Abstract
Pyrolysis of used tires is a promising method for recovering valuable chemicals. However, the conventional high-temperature pyrolysis of natural rubber (polyisoprene)-based tires suffers from a low-selective isoprene recovery, heavy carbon black (CB) damage, and coke formation on the CB. In this paper, we report on characteristics of the low-temperature pyrolysis of CB-containing polyisoprene-based tire rubber that is vulcanized with sulfur. The low-temperature pyrolysis of the tire rubber cleaves the main chain and cross-linking bonds, which allows for the recovery of low-molecular-weight tire rubbers, tire rubber dissolution into the solvent, and CB isolation from the rubber matrix. The maximum liquid rubber recovery rate was 76.7% after 1 h of heating at 282 °C. In addition, the molecular weight of the thermally treated rubber substantially decreased from Mw 340,000 to approximately 20,000 after 1 h of heating at 282 °C. Furthermore, the maximum isoprene skeleton retention rate of the recovered rubber was 83% at 267 °C after 1 h of heating. The remaining rubber matrix on the recovered CB surface was nearly eliminated at temperatures above 320 °C. In conclusion, we believe that the low-temperature pyrolysis of tire rubber is a promising pretreatment method for recovering CB without thermal damage and reducing the molecular weight of tire rubber, which will improve the recovery of isoprene.
1. Introduction
A global study of 45 countries deduced that 29.1 million tons (metric) of used tires are generated annually. These findings revealed that 97% of used tires are repurposed for material and energy recovery, while the remaining 3% are typically subjected to pyrolysis, gasification, or are used in civil engineering applications.1 These outcomes mirror the characteristics of a linear economy (extract-manufacture-consume-dispose), which generates environmental pollutants.2 Furthermore, the production of tires is predicted to increase by approximately 4.11 billion units per year between 2021 and 2026,3 which could result in the consumption of more raw materials. As manufacturers shift their focus to achieve carbon neutrality by 2030, the number of used tires is expected to increase alongside this growth.4 Therefore, reduction, recycling, reuse, renewal, redesign, repair, and recovery are essential to reduce carbon emissions and recover raw materials from used tires. Carbon black (CB) is essential in tire manufacturing because it reinforces rubber and enhances abrasion resistance. Thus, CB is essential for the durability and performance of tires. In addition, recovered CB and rubber are vital for achieving material circularity in tires, emphasizing their importance in the initial manufacturing and recycling processes.5 Pyrolysis is a process that converts waste materials such as used tires into valuable gaseous, liquid, and solid products suitable for energy recovery and alternative fuels through thermal decomposition at elevated temperatures in an inert atmosphere.6 The inert atmosphere used in pyrolysis prevents the production of oxidized compounds that contribute to environmental pollution, unlike landfilling and incineration methods.7,8
Used tires produce oils that can be used as fuel and chemicals. However, the recovery process is influenced by various factors, including the temperature,9 heating rates,10,11 reactors,12,13 and methods that are used.14,15 Additionally, isoprene, a target product, despite its low yield,16 can be obtained from used tires through pyrolysis.17,18
The utilization of recovered CB provides an opportunity to reduce the dependence of the tire industry on petrochemicals by substituting a portion of conventional CB with a sustainable and circular alternative. The surface activity of recovered CB from pyrolysis is weak because of the formation of inorganic ash and carbonaceous deposits at high pyrolysis temperatures,19 which leads to an easy-cluster effect of the CB. Thus, dispersing CB in the polymer matrix is difficult, and its reinforcing properties are weakened.20,21
Pyrolysis conditions and the tire composition also play vital roles in the quality of recovered CB.22 The formation of high ash, oxygen and sulfur contents remain a challenge, as an increase in the pyrolysis temperature leads to increased carbon cluster formation.23 Previous studies have explored the high-temperature degradation of used tires and impact of the heating rates on product distribution including pyrolysis oil, char, and gaseous fractions.24 Fast pyrolysis, characterized by high heating rates and short residence times, typically produces higher yields of pyrolysis oil but the yield of char is reduced.25 Boyu et al.26 observed that increasing the process temperature from 400 to 600 °C during the fast pyrolysis of waste tires in a wire mesh reactor notably decreased the yield of solid products from 37.8 to 26.2 wt %. This decrease in the yield of solid residue was accompanied by an increase in the yield of the gaseous and liquid fractions, suggesting that higher temperatures promote the formation of volatile components and pyrolysis oils. Conversely, slow pyrolysis occurs at temperatures below 450 °C with slow heating and longer residence times. This method allows further breakdown of byproducts, resulting in stable materials such as char.27 Kar28 pyrolyzed 10 g batches of waste tires in a nitrogen-purged fixed bed reactor at a heating rate of 10 °C/min. The study explored pyrolysis temperatures from 375 to 500 °C; a maximum yield of oil (60.0 wt %) was obtained at 425 °C. In our opinion, low-temperature pyrolysis is not sufficiently emphasized to improve the selective recovery of resources from used tires.
