 |
| 12b | c–e | 61 | 9 | 88 | 44 | 610 |
| 12c | c–f | 66 | 15 | 83 | 37 | 392 |
| 14a | c–g | 92 | 54 | 92 | 43 | 103 |
| Lapatinib | 99 | 94 | 96 | 70 | 23 | |
Table 2. In vitro enzymatic kinase assays and anti-proliferative activity of ethynyl-linked isoquinazolines.
| Compound | % inhibition | IC50 (nM) | ||||
|---|---|---|---|---|---|---|
| EGFR | HER2 | SKBR3 | A431 | |||
| 1 μM | 0.1 μM | 1 μM | 0.1 μM | |||
| 13a | 95 | 71 | 95 | 68 | 90 | 850 |
| 13b | 82 | 36 | 88 | 30 | 414 | N.Da |
| 13c | 88 | 49 | 93 | 48 | 567 | N.D |
| 13d | 94 | 73 | 98 | 72 | 125 | N.D |
| 13e | 74 | 29 | 88 | 28 | 427 | N.D |
| 13f | 98 | 89 | 96 | 81 | 12 | 414 |
| Lapatinib | 99 | 94 | 96 | 70 | 23 | 418 |
N.D: not determined.
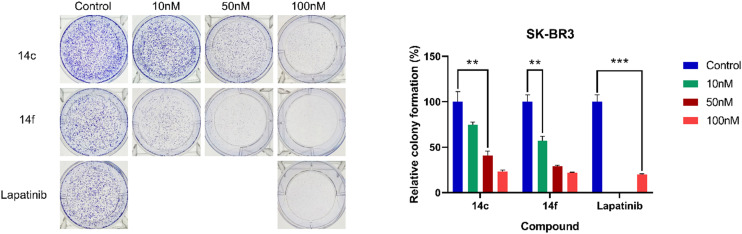
The structure–activity relationship of triazole derivatives and their bioisosteric derivatives was further explored (Table 3). Derivatives 14b–e synthesized using click chemistry exhibited improved inhibitory activity for both kinases compared to the parent compound 14a. Derivatives 14b and 14c showed approximately 10-fold higher potency against SKBR3 compared to A431. However, the anti-proliferative activity of 14d and 14e against SKBR3 cells was slightly reduced. Notably, replacing the triazole ring in 14e with a furan ring (14f) improved cellular activity while maintaining kinase inhibitory activity. Another furan derivative 14g showed potency similar to 14e. Although the alkene derivatives 14h and 14i showed good activity, they did not have a significant advantage. Table 4 lists the IC50 values of the representative compounds. All these derivatives potently inhibited HER2 kinase activity, even exceeding that of lapatinib, whereas the EGFR activity was comparable or slightly reduced. With regard to the selectivity ratio (EGFR/HER2), these derivatives showed an improvement of approximately 7 to 12-fold compared with lapatinib. The metabolic stability of some derivatives was also evaluated (Table 5). Unfortunately, most of the derivatives exhibited poor metabolic stability in human and mouse liver microsomes, except for 14f, which demonstrated good microsomal stability. The furan moiety provided better stability than acetylene and triazole within this series. The potent 14c, bearing an alcohol group at the end of side chain, exhibited extremely poor microsomal stability both in human and mouse liver microsomes.
Table 3. In vitro enzymatic kinase assays and anti-proliferative activity of sp2 carbon-linked quinazolines.
| Compound | % inhibition | IC50 (nM) | ||||
|---|---|---|---|---|---|---|
| EGFR | HER2 | SKBR3 | A431 | |||
| 1 μM | 0.1 μM | 1 μM | 0.1 μM | |||
| 14a | 92 | 54 | 92 | 43 | 103 | 1868 |
| 14b | 100 | 97 | 99 | 91 | 82 | 652 |
| 14c | 98 | 90 | 95 | 71 | 78 | 937 |
| 14d | 98 | 92 | 95 | 73 | 123 | 493 |
| 14e | 99 | 90 | 95 | 88 | 196 | 1346 |
| 14f | 99 | 94 | 94 | 82 | 53 | 639 |
| 14g | 100 | 96 | 98 | 84 | 34 | 623 |
| 14h | 99 | 94 | 98 | 81 | 46 | 505 |
| 14i | 98 | 91 | 98 | 80 | 73 | 693 |
| 14j | 97 | 77 | 95 | 81 | 97 | 1016 |
| Lapatinib | 99 | 94 | 96 | 70 | 23 | 418 |
Table 4. IC50 values of the selected derivatives for EGFR and HER2 in vitro kinase assays.
| Compound | IC50 (nM) | Selectivity ratioa (EGFR/HER2) | |
|---|---|---|---|
| EGFR | HER2 | ||
| 13f | 1.7 | 22 | 0.08 |
| 14b | 1.0 | 2.2 | 0.45 |
| 14c | 3.3 | 8.6 | 0.38 |
| 14f | 2.4 | 6.2 | 0.39 |
| 14g | 3.6 | 6.0 | 0.60 |
| 14h | 4.8 | 13.3 | 0.36 |
| 14i | 4.7 | 8.3 | 0.57 |
| Lapatinib | 1.5 | 31 | 0.05 |
IC50 value for EGFR divided by the IC50 for HER2.
Table 5. Metabolic stability of selected derivatives in human and mouse liver microsomes.
| Compound | Metabolic stability % remaining after 30 min | |
|---|---|---|
| Human | Mouse | |
| 13f | 28.1 | 41.8 |
| 14b | 27.3 | 14.4 |
| 14c | <10 | <10 |
| 14f | 55.7 | 84.9 |
| 14g | 19.0 | 42.3 |
| 14h | 27.7 | 37.9 |
| 14i | 34.9 | 38.2 |
| Verapamil | 25.0 | — |
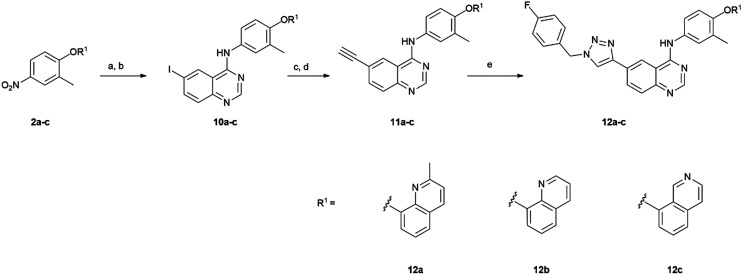
To confirm the cellular activity of the derivatives, western blotting was performed to examine the ability of representative compounds 14c (triazole) and 14f (furan) to inhibit HER2 and EGFR phosphorylation (Fig. 2). Both compounds significantly inhibited HER2 phosphorylation at 100 nM, and showed greater reduction of HER2 phosphorylation than lapatinib at 10 nM, whereas only weak inhibition of EGFR phosphorylation was observed. Interestingly, unlike the results from in vitro kinase assays, cellular experiments demonstrated a clear preference for HER2 inhibition over EGFR, suggesting that these isoquinolines may offer advantages for HER2-targeted cancer therapy while minimizing EGFR inhibition. To validate the cytotoxic effects of both compounds, a colony formation assay was carried out using SK-BR3 cells. Both compounds significantly inhibited cellular growth in a dose dependent manner (Fig. 3). Both derivatives were as potent as the positive control, lapatinib.
Fig. 2. Western blot analysis for compounds 14c and 14f. (a) Lapatinib, 14c and 14f similarly blocked phosphorylation of EGFR at 1000 nM, in the A431 cell line. (b) Lapatinib, 14c, and 14f inhibited HER2 phosphorylation in SKBR3 cells. At 10 nM, 14c and 14f demonstrated more potent inhibition of HER2 phosphorylation compared to lapatinib. β-Actin was used as a loading control. ***p < 0.001, **p < 0.01.
Fig. 3. Colony formation assay of 14c and 14f in SK-BR3 cells. Cells were treated with 14c, 14f or lapatinib (a positive control) for 2 weeks at indicated concentrations. ***p < 0.001, **p < 0.01.
Conclusion
The replacement of the quinoline moiety with isoquinoline led to considerable improvements in cellular activity and HER2 kinase inhibition. Seven isoquinoline derivatives demonstrated greater potency and selectivity for HER2 compared to lapatinib. Notably, compound 14g demonstrated a 12-fold better selectivity ratio for EGFR/HER2 than lapatinib. These isoquinoline derivatives also showed enhanced anti-proliferative activity against SKBR3 cells. In addition, the furan ring was found to be more favorable for cellular activity than the triazole ring within this scaffold. Acetylene derivative 13f displayed stronger activity against SKBR3 cells compared to lapatinib but suffered from poor metabolic stability. The representative derivative 14f, which showed good metabolic stability, demonstrated more potent inhibition of HER2 phosphorylation in SKBR3 cells than lapatinib while maintaining similar anti-proliferative activity, suggesting that it may exhibit reduced off-target cytotoxicity. Based on these findings, compound 14f stands out as a promising candidate for further development, highlighting the potential of isoquinoline-based derivatives as effective HER2 inhibitors.
Experimental
Chemistry
General information
All reagents and solvents were purchased from Alfa Aesar, Combi Blocks, Fisher Scientific, J.T. Baker, Samchun Pure Chemical, Sigma Aldrich, or TCI and used without further purification unless otherwise noted. Reactions were monitored via thin-layer chromatography (TLC) using Merck TLC silica gel 60 F254 250 μm plates. Flash column chromatography was performed using ZEOprep 60 silica gel (Zeochem, 40–63 μm) and a CombiFlash system (Teledyne ISCO) loaded with pre-packed silica gel flash column cartridges (Welux™). 1H and 13C NMR spectra were obtained using a 600 MHz NMR spectrometer (JEOL) using tetramethylsilane (TMS) as an internal standard. Chemical shifts are reported in parts per million (ppm, δ) downfield of TMS, and the coupling constant (J) is reported in hertz (Hz). Splitting patterns are reported with the following abbreviations: s, singlet; d, doublet; t, triplet; q, quartet; p, pentet; dd, doublet of doublets; dt, doublet of triplets; td, triplet of doublets; m, multiplet; br, broad signal. High-resolution mass spectrometry (HRMS) was performed using a Q-Exactive MS (Thermo Scientific) coupled with an Ultimate 3000 LC system (Dionex). A ThermoScientific Hypersil GOLD C18 column (2.1 mm × 50 mm, 1.9 μm) was used for separation.
