Abstract
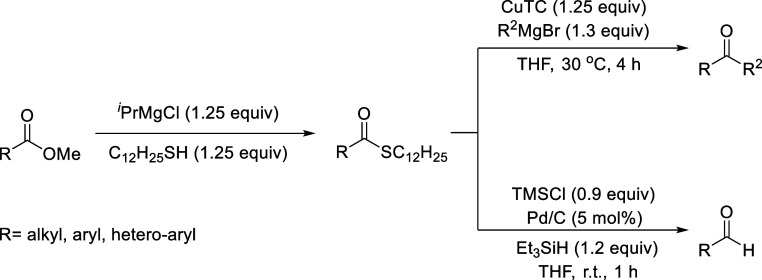
A new protocol for preparing thioesters from the corresponding methyl esters was developed using iPrMgCl and odorless 1-dodecanthiol, nC12H25SH, under mild reaction conditions, during which in situ-generated C12H25SMgCl selectively reacted with the carbonyl group of esters. A variety of aromatic and aliphatic esters were readily converted in up to 99% yield with excellent functional group tolerance. Furthermore, based on the quantitative formation of the thioesters, we successfully applied our method to a one-pot synthesis of ketones and aldehydes from esters.
Introduction
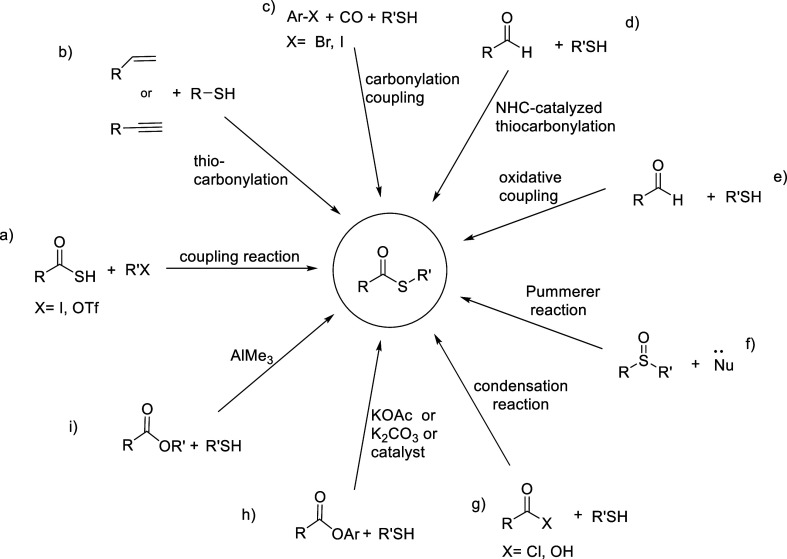
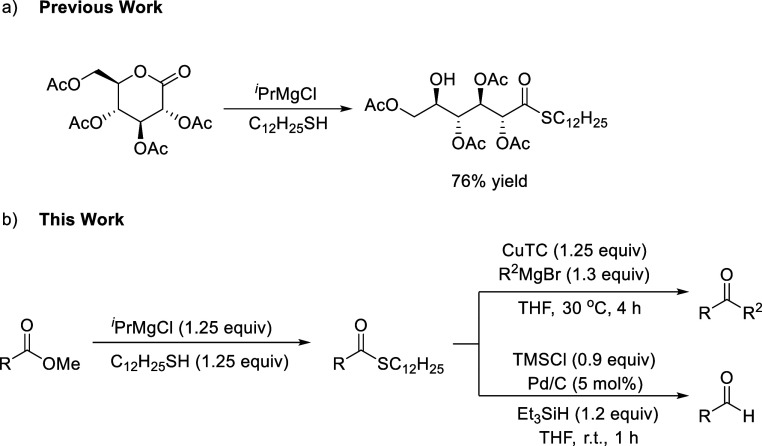
Thioesters are an important class of sulfur-containing organic compounds1−4 and act as higher reactive intermediates than the corresponding esters.5 Thioesters are utilized for preparing aldehydes, ketones, esters, amides, lactams, acylsilanes, benzothiazoles, and so on.6−13 Several synthetic strategies for thioesters have been reported: coupling of thioacid with aryl halide (Scheme 1a),14 transition-metal-catalyzed thiocarbonylation of alkenes and alkynes (Scheme 1b),15 carbonylation coupling of aryl halides and thiols in the presence of carbon monoxide (Scheme 1c),16N-heterocyclic carbene-catalyzed thioesterification of aldehydes (Scheme 1d),17 oxidative coupling of aldehyde and in situ-generated aldehyde with thiol (Scheme 1e),18 the Pummerer reaction (Scheme 1f),19 and condensation of carboxylic acid derivatives with thiols (Scheme 1g).20 Although these methods are efficient, direct thioesterification of esters has attracted much attention due to the easy availability of esters and a straightforward procedure. Although thioesterification of aryl esters with thiol has been achieved owing to the superior leaving ability of the aryloxy group (Scheme 1h),21 thioesterification of the most common and commercially available alkyl esters has been limited to the use of flammable AlMe3 (Scheme 1i).22 The development of new reagents suitable for the thioesterification of alkyl esters is, therefore, in high demand. Recently, we found that the Grignard reagent served as an alternative to AlMe3 for converting the gluconolactone derivative to the corresponding thioester upon treatment with thiol (Scheme 2a).23 As an extension, we report herein the details of applying the Grignard method to the preparation of thioesters from the corresponding methyl esters as well as to a one-pot synthesis of ketones and aldehydes (Scheme 2b).
Scheme 1. Various Synthetic Methods of Thioester.
Scheme 2. Our Works on Thioester Synthesis.