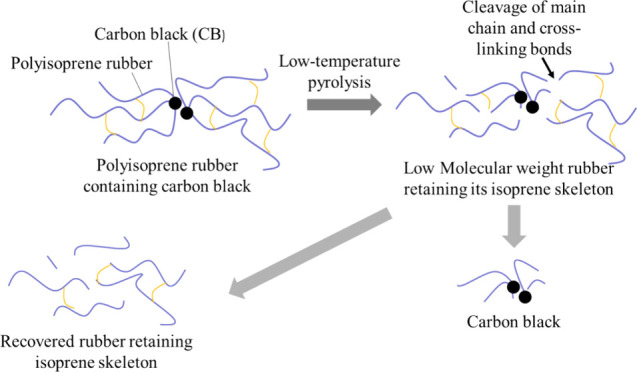
This paper introduces a novel perspective to control the rate of isoprene skeleton degradation during low-temperature pyrolysis; the study aimed to enhance the quality of the obtained liquid rubber and CB, as depicted in Figure 1. The thermal decomposition of polyisoprene rubber at low temperatures was studied using thermogravimetric analysis (TGA) and a nitrogen-substituted electric furnace. In addition, gel permeation chromatography (GPC) and nuclear magnetic resonance (NMR) were used to evaluate the changes in the molecular weight and isoprene skeleton of the rubber. Finally, we investigated the impact of hydrogen-rich oils, such as rapeseed (canola) and palm stearin oils, on the decomposition behavior of polyisoprene rubber through the pyrolysis of swollen rubber.
Figure 1.

Schematic of low-temperature pyrolysis of tire rubber.
2. Results and Discussion
2.1. Weight Loss Behavior of Rubber Samples
Figure 2 shows the thermogravimetric (TG) and derivative TG (DTG) profiles of unswollen and oil-swollen tire rubber. The initial weight loss up to 300 °C suggested the presence of volatile substances. Significant weight loss was observed at temperatures above 310 °C, as indicated by the distinct DTG peak at approximately 380 °C, which revealed thermal degradation of the polymer matrix. The residual weight at 500 °C was attributed to CB and inorganic fillers. Weight loss stopped at approximately 490 °C, which was consistent with the results reported in previous studies that investigated rubber-based polyisoprene.29,30 In addition, the TG/DTG curves shown in Figure 2 show swelling of tire rubber in rapeseed and palm stearin oils. Notably, all rubber samples swollen with oil exhibited weight loss in a single step, similar to that observed for unswollen tire rubber. This observation suggested that rapeseed and palm stearin oils underwent decomposition within a similar temperature range as that of unswollen tire rubber.
Figure 2.
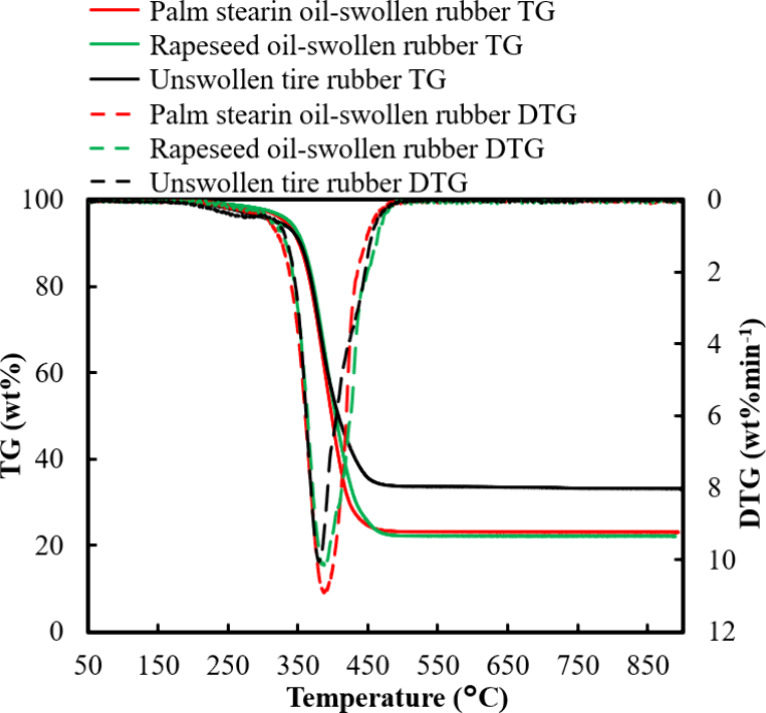
Thermogravimetric (TG) and derivative TG (DTG) curves for tire rubber and tire rubber swollen in rapeseed and palm stearin oils.
The TGA analysis revealed that palm stearin and rapeseed oils had a marked effect on the thermal degradation of tire rubber. The TGA curves indicated that oil-swelling in the rubber further reduced its residual mass, suggesting enhanced volatilization of the rubber matrix. Simultaneously, the DTG peak shifted slightly toward higher temperatures, which indicated an increase in the thermal stability of the swollen rubber. This change suggests that the oils influenced the thermal degradation kinetics of the rubber samples, probably via stabilization of the free radicals that formed during pyrolysis. The modification of the thermal decomposition profile reflects the role of these oils in altering the degradation pathway, enhancing the stability of the rubber during low-temperature pyrolysis.