General procedure A
K2CO3 (3.0 mmol) and ArOH (1.0 mmol) were added to a solution of compound 1 (1.1 mmol) in DMF (3 mL). The solution was stirred at the specified temperature and time. Subsequently, the reaction mixture was diluted with acetone, filtered over celite, and concentrated under reduced pressure. The crude material was purified by MPLC to obtain compounds 2a–f.
General procedure B
A solution of nitro compounds 2a–f (0.05 mmol), Fe (0.25 mmol), and NH4Cl (0.15 mmol) in aqueous EtOH (containing 5% H2O, 5 mL) was stirred at 80 °C for 3 h. After reduction, the reaction mixture was filtered over celite and concentrated under reduced pressure. 4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline or compound 8 (0.05 mmol) was added to a reaction mixture in i-PrOH (1 mL) and stirred at 80 °C for 6 h. The reaction mixture was poured into water, extracted three times with CH2Cl2, washed with brine, filtered, concentrated using a rotary evaporator, and purified over MPLC to obtain compounds 9a–d.
General procedure C
6-Hydroxyquinazoline derivatives 3 (0.1 mmol), alkyl halides (0.13 mmol), K2CO3 (0.5 mmol), and KI (0.02 mmol) were dissolved in DMF (1 mL) and stirred at 70 °C for 5 h. The reaction mixture was poured into water, extracted three times with CH2Cl2, washed with brine, filtered, concentrated using a rotary evaporator, and purified over MPLC to obtain compound 4.
General procedure D
6-Iodoquinazoline derivatives 10a–f (0.1 mmol), alkynes (0.14 mmol), and DIPEA (0.1 mmol) were dissolved in dry DMF (1 mL) and degassed with N2 balloon for 5 min. Pd(PPh3)4 (0.005 mmol) and CuI (0.005 mmol) were added to this mixture, degassed further for 5 min, and stirred at 100 °C using a microwave reactor for 1 h. The reaction mixture was filtered over celite and the filtrate was purified by MPLC to obtain 13a–f.
General procedure E
6-Iodoquinazoline derivatives 10a–f (0.2 mmol), aryl boronic acids (0.28 mmol), and K2CO3 (0.4 mmol) were dissolved in a 1 : 1 mixture of absolute ethanol and dry THF (2 mL) and degassed with N2 balloon for 5 min. Pd(OAc)2 (0.006 mmol) was added to this mixture, degassed further for 5 min, and stirred at 100 °C using a microwave reactor for 1 h. The reaction mixture was filtered over celite and the filtrate was purified by MPLC to obtain 14f–j.
General procedure F
6-Terminal alkyne quinazoline derivatives 11a–c (0.04 mmol), azides (0.048 mmol), sodium ascorbate (0.004 mmol), and copper(ii) sulfate pentahydrate (0.002 mmol) were dissolved in a 2 : 1 mixture of water and isopropanol (0.5 mL) and stirred at 80 °C for 8 h. The reaction mixture was then cooled to RT, diluted with cold water, and filtered under reduced pressure. If necessary, purification was conducted by MPLC to yield 12a–c, 14a–e.
2-Methyl-8-(2-methyl-4-nitrophenoxy)quinoline (2a)
Compound 2a was prepared by general procedure A using 2-fluoro-5-nitrotoluene and 8-hydroxy-2-methylquinoline as starting materials at 100 °C for 20 min in microwave. Yield: 68%. 1H-NMR (600 MHz, CDCl3) δ 8.18 (d, J = 2.6 Hz, 1H), 8.07 (d, J = 8.4 Hz, 1H), 7.92 (dd, J = 9.0, 2.7 Hz, 1H), 7.63 (d, J = 8.2 Hz, 1H), 7.42 (t, J = 7.9 Hz, 1H), 7.32 (d, J = 8.4 Hz, 1H), 7.19 (d, J = 7.6 Hz, 1H), 6.69 (d, J = 9.0 Hz, 1H), 2.65 (s, 3H), 2.52 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 162.2, 159.5, 151.3, 142.8, 140.5, 136.1, 129.9, 128.4, 126.7, 125.6, 124.3, 123.0, 123.0, 118.4, 117.3, 25.7, 16.7; HRMS (ESI): m/z calcd for C17H15N2O3 [M + H]+ 295.1077, found 295.1083.
8-(2-Methyl-4-nitrophenoxy)quinoline (2b)
Compound 2b was prepared by general procedure A using 2-fluoro-5-nitrotoluene and 8-hydroxyquinoline as starting materials at 130 °C for 30 min in microwave. Yield: 44%. 1H-NMR (600 MHz, CDCl3) δ 8.92 (dd, J = 4.1, 1.5 Hz, 1H), 8.23 (dd, J = 8.3, 1.6 Hz, 1H), 8.20 (d, J = 2.6 Hz, 1H), 7.94 (dd, J = 9.0, 2.6 Hz, 1H), 7.70 (dd, J = 8.3, 0.9 Hz, 1H), 7.52 (t, J = 7.8 Hz, 1H), 7.48 (q, J = 4.1 Hz, 1H), 7.23 (dd, J = 7.6, 1.0 Hz, 1H), 6.69 (d, J = 9.0 Hz, 1H), 2.53 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 161.7, 151.7, 150.6, 142.9, 141.0, 136.2, 130.0, 129.8, 126.8, 126.6, 124.5, 123.1, 122.1, 118.3, 117.0, 16.6; HRMS (ESI): m/z calcd for C16H13N2O3 [M + H]+ 281.0921, found 281.0924.
8-(2-Methyl-4-nitrophenoxy)isoquinoline (2c)
Compound 2c was prepared by general procedure A using 2-fluoro-5-nitrotoluene and 8-hydroxyisoquinoline as starting materials at 100 °C for 60 min in microwave. Yield: 63%. 1H-NMR (600 MHz, CDCl3) δ 9.55 (s, 1H), 8.63 (d, J = 6.2 Hz, 1H), 8.23 (d, J = 2.1 Hz, 1H), 8.02 (dd, J = 9.0, 2.8 Hz, 1H), 7.72 (d, J = 5.5 Hz, 1H), 7.68–7.63 (m, 2H), 6.99 (dd, J = 6.9, 1.4 Hz, 1H), 6.88 (d, J = 9.0 Hz, 1H), 2.49 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 160.3, 152.5, 147.2, 144.2, 143.6, 137.4, 130.7, 130.3, 127.1, 123.3, 122.9, 121.5, 120.2, 117.5, 114.5, 16.4; HRMS (ESI): m/z calcd for C16H13N2O3 [M + H]+ 281.0921, found 281.0921.
5-(2-Methyl-4-nitrophenoxy)isoquinoline (2d)
Compound 2d was prepared by the general procedure A using 2-fluoro-5-nitrotoluene and 5-hydroxyisoquinoline as starting materials at 130 °C for 60 min in microwave. Yield: 89%. 1H-NMR (600 MHz, CDCl3) δ 9.35 (s, 1H), 8.58 (d, J = 5.5 Hz, 1H), 8.23 (d, J = 2.8 Hz, 1H), 7.99 (dd, J = 9.0, 2.8 Hz, 1H), 7.87 (d, J = 8.3 Hz, 1H), 7.80 (d, J = 6.2 Hz, 1H), 7.60 (t, J = 7.9 Hz, 1H), 7.21 (d, J = 7.6 Hz, 1H), 6.73 (d, J = 9.0 Hz, 1H), 2.51 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 160.7, 152.5, 150.4, 143.7, 143.2, 129.9, 129.7, 129.2, 127.3, 127.0, 124.2, 123.3, 118.4, 116.5, 114.3, 16.4; HRMS (ESI): m/z calcd for C16H13N2O3 [M + H]+ 281.0921, found 281.0922.
5-(2-Methyl-4-nitrophenoxy)quinoline (2e)
Compound 2e was prepared by general procedure A using 2-fluoro-5-nitrotoluene and 5-hydroxyquinoline as starting materials at 100 °C for 30 min in microwave. Yield: 60%. 1H-NMR (600 MHz, CDCl3) δ 8.99 (q, J = 1.8 Hz, 1H), 8.38 (d, J = 9.6 Hz, 1H), 8.22 (d, J = 2.1 Hz, 1H), 8.01–7.97 (m, 2H), 7.69 (t, J = 7.9 Hz, 1H), 7.45 (q, J = 4.4 Hz, 1H), 7.06 (d, J = 7.6 Hz, 1H), 6.75 (d, J = 9.0 Hz, 1H), 2.51 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 160.9, 151.3, 150.8, 149.4, 143.1, 130.2, 129.6, 129.2, 127.0, 126.3, 123.3, 121.8, 121.4, 116.5, 114.8, 16.4; HRMS (ESI): m/z calcd for C16H13N2O3 [M + H]+ 281.0921, found 281.0922.
8-(2-Chloro-4-nitrophenoxy)isoquinoline (2f)
Compound 2f was prepared by general procedure A using 3-chloro-4-fluoronitrobenzene and 8-hydroxyisoquinoline as starting materials at 100 °C for 30 min in microwave. Yield: 55%. 1H-NMR (600 MHz, CDCl3) δ 9.53 (s, 1H), 8.65 (d, J = 5.5 Hz, 1H), 8.46 (d, J = 2.8 Hz, 1H), 8.09 (dd, J = 9.0, 2.8 Hz, 1H), 7.73 (t, J = 4.1 Hz, 2H), 7.67 (t, J = 7.9 Hz, 1H), 7.08 (d, J = 6.9 Hz, 1H), 6.99 (d, J = 9.0 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 158.2, 151.7, 147.1, 144.4, 143.6, 137.5, 130.6, 126.8, 125.7, 123.8, 123.8, 121.4, 120.2, 118.4, 115.3; HRMS (ESI): m/z calcd for C15H10ClN2O3 [M + H]+ 301.0375, found 301.0375.