Results and Discussion
We first searched for the best reaction conditions for the thioesterification of methyl 4-methoxybenzoate (1a) with odorless 1-dodecanethiol, C12H25SH, as a typical substrate in the presence of any Grignard reagents, and the results are shown in Table 1. When a mixture of C12H25SH and iPrMgCl in THF was added to 1a in THF at room temperature, desired 2a was obtained in a 82% yield within 2 h (entry 1). Extending the reaction time to 4 h led to a slightly higher yield (88%, entry 2), and increasing the reaction temperature to 40 °C for 4 h significantly improved the yield (94%, entry 3). Alternatively, when iPrMgCl was added to the mixture of C12H25SH and 1a at room temperature, almost the same yield of 2a (86%) was obtained (entry 4). Other Grignard reagents, such as EtMgBr and MeMgI, were then evaluated to elucidate the halide effects on the thioesterification: EtMgBr at room temperature and 40 °C gave 2a in comparative yields (80% and 84%, respectively; entries 5 and 6), while MeMgI resulted in a lower yield (73%, entry 7). Inorganic magnesium salts, such as Mg(OEt)2, MgO, and magnesium hydride, did not give 2a at all (entries 8–10). Lithium thiolate derived in situ by treating nBuLi with thiol provided 2a in a lower yield (53%, entry 11). Accordingly, we selected iPrMgCl at 40 °C for 4 h as the best Grignard reagent and reaction conditions for the thioesterification of 1a with C12H25SH.
Table 1. Optimization of the Reaction Conditions for the Thioesterification of Methyl 4-Methoxybenzoate (1a).
| entry | reagents | temp (°C) | time (h) | yield (%)a |
|---|---|---|---|---|
| 1 | iPrMgCl | rt | 2 | 82 |
| 2 | iPrMgCl | rt | 4 | 88 |
| 3 | iPrMgCl | 40 | 4 | 94 |
| 4 | iPrMgCl | rt | 4 | 86b |
| 5 | EtMgBr | rt | 2 | 80 |
| 6 | EtMgBr | 40 | 2 | 84 |
| 7 | MeMgI | rt | 2 | 73 |
| 8 | Mg(OEt)2 | rt | 5 | N.Rc |
| 9 | MgO | rt | 5 | N.Rc |
| 10 | MgH2 | rt | 6 | N.Rc |
| 11 | nBuLi | rt | 2 | 53 |
Isolated yield.
Grignard reagent was added into the mixture of 1a and C12H25SH.
No reaction.
With the best reaction conditions in hand, we examined the substrate scope and functional group tolerance of aromatic methyl esters (Table 2). Methyl benzoate (1g) gave 2g in 93% yield and tolyl derivatives such as p-Me 1d, m-Me 1e, and o-Me 1f afforded 2d, 2e, and 2f in 77%, 97%, and 97% yields, respectively. In addition, para- and meta-MeO derivatives 1a and 1b produced the corresponding derivatives 2a and 2b in high yields (both 94%). In contrast, ortho-MeO derivative 1c resulted in a mixture of 2c and S-dodecyl 2-(dodecylthio)benzothioate (2c′) (see the Supporting Information), the latter of which was a byproduct by replacing the methoxy group of 2c with a sulfur nucleophile.24 Reducing the amounts of 1-dodecanethiol and iPrMgCl to 1.05 equiv improved the yield of 2c to 98%. Halogen substitutions at the para-position of the phenyl group 1h, 1i, 1j, and 1k were also employed for the thioesterification to give the corresponding thioesters 2h, 2i, 2j, and 2k in 99%, 94%, 95%, and 92% yields, respectively, without loss of the halogen moieties. In the case of electron-withdrawing groups, p-CF3-substituted compound 2l was obtained in 96% yield, and p-CN compound 2m was obtained in 89% yield with the cyano group intact. Biphenyl thioester 2n was produced in 96% yield. Methyl 1-naphthoate (1o) and methyl 2-naphthoate (1p) were converted to the corresponding thioesters 2o and 2p in 92% and 99% yields, respectively.
Table 2. Synthesis of Aromatic Thioesters.
Smaller amounts of 1-dodecanethiol (1.05 equiv) and iPrMgCl (1.05 equiv) were used.
1 equiv of iPrMgCl was added to deprotonate the N–H bond at 0 °C prior to the thioesterification.
1.5 equiv of iPrMgCl was added to deprotonate the O–H bond at 0 °C prior to the thioesterification at 60 °C.
Two ester groups at the para-position underwent full conversion to give the corresponding dithioester 2q in 83% yield, while 1r with one ester group and one amide group at the para-position gave monothioester 2r in 90% yield, indicating that the amide group remained intact under the reaction conditions. Protic substrates bearing N–H and O–H bonds are well known to react with Grignard reagents. Accordingly, the N–H moiety of N-Boc-protected ester 1s was deprotonated by 1 equiv of iPrMgCl before adding the mixture of iPrMgCl and 1-dodecanethiol to give 2s in 86% yield. Similarly, substrates containing an O–H moiety, such as 1t, 1u, and 1v, were first deprotonated by 1.5 equiv of iPrMgCl and then treated with the mixture of iPrMgCl and 1-dodecanethiol to afford 2t, 2u, and 2v in 74%, 67%, and 61% yields, respectively.
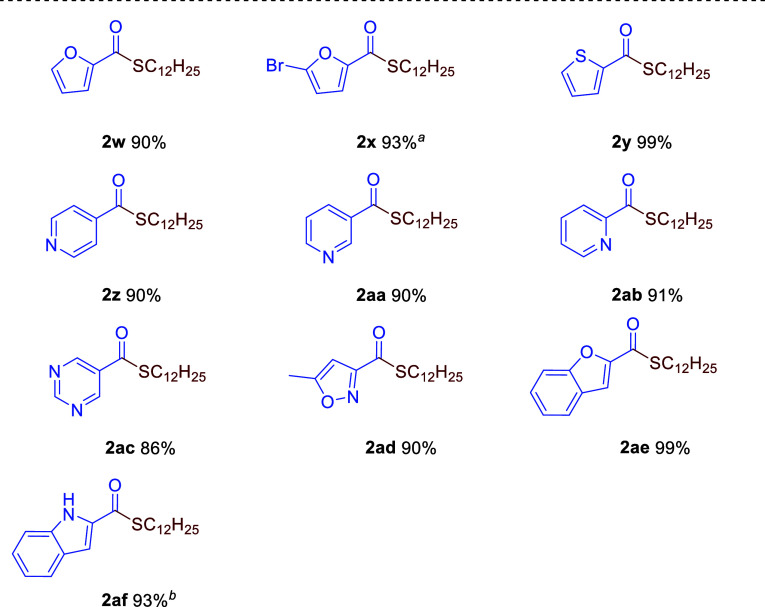
The thioesterification protocol was also applied to heteroaryl derivatives (Table 3). Heterocyclic five-membered substrates, such as furan carboxylates 1w and 1x and thiophene carboxylate 1y, led to the formation of 2w, 2x, and 2y in 90%, 93%, and 99% yields, respectively. Pyridine derivatives, such as isonicotinate (1z), nicotinate (1aa), and picolinate (1ab), furnished the corresponding 2z, 2aa, and 2ab in 90%, 90%, and 91% yields, respectively, while pyrimidine-5-carboxylate 1ac afforded 2ac in 86% yield. 5-Methylisoxazole-3-carboxylate 1ad gave 2ad in 90% yield. Fused heteroaromatic substrates bearing benzofuran and indole units were also applicable to give 2ae and 2af in high yields (99% and 93%), though the latter required deprotonation of the N–H bond by the addition of 1 equiv of iPrMgCl prior to the thioesterification.