2.2. Thermal Decomposition Behavior of Rubber at Low Temperatures
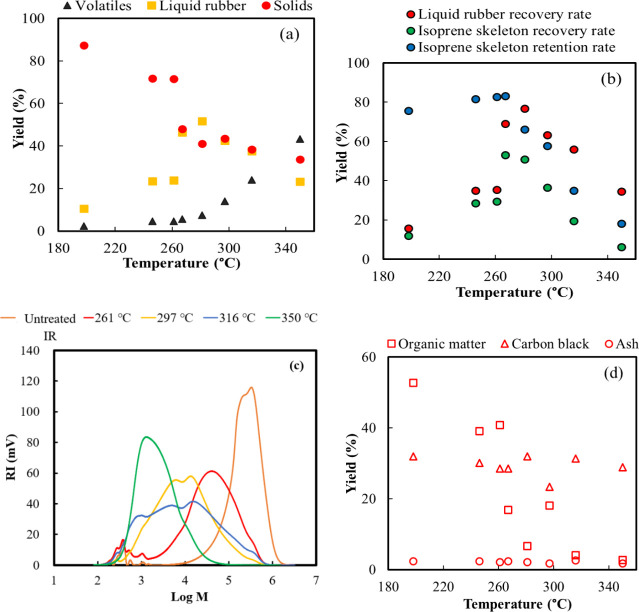
The results of the TGA indicated that the measurable decomposition of tire rubber began and ended at 200 and 500 °C, respectively. Therefore, in this study we defined low-temperature pyrolysis as that occurring between 200 and 400 °C. Figure 3a summarizes the yields of the volatiles produced by the tire rubber when the temperature was increased from 200 to 350 °C in the nitrogen-substituted furnace. The volatile yield of the rubber at 200 °C was 2.3 wt %. As the temperature rose from 250 to 300 °C, the amount of volatile compounds increased. Decomposition occurred without significant degradation of the rubber within this temperature range. At 350 °C, the volatile yield was 43.2 wt %; that of the CB residue, which does not readily evaporate, was 33.6 wt %, and was retained. Higher pyrolysis temperatures enhance the formation of gaseous products at the expense of the liquid and solid fractions.26 This trend aligns with the thermal breakdown of higher molecular weight compounds into smaller, volatile molecules at elevated temperatures.
Figure 3.
Temperature-dependent product yields during the low-temperature pyrolysis of unswollen tire rubber: (a) volatile, liquid rubber, and solid yields (b) liquid rubber content yield; (c) refractive index (mV) versus the logarithm of molecular weight distribution of liquid rubber; and (d) solid content yield.
2.3. Effects of Pyrolysis Conditions on the Properties of Liquid Rubber
2.3.1. Effects of Pyrolysis Temperature
Figure 3a also displays the yields of liquid rubber and solid obtained at different temperatures. As the decomposition temperature increased, the solid yield decreased. The maximum liquid rubber yield was achieved at 280 °C for unswollen tire rubber. However, the liquid rubber was readily converted to volatiles at higher temperatures. Low-temperature pyrolysis led to the degradation of the rubber to low-molecular units that were more readily dissolved in the solvent than vulcanized rubber, making it possible to recover liquid rubber and separate CB. The liquid rubber obtained from the low-temperature pyrolysis of unswollen tire rubber had a high recovery rate of 76.7% at 282 °C. Interestingly, at 267 °C, 83% of the isoprene skeleton in the liquid rubber was retained, but at higher pyrolysis temperatures, only 18% of the isoprene skeleton was retained. The isoprene skeleton recovery rate, which is the product of the liquid rubber recovery rate and its isoprene skeleton retention rate, peaked at 270 °C (Figure 3b). These results indicate that the higher the recovery rate of the isoprene skeleton, the greater the isoprene yield that could be reclaimed when liquid rubber is thermally decomposed at high temperatures in the subsequent pyrolysis stage. The recovered isoprene monomer and other pyrolysis products can be recycled in tire manufacturing and other industrial applications, such as synthetic rubber production and the production of various petrochemical derivatives. The molecular weight distribution of liquid rubber at each thermal decomposition temperature is presented in Figure 3c. The molecular weight of untreated rubber was approximately 340,000. As the pyrolysis temperature increased to approximately 297 °C, the molecular weight decreased to approximately 15,000. Figure 3d shows the composition of the solid content at each pyrolysis temperature. The CB and ash yields were 30 and 2%, respectively. Therefore, the composition of low-temperature treated rubber was similar to that of unpyrolyzed tire rubber, which contained 31.3 and 1.4 wt % of CB and zinc oxide, respectively (Table 1), and did not volatilize during pyrolysis. However, an organic content yield of 40.8% was achieved at 261 °C but decreased with increasing thermal temperature. The separation of CB from organic matter occurred at temperatures exceeding 320 °C, and similar results were obtained at 350 °C. High pyrolysis temperatures often lead to the formation of larger carbon clusters and higher levels of impurities, such as ash, oxygen, and sulfur, which complicate the reuse of CB.23 The CB obtained via low-temperature pyrolysis may be of improved quality and can potentially be recycled via manufacturing of tires.