4-((3-Methyl-4-(quinolin-8-yloxy)phenyl)amino)quinazolin-6-ol (3)
Compound 3 was prepared by general procedure B. Yield: 58%. 1H-NMR (600 MHz, DMSO-d6) δ 10.06 (s, 1H), 9.48 (s, 1H), 8.93 (dd, J = 4.1, 1.6 Hz, 1H), 8.42–8.41 (m, 2H), 7.80 (dd, J = 19.9, 2.3 Hz, 2H), 7.71 (d, J = 8.2 Hz, 1H), 7.68–7.66 (m, 2H), 7.62 (q, J = 4.2 Hz, 1H), 7.52 (t, J = 7.9 Hz, 1H), 7.42 (dd, J = 9.0, 2.4 Hz, 1H), 7.01 (d, J = 7.7 Hz, 1H), 6.86 (d, J = 8.7 Hz, 1H), 2.26 (s, 3H); 13C-NMR (150 MHz, DMSO-d6) δ 156.7, 155.4, 153.2, 151.5, 150.4, 149.8, 143.8, 139.9, 136.0, 135.3, 129.4, 129.2, 128.4, 126.6, 125.1, 123.9, 122.2, 122.1, 121.3, 118.8, 116.2, 114.6, 105.0, 16.0; HRMS (ESI): m/z calcd for C24H20N4O2 [M + H]+ 395.1503, found 395.1519.
N-(3-Methyl-4-(quinolin-8-yloxy)phenyl)-6-(2-morpholinoethoxy)quinazolin-4-amine (4)
Compound 4 was prepared by general procedure C. Yield: 15%. 1H-NMR (600 MHz, CDCl3) δ 9.02 (dd, J = 4.1, 1.6 Hz, 1H), 8.71 (s, 1H), 8.21 (dd, J = 8.3, 1.5 Hz, 1H), 7.86 (d, J = 9.1 Hz, 1H), 7.67 (d, J = 1.9 Hz, 2H), 7.54–7.49 (m, 3H), 7.46 (dd, J = 9.1, 2.3 Hz, 1H), 7.38 (t, J = 8.0 Hz, 1H), 7.02 (d, J = 8.6 Hz, 1H), 6.90 (d, J = 7.7 Hz, 1H), 4.18 (t, J = 5.7 Hz, 2H), 3.74 (t, J = 4.6 Hz, 4H), 2.83 (t, J = 5.7 Hz, 2H), 2.58 (s, 4H), 2.28 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 157.0, 156.9, 154.3, 153.1, 150.8, 149.9, 145.6, 140.3, 136.1, 134.9, 131.3, 130.5, 129.8, 126.7, 124.9, 124.3, 121.9, 121.5, 121.4, 120.9, 115.7, 113.2, 101.1, 66.9, 66.2, 57.4, 54.1, 16.5; HRMS (ESI): m/z calcd for C30H30N5O3 [M + H]+ 508.2343, found 508.2342.
4-Chloro-7-methoxyquinazolin-6-yl acetate
To a solution of 6-hydroxy-7-methoxy-quinazolin-4(1H)-one (384 mg, 2.0 mmol) in acetic anhydride (2.8 ml, 30.0 mmol), pyridine (0.5 ml, 6.0 mmol) was added and the mixture was stirred at 100 °C for 2 h. The solution was cooled down to room temperature and ice was added. The precipitate was filtered, washed with cold water, and dried under high vacuum. To the crude product dissolved in 1 M thionyl chloride in dichloromethane (40 ml, 40 mmol) and toluene (20 ml) was added DMF (40 μl, 0.5 mmol). After stirring at 100 °C for 3 h, the reaction mixture was concentrated under reduced pressure and azeotroped with toluene three times to obtain 6a (400 mg, 79% over 2 steps). 1H-NMR (600 MHz, CDCl3) δ 8.95 (s, 1H), 7.89 (s, 1H), 7.42 (s, 1H), 4.02 (s, 3H), 2.40 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 168.5, 160.8, 157.8, 154.2, 151.5, 142.3, 118.9, 118.4, 108.1, 56.7, 20.6; HRMS (ESI): m/z calcd for C11H10ClN2O3 [M + H]+ 253.0375, found 253.0391.
4-Chloroquinazolin-6-yl acetate (6)
To a solution of 6-hydroxy-4-quinazolone (324 mg, 2.0 mmol) in acetic anhydride (2.8 ml, 30.0 mmol), pyridine (0.5 ml, 6.0 mmol) was added and the mixture was stirred at 100 °C for 2 h. The solution was cooled down to room temperature and ice was added. The precipitate was filtered, washed with cold water, and dried under high vacuum. To the crude product dissolved in 1 M thionyl chloride in dichloromethane (40 ml, 40 mmol) and toluene (20 ml) was added DMF (40 μl, 0.5 mmol). After stirring at 100 °C for 3 h, the reaction mixture was concentrated under reduced pressure and azeotroped with toluene three times to obtain 6b (285 mg, 64% over 2 steps). 1H-NMR (600 MHz, CDCl3) δ 9.05 (s, 1H), 8.11 (d, J = 9.0 Hz, 1H), 8.01 (d, J = 2.5 Hz, 1H), 7.73 (dd, J = 9.0, 2.5 Hz, 1H), 2.41 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 168.9, 162.1, 153.5, 150.2, 149.1, 130.5, 130.5, 124.5, 116.9, 21.1; HRMS (ESI): m/z calcd for C10H8ClN2O2 [M + H]+ 223.0269, found 223.0273.
4-Chloroquinazolin-6-ol (7)
Compound 6 (2.0 mmol, 408 mg) was dissolved in 2 M NH3 in MeOH solution (3 mL) and stirred at RT for 30 min. After concentration under a rotary evaporator, the crude mixture was purified with MPLC to obtain 7 (224 mg, 62%). 1H-NMR (600 MHz, DMSO-d6) δ 10.88 (s, 1H), 8.90 (s, 1H), 7.99 (d, J = 9.0 Hz, 1H), 7.65 (dd, J = 9.1, 2.7 Hz, 1H), 7.42 (d, J = 2.7 Hz, 1H); 13C-NMR (150 MHz, DMSO-d6) δ 159.5, 158.5, 151.2, 146.1, 130.9, 128.5, 125.1, 106.0; HRMS (ESI): m/z calcd for C8H6ClN2O [M + H]+ 181.0163, found 181.0164.
4-(2-((4-Chloroquinazolin-6-yl)oxy)ethyl)morpholine (8)
To a solution of 7 (1.1 mmol, 199 mg), 4-(2-hydroxyethyl)morpholine (3.3 mmol, 400 μL), and triphenylphosphine (346 mg) in DCM (2 mL) was added diethyl azodicarboxylate (1.21 mmol, 550 μL) at 0 °C and stirred at RT for 2 h. The crude mixture was purified over MPLC to obtain 8 (264 mg, 82%). 1H-NMR (600 MHz, CDCl3) δ 8.94 (s, 1H), 7.98 (d, J = 9.6 Hz, 1H), 7.62 (dd, J = 9.6, 2.8 Hz, 1H), 7.45 (d, J = 2.8 Hz, 1H), 4.30 (t, J = 5.9 Hz, 2H), 3.77 (t, J = 4.8 Hz, 4H), 2.92 (t, J = 5.5 Hz, 2H), 2.64–2.63 (m, 4H); 13C-NMR (150 MHz, CDCl3) δ 160.5, 158.6, 151.7, 147.3, 130.3, 128.2, 125.1, 103.5, 66.9, 66.6, 57.4, 54.1; HRMS (ESI): m/z calcd for C14H17ClN3O2 [M + H]+ 294.1004, found 294.1003.
N-(4-(Isoquinolin-8-yloxy)-3-methylphenyl)-6-(2-morpholinoethoxy)quinazolin-4-amine (9a)
Compound 9a was prepared by general procedure B. Yield: 42%. 1H-NMR (600 MHz, CDCl3) δ 9.80 (s, 1H), 8.71 (s, 1H), 8.61 (d, J = 5.5 Hz, 1H), 8.07 (s, 1H), 7.86 (d, J = 9.0 Hz, 1H), 7.68 (t, J = 6.2 Hz, 2H), 7.60 (d, J = 9.0 Hz, 1H), 7.52–7.46 (m, 3H), 7.40 (d, J = 2.1 Hz, 1H), 7.06 (d, J = 8.3 Hz, 1H), 6.73 (d, J = 7.6 Hz, 1H), 4.20 (t, J = 5.9 Hz, 2H), 3.73 (t, J = 4.5 Hz, 4H), 2.84 (t, J = 5.9 Hz, 2H), 2.57 (s, 4H), 2.24 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 157.1, 157.0, 155.1, 153.0, 149.9, 147.6, 145.5, 143.7, 137.2, 135.4, 131.2, 131.0, 130.4, 125.3, 124.4, 121.3, 121.3, 120.9, 120.2, 120.1, 115.7, 110.4, 101.3, 66.7, 66.1, 57.3, 54.0, 16.3; HRMS (ESI): m/z calcd for C30H30N5O3 [M + H]+ 508.2343, found 508.2344.
N-(3-Chloro-4-(isoquinolin-8-yloxy)phenyl)-6-(2-morpholinoethoxy)quinazolin-4-amine (9b)
Compound 9b was prepared by general procedure B. Yield: 72%. 1H-NMR (600 MHz, CDCl3) δ 9.80 (s, 1H), 8.74 (s, 1H), 8.62 (d, J = 5.5 Hz, 1H), 8.48 (s, 1H), 8.05 (d, J = 2.8 Hz, 1H), 7.87 (d, J = 9.0 Hz, 1H), 7.69–7.66 (m, 2H), 7.53 (q, J = 7.6 Hz, 2H), 7.47 (dd, J = 12.4, 3.4 Hz, 2H), 7.15 (d, J = 9.0 Hz, 1H), 6.77 (dd, J = 6.5, 1.7 Hz, 1H), 4.11 (t, J = 5.9 Hz, 2H), 3.69 (t, J = 4.5 Hz, 4H), 2.78 (t, J = 5.5 Hz, 2H), 2.50 (s, 4H); 13C-NMR (150 MHz, acetone-d6) δ 158.2, 155.4, 153.1, 147.8, 147.3, 146.8, 145.0, 138.9, 138.1, 131.7, 131.0, 126.7, 125.3, 124.4, 124.3, 123.4, 122.6, 122.5, 121.8, 121.6, 120.8, 111.5, 103.0, 67.4, 67.4, 58.2, 55.0; HRMS (ESI): m/z calcd for C29H27ClN5O3 [M + H]+ 528.1797, found 528.1793.