Table 3. Synthesis of Heterocyclic Thioesters.
Reaction was conducted at r.t.
1 equiv of iPrMgCl was added at 0 °C to deprotonate a N–H bond prior to the thioesterification.
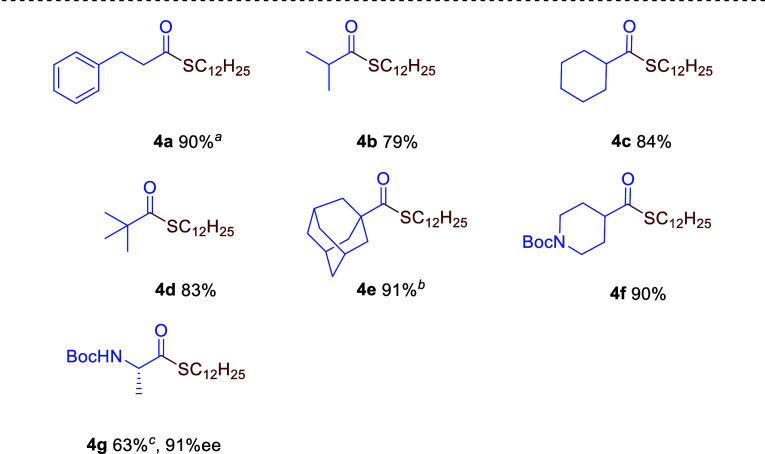
The thioesterification was further conducted for aliphatic esters (Table 4). Primary ester 3a produced 4a in 64% yield under the established thioesterification conditions, while the reaction conducted at −15 °C improved the yield (90%) of 4a through minimizing enolate formation and the Claisen condensation product (4a′) (see the Supporting Information).25 Thioesterification of not only secondary esters 3b and 3c but also sterically demanding tertiary esters 3d and 3e proceeded smoothly to give the corresponding thioesters 4b (79% yield), 4c (84% yield), 4d (83% yield), and 4e (91% yield). Boc-protected piperidine derivative 4f was obtained in 90% yield, while Boc-protected l-alanine derivative 4g was isolated in a lower yield (63%) with a slight loss of enantioselectivity (91% ee) due to the contribution of enolate formation. In sharp contrast to the thioesterification of Ac-protected gluconolactone,23 some lactones (5a, 5b, and 5c) resulted in low yields (37%–57%) (eq 1), probably due to the reverse formation of lactones through nucleophilic attack of the carbonyl group of the thioester by a free alkoxy anion.
 |
1 |
Table 4. Synthesis of Aliphatic Thioesters.
The substrate was added into the mixture of iPrMgCl and C12H25SH at −15 °C and stirred for 2 h.
Reaction was conducted at 50 °C for 1 h.
1 equiv of iPrMgCl was added at −15 °C to deprotonate the N–H bond prior to the thioesterification followed by adding the mixture of 1-dodecanethiol (1.03 equiv) and iPrMgCl (1.00 equiv) at −10 °C for 45 min.
Taking advantage of the easy and direct preparation of thioesters from methyl esters, we applied the method to a one-pot synthesis of ketones and aldehydes. After optimizing the reaction conditions for one-pot benzophenone synthesis from methyl benzoate (seethe Supporting Information), the thioesterification stage was operated at a slightly higher reaction temperature (50 °C) to shorten the reaction time to 1 h, followed by the addition of a mixture of CuTC (1.25 equiv; TC = thiophene-2-carboxylate) and PhMgBr (1.3 equiv) at 30 °C for 4 h as the second stage,26 resulting in the high yield (91%) of 7e without any contamination by triphenylcarbinol. As shown in Table 5, para- and meta-methoxy-substituted benzoate gave the corresponding ketones 7a and 7b in 92% and 88% yields, respectively. According to the result of thioesterification of methyl o-methoxybenzoate (1c) using iPrMgCl (1.05 equiv) and 1-dodecanethiol (1.05 equiv) in the first stage, ketone 7c was obtained in 85%. para-Methylbenzoate 1d afforded 7d in a 90% yield. para-Halogen-substituted benzoates 1f–1i produced the corresponding ketones 7f–7i in high yields (75%–90%) without loss of the C(sp2)-halogen bond. Trifluoromethyl and cyano groups at the para-position of the phenyl moiety efficiently afforded 7j and 7k in 90% and 87% yields, respectively. Biphenyl derivative 7l and naphthalene derivative 7m were obtained in good yields (85% and 88%, respectively). Furthermore, 1q bearing two ester moieties at the para-position underwent full conversion to give the corresponding diketone 7n in 88% yield, while 1r with one amide group at the para-position produced 7o in 89% yield with the amide group intact. In the case of heteroaryl substrates, phenyl(thiophen-2-yl) methanone 7p was formed in 60% yield, likely due to the negative chelation effects from the thiophene moiety and carbonyl group on the copper species. For pyridine derivates, isonicotinate and nicotinate afforded the corresponding ketones 7q and 7r in 81% and 84% yields, respectively, whereas the coupling reaction of picolinate and 5-methylisoxazole-3-carboxylate gave 7s and 7t in lower yields (63% and 70%, respectively), in which similar chelation to the copper species suppressed the ketone formation.27 The present one-pot synthetic protocol was also applicable to aliphatic esters. 3-Phenylpropanoate and cyclohexanecarboxylate afforded the corresponding ketones 7u and 7v in 86% and 78% yields, respectively, by conducting the first thioesterification step at −15 °C. The bulkiness of the tert-butyl and adamantyl groups did not severely decrease the yield of the corresponding ketones 7w (72% yield) and 7x (85% yield). A N-boc-protected piperidine moiety remained intact, resulting in an 81% yield of ketone 7y.