Table 1. Material Composition of Polyisoprene Rubber.
| components | weight composition (wt %) |
|---|---|
| natural rubber | 62.6 |
| carbon black | 31.3 |
| stearic acid | 1.3 |
| zinc oxide | 1.5 |
| N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine | 1.3 |
| N-cyclohexyl-2-benzothiazolylsufenamide | 0.4 |
| sulfur | 1.1 |
| polymerized trimethyl dihydroquinoline | 0.6 |
| total | 100.0 |
2.3.2. Effects of Heating Time
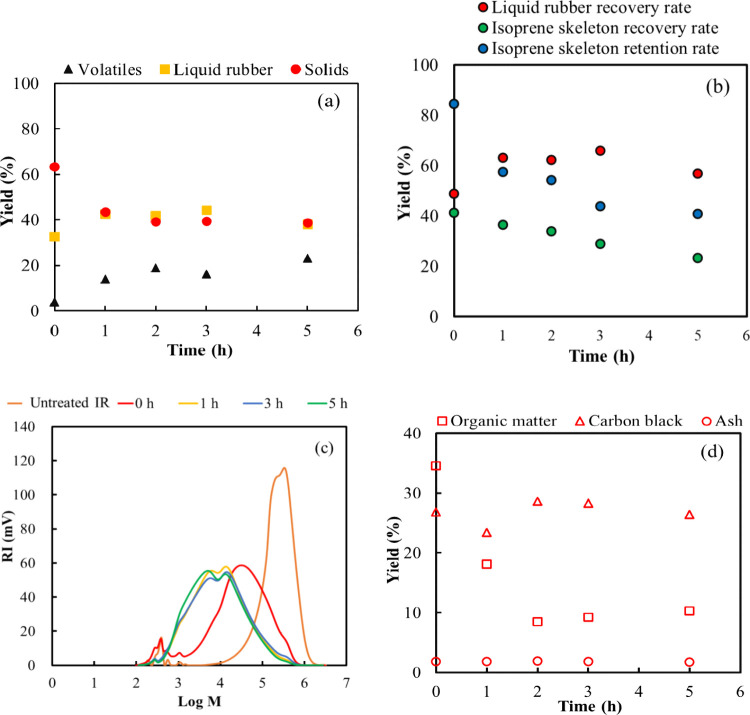
Experiments were conducted at 300 °C with varying thermal decomposition times, as shown in Figure 4. The heating time, 0 h, is the time required to increase the initial temperature to the target temperature. As the pyrolysis time increased, the volatile yield increased while the solid content decreased. The maximum liquid rubber yield was 44.4% after 3 h, as depicted in Figure 4a. In addition, the retention rate of the isoprene skeleton of the liquid rubber decreased as the thermal degradation time increased, with only 40.8% of the isoprene skeleton being retained after 5 h, as shown in Figure 4b. The molecular weight of liquid rubber decreased after 1 h and remained relatively stable as the pyrolysis temperature increased. The organic content decreased as the pyrolysis time increased, with the highest yield of 9.2% being achieved after 3 h. A small change was observed after 2 h, indicating that CB could be separated from the organic matter at 300 °C after this duration.
Figure 4.
Time-dependent product yields during the low-temperature pyrolysis of polyisoprene rubber at 300 °C: (a) volatile, liquid rubber, and solid yields (b) solid yield; (c) liquid rubber yield; and (d) molecular weight distribution of liquid rubber.
2.3.3. Effect of Oil-Swelling
Certain reactions that occur during pyrolysis, such as the formation of aromatic compounds and carbonization, can be inhibited by providing protons to the radicals produced from rubber.31,32 In this study, tire rubber was soaked in rapeseed oil and palm stearin oils (both rich in hydrogen and fatty acids),33 to investigate their swelling effects and assess their ability to act as hydrogen donors during low-temperature pyrolysis. These oils play a dual role: their composition, rich in hydrogen and fatty acids, helps stabilize radical intermediates by donating hydrogen, thereby suppressing secondary reactions and influencing the pyrolytic product distribution. At 300 °C after 1 h, the 1H NMR spectra for oil-swollen rubbers showed that the amount of alkenyl group protons present in the rubbers was significantly decreased. This decrease translated to a reduction in the isoprene skeleton retention rate; furthermore, the isoprene skeleton recovery rate in all oil-swollen rubber samples decreased in the order of unswollen tire rubber > palm stearin oil-swollen tire rubber > rapeseed oil-swollen tire rubber, as shown in Table S1 in the ESI. This result indicated that the presence of double bonds from the oil-swollen samples promoted the denaturation of the isoprene skeleton. The 1H NMR spectrum of each sample is shown in Figure 5.
Figure 5.

1H NMR of (a) unswollen tire rubber and rubber swollen in (b) palm stearin and (c) rapeseed oil at 300 °C for 1 h using hexamethyldisilane (HMDS) as standard.
The signal occurring at 4.1–4.3 ppm was attributed to the methylene protons attached to the central carbon of the glycerol skeleton in the triglycerides; this signal was absent in the spectrum of unswollen tire rubber. The peaks occurring at 5.3–5.5 ppm in the spectra of both oil rubber samples originated from the protons attached to the carbons involved in the double bonds within the unsaturated fatty acid chains in the rapeseed and palm stearin oils; these peaks were also observed in the 1H NMR spectra of rapeseed and palm stearin oils (Figure S3). Figure 6 illustrates the molecular weight distribution of the oil-swollen rubbers. A distinct peak near a molecular weight of 1000 was observed and attributed to the presence of oil in the oil-swollen samples; this peak was absent in the spectrum of unswollen tire rubber.
Figure 6.