N-(4-(Isoquinolin-5-yloxy)-3-methylphenyl)-6-(2-morpholinoethoxy)quinazolin-4-amine (9c)
Compound 9c was prepared by general procedure B. Yield: 42%. 1H-NMR (600 MHz, CDCl3) δ 9.30 (s, 1H), 8.72 (s, 1H), 8.60 (d, J = 6.2 Hz, 1H), 8.13 (d, J = 6.2 Hz, 1H), 8.11 (s, 1H), 7.86 (d, J = 9.0 Hz, 1H), 7.70 (d, J = 2.1 Hz, 1H), 7.66 (d, J = 8.3 Hz, 1H), 7.57 (dd, J = 8.6, 2.4 Hz, 1H), 7.47–7.43 (m, 2H), 7.39 (d, J = 2.8 Hz, 1H), 6.99 (d, J = 8.3 Hz, 1H), 6.90 (d, J = 7.6 Hz, 1H), 4.15 (t, J = 5.9 Hz, 2H), 3.74–3.71 (m, 5H), 2.82 (t, J = 5.5 Hz, 2H), 2.55 (s, 4H), 2.25 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 157.1, 157.0, 153.1, 153.0, 152.1, 150.3, 145.5, 142.9, 135.1, 130.9, 130.3, 129.7, 129.3, 128.5, 127.4, 125.4, 124.4, 121.3, 120.7, 115.7, 115.0, 113.5, 101.3, 66.9, 66.8, 66.1, 57.4, 57.4, 54.0, 54.0, 16.3; HRMS (ESI): m/z calcd for C30H30N5O3 [M + H]+ 508.2343, found 508.2344.
N-(3-Methyl-4-(quinolin-5-yloxy)phenyl)-6-(2-morpholinoethoxy)quinazolin-4-amine (9d)
Compound 9d was prepared by general procedure B. Yield: 81%. 1H-NMR (600 MHz, CDCl3) δ 8.97 (q, J = 1.8 Hz, 1H), 8.73 (s, 1H), 8.70–8.69 (m, 1H), 8.34 (s, 1H), 7.86 (d, J = 9.0 Hz, 1H), 7.81 (d, J = 8.3 Hz, 1H), 7.68 (d, J = 2.8 Hz, 1H), 7.54–7.51 (m, 2H), 7.46 (d, J = 4.1 Hz, 1H), 7.45 (t, J = 3.4 Hz, 2H), 6.96 (d, J = 8.3 Hz, 1H), 6.73 (d, J = 7.6 Hz, 1H), 4.05 (t, J = 5.5 Hz, 2H), 3.70 (t, J = 4.5 Hz, 4H), 2.76 (t, J = 5.9 Hz, 2H), 2.49 (s, 4H), 2.23 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 157.2, 157.1, 153.6, 153.1, 150.9, 150.6, 149.2, 145.5, 135.0, 130.8, 130.8, 130.3, 129.4, 125.4, 124.5, 123.2, 121.3, 121.1, 120.8, 120.7, 115.8, 110.1, 101.4, 66.8, 66.1, 57.4, 54.0, 16.4; HRMS (ESI): m/z calcd for C30H30N5O3 [M + H]+ 508.2343, found 508.2346.
6-Iodo-N-(3-methyl-4-((2-methylquinolin-8-yl)oxy)phenyl)quinazolin-4-amine (10a)
Compound 10a was prepared by general procedure B. Yield: 70%. 1H-NMR (600 MHz, CDCl3) δ 9.45 (s, 1H), 8.80 (d, J = 1.5 Hz, 1H), 8.72 (s, 1H), 8.09 (d, J = 8.4 Hz, 1H), 7.89 (dd, J = 8.8, 1.5 Hz, 1H), 7.55 (d, J = 8.7 Hz, 2H), 7.44–7.41 (m, 2H), 7.35 (d, J = 8.4 Hz, 1H), 7.24 (t, J = 7.9 Hz, 1H), 6.77–6.73 (m, 2H), 2.67 (s, 3H), 2.01 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 158.7, 156.9, 155.5, 153.8, 150.6, 149.2, 141.3, 139.6, 136.5, 135.0, 131.4, 131.1, 130.0, 128.1, 125.8, 125.3, 123.1, 121.4, 121.4, 121.2, 117.4, 113.3, 90.2, 25.1, 16.4; HRMS (ESI): m/z calcd for C25H20IN4O [M + H]+ 519.0676, found 519.0675.
6-Iodo-N-(3-methyl-4-(quinolin-8-yloxy)phenyl)quinazolin-4-amine (10b)
Compound 10b was prepared by general procedure B. Yield: 56%. 1H-NMR (600 MHz, CDCl3) δ 9.11 (s, 1H), 8.93 (dd, J = 4.1, 1.5 Hz, 1H), 8.73 (s, 1H), 8.63 (d, J = 1.5 Hz, 1H), 8.22 (dd, J = 8.3, 1.5 Hz, 1H), 7.92 (dd, J = 8.8, 1.6 Hz, 1H), 7.57 (d, J = 8.8 Hz, 1H), 7.54 (d, J = 2.2 Hz, 1H), 7.51 (d, J = 8.2 Hz, 1H), 7.48 (q, J = 4.2 Hz, 1H), 7.43 (dd, J = 8.6, 2.5 Hz, 1H), 7.35 (t, J = 8.0 Hz, 1H), 6.87 (d, J = 7.7 Hz, 1H), 6.79 (d, J = 8.7 Hz, 1H), 2.12 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 156.9, 155.4, 154.0, 151.0, 149.6, 149.2, 141.4, 140.2, 136.4, 134.7, 131.1, 130.7, 130.1, 129.8, 126.8, 125.4, 122.0, 121.7, 121.4, 120.7, 117.3, 113.8, 90.4, 16.3; HRMS (ESI): m/z calcd for C24H18IN4O [M + H]+ 505.052, found 505.0554.
6-Iodo-N-(4-(isoquinolin-8-yloxy)-3-methylphenyl)quinazolin-4-amine (10c)
Compound 10c was prepared by general procedure B. Yield: 83%. 1H-NMR (600 MHz, CDCl3) δ 9.71 (s, 1H), 9.31 (s, 1H), 8.78 (s, 1H), 8.71 (s, 1H), 8.66 (d, J = 5.5 Hz, 1H), 7.97 (dd, J = 9.0, 1.4 Hz, 1H), 7.69 (d, J = 5.5 Hz, 1H), 7.66 (d, J = 1.4 Hz, 1H), 7.62–7.57 (m, 2H), 7.46 (d, J = 6.2 Hz, 2H), 7.02 (d, J = 8.3 Hz, 1H), 6.67 (q, J = 2.8 Hz, 1H), 2.16 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 157.1, 155.3, 154.8, 150.2, 149.0, 147.5, 143.4, 141.5, 137.1, 135.1, 131.1, 131.0, 130.9, 130.0, 126.2, 122.2, 121.1, 120.8, 120.3, 120.0, 117.2, 110.3, 90.8, 16.2; HRMS (ESI): m/z calcd for C24H18IN4O [M + H]+ 505.052, found 505.0531.
N-(3-Chloro-4-(isoquinolin-8-yloxy)phenyl)-6-iodoquinazolin-4-amine (10d)
Compound 10d was prepared by general procedure B. Yield: 75%. 1H-NMR (600 MHz, CDCl3) δ 9.76 (s, 1H), 8.81 (s, 1H), 8.65 (d, J = 5.5 Hz, 1H), 8.42 (d, J = 1.4 Hz, 1H), 8.10 (d, J = 2.1 Hz, 1H), 8.07–8.06 (m, 2H), 7.75–7.62 (m, 3H), 7.55–7.54 (m, 2H), 7.20 (d, J = 8.3 Hz, 1H), 6.80 (q, J = 2.8 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 155.1, 149.4, 148.2, 147.7, 144.0, 141.9, 137.3, 135.7, 131.0, 130.6, 129.4, 127.2, 124.6, 124.3, 122.5, 121.6, 121.2, 121.1, 120.1, 120.1, 116.8, 111.2, 91.3.
6-Ethynyl-N-(3-methyl-4-((2-methylquinolin-8-yl)oxy)phenyl)quinazolin-4-amine (11a)
Compound 11a was prepared by general procedure D. Yield: 94%. 1H-NMR (600 MHz, CDCl3) δ 8.74 (s, 1H), 8.53 (s, 1H), 8.34 (s, 1H), 8.08 (d, J = 8.3 Hz, 1H), 7.82–7.77 (m, 2H), 7.61 (s, 1H), 7.51 (dd, J = 9.0, 2.1 Hz, 1H), 7.44 (d, J = 7.6 Hz, 1H), 7.35 (d, J = 8.3 Hz, 1H), 7.28 (d, J = 8.3 Hz, 1H), 6.92 (d, J = 8.3 Hz, 1H), 6.81 (d, J = 6.9 Hz, 1H), 3.04 (s, 1H), 2.75 (s, 3H), 2.16 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 158.7, 157.3, 155.7, 153.8, 151.1, 149.6, 139.7, 136.3, 135.7, 134.6, 131.4, 128.6, 128.1, 125.8, 125.6, 125.1, 123.0, 121.8, 121.2, 121.2, 120.2, 115.1, 113.3, 82.5, 78.6, 25.4, 16.4; HRMS (ESI): m/z calcd for C27H21N4O [M + H]+ 417.1715, found 417.1710.
6-Ethynyl-N-(3-methyl-4-(quinolin-8-yloxy)phenyl)quinazolin-4-amine (11b)
Compound 11b was prepared by general procedure D. Yield: 70%. 1H-NMR (600 MHz, CDCl3) δ 8.98 (q, J = 1.8 Hz, 1H), 8.74 (s, 1H), 8.48 (s, 1H), 8.30 (s, 1H), 8.21 (dd, J = 8.3, 1.4 Hz, 1H), 7.82–7.77 (m, 2H), 7.61 (s, 1H), 7.53–7.47 (m, 3H), 7.36 (t, J = 7.9 Hz, 1H), 6.94 (d, J = 8.3 Hz, 1H), 6.86 (d, J = 7.6 Hz, 1H), 3.06 (s, 1H), 2.20 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 157.4, 155.7, 154.2, 151.0, 149.7, 149.7, 140.2, 136.2, 135.7, 134.6, 132.1, 131.2, 129.8, 128.7, 126.7, 125.7, 125.2, 121.9, 121.5, 121.3, 120.2, 115.1, 113.3, 82.6, 78.7, 16.4; HRMS (ESI): m/z calcd for C26H19N4O [M + H]+ 403.1553, found 403.1553.