Table 5. One-Pot Synthesis of Ketones via Thioesters.
Smaller amounts of 1-dodecanethiol (1.05 equiv) and iPrMgCl (1.05 equiv) were used.
Reaction was conducted at −15 °C for 2 h in the first step.
Aldehyde is one of the most important carbonyl compounds as a versatile intermediate in organic synthesis. We applied this thioester synthesis method to aldehyde synthesis from methyl esters in a one-pot manner. The first thioesterification step was carried out under the same high temperatures (50 °C) for a shorter time (1 h). After optimization for the one-pot synthesis of p-methoxybenzaldehyde (8a) (see the Supporting Information), we selected TMSCl (0.9 equiv; TMSCl = trimethylsilyl chloride), Pd/C (5 mol %), and Et3SiH (1.2 equiv) as the conditions for the second stage,28 in which TMSCl was essential for aldehyde formation through trapping an excess amount of magnesium thiolate as a thiolate scavenger of palladium; the results are summarized in Table 6. Methyl benzoate (1g) gave 8g in an 85% yield. Methylbenzoates bearing a Me group at the para, meta, and ortho positions produced the corresponding aldehydes 8d, 8e, and 8f in 81%, 85%, and 68% yields. Anisate derivatives were also examined: p-anisaldehyde 8a and m-anisaldehyde (8b) were both obtained in 86% yield, whereas o-anisaldehyde (8c) was formed in 72% yield by first treating it with 1.05 equiv of iPrMgCl and 1-dodecanethiol followed by silane reduction. With regard to para-halogen-substituted substrates, p-fluorobenzaldehyde (8h) was obtained in 62% yield without contamination by dehalogenated byproduct 8g, whereas dehalogenation of 1i and 1j concomitantly occurred to give the corresponding p-chlorobenzaldehyde 8i (77% yield) and p-bromobenzaldehyde (8j) (20% yield), together with the dehalogenated product 8g. p-CF3-Benzaldehyde (8k) was obtained in a 49% yield. This protocol is also applicable to secondary and tertiary aliphatic substrates: both cyclohexanecarbaldehyde (8l) and adamantane-1-carbaldehyde (8m) were obtained in 69% yields.
Table 6. One-Pot Synthesis of Aldehyde via Thioestersa,b.
Isolated yields after chromatographic purification.
Isolated as tosylhydrazone.
Smaller amounts of 1-dodecanethiol (1.05 equiv) and iPrMgCl (1.05 equiv) were used in the first step.
The second step was conducted for 18 h.
Determined by 1H NMR spectroscopy.
Conclusions
A convenient and useful synthetic method for the thioesterification of methyl esters was developed using iPrMgCl and C12H25SH as reagents, by which in situ-generated C12H25SMgCl efficiently facilitates the conversion of a wide range of esters, including aliphatic, aromatic, and heterocyclic esters, into thioesters under mild reaction conditions with an excellent functional group tolerance. Compared with the widely used alkyl aluminum reagents, such as AlMe3, Grignard reagents, such as iPrMgCl, offer significant advantages, ease of preparation, and improved safety in handling. The practicality of this methodology was further demonstrated by the successful one-pot synthesis of ketones and aldehydes, highlighting the ready applicability of the resulting thioesters.
General Information
All reactions were performed under an argon atmosphere using the standard Schlenk technique and argon-filled glovebox. Anhydrous solvents were purchased from Sigma-Aldrich Chemical. All chemicals were purchased from commercial suppliers and used directly without further purification. Progress of reactions was monitored by thin-layer chromatography on a silica gel 60 F-254 plate and visualized under UV illumination and/or by staining with acidic ceric ammonium molybdate or dinitrophenylhydrazine. Silica gel (Geduran Si-60, 0.063–0.200 mm) for chromatography was obtained from Merck. NMR spectra were recorded at 300 MHz (1H), 75 MHz (13C), and 282 MHz (19F) spectrometers in a Jeol console, 400 MHz (1H), 100 MHz (13C), and 376 MHz (19F) spectrometers in a Jeol console, or 500 MHz (1H), 125 MHz (13C), and 471 MHz (19F) spectrometers in a Jeol console as specified. Coupling constants in Hz was calculated from chemical shifts of 1H NMR spectra.
Experimental Section
General Procedure for the Preparation of Thioesters from Methyl Esters
Typical reaction of preparation of S-dodecyl 4-methoxybenzothiolate (2a). To a solution of 1-dodecanethiol (762 mg, 3.76 mmol, 1.25 equiv) in THF (7 mL) at 0 °C was added iPrMgCl (2.0 M in THF) (1.88 mL, 3.76 mmol, 1.25 equiv) dropwise via a syringe. A solution of methyl 4-methoxybenzoate (1a) (500 mg, 3.01 mmol, 1 equiv) in THF (2 mL) charged in the other Schlenk tube was added dropwise via a syringe to the solution of thiolate in THF at 0 °C followed by washing the Schlenk flask of the ester with THF (2 mL). After the reaction mixture was stirred at 40 °C for 4 h, the reaction was quenched at room temperature by aq. 1 M HCl (5 mL) and then extracted with EtOAc (25 mL). The organic extract was treated with sat. aq. NaHCO3 (2 × 15 mL) and brine (2 × 20 mL) and then dried over anhydrous Na2SO4. Concentration under a rotary evaporator followed by silica gel column chromatography afforded 2a as a white solid (897 mg, 96% yield).