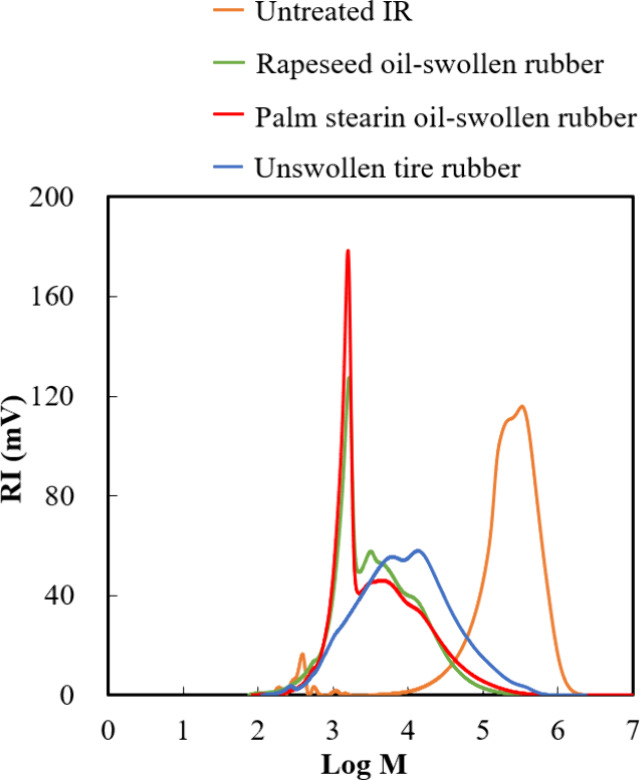
Refractive index (in mV) vs logarithm of molecular weight for low-temperature pyrolyzed oil-swollen, unswollen tire rubber, and untreated isoprene rubber.
The molecular weight of the oil-swollen rubbers significantly decreased as the decomposition temperature increased, thus emphasizing the influence of oil in promoting the reduction of the molecular weight of rubber compared with that of tire rubber (without oil). Furthermore, oil-swollen rubber samples had a lower organic content than unswollen tire rubber, as shown in Table S1. Functional groups containing hydrogen and oxygen compounds exist on the surface of CB, known as the carbon gel layer, and are used for tire manufacturing to enhance the properties of tires.34,35 This result suggested that the oil in the swollen rubber samples penetrated the layer, weakening the intermolecular force between CB and the gel layer and promoting the decomposition of rubber components. Therefore, oil-swelling was an effective method for separating CB from rubber components.
3. Conclusions
Low-temperature pyrolysis was used to thermally decompose polyisoprene rubber by reducing its molecular weight while selectively retaining its isoprene skeleton. Heat-treated tire rubber was pyrolyzed using a nitrogen-substituted electric furnace. The recovery rate of isoprene in the liquid rubber directly correlates with the amount that can be reclaimed in the next high-temperature pyrolysis stage. Residence time experiments showed that the molecular weight distribution of the rubber barely changed after 1 h, and the isoprene skeleton retention rate tended to decrease during extended residence times. In addition, CB separation was achieved at 300 °C after 2 h. Notably, the introduction of oil-swollen rubber in low-temperature pyrolysis exhibited a characteristic influence on rubber degradation. However, the ingress of oil molecules in the rubber layer led to an expansion of the intermolecular distance, thereby facilitating bond cleavage during pyrolysis, further reducing the molecular weight and organic content. Furthermore, the anticipated role of the oil-swollen rubber in mitigating aromatization and carbonization reactions was substantiated by the observed suppression of the isoprene skeleton. Rubber swelling using saturated oil promoted decomposition without altering the isoprene skeleton.
The findings presented herein should contribute to a broader understanding of the thermal decomposition behavior of polyisoprene rubber at low-temperatures that reduces its molecular weight and simultaneously retains the isoprene skeleton to produce a liquid rubber suitable for the recovery and recycling of isoprene monomer and CB in the tire industry.
4. Methods
4.1. Materials
Bridgestone Corporation (Tokyo, Japan) synthesized an NR-based sample from polyisoprene rubber-containing CB and tire rubber swelled in rapeseed and palm stearin oils. The material composition is summarized in Table 1. Rapeseed and palm stearin oils were delivered from Bridgestone Corporation. Other chemicals and standard gases were procured from Kanto Chemical (Tokyo, Japan) or Tokyo Chemical Industry (Tokyo, Japan).
Proximate and ultimate analyses of the tire rubber material are provided in the electronic Supporting Information.
4.2. Preparation of Oil-Swollen Tire Rubber
Tire rubber (15 g) was soaked in rapeseed and palm stearin oil (200 g each) for 24 h to prepare the swollen tire rubber samples. The swelling ratios were calculated as the difference between the weight measurements taken before and after soaking. This process was repeated for multiple samples to ensure accuracy, resulting in swelling ratios of 1.53 and 1.52 for the rapeseed and palm stearin oils, respectively.
4.3. Thermogravimetric Analysis
The thermal decomposition temperature range of the rubber samples was investigated using a TG conducted with an STA7200RV instrument (Hitachi High-Tech Science Corporation, Japan). Each sample (10 mg) was loaded into a Pt pan, heated from 50 to 900 °C at a heating rate of 10 °C min–1, and maintained in a nitrogen atmosphere for 30 min at the final temperature. In addition, rubber samples underwent heating from 200 to 400 °C at a rate of 25 °C min–1. Thereafter, the final temperature was maintained for 1–15 h to investigate the behavior of rubber at low temperatures.