6-Ethynyl-N-(4-(isoquinolin-8-yloxy)-3-methylphenyl)quinazolin-4-amine (11c)
Compound 12c was prepared by general procedure D. Yield: 83%. 1H-NMR (600 MHz, CDCl3) δ 9.71 (s, 1H), 8.85 (s, 1H), 8.77 (s, 1H), 8.67 (d, J = 5.5 Hz, 1H), 8.43 (s, 1H), 7.84 (t, J = 10.0 Hz, 2H), 7.68 (d, J = 6.2 Hz, 2H), 7.60 (d, J = 7.6 Hz, 1H), 7.47 (q, J = 8.0 Hz, 2H), 7.06 (d, J = 8.3 Hz, 1H), 6.70 (d, J = 6.9 Hz, 1H), 3.18 (s, 1H), 2.20 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 157.7, 155.8, 154.9, 150.4, 149.8, 147.7, 143.6, 137.2, 135.8, 135.1, 131.3, 131.0, 128.8, 126.2, 125.8, 122.2, 121.3, 120.9, 120.3, 120.3, 120.1, 115.1, 110.3, 82.7, 78.8, 16.3; HRMS (ESI): m/z calcd for C26H19N4O [M + H]+ 403.1553, found 403.1552.
6-(1-(4-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)-N-(3-methyl-4-((2-methylquinolin-8-yl)oxy)phenyl)quinazolin-4-amine (12a)
Compound 12a was prepared by general procedure F. Yield: 41%. 1H-NMR (600 MHz, CDCl3) δ 8.81 (s, 1H), 8.74 (s, 1H), 8.08 (d, J = 8.3 Hz, 1H), 8.04 (d, J = 9.0 Hz, 1H), 7.91 (d, J = 9.0 Hz, 1H), 7.83 (s, 1H), 7.70 (s, 1H), 7.61 (d, J = 7.6 Hz, 1H), 7.44 (d, J = 6.9 Hz, 1H), 7.36 (d, J = 8.3 Hz, 1H), 7.33–7.28 (m, 3H), 7.08 (t, J = 8.6 Hz, 2H), 7.01 (q, J = 2.8 Hz, 1H), 6.84 (d, J = 7.6 Hz, 1H), 5.55 (s, 2H), 2.80 (s, 3H), 2.22 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 163.8, 162.2, 158.8, 157.8, 154.9, 153.9, 151.1, 147.2, 139.7, 136.2, 134.7, 131.5, 130.4, 130.2, 130.1, 130.0, 128.9, 128.5, 128.0, 125.6, 125.1, 122.9, 121.9, 121.9, 121.2, 120.1, 117.8, 116.4, 116.3, 116.2, 115.6, 113.3, 53.7, 25.6, 16.5; HRMS (ESI): m/z calcd for C34H27FN7O [M + H]+ 568.2256, found 568.2251.
6-(1-(4-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)-N-(3-methyl-4-(quinolin-8-yloxy)phenyl)quinazolin-4-amine (12b)
Compound 12b was prepared by general procedure F. Yield: 48%. 1H-NMR (600 MHz, CDCl3) δ 9.02 (q, J = 2.1 Hz, 1H), 8.74 (s, 2H), 8.21 (dd, J = 8.3, 1.4 Hz, 1H), 8.03 (d, J = 9.0 Hz, 1H), 7.90 (d, J = 9.0 Hz, 1H), 7.83 (s, 1H), 7.71 (s, 1H), 7.62 (d, J = 8.3 Hz, 1H), 7.50 (q, J = 4.1 Hz, 2H), 7.38 (t, J = 7.9 Hz, 1H), 7.32 (dd, J = 8.3, 5.5 Hz, 2H), 7.08 (t, J = 8.6 Hz, 2H), 7.03 (d, J = 9.0 Hz, 1H), 6.88–6.86 (m, 1H), 5.55 (s, 2H), 2.26 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 163.8, 162.2, 157.8, 155.1, 154.3, 150.9, 149.8, 147.2, 140.2, 136.1, 134.8, 131.4, 130.4, 130.1, 130.1, 130.0, 129.8, 129.2, 128.5, 126.7, 125.0, 124.9, 121.9, 121.7, 121.3, 121.1, 120.1, 117.6, 116.4, 116.3, 115.5, 113.0, 53.7, 16.4; HRMS (ESI): m/z calcd for C33H25FN7O [M + H]+ 554.2099, found 554.2095.
6-(1-(4-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)-N-(4-(isoquinolin-8-yloxy)-3-methylphenyl)quinazolin-4-amine (12c)
Compound 12c was prepared by general procedure F. Yield: 54%. 1H-NMR (600 MHz, CDCl3) δ 9.79 (s, 1H), 8.75 (d, J = 4.8 Hz, 2H), 8.62 (d, J = 5.5 Hz, 1H), 8.16 (s, 1H), 8.03 (d, J = 9.0 Hz, 1H), 7.91 (d, J = 8.3 Hz, 1H), 7.86 (s, 1H), 7.73 (s, 1H), 7.68–7.65 (m, 2H), 7.53–7.47 (m, 2H), 7.32 (dd, J = 8.3, 5.5 Hz, 2H), 7.08 (t, J = 8.6 Hz, 3H), 6.73 (d, J = 7.6 Hz, 1H), 5.56 (s, 2H), 2.26 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 163.7, 162.1, 158.0, 154.9, 154.0, 150.3, 147.3, 146.9, 143.1, 137.3, 134.7, 131.3, 131.1, 130.8, 130.2, 130.2, 130.0, 130.0, 129.1, 127.6, 125.7, 121.7, 121.0, 120.9, 120.7, 120.4, 120.2, 118.3, 116.3, 116.1, 115.2, 110.6, 53.6, 16.3; HRMS (ESI): m/z calcd for C33H25FN7O [M + H]+ 554.2099, found 554.2094.
N-(4-(Isoquinolin-8-yloxy)-3-methylphenyl)-6-(3-morpholinoprop-1-yn-1-yl)quinazolin-4-amine (13a)
Compound 13a was prepared by general procedure D. Yield: 51%. 1H-NMR (600 MHz, CDCl3) δ 9.76 (s, 1H), 8.77 (s, 1H), 8.64 (d, J = 6.2 Hz, 1H), 8.20 (s, 1H), 8.13 (s, 1H), 7.85 (d, J = 8.3 Hz, 1H), 7.81–7.79 (m, 1H), 7.71 (s, 1H), 7.68 (d, J = 5.5 Hz, 1H), 7.62 (dd, J = 8.3, 2.1 Hz, 1H), 7.52–7.47 (m, 2H), 7.08 (d, J = 9.0 Hz, 1H), 6.74 (d, J = 7.6 Hz, 1H), 3.78 (t, J = 4.1 Hz, 4H), 3.56 (s, 2H), 2.66 (s, 4H), 2.26 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 157.2, 155.5, 154.9, 150.2, 149.4, 147.6, 143.6, 137.2, 135.7, 134.9, 131.3, 131.0, 128.9, 125.5, 124.3, 121.5, 121.3, 121.3, 120.9, 120.2, 120.1, 115.0, 110.3, 86.0, 84.7, 66.8, 52.4, 48.0, 16.3; HRMS (ESI): m/z calcd for C31H28N5O2 [M + H]+ 502.2238, found 502.2235.
N-(4-(Isoquinolin-8-yloxy)-3-methylphenyl)-6-(3-(piperidin-1-yl)prop-1-yn-1-yl)quinazolin-4-amine (13b)
Compound 13b was prepared by general procedure D. Yield: 34%. 1H-NMR (600 MHz, CDCl3) δ 9.78 (s, 1H), 8.76 (s, 1H), 8.63 (d, J = 6.2 Hz, 1H), 8.19 (s, 1H), 7.96 (s, 1H), 7.84 (d, J = 9.0 Hz, 1H), 7.81–7.79 (m, 1H), 7.74 (d, J = 2.1 Hz, 1H), 7.67–7.63 (m, 2H), 7.51–7.46 (m, 2H), 7.08 (d, J = 8.3 Hz, 1H), 6.73 (d, J = 7.6 Hz, 1H), 3.54 (s, 2H), 2.65 (s, 4H), 2.27 (s, 3H), 1.71–1.67 (m, 4H), 1.48 (s, 2H); 13C-NMR (150 MHz, CDCl3) δ 157.2, 155.5, 155.0, 150.3, 149.5, 147.7, 143.8, 137.2, 135.7, 135.0, 131.3, 130.9, 130.8, 128.9, 125.5, 124.4, 121.5, 121.4, 121.4, 120.9, 120.1, 115.0, 110.4, 86.8, 84.4, 53.7, 48.5, 25.8, 23.7, 16.3; HRMS (ESI): m/z calcd for C32H30N5O [M + H]+ 500.2445, found 500.2442.
N-(4-(Isoquinolin-8-yloxy)-3-methylphenyl)-6-(3-(pyrrolidin-1-yl)prop-1-yn-1-yl)quinazolin-4-amine (13c)
Compound 13c was prepared by general procedure D. Yield: 7%. 1H-NMR (600 MHz, CDCl3) δ 9.80 (s, 1H), 8.77 (s, 1H), 8.62 (d, J = 4.8 Hz, 1H), 8.03 (s, 1H), 7.85 (d, J = 9.0 Hz, 1H), 7.82–7.81 (m, 1H), 7.73 (d, J = 2.1 Hz, 1H), 7.67 (d, J = 5.5 Hz, 1H), 7.64 (dd, J = 9.0, 2.8 Hz, 1H), 7.53–7.48 (m, 3H), 7.11 (d, J = 8.3 Hz, 1H), 6.75 (d, J = 7.6 Hz, 1H), 3.69 (s, 2H), 2.75 (s, 4H), 2.31 (s, 3H), 1.89 (d, J = 5.9 Hz, 4H), 1.71 (s, 4H); 13C-NMR (150 MHz, CDCl3) δ 157.9, 156.9, 155.4, 155.0, 150.3, 149.5, 147.7, 143.9, 137.2, 135.8, 134.8, 131.4, 130.8, 129.1, 125.1, 123.8, 121.7, 121.4, 121.1, 120.2, 120.1, 114.9, 110.4, 87.6, 83.5, 53.1, 44.1, 23.8, 16.4; HRMS (ESI): m/z calcd for C31H28N5O [M + H]+ 486.2288, found 486.2287.