General Procedure for the Preparation of Ketones from Methyl Esters
Typical reaction of preparation of (4-methoxyphenyl)(phenyl)methanone (7a). To a solution of 1-dodecanethiol (253 mg, 1.25 mmol, 1.25 equiv) in THF (2.6 mL) at 0 °C was added iPrMgCl (2.0 M in THF) (0.625 mL, 1.25 mmol, 1.25 equiv) dropwise over 5 min. The mixture was stirred for 15 min and then slowly transferred into a solution of methyl 4-methoxybenzoate (1a) (166 mg, 1.00 mmol, 1 equiv) in THF (1.6 mL) charged in the other Schlenk tube, followed by washing the Schlenk flask by THF (1.2 mL). The resulting reaction mixture was stirred at 50 °C for 1 h. Concurrently, a solution of PhMgBr (1.0 M in THF) (1.30 mL, 1.30 mmol, 1.3 equiv) was added dropwise over 5 min at room temperature to CuTC (TC = thiophene-2-carboxylate) (238 mg, 1.25 mmol,1.25 equiv) in THF (4 mL). The resulting mixture was stirred for 10 min and then transferred into the solution of thioester. After the reaction mixture was stirred at 30 °C for 4 h, the reaction was quenched by adding aq. 1 M HCl (4 mL). The mixture was filtered through a Celite pad and then concentrated. The resulting residue was extracted with EtOAc (40 mL). The extract was washed with aq 1 M HCl (3 × 20 mL) followed by brine (20 mL) and then dried over anhydrous Na2SO4. Concentration and purification by silica gel column chromatography gave 7a as a white solid (196 mg, 92% yield).
General Procedure for the Preparation of Aldehydes from Esters
Typical reaction of preparation of 4-methoxybenzaldehyde (8a). To a solution of 1-dodecanethiol (761 mg, 3.76 mmol, 1.25 equiv) in THF (7 mL) at 0 °C was added iPrMgCl (2.0 M in THF) (1.88 mL, 3.76 mmol, 1.25 equiv) dropwise. A solution of methyl 4-methoxybenzoate (1a) (500 mg, 3.01 mmol, 1 equiv) in THF (3 mL) was added to the mixture of thiolate at 0 °C dropwise via a syringe followed by washing with THF (2 × 1 mL). After the reaction mixture was stirred at 50 °C for 1 h, TMSCl (0.344 mL, 2.71 mmol, 0.9 equiv) was added dropwise at room temperature. The resulting reaction mixture was further stirred for 15 min and then 10% Pd/C (160 mg, 0.150 mmol, 5 mol %) was added. After 5 min, triethylsilane (0.577 mL, 3.61 mmol, 1.2 equiv) was added dropwise. The reaction mixture was stirred for 1 h and quenched by adding aq. 0.2 M HCl (10 mL). Then, the mixture was filtered through the Celite pad and washed with EtOAc. The filtrate was treated with sat. aq. sodium bicarbonate (4 mL). After the organic layer was collected, the aqueous layer was further extracted with EtOAc (3 × 10 mL). The combined organic layer was washed with brine (10 mL) and dried over anhydrous Na2SO4 and then evaporated using a rotary evaporator. 1,3,5-Trimethoxybenzene (202 mg, 1.20 mmol, 0.4 equiv) was added into the crude mixture as the internal standard and subjected to take 1H NMR yield as calculated to be 90%.
The crude mixture was added to a solution of TsNHNH2 (616 mg, 3.31 mmol, 1.1 equiv) in MeOH (3 mL) followed by washing the flask of the crude mixture with MeOH (3 × 2 mL). After the resulting reaction mixture was stirred for 1 h, the solvent was removed. The crude product was purified by silica gel chromatography to give N′-(4-methoxybenzylidene)-4-methylbenzenesulfonohydrazide (8a′) as a white solid (788 mg, 86% yield).
Acknowledgments
This work was partially supported by JSPS KAKENHI, Grant-in-Aid for Scientific Research (B), Grant Number: JP22H02076.
Data Availability Statement
The data underlying this study are available in the manuscript and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c10622.
Optimization tables, characterization data, and NMR spectra of compounds (PDF)
Author Contributions
§ S.K.M., J.K., and R.Y. contributed equally to this work. The manuscript was written through the contributions of all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- a Schaumann E. Sulfur is more than the fat brother of oxygen. An overview of organosulfur chemistry. Sulfur-Mediated Rearrangements I. Top. Curr. Chem. 2007, 274, 1–34. 10.1007/128_2006_105. [DOI] [Google Scholar]; b Du Y. E.; Byun W. S.; Lee S. B.; Hwang S.; Shin Y.-H.; Shin B.; Jang Y. J.; Hong S.; Shin J.; Lee S. K.; Oh D. C. Formicins, N-acetylcysteamine-bearing indenone thioesters from a wood ant-associated bacterium. Org. Lett. 2020, 22, 5337–5341. 10.1021/acs.orglett.0c01584. [DOI] [PubMed] [Google Scholar]
- Ilardi E. A.; Vitaku E.; Njardarson J. T. Data mining for sulfur and fluorine: An evaluation of pharmaceuticals to reveal opportunities for drug design and discovery. J. Med. Chem. 2014, 57, 2832–2842. 10.1021/jm401375q. [DOI] [PubMed] [Google Scholar]
- a Dawson P.; Muir T.; Clark-Lewis I.; Kent S. Synthesis of proteins by native chemical ligation. Science 1994, 266, 776–779. 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]; b Staunton J.; Weissman K. J. Polyketide biosynthesis: a millennium review. Nat. Prod. Rep. 2001, 18, 380–416. 10.1039/a909079g. [DOI] [PubMed] [Google Scholar]