4.4. Low-Temperature Pyrolysis Treatment and Product Separation
Figures S1 and S2 show the schematics of the nitrogen displacement electric furnace used in the study and flowchart for separating thermally treated rubber, respectively. A 1.5-g sample with dimensions of approximately 2 × 2 × 2 mm was placed in a sample boat with dimensions of 9.7 × 1.5 × 1.2 cm. The weight was measured, and the sample quantity was calculated based on the weight before and after loading. The sample boats were preconditioned at 80 °C for 3 h to reduce the risk of moisture-induced weight changes. The sample boat, with a thermocouple installed above it, was placed in an electric furnace (ROP-001PG, As One, Japan). Thereafter, nitrogen purging was conducted at 5 L min–1 for 30 min. The gas pipe was preheated to 100 °C during nitrogen introduction to prevent the furnace temperature from decreasing. After nitrogen substitution, an electric furnace temperature program was started. The internal and sample temperatures were recorded every minute during the temperature rise and every 5–10 min during the temperature hold. The average temperature and standard deviation during the temperature hold were calculated. After pyrolysis, the temperature inside the furnace was allowed to decrease to below 200 °C before the nitrogen flow was stopped. The volatile yields were determined from the weight changes in the sample before and after heating (eq 1). The nonvolatile part was termed ‘heat-treated rubber.’ The heat-treated rubber was dissolved in deuterated chloroform (CDCl3) and divided into soluble (liquid rubber) and insoluble fractions (solid) that mainly consisted of CB and unreacted rubber. The liquid rubber and solid yields were expressed using eqs 2 and 3, respectively:
| 1 |
| 2 |
| 3 |
where Wvolatile (g), Wliq. rubber (g), Wsolid (g), and Wsample (g) are the weights of volatiles, liquid rubber, solids, and the sample used in the pyrolysis reaction, respectively.
4.5. Characterization of Liquid Rubber by 1H NMR and Gas Permeation Chromatography
A 500-MHz NMR (JNM-ECA500, JEOL Ltd., Japan) was used to determine how much of the rubber skeleton was retained in the liquid rubber. Thermally treated rubber (20 mg) and hexamethyldisilane (HMDS, 0.1 g) were added to CDCl3 (2 g) and dispersed. The dispersed liquid was centrifuged using an ultracentrifuge (CP 80NX, Himac, Eppendorf Group) at a rotation speed of 35,000 rpm and temperature of 10 °C for 1 h. The supernatant solution was collected and filtered through a 0.45-μm mesh polytetrafluoroethylene (PTFE) syringe filter, and the filtrate was used for NMR measurements. HMDS was added as an internal standard, and the weight of the isoprene skeleton in the sample was calculated by determining the integral ratio of the alkenyl group proton of the isoprene skeleton to the methyl group proton of HMDS. This was defined as the isoprene skeleton retention rate (Riso. skel. (%)), which is expressed by eq 4:
| 4 |
where S, N, m, M, and P represent the integrated area, number of hydrogen atoms in the alkenyl group, mass, molecular weight, and purity, respectively, while the subscripts sample and HMDS represent the heat-treated rubber and HMDS, respectively.
The liquid rubber recovery rate was defined as the percentage of the liquid rubber recovered from the initial rubber sample after pyrolysis. Given the initial polymer content of 107.59 phr in a total formulation of 159.65 phr, this rate provided a crucial measure of the degradation process in converting a solid polymer to liquid rubber, as indicated by eq 5.
| 5 |
The isoprene skeleton recovery rate was defined as the efficiency of recovering isoprene units from a polymer after pyrolysis, and considers both the rate of isoprene skeleton retention in the recovered liquid rubber and overall recovery rate of the liquid rubber from the polymer (eq 6).
| 6 |
The molecular weight distribution of the heat-treated rubber was analyzed by GPC. Tetrahydrofuran (THF, 5 mL) was added to the thermally treated rubber (65 mg) which was allowed to dissolve overnight. The dispersed liquid was centrifuged at a rotation speed of 35,000 rpm and temperature of 10 °C for 1 h. The supernatant solution was collected and filtered through a 0.45-μm PTFE syringe filter, and the filtrate was used for GPC measurements (HLC-8320GPC, Tosoh Corporation, Japan). Calibration curves were prepared using polystyrene (PS) standard solutions. The standard solutions were prepared by adding THF (40 mL) to each PS standard (10 mg) and centrifuging under the same conditions used for the sample.
4.6. Solid Characterization via Thermogravimetric Analysis
TGA was conducted on the recovered insoluble fraction (solid) using a TGA instrument (TG/DTA 7220, Hitachi High-Tech Science Co. Ltd., Japan) under both nitrogen and atmospheric conditions. The weight loss observed between 125 and 550 °C under nitrogen was used to determine the amount of organic matter. The weight loss of the CB content was observed between 300 and 550 °C in the presence of air, and the ash content was calculated from the weight of the residue.
Acknowledgments
This study was commissioned by the New Energy and Industrial Technology Development Organization (Grant number: JPNP21021). The authors express their gratitude to Hiroshi Yamashita and Norihisa Fukaya from the National Institute of Advanced Industrial Science and Technology, Japan (AIST) for their valuable contributions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c09456.
Comprehensive proximate and ultimate analyses of the rubber material, supplementary figures and tables illustrating extended experimental results, schematics of nitrogen-substituted electric furnaces used in low-temperature pyrolysis studies, and a flowchart of the product separation (PDF)
Author Contributions
E.A.: data analysis and manuscript drafting. Y.H.: pyrolysis experiments using a nitrogen electric furnace. S.K.: conceptualization, supervision, and manuscript drafting. Y.S., S.T., T.N., M.H., M.H., and T.Y.: GPC, NMR, and TGA studies, as well as supervision and introduction drafting.