1-(4-(3-(4-((4-(Isoquinolin-8-yloxy)-3-methylphenyl)amino)quinazolin-6-yl)prop-2-yn-1-yl)piperazin-1-yl)ethanone (13d)
Compound 13d was prepared by general procedure D. Yield: 63%. 1H-NMR (600 MHz, CDCl3) δ 9.77 (s, 1H), 8.76 (s, 1H), 8.62 (d, J = 6.2 Hz, 1H), 8.37 (s, 1H), 8.24 (s, 1H), 7.83 (d, J = 8.3 Hz, 1H), 7.78 (dd, J = 9.0, 1.4 Hz, 1H), 7.72 (s, 1H), 7.67 (d, J = 6.2 Hz, 1H), 7.63 (dd, J = 8.3, 2.1 Hz, 1H), 7.51–7.47 (m, 2H), 7.07 (d, J = 8.3 Hz, 1H), 6.73 (d, J = 6.9 Hz, 1H), 3.69 (s, 2H), 3.59 (s, 2H), 3.53 (t, J = 5.2 Hz, 2H), 2.64 (t, J = 4.8 Hz, 2H), 2.59 (t, J = 5.2 Hz, 2H), 2.26 (s, 3H), 2.10 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 169.1, 157.4, 155.6, 155.0, 150.3, 149.6, 147.6, 143.8, 137.2, 135.7, 135.0, 131.2, 130.9, 128.9, 125.7, 124.7, 121.7, 121.2, 121.0, 120.9, 120.2, 120.1, 115.1, 110.4, 85.6, 85.0, 52.1, 51.6, 47.7, 46.2, 41.4, 21.4, 16.3; HRMS (ESI): m/z calcd for C33H31N6O2 [M + H]+ 543.2503, found 543.2498.
1-(3-(4-((4-(Isoquinolin-8-yloxy)-3-methylphenyl)amino)quinazolin-6-yl)prop-2-yn-1-yl)pyrrolidin-2-one (13e)
Compound 13e was prepared by general procedure D. Yield: 39%. 1H-NMR (600 MHz, CDCl3) δ 9.78 (s, 1H), 8.76 (s, 1H), 8.62 (d, J = 5.5 Hz, 1H), 8.54 (s, 1H), 8.29 (s, 1H), 7.82 (d, J = 8.3 Hz, 1H), 7.78 (d, J = 2.1 Hz, 1H), 7.76 (dd, J = 9.0, 1.4 Hz, 1H), 7.70–7.67 (m, 2H), 7.49 (q, J = 8.3 Hz, 2H), 7.08 (d, J = 8.3 Hz, 1H), 6.72 (dd, J = 6.9, 1.4 Hz, 1H), 4.41 (s, 2H), 3.60 (t, J = 7.2 Hz, 2H), 2.45 (t, J = 7.9 Hz, 2H), 2.27 (s, 3H), 2.12 (q, J = 7.6 Hz, 2H); 13C-NMR (150 MHz, CDCl3) δ 174.9, 157.4, 155.7, 155.0, 150.2, 149.7, 147.6, 143.8, 137.2, 135.5, 135.2, 131.2, 130.9, 128.8, 125.5, 125.3, 121.5, 121.2, 120.9, 120.5, 120.2, 120.1, 115.2, 110.4, 84.7, 83.3, 46.7, 32.9, 30.7, 17.7, 16.4; HRMS (ESI): m/z calcd for C31H26N5O2 [M + H]+ 500.2081, found 500.2079.
N-(3-Chloro-4-(isoquinolin-8-yloxy)phenyl)-6-(3-morpholinoprop-1-yn-1-yl)quinazolin-4-amine (13f)
Compound 13f was prepared by general procedure D. Yield: 18%. 1H-NMR (600 MHz, CDCl3) δ 9.80 (s, 1H), 8.80 (s, 1H), 8.65 (s, 1H), 8.13–8.10 (m, 2H), 7.88–7.82 (m, 3H), 7.68 (dd, J = 8.6, 2.4 Hz, 2H), 7.54 (q, J = 7.3 Hz, 2H), 7.21 (d, J = 9.0 Hz, 1H), 6.79 (dd, J = 6.5, 1.7 Hz, 1H), 3.79 (t, J = 4.1 Hz, 4H), 3.57 (s, 2H), 2.68–2.64 (m, 4H); 13C-NMR (150 MHz, CDCl3) δ 156.8, 155.2, 154.4, 149.6, 147.8, 147.7, 143.9, 143.8, 137.2, 135.9, 135.8, 130.7, 129.2, 127.1, 124.3, 123.9, 122.5, 121.7, 121.6, 121.0, 120.2, 114.8, 111.0, 86.4, 84.6, 66.9, 52.5, 48.1; HRMS (ESI): m/z calcd for C30H25ClN5O2 [M + H]+ 522.1691, found 522.1691.
N-(3-Chloro-4-(isoquinolin-8-yloxy)phenyl)-6-(1-(4-fluorobenzyl)-1H-1,2,3-triazol-4-yl)quinazolin-4-amine (14a)
Compound 14a was prepared by general procedure F. Yield: 87%. 1H-NMR (600 MHz, CDCl3) δ 9.79 (s, 1H), 8.78 (s, 1H), 8.77 (s, 1H), 8.62 (s, 1H), 8.28 (1H), 8.12 (d, J = 2.8 Hz, 1H), 8.05–7.95 (m, 1H), 7.91 (d, J = 9.0 Hz, 1H), 7.78 (s, 1H), 7.68 (dd, J = 8.6, 2.4 Hz, 2H), 7.53 (q, J = 7.3 Hz, 2H), 7.30 (q, J = 4.6 Hz, 2H), 7.17 (d, J = 9.0 Hz, 1H), 7.11–7.01 (m, 2H), 6.77 (dd, J = 6.5, 1.7 Hz, 1H), 5.55 (s, 2H); 13C-NMR (150 MHz, CDCl3) δ 163.8, 162.2, 157.8, 154.4, 154.4, 147.5, 147.4, 147.1, 143.4, 137.3, 136.2, 131.1, 130.8, 130.2, 130.2, 130.1, 130.1, 129.1, 128.5, 126.8, 124.5, 122.4, 121.9, 121.0, 120.6, 120.5, 120.4, 118.1, 116.4, 116.3, 115.5, 111.2, 53.8; HRMS (ESI): m/z calcd for C32H22ClFN7O [M + H]+ 574.1553, found 574.1547.
N-(3-Chloro-4-(isoquinolin-8-yloxy)phenyl)-6-(1-(3-morpholinopropyl)-1H-1,2,3-triazol-4-yl)quinazolin-4-amine (14b)
Compound 14b was prepared by general procedure F. Yield: 77%. 1H-NMR (600 MHz, CDCl3) δ 9.80 (s, 1H), 8.79 (d, J = 10.3 Hz, 2 H), 8.63 (d, J = 5.5 Hz, 1H), 8.23 (s, 1H), 8.17 (d, J = 2.1 Hz, 1H), 8.07–8.00 (m, 1H), 7.95 (d, J = 9.0 Hz, 1H), 7.92 (s, 1H), 7.71 (dd, J = 8.6, 2.4 Hz, 1H), 7.68 (d, J = 5.5 Hz, 1H), 7.53 (q, J = 7.1 Hz, 2H), 7.19 (d, J = 8.3 Hz, 1H), 6.78 (dd, J = 6.9, 2.1 Hz, 1H), 4.52 (t, J = 6.9 Hz, 2H), 3.72 (t, J = 4.5 Hz, 4H), 2.44 (s, 4H), 2.40 (t, J = 6.9 Hz, 2H), 2.22–2.09 (m, 2H); 13C-NMR (150 MHz, CDCl3) δ 157.5, 154.9, 154.4, 149.8, 147.7, 147.5, 146.5, 143.9, 137.2, 136.2, 130.7, 130.5, 129.6, 128.9, 127.0, 124.1, 122.5, 121.5, 120.9, 120.6, 120.6, 120.1, 117.2, 115.5, 110.9, 66.9, 55.0, 53.6, 48.5, 27.0; HRMS (ESI): m/z calcd for C32H30ClN8O2 [M + H]+ 593.2175, found 593.2172.
3-(4-(4-((3-Chloro-4-(isoquinolin-8-yloxy)phenyl)amino)quinazolin-6-yl)-1H-1,2,3-triazol-1-yl)propan-1-ol (14c)
Compound 14c was prepared by general procedure F. Yield: 36%. 1H-NMR (600 MHz, CDCl3) δ 9.75 (s, 1H), 8.76 (s, 1H), 8.73 (s, 1H), 8.59 (d, J = 5.5 Hz, 1H), 8.43 (s, 1H), 8.12 (d, J = 2.1 Hz, 1H), 8.04–7.95 (m, 1H), 7.91 (s, 1H), 7.88 (d, J = 9.0 Hz, 1H), 7.74–7.68 (m, 1H), 7.66 (d, J = 5.5 Hz, 1H), 7.53–7.50 (m, 2H), 7.13 (d, J = 9.0 Hz, 1H), 6.76 (q, J = 2.8 Hz, 1H), 4.59 (t, J = 6.9 Hz, 2H), 3.73 (t, J = 5.9 Hz, 2H), 2.26–2.12 (m, 3H); 13C-NMR (150 MHz, CDCl3) δ 157.6, 154.9, 154.4, 149.7, 147.6, 147.5, 146.5, 143.7, 137.2, 136.2, 130.8, 130.5, 129.3, 129.3, 128.8, 126.8, 124.1, 122.4, 121.5, 121.0, 120.9, 120.2, 117.4, 115.5, 111.1, 58.6, 47.4, 32.5; HRMS (ESI): m/z calcd for C28H23ClN7O2 [M + H]+ 524.1596, found 524.1596.