- a Wang X.; Dong Z.-B. A Recent Progress for the Synthesis of Thiocarboxylates. Eur. J. Org Chem. 2022, 2022, e202200452 10.1002/ejoc.202200452. [DOI] [Google Scholar]; b Zhou J.; Jin C.; Su W. Improved synthesis of fluticasone propionate. Org. Process Res. Dev. 2014, 18, 928–933. 10.1021/op5001226. [DOI] [Google Scholar]; c Chisuga T.; Miyanaga A.; Kudo F.; Eguchi T. Structural analysis of the dual-function thioesterase SAV606 unravels the mechanism of Michael addition of glycine to an α, β-unsaturated thioester. J. Biol. Chem. 2017, 292, 10926–10937. 10.1074/jbc.M117.792549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayden J.; Greeves N.; Warren S.. Organic Chemistry, 2nd ed.; Oxford University Press: New York, 2001. [Google Scholar]
- Bannin T. J.; Kiesewetter M. K. Poly(thioester) by organocatalytic ring-opening polymerization. Macromolecules 2015, 48, 5481–5486. 10.1021/acs.macromol.5b01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Marlier J. F.; Fogle E. J.; Redman R. L.; Stillman A. D.; Denison M. A.; Robins L. I. A mechanistic study of thioester hydrolysis with heavy atom kinetic isotope effects. J. Org. Chem. 2015, 80, 1905–1908. 10.1021/jo502472m. [DOI] [PubMed] [Google Scholar]; b Yang W.; Drueckhammer D. G. Understanding the relative acyl-transfer reactivity of oxoesters and thioesters: computational analysis of transition statedelocalization effects. J. Am. Chem. Soc. 2001, 123, 11004–11009. 10.1021/ja010726a. [DOI] [PubMed] [Google Scholar]
- Miyazaki T.; Han-ya Y.; Tokuyama H.; Fukuyama T. New odorless protocols for the synthesis of aldehydes and ketones from thiol esters. Synlett 2004, 2004, 477–480. 10.1055/s-2004-815427. [DOI] [Google Scholar]
- a Mukaiyama T.; Araki M.; Takei H. Reaction of S-(2-pyridyl) thioates with Grignard reagents. Convenient method for the preparation of ketones. J. Am. Chem. Soc. 1973, 95, 4763–4765. 10.1021/ja00795a055. [DOI] [Google Scholar]; b Wang J.; Cary B. P.; Beyer P. D.; Gellman S. H.; Weix D. J. Ketones from nickel-catalyzed decarboxylative, non-symmetric cross-electrophile coupling of carboxylic acid esters. Angew. Chem., Int. Ed. 2019, 58, 12081–12085. 10.1002/anie.201906000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iimura S.; Manabe K.; Kobayashi S. Hydrophobic polymer-supported catalyst for organic reactions in water: acid-catalyzed hydrolysis of thioesters and transprotection of thiols. Org. Lett. 2003, 5, 101–103. 10.1021/ol026906m. [DOI] [PubMed] [Google Scholar]
- Davis A. P.; Walsh J. J. Amide bond formation via pentafluorothiophenyl active esters. Tetrahedron Lett. 1994, 35, 4865–4868. 10.1016/S0040-4039(00)76989-9. [DOI] [Google Scholar]
- Azuma H.; Okano K.; Tokuyama H. Synthesis of acylsilanes by palladium-catalyzed cross-coupling reaction of thiol esters and silylzinc chlorides. Chem. Lett. 2011, 40, 959–961. 10.1246/cl.2011.959. [DOI] [Google Scholar]
- Chakraborti A. K.; Rudrawar S.; Kaur G.; Sharma L. An efficient conversion of phenolic esters to benzothiazoles under mild and virtually neutral conditions. Synlett 2004, 9, 1533–1536. 10.1055/s-2004-829089. [DOI] [Google Scholar]
- a Zheng T.-C.; Burkart M.; Richardson D. E. A general and mild synthesis of thioesters and thiols from halides. Tetrahedron Lett. 1999, 40, 603–606. 10.1016/S0040-4039(98)02545-3. [DOI] [Google Scholar]; b Sawada N.; Itoh T.; Yasuda N. Efficient copper-catalyzed coupling of aryl iodides and thiobenzoic acid. Tetrahedron Lett. 2006, 47, 6595–6597. 10.1016/j.tetlet.2006.07.008. [DOI] [Google Scholar]; c Lai C.; Backes B. J. Efficient preparation of S-aryl thioacetates from aryl halides and potassium thioacetate. Tetrahedron Lett. 2007, 48, 3033–3037. 10.1016/j.tetlet.2007.02.128. [DOI] [Google Scholar]; d Sanden S. A.; Yi R.; Hara M.; McGlynn S. E. Simultaneous synthesis of thioesters and iron sulfur clusters in water: two universal components of energy metabolism. Chem. Commun. 2020, 56, 11989–11992. 10.1039/D0CC04078A. [DOI] [PubMed] [Google Scholar]
- a Xiao W. J.; Alper H. The first examples of the palladium-catalyzed thiocarbonylation of propargylic alcohols with thiols and carbon monoxide. J. Org. Chem. 1997, 62, 3422–3423. 10.1021/jo970126n. [DOI] [Google Scholar]; b Ogawa A.; Kawakami J.; Mihara M.; Ikeda T.; Sonoda N.; Hirao T. Highly regioselective hydrothiocarboxylation of acetylenes with carbon monoxide and thiols catalyzed by Pt(PPh3)4. J. Am. Chem. Soc. 1997, 119, 12380–12381. 10.1021/ja9726973. [DOI] [Google Scholar]; c Li C. F.; Xiao W. J.; Alper H. Palladium-catalyzed ring opening thiocarbonylation of vinylcyclopropanes with thiols and carbon monoxide. J. Org. Chem. 2009, 74, 888–890. 10.1021/jo801725j. [DOI] [PubMed] [Google Scholar]; d Hirschbeck V.; Gehrtz P. H.; Fleischer I. Regioselective thiocarbonylation of vinyl arenes. J. Am. Chem. Soc. 2016, 138, 16794–16799. 10.1021/jacs.6b11020. [DOI] [PubMed] [Google Scholar]; e Wang X.; Wang B.; Yin X.; Yu W.; Liao Y.; Ye J.; Wang M.; Hu L.; Liao J. Palladium-catalyzed enantioselective thiocarbonylation of styrenes. Angew. Chem., Int. Ed. 2019, 58, 12264–12270. 10.1002/anie.201905905. [DOI] [PubMed] [Google Scholar]