The authors declare no competing financial interest.
Supplementary Material
References
- Cummins A.Global ELT Management–A global state of knowledge on regulation, management systems, impacts of recovery and, World Business Council for Sustainable Development, Switzerland, 2019. Available from: https://policycommons.net/artifacts/3130911/global-elt-management/3924143 (accessed Apr 27, 2024).
- Araujo-Morera J.; Verdejo R.; López-Manchado M.-A; Santana M.-H. Sustainable mobility: The route of tires through the circular economy model. Waste Manage. 2021, 126, 309–322. 10.1016/j.wasman.2021.03.025. [DOI] [PubMed] [Google Scholar]
- Antony A.; Provodnikova A.; Kumar S.; Balachandan B.; Sustainable materials in tire industry: A comparative study of Europe and Asian markets. Global J. Business Integr. Secur. 2021. [Google Scholar]
- Bridgestone Corporation Long-term environmental vision (2050 and beyond): Contribute to globally agreed target (towards carbon neutral society), 2021. URL: https://www.bridgestone.com/responsibilities/environment/reduce_co2/ (accessed Feb 16, 2024).
- https://www.bridgestoneamericas.com/en/newsroom/press-releases/2023/bridgestone-michelin-publish-rcb-white-paper# (accessed Feb 19 2024).
- Soprych P.; Czerski G.; Grzywacz P. Studies on the Thermochemical Conversion of Waste Tyre Rubber—A Review. Energies 2023, 17, 14. 10.3390/en17010014. [DOI] [Google Scholar]
- Czajczyńska D.; Czajka K.; Krzyżyńska R.; Jouhara H. Waste tyre pyrolysis–Impact of the process and its products on the environment. Thermal Science and Engineering Progress. 2020, 20, 100690 10.1016/j.tsep.2020.100690. [DOI] [Google Scholar]
- Czajczyńska D.; Krzyżyńska R.; Jouhara H.; Spencer N. Use of pyrolytic gas from waste tires as a fuel: A review. Energy. 2017, 134, 1121–1131. 10.1016/j.energy.2017.05.042. [DOI] [Google Scholar]
- Zhang L.; Zhou B.; Duan P.; Wang F.; Xu Y. Hydrothermal conversion of scrap tire to liquid fuel. Chemical Engineering Journal. 2016, 285, 157–163. 10.1016/j.cej.2015.10.001. [DOI] [Google Scholar]
- Aydın H.; İlkılıç C. Optimization of fuel production from waste vehicle tires by pyrolysis and resembling diesel fuel by various desulfurization methods. Fuel. 2012, 102, 605–612. 10.1016/j.fuel.2012.06.067. [DOI] [Google Scholar]
- Cunliffe A.-M.; Williams P.-T. Composition of oils derived from the batch pyrolysis of tires. Journal of Analytical and Applied Pyrolysis. 1998, 44 (2), 131–152. 10.1016/S0165-2370(97)00085-5. [DOI] [Google Scholar]
- Galvagno S.; Casu S.; Casabianca T.; Calabrese A.; Cornacchia G. Pyrolysis process for the treatment of scrap tires: preliminary experimental results. Waste management. 2002, 22 (8), 917–923. 10.1016/S0956-053X(02)00083-1. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Chen Y.; Chen G.; Sun T.; Zhang M.; Wang Q.; Wu M.; Guo S.; Yang S.; Lei T.; Burra K. G.; et al. Products Distribution and Synergistic Effects Analysis During Co-Pyrolysis of Agricultural Residues and Waste Tire Using Gas Chromatography/Mass Spectrometry. Trans. ASME: J. Energy Resour. Technol. 2023, 145 (8), 081501 10.1115/1.4056940. [DOI] [Google Scholar]
- Leung D.-Y.; Yin X.-L.; Zhao Z.-L.; Xu B.-Y.; Chen Y. Pyrolysis of tire powder: influence of operation variables on the composition and yields of gaseous product. Fuel Process. Technol. 2002, 79 (2), 141–155. 10.1016/S0378-3820(02)00109-1. [DOI] [Google Scholar]
- Miranda M.; Pinto F.; Gulyurtlu I.; Cabrita I. Pyrolysis of rubber tire wastes: A kinetic study. Fuel. 2013, 103, 542–552. 10.1016/j.fuel.2012.06.114. [DOI] [Google Scholar]
- Rahman M.-M.; Yu Y.; Wu H. Method for the Capture and Quantification of Isoprene and the Determination of Oil Yield during Waste Tire Pyrolysis. Energy & Fuels. 2023, 37 (23), 18911–18918. 10.1021/acs.energyfuels.3c03129. [DOI] [Google Scholar]
- Kaminsky W.; Mennerich C.; Zhang Z. Feedstock recycling of synthetic and natural rubber by pyrolysis in a fluidized bed. Journal of Analytical and Applied Pyrolysis. 2009, 85 (1–2), 334–337. 10.1016/j.jaap.2008.11.012. [DOI] [Google Scholar]
- Lopez G.; Olazar M.; Amutio M.; Aguado R.; Bilbao J. Influence of tire formulation on the products of continuous pyrolysis in a conical spouted bed reactor. Energy & Fuels. 2009, 23 (11), 5423–5431. 10.1021/ef900582k. [DOI] [Google Scholar]