N-(3-Chloro-4-(isoquinolin-8-yloxy)phenyl)-6-(1-(2-morpholinoethyl)-1H-1,2,3-triazol-4-yl)quinazolin-4-amine (14d)
Compound 14d was prepared by general procedure F. Yield: 94%. 1H-NMR (600 MHz, CDCl3) δ 9.79 (s, 1H), 8.80 (d, J = 5.5 Hz, 2H), 8.63 (d, J = 5.5 Hz, 1H), 8.24 (s, 1H), 8.15 (d, J = 1.0 Hz, 1H), 8.10–8.01 (m, 2H), 7.96 (d, J = 9.0 Hz, 1H), 7.75–7.62 (m, 2H), 7.59–7.47 (m, 2H), 7.19 (d, J = 9.0 Hz, 1H), 6.77 (q, J = 2.8 Hz, 1H), 4.54 (t, J = 6.2 Hz, 2H), 3.71 (t, J = 4.5 Hz, 4H), 2.88 (t, J = 6.2 Hz, 2H), 2.53 (m, 4H); 13C-NMR (150 MHz, CDCl3) δ 157.5, 154.8, 154.4, 149.8, 147.7, 147.5, 146.5, 143.9, 137.2, 136.1, 130.7, 130.6, 129.5, 129.0, 126.9, 124.2, 122.5, 121.5, 120.9, 120.9, 120.8, 120.1, 117.2, 115.5, 110.9, 66.9, 57.9, 53.5, 47.7; HRMS (ESI): m/z calcd for C31H28ClN8O2 [M + H]+ 579.2018, found 579.2012.
N-(3-Chloro-4-(isoquinolin-8-yloxy)phenyl)-6-(1-(2-((2-(methylsulfonyl)ethyl)amino)ethyl)-1H-1,2,3-triazol-4-yl)quinazolin-4-amine (14e)
Compound 14e was prepared by general procedure F. Yield: 50%. 1H-NMR (600 MHz, Methanol-d4) δ 9.68 (s, 1H), 8.78 (d, J = 1.4 Hz, 1H), 8.58 (s, 1H), 8.52 (d, J = 4.8 Hz, 1H), 8.50 (s, 1H), 8.27 (dd, J = 8.6, 1.7 Hz, 1H), 8.22 (d, J = 2.1 Hz, 1H), 7.86–7.84 (m, 2H), 7.82 (d, J = 8.3 Hz, 1H), 7.63 (t, J = 3.1 Hz, 2H), 7.32 (d, J = 8.3 Hz, 1H), 6.82 (q, J = 2.8 Hz, 1H), 4.61 (t, J = 5.9 Hz, 2H), 3.31–3.29 (m, 2H), 3.23 (t, J = 5.9 Hz, 2H), 3.17 (t, J = 6.2 Hz, 2H), 2.97 (s, 3H); 13C-NMR (150 MHz, methanol-d4) δ 159.7, 159.7, 156.0, 155.6, 150.1, 148.5, 147.9, 147.7, 143.8, 139.0, 138.5, 132.9, 132.2, 130.9, 128.9, 127.7, 126.1, 124.0, 124.0, 123.7, 122.2, 122.0, 120.0, 116.9, 112.0, 55.0, 51.2, 49.9, 43.7, 42.1; HRMS (ESI): m/z calcd for C30H28ClN8O3S [M + H]+ 615.1688, found 613.1523.
N-(3-Chloro-4-(isoquinolin-8-yloxy)phenyl)-6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)furan-2-yl)quinazolin-4-amine (14f)
Compound 14f was prepared by general procedure E. Yield: 64%. 1H-NMR (600 MHz, CDCl3) δ 9.81 (s, 1H), 8.76 (s, 1H), 8.61 (d, J = 5.5 Hz, 1H), 8.55 (s, 1H), 8.47 (s, 1H), 8.18 (d, J = 2.8 Hz, 1H), 7.98 (dd, J = 9.0, 1.4 Hz, 1H), 7.94–7.83 (m, 2H), 7.67 (d, J = 5.5 Hz, 1H), 7.54–7.53 (m, 2H), 7.21 (d, J = 8.3 Hz, 1H), 6.79 (dd, J = 6.9, 1.4 Hz, 1H), 6.71 (d, J = 2.8 Hz, 1H), 6.31 (d, J = 3.4 Hz, 1H), 3.92 (s, 2H), 3.32 (s, 4H), 2.97 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 157.7, 154.7, 154.6, 154.0, 152.4, 149.6, 147.8, 147.4, 143.9, 137.3, 136.7, 130.8, 129.3, 129.1, 129.0, 126.8, 124.4, 122.5, 122.0, 121.0, 120.9, 120.1, 115.7, 115.3, 110.9, 110.0, 107.4, 54.8, 45.5, 42.2, 42.1; HRMS (ESI): m/z calcd for C31H27ClN5O4S [M + H]+ 600.1467, found 600.1461.
N-(3-Chloro-4-(isoquinolin-8-yloxy)phenyl)-6-(5-(morpholinomethyl)furan-2-yl)quinazolin-4-amine (14g)
Compound 14g was prepared by general procedure E. Yield: 68%. 1H-NMR (600 MHz, CDCl3) δ 9.79 (s, 1H), 8.78 (s, 1H), 8.62 (d, J = 6.2 Hz, 1H), 8.41 (s, 1H), 8.39 (s, 1H), 8.13 (d, J = 2.1 Hz, 1H), 8.03 (dd, J = 8.6, 1.7 Hz, 1H), 7.90 (d, J = 8.3 Hz, 1H), 7.73 (dd, J = 8.6, 2.4 Hz, 1H), 7.67 (d, J = 6.2 Hz, 1H), 7.53–7.52 (m, 2H), 7.18 (d, J = 8.3 Hz, 1H), 6.77 (q, J = 3.0 Hz, 1H), 6.72 (d, J = 2.8 Hz, 1H), 6.37 (d, J = 2.8 Hz, 1H), 3.76 (t, J = 4.5 Hz, 4H), 3.59 (s, 2H), 2.53 (s, 4H); 13C-NMR (150 MHz, CDCl3) δ 157.5, 154.5, 154.4, 152.6, 151.8, 149.4, 147.7, 147.5, 143.8, 137.2, 136.3, 130.7, 129.3, 129.2, 129.1, 127.9, 124.4, 122.5, 121.8, 120.9, 120.9, 120.1, 115.4, 114.7, 111.9, 110.9, 107.5, 66.8, 55.5, 53.4; HRMS (ESI): m/z calcd for C32H27ClN5O3 [M + H]+ 564.1797, found 564.1793.
(E)-N-(3-Chloro-4-(isoquinolin-8-yloxy)phenyl)-6-(3-morpholinoprop-1-en-1-yl)quinazolin-4-amine (14h)
Compound 14h was prepared by general procedure E. Yield: 28%. 1H-NMR (600 MHz, Acetone-d6) δ 9.74 (s, 1H), 9.38 (s, 1H), 8.69 (s, 1H), 8.62 (d, J = 5.5 Hz, 1H), 8.47–8.46 (m, 2H), 8.02 (td, J = 5.5, 3.0 Hz, 2H), 7.88–7.77 (m, 2H), 7.68–7.67 (m, 2H), 7.38 (d, J = 9.0 Hz, 1H), 6.84 (q, J = 3.0 Hz, 1H), 6.76 (d, J = 15.8 Hz, 1H), 6.53 (dt, J = 15.8, 6.5 Hz, 1H), 3.64 (t, J = 4.5 Hz, 4H), 3.19 (dd, J = 6.2, 1.4 Hz, 2H), 2.47 (s, 4H); 13C-NMR (150 MHz, Acetone-d6) δ 158.4, 155.3, 154.9, 150.8, 147.8, 147.3, 144.9, 138.7, 138.1, 136.5, 132.2, 131.9, 131.7, 129.9, 129.6, 126.7, 124.4, 123.5, 122.6, 121.8, 121.5, 120.8, 120.1, 116.3, 111.4, 67.5, 61.8, 54.5; HRMS (ESI): m/z calcd for C30H27ClN5O2 [M + H]+ 524.18478, found 524.18439.
(E)-N-(3-(4-((3-Chloro-4-(isoquinolin-8-yloxy)phenyl)amino)quinazolin-6-yl)allyl)-2-methoxyacetamide (14i)
Compound 14i was prepared by general procedure E. Yield: 27%. 1H-NMR (600 MHz, CDCl3) δ 9.81 (s, 1H), 8.78 (s, 1H), 8.62 (d, J = 5.5 Hz, 1H), 8.15 (d, J = 2.8 Hz, 1H), 7.88 (s, 2H), 7.83 (s, 1H), 7.76–7.73 (m, 2H), 7.68 (d, J = 5.5 Hz, 1H), 7.55 (d, J = 6.9 Hz, 2H), 7.22 (d, J = 9.0 Hz, 1H), 6.84 (s, 1H), 6.79 (dd, J = 6.3, 2.1 Hz, 1H), 6.70 (d, J = 15.8 Hz, 1H), 6.38 (dt, J = 16.2, 6.0 Hz, 1H), 4.17 (t, J = 5.9 Hz, 2H), 3.98 (s, 2H), 3.47 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 169.7, 157.1, 154.5, 154.4, 149.8, 147.6, 143.9, 137.2, 136.1, 135.3, 131.0, 130.8, 130.7, 129.3, 127.8, 127.1, 125.8, 124.0, 122.6, 121.4, 121.0, 120.1, 118.1, 115.8, 115.1, 110.9, 72.0, 59.3, 40.8; HRMS (ESI): m/z calcd for C29H25ClN5O3 [M + H]+ 526.1641, found 526.1658.