- a Cao H.; McNamee L.; Alper H. Palladium-catalyzed thiocarbonylation of iodoarenes with thiols in phosphonium salt ionic liquids. J. Org. Chem. 2008, 73, 3530–3534. 10.1021/jo800287s. [DOI] [PubMed] [Google Scholar]; b Nakaya R.; Yorimitsu H.; Oshima K. Bis(cyclopentadienyldicarbonyliron) as a convenient carbon monoxide source in palladium-catalyzed carbonylative coupling of aryl iodides with amines, alcohols, and thiols. Chem. Lett. 2011, 40, 904–906. 10.1246/cl.2011.904. [DOI] [Google Scholar]; c Islam S. M.; Molla R. A.; Roy A. S.; Ghosh K. Polymer supported Pd catalyzed thioester synthesis via carbonylation of aryl halides under phosphine free conditions. RSC Adv. 2014, 4, 26181–26192. 10.1039/C4RA03338H. [DOI] [Google Scholar]; d Burhardt M. N.; Ahlburg A.; Skrydstrup T. Palladium-catalyzed thiocarbonylation of aryl, vinyl, and benzyl bromides. J. Org. Chem. 2014, 79, 11830–11840. 10.1021/jo5009965. [DOI] [PubMed] [Google Scholar]
- a Uno T.; Inokuma T.; Takemoto Y. NHC-catalyzed thioesterification of aldehydes by external redox activation. Chem. Commun. 2012, 48, 1901–1903. 10.1039/c2cc17183j. [DOI] [PubMed] [Google Scholar]; b Man Y.; Zeng X.; Xu B. Synthesis of thioesters from aldehydes via n-heterocyclic carbene (NHC) catalyzed radical relay. Chem.—Eur. J. 2023, 29, e202203716 10.1002/chem.202203716. [DOI] [PubMed] [Google Scholar]
- a Ogawa K. A.; Boydston A. J.; Boydston A. J. Organocatalyzed anodic oxidation of aldehydes to thioesters. Org. Lett. 2014, 16, 1928–1931. 10.1021/ol500459x. [DOI] [PubMed] [Google Scholar]; b Mupparapu N.; Khushwaha M.; Gupta A. P.; Singh P. P.; Ahmed Q. N. Amino catalytic oxidative thioesterification approach to α ketothioesters. J. Org. Chem. 2015, 80, 11588–11592. 10.1021/acs.joc.5b02176. [DOI] [PubMed] [Google Scholar]; c Chung Y.; Seo U. R.; Chun S.; Chung Y. K. Poly(3,4-dimethyl-5-vinylthiazolium)/DBU-catalyzed thioesterification of aldehydes with thiols. ChemCatChem 2016, 8, 318–321. 10.1002/cctc.201501140. [DOI] [Google Scholar]; d Zhang Y.; Ji P.; Hu W.; Wei Y.; Huang H.; Wang W. Organocatalytic transformation of aldehydes to thioesters with visible light. Chem.—Eur. J. 2019, 25, 8225–8228. 10.1002/chem.201900932. [DOI] [PubMed] [Google Scholar]; e Bołt M.; Hanek K.; Żak P. Metal-free thioesterification of α, β-unsaturated aldehydes with thiols. Org. Chem. Front. 2022, 9, 4846–4853. 10.1039/D2QO00678B. [DOI] [Google Scholar]; f Ismaeel N.; Imran S.; Zhu X.; Chen J.; Yuan D.; Yao Y. Rare earth amide-catalyzed direct thioesterification of aldehydes with thiols under mild conditions. Org. Lett. 2023, 25, 8672–8676. 10.1021/acs.orglett.3c03497. [DOI] [PubMed] [Google Scholar]; g Wang L.; Cao J.; Chen Q.; He M. Iron-catalyzed thioesterification of methylarenes with thiols in water. Tetrahedron Lett. 2014, 55, 7190–7193. 10.1016/j.tetlet.2014.10.155. [DOI] [Google Scholar]; h Feng J.; Lu G. P.; Cai C. Selective approach to thioesters and thioethers via sp3 C-H activation of methylarenes. RSC Adv. 2014, 4, 54409–54415. 10.1039/C4RA09450F. [DOI] [Google Scholar]; i Ali W.; Guin S.; Rout S. R.; Gogoi A.; Patel B. K. Thioesterification of alkylbenzenes with thiols via copper-catalyzed cross-dehydrogenative coupling without a directing group. Adv. Synth. Catal. 2014, 356, 3099–3105. 10.1002/adsc.201400360. [DOI] [Google Scholar]
- Murakami K.; Imoto J.; Matsubara H.; Yoshida S.; Yorimitsu H.; Oshima K. Copper-catalyzed extended Pummerer reactions of ketene dithioacetal monoxides with alkynyl sulfides and ynamides with an accompanying oxygen rearrangement. Chem.—Eur. J. 2013, 19, 5625–5630. 10.1002/chem.201204072. [DOI] [PubMed] [Google Scholar]
- a Funatomi T.; Wakasugi K.; Misaki T.; Tanabe Y. Pentafluorophenylammonium triflate (PFPAT): an efficient, practical, and cost-effective catalyst for esterification, thioesterification, transesterification, and macrolactone formation. Green Chem. 2006, 8, 1022–1027. 10.1039/b609181b. [DOI] [Google Scholar]; b Iranpoor N.; Firouzabadi H.; Davan E. E. In situ generated Ph3P(OAc)2 as a novel reagent for the efficient acetylation of alcohols and thiols at room temperature. Tetrahedron Lett. 2013, 54, 1813–1816. 10.1016/j.tetlet.2013.01.068. [DOI] [Google Scholar]; c Kazemi M.; Shiri L. Thioesters synthesis: recent adventures in the esterification of thiols. J. Sulphur Chem. 2015, 36, 613–623. 10.1080/17415993.2015.1075023. [DOI] [Google Scholar]; d Wakayama F.; Ito R.; Park K.; Ishida M.; Yamada Y.; Ichihara S.; Takada H.; Nakamura S.; Kato A.; Yamada T.; Sajiki H.; Monguchi Y. Esterification or thioesterification of carboxylic acids with alcohols or thiols using amphipathic monolith-SO3H resin. Bull. Chem. Soc. Jpn. 2021, 94, 2702–2710. 10.1246/bcsj.20210266. [DOI] [Google Scholar]