- Li Q.; Li F.; Meng A.; Tan Z.; Zhang Y. Thermolysis of scrap tire and rubber in sub/supercritical water. Waste Management. 2018, 71, 311–319. 10.1016/j.wasman.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Sidor J.; Budzyń S.; Czosnek C.; Kozub-Budzyń G.; Tora B. Investigation of the process of grinding of a char coal obtained from tires waste pyrolysis. IOP Conf. Ser.: Mater. Sci. Eng. 2019, 641 (1), 012022 10.1088/1757-899X/641/1/012022. [DOI] [Google Scholar]
- Pazat A.; Barrès C.; Bruno F.; Janin C.; Beyou E. Preparation and properties of elastomer composites containing “graphene”-based fillers: a review. Polymer Reviews. 2018, 58 (3), 403–443. 10.1080/15583724.2017.1403446. [DOI] [Google Scholar]; Jul,
- Yu J.; Xu J.; Li Z.; He W.; Huang J.; Xu J.; Li G. Upgrading pyrolytic carbon-blacks (CBp) from end-of-life tires: Characteristics and modification methodologies. Front. Environ. Sci. Eng. 2020, 14, 19. 10.1007/s11783-019-1198-0. [DOI] [Google Scholar]
- Jiang H.; Shao J.; Hu Q.; Zhu Y.; Cheng W.; Zhang J.; Fan T.; Yu J.; Yang H.; Zhang X.; Chen H. Carbon black production characteristics and mechanisms from pyrolysis of rubbers. Fuel Process. Technol. 2024, 253, 108011 10.1016/j.fuproc.2023.108011. [DOI] [Google Scholar]
- Mkhize N. M.; Van der Gryp P.; Danon B.; Görgens J. F. Effect of temperature and heating rate on limonene production from waste tyre pyrolysis. Journal of analytical and applied pyrolysis. 2016, 120, 314–320. 10.1016/j.jaap.2016.04.019. [DOI] [Google Scholar]
- Qu B.; Liu C.; Wang Y.; Li A.; Qu Y.; Zhang Y. S.; Ji G. Fast pyrolysis kinetics of waste tires and its products studied by a wireless-powered thermo-balance. Journal of Hazardous Materials. 2023, 460, 132494 10.1016/j.jhazmat.2023.132494. [DOI] [PubMed] [Google Scholar]
- Bing W.; Hongbin Z.; Zeng D.; Yuefeng F.; Yu Q.; Rui X. Microwave fast pyrolysis of waste tires: Effect of microwave power on product composition and quality. Journal of Analytical and Applied Pyrolysis 2021, 155, 104979 10.1016/j.jaap.2020.104979. [DOI] [Google Scholar]
- Silva W. O.; Nagar B.; Ellersiek D.; Bondaz L.; Espín J.; Soutrenon M.; Girault H. H. Hydrogen production by waste tire recycling by photo-pyrolysis. Sustainable Energy & Fuels 2023, 7 (24), 5693–5703. 10.1039/D3SE01319G. [DOI] [Google Scholar]
- Kar Y. Catalytic pyrolysis of car tire waste using expanded perlite. Waste Management. 2011, 31 (8), 1772–1782. 10.1016/j.wasman.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Williams P.-T.; Besler S.; Taylor D.-T. The pyrolysis of scrap automotive tires: The influence of temperature and heating rate on product composition. Fuel. 1990, 69 (12), 1474–1482. 10.1016/0016-2361(90)90193-T. [DOI] [Google Scholar]
- Yao E.; Wang Y.; Yang Q.; Zhong H.; Liu T.; Zou H.; Zhang Y. Co-pyrolysis mechanism of natural rubber and cellulose-based on thermogravimetry–gas chromatography and molecular dynamics simulation. Energy & fuels. 2019, 33 (12), 12450–12458. 10.1021/acs.energyfuels.9b02918. [DOI] [Google Scholar]
- Kasataka K.; Kumagai S.; Kameda T.; Saito Y.; Yoshioka T. Enhancement of gasification and liquefaction during fast co-pyrolysis of cedar wood and polyethylene through control of synergistic interactions. Bioresource Technology Reports. 2020, 11, 100431 10.1016/j.biteb.2020.100431. [DOI] [Google Scholar]
- Wu L.; Liu J.; Xu P.; Zhou J.; Yang F. Biomass hydrogen donor assisted microwave pyrolysis of low-rank pulverized coal: Optimization, product upgrade and synergistic mechanism. Waste Management. 2022, 143, 177–185. 10.1016/j.wasman.2022.02.020. [DOI] [PubMed] [Google Scholar]
- Dos Santos M.-T.; Viana I.-S.; Ract J.-N.; Le Roux G.-A. Thermal properties of palm stearin, canola oil, and fully hydrogenated soybean oil blends: coupling experiments and modeling. J. Food Eng. 2016, 185, 17–25. 10.1016/j.jfoodeng.2016.03.029. [DOI] [Google Scholar]
- Nishi T. Heterogeneous Structure of Rubber and Polymer Nanotechnology, Part 1: Research on Heterogeneous Structure of Rubber and Its Development. Journal of the Society of Rubber Industry, Japan. 2019, 92 (1), 38–44. 10.2324/gomu.92.38. [DOI] [Google Scholar]
- Kiuchi Y.; Ito M. Microstructural analysis of carbon gel. Journal of the Society of Rubber Industry, Japan. 1999, 72 (10), 599–605. 10.2324/gomu.72.599. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.