N-(4-(Isoquinolin-8-yloxy)-3-methylphenyl)-6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)furan-2-yl)quinazolin-4-amine (14j)
Compound 14j was prepared by general procedure E. Yield: 66%. 1H-NMR (600 MHz, CDCl3) δ 9.80 (s, 1H), 8.74 (s, 1H), 8.61 (d, J = 5.5 Hz, 1H), 8.48 (s, 2H), 7.97 (d, J = 9.0 Hz, 1H), 7.88 (d, J = 9.0 Hz, 1H), 7.81 (s, 1H), 7.74 (d, J = 8.3 Hz, 1H), 7.67 (d, J = 5.5 Hz, 1H), 7.52–7.46 (m, 2H), 7.09 (d, J = 9.0 Hz, 1H), 6.75 (d, J = 7.6 Hz, 1H), 6.71 (d, J = 3.4 Hz, 1H), 6.32 (d, J = 3.4 Hz, 1H), 3.93 (s, 2H), 3.30 (s, 4H), 2.94 (s, 3H), 2.28 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 157.9, 155.1, 154.9, 153.7, 152.4, 150.0, 149.4, 147.7, 143.8, 137.2, 135.4, 131.1, 130.9, 129.1, 128.8, 128.8, 125.6, 121.6, 121.2, 120.9, 120.1, 120.0, 115.7, 115.3, 110.3, 109.6, 107.2, 54.7, 45.5, 45.5, 42.0, 16.3; HRMS (ESI): m/z calcd for C32H30N5O4S [M + H]+ 580.2013, found 580.2033.
In vitro kinase assay
Kinase profiles (IC50 or % inhibition) of final compounds were obtained using the kinase HotSpot Profiling service of Reaction Biology Corporation (USA). All kinase assays were performed in the presence of Km concentration of ATP.
Cell culture
A431 cells (ATCC® CRL-1555™) and SKBR3 cells (ATCC® HTB-30™) were obtained from the American Type Culture Collection (ATCC). Cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C with 5% CO2 in a humidified atmosphere.
Cell viability assay
A431 cells (3000 cells per 90 μL) and SKBR3 cells (5000 cells per 90 μL) were seeded in each well of 96-well plates and incubated for 24 h. Cells were treated with 10 μL of diluted DMSO stock solution containing the corresponding compounds. The final concentration was 0.1% in all the samples and the control. After 72 h, 10 μL of EZ-Cytox reagent (Daeil Lab Service Co., Seoul, Republic of Korea) was added to each well and A431 and SKBR3 cells were incubated for 1 h and 2 h, respectively. Cell viability was assessed by measuring absorbance at 450 nm using FlexStation 3 Multi-Mode microplate readers (Molecular Devices). The IC50 values were calculated using GraphPad Prism 5. All the assays were performed as three independent experiments.
Western blot
A431 cells (500 000 cells per 2 mL) and A431 cells (800 000 cells per 2 mL) were seeded in each well of 6-well plates and incubated for 24 h. Cells were treated with 20 μL of DMSO stock solution of corresponding compounds. After 4 h, cells were washed twice with cold DPBS and lysed on ice with RIPA buffer supplemented with protease inhibitor and phosphatase inhibitor cocktails on ice. Equal amounts of the protein sample were boiled with 5× SDS-PAGE loading buffer, separated by 10% SDS-PAGE gels, and transferred onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 5% BSA in 1× TBST (1× TBS with 0.1% Tween-20) for 1 h and incubated overnight at 4 °C with 1 : 1000 dilution of the following primary antibodies in blocking buffer: pHER2 (Tyr1121/1222, CST #2243), HER2 (CST #2232), pEGFR (Tyr1068, CST #3777), EGFR (CST #2232), and β-actin (SC #SC-47778). Membranes were washed three times with 1× TBST and incubated with a 1 : 2000 dilution of the following secondary antibodies at RT for 1 h, followed by three extensive washings. Secondary antibodies were anti-rabbit IgG, HRP-linked antibody (CST #7074S), and anti-mouse IgG, HRP-linked antibody (CST #7076S). Antibodies were visualized using an enhanced chemiluminescence (ECL) system (Bio-Rad, Clarity Western ECL Substrate, 500 ml #1705061) and VILBER FUSION SOLO X. Antibodies were purchased from Cell Signaling Technology (CST) and Santa Cruz Biotechnology (SC). β-actin was used as a loading control, and all the assays were performed using three independent experiments.
Metabolic stability assay
Human and mouse liver microsomes (0.5 mg ml−1, Corning, cat No. #452117, #452701) were pre-incubated with compounds (1 μM) in 0.1 M phosphate buffer (pH 7) at 37 °C for 5 min. The reaction was initiated by adding the NADPH Regeneration System solution (Promega, #V9510) and incubating for 30 min at 37 °C. To stop the reaction, an acetonitrile solution containing chlorpropamide as an internal standard was added, followed by centrifugation at 15 000 rpm and 4 °C for 15 min. The supernatant was injected into the LC–MS/MS system (Shimadzu Nexera XR) for analysis. Experiments were conducted in duplicate with variation within 15%. The results were quantified by comparing the values obtained after 30 min to those obtained at 0 min, expressed as % remaining. A positive control group using verapamil (1 μM) in human microsomes showed internal reference values within 15% (±1), confirming the experimental accuracy.
Colony formation assay
In the colony formation assay, SK-BR3 cells were initially seeded at a density of 1 × 104 cells per well in 6-well plates. Cells were treated with either 14c compound, 14f compound, or lapatinib for 2 weeks. Post-treatment, cells were fixed using 4% paraformaldehyde (PFA) and stained with a 0.1% crystal violet solution in 75% ethanol. The colonies were subsequently counted, and images of the stained colonies were taken with a microscope for further analysis.
Data availability
The data supporting this article have been included as part of the ESI.†
Author contributions
J. L. mainly conducted the experimental work with support from C. G. I., J. M. L., M. C., and M. K. who assisted in the preparation of some derivatives, contributed to the data collection and interpretation. K. L. was involved in assessing metabolic stability. H. N., J. S. and J. H. S. conducted the colony formation assay and western blotting. K. H. M. conceptualized and designed the experiment, and also prepared the manuscript.
Conflicts of interest
No potential conflict of interest was reported by the authors.
Supplementary Material
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (2022R1A2C2010824).
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5md00025d
References
- Swain S. M. Shastry M. Hamilton E. Nat. Rev. Drug Discovery. 2023;22:101–126. doi: 10.1038/s41573-022-00579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaltriti M. Rojo F. Ocaña A. Anido J. Guzman M. Cortes J. Di Cosimo S. Matias-Guiu X. Cajal S. R. Y. Arribas J. Baselga J. J. Natl. Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- Scaltriti M. Chandarlapaty S. Prudkin L. Aura C. Jimenez J. Angelini P. D. Sánchez G. Guzman M. Parra J. L. Ellis C. Gagnon R. Koehler M. Gomez H. Geyer C. Cameron D. Arribas J. Rosen N. Baselga J. Clin. Cancer Res. 2010;16:2688–2695. doi: 10.1158/1078-0432.CCR-09-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J. Jang J. Beyett T. S. Eum Y. Haikala H. M. Verano A. Lin M. K. Hatcher J. M. Kwiatkowski N. P. Eser P. Ö. Poitras M. J. Wang S. Xu M. Gokhale P. C. Cameron M. D. Eck M. J. Gray N. S. Jänne P. A. Cancer Res. 2022;82:1633–1645. doi: 10.1158/0008-5472.CAN-21-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D. Hong J. Eur. J. Med. Chem. 2019;170:55–72. doi: 10.1016/j.ejmech.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Li Y. J. Li J. X. Zhou H. X. Liu C. Y. L. Liu Z. Ying B. W. Xie Y. M. Hu M. X. Gong Y. L. Eur. J. Med. Chem. 2022;244:114775. doi: 10.1016/j.ejmech.2022.114775. [DOI] [PubMed] [Google Scholar]
- Medina P. J. Goodin S. Clin. Ther. 2008;30:1426–1447. doi: 10.1016/j.clinthera.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Chatsiproios D. Breast Care. 2010;5:16–21. doi: 10.1159/000285776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy R. K. Loi S. Okines A. Paplomata E. Hamilton E. Hurvitz S. A. Lin N. U. Borges V. Abramson V. Anders C. Bedard P. L. Oliveira M. Jakobsen E. Bachelot T. Shachar S. S. Müller V. Braga S. Duhoux F. P. Greil R. Cameron D. Carey L. A. Curigliano G. Gelmon K. Hortobagyi G. Krop I. Loibl S. Pegram M. Slamon D. Palanca-Wessels M. C. Walker L. Feng W. Winer E. P. N. Engl. J. Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- Nakamura Y. Mizuno N. Sunakawa Y. Canon J. L. Galsky M. D. Hamilton E. Hayashi H. Jerusalem G. Kim S. T. Lee K. W. Fonkoua L. A. K. Monk B. J. Nguyen D. Oh D. Y. Okines A. O'Malley D. M. Pohlmann P. Reck M. Shin S. J. Sudo K. Takahashi S. Van Marcke C. Yu E. Y. Groisberg R. Ramos J. Tan S. Stinchcombe T. E. Bekaii-Saab T. J. Clin. Oncol. 2023;41:5569–5578. doi: 10.1200/JCO.23.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler J. H. Cercek A. Siena S. Andre T. Ng K. Van Cutsem E. Wu C. Paulson A. S. Hubbard J. M. Coveler A. L. Fountzilas C. Kardosh A. Kasi P. M. Lenz H. J. Ciombor K. K. Elez E. Bajor D. L. Cremolini C. Sanchez F. Stecher M. Feng W. T. Bekaii-Saab T. S. Investigators M. Lancet Oncol. 2023;24:496–508. doi: 10.1016/S1470-2045(23)00150-X. [DOI] [PubMed] [Google Scholar]
- Robinson H. R. Messersmith W. A. Lentz R. W. Curr. Treat. Options Oncol. 2024;25:585–604. doi: 10.1007/s11864-024-01183-7. [DOI] [PubMed] [Google Scholar]
- Lee J. W. Choi C. Kim J. Lee S. Kim J. Lee Y. Min K. H. Arch. Pharmacal Res. 2022;45:123–141. doi: 10.1007/s12272-022-01376-4. [DOI] [PubMed] [Google Scholar]
- Appukkuttan P. Dehaen W. Fokin V. V. Van der Eycken E. Org. Lett. 2004;6:4223–4225. doi: 10.1021/ol048341v. [DOI] [PubMed] [Google Scholar]
- Erdélyi M. Gogoll A. J. Org. Chem. 2001;66:4165–4169. doi: 10.1021/jo0057250. [DOI] [PubMed] [Google Scholar]
- Sharma A. K. Gowdahalli K. Krzeminski J. Amin S. J. Org. Chem. 2007;72:8987–8989. doi: 10.1021/jo701665j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been included as part of the ESI.†