- a Chakraborti A. K.; Rudrawar S.; Kaur G.; Sharma L. An Efficient Conversion of Phenolic Esters to Benzothiazoles under Mild and Virtually Neutral Conditions. Synlett 2004, 9, 1533–1536. 10.1055/s-2004-829089. [DOI] [Google Scholar]; b Watson D. A.; Fan X.; Buchwald S. L. Carbonylation of Aryl Chlorides with Oxygen Nucleophiles at Atmospheric Pressure. Preparation of Phenyl Esters as Acyl Transfer Agents and the Direct Preparation of Alkyl Esters and Carboxylic Acids. J. Org. Chem. 2008, 73, 7096–7101. 10.1021/jo800907e. [DOI] [PubMed] [Google Scholar]; c Os’kina I. A.; Vlasov V. M. Kinetics of the Reaction of Substituted 4-Nitrophenyl Benzoates with Benzenethiol in the Presence of Potassium Carbonate in Dimethylformamide. Russ. J. Org. Chem. 2008, 44, 561–569. 10.1134/S1070428008040167. [DOI] [Google Scholar]; d Os’kina I. A.; Vlasov V. M. Effect of Nucleophile on the Activation Parameters of Transesterification of 4-Nitrophenyl Benzoates. Russ. J. Org. Chem. 2010, 46, 326–330. 10.1134/S1070428010030048. [DOI] [Google Scholar]; e Magens S.; Plietker B. Fe-Catalyzed Thioesterification of Carboxylic Esters. Chem.—Eur. J. 2011, 17, 8807–8809. 10.1002/chem.201101073. [DOI] [PubMed] [Google Scholar]; f Iglesias E.; Brandariz I. Captopril as a nucleophile for ester cleavage. Formation of the thiolester S-benzoylcaptopril. RSC Adv. 2015, 5, 45740–45748. 10.1039/C5RA06900A. [DOI] [Google Scholar]; g Shi Y.; Liu X.; Cao H.; Bie F.; Han Y.; Yan P.; Szostak R.; Szostak M.; Liu C. Conversion of esters to thioesters under mild conditions. Org. Biomol. Chem. 2021, 19, 2991–2996. 10.1039/D1OB00187F. [DOI] [PubMed] [Google Scholar]
- a Corey E. J.; Beames D. J.; Beames D. J. Method for the protection of lactones and esters against nucleophilic attack. J. Am. Chem. Soc. 1973, 95, 5829–5831. 10.1021/ja00798a100. [DOI] [Google Scholar]; b Gierasch T. M.; Shi Z.; Verdine G. L. Extensively stereodiversified scaffolds for use in dversity-oriented library synthesis. Org. Lett. 2003, 5, 621–624. 10.1021/ol027116f. [DOI] [PubMed] [Google Scholar]; c Lindgren C.; Andersson I. E.; Berg L.; Dobritzsch D.; Ge C.; Haag S.; Uciechowska U.; Holmdahl R.; Kihlberg J.; Linusson A. Hydroxyethylene isosteres introduced in type II collagen fragments substantially alter the structure and dynamics of class IIMHCAq/glycopeptidecomplexes. Org. Biomol. Chem. 2015, 13, 6203–6216. 10.1039/C5OB00395D. [DOI] [PubMed] [Google Scholar]; d Talode J.; Kato D.; Nagae H.; Tsurugi H.; Seki M.; Mashima K. Syntheses of SGLT2 inhibitors by Ni- and Pd-catalyzed Fukuyama coupling reactions. J. Org. Chem. 2020, 85, 12382–12392. 10.1021/acs.joc.0c01635. [DOI] [PubMed] [Google Scholar]
- Seki M.; Tapkir S. R.; Nadiveedhi M. R.; Kalita S. J.; Mulani S. K.; Mashima K. Synthesis of SGLT2 Inhibitors by Means of Fukuyama Coupling Reaction. J. Org. Chem. 2023, 88, 15367–15373. 10.1021/acs.joc.3c01873. [DOI] [PubMed] [Google Scholar]
- Choi H.; Shirley H. J.; Hume P. A.; Brimble M. A.; Furkert D. P. Angew. Chem., Int. Ed. 2017, 56, 7420–7424. 10.1002/anie.201702727. [DOI] [PubMed] [Google Scholar]
- Hattori T.; Sato S.; Miyano S. Facile Alkoxyl Exchange of 2-Methoxybenzoates via Nucleophilic Aromatic Substitution with Sodium Alkoxides in Dimethylformamide. B. Chem. Soc. Jpn. 1993, 66, 3840–3842. 10.1246/bcsj.66.3840. [DOI] [Google Scholar]
- Kato D.; Murase T.; Talode J.; Nagae H.; Tsurugi H.; Seki M.; Mashima K. Diarylcuprates for Selective Syntheses of Multifunctionalized Ketones from Thioesters under Mild Conditions. Chem.—Eur. J. 2022, 28, e202200474 10.1002/chem.202200474. [DOI] [PubMed] [Google Scholar]
- Hanh Nguyen D.; Lassauque N.; Vendier L.; Mallet-Ladeira S.; Le Berre C.; Serp P.; Kalck P. Reductive Elimination of Anhydrides from Anionic Iodo Acetyl Carboxylato Rhodium Complexes. Eur. J. Inorg. Chem. 2014, 2014, 326–336. 10.1002/ejic.201300933. [DOI] [Google Scholar]
- a Fukuyama T.; Lin S. C.; Li L. Facile reduction of ethyl thiol esters to aldehydes: application to a total synthesis of (+)-neothramycin A methyl ether. J. Am. Chem. Soc. 1990, 112, 7050–7051. 10.1021/ja00175a043. [DOI] [Google Scholar]; b Kimura M.; Seki M. A practical procedure for the synthesis of multifunctional aldehydes through the Fukuyama reduction and elucidation of the reaction site and mechanism. Tetrahedron Lett. 2004, 45, 3219–3223. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the manuscript and its Supporting Information.